Abstract
Background:
Congestion is a common cause of symptoms in heart failure (HF). Yet, intrathoracic impedance, an objective marker of cardiopulmonary congestion, has not been examined in relation to HF symptoms.
Objective:
To quantify relationships among physical and affective symptoms, health-related quality of life (HRQOL) and device-detected cardiopulmonary congestion in adults with HF over 3 months.
Methods:
Multivariate generalized linear modeling was used to quantify the association of cardiopulmonary congestion (Optivol® Index exceeding 60 Ω threshold) with HRQOL (12-item Kansas City Cardiomyopathy Questionnaire) and both physical symptoms (Functional Assessment of Chronic Illness Therapy-Fatigue Scale; HF Somatic Perception Scale Dyspnea and Early & Subtle Symptoms subscales) and affective symptoms (9-item Patient Health Questionnaire; 6-item Patient-Reported Outcomes Measurement Information System Anxiety Scale).
Results:
The mean age of the sample (n=49) was 62 years old, 39% were women, and 63% had NYHA class III/IV HF. Participants who experienced threshold crossings in the previous 90 days reported on average, 130% higher dyspnea (p=0.017; confidence interval (CI) 10.2%, 437%), 40% higher early & subtle symptoms (p=0.029; CI 3.4%, 89.7%), 106% higher depressive symptoms (p=0.003; CI 19.1%, 257%) and 40% higher anxiety (p=0.028; CI 3.7%, 89.1%). Threshold crossings in the previous 90 days were also significantly associated with a clinically meaningful decrease in HRQOL (β=16.16±6.32; p=0.01).
Conclusions:
Intrathoracic impedance measured with the Optivol Index can provide additional information regarding the patient experience of hallmark physical and psychological HF symptoms and HRQOL over 3 months.
Keywords: Heart failure, Symptom, Quality of life, Intrathoracic impedance, Pulmonary Congestion
Introduction
Heart failure (HF) is a distressing cardiovascular syndrome that is continuing to increase in prevalence in the US. Currently, 6.5 million Americans are diagnosed with HF and that number is projected to increase by 46% by 2030.1 In spite of recent improvements in medical management and technological advances in the treatment of HF, patients with HF continue to experience poor quality of life due to distressing symptoms such as dyspnea, fatigue, depression and anxiety,2–4 and HF remains the leading cause of hospital admission for older adults.
Cardiopulmonary congestion resulting from fluid overload and elevated pressures in the heart is a common cause of symptoms and it is a primary reason for HF hospitalization.5–7 Remote monitoring of pulmonary congestion has been introduced into implantable defibrillator/pacemakers that many patients with HF receive. This device-detected congestion has been associated with increased HF events and worse mortality.8,9 One area of study that has received little attention with this technology is the association between device-detected cardiopulmonary congestion and patients’ experience of HF symptoms. Patient-reported symptoms in HF are key drivers for healthcare utilization and quality of life.4,6,10 However, patients are often limited in their ability to recognize and respond to symptoms (i.e. self-care management).11,12 These limitations include 1) difficulty in identifying the symptoms as related to HF especially in the presence of multiple co-morbidities 13, 2) symptoms that are subtle and difficult to detect 14 and 3) late recognition of symptoms.11
Further complicating the identification of symptoms for patients and clinicians is a dearth of objective markers of heart function related to patient HF symptoms.15–19 For patients, daily weights are poorly associated with clinical deterioration20 and for clinicians few hemodynamic indicators of HF are related to symptoms.15 In a recent study, Lee et al.21 describes multiple groups with differing profiles of hemodynamic-symptoms mismatch. One group of HF patients experienced very poor hemodynamics with only moderate levels of symptom burden and exhibited an increased clinical event risk compared to patients whose hemodynamics and symptoms were congruent. This study, along with evidence of patient difficulty identifying and managing symptoms, highlights the need to find better methods to monitor symptoms both as an indicator of progressing HF and as a means to improve patient-reported outcomes such as symptoms and HRQOL. The purpose of this study is to quantify the relationships among symptoms, HRQOL and device-detected cardiopulmonary congestion in patients with HF over 3 months. A better understanding of how an objective measure of worsening HF is associated with changes in symptoms and HRQOL may enhance more patient-specific interventions; reducing costly readmission and improving quality of life.
Materials and Methods
This was a National-Institutes of Health-sponsored observational study examining 3 months of device-detected cardiopulmonary congestion data in 49 patients with symptomatic HF. Patients from a HF clinic associated with an academic medical center in the Pacific Northwest were identified by their cardiologist as having symptomatic HF (NYHA Class II-IV) and an Optivol® (Medtronic, Minneapolis) enabled device. Between January 2017 and December 2017, identified patients were approached at a pacemaker/ICD clinic visit by a member of the research team not directly involved with HF care for participation in the study. The study was approved by the medical center institutional review board and all participants were provided written and informed consent. At enrollment, the prior 3 months of device data were downloaded and a questionnaire was completed either during the visit or by mail per the participants’ preference. The questionnaire collected data on demographic, socioeconomics and information asking patients to evaluate their HF symptoms and HRQOL over the past 3 months. In addition, an assessment of mild cognitive function was administered at the time of enrollment. Clinical information regarding the patient’s HF treatment and co-morbidities were obtained from an in-depth review of the participants’ medical record at the time of enrollment.
