Abstract
Background
Managing severe asthma during the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic is challenging, particularly due to safety concerns regarding the use of systemic corticosteroids and biologics.
Objectives
We sought to determine the association between biologics or systemic corticosteroids use and PCR positivity for SARS-CoV-2 and coronavirus disease 2019 (COVID-19) outcomes among asthmatic patients.
Methods
We used the computerized database of Clalit Health Services, the largest health care provider in Israel, to identify all asthmatic adult patients who underwent PCR testing for SARS-CoV-2, between March 1, 2020, and December 7, 2020. A cohort approach was used to assess the association between biologics use and steroids treatment and COVID-19 severity and 90-day mortality.
Results
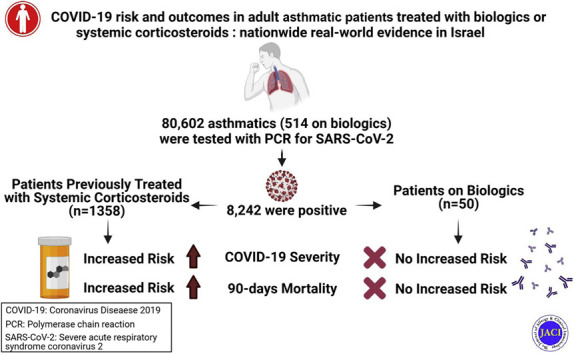
Overall, 8,242 of 80,602 tested asthmatic patients had positive PCR testing result for SARS-CoV-2. Both biologics and systemic corticosteroids were not associated with increased risk of SARS-CoV-2 infection. Multivariate analyses revealed that biologics were not associated with a significantly increased risk of moderate to severe COVID-19, nor with the composite end point of moderate to severe COVID-19 or all-cause mortality within 90 days. Chronic systemic corticosteroid use was associated with significantly increased risk of all tested outcome. Recent (within the previous 120 days) systemic corticosteroid use, but not former use, was significantly associated with increased risk of both moderate to severe COVID-19 and the composite of moderate to severe COVID-19 or all-cause mortality.
Conclusions
Biologics approved for asthma and systemic corticosteroids are not associated with increased risk of SARS-CoV-2 infection. In contrast, systemic corticosteroids are an independent risk factor for worst COVID-19 severity and all-cause mortality. Our findings underscore the risk of recent or current exposure to systemic corticosteroids in asthmatic patients infected with SARS-CoV-2.
Key words: COVID-19, asthma, systemic corticosteroids, biologics
Abbreviations used: COVID-19, Coronavirus disease 2019; HR, Hazard ratio; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; SCS, Systemic corticosteroid
Graphical abstract
Several respiratory viral infections such as rhinovirus or influenza virus are definite risk factors for acute asthma exacerbations.1 , 2 Intriguingly, recent epidemiologic studies suggest that patients with asthma are not at increased risk of exacerbations when infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and that asthmatic patients are not more susceptible to coronavirus disease 2019 (COVID-19) or to the development of severe COVID-19.3, 4, 5, 6, 7 The management of COVID-19 in severe asthma remains challenging, and it is unclear whether patients with severe asthma could be at a higher risk of worst outcomes at least in part because of safety concerns associated with therapies such as biologics or systemic corticosteroids (SCSs).8, 9, 10, 11, 12, 13, 14 Previous studies have suggested that the use of biologics for severe allergic and eosinophilic asthma was not associated with COVID-19 severity,13, 14, 15 but the number of patients included in the studies was small. Furthermore, an association has been suggested between recent SCS use and poor outcomes in asthmatic patients with COVID-19.16
In the current study, we used a computerized database covering half of the Israeli population to evaluate the association between biologics or SCS use and PCR positivity for SARS-CoV-2 and COVID-19 severity and mortality among asthmatic patients.
Methods
Source of data
This study is based on data from the computerized database of Clalit Health Services, which provides inclusive health care for more than half of the Israeli population (∼4.6 million). Health care coverage in Israel is mandatory according to the National Health Insurance Law and is provided by 4 groups akin to not-for-profit health maintenance organizations. All members of the different health maintenance organizations have a similar health insurance plan and similar access to health services, including low medications co-payment. The electronic medical records of Clalit Health Services include data from multiple sources: records of primary care physicians, community specialty clinics, hospitalizations, laboratories, and pharmacies. A registry of chronic diseases diagnoses is compiled from these data sources. Diagnoses are captured in the registry by diagnosis-specific algorithms, using International Classification of Diseases Ninth Revision code reading, text reading, laboratory test results, and disease-specific drug usage. The first record of each data source is kept in the database, and the earliest recorded date, from any source, is used to define the starting date of the diagnosis. Several high-quality, population-based studies have been conducted on the basis of data retrieved from Clalit Health Services database.17 , 18
Since the start of the COVID-19 pandemic, the Israeli Ministry of Health has been collecting all COVID-19–related data and activities to a national database. Among these activities are active surveillance for all laboratory-confirmed SARS-CoV-2 infections, with mandatory daily reporting of PCR results, and active surveillance of COVID-19–associated hospitalizations by daily updates from all hospitals, including daily status definitions during hospitalization. The collected data are transferred daily to the health care providers.
Selection of study population and study design
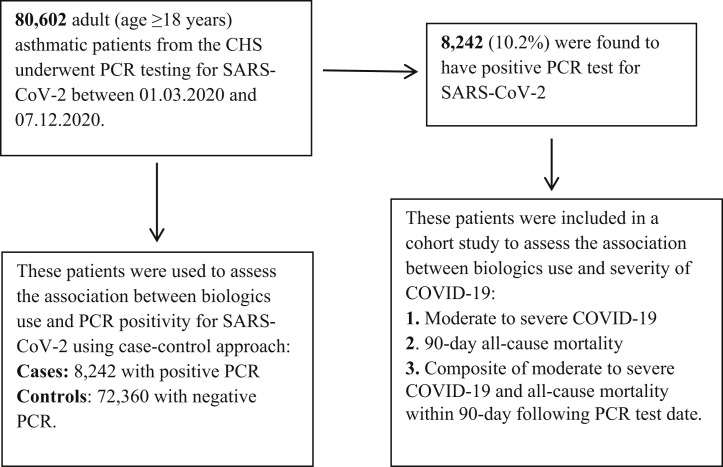
We used the computerized database of Clalit Health Services to retrospectively identify all adult (≥18 years) asthmatic patients (International Classification of Diseases Ninth Revision, 493.xx) who underwent PCR testing for SARS-CoV-2 between March 1, 2020, and December 7, 2020. Identified patients served to assess the association between biologics or SCS use and PCR positivity for SARS-CoV-2, using case-control study approach in which patients with positive PCR test result constituted the cases and patients with negative PCR test result constituted the control group. In addition, a cohort approach was used to assess the association between biologics or SCS use and COVID-19 severity, among patients with positive PCR test result for SARS-CoV-2 (see Fig E1 in this article’s Online Repository at www.jacionline.org).
Fig E1.
Flowchart describing the selection process and evaluation of the study population. CHS, Clalit Health Services.
Study variables
PCR test samples for SARS-CoV-2 are obtained from nasopharyngeal swabs. PCR testing is offered free of charge for all the population without a need for referral.
