Abstract
Objective
To delineate research priorities for improving clinical management of laryngeal dystonia, the NIH convened a multidisciplinary panel of experts for a 1-day workshop to examine the current progress in understanding its etiopathophysiology and clinical care.
Methods
The participants reviewed the current terminology of disorder and discussed advances in understanding its pathophysiology since a similar workshop was held in 2005. Clinical and research gaps were identified, and recommendations for future directions were delineated.
Results
The panel unanimously agreed to adopt the term “laryngeal dystonia” instead of “spasmodic dysphonia” to reflect the current progress in characterizations of this disorder. Laryngeal dystonia was recognized as a multifactorial, phenotypically heterogeneous form of isolated dystonia. Its etiology remains unknown, whereas the pathophysiology likely involves large-scale functional and structural brain network disorganization. Current challenges include the lack of clinically validated diagnostic markers and outcome measures and the paucity of therapies that address the disorder pathophysiology.
Conclusion
Research priorities should be guided by challenges in clinical management of laryngeal dystonia. Identification of disorder-specific biomarkers would allow the development of novel diagnostic tools and unified measures of treatment outcome. Elucidation of the critical nodes within neural networks that cause or modulate symptoms would allow the development of targeted therapies that address the underlying pathophysiology. Given the rarity of laryngeal dystonia, future rapid research progress may be facilitated by multicenter, national and international collaborations.
Isolated dystonia is a neurologic disorder characterized by sustained or intermittent contractions causing abnormal, often repetitive movements, postures, or both. It is a rare disorder, with the incidence of up to 35.1 per 100,000 in the general population. Focal dystonias affect the muscle groups in a single body region and are the most common form of this disorder. Among these is the laryngeal form of dystonia characterized by task specificity and selective impairment of speaking but not whispering or innate vocal behaviors, such as laughing, crying, or yawning. Its clinical management is challenging due to the lack of established diagnostic markers and validated outcome measures, resulting in prolonged diagnostic delays and suboptimal therapies. Our ability to improve the patient care relies on scientific progress toward identification of its causative pathophysiology. If identified and validated, pathophysiologic markers will be critical for objective measures of early and accurate disorder detection and diagnosis and the assessment of efficacy of existing and novel therapeutic options.
This report outlines the consensus outcome of a multidisciplinary panel of experts from the fields of neurology, otolaryngology, speech-language pathology, neurosurgery, genetics, and neuroscience who reviewed the clinical definition of the laryngeal form of dystonia and discussed progress in understanding its pathophysiology. The workshop was organized by the National Institute on Deafness and Other Communication Disorders (NIDCD) and held in August of 2019. The panel participants were selected based on the expertise in their respective fields and the ability to provide a broad overview of dystonia and related disorders. Clinical and research gaps were examined, and recommendations for future directions were delineated. Other workshop attendees included additional experts in the field, NIDCD program directors, and patient representatives who participated in the discussions of the panel.
Updated Terminology: Laryngeal Dystonia
The panel of experts discussed the need for updated terminology that would more inclusively and accurately define the clinical phenomenology of dystonia affecting the laryngeal muscles. The proposed adoption of the term “laryngeal dystonia (LD)” instead of the more frequently used “spasmodic dysphonia” was unanimously agreed upon to reflect the current progress in scientific and clinical characterization of this disorder. LD was classified into adductor (ADLD), abductor (ABLD), singer's LD (SLD), mixed, and adductor respiratory (ARLD) forms. ADLD is the most common form characterized by strained-strangled quality of voice with intermittent voice stoppages during vowel production. Much rarer ABLD is characterized by intermittent breathy voice breaks, occurring predominantly on voiceless consonants. Mixed LD combines the features of both ADLD and ABDL. SLD is a rare form that can be considered as a subtype of both LD and musician's dystonia. It affects professional singers and has symptoms characteristic of either ADLD or ABLD occurring selectively during singing. ARLD involves adductor laryngeal spasms during inspiration, causing stridor, dyspnea, or obstruction. This new terminology more accurately reflects the current movement disorder nomenclature of other forms of dystonia.
Multidisciplinary Clinical Assessment of Laryngeal Dystonia
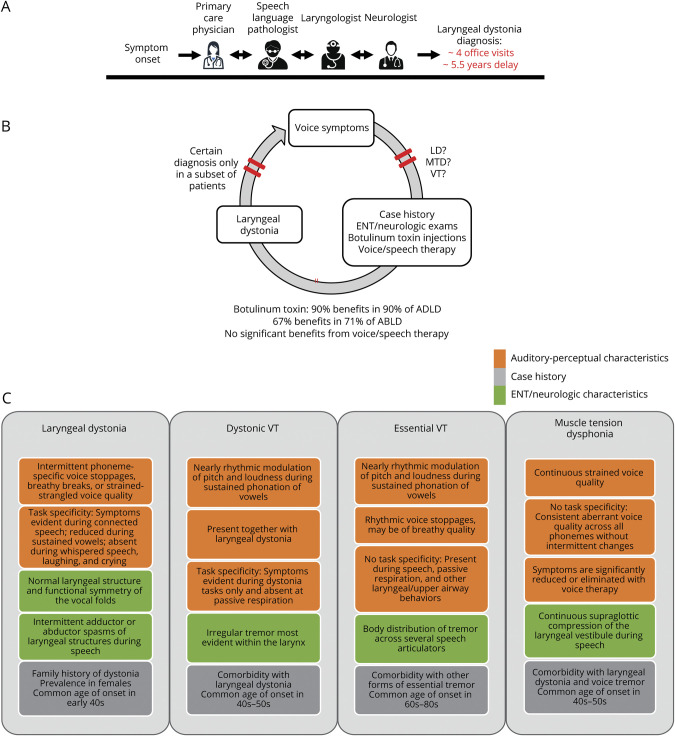
Similar to other forms of isolated dystonia, there are no biomarkers of LD that are implemented in clinical setting as objective diagnostic tests. Diagnosis continues to be based on qualitative and phenomenological assessments, predominantly rendered by laryngologists or speech-language pathologists, in some cases, in consultation with movement disorder neurologists (figure 1A). This approach is, however, not reliable, as recent data from a multicenter study showed a discouraging 34% agreement rate on LD diagnosis with nil to minimal agreement at Cohen κ = 0.05–0.26 between laryngologists, speech-language pathologists, and neurologists.1 The low diagnostic accuracy of LD is not an outlier among other forms of dystonia. Earlier studies found a minimal to weak agreement rate between neurologists (Cohen κ = 0.20–0.52) on the diagnosis of oromandibular dystonia, writer's cramp, blepharospasm, and cervical dystonia.2 These findings point to a persisting clinical challenge in diagnosing isolated dystonia, independent of its form, in the absence of a clinically applicable biomarker and its diagnostic test. Consequently, it is estimated that an average delay of LD diagnosis is 5.5 years, with an average of 4 office visits3 (figure 1A). Until accurate, objective diagnostic tests of LD are available, the common elements of clinical assessment should incorporate a detailed case history, auditory-perceptual testing, nasoendoscopy, and neurologic examination. This combined evaluation is essential for improving diagnostic precision and reducing delays in treatment.
