Abstract
Background
Past survey studies document that people strongly prefer Covid-19 vaccines developed domestically over those developed abroad. Available evidence suggests that this preference for domestic vaccines over foreign ones may stem from prejudice against foreign countries, but identifying prejudice-based vaccine preferences is difficult because people also draw inferences about the quality of vaccines based on country of origin. We exploit a unique opportunity provided by the announcement of a viable vaccine by a bi-national venture, BioNTech and Pfizer, to examine the effect of such prejudice on vaccination intentions while controlling for beliefs about the vaccine quality.
Methods
We implemented a survey experiment in Germany and the United States (n = 582, 661 respectively) a few days after the BioNTech/Pfizer announcement of a viable vaccine. We randomized the identified company (and country) responsible for the vaccine development between BioNTech (Germany) and Pfizer (U.S.) and asked respondents when they would take said vaccine.
Results
In either the German and U.S. samples, we find little evidence that a country of origin of the vaccine makes a difference in when respondents intend to get vaccinated. We also see no evidence that those with a general animus toward the other foreign country would be more biased against a foreign vaccine.
Conclusion
Our findings suggest that prejudice against foreign countries may be less of a concern for vaccine hesitancy and that its effect may be highly context specific.
Keywords: Covid-19, Vaccine hesitancy, Survey research, Health behavior, Vaccine uptake
1. Introduction
Studies show little progress in global immunization efforts despite a surge in research and resources (de Figueiredo et al., 2016). Even long-established vaccines, like those for measles, mumps, and rubella, saw their adoption drop below herd immunity levels in many areas around the globe in the 2010s (The Lancet Child and Adolescent Health, 2019). Indeed, vaccine hesitancy—the delay or refusal of vaccination despite availability—was named one of the top ten threats to global health by the World Health Organization (WHO) in 2019. In the case of the Covid-19 pandemic, a growing body of research suggests that vaccine hesitancy will present serious obstacles to achieving community immunity in many countries (Lazarus et al., 2020; Lunz Trujillo and Motta, 2020; Neumann-Böhme et al., 2020).
People choose not to vaccinate for many reasons, primarily for efficacy and safety concerns (Callaghan et al., 2021; Lane et al., 2018). Presumably because people often lack information about, or the ability to evaluate, the efficacy and safety of vaccines, they rely on external cues. Previous work suggests that people take endorsements by families, physicians, and political leaders as cues when deciding to vaccinate (e.g. Determann et al., 2014; Determann et al., 2016; Kreps et al., 2020). Another such cue may be a vaccine's country of origin—that is, in which country the vaccine was developed. A large multi-country survey by YouGov in December 2020 shows that people strongly prefer Covid-19 vaccines developed domestically to those developed abroad (Smith, 2021). For example, strikingly, Germans are 28 percentage-points more favorable toward a German vaccine compared to the second highest (Canada). For the United Kingdom, the corresponding gap is 10 percentage-points, United States 12, Australia 20, China 59, Singapore 24, and India 19.
Why do people prefer domestic vaccines to foreign ones? The literatures on international marketing and agricultural economics provide two intertwined explanations (e.g. Elliott and Cameron, 1994; Lusk et al., 2006). First, people may use country of origin as a signal of vaccine quality. This quality-based account can explain why, for example, every country in the YouGov survey (with an exception of China) prefer German- and UK-developed vaccines to Chinese- or Russian-developed vaccines. However, this explanation alone is unsatisfactory in accounting for the sizable home bias found in the YouGov's survey—i.e. people strictly and universally prefer domestic vaccines to those developed by foreign countries, including those whose products have reputation for high product quality. Perhaps more puzzling is that Chinese rate an Iranian-developed vaccine more positively than an American-developed one, which is just the opposite of what the quality-based explanation would predict. The second explanation is that country of origin triggers people's affinity for their home and/or animosity toward other countries, which, in turn, influences their vaccination intentions. We refer to this as state bias, and it is the focus of our study. This explanation can account for the strong home bias across countries as well as for Chinese preferences for Iranian vaccines over American vaccines.
In this paper, we examine state bias in vaccine preferences. In the context of the Covid-19 pandemic, the question about state bias is particularly salient. As soon as the pandemic hit, many projects began developing vaccines in several countries. However, with successful vaccines being developed in just a few countries, most people in the world will be receiving a vaccine developed abroad. If state bias indeed effects greater vaccine hesitancy, it could greatly delay global control of the pandemic. Further, state bias may be at play in governments' and companies’ decisions to manufacture, purchase, approve, and distribute vaccines (Abbas, 2020; Burki, 2020; Ghosal and SaaliQ, 2021). That way, state bias might have already or could further complicate fierce disputes between countries over vaccine distributions that we have witnessed (Colchester and Norman, 2021; Mayes, 2021). Indeed, Covid-19 has been politicized both domestically (Hart et al., 2020; Wadman, 2020) and internationally (De Vries et al., 2020), which should increase the salience of any state bias. As politicians insert nationalism into healthcare debates, it follows that citizens would take that issue frame into consideration.
However, studying state bias is difficult because country of origin would lead people to draw inferences about vaccine quality. A survey such as YouGov's that simply changes country names of origin may evoke a slew of beliefs about vaccine quality, which can confound the estimates of state bias. One strategy to control for those beliefs is to create a scenario where there is no need for people to draw inferences about vaccine quality from country of origin. Researchers could achieve this by designing an experiment in which participants receive information about the quality of vaccines and therefore see little reason to draw inferences about vaccine quality. Employing a conjoint design, several recent studies have asked survey-takers to rate hypothetical vaccines that are characterized by key features of a vaccine, such as its efficacy, side effects, and technology (e.g. mRNA, adenovirus), as well as country of origin (Kreps et al., 2020; Motta, 2021; Pogue et al., 2020). Even when information about vaccine quality is given, these studies consistently find evidence for state bias among Americans—that is, Americans prefer domestic vaccines to those developed by China, Russia, and even the United Kingdom and European countries.
We propose an alternative, complementary approach to shed new light on the question of state bias. Researchers can look for a real-world situation where an available vaccine can plausibly be framed as developed either domestically or abroad. By asking survey-takers to evaluate the vaccine (randomly) labeled as domestic or foreign, we could examine whether country-of-origin affects their vaccine preferences. If the real-world vaccine was widely known and therefore people had already developed some beliefs about its quality, we could interpret the country-of-origin effect as state bias. That is, the use of a (salient) real-world vaccine allows us to exploit beliefs about vaccine quality that are naturally formed in a real-world environment rather than experimentally controlling for beliefs about vaccine quality. This approach has an additional advantage in that state bias is studied in a more realistic environment and therefore external validity of the study is improved.