Biologics or SCS use was determined on the basis of Clalit Health Services pharmacy records using the Anatomical Therapeutic Chemical classification codes. The following biologics approved for asthma were included: benralizumab (anti–IL-5 receptor mAb), dupilumab (anti–IL-4 receptor alpha chain), mepolizumab (anti–IL-5), omalizumab (anti-IgE), and reslizumab (anti–IL-5). A patient was defined as biologics user if he filled at least 1 prescription in the 120 days before the PCR test. The number and the timing of SCS prescriptions filled in the previous year was used to examine the SCS exposure, using different definitions: (1) users versus nonusers in the previous year, (2) number of prescriptions in the previous year (none vs 1 vs 2 vs ≥3 prescriptions), (3) timing of SCS prescriptions filled in the previous year (none vs recent [≤120 days] vs former [120-365 days]), and (4) chronic SCS use defined as purchasing 6 or more prescriptions in the previous year.
The association of biologics or SCS was assessed with the following outcomes: (1) PCR positivity among asthmatic patients who were tested for SARS-CoV-2, (2) 90-day all-cause mortality, (3) moderate to severe COVID-19 as defined on the Israeli Ministry of Health’s guidelines, which are in accordance with the World Health Organization definitions,19 and (4) composite of moderate to severe COVID-19 or 90-day all-cause mortality.
In addition, for each patient the following baseline data were retrieved from the computerized database of the Clalit Health Services: demographic and other descriptive variables, smoking status (smoker, never smoker), and presence of selected chronic medical conditions including diabetes, hypertension, obesity, and ischemic heart disease.
Statistical methods
Statistical analyses were performed using IBM SPSS Statistics 24.0 (IBM, New York, NY). For all analyses, P less than .05 for the 2-tailed tests was considered statistically significant. Continuous variables were summarized with means and SD, and categorical variables were summarized with counts and proportions. Comparisons of baseline characteristics between patients with positive PCR test result and patients with negative PCR test result, and between patients on biologics and patients without biologics, were performed using the chi-square test for categorical variables and using the independent samples student t test for continuous variables.
Logistic regression models were used to examine the association between biologics or SCS use and PCR positivity among asthmatic patients who underwent PCR testing for SARS-CoV-2. Cox proportional hazard regression models were used to assess the association between recent biologics use or SCS use, among patients with positive PCR test result, and each of the following outcomes: (1) moderate to severe COVID-19, (2) 90-day all-cause mortality, and (3) the composite of moderate to severe COVID-19 or 90-day all-cause mortality. To examine the independent association of biologics and SCS use, the multivariate regression models were adjusted for age, sex, ethnicity, diabetes, hypertension, ischemic heart disease, obesity, and smoking. Time to event was defined as the time that elapsed from the date of positive PCR test result (date of cohort entry) until the first occurrence of study outcomes, death, or end of follow-up, whichever came first. Multivariate Cox regression models were used to depict the adjusted cumulative incidence curves of the study outcomes. An interaction between biologics and SCS use was tested by including an interaction factor of both variables into the multivariate Cox regression model.
Results
Overall, 80,602 adult asthmatic patients (age ≥18 years) underwent PCR testing for SARS-CoV-2 between March 1, 2020, and December 7, 2020. For patients with at least 1 positive PCR test result, the first dated positive test was selected. For patients with consistently negative PCR test results, the first dated test was selected. Of them, 8242 (10.2%) were found to be positive for SARS-CoV-2 (Fig E1). The distribution of demographic and clinical baseline characteristics by PCR status (positive vs negative) is presented in Table E1 in this article’s Online Repository at www.jacionline.org. Asthmatic patients who tested positive for SARS-CoV-2 were more likely to be younger, female, of an Arabic origin, and with significantly higher prevalence of obesity and diabetes, as compared with those who tested negative. No significant differences in SCS and biologics use were found between the 2 groups. Only 464 (0.6%) patients with negative PCR test result and 50 (0.6%) patients with positive PCR test result were biologics users. The distribution of the different types of biologics was similar in both groups, with omalizumab being the most frequently used, followed by mepolizumab, benralizumab, dupilumab, and reslizumab (Table E1).
Biologics and SCS use was not associated with an increased risk of infection with SARS-CoV-2 in multivariate analyses (for biologics use: adjusted odds ratio, 0.99; 95% CI, 0.73-1.33; for SCS use: adjusted odds ratio, 0.96; 95% CI, 0.90-1.03), as compared with no use (see Tables E2 and Table E3 in this article’s Online Repository at www.jacionline.org).
The second phase of the analysis was restricted to the 8242 adult asthmatic patients with positive PCR test result for SARS-CoV-2 and aimed to assess the association of biologics or SCS use and outcomes. The baseline characteristics of biologics users (n = 50) and nonusers (n = 8192) are reported in Table I . Patients on biologics were older, mainly female with a significantly higher prevalence of diabetes, obesity, and hypertension, and had a significantly higher use of SCSs (Table I). Blood eosinophils count was available in 90% of biologics users. Among anti–IL-5 users, the mean absolute eosinophils count was 42 ± 39/μL.
Table I.
Baseline characteristics of the study population
| Variable | Biologics use∗ (n = 50) | No biologics use (n = 8192) | P value |
|---|---|---|---|
| Age (y) | <.001 | ||
| Mean ± SD | 55.3 ± 14.4 | 43.2 ± 20.5 | |
| Median (interquartile range) | 56.5 (46.0-65.4) | 37.3 (25.3-59.0) | |
| Female sex | 35 (70.0) | 4308 (52.6) | .014 |
| Ethnicity | .549 | ||
| Jews | 35 (70.0) | 6041 (73.7) | |
| Arabs | 15 (30.0) | 2151 (26.3) | |
| Diabetes | 14 (28.0) | 1303 (15.9) | .020 |
| Hypertension | 16 (32.0) | 1697 (20.7) | .050 |
| Obesity | 25 (50) | 2653 (32.4) | .008 |
| Ischemic heart disease | 7 (14.0) | 620 (7.6) | .087 |
| Smoking (ever) | 14 (28.0) | 2170 (26.5) | .809 |
| Steroids use in the previous year | <.001 | ||
| Yes | 34 (68.0) | 1324 (16.2) | |
| No | 16 (32.0) | 6868 (83.8) | |
| Steroids use in the previous year | <.001 | ||
| No | 16 (32.0) | 6868 (83.8) | |
| Recent (≤120 d) | 21 (42.0) | 569 (6.9) | |
| Former (120-365 d) | 13 (26.0) | 755 (9.2) | |
| Chronic steroids treatment (≥6 prescriptions in the previous year) | <.001 | ||
| Yes | 10 (20.0) | 152 (1.9) | |
| No | 40 (80.0) | 8040 (98.1) | |
| Steroid use in the previous year (no. of filled prescriptions) | <.001 | ||
| 0 prescription | 16 (32.0) | 6868 (83.8) | |
| 1 prescription | 6 (12.0) | 721 (8.8) | |
| 2 prescriptions | 11 (22.0) | 265 (3.2) | |
| ≥3 prescriptions | 17 (34.0) | 338 (4.1) | |
| Biologics use∗ | |||
| Omalizumab | 24 (48.0) | ||
| Benralizumab | 7 (14.0) | ||
| Mepolizumab | 13 (26.0) | ||
| Reslizumab | 3 (6.0) | ||
| Dupilumab | 3 (6.0) |
Biologics use was defined as the documentation of filling at least 1 prescription of omalizumab, benralizumab, mepolizumab, reslizumab, or dupilumab in the 120 d before the positive PCR test result date.