Measurement
Device-detected cardiopulmonary congestion
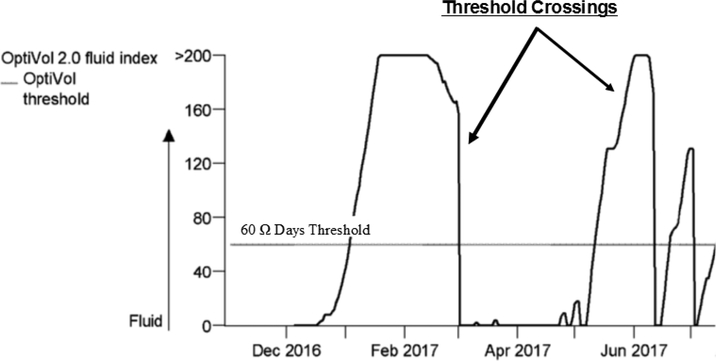
Cardiopulmonary congestion was quantified using data that is already generated and stored in patients’ implanted Optivol® therapeutic devices. Devices send a high frequency, low amperage, alternating current from the device generator to the right ventricular coil, tip, or ring electrode to measure changes in electronic resistance. Increasing congestion within the lungs results in a reduction in the resistance/impedance to the electronic current.22,23 Average raw daily impedance is quantified and stored in the device computer; raw average daily impedance is calculated in an identical fashion among all commercially-available Optivol® devices. Post-hoc, several metrics of congestion are calculated including the Optivol® fluid index, which represents day-to-day differences between impedance and a reference impedance threshold of 60 Ω days (Figure 1). In our study as well as others24,25, an Optivol® Index exceeding the 60 Ω threshold constitutes a “threshold crossing”, an indicator of a cardiopulmonary congestion event. The data extracted from the devices included the frequency and duration of all threshold crossings in the previous 3 months. Device data is collected beginning 34 days after implantation as pocket healing interferes with impedance. Major surgical procedures within 6 weeks may interfere with impedance; hence, this was an important exclusion criterion.
Figure 1. Device-detected Cardiopulmonary Congestion with Optivol® Index.
Optivol® fluid index threshold crossings occur when Optivol® fluid index exceeds 60 Ω days. Threshold crossings are indications of a cardiopulmonary congestion event. The precise number and duration of the threshold crossings were determined.
Physical HF Symptoms
Fatigue was measured using the Functional Assessment of Chronic Illness Therapy Fatigue Scale (FACIT-F v. 4).26 The FACIT-F assesses patients’ tiredness, weakness, and difficulty conducting usual activities due to fatigue.26,27 Scores of the 13 items range from 0–52 with higher scores indicating more fatigue. The FACIT-F has excellent concordant validity with the Piper fatigue scale (r = 0.83) and Profile of Mood States fatigue scale (r =0.77).28 The scale has also good reliability and validity in adults.29
Dyspnea was measured with the 6-item dyspnea subscale of the Heart Failure Somatic Perception Scale (HFSPS Dyspnea).30 The HFSPS is rooted in the Theory of Unpleasant Symptoms which posits that the patient’s physiology and multiple symptoms are important factors in the symptom experience.31 The HFSPS asks about how much the participant was bothered by HF symptoms related to shortness of breath or other breathing difficulties and provides six response options ranging from 0 (not at all) to 5 (extremely bothersome). The HFSPS dyspnea has excellent internal consistency (alpha = 0.89) concordant validity with the KCCQ functional limitations scale (r = 0.53) and independently predicts HF-related clinical events (per point HR = 1.031, p=0.031).31
Early and Subtle symptoms were measured with the 7-item early and subtle subscale of the HFSPS (HFSPS E&S).31 The HFSPS asks about how much the participant was bothered by HF symptoms that can occur prior to more noticeable symptoms such as dyspnea and fatigue. For example, the HFSPS E&S asks patients to rate how much they were bothered by tight clothing, stomach ache, waking to urinate and needing more rest during the day. The HFSPS E&S provides six response options ranging from 0 (not at all) to 5 (extremely bothersome). The HFSPS E&S has good internal consistency (alpha = 0.75) moderate concordant validity with the KCCQ functional limitations scale (r = 0.39) and independently predicts HF-related clinical events (per point HR = 1.030, p=0.028).31
Psychological HF Symptoms
Depressive symptoms were measured using the 9-item Patient Health Questionnaire (PHQ9). The PHQ9 has 4 response options ranging from 0 (not at all) to 3 (nearly every day).32 Higher scores (range 0–27) indicate more depression. The PHQ9 has been validated in the HF population. The PHQ9 is 87% sensitive and 76% specific in detecting depressive disorder in the general population.33
Symptoms of anxiety were measured with the 6-item patient reported outcomes measurement information system (PROMIS) short form anxiety scale. The PROMIS anxiety SF-6 asks respondents to rate level of fear, anxiousness and worry with 5 response options of never, rarely, sometimes, often, always (scale of 1 −5). A raw score is calculated by summing the 6-items with a range of 6–30. Raw scores correspond to T-scores based on large nationally representative samples.34
Mild Cognitive Dysfunction
Mild cognitive dysfunction was measured with the Montreal Cognitive Assessment (MoCA). The MoCA assesses a number of cognitive functions including short-term verbal memory recall, visuospatial ability, executive function, attention, concentration, working memory, language, and orientation. The MoCA has been shown to be sensitive and specific to mild cognitive function with a cut-off score of <26 and has been used in patients with HF.35,36 The MoCA was assessed in person following the detailed instructions.