In this study, we leverage the first announcement of a viable vaccine against Covid-19 by BioNTech and Pfizer on November 9, 2020. This announcement is serendipitous for us for three reasons. First, the vaccine is a product of a bi-national venture by BioNTech (Germany) and Pfizer (U.S.). As such, we could portray this vaccine credibly and correctly as either of German or U.S. origin to survey-takers. Second, the surprisingly high efficacy of the vaccine was widely covered by the media, so we can safely assume that many of survey-takers have formed beliefs about the vaccine quality. Third, both Germany and the United States have strong global reputations for their pharmaceutical industries and regulations as well as product quality more generally. This will further help us implicitly control for inferences about vaccine quality based on country of origin. In our experiment, we presented survey-takers with a short news story that mimics the kicker of actual news stories about the vaccine results. In it, we randomized the identified company responsible for the vaccine between BioNTech (Germany) and Pfizer (U.S.) and asked people when (if ever) they would take said vaccine. The survey was fielded in Germany and the United States just a few days after the announcement among people recruited via Prolific, an Oxford-based company for opt-in survey research (Palan and Schitter, 2018).
We examined three aspects of state bias through our survey experiment. First, we studied whether people prefer to take a vaccine portrayed as domestic to one depicted as foreign. We find no evidence that Germans or Americans care about either country of origin label. In the German and U.S. samples, there is no statistically significant effect when comparing a “domestic” origin to a “foreign” origin. Second, if state bias plays an important role, we would expect that people with a less favorable disposition toward the foreign country would be more biased against the foreign vaccine compared to the domestic one. Therefore, we examined whether warmer general feelings toward the other foreign country moderate the country-of-origin effect. We find no evidence consistent with this expectation. Third and last, we suspected that the country-of-origin treatment effect may be moderated by a person's demand for a vaccine. We asked people about their fear of Covid-19 and examined the associated treatment moderation. While more fearful people tend to say they would take the vaccine sooner, the answers differ little based on the origin of the vaccine. There is no evidence for such a treatment moderation effect.
By leveraging a real-world successful vaccine that could be framed both as domestic and foreign and the fact that both Germany and the United States have strong quality reputations, we were able to isolate state bias from quality inferences as the beliefs over vaccine quality occurred naturally. Our results consistently show that country of origin is not salient to either Americans or Germans when deciding when to get vaccinated and that this result is not an artifact from the heterogenous treatment effects we studied. These findings suggest that when a vaccine is developed by reputable countries like the United States and Germany and becomes available in the real-world setting, state bias does not seem particularly worrying.
However, the serendipity of the BioNTech/Pfizer announcement comes with a limit to the generalizability of our results. It would be premature to draw too strong a conclusion that state bias is absent more generally across vaccines, countries, and contexts. For example, the announcement garnered a tremendous amount of media attention not only because the vaccine was the first, highly successful one, but also because the results of the trial were much better than expected. We speculate that the salience of the vaccine news would provoke or reduce certain emotions (e.g. anxiety, enthusiasm), which might have systematically affected how people seek and perceive information and played a role in reducing state bias (Brader, 2005; Marcus and MacKuen, 1993; Marcus et al., 2000). This implies that when a vaccine is not as successful as BioNTech/Pfizer vaccine or when other viable vaccines are available, people may use country of origin as a cue to decide whether to get vaccinated or which vaccine to take. In addition, our case involves a pair of countries with strong cultural, business, and political ties. Thus, we need to be cautious about the extent to which our findings extend beyond pairs that do not share these characteristics.
With these caveats in mind, our results are useful and important for suggesting that state bias may be highly context dependent (Kennedy, 2020; MacDonald, 2015) and may be overstated in previous studies. Prior work suggests Americans are more willing to take a vaccine of U.S. origin than one of U.K., European, China, and Russia origin, even when some of the key vaccine attributes are accounted for (Kreps et al., 2020; Motta, 2021; Pogue et al., 2020). Our evidence suggests people may just not pay much attention to country of origin in real-world situations, especially when a successful vaccine is the first viable vaccine and/or is developed by countries like the United States and Germany with reputation of quality products. In the discussion section, we discuss in detail what may account for the discrepancy between our results and these previous findings.
In the next section, we will introduce our sample and experimental design. Then, we will present the results and discuss their implications and limitations. We conclude by drawing lessons for the Covid-19 pandemic and future vaccines.
2. Methods
2.1. Experimental design
The BioNTech/Pfizer announcement of a viable Covid-19 vaccine, the first of its kind, which the two companies jointly produced, created a unique opportunity to test for state bias in vaccination intentions. Three features of this case are particularly important. First, the results were widely and immediately covered in the media. This allows us to assume that people were aware of the vaccine quality and had formed some beliefs about the vaccine quality. Second, the vaccine was co-developed by two companies from separate countries, Germany (BioNTech) and the United States (Pfizer). This allows us to frame the vaccine as both domestic and foreign for Germans and Americans while naturally controlling for beliefs about the vaccine quality based on the ample media coverage of the vaccine efficacy. Third, the countries that produced the vaccine (Germany and the United States) have similar reputations for high quality (pharmaceutical, medical) products. This further ensures that beliefs about vaccine quality is held comparable when we frame the country name of origin as either German or the United States. In short, these three features allow us to better isolate state bias from inferences about the vaccine quality based on origin labels and thus better attribute any country-of-origin effect to state bias.
We designed our survey experiment with a news story about the results from the Phase-3 trial by BioNTech and Pfizer at its core. Specifically, we introduced the results of a 90%-effectiveness of the vaccine at preventing infections becoming severe. By varying the identified company, CEO, and country name that appear in the news story, we arrive at two scenarios that frame the vaccine as either German or American. The (hypothetical) news story reads as follows, with respondents always seeing either all first italic phrases or all seconds.
Early Data of COVID-19 Vaccine Shows It Is 90% Effective
In a major boost to vaccine development, [BioNTech/Pfizer] released early study results on Monday, November 10th, indicating that their vaccine prevented more than 90% of infections with the virus that causes COVID-19. The company based in [Germany/the United States] said they hoped to have authorization to roll out the vaccine as soon as December. The authorization must be granted by the Food and Drug Administration, which regulates vaccines in the United States. If granted, [BioNTech/Pfizer] estimates they can produce up to 50 million doses this year, enough to protect 25 million people, and then provide up to 1.3 billion doses in 2021.