Multivariate analyses revealed that biologics use was not associated with a significantly increased risk of moderate to severe COVID-19 (adjusted hazard ratio [HR], 1.28; 95% CI, 0.60-2.73; Table II ), nor with the composite end point of moderate to severe COVID-19 or all-cause mortality within 90 days (adjusted HR, 1.42; 95% CI, 0.70-2.88; Table III ), or all-cause mortality within 90 days (adjusted HR, 1.04; 95% CI, 0.14-7.59; see Table E4 in this article’s Online Repository at www.jacionline.org). The adjusted cumulative incidence curves of the composite end point are depicted, by biologics use status, in Fig E2, A, in this article’s Online Repository at www.jacionline.org. No significant interaction was found between biologics use and SCS use on their association with the study outcomes. The adjusted HRs for the association of biologics use and study outcomes among SCS users were as follows: 1.15 (95% CI, 0.47-2.81) for moderate to severe COVID-19 (P for interaction = .531), 0.92 (95% CI, 0.12-6.85) for all-cause mortality within 90 days (P for interaction = .956), and 1.32 (95% CI, 0.58-2.99) for the composite end point of moderate to severe COVID-19 or all-cause mortality within 90 days (P for interaction = .726). In a restricted analysis to biologics users, compared with anti-IgE (omalizumab), the adjusted HR of anti–IL-5 (benralizumab, mepolizumab, reslizumab) was 2.45 (95% CI, 0.32-18.87) for the association with moderate to severe COVID-19, and 1.77 (95% CI, 0.29-10.9) for the composite end point of moderate to severe COVID-19 or all-cause mortality within 90 days.
Table II.
Multivariate analysis for the association between biologics use and COVID-19 severity (moderate-severe) among adult asthmatic patients with positive PCR test result for SARS-CoV-2 (n = 8242)
| Variable | Adjusted∗ HR (95% CI) | P value |
|---|---|---|
| Age (for each year increase) | 1.053 (1.050-1.060) | <.001 |
| Sex | ||
| Males | 1.23 (1.02-1.48) | .033 |
| Females | Reference | |
| Ethnicity | ||
| Jews | Reference | |
| Arabs | 1.67 (1.38-2.01) | <.001 |
| Diabetes | 1.30 (1.07-1.57) | .009 |
| Hypertension | 1.36 (1.07-1.73) | .012 |
| Obesity | 1.40 (1.16-1.70) | .001 |
| IHD | 1.33 (1.09-1.63) | .006 |
| Smoking (ever) | 1.09 (0.90-1.32) | .381 |
| Steroids use in the previous year (no. of filled prescriptions) | ||
| None | Reference | |
| 1 prescription | 1.06 (0.81-1.39) | .655 |
| 2 prescriptions | 1.54 (1.10-2.15) | .012 |
| ≥3 prescriptions | 2.09 (1.65-2.65) | <.001 |
| Biologics use (at least 1 prescription filled in the previous 120 d) | ||
| None | Reference | |
| Yes | 1.28 (0.60-2.73) | .519 |
IHD, Ischemic heart disease.
Adjusted for age, sex, ethnicity, diabetes, hypertension, IHD, obesity, smoking, and steroids and biologics use.
Table III.
Multivariate analysis for the association between biologics use and the composite of moderate to severe COVID-19 or all-cause mortality within 90 d following PCR date among adult asthmatic patients with positive PCR test result for SARS-CoV-2 (n = 8242)
| Variable | Adjusted∗ HR (95% CI) | P value |
|---|---|---|
| Age (for each year increase) | 1.057 (1.050-1.063) | <.001 |
| Sex | ||
| Males | 1.23 (1.03-1.48) | .023 |
| Females | Reference | |
| Ethnicity | ||
| Jews | Reference | |
| Arabs | 1.56 (1.30-1.88) | <.001 |
| Diabetes | 1.36 (1.13-1.63) | .001 |
| Hypertension | 1.35 (1.07-1.70) | .010 |
| Obesity | 1.36 (1.13-1.63) | .001 |
| IHD | 1.37 (1.13-1.67) | .001 |
| Smoking (ever) | 1.05 (0.87-1.26) | .590 |
| Steroids use in the previous year (no. of filled prescriptions) | ||
| None | Reference | |
| 1 prescription | 1.01 (0.78-1.30) | .955 |
| 2 prescriptions | 1.39 (1.00-1.93) | .049 |
| ≥3 prescriptions | 1.92 (1.52-2.41) | <.001 |
| Biologics use (at least 1 prescription filled in the previous 120 d) | ||
| None | Reference | |
| Yes | 1.42 (0.70-2.88) | .332 |
IHD, Ischemic heart disease.
Adjusted for age, sex, ethnicity, diabetes, hypertension, IHD, obesity, smoking, and steroids and biologics use.
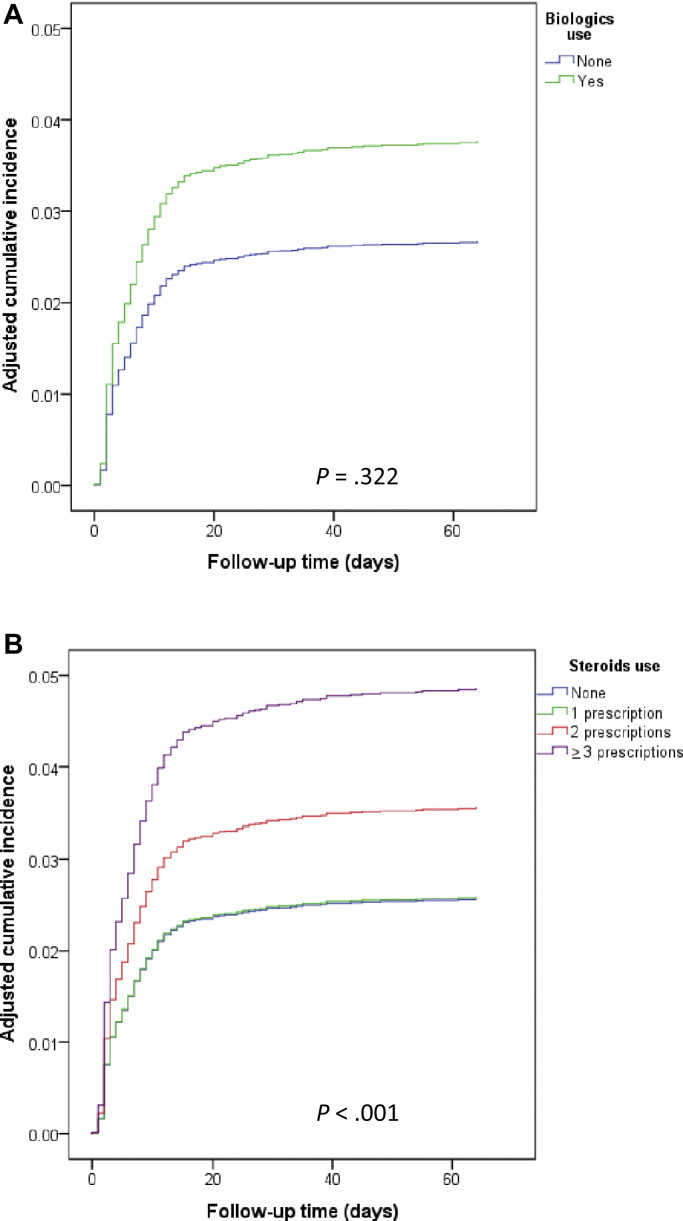
Fig E2.
Adjusted∗ cumulative incidence curves, (A) for biologics use and (B) for steroids use, of the composite of moderate to severe COVID-19 and all-cause mortality within 90 days following PCR test date among adult asthmatic patients with positive PCR test result for SARS-CoV-2 (n = 8242).