Figure 1. Standard Clinical Management and Clinical Characteristics and of Laryngeal Dystonia.
(A) The current standard clinical management of laryngeal dystonia. The patient undergoes multiple assessments by several specialists until the final diagnosis can be reached, often delaying the overall time-to-diagnosis for several years. Multidisciplinary team evaluations of a patient are recommended to facilitate the diagnosis and initiate the treatment. (B) Clinical diagnosis is based on a syndromic approach, using (C) a combination of case history, auditory-perceptual characteristics, and laryngeal/neurologic examinations. Red bars in (B) indicate different stages in the diagnostic process when the clinical decision is refined based on additional evaluations. AD = autosomal dominant; ABLD = abductor form of laryngeal dystonia; ADLD = adductor form of laryngeal dystonia; LD = laryngeal dystonia; MTD = muscle tension dysphonia; VT = voice tremor.
LD affects more women than men (4:1 ratio), with the average onset around 40 years of age.4,5 About 55% of patients with LD report gradual symptom development, whereas the other half (45%) experience a sudden onset, often associated with stress or upper respiratory infection. The majority of patients (82.4%) have a focal laryngeal presentation, whereas 17.6% of patients exhibit a spread of dystonia to other body regions.4 Over 55% of patients report symptom improvement after ingesting alcohol,6 and some report the presence of geste antagoniste or sensory tricks, such as touching the throat, head, and abdomen and laughing or humming before speaking, that temporarily reduce symptoms.4 One of the important clinical characteristics of LD is its task specificity, that is, LD symptoms are defined by selective impairment of speaking in ADLD, ABLD, and mixed LD, singing in SLD, and inspiration in ARLD. Patients with ADLD exhibit worse symptoms on voiced phonemes during counting from 80 to 90, whereas patients with ABLD have more difficulties with voiceless phonemes during counting from 60 to 70. In addition, shouting may differentiate between LD subtypes as it is more challenging for those with ADLD due to increased effort for voice projection. Conversely, whispered speech, overt emotional speech, innate vocalizations (e.g., crying, laughing, and yawning), and other upper respiratory behaviors (e.g., coughing and sniffing) remain intact4,5 (figure 1C).
Based on LD task specificity, a series of vocal tasks, including sustained and repetitive phonations of vowels, pitch glides, shouting, counting, and overt and whispered production of sentences loaded with voiced or voiceless phonemes, are recommended for defining LD and differentiating it from voice tremor and muscle tension dysphonia.4,5,7 Both voice tremor and muscle tension dysphonia affect up to one-third of patients with LD and are often misdiagnosed as LD or vice versa. The central vs peripheral origin of hyperfunctional voice in muscle tension dysphonia remains unclear,8 whereas understanding of the voice tremor spectrum is still being developed.9,10 A recent consensus on tremor classification listed voice tremor as an additional clinical phenotype beyond the core criteria used to classify essential and dystonic tremor.9 This is despite the fact that specific clinical characteristics that differentiate between those with dystonic and essential voice tremor were published earlier by the Neurolaryngology Committee of the American Academy of Otolaryngology—Head and Neck Surgery.10 We view voice tremor as an umbrella diagnosis where dystonic voice tremor is characterized by task-specific laryngeal tremor that co-occurs with LD, whereas essential voice tremor affects laryngeal muscles either in isolation or in combination with other upper airway structures and/or extremities, is not task specific, and may be present independent of LD (figure 1C).
In the majority of cases, voice and speech therapy and botulinum toxin injections into the laryngeal muscles are tried to help with differential diagnostics (figure 1B). LD symptoms typically do not respond well to voice and speech therapy, although individuals can benefit from treatment focusing on education, counseling, and effective speaking strategies to address their heightened anxiety regarding social and occupational communication situations. Botulinum toxin injections are more effective in ADLD than any other form of LD or voice tremor. Voice tremor symptoms may exhibit reduced symptom severity with behavioral therapy if able to modify their speaking patterns to shorten voicing duration. Conversely, symptoms of muscle tension dysphonia characterized by significant vocal effort due to excessive tension in laryngeal and extralaryngeal muscles are typically relieved with behavioral therapy, although more severe cases may benefit from botulinum toxin injections, which may lead to resolution of symptoms after a single treatment.
Other methods probed for diagnosis and differentiation of LD from other voice disorders include high-speed videoendoscopy and laryngeal EMG. High-speed videoendoscopy showed promise for detection of distinct patterns of spasms affecting vocal fold vibratory motion in LD vs muscle tension dysphonia and voice tremor.11,12 However, its sensitivity and specificity need to be established before the wider application in clinical settings. Similar to other forms of dystonia, EMG is not used for LD diagnosis.4 It offers a qualitative rather than definitive diagnostic value due to the fact that potentials are typically normal. However, laryngeal EMG combined with an acoustic channel may show a marked delay from the onset of an electrical signal to the onset of acoustic output and as such might be useful in aiding the differential diagnosis between LD, voice tremor, and muscle tension dysphonia.
Summary, Gaps, and Priorities for Multidisciplinary Clinical Assessment and Diagnosis of LD
LD is a phenotypically complex and heterogeneous disorder that requires a multidisciplinary clinical approach for accurate diagnosis.
The current diagnosis of LD is based on a syndromic approach that is open to bias; thus, a diagnostic consensus between clinicians is hard to achieve.
Clinical diagnosis is affected by the variability of LD symptoms, co-occurring conditions that mimic LD symptoms, and the experience and expertise of the clinician.
The access to health care professionals with the necessary knowledge and skills is a significant barrier. Less than 6% of speech-language pathologists work in a health care setting where patients with LD are likely to be seen. Only 2% of otolaryngologists are trained and specialized in laryngology. The proportion of movement disorder neurologists specialized in LD is likely far smaller. Specialized training of clinicians in LD and related disorders is critical for reducing misdiagnosis and delayed diagnosis.
Ultimately, the highest priority is clinical implementation of LD-specific, pathophysiologically relevant biomarkers that are accurate, fast, objective, and cost-effective in diagnosing LD and differentiating it from other similar conditions.
Acceleration of a biomarker-based LD diagnosis necessitates the identification of etiology and pathophysiology of this disorder.
Etiology of Laryngeal Dystonia
Genes and Genetic Risk Factors
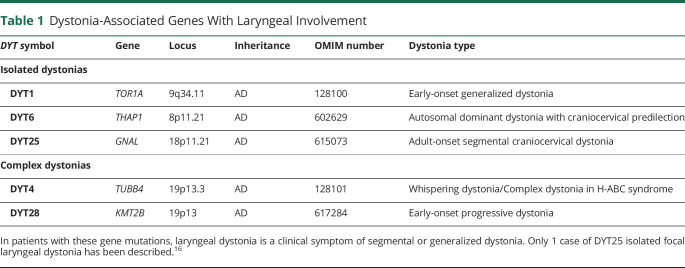
LD is characteristically multifactorial in its etiology, and genetic variants are considered a significant risk factor for disorder development. It has been reported that up to 25.3% of patients with LD have a family history of dystonia, and up to 11.8% of patients have a family history of other movement disorders.4-6 However, traditional linkage studies in LD have been severely limited by rare availability of large families, phenotypic discordance between affected family members, low penetrance of dystonia, and late age at onset. As such, causative gene mutations of isolated focal LD remain unknown.