“Today is a great day for science and humanity. The first set of results from our Phase 3 COVID-19 vaccine trial provides the initial evidence of our vaccine's ability to prevent COVID-19,” said Dr. [Ugur Sahin/Albert Bourla], chief executive at [BioNTech/Pfizer]. “With today's news, we are a significant step closer to providing people around the world with a much-needed breakthrough to help bring an end to this global health crisis.
The above text in English is the version shown to survey-takers in the United States. We also prepared one translated into German for our German survey-takers, in which the authorization must be granted by the Paul-Ehrlich-Institut, the German federal medical agency. We opted for the Paul-Ehrlich-Institut instead of the European Medicines Agency (EMA) as the entity giving approval. While the German government had pledged to go the European route to obtain vaccine approval, we did not want to trigger sentiments about the European Union. The approval process via the EMA became only a widespread (negative) news story in Germany about a month after our experiment was fielded, however.
2.2. Outcome and additional variables
To measure the willingness to take the vaccine (if at all), we asked survey-takers to indicate how long they would wait before taking it. We asked: “[s]uppose the vaccine against Coronavirus by [BioNTech (Germany)/Pfizer (United States)] that was described in the news story on the previous page was available to you. When would you take it, if at all?” Answer options were “Within a month”, “Within 2–3 months”, “Within 4–6 months”, “Within 7–12 months”, “After at least a year”, and “Never”. An outcome question measuring intent to take a vaccine this way allows us to measure both a delay in acceptance and refusal of vaccination, not just refusal, more richly and naturally than a binary question of whether one would take it or not can.
We are also interested in treatment moderation—the extent to which attributes of people may influence the effects of different country-of-origin frames. First, we examine whether fear of Covid-19 has any impact on treatment effects. We measure a survey-taker's fear of Covid-19 by asking, prior to the experiment, “[h]ow afraid are you that Covid-19 will harm you and people close to you?” with five-point Likert scale responses from “not at all” to “very” in five steps. For simplicity, we treat the variable as linear and rescale it to the unit interval for our statistical analysis.
Second, we also investigate whether a feeling toward the other country has any impact on treatment effects. We obtain a feeling thermometer score for the other country (i.e. Germany for U.S. survey-takers and the United States for German survey-takers) to measure someone's stance to other country from where the vaccine could come. Survey-takers could select any integer between 0 (“cold”) and 100 (“warm”) via a slider, which we also rescale into the unit interval.
We collected a series of pre-treatment demographic and attitudinal variables that we include in our statistical models. These not only help improve the precision of our estimates, but help model the treatment moderation effects, which themselves are not identified by via randomization (Bansak, 2021). These additional variables include a survey-taker's gender, age, education, ideology, and general attitudes about vaccines. The German models include a variable about which religion one belongs to, which matters for attitudes toward Americans (Allen et al., 2020), and the U.S. models whether one claims German ancestry to capture broader family ties. Out of space concerns, we report details on the exact question wording, response options, and coding in Section A in the appendix. The pre-treatment variables, some of which touch upon Covid-19 and vaccination, are followed by a distraction task before the experimental manipulation was given.
2.3. Sample
The survey was implemented in Germany and the United States, with sample sizes of 582 and 661 respectively, from November 13–16, 2020, starting just four days after the BioNTech/Pfizer announcement of their Covid-19 vaccine. Participants were recruited via Prolific, a survey platform out of Oxford University that enables researchers advertise surveys which participants can opt to take in exchange for commensurate payment. Prolific is similar to other online recruitment platforms like Amazon's MTurk but is designed specifically for the purpose of academic survey research. Studies have shown that participants on Prolific are more diverse and more naive and give better quality sample and response than other online platforms such as Amazon's MTurk (Palan and Schitter, 2018; Peer et al., 2017).
Recruitment of participants via Prolific was not set up to be representative of the two countries' adult populations. Our own samples' demographics differ in unsurprising ways from the target populations' as we show in Figure A.3 in the appendix: our survey takers are younger and contain more left/liberal, less right/conservative, and more male people. These are well-known patterns in samples recruited via online recruitment platforms, like Amazon's MTurk and Prolific (Huff and Tingley, 2015). However, extensive previous validation efforts show that experimental results using opt-in survey-takers almost always replicate the qualitative result of benchmark studies based on more traditional, nationally representative samples (significant and same sign of effect; insignificant) (Berinsky et al., 2012; Mullinix et al., 2015). Further, some studies show that reweighting opt-in survey data to match its demographic moments to the target population's can recover even the magnitudes of treatment effects (Hainmueller et al., 2015). Therefore, we rely on entropy balancing to reweight our samples (Hainmueller, 2012). Entropy balancing is an approach that obtains (non-zero) weights for the survey data that make means of pre-specified, demographic variable match those of a target dataset. At the same time, it punishes large weights (in the entropy sense) to reduce subsequence model dependence. See also Zhao and Percival (2016). As re-balancing demographics, we use age, gender, and indicator variables for whether one self-identifies as left/liberal or right/conservative. The target datasets are the Cooperative Congressional Election Study 2018 (U.S.) and the (pooled) 2020 January, May, and September waves of the German Longitudinal Election Study. We make use of the trimmed-weights approach offered by entropy balancing. In all analyses, we use the re-weighted data.
2.4. Statistical model
The outcome of interest is the time until one is willing to take the shown vaccine. We use an interval-censored regression model with a Weibull distribution function to link our treatments and moderators to the (latent) continuous duration until one is willing to take a vaccine. Although our outcome variable is ordinal, we know the cut-points in days. For example, when someone selects “Within 2–3 months” (“Within a month”), we know the expressed choice is between 30 and 90 days (0–30 days). These cut-points allow us to model the time until one is willing to take the vaccine. Specifically, let Y i be a discrete variable corresponding to the ordinal response levels, A l,i and A u,i be the lower/upper censoring points (in days) implied by Y i. The probability of person i choosing level k is:
with being the cumulative density function (CDF) of the Weibull distribution. The shape and scale of the CDF are parameterized via a (exponentiated) linear predictor (x i β) and a scalar parameter (η), respectively. For “After at least a year”, we set A l,i and A u,i to 360 and 720 days, respectively. For “Never” to 720 and + ∞. All estimates are based on summaries of 10,000 non-parametric bootstrap draws. (In results available from the authors upon request, we replicate the main models using an ordered probit, which leads to the same qualitative results.)