The number of filled SCS prescriptions in the previous year was associated with a statistically significant dose-response increase in the risk of tested outcomes (Tables II and III; see Table E4; Fig 1 ). The adjusted cumulative incidence curves of the composite end point are depicted, by the number of filled SCS prescriptions, in Fig E2, B. Chronic SCS use was associated with significantly increased risk of all tested outcomes: adjusted HR 2.19 (95% CI, 1.63-2.94) for moderate to severe COVID-19, HR 2.00 (1.18-3.40) for all-cause mortality, and HR 2.07 (95% CI, 1.55-2.76) for the composite of moderate to severe COVID-19 or all-cause mortality (Tables IV and V ; see Table E5, Table E6, Table E7 in this article’s Online Repository at www.jacionline.org ). Recent (within the previous 120 days) SCS use, but not former use, was significantly associated with increased risk of both moderate to severe COVID-19, HR 1.92 (95% CI, 1.55-2.38), and the composite of moderate to severe COVID-19 or all-cause mortality, HR 1.76 (95% CI, 1.43-2.17) (Tables IV and V; see Tables E8 and E9 in this article’s Online Repository at www.jacionline.org).
Fig 1.
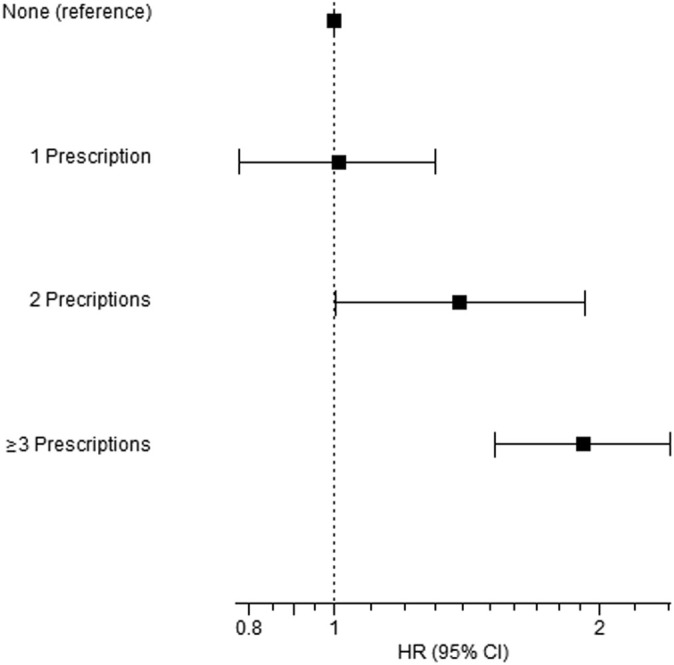
Adjusted∗ HRs (95% CI) for the association between the number of filled steroid prescriptions in the previous years and the composite of moderate to severe COVID-19 or all-cause mortality within 90 days following PCR date among adult asthmatic patients with positive PCR for SARS-CoV-2 (n = 8242). ∗Adjusted for age, sex, ethnicity, diabetes, hypertension, ischemic heart disease, obesity, smoking, and biologics use.
Table IV.
Multivariate∗ analysis for the association between steroids use and COVID-19 severity (moderate-severe) among adult asthmatic patients with positive PCR test result for SARS-CoV-2, using different specifications of steroid use (n = 8242)
| Variable | Adjusted∗ HR (95% CI) | P value |
|---|---|---|
| Steroids use in the previous year | ||
| None | Reference | |
| Yes | 1.49 (1.24-1.79) | <.001 |
| Steroids use in the previous year | ||
| None | Reference | |
| Recent (≤120 d) | 1.92 (1.55-2.38) | <.001 |
| Former (120-365 d) | 1.16 (0.87-1.43) | .390 |
| Chronic steroids treatment (≥6 prescriptions in the previous year) | ||
| None | Reference | |
| Yes | 2.19 (1.63-2.94) | <.001 |
| Steroids use in the previous year (no. of filled prescriptions) | ||
| None | Reference | |
| 1 prescription | 1.06 (0.81-1.39) | .655 |
| 2 prescriptions | 1.54 (1.10-2.15) | .012 |
| ≥3 prescriptions | 2.09 (1.65-2.65) | <.001 |
Table V.
Multivariate∗ analysis for the association between steroids use and the composite of moderate to severe COVID-19 or all-cause mortality within 90 d following PCR date among adult asthmatic patients with positive PCR test result for SARS-CoV-2, using different specifications of steroid use (n = 8242)
| Variable | Adjusted∗ HR (95% CI) | P value |
|---|---|---|
| Steroids use in the previous year | ||
| None | Reference | |
| Yes | 1.38 (1.16-1.64) | <.001 |
| Steroids use in the previous year | ||
| None | Reference | |
| Recent (≤120 d) | 1.76 (1.43-2.17) | <.001 |
| Former (120-365 d) | 1.04 (0.82-1.33) | .734 |
| Chronic steroids treatment (≥6 prescriptions in the previous year) | ||
| None | Reference | |
| Yes | 2.07 (1.55-2.76) | <.001 |
| Steroids use in the previous year (no. of filled prescriptions) | ||
| None | Reference | |
| 1 prescription | 1.01 (0.78-1.30) | .955 |
| 2 prescriptions | 1.39 (1.001-1.93) | .049 |
| ≥3 prescriptions | 1.92 (1.52-2.41) | <.001 |
Detailed multivariable models are shown in Tables III, Table E7, Table E9, and E11.
Adjusted for age, sex, ethnicity, diabetes, hypertension, ischemic heart disease, obesity, smoking, and biologics use.
The independent association of the other covariates with the examined outcomes is presented in Tables II and III, and in Tables E4 and Table E10, Table E11, Table E6, Table E7, Table E8, Table E9 in this article’s Online Repository at www.jacionline.org. In general, male sex, Arabic origin, diabetes, hypertension, obesity, and ischemic heart disease were all significantly associated with increased risk of moderate to severe COVID-19 and the composite of COVID-19 severity or all-cause mortality (Tables II and III and Table E10, Table E11, Table E6, Table E7, Table E8, Table E9).
Discussion
Whether biologic therapies approved for severe allergic and eosinophilic asthma are risk factors for poor COVID-19 outcomes is still debated.20 Indeed, eosinophils have an active role in the innate immunity against respiratory viral infections, and previous studies have reported that eosinopenia was associated with COVID-19 severity.21, 22, 23, 24, 25, 26 In theory, type 2 characteristic of allergic and/or eosinophilic asthma has opposite effects on SARS-CoV-2 receptors. On one hand, it enhances transmembrane serine protease 2 expression, but on the other hand, it reduces angiotensin-converting enzyme 2 epithelial expression, thus making it difficult to predict how this could influence SARS-CoV-2 infection and subsequent COVID-19 severity and outcomes.24
The role of inhaled corticosteroids and SCSs in risk of SARS-CoV-2 infection and COVID-19 severity is not clear. Schultze et al27 using the OpenSAFELY platform reported an increased risk of death from COVID-19 among people with asthma prescribed high-dose inhaled corticosteroids; however, various sensitivity analyses indicated that this increased mortality risk could be explained by unmeasured confounders. In contrast, a large multicenter prospective cohort study by Bloom et al28 reported that patients with severe asthma were significantly more likely than those with no underlying respiratory condition to receive critical care and ventilatory support even after adjusting for severity on admission, age, and comorbidities. Interestingly, the use of inhaled corticosteroids in patients aged 50 years and older within 2 weeks of admission was associated with decreased mortality. Other studies did not provide clear evidence of increased risk of COVID-19 severity, hospitalization, or mortality in asthmatic patients.29, 30, 31 The data on SCSs in asthma and COVID-19 are scarce. The results of the Randomized Evaluation of Covid-19 Therapy (RECOVERY) trial32 showed that oral or intravenous administration of dexamethasone significantly reduces 28-day mortality among patients admitted to hospital with COVID-19 receiving invasive mechanical ventilation or oxygen, whereas Williamson et al16 using the OpenSAFELY platform to examine factors associated with COVID-19–related death reported that severe asthma defined by recent SCS use was associated with increased mortality. In a smaller group of 15 asthmatic patients who received SCSs (13 of them in the 2 weeks before COVID-19 diagnosis), Chhiba et al31 reported that SCS use was not associated with COVID-19–related hospitalization.