Among the verified gene mutations causing other forms of dystonia, laryngeal involvement is reported in patients with generalized and segmental dystonias who are carriers of DYT1, DYT4, DYT6, DYT25, and DYT28 mutations13-15 (table 1). Only 1 case of focal ADLD with DYT25 (GNAL) mutation and without any other co-occurring forms of dystonia, a family history of dystonia, or other movement disorders was identified to date.16 This finding pointed to the genetic overlap between LD and other forms of dystonia as well as suggested that gene mutations may underlie even sporadic LD presentations as a result of reduced penetrance. It was proposed that stratification of patients into truly sporadic and familial cases would remain arbitrary, pending the discovery of causative gene mutations specific to focal LD.16
Table 1.
Dystonia-Associated Genes With Laryngeal Involvement
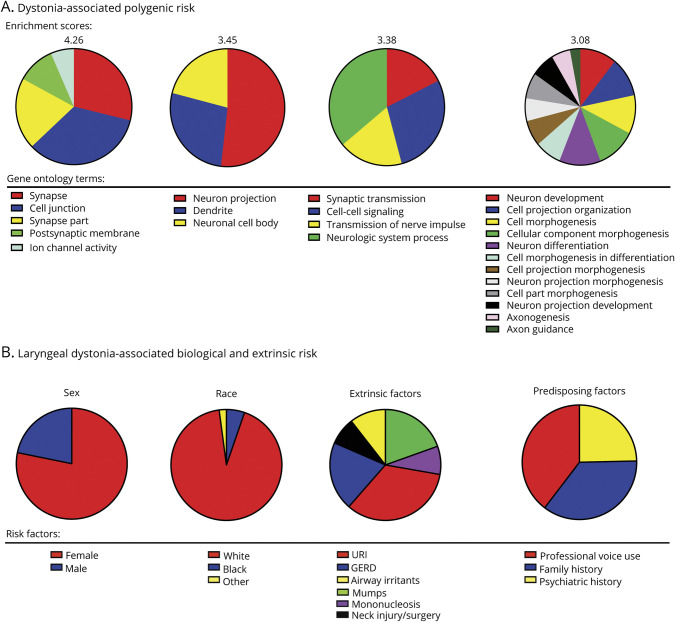
Other efforts in the field of LD genetics have been directed toward the identification of polygenic risk that affects disorder development. Although traditional genome-wide association studies typically lack power given the limited number of DNA samples available from patients with LD, the polygenic risk analysis found a significant number of genetic variants lying near genes related to synaptic transmission and neural development17 (figure 2A). The enrichment of genes related to synaptic transmission is in line with alterations of dopaminergic and GABAergic neurotransmission in LD, as discussed below. In parallel, the DYT1 and DYT11 genes were found to be highly expressed during early brain development, consistent with the view that dystonia, including LD, may be a neurodevelopmental disorder.
Figure 2. Risk Factors for the Development of Laryngeal Dystonia.
(A) Dystonia-associated polygenic risk and the contribution of different gene ontology terms to the enrichment score based on data described in Ref. 17. (B) Distribution of laryngeal dystonia-associated biological and extrinsic risk factors based on data described in Ref. 3.
Endophenotypic Traits
Intermediate, or mediational, endophenotypes reflect gene expression and share common pathogenic mechanisms with phenotype, thus linking genotype with a disorder phenotype. Conversely, secondary endophenotypes arise solely through disease manifestation with adaptive, compensatory neural changes and are found in the disease state only. Thus, the identification of LD endophenotypes is critical for a better understanding of its causes. Furthermore, because prevention of the endophenotype progression is thought to prevent the disorder, the establishment of LD endophenotypes through the examination of unaffected family members would allow identification of a much-needed biomarker of LD development and estimation of the trajectory of symptom manifestation in at-risk individuals.
Recent research disclosed that temporal discrimination, measured as a time interval at which an individual perceives 2 stimuli as being asynchronous, is abnormal across different forms of dystonia and may represent a mediational endophenotype.18 In LD, abnormalities in visual temporal discrimination threshold (TDT) were found with both higher frequency and higher penetrance in familial than sporadic LD.19 In contrary, abnormal TDT frequency rates did not differ in clinically distinct ADLD and ABLD,19 whereas SLD, together with musician's focal hand dystonia, showed normal TDT ranges.20 The latter may be either due to patients with SLD harnessing inherently superior timing abilities as a result of long-term musical skill acquisition or lesser role of maladaptive plasticity in shaping TDT alterations.20 It was proposed that abnormal TDT as the mediational endophenotype in nonmusician forms of LD has a closer, more upstream relationship with the underlying (yet unknown) gene mutation than its variable clinical phenotype.19 Overall, this line of research concluded that broad genetic influences are greater in patients with familial LD, which may prime them to develop dystonia triggered by intrinsic risk factors. On the other hand, largely similar TDT abnormalities across the LD phenotypical spectrum pointed to the influence of extrinsic risk factors.
Extrinsic Risk Factors
Extrinsic risk factors are exogenous to the individual but may interact with genetic or other intrinsic factors to predispose and trigger the disease onset. Studying extrinsic risk in epidemiologic studies of LD is not trivial given its low prevalence, relatively small research cohorts, recall bias, and frequent LD diagnostic errors.
Although there is no direct evidence for isolated focal LD to occur due to the causative influence of an extrinsic factor alone, case-control studies point to significantly higher frequency of some health and environmental events in patients with LD vs the general population3,21 (figure 2B). White females have been identified at a higher risk of developing LD, which combined with a higher frequency of a family history of dystonia points to a possible interaction between predisposing risk factors. Similarly, a significant history of anxiety, depression, and stress before LD symptom onset suggests the potential risk of a psychiatric dimension in its pathophysiology.
Professional voice use was reported as another prevalent factor among patients with LD and most relevant to SLD, with parallels drawn with repetitive hand motor tasks, such as strenuous fine motor training in musician's dystonia.21,22 Recent research further showed that stressors altering sensory feedback from the larynx (i.e., recurrent upper respiratory infections, gastroesophageal reflux, and neck injury) may represent an extrinsic risk for LD and contribute to altered sensorimotor preparation and integration in susceptible individuals.3
Summary, Gaps, and Priorities for Understanding the Etiology of LD
Although LD genetics presents unprecedented challenges for the discovery of a causative mutation, a single case of isolated focal LD with DYT25 (GNAL) mutation has been identified, and the polygenic risk of dystonia, including LD and involving genes implicated in synaptic transmission and neural development, has been determined.
Abnormal sensory discrimination may be considered as an LD endophenotype.
Certain extrinsic risk factors may trigger LD manifestation in susceptible individuals.
Multi-institutional studies are needed to overcome challenges associated with the sample size required for conducting large-scale genomic studies in LD. A cross-disciplinary approach should integrate LD genetics, endophenotypes, and extrinsic triggers with the disorder pathophysiology and symptomatology. Until then, caution should be exercised when stratifying sporadic and familial LD cases.
Novel approaches to LD prevention, diagnostics, and treatment may be developed based on enhanced understanding of the interplay between genetic and extrinsic risk factors.
Pathophysiology of Laryngeal Dystonia
Brain Structure and Function
LD, as all other forms of isolated dystonia, has long been considered a textbook example of a basal ganglia disorder. This notion was an approximation made on the initial observation that striatal lesions most often trigger the development of secondary or combined dystonias.23 Recent advanced neuroimaging studies have been instrumental in expanding our understanding of dystonia pathophysiology by determining that LD is a functional and structural neural network disorder, which commonly encompasses abnormalities in primary sensorimotor and higher-order motor and associative cortical areas, thalamus, and cerebellum, in addition to the basal ganglia24 (figure 3A). Specifically, robust structural and functional abnormalities were mapped not only in the laryngeal region of the primary sensorimotor cortex but also premotor and inferior parietal areas.25-28 Vulnerable parietal-premotor function was linked to the polygenic risk of LD17 and found to be influenced by the extrinsic risk factors altering laryngeal sensory feedback.3 Neural alterations in LD were further found in cortical areas that are explicitly associated with the control of speech processing, motor preparation, and executive functions, such as inferior/middle frontal gyri, superior/middle temporal gyri, and parietal operculum.26,27,29-34
Figure 3. Characteristic Brain Alterations in Laryngeal Dystonia.
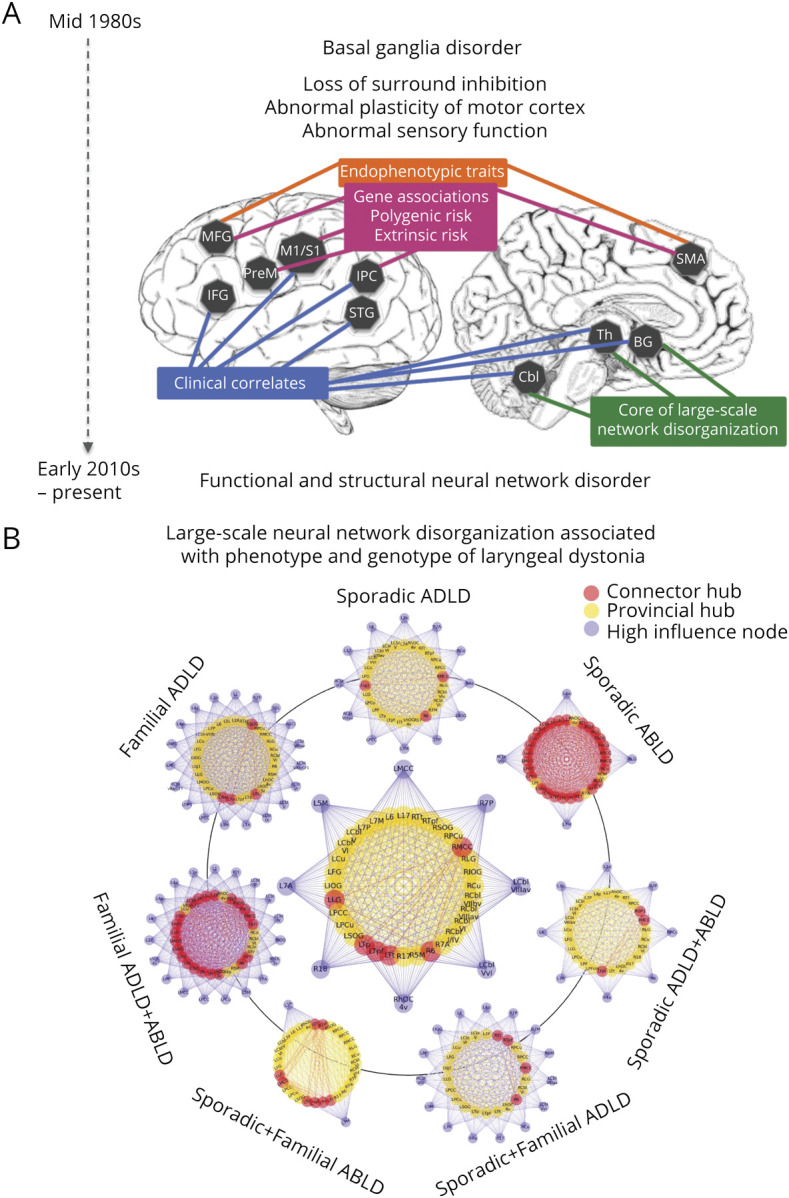
(A) Schematic of large-scale neural network alterations in laryngeal dystonia, with associations between regional changes, clinical features, endophenotypic traits, genetic mutations, polygenic risk, and extrinsic risk. The timeline shows the evolution of understanding of the pathophysiology of dystonia from a basal ganglia disorder to a functional and structural neural network disorder. This figure was modified from Ref. 24 to represent the neuroimaging literature in laryngeal dystonia. (B) Common features of large-scale neural network disorganization in patients across different phenotypes and genotypes of laryngeal dystonia (middle circular plot) and the distinct features of the large-scale network architecture based on the disorder phenotype and genotype. The figure was modified from Ref. 45. The inner circle in each graph represents the network hubs (red—connector hubs; yellow—provincial hubs); the outer circle in each group represents high-influence network nodes; lines represent connections of each node with the network. For detailed information on network node/hub participation, see original research study.45 ABLD = abductor form of laryngeal dystonia; ADLD = adductor form of laryngeal dystonia.
Studies examining brain structure-function relationship demonstrated that abnormal activity in the primary sensorimotor cortex, inferior parietal cortex, putamen, and cerebellum is associated with underlying gray matter structural disorganization.27,29 Using diffusion-weighted imaging combined with postmortem neuropathology, reduced white matter integrity in the descending corticobulbar tract was attributed to regional axonal demyelination, whereas increased water diffusivity in the basal ganglia and cerebellum was related to clusters of iron, calcium and phosphate precipitates.28
Other studies determined that increased activity in the left primary sensorimotor cortex and cerebellum and abnormal gray matter organization in the right inferior frontal gyrus, left parietal operculum, insula, and cerebellum were associated with LD symptom severity.27,33 Altered functional connectivity of the left thalamus with caudate nucleus and of the inferior parietal cortex with supplementary motor area was correlated with LD clinical characteristics,17,34,35 whereas abnormal structural connectivity of the left caudate nucleus and insula was associated with LD duration and symptom onset.36 However, it remains unclear whether the relationship between brain changes and clinical features is primary to disorder pathophysiology or compensatory due to the presence of LD symptoms.
Brain Plasticity and Neurotransmission
LD shares several pathophysiologic features with other focal dystonias, including loss of inhibition and abnormal neurotransmitter function. Loss of inhibition in dystonia involves both the motor and sensory systems at the spinal, brainstem, and cortical levels. Loss of inhibition leads to loss of surround inhibition in the motor command, predisposing to overflow movements. Moreover, sensory abnormalities may arise from loss of short-latency inhibitory processes. Loss of inhibition has been consistently documented as a decrease in the cortical process of short intracortical inhibition and loss of inhibition of the blink reflex recovery curve37-40 and shown to differentiate between LD and muscle tension dysphonia.41
Derangement of neurotransmitters in LD was characterized by a deficiency of a major inhibitory neurotransmitter and its GABA-A receptors,42 a deficiency of dopamine D2 receptors within the indirect basal ganglia pathway, an excess of dopamine D1 receptors within the direct basal ganglia pathway, and an abnormal nigrostriatal dopamine release.43,44 Loss of GABAergic function together with D1/D2 imbalance favors the direct pathway over the indirect pathway hypothesis, potentially leading to excess (dystonic) movement. Notably, neurotransmitter abnormalities were found within the speech motor system, pointing to their contribution to task-specific impairment of speech in LD. Given that the brain operates in networks, these pathophysiologic features would likely contribute to abnormalities of brain network function, and their malfunction would lead to clinical symptoms of dystonia.
Brain Networks
Advances in network neuroscience led to important discoveries about the global disorganization of functional and structural neural networks in LD. Studies using graph theoretical analysis showed that functional and structural connectomes in LD are characterized by a breakdown of the basal ganglia-thalamo-cerebellar community, loss of regions of information transfer (hubs) in sensorimotor and parietal cortical regions, and loss of hemispheric lateralization of neural communities.35,36,45,46 Different phenotypes and putative genotypes of LD were further characterized based on their unique network architecture45 (figure 3B). Other studies using independent component analysis confirmed the presence of sensorimotor and frontoparietal network alterations, with phenotype- and genotype-based distinct changes involving primary somatosensory, premotor, and parietal cortices.47 Investigation of regional influences within these networks in LD determined that alterations are due to abnormally increased excitatory influence of the left inferior parietal cortex onto the left putamen and of the right premotor cortex onto its left homolog.48 A conceptually novel, mechanistic model of LD network alterations was formulated, where disruption of sensorimotor regions controlling movement planning and execution is instigated by hyperexcitable premotor interhemispheric communication and top-down parietal to putaminal influence.48 This pathophysiologic cascade is likely staged in inferior parietal and premotor cortical areas before the output of dystonic speech by primary motor cortex. From a clinical point of view, the significance of alterations in these regions is apparent from their diagnostic potential in successful machine-learning classification of LD, achieving up to 98.8% accuracy in objectively diagnosing this disorder.47,49
Summary, Gaps, and Priorities for Understanding the Pathophysiology of LD
Neuroimaging studies determined that LD pathophysiology involves widespread alterations of network function and structure, which comprise not only the basal ganglia but also higher-order motor and associative cortical regions, thalamus and cerebellum. Alterations of premotor and parietal cortices are of critical importance as they are influenced by external and polygenic risk factors, likely triggering symptoms in susceptible individuals.
Altered brain plasticity and neurotransmission in LD points to other mechanisms in dystonia pathophysiology, including abnormal dopaminergic and GABAergic function and maladaptive plasticity.
The knowledge gap includes the understanding of primary vs compensatory neural abnormalities, which play a mechanistic role in the pathophysiology of LD.
The identification of complex pathophysiologic mechanisms underlying the development of LD symptoms necessitates the use of complex cross-disciplinary and multimodal methodologies to assess different aspects of pathophysiology. Identification of LD mechanistic pathophysiology would make attainable the formulation of novel diagnostic and treatment opportunities for these patients.
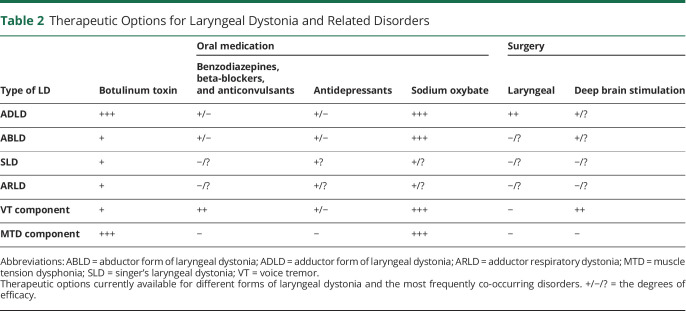
Existing and Experimental Treatment Approaches in Laryngeal Dystonia
Currently, there are no established therapies for successful treatment of LD other than temporary management of its symptoms (table 2). In parallel, unified outcome measures are not determined, with as many as 220 different objective and subjective instruments being used to evaluate the outcome across studies. Auditory-perceptual measures of voice quality are the most frequently used approach, with acoustics being most often used to quantify voice characteristics and their change following treatment. Nearly 80 different acoustic parameters have been published; however, none were identified as highly sensitive or specific to LD. Without a consensus on specific benchmark outcome measures for LD, a meaningful and timely decision regarding symptom management in the clinical setting remains challenging. We review the existing and experimental treatments of LD with this caveat in mind.
Table 2.
Therapeutic Options for Laryngeal Dystonia and Related Disorders
Pharmacologic Therapies and Laryngeal Surgery
For the past 3 decades, standard of LD care has been largely limited to symptom management with botulinum toxin (BoNT) injections into the laryngeal muscles.4 One study reported that BoNT may influence brain activity in LD,31 whereas others found no direct central effects,32,50 suggesting that toxin-induced changes in laryngeal physiology may have compensatory mechanisms in influencing brain activity via modulated feedback loops. In the absence of better therapies, BoNT is a treatment of choice that is tried at least once in the majority of patients with LD. It, however, provides only temporary relief and shows narrow benefits due to its ineffectiveness in nearly 40% of patients.51 On the other hand, the short-term action of BoNT presents an advantage over more permanent laryngeal surgery as the effects of injection are easily reversible when new therapies of the underlying pathology become available. BoNT is predominantly effective in ADLD compared with any other form of disorder. In treatment-responsive patients, benefits are seen for approximately 30% of the injection cycle, with 51% of patients experiencing prolonged side effects that often interfere with breathing and swallowing.4 Treatment efficacy may gradually decrease over time as some patients develop resistance to BoNT.52 Injections are burdensome psychologically and financially as they are expensive and must be repeated every 3–4 months throughout the patient's life.
Other pharmacologic or surgical therapies have not yet been established for the long-term treatment of LD. On empirical bases, about 6% of patients receive off-label medications, such as beta-blockers, benzodiazepines, or anticonvulsants, which provide only mild, if any, benefits.51 Various surgical approaches for anatomic remodeling of the larynx as the affected end organ or its peripheral nerves are probed in ADLD, although their long-term efficacy is not established. Among these are the recurrent nerve section procedure that provided initially promising results but had many failures over time; selective laryngeal denervation-reinnervation; type 2 thyroplasty with an implant; laser myectomy of thyroarytenoid or posterior cricoarytenoid muscles, and implanted peripheral nerve stimulators.4
Considering the alcohol responsiveness of LD symptoms in more than 55% of patients6 and pathophysiologically relevant abnormal GABAergic neurotransmission with loss of inhibition,42 a centrally acting oral drug, sodium oxybate, has been experimentally tried in the open-label study in patients with LD.53 Sodium oxybate is a schedule III controlled substance, chemically identical to gamma-hydroxybutyric acid that crosses the blood-brain barrier and converts into GABA. Sodium oxybate was found to significantly reduce symptom severity in the majority (82.2%) of alcohol-responsive patients, with the effects lasting about 4 hours. Its short-lived but fast-acting mechanism may pose both benefits (e.g., self-administration at home and on demand) and drawbacks (e.g., repeated ingestion) for the patient. Importantly, sodium oxybate treatment showed direct modulatory effects on LD pathophysiology by attenuating hyperfunctional activity in cerebellar, thalamic, and sensorimotor cortical regions.51 Currently ongoing double-blind placebo-controlled randomized crossover study of sodium oxybate (NCT03292458) will provide more in-depth understanding of the benefits of this drug for its wider recommendation as a treatment choice for alcohol-responsive LD.
Laryngeal Modulation as Experimental Therapy
Improved understanding of LD pathophysiology has led to the development of paradigms for experimental laryngeal stimulation as alternative clinical management strategies of this disorder. Vibrotactile and electrical stimulation approaches have been used to target the laryngeal proprioceptive system. One study evaluating electrical stimulation of the left thyroarytenoid muscle reported symptom improvement in 4 of 5 patients, with a carryover effect of 3–12 days.54 In another study, vibrotactile stimulation over the thyroid cartilage showed reduction of LD symptoms in 69% of patients, with a carryover effect of 20 minutes.55 Vibrotactile stimulation suppressed theta activity (4–8 Hz) over the left sensorimotor cortex and increased low gamma activity (30–49 Hz) over the right sensorimotor cortex. Although tested in small cohorts, noninvasive neuromuscular modulation may have a temporary effect by influencing the laryngeal afferent feedback. It remains unclear what type of receptors play a role in laryngeal feedback, with some implying the possibility for mucosal mechanoreceptors and muscle spindles. The next phase of this research is currently underway in an attempt to define the optimal stimulation parameters, vibration frequency, duration, and frequency of applications, as well as to optimize the implantable stimulator.
Brain Modulation as Experimental Therapy
Noninvasive neuromodulation with repetitive TMS (rTMS) and transcranial direct current stimulation (tDCS) of the motor cortex or cerebellum has been used in other forms of dystonia, with a therapeutic range from none to significant symptom improvement.56 Regrettably, much less is known to date whether noninvasive neuromodulation is an effective therapeutic option in LD as these therapeutic approaches have yet to be probed in this disorder.
Invasive brain modulation with deep brain stimulation (DBS) of unilateral or bilateral stimulation of the globus pallidus internus (GPi) or subthalamic nucleus (STN) has been approved by the Food and Drug Administration for the treatment of drug-refractory generalized, segmental and cervical dystonias and hemidystonia. Its therapeutic effects are thought to be due to disruption of increased synchronization between the basal ganglia and motor cortex. Limited case studies reported some positive DBS effects on LD symptoms in patients with concurrent DYT6 dystonia, cervical dystonia, and cricopharyngeal dystonia.57,58 Limited case studies reported potential therapeutic benefits of thalamic DBS in patients with essential tremor with co-occurring ADLD.59
Summary, Gaps, and Priorities for Treatment of LD
BoNT injections continue to prevail as clinical choice for temporary symptom management of LD. However, the benefits are limited, with more than 40% of patients remaining untreated. Longitudinal studies of botulinum toxin effect on central brain activity are warranted to help clarify the nature of its benefits.
A novel centrally acting oral drug, sodium oxybate, showed initial efficacy in alcohol-responsive LD and is currently being tested in a clinical trial to determine its benefits and mechanisms of action.
Therapeutic approaches to laryngeal modulation using vibrotactile or electrical stimulation are being explored, whereas targeted noninvasive or invasive brain stimulation remains scarce.
Future research needs to examine parallel avenues for drug development, both through targeting known pathophysiologic mechanisms and repurposing existing drugs. Similarly, laryngeal modulation may show greater therapeutic benefits when paired with brain stimulation.
Novel LD-specific neural targets of a therapeutic potential need to be defined based on disorder pathophysiology for both invasive and noninvasive brain stimulation. These studies require carefully designed and controlled clinical trials that use validated, unified outcome measures and include deeply phenotyped patients.
Summary
Since a similar NIDCD workshop was held in 2005, numerous advances have been made to clinically delineate LD and investigate its genetics and pathophysiology. Based on this collective knowledge, we recommend the revised use of terminology of “laryngeal dystonia,” instead of “spasmodic dysphonia,” that is inclusive of several related forms of this disorder. LD is currently considered a multifactorial, phenotypically heterogeneous form of isolated focal dystonia. Its etiology, including genetic causes, remains unknown, whereas the pathophysiology likely involves large-scale functional and structural brain network disorganization. In addition, endophenotypic traits, extrinsic and polygenic risk factors of LD have been identified and their influence on disorder pathophysiology has been described. Despite this progress, current clinical challenges include the lack of objective, clinically validated markers for LD diagnosis and the paucity of long-tern efficacious therapeutic options that address LD pathophysiology. The goal to improve LD diagnostics and treatment should guide the prioritization of future research endeavors. Clinical translation and implementation of highly sensitive and specific biomarkers47,49 would not only enable the development of novel diagnostic tools but also define unified clinical outcome measures of treatment effects. With more precise objective diagnostic tests, specific targeted therapy can be developed that addresses the underlying pathogenesis for each patient, including drugs and targeted neuromodulation. Research elucidating critical hubs of neural networks that cause or modulate LD symptoms would lead to the development of novel treatments that address the underlying pathophysiology of this disorder. Given the rarity of LD, the achievement of these ambitious goals may be facilitated by multicenter national and international collaborations, with teams including clinicians and researchers across different disciplines.
Acknowledgment
The authors thank Kaitlyn Dwenger, BS, Stefan Fuertinger, PhD, and Davide Valeriani, PhD, for their help with figure panels.
Glossary
- ABLD
abductor laryngeal dystonia
- ADLD
adductor laryngeal dystonia
- ARLD
adductor respiratory
- LD
laryngeal dystonia
- NIDCD
National Institute on Deafness and Other Communication Disorders
- SLD
singer's laryngeal dystonia
- TDT
temporal discrimination threshold
Appendix 1. Authors
Appendix 2. Coinvestigators
Contributor Information
Collaborators: Gerald Berke, Tanya Eadie, Jeremy Greenlee, Michael Hammer, Michael Johns, Juergen Konczak, Christy Ludlow, Srikantan Nagarajan, Callum Ross, Phillip Song, and Cara Stepp
Study Funding
The workshop generating this article was organized and funded by the National Institute on Deafness and Other Communication Disorders, NIH.
Disclosure
Dr. Simonyan reports no relevant disclosures. She receives funding from the NIH (R01NS088160, R01DC011805, and R01DC012545), Department of Defense (W911NF1810434), and Amazon Web Services and serves on the Scientific Advisory Board of the Tourette Association of America. Dr. Barkmeier-Kraemer reports no relevant disclosures. She receives funding from the NIH (R01DC016838). Dr. Blitzer has no relevant disclosures. He received research grants from Allergan, Inc., and Merz Pharmaceuticals. Dr. Hallett holds patents for an immunotoxin for the treatment of focal movement disorders and the H-coil for magnetic stimulation; in relation to the latter, he has received license fee payments from the NIH (from Brainsway). He is on the Medical Advisory Boards of CALA Health, Brainsway, and Cadent. He receives royalties and/or honoraria from publishing from Cambridge University Press, Oxford University Press, Springer, and Elsevier. He has research grants from Allergan for studies of methods to inject botulinum toxins, Medtronic, Inc., for a study of DBS for dystonia, and CALA Health for studies of a device to suppress tremor. The work was conducted in the course of employment for the NIH, an agency of the US Government. Dr. Houde, Dr. Kimberley, Dr. Ozelius, Dr. Pitman, and Dr. Richardson report no relevant disclosures. He served as a consultant for NeuroPace, Medtronic, and Zimmer Biomet. Dr. Sharma and Dr. Tanner report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Ludlow CL, Domangue R, Sharma D, et al. Consensus-based attributes for identifying patients with spasmodic dysphonia and other voice disorders. JAMA Otolaryngol Head Neck Surg 2018;144:657–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Logroscino G, Livrea P, Anaclerio D, et al. Agreement among neurologists on the clinical diagnosis of dystonia at different body sites. J Neurol Neurosurg Psychiatry 2003;74:348–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Lima Xavier L, Simonyan K. The extrinsic risk and its association with neural alterations in spasmodic dysphonia. Parkinsonism Relat Disord 2019;65:117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blitzer A, Brin MF, Simonyan K, Ozelius LJ, Frucht SJ. Phenomenology, genetics, and CNS network abnormalities in laryngeal dystonia: a 30-year experience. Laryngoscope 2018;128(Suppl 1):S1–S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guiry S, Worthley A, Simonyan K. A separation of innate and learned vocal behaviors defines the symptomatology of spasmodic dysphonia. Laryngoscope 2019;129:1627–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirke DN, Frucht SJ, Simonyan K. Alcohol responsiveness in laryngeal dystonia: a survey study. J Neurol 2015;262:1548–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barkmeier JM, Case JL, Ludlow CL. Identification of symptoms for spasmodic dysphonia and vocal tremor: a comparison of expert and nonexpert judges. J Commun Disord 2001;34:21–37. [DOI] [PubMed] [Google Scholar]
- 8.Roy N, Dietrich M, Blomgren M, Heller A, Houtz DR, Lee J. Exploring the neural bases of primary muscle tension dysphonia: a case study using functional magnetic resonance imaging. J Voice 2019;33:183–194. [DOI] [PubMed] [Google Scholar]
- 9.Bhatia KP, Bain P, Bajaj N, et al. Consensus statement on the classification of tremors. From the task force on tremor of the international Parkinson and movement disorder society. Mov Disord 2018;33:75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merati AL, Heman-Ackah YD, Abaza M, Altman KW, Sulica L, Belamowicz S. Common movement disorders affecting the larynx: a report from the neurolaryngology committee of the AAO-HNS. Otolaryngol Head Neck Surg 2005;133:654–665. [DOI] [PubMed] [Google Scholar]
- 11.Patel RR, Liu L, Galatsanos N, Bless DM. Differential vibratory characteristics of adductor spasmodic dysphonia and muscle tension dysphonia on high-speed digital imaging. Ann Otol Rhinol Laryngol 2011;120:21–32. [DOI] [PubMed] [Google Scholar]
- 12.Parker LA, Kunduk M, Fink DS, McWhorter A. Reliability of high-speed videoendoscopic ratings of essential voice tremor and adductor spasmodic dysphonia. J Voice 2019;33:16–26. [DOI] [PubMed] [Google Scholar]
- 13.Parker N. Hereditary whispering dysphonia. J Neurol Neurosurg Psychiatry 1985;48:218–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ozelius LJ, Lubarr N, Bressman SB. Milestones in dystonia. Mov Disord 2011;26:1106–1126. [DOI] [PubMed] [Google Scholar]
- 15.Fuchs T, Saunders-Pullman R, Masuho I, et al. Mutations in GNAL cause primary torsion dystonia. Nat Genet 2013;45:88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Putzel GG, Fuchs T, Battistella G, et al. GNAL mutation in isolated laryngeal dystonia. Movement Disord 2016;31:750–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Putzel GG, Battistella G, Rumbach AF, Ozelius LJ, Sabuncu MR, Simonyan K. Polygenic risk of spasmodic dysphonia is associated with vulnerable sensorimotor connectivity. Cereb Cortex 2018;28:158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hutchinson M, Kimmich O, Molloy A, et al. The endophenotype and the phenotype: temporal discrimination and adult-onset dystonia. Mov Disord 2013;28:1766–1774. [DOI] [PubMed] [Google Scholar]
- 19.Termsarasab P, Ramdhani RA, Battistella G, et al. Neural correlates of abnormal sensory discrimination in laryngeal dystonia. NeuroImage Clin 2016;10:18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maguire F, Reilly RB, Simonyan K. Normal temporal discrimination in musician's dystonia is linked to aberrant sensorimotor processing. Mov Disord 2020;35:800–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanner K, Roy N, Merrill RM, et al. Risk and protective factors for spasmodic dysphonia: a case-control investigation. J Voice 2011;25:e35–46. [DOI] [PubMed] [Google Scholar]
- 22.Childs L, Rickert S, Murry T, Blitzer A, Sulica L. Patient perceptions of factors leading to spasmodic dysphonia: a combined clinical experience of 350 patients. Laryngoscope 2011;121:2195–2198. [DOI] [PubMed] [Google Scholar]
- 23.Marsden CD, Obeso JA, Zarranz JJ, Lang AE. The anatomical basis of symptomatic hemidystonia. Brain 1985;108(Pt 2):463–483. [DOI] [PubMed] [Google Scholar]
- 24.Simonyan K. Neuroimaging applications in dystonia. Int Rev Neurobiol 2018;143:1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bianchi S, Battistella G, Huddleston H, et al. Phenotype- and genotype-specific structural alterations in spasmodic dysphonia. Mov Disord 2017;32:560–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kostic VS, Agosta F, Sarro L, et al. Brain structural changes in spasmodic dysphonia: a multimodal magnetic resonance imaging study. Parkinsonism Relat Disord 2016;25:78–84. [DOI] [PubMed] [Google Scholar]
- 27.Simonyan K, Ludlow CL. Abnormal structure-function relationship in spasmodic dysphonia. Cereb Cortex 2012;22:417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simonyan K, Tovar-Moll F, Ostuni J, et al. Focal white matter changes in spasmodic dysphonia: a combined diffusion tensor imaging and neuropathological study. Brain 2008;131:447–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kirke DN, Battistella G, Kumar V, et al. Neural correlates of dystonic tremor: a multimodal study of voice tremor in spasmodic dysphonia. Brain Imaging Behav 2017;11:166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bianchi S, Fuertinger S, Huddleston H, Frucht SJ, Simonyan K. Functional and structural neural bases of task specificity in isolated focal dystonia. Mov Disord 2019;34:555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ali SO, Thomassen M, Schulz GM, et al. Alterations in CNS activity induced by botulinum toxin treatment in spasmodic dysphonia: an H215O PET study. J Speech Lang Hear Res 2006;49:1127–1146. [DOI] [PubMed] [Google Scholar]
- 32.Haslinger B, Erhard P, Dresel C, Castrop F, Roettinger M, Ceballos-Baumann AO. “Silent event-related” fMRI reveals reduced sensorimotor activation in laryngeal dystonia. Neurology 2005;65:1562–1569. [DOI] [PubMed] [Google Scholar]
- 33.Simonyan K, Ludlow CL. Abnormal activation of the primary somatosensory cortex in spasmodic dysphonia: an fMRI study. Cereb Cortex 2010;20:2749–2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kiyuna A, Kise N, Hiratsuka M, et al. Brain activity in patients with adductor spasmodic dysphonia detected by functional magnetic resonance imaging. J Voice 2017;31:379 e371–379 e311. [DOI] [PubMed] [Google Scholar]
- 35.Battistella G, Termsarasab P, Ramdhani RA, Fuertinger S, Simonyan K. Isolated focal dystonia as a disorder of large-scale functional networks. Cereb Cortex 2017;27:1203–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanekamp S, Simonyan K. The large-scale structural connectome of task-specific focal dystonia. Hum Brain Mapp 2020;41:3253–3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kimberley TJ, Borich MR, Prochaska KD, Mundfrom SL, Perkins AE, Poepping JM. Establishing the definition and inter-rater reliability of cortical silent period calculation in subjects with focal hand dystonia and healthy controls. Neurosci Lett 2009;464:84–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Samargia S, Schmidt R, Kimberley TJ. Shortened cortical silent period in adductor spasmodic dysphonia: evidence for widespread cortical excitability. Neurosci Lett 2014;560:12–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quartarone A, Sant'Angelo A, Battaglia F, et al. Enhanced long-term potentiation-like plasticity of the trigeminal blink reflex circuit in blepharospasm. J Neurosci 2006;26:716–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murase N, Rothwell JC, Kaji R, et al. Subthreshold low-frequency repetitive transcranial magnetic stimulation over the premotor cortex modulates writer's cramp. Brain 2005;128:104–115. [DOI] [PubMed] [Google Scholar]
- 41.Samargia S, Schmidt R, Kimberley TJ. Cortical silent period reveals differences between adductor spasmodic dysphonia and muscle tension dysphonia. Neurorehabil Neural Repair 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simonyan K. Inferior parietal cortex as a hub of loss of inhibition and maladaptive plasticity. Annual Meeting of Americal ACademy of Neurology; 2017; Boston.
- 43.Simonyan K, Berman BD, Herscovitch P, Hallett M. Abnormal striatal dopaminergic neurotransmission during rest and task production in spasmodic dysphonia. J Neurosci 2013;33:14705–14714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simonyan K, Cho H, Hamzehei Sichani A, Rubien-Thomas E, Hallett M. The direct basal ganglia pathway is hyperfunctional in focal dystonia. Brain 2017;140:3179–3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fuertinger S, Simonyan K. Connectome-wide phenotypical and genotypical associations in focal dystonia. J Neurosci 2017;37:7438–7449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fuertinger S, Simonyan K. Task-specificity in focal dystonia is shaped by aberrant diversity of a functional network kernel. Mov Disord 2018;33:1918–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Battistella G, Fuertinger S, Fleysher L, Ozelius LJ, Simonyan K. Cortical sensorimotor alterations classify clinical phenotype and putative genotype of spasmodic dysphonia. Eur J Neurol 2016. doi: 10.1111/ene.13067.[Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Battistella G, Simonyan K. Top-down alteration of functional connectivity within the sensorimotor network in focal dystonia. Neurology 2019;92:e1843–e1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Valeriani D, Simonyan K. A microstructural neural network biomarker for dystonia diagnosis identified by a DystoniaNet deep learning platform. Proc Natl Acad Sci U S A 2020;117:26398–26405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simonyan K, Frucht SJ, Blitzer A, Sichani AH, Rumbach AF. A novel therapeutic agent, sodium oxybate, improves dystonic symptoms via reduced network-wide activity. Sci Rep 2018;8:16111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pirio Richardson S, Wegele AR, Skipper B, Deligtisch A, Jinnah HA. Dystonia Coalition I. Dystonia treatment: patterns of medication use in an international cohort. Neurology 2017;88:543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Park JB, Simpson LL, Anderson TD, Sataloff R. Immunologic characterization of spasmodic dysphonia patients who develop resistance to botulinum toxin. J Voice 2003;17:255–264. [DOI] [PubMed] [Google Scholar]
- 53.Rumbach AF, Blitzer A, Frucht SJ, Simonyan K. An open-label study of sodium oxybate in spasmodic dysphonia. Laryngoscope 2017;127:1402–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pitman MJ. Treatment of spasmodic dysphonia with a neuromodulating electrical implant. Laryngoscope 2014;124:2537–2543. [DOI] [PubMed] [Google Scholar]
- 55.Khosravani S, Mahnan A, Yeh IL, et al. Atypical somatosensory-motor cortical response during vowel vocalization in spasmodic dysphonia. Clin Neurophysiol 2019;130:1033–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cho HJ, Hallett M. Non-invasive brain stimulation for treatment of focal hand dystonia: update and future direction. J Mov Disord 2016;9:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Risch V, Staiger A, Ziegler W, et al. How does GPi-DBS affect speech in primary dystonia?. Brain Stimul 2015;8:875–880. [DOI] [PubMed] [Google Scholar]
- 58.Reese R, Gruber D, Schoenecker T, et al. Long-term clinical outcome in Meige syndrome treated with internal pallidum deep brain stimulation. Mov Disord 2011;26:691–698. [DOI] [PubMed] [Google Scholar]
- 59.Evidente VGHPF, Evidente MH, Lambert M, Garrett R, Sugumaran M, Lott DG. Adductor spasmodic dysphonia improves with bilateral thalamic deep brain stimulation: report of 3 cases done asleep and review of literature. Tremor and Other Hyperkinetic Movements 2020;10:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]