3. Results
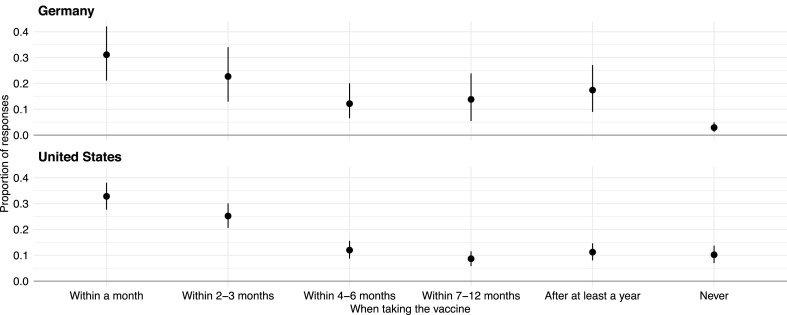
First, we show the descriptive statistic of answers pooled across the “domestic” and “foreign” treatments. Fig. 1 gives the proportion of each answer with 95% confidence intervals using 2000 non-parametric bootstrap draws. Consider the top panel for German survey-takers, which shows that roughly 30% of respondents would vaccinate within a month of the vaccine's availability and 23% within 2–3 months. About 3% of respondents would never take the vaccine. The overall distribution for American survey-takers looks very similar to that of Germans although about 10% of American respondents would never take the vaccine. It is worth noting that the proportion of those would categorically reject the vaccine in our American sample is lower than those found in typical surveys. For example, a poll by the Kaiser Family Foundation in late November-early December 2020 finds that 15% of respondents would definitely not get a vaccine and 12% would probably not even if it were available for free and deemed safe (Hamel et al., 2020).
Fig. 1.
Proportions of answers for when one would take the vaccine (if ever). The range represent 95% confidence intervals obtained via 2000 non-parametric bootstrap draws. Entropy balancing weights were used.
Table 1 shows the estimates of the country-of-origin treatment effects from the interval-censored regressions for Germany and the United States. First, consider the first column (“All”) for each country. The coefficients on a vaccine of “domestic” origin are positive (Germany) and negative (U.S.), giving different mean response tendencies for the country-of-origin treatment based on the survey country. However, both confidence intervals for the coefficients contain zero, which means that we cannot reject the null hypothesis of no country-of-origin effect. Both coefficients are not precisely estimated as the widths of the confidence intervals are several times the mean estimates. Therefore, examining the treatment moderation for heterogeneous effects is particularly called for, which we will turn to later in this section.
Table 1.
Simple treatment effects, Weibull interval censored model. Each estimate shows results for a different model by country and sample. The first number gives the mean estimate, the range below the 95% confidence interval. Uncertainty comes from 2000 non-parametric bootstrap replications.
| Germany | United States | |||||
|---|---|---|---|---|---|---|
| All | Take at all | Drop ‘Never’ | All | Take at all | Drop ‘Never’ | |
| Vaccine, domestic | 0.18 | 0.30 | 0.28 | −0.12 | −0.05 | 0.05 |
| (-0.46, 0.77) | (-1.06, 1.86) | (-0.19, 0.75) | (-0.43, 0.18) | (-0.58, 0.50) | (-0.25, 0.34) | |
| Age | −2.32 | 4.81 | −0.46 | −0.74 | 0.43 | 0.17 |
| (-4.80, −0.73) | (0.56, 9.25) | (-2.28, 1.19) | (-1.67, 0.14) | (-1.19, 2.26) | (-0.64, 0.95) | |
| Gender, male | −0.58 | 0.81 | −0.30 | −0.83 | 0.35 | −0.66 |
| (-1.21, 0.02) | (-0.52, 2.18) | (-0.70, 0.14) | (-1.16, −0.51) | (-0.16, 0.92) | (-0.97, −0.35) | |
| Education, university | −0.47 | 0.20 | 0.20 | −0.21 | 0.34 | 0.12 |
| (-1.20, 0.28) | (-1.39, 1.70) | (-0.27, 0.72) | (-0.53, 0.11) | (-0.21, 0.90) | (-0.17, 0.43) | |
| Ideology, don't know | 0.68 | −0.35 | 0.11 | |||
| (-0.53, 1.95) | (-1.96, 2.49) | (-1.10, 1.16) | ||||
| Ideology, left/liberal | −0.58 | 0.54 | −1.08 | −0.26 | −0.06 | −0.08 |
| (-1.19, 0.24) | (-0.74, 2.25) | (-1.63, −0.51) | (-0.61, 0.08) | (-0.78, 0.69) | (-0.43, 0.28) | |
| Ideology, right/conservative | −0.52 | −0.20 | −1.55 | 0.34 | −0.89 | −0.16 |
| (-1.60, 0.35) | (-2.26, 2.12) | (-2.34, −0.75) | (-0.13, 0.81) | (-1.58, −0.23) | (-0.56, 0.24) | |
| Pro-vaccine | −0.64 | 0.94 | −0.56 | −0.94 | 0.80 | −0.74 |
| (-1.05, −0.29) | (0.49, 1.77) | (-0.90, −0.28) | (-1.13, −0.75) | (0.57, 1.09) | (-0.92, −0.58) | |
| Feeling, business | −1.06 | 0.54 | −0.16 | −0.62 | 1.12 | −0.43 |
| (-2.39, 0.44) | (-1.84, 2.97) | (-1.37, 1.01) | (-1.41, 0.18) | (-0.07, 2.38) | (-1.01, 0.17) | |
| Afraid of Covid | −1.27 | 3.45 | 0.02 | −0.63 | 1.44 | −0.09 |
| (-2.41, −0.18) | (1.42, 8.26) | (-0.79, 0.81) | (-1.29, 0.00) | (0.53, 2.49) | (-0.65, 0.47) | |
| Intercept | 7.49 | −0.77 | 5.47 | 6.20 | 0.77 | 5.07 |
| (6.16, 8.93) | (-3.05, 1.29) | (4.55, 6.51) | (5.61, 6.81) | (-0.08, 1.71) | (4.50, 5.64) | |
| Scale | 1.08 | 0.89 | 1.25 | 1.13 | ||
| (0.85, 1.32) | (0.72, 1.08) | (1.13, 1.37) | (1.02, 1.24) | |||
| Observations | 582 | 582 | 560 | 661 | 661 | 610 |
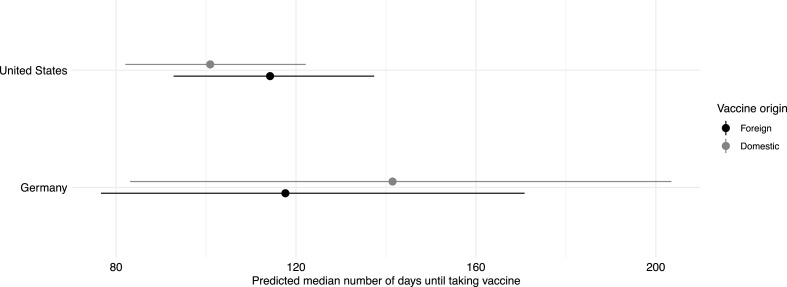
Coefficient estimates only poorly convey the magnitude of effects in non-linear models like our interval-censored Weibull model. Therefore, we turn to simulations of treatment effects on the outcome scale by setting the auxiliary demographics to the observed values of the variables, preserving the coherence and structure of the demographic variables (Hanmer and Ozan Kalkan, 2013). This approach gives us the predicted time until each person in our dataset takes the vaccine, synthetically assigning either treatment status. Then, we save the (weighted) median estimate across all the synthetic observations. We repeat this process for each country and each non-parametric bootstrap coefficient estimate. Fig. 2 gives the summary (mean, 95% confidence interval) of these median predictions by country (y-axis) and treatment condition (black for foreign, gray for domestic). As we can see, the differences between the domestic and foreign conditions are small for the sizes of confidence intervals. The mean estimates of the four medians lie between (about) 100 and 150 days.