Our large nationwide study of 80,602 adult asthmatic patients shows that patients treated with biologics or SCS are not at a higher risk of SARS-CoV-2 infection. In addition, there was no significant risk of moderate to severe COVID-19 and mortality in severe asthmatic patients treated with biologics, when compared with those not receiving biologics. In contrast, SCS use was an independent risk factor for worst COVID-19 severity and all-cause mortality. Therefore, our findings underscore the risk of recent or chronic SCS use in asthmatic patients infected with SARS-CoV-2.
Two recent studies had suggested a higher susceptibility of SARS-CoV-2 infection in asthmatic patients, when compared with the general population, especially in those with severe asthma on biologic therapy.15 , 32 In contrast, data from the Belgian Severe Asthma Registry reported a relatively low incidence of COVID -19 in patients with severe asthma and no association with a higher risk of SARS-CoV-2 infection.13 Moreover, asthmatic patients were not overrepresented in a cohort of consecutive patients with severe pneumonia due to SARS-CoV-2 infection who required hospitalization during the Spring 2020 outbreak in Paris.14 Our study shows that severe asthmatic patients treated with biologic therapies for severe allergic and eosinophilic asthma are not more likely to be infected with SARS-CoV-2, as compared with asthmatic patients who were not treated with biologics. Importantly, in our study, all cases of COVID-19 were diagnosed by positive PCR test result for SARS-CoV-2, whereas others included patients with COVID-19 diagnosis based on either PCR or clinical and/or radiological parameters.13 , 14
COVID-19 severity and mortality are highly dependent on age and comorbidities, whereas asthma was not found to be an independent risk factor for severe COVID-19 or worst outcome.3, 4, 5, 6, 7 Interestingly, although viral respiratory tract infections are an important cause of asthma exacerbations, it appears that SARS-CoV-2 infection is not associated with asthma exacerbation.13 , 14 In the Belgian Severe Asthma Registry, biologic therapy for severe allergic or eosinophilic asthma was not associated with COVID-19 severity.13 In addition, Izquierdo et al15 reported that COVID-19–related hospital admission was low among asthmatic patients treated by biologics in a large Spanish database. Interestingly, a retrospective study by Ferastraoaru et al32 has suggested that preexisting eosinophil count greater than or equal to 150/μL was protective from future COVID-19–associated hospitalization, and that development of eosinophil count greater than or equal to 150 /μL during hospitalization was associated with decreased mortality in a cohort of patients with asthma with COVID-19. However, this may not apply to patients treated with biologics; indeed, in our study, a significantly lower eosinophils count (42 ± 39/μL) was not found to be associated with increased COVID-19 severity and mortality in patients treated with anti–IL-5. Although our data are reassuring, additional analyses of a higher number of patients on biologics are needed to definitively conclude on the lack of risk associated with the use of biologics in severe asthmatic patients infected with SARS-CoV-2.
In contrast, our study underscores the risk of recent or current exposure to SCSs in asthmatic patients infected with SARS-CoV-2. Indeed, SCS use decreases innate and acquired immunity and predisposes to infection. Therefore, it is recommended to avoid chronic or repeated SCS use whenever possible, and to prescribe the lowest possible dose of SCS in the subgroup of severe asthmatic patients requiring long-term treatment with oral corticosteroids.33 A major finding of our study is that SCS treatment whether chronic or recent (defined as within 120 days before being infected with SARS-CoV-2) is associated with increased COVID-19 severity and 90-day mortality. Furthermore, COVID-19 severity and 90-day mortality increased in a dose-response manner with the number of SCS prescriptions in the previous year (Fig 1 and Fig E2, B). Conversely, SCS use did not increase the likelihood of being infected with SARS-CoV-2. Other studies have also reported that recent SCS use in asthmatic patients was associated with increased COVID-19 mortality.16 Therefore, severe asthmatic patients treated with chronic or recurrent SCS therapy to treat and/or prevent exacerbations and improve asthma control are at increased risk for severe COVID-19 and worst outcomes. Our results emphasize the need for optimized management of asthmatic patients to achieve asthma control and avoid whenever possible the need for chronic or recurrent use of SCSs. In patients with severe uncontrolled asthma requiring chronic or recurrent use of SCSs, steroid-sparing approaches are desirable alternatives. These include interventional and medical options, such as biologics in eligible allergic and/or eosinophilic patients. Our data suggest that these treatments may help in achieving asthma control and by inference prevent worst outcomes when patients are infected with SARS-CoV-2.
In summary, biological treatment for severe allergic and eosinophilic asthma does not increase the risk of being infected with SARS-CoV-2 or COVID-19 severity. Chronic or recurrent use of SCSs before SARS-CoV-2 infection is a major risk factor of poor outcomes and worst survival in asthmatic patients. We conclude that treating physicians should follow carefully current guidelines34 to achieve asthma control and reduce the need for chronic or recurrent SCS therapy.
Clinical implication.
Our results emphasize the need for optimized management of asthma to achieve disease control and avoid whenever possible the need for chronic or recurrent use of SCSs.
Footnotes
Disclosure of potential conflict of interest: Y. Adir reports personal fees from Teva and Sanofi and grants and personal fees from Glaxo Smith Kline (GSK) and Astra Zeneca, outside the submitted work. M. Humbert reports personal fees from Acceleron, GSK, Merck, Novartis, Astra Zeneca, and Sanofi and grants and personal fees from Actelion and Bayer, outside the submitted work. W. Saliba has nothing to disclose.
Appendix
Table E1.
Baseline characteristics of the study population
| Variable | PCR for SARS-CoV-2 status |
P value | |
|---|---|---|---|
| Positive (n = 8,242) | Negative (n = 72,360) | ||
| Age (y) | <.001 | ||
| Mean ± SD | 43.3 ± 20.4 | 44.9 ± 20.4 | |
| Median (interquartile range) | 37.5 (25.4-59.1) | 39.1 (27.7-60.6) | |
| Female sex | 3,899 (47.3) | 32,384 (45.4) | .001 |
| Ethnicity | <.001 | ||
| Jews | 6,076 (73.7) | 60,907 (84.2) | |
| Arabs | 2,166 (26.3) | 11,453 (15.8) | |
| Diabetes | 1,316 (16.0) | 10,062 (13.9) | <.001 |
| Hypertension | 1,709 (20.7) | 15,044 (20.8) | .907 |
| Obesity | 2,673 (32.4) | 20,846 (28.8) | <.001 |
| Ischemic heart disease | 627 (7.6) | 6,051 (8.4) | .018 |
| Steroids use in the previous year | .082 | ||
| Yes | 1,358 (16.5) | 12,474 (17.2) | |
| No | 6,884 (83.5) | 59,886 (82.8) | |
| Steroids use in the previous year | .074 | ||
| No | 6,884 (83.5) | 59,886 (82.8) | |
| Recent (≤120 d) | 590 (7.2) | 5,687 (7.9) | |
| Former (120-365 d) | 768 (9.3) | 6,787 (9.4) | |
| Chronic steroids treatment (≥6 prescriptions in the previous year) | .645 | ||
| Yes | 162 (2.0) | 1,477 (2.0) | |
| No | 8,080 (98.0) | 70,883 (98.0) | |
| Steroid use in the previous year (no. of filled prescriptions) | .222 | ||
| 0 prescription | 6,884 (83.5) | 59,886 (82.8) | |
| 1 prescription | 727 (8.8) | 6,730 (9.3) | |
| 2 prescriptions | 276 (3.3) | 2,376 (3.3) | |
| ≥3 prescriptions | 355 (4.3) | 3,368 (4.7) | |
| Biologics use∗ | .881 | ||
| None | 8,192 (99.4) | 71,896 (99.4) | |
| Omalizumab | 24 (0.3) | 200 (0.3) | |
| Benralizumab | 7 (0.1) | 71 (0.1) | |
| Mepolizumab | 13 (0.2) | 122 (0.2) | |
| Reslizumab | 3 (0.04) | 17 (0.02) | |
| Dupilumab | 3 (0.04) | 54 (0.1) | |
Biologics use was defined as the documentation of filling at least 1 prescription of omalizumab, benralizumab, mepolizumab, reslizumab, or dupilumab in the 120 d before the PCR date.