Health-Related Quality-of-Life
Heart failure-specific health-related quality of life was measured with the Kansas City Cardiomyopathy Questionnaire Short Version (KCCQ-12). The KCCQ-12 is a 12-item Likert scale comprised of 4 sub-scales; symptom frequency, physical and social function and quality-of-life. Scores range from 0–100 with higher scores reflecting better function. A KCCQ-12 summary score is created by calculating the mean of the 4 sub-scores. The KCCQ-12 has excellent concordant validity (0.93–0.99) with the original well-validated and reliable item measure and good test-re-test reliability (0.76–0.92).37
Statistical Procedure
Means, standard deviations, frequency, and percentages were used to describe the sample using Stata/IC v14.2 (Texas). The Student’s t test, Mann-Whitney U, analysis of variance (ANOVA), Kruskal-Wallis test, Chi-square or Fischer’s exact tests were used to examine differences between participants with no threshold crossings and those with varying frequency and duration of threshold crossings over the previous 3 months. Two different variables were created to describe the Optivol® threshold crossings in this study. One described the presence (or not) of a threshold crossing in the previous 90 days and to paint a broader picture of the variability in congestion the second was a categorical variable with one group having no days above threshold and the following two groups split by the median days above the Optivol® Index threshold. Given some of the symptom and HRQOL variables were modestly skewed, both parametric and non-parametric tests were used to evaluate the differences between the frequency and duration of threshold crossings. Since the levels of significance between the parametric and non-parametric tests were similar, the parametric tests (t tests and ANOVA) were used to describe the differences in symptoms and HRQOL as a function of threshold crossings. Hedge’s g was calculated to determine effect sizes between the presence or duration of threshold crossing and symptoms and HRQOL. Generalized linear modeling using either a gamma or normal distribution, depending on the shape of these data, was used to quantify the association of cardiopulmonary congestion (Optivol® Index exceeding 60 Ω threshold) with HRQOL and physical and psychological symptoms. The log likelihood and AIC were used to compare models with lower values indicative of better fit. A p-value <0.05 was considered significant. For HRQOL models, estimates are in units of the KCCQ summary score. In order to facilitate comparisons across symptoms, the relative difference in symptoms are reported for differing levels of the presence and duration of threshold crossings. The relative differences are reported as a percentage increase or decrease in the symptom score compared to the referent (0 threshold crossings and 0 days above threshold).
In order to avoid oversaturation of multivariate models due to small sample sizes, a limited number of covariates were identified using an empirical/theoretical approach. Covariates significantly different between those with threshold crossing and none (empirical) as well as variables shown by others to be significantly associated with HRQOL or symptoms from the literature were included to adjust the analyses. Specifically, NYHA class4,38 for HRQOL models and comorbidities39,40 for symptom models were used as the theoretical covariates. New York Heart Association class was not used as a covariate in the symptom models to avoid overlap in symptoms assessed with NYHA class and symptoms measured in the current study (fatigue and dyspnea).
Results
Participants in the study were on average 62 ± 13.5 years old, predominantly white (86%), and married or living with their partners (63%) (Table 1). Women comprised 39% of the sample. Most participants had moderate to severe functional limitation due to HF (63 % NYHA III/IV) and a non-ischemic HF etiology (80%).
Table 1.
Characteristics of the Sample
| -----------Threshold (TH) Crossings ----------- | ||||
|---|---|---|---|---|
| Sample (n = 49) | +TH Crossing (n= 22) | −TH Crossing(n = 27) | p value | |
| Age (in years) | 62.4 ± 13.5 | 63.0 ± 13.1 | 62.0 ± 14.0 | 0.801 |
| Female | 19 (38.8) | 10 (45.5) | 9 (33.3) | 0.386 |
| Caucasian | 42 (85.7) | 20 (90.9) | 22 (81.5) | 0.624 |
| Educational Status | ||||
| High School or less | 15 (31.9) | 6 (27.3) | 9 (33.3) | 0.659 |
| Financial Status | ||||
| More than enough | 8 (17.4) | 3 (13.6) | 5 (18.5) | 0.429 |
| Marital Status | ||||
| Married or living with partner | 31 (63.3) | 14 (63.6) | 17 (63.0) | 0.961 |
| Charlson Co-morbidity category | ||||
| low (score of 1 or 2) | 27 (55.1) | 9 (40.9) | 18 (66.7) | |
| medium (score of 3 or 4) | 17 (34.7) | 10 (45.5) | 7 (25.9) | |
| high (score of 5 or more) | 5 (10.2) | 3 (13.6) | 2 (7.4) | 0.174 |
| BMI | 30.9 ± 6.7 | 31.0 ± 5.6 | 30.8 ± 7.6 | 0.914 |
| MOCA | 25.8 ± 2.3 | 25.5 ± 2.6 | 26.0 ± 2.2 | 0.473 |
| Heart Failure Characteristics: | ||||
| NYHA III/IV | 31 (63.3) | 15 (68.2) | 16 (59.2) | 0.519 |
| Ejection Fraction % | 33.8 ± 14.2 | 29.4 ± 14.1 | 37.4 ± 13.4 | 0.048 |