Fig. 2.
Estimate of median time until taking vaccine under each treatment condition. Estimates of (entropy-balanced) median duration across the synthetic data set. The dot gives the mean estimate, the line the 95% confidence interval. Gray dots and lines signify the home treatment condition, black the foreign counterpart.
We conduct two additional analyses to probe robustness of our main finding. First, so far, we have assumed a response of “Never” to represent a choice between 720 days and +∞. Under this perspective, the foreign/domestic distinction could still matter to those who chose “Never”. Alternatively, we could treat a “Never” response as indicating a categorical rejection of any vaccine regardless of its quality or origin. One implication of this alternative assumption is that for those who chose “Never”, the country of origin cannot influence their vaccination intentions, and therefore the treatment effect is a priori zero for them. We examine if this alternative assumption of “Never” answers changes our main results when using statistical models that account for the alternative process.
We first use a Bernoulli-probit model to examine whether the country of origin matters for the decision to answer “Never” as opposed to any duration. The second column (“Take at all”) for each country in Table 1 shows the results. The coefficient estimates show no evidence of a country-of-origin effect on whether survey-takers answer “Never”. Second, we repeat interval-censored regressions on subsets of those who chose any response option other than “Never”, for whom the country of origin could matter under this alternative assumption of the “Never” response. The results are shown in the third column (“Drop ‘Never”‘) for each country. We find that the results are similar to those from the original model in the “All” columns. For those that contemplate taking the vaccine at all, we find no evidence for a country-of-origin effect.
Next, we examine whether the country-of-origin treatment effect is moderated by one's feeling towards the foreign country and one's fear of Covid-19. After all, the treatment coefficient estimates and the substantive simulations suggest noisiness which might stem from heterogeneity of treatment effects. Following an approach proposed by (Bansak, 2021), we first subset the data by the “domestic” and “foreign” vaccine treatment status and then calculate the effect of the moderator on the outcome (for each treatment/country subset); for details, see (Bansak, 2021). This strategy lets us calculate the changes to vaccine uptake due to feelings toward the foreign country and one's fear of Covid-19, respectively, under each treatment, as we introduced and discussed earlier in the paper. In Section B, we show the empirical distribution of the moderators for reference.
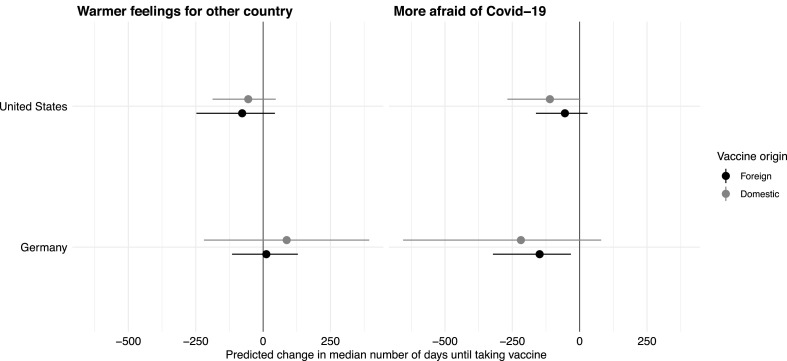
Because our quantity of interest is the difference of two non-linear functions, we will show the moderation effects using the same simulation approach involving synthetic data as before. We relegate the detailed tables of the interval-censored Weibull regressions to the appendix in Section D. Fig. 3 shows the estimated effects of the two moderators—changes to the predicted (median) duration when moderators change from the minimum value to the maximum—under each treatment case by country.
Fig. 3.
Treatment moderation effects by fear of COVID-19 and by feelings toward the foreign vaccine producer. Each panel shows the changes in the predicted (median) numbers of days when feeling toward the foreign country (left panel) and fear of Covid-19 change from the minimum to the maximum. The dot gives mean estimate, the line the 95% confidence interval. Gray dots/lines denote cases with vaccine produced domestically, black when in the foreign country.
First, consider the left panel of Fig. 3. Moving the feelings towards the foreign country from the minimum to the maximum does not significantly affect the predicted (median) number of days to vaccinate regardless of whether the vaccine was domestic (gray dot and line) or foreign (black). Neither is statistically significantly different zero in either Germany or the United States. Since the foreign and domestic effects are not different from each other, we find no evidence that feelings towards the foreign country moderate the country-of-origin treatment effect.
Second, in the right panel, we show the analogous result when fear of Covid-19 changes from “not at all afraid” to “very afraid”. In both countries, greater fear is consistently but somewhat noisily associated with less wait time, and more strongly so in Germany. However, as before, the effects do not differ by treatment status. Again, there is no evidence that Covid-19 fear moderates the treatment effects.