Table E2.
Multivariate analysis for the association between biologics use and PCR positivity among adult asthmatic patients who underwent PCR testing for SARS-CoV-2 (n = 80,602)
| Variable | Adjusted odds ratio (95% CI) | P value |
|---|---|---|
| Age (for each year increase) | 0.997 (0.995-0.998) | <.001 |
| Sex | ||
| Males | 1.14 (1.08-1.19) | <.001 |
| Females | Reference | |
| Ethnicity | ||
| Jews | Reference | |
| Arabs | 1.74 (1.64-1.83) | <.001 |
| Diabetes | 1.27 (1.18-1.38) | <.001 |
| Hypertension | 1.06 (0.98-1.15) | .150 |
| Obesity | 1.16 (1.10-1.22) | <.001 |
| Ischemic heart disease | 0.93 (0.84-1.02) | .139 |
| Steroids use in the previous year (no. of filled prescriptions) | ||
| None | Reference | |
| 1 prescription | 0.94 (0.87-1.02) | .163 |
| 2 prescriptions | 1.04 (0.91-1.18) | .564 |
| ≥3 prescriptions | 0.95 (0.84-1.06) | .343 |
| Biologics use (at least 1 prescription filled in the previous 120 d) | ||
| None | Reference | |
| Yes | 0.99 (0.73-1.33) | .936 |
Table E3.
Multivariate analysis for the association between steroid use and PCR positivity among adult asthmatic patients who underwent PCR testing for SARS-CoV-2 (n = 80,602)
| Variable | Adjusted odds ratio (95% CI) | P value |
|---|---|---|
| Steroids use in the previous year | ||
| None | Reference | |
| Yes | 0.96 (0.90-1.03) | .234 |
| Steroids use in the previous year | ||
| None | Reference | |
| Recent (≤120 d) | 0.92 (0.84-1.01) | .084 |
| Former (120-365 d) | 0.99 (0.92-1.08) | .862 |
| Chronic steroids treatment (≥6 prescriptions in the previous year) | ||
| None | Reference | |
| Yes | 1.00 (0.85-1.19) | .967 |
| Steroids use in the previous year (no. of filled prescription) | ||
| None | Reference | |
| 1 prescription | 0.94 (0.87-1.02) | .163 |
| 2 prescriptions | 1.04 (0.91-1.18) | .564 |
| ≥3 prescriptions | 0.94 (0.84-1.06) | .343 |
Presented are 4 models that include different classification of steroids treatment.
Table E4.
Multivariate analysis for the association between biologics use and all-cause mortality within 90 d following PCR test date among adult asthmatic patients with positive PCR test result for SARS-CoV-2 (n = 8242)
| Variable | Adjusted∗ HR (95% CI) | P value |
|---|---|---|
| Age (for each year increase) | 1.11 (1.09-1.12) | <.001 |
| Sex | ||
| Males | 1.63 (1.14-2.33) | .008 |
| Females | Reference | |
| Ethnicity | ||
| Jews | Reference | |
| Arabs | 1.07 (0.71-1.63) | .723 |
| Diabetes | 1.73 (1.22-2.47) | .002 |
| Hypertension | 1.44 (0.87-2.37) | .154 |
| Obesity | 1.12 (0.79-1.59) | .514 |
| IHD | 1.85 (1.31-2.60) | <.001 |
| Smoking (ever) | 0.74 (0.50-1.09) | .124 |
| Steroids use in the previous year (no. of filled prescriptions) | ||
| None | Reference | |
| 1 prescription | 0.91 (0.53-1.56) | .733 |
| 2 prescriptions | 0.86 (0.42-1.78) | .694 |
| ≥3 prescriptions | 1.64 (1.05-2.59) | .032 |
| Biologics use (at least 1 prescription filled in the previous 120 d) | ||
| None | Reference | |
| Yes | 1.04 (0.14-7.59) | .969 |
IHD, Ischemic heart disease.
Adjusted for age, sex, ethnicity, diabetes, hypertension, IHD, obesity, smoking, and steroids and biologics use.
Table E5.
Multivariate∗ analysis for the association between steroids use and all-cause mortality within 90 d following PCR test date among adult asthmatic patients with positive PCR test result for SARS-CoV-2, using different specifications of steroid use (n = 8242)
| Variable | Adjusted∗ HR (95% CI) | P value |
|---|---|---|
| Steroids use in the previous year | ||
| None | Reference | |
| Yes | 1.16 (0.81-1.64) | .418 |
| Steroids use in the previous year | ||
| None | Reference | |
| Recent (≤120 d) | 1.40 (0.92-2.15) | .120 |
| Former (120-365 d) | 0.93 (0.57-1.51) | .769 |
| Chronic steroids treatment (≥6 prescriptions in the previous year) | ||
| None | Reference | |
| Yes | 2.00 (1.18-3.40) | .010 |
| Steroids use in the previous year (no. of filled prescriptions) | ||
| None | Reference | |
| 1 prescription | 0.91 (0.53-1.56) | .733 |
| 2 prescriptions | 0.86 (0.42-1.78) | .694 |
| ≥3 prescriptions | 1.64 (1.04-2.59) | .032 |
Adjusted for age, sex, ethnicity, diabetes, hypertension, ischemic heart disease, obesity, smoking, and biologics use.
Table E6.
Multivariate∗ analysis for the association between chronic steroids use and COVID-19 severity (moderate-severe) among adult asthmatic patients with positive PCR test result for SARS-CoV-2 (n = 8242)
| Variable | Adjusted∗ HR (95% CI) | P value |
|---|---|---|
| Age (for each year increase) | 1.054 (1.047-1.060) | <.001 |
| Sex | ||
| Males | 1.23 (1.02-1.49) | .029 |
| Females | Reference | |
| Ethnicity | ||
| Jews | Reference | |
| Arabs | 1.72 (1.43-2.08) | <.001 |
| Diabetes | 1.32 (1.08-1.60) | .005 |
| Hypertension | 1.37 (1.07-1.74) | .011 |
| Obesity | 1.41 (1.16-1.71) | <.001 |
| IHD | 1.32 (1.07-1.61) | .008 |
| Smoking (ever) | 0.90 (0.74-1.09) | .285 |
| Chronic steroids treatment (≥6 prescriptions in the previous year) | ||
| None | Reference | |
| Yes | 2.19 (1.63-2.94) | <.001 |
| Biologics use (at least 1 prescription filled in the previous 120 d) | ||
| None | Reference | |
| Yes | 1.39 (0.65-2.97) | .391 |
IHD, Ischemic heart disease.
Adjusted for age, sex, ethnicity, diabetes, hypertension, IHD, obesity, smoking, and steroids and biologics use.
Table E7.