| LVID | 5.8 ± 1.2 | 5.9 ± 1.1 | 5.8± 1.3 | 0.856 |
| Primary Etiology | ||||
| Ischemic | 10 (20.4) | 7 (31.8) | 3 (11.1) | 0.090 |
| Systolic BP | 113.6 ± 15.3 | 112.9 ± 13.9 | 114.2 ± 16.6 | 0.763 |
| Aldosterone Antagonist | 28 (57.1) | 11 (50.0) | 17 (63.0) | 0.362 |
| Diuretic | 39 (79.6) | 19 (86.4) | 20 (74.1) | 0.288 |
| ACE/ARB | 43 (87.8) | 17 (77.3) | 26 (96.3) | 0.077 |
| Beta Blocker | 46 (93.9) | 20 (90.9) | 26 (96.3) | 0.581 |
| Hemoglobin | 90.013.3 ± 2.0 | 13.5 ± 1.8 | 13.1± 2.1 | 0.722 |
| Serum Sodium | 138.0 ± 3.1 | 138.5 ± 3.0 | 137.5 ± 3.2 | 0.143 |
| Years with HF | ||||
| >7 years | 24 (49.0) | 15 (68.2) | 10 (37.0) | 0.030 |
Sample characteristics comparing those with a threshold crossing (+) to those with no threshold crossings in the previous 90 days (−). Threshold crossing = exceeding the 60 Ω Opitvol® Index threshold in the 3 months prior to study enrollment. Abbreviations: ACE/ARBangiotensin converting enzyme inhibitor/angiotensin receptor blocker, BMI - body mass index, HF-heart failure, LVID- left ventricular internal diameter MOCA - Montreal cognitive assessment, NYHA- New York heart association functional class
A slight majority (55%) of the sample did not have a threshold crossing in the previous three months from enrollment. Having ≥ one threshold crossing in the previous three months was significantly associated with lower ejection fraction and longer duration of HF (Table 1). There were no statistically significant diffeernces in symtpoms comparing patients with and without threshold crossings (Table 2A), or among patients with 0, 4–22 or 23–56 days above threshold (Table 2B) in the previous 90 days.
Table 2.
Unadjusted Differences in HF Symptoms and Health-related Quality-of-life (HRQOL) as a Function of the Presence and Duration of Threshold (TH) Crossings
 |
Effect sizes in 2B show the mean comparisons between 0 days and 4–22 days above threshold and 0 days above threshold and 23–56 days above threshold. Threshold crossing = exceeding the 60 Ω Opitvol® Index threshold in the 3 months prior to study enrollment. Abbreviations: KCCQ-Kansas City Cardiomyopathy Questionnaire, HF-heart failure, HRQOL-health-related quality of life, TH-threshold crossing
In multivariate models, the presence of at least one threshold crossing and a duration of 4–22 days above threshold were associated with worse HRQOL (Table 3). Adjusted multivariate models of physical and psychological symptoms showed that dyspnea, early & subtle, depressive and anxiety symptoms were significantly worse for those who experienced threshold crossings or had threshold crossings between 4 and 22 days (Table 4). Only depressive symptoms were also significantly worse for participants with threshold crossing of 23 days or more.
Table 3:
Generalized Linear Models of Objective Marker of Cardiopulmonary Congestion Associated with Health-Related Quality of Life (KCCQ) in Patients with HF.
| A. KCCQ Summary Score | β ± Standard Error | p value |
| Threshold crossing | −11.78 ± 5.08 | 0.020 |
| >7 years with HF | 9.99 ± 4.89 | 0.041 |
| NYHA III/IV | −23.92 ± 5.09 | <0.001 |
| LVEF | −0.11 ± 0.19 | 0.539 |
| B. KCCQ Summary Score | β ± Standard Error | p value |
| < median days above threshold (4–22 days)1 | −16.16 ± 6.32 | 0.011 |
| ≥ median days above threshold (23–56 days)1 | −7.90 ± 6.05 | 0.193 |
| > 7 years with HF | 9.32 ± 4.90 | 0.057 |
| NYHA III/IV | −23.76 ± 5.07 | <0.001 |
| LVEF | −0.11 ± 0.19 | 0.562 |
Estimates are in units of the KCCQ summary score. Higher values indicate better HRQOL. An empirical and theoretical approach was used to identify model covariates for adjusted analysis. Empirical covariates were shown to be significantly different between those with and without threshold crossings (years of HF and LVEF) in the current study. The theoretical covariate was NYHA class, which has been shown by others to be consistently associated with HRQOL in HF. Threshold crossing = exceeding the 60 Ω Optivol® Index threshold in the 3 months prior to study enrollment. Abbreviations: HF-heart failure, KCCQ-Kansas City Cardiomyopathy Questionnaire, LVEF=left ventricular ejection fraction, NYHA-New York Heart Association
Table 4:
Generalized Linear Models of an Objective Marker of Cardiopulmonary Congestion Associated with Patient-reported Symptoms
| A. HF Symptoms | Fatigue | Dyspnea | Early/Subtle | Depressive | Anxiety |
| Relative Difference (95% CI) | Relative Difference (95% CI) | Relative Difference (95% CI) | Relative Difference (95% CI) | Relative Difference (95% CI) | |
| Threshold crossing | +34.7% (−6.0%, 93.1%) | +130% * (15.8%, 356%) | +25.0% (−5.4%, 65.1%) | +95.0% ** (25.9%, 202%) | +20.0% (−9.3%, 59.0%) |
| B. HF Symptoms | Fatigue | Dyspnea | Early/Subtle | Depressive | Anxiety |
| Relative Difference (95% CI) | Relative Difference (95% CI) | Relative Difference (95% CI) | Relative Difference (95% CI) | Relative Difference (95% CI) | |
| < median days above threshold (4–22 days) | +47.3% (−1.8%, 121%) | +143% * (10.2%, 437%) | +40.0% * (3.4%, 89.7%) | +83.9% * (6.40%, 218%) | +40.1% * (3.7%, 89.1%) |
| ≥ median days above threshold (23–56 days) | +15.3% (−28.0%, 84.6%) | +114% (−6.60%, 390%) | +10.2% (−21.9%, 55.5%) | +106% ** (19.1%, 257%) | −1.0% (−32.2%, 44.5%) |
<0.05
≤0.01
In order to facilitate comparisons across symptoms, the relative difference in symptoms are reported for differing levels of the presence and duration of threshold crossings. The relative differences are reported as a percentage increase or decrease in the symptom score compared to 0 threshold crossings. An empirical and theoretical approach was used to identify model covariates for adjusted analysis. Empirical covariates were shown to be significantly different between those with and without threshold crossings (years of HF and LVEF) in the current study. The theoretical covariate was co-morbidities, which has been shown by others to be associated with symptom perception in HF. Threshold crossing = exceeding the 60 Ω Optivol® Index threshold in the 3 months prior to study enrollment. Abbreviations: HF-heart failure, KCCQ-Kansas City Cardiomyopathy Questionnaire, LVEF=left ventricular ejection fraction, NYHA-New York Heart Association
Discussion
In this prospective observational study of 49 patients with symptomatic HF and Optivol®-enabled pacemarker/ICDs, we found that the presence and duration of a device-detected cardiopulmonary congestion event in the previous 3 months was associated with higher physical and psychological symptom burden and worse HRQOL. Highlighting the need to better understand objective indicators of HF symptoms, a recent study by Riegel et al.41 showed that the perception of fluid retention in 44% of patients with symptomatic HF did not match objective measures of fluid congestion with the Optivol® Index. The results of our study adds to our understanding of HF symptomology by identifying an objective measure of HF pathophysiology associated with worse hallmark HF symptoms and HRQOL. Furthermore, our study suggests monitoring of intrathoracic impedance may be a valuable tool in addressing symptom burden and quality of life in patients with symptomatic HF.
Intrathoracic impedance, quantified using the Opitvol® Index, has been considered a gold standard in detecting fluid retention events in patients with HF.41 Although lower intrathoracic impedance (higher Optivol® Index) has been associated with less engagement in HF self-care behaviors, higher clinical event risk and mortality, our study demonstrated a significant relationship between intrathoracic impedance and patient-reported outcomes of physical and psychological HF symptoms and a clinically meaningful difference in HRQOL.8,25 Thus, this is an important finding in our understanding of symptom biology in HF. First, physical symptoms such as dyspnea are the primary reasons patients with HF seek care.6 Physical and psychological symptoms are also significant drivers of HRQOL. Furthermore, patients often delay seeking treatment for worsening symptoms due to difficulty in the detection or interpretation of symptoms.14 Our data suggests objective measures of intrathoracic fluid, such as the Optivol® Index, may provide additional information regarding underlying changes in physiology that may portend worsening symptoms and allow for earlier detection and treatment of burdensome symptoms.
Second, our study suggests intrathoracic impedance may not be a reliable indicator of fatigue in HF. The experience of fatigue in our sample was not significantly related to cardiopulmonary congestion events. One explanation for the lack of association between an objective measure of cardiopulmonary congestion and fatigue may be that intrathoracic impedance is simply not particularly sensitive to fatigue. Although the pathophysiology of fatigue has not been fully elucidated, it is believed to stem from both physiological sources (inflammation, muscle dysfunction and anemia) and from psychological sources such as depression.42,43 Cardiopulmonary congestion, on the other hand, results from elevated pressures in the heart and pulmonary systems that often result in dyspnea.44 Second, fatigue is a common and generalized symptom that may be less HF-specific.45,46 Patients may experience a response shift bias47 with the perception of fatigue. In other words, patients may recalibrate their perceptions of fatigue as they adapt to symptoms over time. Further research is needed to identify objective HF-related measures associated with fatigue in patients with HF.
Third, an unexpected finding from our study was that patients with the most severe marker of cardiopulmonary congestion (longest duration of threshold crossings) did not report the worst physical or psychological symptom burden or worst HRQOL. Although comparisons were not statistically significant at a p value of 0.05 (likely due to small sample sizes and large standard deviations), based on substantial differences in effect sizes patients who experienced more severe congestion (23–56 days), reported, on average, milder symptoms and better HRQOL compared to patients with less severe cardiopulmonary congestion (4–22 days above threshold).
This intriguing finding is consistent with two recent studies which found a mismatch between objective metrics of HF pathophysiology and subjectively-reported HF symptoms.21,41 Both studies showed that there are substantial proportions of patients whose perception of HF symptoms are discordant with objectively measured metrics of HF pathophysiology including intrathoracic impedance.41 The discordant groups include 1) patients who had mild objective markers of HF with more severe symptoms and 2) patients with more severe objective markers but less severe symptoms. Of particular importance was the observation that those, with what Lee et al. termed “symptom-hemodynamic mismatch”, were at higher risk for adverse clinical events than those with concordant symptoms and hemodynamics.21 Our study may also reflect a similar mismatch between an objective metric of worsening cardiopulmonary congestion and patient-reported symptoms and HRQOL. One reason for the mismatch may be related to response shift bias.47 Response shift bias may occur as individuals learn to adapt and compensate for declines in physical or psychological function that often occur in the trajectory of HF. As function declines over time, people with HF may continually develop a “new normal” from which they evaluate symptoms that eventually results in a mismatch between the patient’s subjective interpretation of symptoms and the worsening HF pathophysiology. Alternatively, patients may improve self-care as symptoms worsen, the improved self-care may result in lower symptom burden over time.48 Further research is needed to more clearly determine the causes, characteristics and outcomes associated with symptom-hemodynamic mismatch.
Finally, this study suggests the use of validated and reliable instruments to assess patient-reported outcomes may enhance the research of symptom biology in HF. Previous studies, using predominantly single-item symptoms measures, have shown little to no association between metrics of cardiopulmonary function and subjectively-reported HF metrics of symptoms and HRQOL.15,17,18 Since this study is one of the first to show moderate to strong effect sizes, although non-significant unadjusted findings, between an objectives metric of cardiopulmonary congestions and patient-reported outcomes in HF, it may offer insights into the study of HF symptomology that may enhance future research. For example, the use of reliable and validated symptom and HRQOL measures may have enhanced the ability of our study to detect significant associations. The symptom experience is often multifaceted and can be experienced differently by differing patient populations.49 Single-item descriptions of symptoms such as dyspnea and fatigue may capture a narrower range of the symptom experience making it more difficult to detect associations with physiologic markers. The systematic collection of patient-reported data with valid and reliable instruments that assess a wider range of the patient’s experience may prove useful in clinical practice and research to identify objective markers of HF that may better predict HF symptoms and HRQOL.
Strengths and Limitations
Our study had several strengths. First, the study examined the association of intrathoracic impedance with both physical and psychological symptoms. The use of physical and psychological symptoms provides a more comprehensive understanding of the patient symptom experience. Second, the use of GLM allowed non-normally distributed variables to be used without transformation. In addition to strengths, there a number of limitations to the study. The sample size was small, limiting the number of covariates and the ability to detect smaller effects. Additionally, the sample had little variation in self-reported race and the use of certain medications such as beta-blockers and ACE/ARBs. This prevents our study from making inferences about how these variables may have affected the results. Finally, the sample was comprised predominantly of non-ischemic, married, self-described white participants with more severe functional limitations. These characteristics of the sample hinder the generalizability of the results to the general HF population.
Conclusion
Optivol® fluid index threshold crossings over 6 months were significantly associated with physical and psychological HF symptoms and HRQOL. Optivol® threshold crossings can provide additional information regarding symptom burden and HRQOL that may enhance clinical assessment of symptoms and facilitate earlier treatment to improve patient outcomes.
Acknowledgments
Funding: This work was supported by the National Institute of Nursing Research (F31NR016660). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jonathan P. Auld, Oregon Health & Science University School of Nursing, 3455 SW US Veterans Hospital Rd., SN-ADM, Portland, OR 97239-2941 USA auldja@uw.edu.
James O. Mudd, Oregon Health & Science University, Knight Cardiovascular Institute, 3181 S.W. Sam Jackson Park Rd., Portland, Oregon 97239-3098 USA mudd@ohsu.edu.
Jill M. Gelow, Oregon Health & Science University, Knight Cardiovascular Institute, 3181 S.W. Sam Jackson Park Rd., Portland, Oregon 97239-3098 USA Jill.Gelow@providence.org.
Karen S. Lyons, Oregon Health & Science University School of Nursing, 3455 SW US Veterans Hospital Rd., SN-ADM, Portland, OR 97239-2941 USA lyonskw@bc.edu.
Shirin O. Hiatt, Oregon Health & Science University School of Nursing, 3455 SW US Veterans Hospital Rd., SN-ADM, Portland, OR 97239-2941 USA hiatts@ohsu.edu.
Christopher S. Lee, William F. Connell School of Nursing, Boston College Maloney Hall, 231140 Commonwealth Avenue, Chestnut Hill, MA, 02467 USA leeddo@bc.edu.
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135(10):e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. The New England journal of medicine. 2009;360(14):1418–1428. [DOI] [PubMed] [Google Scholar]
- 3.Heo S, Moser DK, Lennie TA, Zambroski CH, Chung ML. A comparison of health-related quality of life between older adults with heart failure and healthy older adults. Heart & lung : the journal of critical care. 2007;36(1):16–24. [DOI] [PubMed] [Google Scholar]
- 4.Zambroski CH, Moser DK, Bhat G, Ziegler C. Impact of symptom prevalence and symptom burden on quality of life in patients with heart failure. European Journal of Cardiovascular Nursing. 2005;4(3):198–206. [DOI] [PubMed] [Google Scholar]
- 5.Parrinello G, Greene SJ, Torres D, et al. Water and Sodium in Heart Failure: A Spotlight on Congestion. Heart Failure Reviews. 2015;20(1):13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Negi S, Sawano M, Kohsaka S, et al. Prognostic Implication of Physical Signs of Congestion in Acute Heart Failure Patients and Its Association with Steady-State Biomarker Levels. PLoS ONE. 2014;9(5):e96325–e96325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adams KF Jr., Fonarow GC, Emerman CL, et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J. 2005;149(2):209–216. [DOI] [PubMed] [Google Scholar]
- 8.Tang WH, Warman EN, Johnson JW, Small RS, Heywood JT. Threshold crossing of device-based intrathoracic impedance trends identifies relatively increased mortality risk. Eur Heart J. 2012;33(17):2189–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Small RS, Whellan DJ, Boyle A, et al. Implantable device diagnostics on day of discharge identify heart failure patients at increased risk for early readmission for heart failure. Eur J Heart Fail. 2014;16(4):419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwarz KA, Elman CS. Identification of factors predictive of hospital readmissions for patients with heart failure. Heart Lung. 2003;32(2):88–99. [DOI] [PubMed] [Google Scholar]
- 11.Jurgens CY. Somatic Awareness, Uncertainty, and Delay in Care-Seeking in Acute Heart Failure. Research in nursing & health. 2006;29:74–86. [DOI] [PubMed] [Google Scholar]
- 12.Lee CS, Gelow JM, Mudd JO, et al. Profiles of self-care management versus consulting behaviors in adults with heart failure. Eur J Cardiovasc Nurs. 2015;14(1):63–72. [DOI] [PubMed] [Google Scholar]
- 13.Darling C, Saczynski JS, McManus DD, Lessard D, Spencer FA, Goldberg RJ. Delayed hospital presentation in acute decompensated heart failure: Clinical and patient reported factors. Heart & Lung: The Journal of Acute and Critical Care. 2013;42(4):281–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jurgens CY, Hoke L, Byrnes J, Riegel B. Why do elders delay responding to heart failure symptoms? Nursing research. 2009;58(4):274–282. [DOI] [PubMed] [Google Scholar]
- 15.Guglin M, Patel T, Darbinyan N. Symptoms in heart failure correlate poorly with objective haemodynamic parameters. International Journal of Clinical Practice. 2012;66(12):1224–1229. [DOI] [PubMed] [Google Scholar]
- 16.Rector TS, Anand IS, Cohn JN. Relationships between clinical assessments and patients’ perceptions of the effects of heart failure on their quality of life. Journal of cardiac failure. 2006;12(2):87–92. [DOI] [PubMed] [Google Scholar]
- 17.Shah MR, Hasselblad V, Stinnett SS, et al. Dissociation between hemodynamic changes and symptom improvement in patients with advanced congestive heart failure. European journal of heart failure : journal of the Working Group on Heart Failure of the European Society of Cardiology. 2002;4(3):297–304. [DOI] [PubMed] [Google Scholar]
- 18.Lewis EF, Lamas Ga, O’Meara E, et al. Characterization of health-related quality of life in heart failure patients with preserved versus low ejection fraction in CHARM. European journal of heart failure. 2007;9(1):83–91. [DOI] [PubMed] [Google Scholar]
- 19.Bhardwaj A, Rehman SU, Mohammed AA, et al. Quality of life and chronic heart failure therapy guided by natriuretic peptides: results from the ProBNP Outpatient Tailored Chronic Heart Failure Therapy (PROTECT) study. Am Heart J. 2012;164(5):793–799 e791. [DOI] [PubMed] [Google Scholar]
- 20.Abraham WT, Compton S, Haas G, et al. Intrathoracic Impedance vs Daily Weight Monitoring for Predicting Worsening Heart Failure Events: Results of the Fluid Accumulation Status Trial (FAST). Congestive Heart Failure. 2011;17(2):51–55. [DOI] [PubMed] [Google Scholar]
- 21.Lee CS, Hiatt SO, Denfeld QE, Mudd JO, Chien C, Gelow JM. Symptom-Hemodynamic Mismatch and Heart Failure Event Risk. The Journal of Cardiovascular Nursing. 2015;30(5):394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu CM, Wang L, Chau E, et al. Intrathoracic impedance monitoring in patients with heart failure: correlation with fluid status and feasibility of early warning preceding hospitalization. Circulation. 2005;112(6):841–848. [DOI] [PubMed] [Google Scholar]
- 23.Ypenburg C, Bax JJ, van der Wall EE, Schalij MJ, van Erven L. Intrathoracic impedance monitoring to predict decompensated heart failure. The American journal of cardiology. 2007;99(4):554–557. [DOI] [PubMed] [Google Scholar]
- 24.Small RS, Wickemeyer W, Germany R, et al. Changes in intrathoracic impedance are associated with subsequent risk of hospitalizations for acute decompensated heart failure: clinical utility of implanted device monitoring without a patient alert. J Card Fail. 2009;15(6):475–481. [DOI] [PubMed] [Google Scholar]
- 25.Rathman LD, Lee CS, Sarkar S, Small RS. A critical link between heart failure self-care and intrathoracic impedance. The Journal of cardiovascular nursing. 2011;26(4):E20–E26. [DOI] [PubMed] [Google Scholar]
- 26.Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring Fatigue and Other Anemia-Related Symptoms with the Functional Assessment of Cancer Therapy (FACT) Measurement System. Journal of Pain and Symptom Management. 1997;13(2):63–74. [DOI] [PubMed] [Google Scholar]
- 27.Hjollund NH, Andersen JH, Bech P. Assessment of fatigue in chronic disease: a bibliographic study of fatigue measurement scales. Health and quality of life outcomes. 2007;5:12–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cella D, Y. S, Sorensen M, Chartash E, Sengupta N, Grober J. Validation of the Functional Assessment of Chronic Illness Therapy Fatigue Scale relative to other instrumentation in patients with rheumatoid arthritis. J Rheumatol. 2005;32:811–819. [PubMed] [Google Scholar]
- 29.Cella D, Lai J-s, Chang C-H, Peterman A, Slavin M. Fatigue in cancer patients compared with fatigue in the general United States population. Cancer. 2002;94(2):528–538. [DOI] [PubMed] [Google Scholar]
- 30.Jurgens CY, Fain JA, Riegel B. Psychometric testing of the heart failure somatic awareness scale. The Journal of cardiovascular nursing. 2006;21(2):95–102. [DOI] [PubMed] [Google Scholar]
- 31.Jurgens CY, Lee CS, Riegel B. Psychometric Analysis of the Heart Failure Somatic Perception Scale as a Measure of Patient Symptom Perception. J Cardiovasc Nurs. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spitzer RL, Kroenke K, Williams JDW. Validation and Utility of a Self-report Version of PRIME-MD: The PHQ Primary Care Study. JAMA. 1999;282(18):1737–1744. [DOI] [PubMed] [Google Scholar]
- 33.Kroenke K, Spitzer RL, Willaims JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pilkonis PA, Choi SW, Reise SP, et al. Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement Information System (PROMIS(R)): depression, anxiety, and anger. Assessment. 2011;18(3):263–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool For Mild Cognitive Impairment. Journal of the American Geriatrics Society. 2005;53:695–699. [DOI] [PubMed] [Google Scholar]
- 36.Harkness K, Heckman GA, Akhtar-Danesh N, Demers C, Gunn E, McKelvie RS. Cognitive function and self-care management in older patients with heart failure. Eur J Cardiovasc Nurs. 2014;13(3):277–284. [DOI] [PubMed] [Google Scholar]
- 37.Spertus JA, Jones PG. Development and Validation of a Short Version of the Kansas City Cardiomyopathy Questionnaire. Circulation: Cardiovascular Quality and Outcomes. 2015;8(5):469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu MH, Wang CH, Huang YY, Cherng WJ, Wang KW. A correlational study of illness knowledge, self-care behaviors, and quality of life in elderly patients with heart failure. J Nurs Res. 2014;22(2):136–145. [DOI] [PubMed] [Google Scholar]
- 39.Fry M, McLachlan S, Purdy S, Sanders T, Kadam UT, Chew-Graham CA. The implications of living with heart failure; the impact on everyday life, family support, co-morbidities and access to healthcare: a secondary qualitative analysis. BMC Fam Pract. 2016;17(1):139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dickson VV, Buck H, Riegel B. A qualitative meta-analysis of heart failure self-care practices among individuals with multiple comorbid conditions. J Card Fail. 2011;17(5):413–419. [DOI] [PubMed] [Google Scholar]
- 41.Riegel B, Dickson VV, Lee CS, et al. A mixed methods study of symptom perception in patients with chronic heart failure. Heart Lung. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Witte KK, Clark AL. Why does chronic heart failure cause breathlessness and fatigue? Progress in Cardiovascular Diseases. 2007;49(5):366–384. [DOI] [PubMed] [Google Scholar]
- 43.Fink AM, Sullivan SL, Zerwic JJ, PM R Fatigue With Systolic Heart Failure. Journal of Cardiovascular Nursing. 2009;24(5):410–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gheorghiade M, Filippatos G, De Luca L, Burnett J. Congestion in acute heart failure syndromes: an essential target of evaluation and treatment. Am J Med. 2006;119(12 Suppl 1):S3–S10. [DOI] [PubMed] [Google Scholar]
- 45.Stephen SA. Fatigue in older adults with stable heart failure. Heart Lung. 2008;37(2):122–131. [DOI] [PubMed] [Google Scholar]
- 46.Matura LA, Malone S, Jaime-Lara R, Riegel B. A Systematic Review of Biological Mechanisms of Fatigue in Chronic Illness. Biol Res Nurs. 2018;20(4):410–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosenman R, Tennekoon V, Hill LG. Measuring bias in self-reported data. Int J Behav Healthc Res. 2011;2(4):320–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Riegel B, Dickson VV, Faulkner KM. The Situation-Specific Theory of Heart Failure Self-Care. The Journal of Cardiovascular Nursing. 2015;31(3):226–235. [DOI] [PubMed] [Google Scholar]
- 49.Moser DK, Lee KS, Wu JR, et al. Identification of symptom clusters among patients with heart failure: an international observational study. Int J Nurs Stud. 2014;51(10):1366–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]