4. Discussion
We hypothesized that if state bias—one's affinity for one's own country and animosity toward other countries—is salient to vaccination intentions, switching the country-of-origin label of the BioNTech/Pfizer vaccine should affect individual decisions to take the vaccine. We find virtually no evidence that framing the BioNTech/Pfizer vaccine as domestic or foreign matters to vaccination intentions in either Germany or the United States. As we demonstrate, the absence of evidence for an effect is also not an artifact of a particular interpretation of vaccine refusers or (plausible) heterogeneous effects going into opposite directions.
Unlike prior experiments that have asked survey-takers to evaluate hypothetical vaccines, our experiment mimics the real-world settings in that our survey-takers evaluate the real-world vaccine. While the use of the real-world vaccine is advantageous in increasing realism and external validity of our experiment, one disadvantage of our approach derives from its very nature: we had little control over what our survey-takers knew and believed at the time of our survey. We clarify and address some of issues and limitations emanating from this research design choice.
First, our inference depends in part on the assumption that our survey-takers were aware of the announcement of the new, viable vaccine at the time of our survey. This assumption allows us to naturally controlling for beliefs about the vaccine quality. We are fairly confident that most of our survey-takers knew about the news of the vaccine results and therefore held some beliefs about the vaccine quality at the time of the survey. First, we asked our survey-takers after the experiment if they had heard of the news about the new vaccine. More than 65% in our samples reported having had heard of the news. Additionally, we gathered data on web search volumes for the United States, Germany, and the world. Across these cases, interest in the vaccine spiked with the announcement (see Figure A.4 in the appendix).
Yet, some of our survey-takers learned about the vaccine in our survey for the first time and therefore might have drawn inferences about the quality of the vaccine during the experiment. This could potentially threaten our inference about state bias. However, we are not very concerned about this issue because both Germany and the United States have similar reputations for their strong pharmaceutical industries and regulations, product quality, etc. Therefore, even if some of our survey-takers were not aware of the new vaccine, this unique feature of our case helps us control for their beliefs about vaccine quality to a large extent.
Second, our inference also assumes that our survey-takers either were not aware of the vaccine's country of origin or cared little about it prior to the survey. It is possible that some of the survey-takers believed that the vaccine was developed by their countries and therefore might not have taken the experiment in the “foreign” treatment condition seriously. If true, this may explain why we found little evidence of state bias. However, we are fairly convinced that this is not a serious issue. Additional evidence indicates that people cared relatively little about the vaccine's country of origin. When asked to summarize the key points of the news article in our survey, our survey-takers mentioned surprisingly little about the vaccine's country of origin compared to the news about the vaccine's effectiveness. Only 6% of German survey-takers in the treatment group (those who were assigned the vaccine labeled as American) and 32% of American survey-takers in the treatment group (those who were assigned German vaccines) mentioned the country of origin in their summaries. In contrast, 66% in the same subsets mentioned that the vaccine was “90% effective,” bolstering the assumptions of our study. In addition, if our survey-takers believed the vaccine was developed in their country and this fact was salient to them, at least some would have written comments challenging the premise of the news story. Nobody mentions this in their written summaries. All in all, we are fairly confident that country of origin was not salient to our survey-takers when evaluating the presented vaccine, corroborating our statistical results and interpretation.
The discussion so far gives us a good amount of confidence in the validity of our estimates of state bias. Yet, it would be premature to conclude that state bias is absent more generally across countries, vaccines, and contexts. First, our case may be an a priori hard case in which to find strong state bias in vaccination intentions. The multi-national survey by YouGov in December 2020 shows that Germans give a net favorability rating of +44 to a German vaccine while −10 to a vaccine of U.S. origin. U.S. survey-takers rate a U.S. vaccine +36 but a German one as +15 (Smith, 2021). Yet, notwithstanding U.S.-German highest-level political discord in 2020, cultural, scientific, business, and academic ties have remained close and strong between the two countries. This may suggest diminished reasons to expect particularly strong state bias. We need to be cautious about the extent to which our findings generalize beyond the U.S.-Germany pair. We believe our results are highly applicable to pairs of countries, such as U.S.-U.K, U.K.-Switzerland, and Germany-Australia, with reputations for quality products and strong regulatory mechanisms. Outside of such pairs, quality concerns may kick in, and state bias may become more salient to citizens’ willingness to take foreign vaccines.
That said, we would like to highlight our additional results suggesting prejudice against foreign countries may be overstated. In our survey, we find that a significant contingent of German survey-takers has cold to neutral feelings toward the United States (see Section B in the appendix). Nonetheless, our results show these feeling thermometer scores do not generate state bias in either German or U.S. samples. This gives suggestive evidence that prejudice against a foreign country might matter little even outside our particular context. Clearly, more research is needed to establish such conclusions firmly.
Second, the BioNTech/Pfizer vaccine not only was the first viable vaccine for Germans and Americans but also was celebrated for its unexpectedly high efficacy. The high efficacy might have outshone any state bias as less anxious or enthusiastic citizens focused their attention on that aspect of the announcement. This may suggest that if a vaccine was not as efficacious and/or the first vaccine, country of origin may have been more salient. If true, our findings may be limited to vaccines that are highly successful and/or to a situation where alternative viable vaccines are not readily available. It would be interesting to see if state bias is greater when the vaccine quality is low or the effect depends on the availability of other vaccines.
Third, our results rely on samples collected via Prolific. Our samples are not nationally representative of the German and U.S. populations. Therefore we should be cautious about the extent to which our results generalize to these populations. That said, studies have shown that experimental findings from nationally representative samples are similar to those from samples from online recruitment platforms like Amazon's MTurk (Berinsky et al., 2012; Mullinix et al., 2015). We have also taken additional steps to mitigate issue by re-weighing the samples using entropy balancing in the spirit of (Hainmueller et al., 2015).
Despite these caveats, our findings are useful in suggesting that state bias may be overstated in previous research. Prior work finds that Americans prefer U.S.-made vaccines over those developed in the U.K. and Europe, a group of countries with as (generally) friendly ties to the U.S as Germany (Kreps et al., 2020; Motta, 2021; Pogue et al., 2020; Smith, 2021). Yet, our survey finds little evidence that country of origin matters in the U.S-Germany pair. What accounts for these discrepancies between ours and their findings? We point to a few potential reasons. First, the previous studies were administered in the summer of 2020 when the public awareness, knowledge, and anticipation of Covid-19 vaccines was not high. In such a low-information, high-uncertainty environment, we would expect people to rely heavily on country origins as an informational shortcuts to reach a decision about vaccination. Therefore, the timing of these prior surveys may explain the consistent evidence showing the country-of-origin effect even for country-pairs like U.S.-U.K. By contrast, our survey was conducted a few days after the announcement of the BioNTech/Pfizer vaccine. Survey-takers made the hypothetical vaccination decisions in a rich-information environment where a successful, concrete vaccine was going to be available in the near future.
Second, survey-takers in previous studies were asked to evaluate purely hypothetical vaccines. In a relatively unrealistic and uncertain environment, we would also expect survey-takers to rely on cues that they are more familiar with (i.e. country origins) in evaluating vaccine profiles. However, in our setting, survey-takers evaluate a vaccine that was just announced to be successful and which draw substantial interest on Google (see Figure A.4 in the appendix). These differences in the timing and setting might have contributed to the differences in the results.
5. Conclusion
The struggle to combat vaccine hesitancy increased in saliency during the Covid-19 pandemic, with lives and economies dependent on the development and successful implementation of an effective vaccination against the virus. In addition to the known hazards with overcoming vaccine hesitancy, inward-looking policies, “national solo runs” nationale Alleingänge, and reduced international cooperation created additional worries for public health. Leveraging a serendipitous opportunity—the opportunity to meaningfully evaluate the impacts of prejudice on a real-world vaccine developed jointly in two countries—we endeavored to estimate the causal effect of state bias on people's willingness to take the vaccine against Covid-19 by BioNTech and Pfizer. Although previous studies consistently report strong preferences for domestic vaccines over foreign ones, we find little evidence for state bias in a pair of populations that are mutually amicable in the real world settings. Recognizing the caveats spelled out above, this implies that state bias may not be as strong as suggested by prior work when individuals face decisions to take a successful vaccine in the real-world settings. Though countries may adopt more nationalistic policies, when it comes to individual health preferences, state bias may be less salient and subsumed by more proximate concerns. This implies that in terms of public messaging on new vaccines, positive news touting efficacy and approval may stick with people than other cues like the country of origin. Overall, maintaining optimistic messaging about vaccine development may be an effective communication strategy to minimize the bias around the globe that prior studies found as being potential hurdles to Covid-19 vaccine acceptance.
Credit author statement
All authors were involved in the conceptualization of the manuscript and the survey design. TH was responsible for the administration of the survey and the analysis of the data. CH led the initial drafting of the manuscript. All authors provided critical revisions to manuscript drafts. All authors approve of the final version of the manuscript.
Funding statement
The authors have no funding to report for this project.
Institutional Review Board
Ethics approval was given by the Office of Research Compliance at the University of South Carolina, Columbia, SC, United States (#Pro00105966).
Declaration of competing interest
The authors have no conflicts of interests to declare.
Acknowledgement
We thank Stephanie Davis, Lydia Howell, Katie Jones, Kate Musliner, two referees, and the editor for feedback.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.socscimed.2021.114115.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- Abbas M.Z. Practical implications of ‘vaccine nationalism’: a short-sighted and risky approach in response to covid-19. 2020. https://eprints.qut.edu.au/206694/ URL:
- Allen M.A., Flynn M.E., Machain C.M., Stravers A. Outside the wire: US military deployments and public opinion in host states. Am. Polit. Sci. Rev. 2020;114(2):326–341. [Google Scholar]
- Bansak K. Estimating causal moderation effects with randomized treatments and non-randomized moderators. J. Roy. Stat. Soc. 2021;184(1):65–86. https://rss.onlinelibrary.wiley.com/doi/abs/10.1111/rssa.12614 URL: [Google Scholar]
- Berinsky A.J., Huber G.A., Lenz G.S. Evaluating online labor markets for experimental research: Amazon. com's mechanical turk. Polit. Anal. 2012;20(3):351–368. [Google Scholar]
- Brader T. Striking a responsive chord: how political ads motivate and persuade voters by appealing to emotions. Am. J. Polit. Sci. 2005;49(2):388–405. [Google Scholar]
- Burki T.K. The Russian vaccine for covid-19. The Lancet Respiratory Medicine. 2020;8(11):e85–e86. doi: 10.1016/S2213-2600(20)30402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan T., Moghtaderi A., Lueck J.A., Hotez P., Strych U., Dor A., Fowler E.F., Motta M. Correlates and disparities of intention to vaccinate against covid-19. Soc. Sci. Med. 2021;272:113638. doi: 10.1016/j.socscimed.2020.113638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colchester M., Norman L. The Wall Street Journal; 2021. Vaccine-export fight hastens decline in EU-U.K. relations.https://www.wsj.com/articles/eu-threatens-tightening-vaccine-export-ban-11615985024 URL: [Google Scholar]
- de Figueiredo A., Johnston I.G., Smith D.M.D., Agarwal S., Larson H.J., Jones N.S. Forecasted trends in vaccination coverage and correlations with socioeconomic factors: a global time-series analysis over 30 years. The Lancet Global Health. 2016;4(10):e726–e735. doi: 10.1016/S2214-109X(16)30167-X. [DOI] [PubMed] [Google Scholar]
- De Vries C.E., Hobolt S., Walter S. International Organization; 2020. Politicizing International Cooperation: the Mass Public, Political Entrepreneurs and Political Opportunity Structures. [Google Scholar]
- Determann D., Korfage I., Fagerlin A., Steyerberg E., Bliemer M.C., Voeten H., Richardus J.H., Lambooij M., de Bekker-Grob E. Public preferences for vaccination programmes during pandemics caused by pathogens transmitted through respiratory droplets-a discrete choice experiment in four european countries. Euro Surveill. 2016;21(22) doi: 10.2807/1560-7917.ES.2016.21.22.30247. [DOI] [PubMed] [Google Scholar]
- Determann D., Korfage I., Lambooij M., Bliemer M., Richardus J., Steyerberg E., et al. Acceptance of vaccinations in pandemic outbreaks: a discrete choice experiment. PloS One. 2014;9(7) doi: 10.1371/journal.pone.0102505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott G.R., Cameron R.C. Consumer perception of product quality and the country-of-origin effect. J. Int. Market. 1994;2(2):49–62. doi: 10.1177/1069031X9400200204. URL: [DOI] [Google Scholar]
- Ghosal A., SaaliQ S. Associated Press; 2021. India's Quick Nod to Homegrown Covid-19 Vaccine Seeds Doubt.https://bit.ly/2MX9z4Q URL: [Google Scholar]
- Hainmueller J. Entropy balancing for causal effects: a multivariate reweighting method to produce balanced samples in observational studies. Polit. Anal. 2012;20(1):25–46. [Google Scholar]
- Hainmueller J., Hangartner D., Yamamoto T. Validating vignette and conjoint survey experiments against real-world behavior. Proc. Natl. Acad. Sci. Unit. States Am. 2015;112(8):2395–2400. doi: 10.1073/pnas.1416587112. https://www.pnas.org/content/112/8/2395 URL: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel L., Kirzinger A., Muñana C., Brodie M. 2020. Kff Covid-19 Vaccine Monitor: December 2020.https://www.kff.org/coronavirus-covid-19/report/kff-covid-19-vaccine-monitor-december-2020/ URL: [Google Scholar]
- Hanmer M.J., Ozan Kalkan K. Behind the curve: clarifying the best approach to calculating predicted probabilities and marginal effects from limited dependent variable models. Am. J. Polit. Sci. 2013;57(1):263–277. [Google Scholar]
- Hart P.S., Chinn S., Soroka S. Politicization and polarization in covid-19 news coverage. Sci. Commun. 2020;42(5):679–697. doi: 10.1177/1075547020950735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff C., Tingley D. “Who are these people?” Evaluating the demographic characteristics and political preferences of MTurk survey respondents. Research & Politics. 2015;2(3) doi: 10.1177/2053168015604648. URL: [DOI] [Google Scholar]
- Kennedy J. Vaccine hesitancy: a growing concern. Pediatr. Drugs. 2020;22(2):105–111. doi: 10.1007/s40272-020-00385-4. [DOI] [PubMed] [Google Scholar]
- Kreps S., Prasad S., Brownstein J.S., Hswen Y., Garibaldi B.T., Zhang B., Kriner D.L. Factors associated with US adults' likelihood of accepting COVID-19 vaccination. JAMA network open. 2020;3(10) doi: 10.1001/jamanetworkopen.2020.25594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane S., MacDonald N.E., Marti M., Dumolard L. Vaccine hesitancy around the globe: analysis of three years of WHO/UNICEF Joint Reporting Form data-2015-2017. Vaccine. 2018;36(26):3861–3867. doi: 10.1016/j.vaccine.2018.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus J.V., Ratzan S.C., Palayew A., Gostin L.O., Larson H.J., Rabin K., Kimball S., El-Mohandes A. A global survey of potential acceptance of a covid-19 vaccine. Nat. Med. 2020:1–4. doi: 10.1038/s41591-020-1124-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunz Trujillo K., Motta M. Why are wealthier countries more vaccine skeptical?: how internet access drives global vaccine skepticism. APSA Preprints. 2020 [Google Scholar]
- Lusk J.L., Brown J., Mark T., Proseku I., Thompson R., Welsh J. Consumer behavior, public policy, and country-of-origin labeling. Rev. Agric. Econ. 2006;28(2):284–292. [Google Scholar]
- MacDonald N.E. Vaccine hesitancy: definition, scope and determinants. Vaccine. 2015;33(34):4161–4164. doi: 10.1016/j.vaccine.2015.04.036. [DOI] [PubMed] [Google Scholar]
- Marcus G.E., MacKuen M.B. Anxiety, enthusiasm, and the vote: the emotional underpinnings of learning and involvement during presidential campaigns. Am. Polit. Sci. Rev. 1993:672–685. [Google Scholar]
- Marcus G.E., Neuman W.R., MacKuen M. University of Chicago Press; 2000. Affective Intelligence and Political Judgment. [Google Scholar]
- Mayes J. U.K. and E.U. escalate their dispute over vaccine shipments, Bloomberg. 2021. https://bloom.bg/3tO5jVI URL:
- Motta M. Can a covid-19 vaccine live up to americans' expectations? a conjoint analysis of how vaccine characteristics influence vaccination intentions. Soc. Sci. Med. 2021;272:113642. doi: 10.1016/j.socscimed.2020.113642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullinix K.J., Leeper T.J., Druckman J.N., Freese J. The generalizability of survey experiments. Journal of Experimental Political Science. 2015;2(2):109–138. [Google Scholar]
- Neumann-Böhme S., Elsem V.N., Iryna S., Barros P.P., Brouwer W., Jonas S., Stargardt T., et al. Once we have it, will we use it? A European survey on willingness to be vaccinated against covid-19. Eur. J. Health Econ.: HEPAC. 2020;21(7):977–982. doi: 10.1007/s10198-020-01208-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palan S., Schitter C. Prolific.ac – a subject pool for online experiments. Journal of Behavioral and Experimental Finance. 2018;17:22–27. [Google Scholar]
- Peer E., Brandimarte L., Samat S., Acquisti A. Beyond the turk: alternative platforms for crowdsourcing behavioral research. J. Exp. Soc. Psychol. 2017;70:153–163. [Google Scholar]
- Pogue K., Jensen J.L., Stancil C.K., Ferguson D.G., Hughes S.J., Mello E.J., Burgess R., Berges B.K., Quaye A., Poole B.D. Influences on attitudes regarding potential covid-19 vaccination in the United States. Vaccines. 2020;8(4):582. doi: 10.3390/vaccines8040582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. How much difference does it make to people where a covid vaccine was developed? 2021. https://today.yougov.com/topics/health/articles-reports/2021/01/15/how-much-difference-does-it-make-people-where-covi URL:
- The Lancet Child & Adolescent Health Vaccine hesitancy: a generation at risk. The Lancet Child & Adolescent Health. 2019;3(5):281. doi: 10.1016/S2352-4642(19)30092-6. [DOI] [PubMed] [Google Scholar]
- Wadman M. Public needs to prep for vaccine side effects. Science. 2020;370(6520) doi: 10.1126/science.370.6520.1022. https://science.sciencemag.org/content/370/6520/1022 URL: [DOI] [PubMed] [Google Scholar]
- Zhao Q., Percival D. Entropy balancing is doubly robust. J. Causal Inference. 2016;5(1) doi: 10.1515/jci-2016-0010. URL: [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.