Multivariate∗ analysis for the association between chronic steroids use and the composite of moderate to severe COVID-19 or all-cause mortality within 90 d following PCR date among adult asthmatic patients with positive PCR test result for SARS-CoV-2 (n = 8242)
| Variable | Adjusted∗ HR (95% CI) | P value |
|---|---|---|
| Age (for each year increase) | 1.057 (1.051) | <.001 |
| Sex | ||
| Males | 1.24 (1.03-1.48) | .020 |
| Females | Reference | |
| Ethnicity | ||
| Jews | Reference | |
| Arabs | 1.60 (1.33-1.92) | <.001 |
| Diabetes | 1.38 (1.14-1.66) | .001 |
| Hypertension | 1.36 (1.08-1.72) | .009 |
| Obesity | 1.37 (1.14-1.64) | .001 |
| IHD | 1.36 (1.12-1.65) | .002 |
| Smoking (ever) | 0.93 (0.78-1.12) | .475 |
| Chronic steroids treatment (≥6 prescriptions in the previous year) | ||
| None | Reference | |
| Yes | 2.07 (1.55-2.76) | <.001 |
| Biologics use (at least 1 prescription filled in the previous 120 d) | ||
| None | Reference | |
| Yes | 1.50 (0.74-3.05) | .259 |
IHD, Ischemic heart disease.
Adjusted for age, sex, ethnicity, diabetes, hypertension, IHD, obesity, smoking, and steroids and biologics use.
Table E8.
Multivariate∗ analysis for the association between steroids use in the prior year (none/recent/former) and COVID-19 severity (moderate-severe) among adult asthmatic patients with positive PCR test result for SARS-CoV-2 (n = 8242)
| Variable | Adjusted∗ HR (95% CI) | P value |
|---|---|---|
| Age (for each year increase) | 1.053 (1.047-1.060) | <.001 |
| Sex | ||
| Males | 1.23 (1.02-1.48) | .031 |
| Females | Reference | |
| Ethnicity | ||
| Jews | Reference | |
| Arabs | 1.66 (1.37-2.01) | <.001 |
| Diabetes | 1.30 (1.07-1.58) | .009 |
| Hypertension | 1.36 (1.07-1.73) | .013 |
| Obesity | 1.40 (1.15-1.70) | .001 |
| IHD | 1.34 (1.09-1.64) | .005 |
| Smoking (ever) | 0.90 (0.75-1.09) | .295 |
| Steroids use in the previous year | ||
| None | Reference | |
| Recent (≤120 d) | 1.92 (1.55-2.38) | <.001 |
| Former (120-365 d) | 1.16 (0.87-1.43) | .390 |
| Biologics use (at least 1 prescription filled in the previous 120 d) | ||
| None | Reference | |
| Yes | 1.46 (0.67-3.09) | .325 |
IHD, Ischemic heart disease.
Adjusted for age, sex, ethnicity, diabetes, hypertension, IHD, obesity, smoking, and steroids and biologics use.
Table E9.
Multivariate∗ analysis for the association between steroids use in the prior year (none/recent/former) and the composite of moderate to severe COVID-19 or all-cause mortality within 90 d following PCR date among adult asthmatic patients with positive PCR test result for SARS-CoV-2 (n = 8242)
| Variable | Adjusted∗ HR (95% CI) | P value |
|---|---|---|
| Age (for each year increase) | 1.057 (1.050-1.063) | <.001 |
| Sex | ||
| Males | 1.23 (1.03-1.48) | .022 |
| Females | Reference | |
| Ethnicity | ||
| Jews | Reference | |
| Arabs | 1.55 (1.29-1.87) | <.001 |
| Diabetes | 1.36 (1.13-1.63) | .001 |
| Hypertension | 1.35 (1.07-1.70) | .011 |
| Obesity | 1.35 (1.13-1.63) | .001 |
| IHD | 1.38 (1.14-1.68) | .001 |
| Smoking (ever) | 0.94 (0.80-1.12) | .480 |
| Steroids use in the previous year | ||
| None | Reference | |
| Recent (≤120 d) | 1.76 (1.43-2.17) | <.001 |
| Former (120-365 d) | 1.04 (0.82-1.33) | .734 |
| Biologics use (at least 1 prescription filled in the previous 120 d) | ||
| None | Reference | |
| Yes | 1.61 (0.80-3.25) | .185 |
IHD, Ischemic heart disease.
Adjusted for age, sex, ethnicity, diabetes, hypertension, IHD, obesity, smoking, and steroids and biologics use.
Table E10.
Multivariate∗ analysis for the association between steroids use in the prior year (yes/no) and COVID-19 severity (moderate-severe) among adult asthmatic patients with positive PCR test result for SARS-CoV-2 (n = 8242)
| Variable | Adjusted∗ HR (95% CI) | P value |
|---|---|---|
| Age (for each year increase) | 1.054 (1.047-1.060) | <.001 |
| Sex | ||
| Males | 1.25 (1.03-1.50) | .021 |
| Females | Reference | |
| Ethnicity | ||
| Jews | Reference | |
| Arabs | 1.66 (1.38-2.01) | <.001 |
| Diabetes | 1.30 (1.07-1.57) | .009 |
| Hypertension | 1.37 (1.08-1.75) | .010 |
| Obesity | 1.40 (1.16-1.70) | .001 |
| IHD | 1.31 (1.07-1.61) | .009 |
| Smoking (ever) | .247 | |
| Steroids use in the previous year | ||
| None | Reference | |
| Yes | 1.49 (1.24-1.79) | <.001 |
| Biologics use (at least 1 prescription filled in the previous 120 d) | ||
| None | Reference | |
| Yes | 1.50 (0.71-3.18) | .290 |
IHD, Ischemic heart disease.
Adjusted for age, sex, ethnicity, diabetes, hypertension, IHD, obesity, smoking, and steroids and biologics use.
Table E11.
Multivariate∗ analysis for the association between steroids use in the previous year (yes/no) and the composite of moderate to severe COVID-19 or all-cause mortality within 90 d following PCR date among adult asthmatic patients with positive PCR test result for SARS-CoV-2 (n = 8242)
| Variable | Adjusted∗ HR (95% CI) | P value |
|---|---|---|
| Age (for each year increase) | 1.057 (1.051-1.063) | <.001 |
| Sex | ||
| Males | 1.25 (1.04-1.50) | .015 |
| Females | Reference | |
| Ethnicity | ||
| Jews | Reference | |
| Arabs | 1.56 (1.30-1.87) | <.001 |
| Diabetes | 1.36 (1.13-1.63) | .001 |
| Hypertension | 1.36 (1.08-1.72) | .008 |
| Obesity | 1.36 (1.13-1.63) | .001 |
| IHD | 1.36 (1.12-1.65) | .002 |
| Smoking (ever) | 0.93 (0.77-1.11) | .418 |
| Steroids use in the previous year | ||
| None | Reference | |
| Yes | 1.38 (1.16-1.64) | <.001 |
| Biologics use (at least 1 prescription filled in the previous 120 d) | ||
| None | Reference | |
| Yes | 1.65 (0.82-3.33) | .164 |
IHD, Ischemic heart disease.
Adjusted for age, sex, ethnicity, diabetes, hypertension, IHD, obesity, smoking, and steroids and biologics use.
References
- 1.Satia I., Cusack R., Greene J.M., O’Byrne P.M., Killian K.J., Johnston N. Prevalence and contribution of respiratory viruses in the community to rates of emergency department visits and hospitalizations with respiratory tract infections, chronic obstructive pulmonary disease and asthma. PLoS One. 2020;15 doi: 10.1371/journal.pone.0228544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Busse W.W., Lemanske R.F., Jr., Gern J.E. Role of viral respiratory infections in asthma and asthma exacerbations. Lancet. 2010;376:826–834. doi: 10.1016/S0140-6736(10)61380-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q., et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;23:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 5.COVID View. 2020.. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/pdf/covidview-05-01-2020.pdf Available at:
- 6.Heffler E., Detoraki A., Contoli M., Papi A., Paoletti G., Malipiero G., et al. COVID-19 in Severe Asthma Network in Italy (SANI) patients: clinical features, impact of comorbidities and treatments. Allergy. 2021;76:887–892. doi: 10.1111/all.14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maes T., Bracke K., Brusselle G.G. Reply to: inhaled corticosteroids and COVID-19. Am J Respir Crit Care Med. 2020;202:900–902. doi: 10.1164/rccm.202006-2129LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi H.G., Wee J.H., Kim S.Y., Kim J.-H., Kim H.I., Park J.-Y., et al. Association between asthma and clinical mortality/morbidity in COVID-19 patients using clinical epidemiologic data from Korean Disease Control & Prevention. Allergy. 2021;76:921–924. doi: 10.1111/all.14675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu Z., Hasegawa K., Ma B., Fujiogi M., Camargo C.A., Liang L. Association of asthma and its genetic predisposition with the risk of severe COVID-19. J Allergy Clin Immunol. 2020;146:327–329. doi: 10.1016/j.jaci.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green I., Merzon E., Vinker S., Golan-cohen A., Magen E. COVID-19 susceptibility in bronchial asthma. J Allergy Clin Immunol Pract. 2020;9:684–692. doi: 10.1016/j.jaip.2020.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lieberman-Cribbin W., Rapp J., Alpert N., Tuminello S., Taioli E. The impact of asthma on mortality in patients with COVID-19. Chest. 2020;194:2019–2020. doi: 10.1016/j.chest.2020.05.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanon S., Brusselle G., Deschampheleire M., Louis R., Michils A., Peché R., et al. COVID-19 and biologics in severe asthma: data from the Belgian Severe Asthma Registry. Eur Respir J. 2020;56 doi: 10.1183/13993003.02857-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beurnier A., Jutant E.M., Jevnikar M., Boucly A., Pichon J., Preda M., et al. Characteristics and outcomes of asthmatic patients with COVID-19 pneumonia who require hospitalisation. Eur Respir J. 2020;56 doi: 10.1183/13993003.01875-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Izquierdo J.L., Almonacid C., González Y., Del Rio-Bermudez C., Ancochea J., Cárdenas R., et al. The impact of COVID-19 on patients with asthma. Eur Respir J. 2021;57 doi: 10.1183/13993003.03142-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williamson E.J., Walker A.J., Bhaskaran K., Bacon S., Bates C., Morton C.E., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Preis M., Hirsch J., Kotler A., Zoabi A., Stein N., Rennert G., et al. Factor XI deficiency is associated with lower risk for cardiovascular and venous thromboembolism events. Blood. 2017;129:1210–1215. doi: 10.1182/blood-2016-09-742262. [DOI] [PubMed] [Google Scholar]
- 18.Saliba W., Barnett-Griness O., Gronich N., Molad J., Naftali J., Rennert G., et al. Association of diabetes and glycated hemoglobin with the risk of intracerebral hemorrhage: a population-based cohort study. Diabetes Care. 2019;42:682–688. doi: 10.2337/dc18-2472. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization Clinical management of COVID-19. 2020.. https://www.who.int/emergencies/diseases/novel-coronavirus-2019?gclid=EAIaIQobChMIo_XM_pik6wIVyrHtCh2-NA61EAAYASAAEgKZRPD_BwE Available at: [PubMed]
- 20.Morais-Almeida M., Aguiar R., Martin B., Ansotegui I.J., Ebisawa M., Arruda L.K., et al. COVID-19, asthma, and biological therapies: what we need to know. World Allergy Organ J. 2020;13 doi: 10.1016/j.waojou.2020.100126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang J.J., Dong X., Cao Y.Y., Yuan Y.D., Yang Y.B., Yan Y.Q., et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75:1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 22.Lindsley A.W., Schwartz J.T., Rothenberg M.E. Eosinophil responses during COVID-19 infections and coronavirus vaccination. J Allergy Clin Immunol. 2020;46:1–7. doi: 10.1016/j.jaci.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Du Y., Tu L., Zhu P., Mu M., Wang R., Yang P., et al. Clinical features of 85 fatal cases of COVID-19 from Wuhan: a retrospective observational study. Am J Respir Crit Care Med. 2020;201:1372–1379. doi: 10.1164/rccm.202003-0543OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sajuthi S.P., DeFord P., Li Y., Jackson N.D., Montgomery M.T., Everman J.L., et al. Type 2 and interferon inflammation regulate SARS-CoV-2 entry factor expression in the airway epithelium. Nat Commun. 2020;11:5139. doi: 10.1038/s41467-020-18781-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A., et al. COVID-19 Lombardy ICU Network. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region. Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu J., Ji P., Pang J., Zhong Z., Li H., He C., et al. Clinical characteristics of 3,062 COVID-19 patients: a meta-analysis. J Med Virol. 2020;92:1902–1914. doi: 10.1002/jmv.25884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schultze A., Walker A.J., MacKenna, Morton C.E., Bhaskaran K., Brown J.P., et al. Risk of COVID-19-related death among patients with chronic obstructive pulmonary disease or asthma prescribed inhaled corticosteroids: an observational cohort study using the OpenSAFELY platform. Lancet Respir Med. 2020;8:1106–1120. doi: 10.1016/S2213-2600(20)30415-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bloom CI, Drake TM, Docherty AB, Lipworth BJ, Johnston SL, Nguyen-Van-Tam JS, et al. Risk of adverse outcomes in patients with underlying respiratory conditions admitted to hospital with COVID-19: a national, multicentre prospective cohort study using the ISARIC WHO Clinical Characterisation Protocol UK [published online ahead of print Match 4, 2021]. Lancet Respir Med. 10.1016/S2213-2600(21)00013-8. [DOI] [PMC free article] [PubMed]
- 29.Terry PD, Heidel RE, Dhand R. Asthma in adult patients with COVID-19: prevalence and risk of severe disease [published online ahead of print January 25, 2021]. Am J Respir Crit Care Med. https://doi.org/10.1164/rccm.202008-3266OC. [DOI] [PMC free article] [PubMed]
- 30.Lovinsky-Desir S., Deshpande D.R., De A., Murray L., Stingone J.A., Chan A., et al. Asthma among hospitalized patients with COVID-19 and related outcomes. J Allergy Clin Immunol. 2020;146:1027–1034. doi: 10.1016/j.jaci.2020.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chhiba K.D., Patel G.B., Vu T.H.T., Chen M.M., Guo A., Kudlaty E., et al. Prevalence and characterization of asthma in hospitalized and nonhospitalized patients with COVID-19. J Allergy Clin Immunol. 2020;146:307–314. doi: 10.1016/j.jaci.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferastraoaru D., Hudes G., Jerschow E., Jariwala S., Karagic M., de Vos G., et al. Eosinophilia in asthma patients is protective against severe COVID-19 illness. J Allergy Clin Immunol Pract. 2021;9:1152–1162. doi: 10.1016/j.jaip.2020.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi Y.J., Park J.-Y., Lee H.S., Suh J., Song J.Y., Byun M.K., et al. Effect of asthma and asthma medication on the prognosis of patients with COVID-19. Eur Respir J. 2021;57 doi: 10.1183/13993003.02226-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Global INitiative for Asthma Global strategy for asthma management and prevention, 2020. www.ginasthma.org Available at: