Abstract
Objective
To identify the molecular signaling pathways underlying sudden unexpected death in epilepsy (SUDEP) and high-risk SUDEP compared to control patients with epilepsy.
Methods
For proteomics analyses, we evaluated the hippocampus and frontal cortex from microdissected postmortem brain tissue of 12 patients with SUDEP and 14 with non-SUDEP epilepsy. For transcriptomics analyses, we evaluated hippocampus and temporal cortex surgical brain tissue from patients with mesial temporal lobe epilepsy: 6 low-risk and 8 high-risk SUDEP as determined by a short (<50 seconds) or prolonged (≥50 seconds) postictal generalized EEG suppression (PGES) that may indicate severely depressed brain activity impairing respiration, arousal, and protective reflexes.
Results
In autopsy hippocampus and cortex, we observed no proteomic differences between patients with SUDEP and those with non-SUDEP epilepsy, contrasting with our previously reported robust differences between epilepsy and controls without epilepsy. Transcriptomics in hippocampus and cortex from patients with surgical epilepsy segregated by PGES identified 55 differentially expressed genes (37 protein-coding, 15 long noncoding RNAs, 3 pending) in hippocampus.
Conclusion
The SUDEP proteome and high-risk SUDEP transcriptome were similar to those in other patients with epilepsy in hippocampus and cortex, consistent with diverse epilepsy syndromes and comorbid conditions associated with SUDEP. Studies with larger cohorts and different epilepsy syndromes, as well as additional anatomic regions, may identify molecular mechanisms of SUDEP.
Sudden unexpected death in epilepsy (SUDEP) affects 1 in 1,000 patients with epilepsy annually and is the leading cause of epilepsy-related deaths.1 SUDEP most often follows a generalized tonic-clonic seizure (GTCS) and excludes trauma, drowning, status epilepticus, or other causes. Most deaths are unwitnessed and occur during sleep, and the patient is found prone.
Studies on SUDEP epidemiology, risk factors, mechanisms, and prevention have advanced our understanding, although pathophysiologic understanding remains limited.2,3 After a GTCS, prolonged (>50 seconds) postictal generalized EEG suppression (PGES) may increase SUDEP risk and may be a SUDEP biomarker because severe, prolonged reduced brain activity impairs arousal, respiration, and other autonomic functions.4 However, we cannot predict why some low-risk patients become patients with SUDEP, high-risk patients survive for decades, and other patients succumb to SUDEP despite recovering from many earlier GTCS. Patients with SUDEP may harbor pathogenic gene variants in brain and heart ion channels,5-7 but a role in SUDEP pathogenesis remains speculative. Animal models of genetic epilepsies and chemo-induced seizures implicate abnormalities in respiration, arousal, and parasympathetic hyperactivity in SUDEP pathogenesis.1,8-10 However, the neuropathology of SUDEP parallels findings in patients with non-SUDEP epilepsy.11,12 Potential proteomic and transcriptional molecular signatures associated with SUDEP have not been studied.
Our study investigated the molecular signaling networks associated with SUDEP in brain regions implicated in ictogenesis,13 from localized proteomics in autopsy hippocampal CA1-3, dentate gyrus, and frontal cortex from patients with SUDEP and patients with non-SUDEP epilepsy and transcriptomics in hippocampus and temporal cortex from low- and high-risk SUDEP (PGES <50 or ≥50 seconds) epilepsy surgical tissue.
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
Autopsy brain tissue and clinical information from patients with SUDEP or non-SUDEP epilepsy were obtained with approval by the New York University School of Medicine Institutional Review Board. All next of kin provided written informed consent.
Human Brain Tissue for Proteomics
Postmortem brain tissue from patients with epilepsy who died of SUDEP or other causes was obtained through the North American SUDEP Registry (NASR), which began enrolling patients in October 2011,2 with approval by the New York University School of Medicine Institutional Review Board. Causes of death were classified (O.D., D.F.) into non-SUDEP epilepsy and SUDEP (definite SUDEP, definite SUDEP plus, and probable SUDEP).1,2 Lifetime GTCS history was determined from interviews and medical records, representing the best estimate for each patient and as described previously for these patients.2 After neuropathologic review (T.W., A.F.), brain tissue was processed into formalin-fixed paraffin-embedded (FFPE) blocks and sections were stained with luxol fast blue counterstained with hematoxylin & eosin. Archival time for brain tissue storage in formalin was ≤3 years; thus, patients were chosen from those who were enrolled in NASR between July 2014 to March 2017. Patients were age and sex matched from available NASR cases. There were no significant differences in age at death (p = 0.9190, unpaired t test), disease duration (p = 0.7295), disease onset (p = 0.4797), or sex (p > 0.9999). Clinical and neuropathologic data on the 14 patients with non-SUDEP epilepsy and 12 with SUDEP are summarized in table 1. Group sizes were determined from the number of patients with significant findings as previously reported,14-16 including our earlier studies in patients with epilepsy with similar methods.17,18
Table 1.
Patients With Epilepsy and SUDEP in Proteomics Analyses
Laser Capture Microdissection for Proteomics
FFPE brain tissue blocks containing either hippocampus (lateral geniculate nucleus level)19 or superior frontal gyrus were sectioned at 8 µm and collected onto laser capture microdissection (LCM)–compatible PET slides (Leica, Newcastle, UK). Sections were stained with cresyl violet to localize regions of interest for LCM20 and air dried overnight in a loosely closed container. LCM was used to individually microdissect 10 mm2 from the hippocampal CA1-3 region and superior frontal cortex (layers I–IV), and 4 mm2 from the hippocampal dentate gyrus into liquid chromatography (LC)–mass spectrometry (MS)–grade water (Thermo Scientific, Waltham, MA). Microdissected samples were centrifuged for 2 minutes at 14,000g and stored at −80°C. LCM was performed at 5× magnification with an LMD6500 microscope equipped with an ultraviolet laser (Leica).
Label-Free Quantitative MS Proteomics
Label-free quantitative MS assessed differential protein expression as described previously.18,21,22 FFPE cuts were incubated in 50 mM ammonium bicarbonate solution containing 20% (vol/vol) acetonitrile for 1 hour at 95°C followed by 2 hours at 65°C. Disulfide bonds were reduced with 10 mM DTT (1 hour at 57°C) and alkylated with 30 mM iodoacetamide (45 minutes at room temperature in the dark). Proteins were enzymatically digested into peptides with 300 ng trypsin (sequencing grade, Promega, Madison, WI) overnight at room temperature. Digestions were quenched by acidification with trifluoroacetic acid, and peptides were concentrated and desalted on POROS R2 C18 beads. Eluates were dried in a speedvac and resuspended in 0.5% acetic acid (AcOH). LC separation was performed online on EASY-nLC 1200 (Thermo Scientific) with the use of an Acclaim PepMap 100 (75 μm × 2 cm) precolumn and a PepMap RSLC C18 (2 μm, 100 A × 50 cm) analytical column. Peptides were gradient eluted from the column directly into the Orbitrap Fusion Lumos mass spectrometer using a 165-minute acetonitrile gradient (A = 2% acetonitrile in 0.5% AcOH/B = 80% acetonitrile in 0.5% AcOH). The flow rate was set at 200 nL/min. The mass spectrometer was operated in a data-dependent acquisition mode. High-resolution full MS spectra were acquired with a resolution of 240,000, an automatic gain control (AGC) target of 1e6, with a maximum ion injection time of 50 milliseconds, and scan range of 400 to 1,500 m/z. After each full MS scan, data-dependent HCD MS/MS scans were acquired in the ion trap (scan rate rapid, AGC target of 2e4, normalized collision energy of 32). Precursor isolation window was set at 2 Da.
Proteomics Computational Analysis
MS data were analyzed as previously described.18,21,22 Raw MS data were processed using the MaxQuant23 software (version 1.6.3.4) and the SwissProt human protein database (uniprot.org) containing 20,421 entries. A database including a common list of common laboratory contaminants (248 entries) was also used in the search. All peptide-spectrum matches and peptide and protein identifications were filtered to get a desired false discovery rate (FDR) level <1% (calculated with the decoy database approach). For the MS/MS search, enzyme specificity was set to trypsin (up to 2 miscleavages), and precursor mass tolerance was set to 20 ppm with subsequent nonlinear mass recalibration. Carbamidomethylation of cysteine was set as a fixed modification; protein N-term acetylation and methionine oxidation were set as variable modifications. Match between runs algorithm was enabled to transfer peptide feature identifications between MS runs based on LC retention time (0.7-minute tolerance after initial recalibration) and precursor mass tolerance. Label-free quantification (LFQ) was performed with a built-in maxLFQ algorithm,24 and normalization was performed separately for all samples within each region of interest.
Data analysis was performed in Perseus framework25 (perseus-framework.org/), R environment (r-project.org/, Vienna, Austria), or GraphPad Prism (La Jolla, CA).
Proteomics Statistical Analyses
The protein expression matrix (n = 4,129) was filtered to contain only proteins that were quantified in ≥8 replicates in at least 1 condition (SUDEP or non-SUDEP epilepsy) in any brain region (n = 2,847). Subsequently missing values were imputed from the intensity distribution–simulated low-intensity protein features (width of 0.3 and downshift of 1.8 relative to measured protein intensity distribution). An unpaired 2-tailed t test was performed for PCA1 in each brain region to determine the significance of separation in the patients with SUDEP and non-SUDEP epilepsy. All other analyses were done using nonimputed data. A Student 2-sample t test was used to assess statistical significance of the changes in protein abundance between conditions. Obtained p values were adjusted for multiple hypothesis testing using permutation-based FDR to a cutoff of 5%. Cell type–specific annotations were included in the data available on Dryad (table e-3, doi.org/10.5061/dryad.dfn2z3508) and on volcano plots in figure 1, F through H, derived from previous data.26 Annotations were included when a protein had only 1 associated cell type after removing cerebellar annotations and when the annotation included >1 associated cell type (both excitatory and inhibitory neuron annotations) and were thus assigned a general neuron annotation, for a total of 1,066 possible annotations.
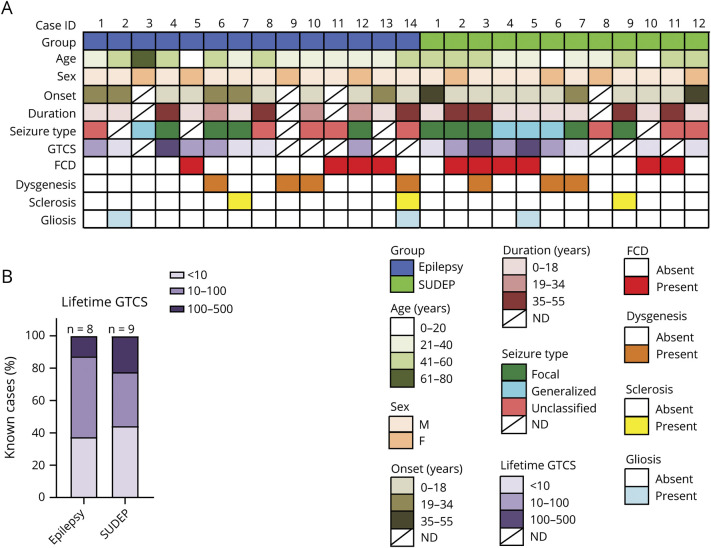
Figure 1. History of Patients With SUDEP and Non-SUDEP Epilepsy.
(A) Patient history is summarized for patients with sudden unexpected death in epilepsy (SUDEP) and non-SUDEP epilepsy. (B) A summary of lifetime generalized tonic-clonic seizure (GTCS) history burden for the patients in this study with known information. ID = identification.
Proteomics Correlation
For the correlation in protein abundance between conditions and brain regions, we used averaged LFQ values. A Pearson correlation was calculated for proteins detected in both patients with SUDEP and those with non-SUDEP epilepsy for each brain region, with 2,715 proteins for hippocampal CA1-3, 2,464 proteins for dentate gyrus, and 2,695 proteins for the frontal cortex.
Immunohistochemistry
Immunohistochemistry was performed to validate the identified protein of interest, ermin (ERMN) as previously described.18,27 Briefly, FFPE sections (8 µm) were deparaffinized and rehydrated through a series of xylenes and ethanol dilutions. Heat-induced antigen retrieval was performed with 10 mM sodium citrate and 0.05% triton-x 100, pH6. Blocking with 10% normal donkey serum was followed by ERMN primary antibody (1:200, Sigma HPA038295) overnight at 4°C. Sections were incubated with donkey anti-rabbit Alexa-Fluor 647 secondary antibody (1:500, ThermoFisher Invitrogen, Carlsbad, CA) and coverslipped.
Image Semiquantitative Analysis
Whole slide scanning was performed at 20× magnification with a NanoZoomer HT2 (Hamamatsu) microscope using the same settings for each slide. One image containing the hippocampal CA1-3 region was collected for each patient, 11 with non-SUDEP epilepsy and 11 with SUDEP. Images were analyzed in Fiji ImageJ to compare the amount of ERMN in patients with SUDEP and non-SUDEP epilepsy. The same binary threshold was used for all images to determine the number of ERMN-positive pixels in each image, which was reported as a percentage of the total image area. An unpaired t test was performed for statistical analysis; a value of p < 0.05 was considered significant.
Confocal imaging was used to collect representative images of ERMN immunohistochemistry using a Zeiss LSM880 confocal microscope with the same settings on each slide with a Plan-Apochromat 20×/0.8 M27 objective and a pinhole of 38 μm.
RNA-Sequencing Datasets
Small RNA-sequencing (RNAseq) and RNAseq datasets were retrieved form the European Genome-Phenome Archive (accession No. EGAS00001003922) from patients with mesial temporal lobe epilepsy (MTLE) undergoing surgical resection and with available PGES duration >1 second.17 The patients were age and sex matched, with no significant differences in age at surgery (p = 0.6622, unpaired t test), disease duration (p = 0.4391), disease onset (p = 0.4612), or sex (p > 0.9999). Small RNAseq and RNAseq data were retrieved for 6 patients with PGES <50 seconds, indicating a potential low risk for SUDEP, and 8 patients with PGES ≥50 seconds, indicating a potential high risk for SUDEP as previously described.4 Table 2 summarizes the clinical characteristics of these patients. PGES occurrence and duration was assessed by 2 epileptologists (C.S., R.T.).
Table 2.
Epilepsy Patients With Low or High Risk of SUDEP in RNAseq Analyses
Bioinformatic Analysis of RNAseq Data
Bioinformatic analysis was performed as described previously.17 Briefly, library normalization and differential expression testing were carried out with the R package DESeq2. The Wald test identified differentially expressed genes using a Benjamini-Hochberg–adjusted value of p < 0.05 for significance. Cell type–specific annotations were included (Dryad tables e-4 and e-5, doi.org/10.5061/dryad.dfn2z3508) and on volcano plots in figures 2, C and E, derived from previous data.26 Annotations were included when a gene had only 1 associated cell type after removal of cerebellar annotations and when the annotation included >1 associated cell type (both excitatory and inhibitory neuron annotations) and were thus assigned a general neuron annotation, for a total of 1,066 possible annotations.
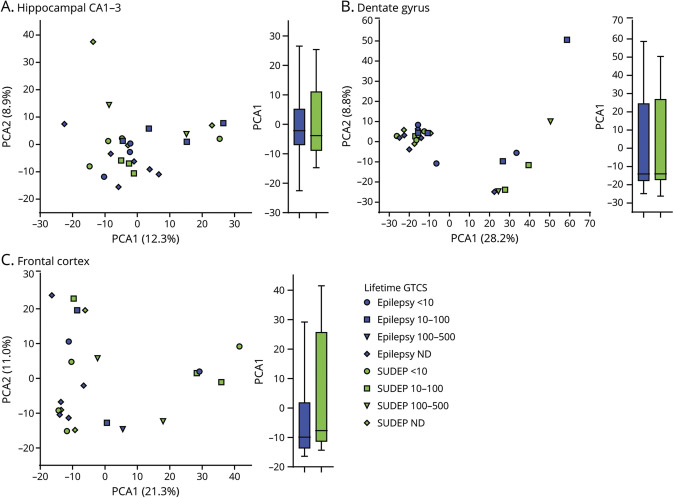
Figure 2. Proteomics PCA Analyses in Hippocampus, Dentate Gyrus, and Frontal Cortex of Patients With SUDEP and Non-SUDEP Epilepsy.
(A–C) A principal component analysis (PCA) of the proteomics analyses shows the indicated variation in each brain region of patients with sudden unexpected death in epilepsy (SUDEP) (n = 12) and non-SUDEP epilepsy (n = 14). There is no separation by SUDEP status or lifetime generalized tonic-clonic seizure (GTCS) history burden. An unpaired 2-tailed t test of PCA1 between the SUDEP and non-SUDEP epilepsy groups in each brain region was not significant, as depicted by a boxplot with bars indicating minimum and maximum values. ND = not determined.
A Reactome pathway enrichment analysis was performed with the R package ReactomePA. The differentially expressed genes from the RNAseq differential expression analysis were put into R and tested for overrepresentation of enriched Reactome pathways using hypergeometric testing. Pathways with a Benjamini-Hochberg–corrected value of p < 0.05 were considered significantly enriched.
Bioinformatic Analysis of Small RNAseq Data
Bioinformatic analysis of the small RNAseq data was performed as described previously.17 Briefly, library normalization and differential expression testing were carried out with the R package DESeq2. The Wald test identified differentially expressed genes with a Benjamini-Hochberg–adjusted value of p < 0.05 considered significant.
RNAseq Validation by Quantitative PCR
The gene expression of glial cell-derived neurotrophic factor (GDNF) family receptor alpha 1 (GFRA1) was assessed in the same cohort of samples used in the RNAseq analysis for which sufficient RNA remained (PGES <50 seconds, n = 4, PGES ≥50 seconds, n = 7). PCR primers based on the reported cDNA sequences were designed using the National Center for Biotechnology Information primer design tool.28 The sequences for the forward and reverse primers of GFRA1 were 5′-TCT TCC AGC CGC AGA AGA AC-3′ and 5′-AAC AGT GGG GAC AAA CTG GG-3′, respectively. Total RNA (700 ng) was reverse transcribed into cDNA using oligodT primers. For each quantitative PCR reaction, a mastermix was prepared as follows: 1 µL cDNA, 2.5 µL of 2× SensiFAST SYBR Green Reaction Mix (Bioline Inc, Taunton, MA), and 0.2 µM of both the reverse and forward primers. The PCRs were run on a Roche Lightcycler 480 thermocycler (Roche Applied Science, Basel, Switzerland). Each sample and primer pair were run in triplicates. Data quantification was performed as previously described17 relative to the reference genes, eukaryotic translation elongation factor 1 alpha 1 (EEF1A1) and chromosome 1 open reading frame 43 (C1orf43). The normalized ratio was compared between the 2 groups (Mann-Whitney U test); values of p < 0.05 were considered significant.
Data Availability
All data needed to evaluate the conclusions in the paper are present in the paper and on Dryad at doi.org/10.5061/dryad.dfn2z3508. Additional data related to this article may be requested from the authors.
Results
Proteome of Patients With SUDEP and Non-SUDEP Epilepsy Autopsy
The differential expression of proteins in patients with SUDEP (n = 12) and non-SUDEP (n = 14) was evaluated in autopsy tissue using label-free quantitative MS in the microdissected hippocampal CA1-3 region, dentate gyrus, and frontal cortex because these regions have been implicated in ictogenesis and may also be influenced by seizure activity.13 Patient histories are summarized in table 1 and figure 1, A and B. A principal component analysis (PCA) did not distinguish patients with SUDEP and those with non-SUDEP epilepsy in any of the studied brain regions (figure 2, A–C). The main source of variation in these patients, PCA1, did not show a significant difference when patients with SUDEP and non-SUDEP were compared in each brain region by an unpaired 2-tailed t test, as depicted by a box plot in figure 2, A through C. Lifetime GTCS burden, associated with an increased SUDEP risk,1 was evaluated to determine whether this factor may contribute to protein differences as seen by a separation of groups. From patients with available data (9 with SUDEP and 8 with non-SUDEP epilepsy), 55.6% of those with SUDEP and 62.5% of those with non-SUDEP epilepsy had >10 lifetime GTCS, and 22.2% of those with SUDEP and 12.5% of patients with non-SUDEP epilepsy had >100 lifetime GTCS. Lifetime GTCS frequency did not contribute to group differences in the PCA (figure 2, A–C). There was no enrichment in patients with SUDEP or non-SUDEP epilepsy with >10 or >100 lifetime GTCS by a Fisher exact test. Furthermore, in the PCA, there was no relationship of SUDEP status to neuropathology (focal cortical dysplasia [FCD, n = 10], hippocampal dentate gyrus dysgenesis [n = 7], hippocampal sclerosis [n = 3], and gliosis [n = 3]). Of note, microdissected regions did not necessarily contain observed FCD because it may have been present in other brain regions. Similarly, neuropathology was unrelated to SUDEP status (FCD in 50% of patients with SUDEP vs 28.6% of patients with non-SUDEP epilepsy, Fisher exact test, p = 0.4216).
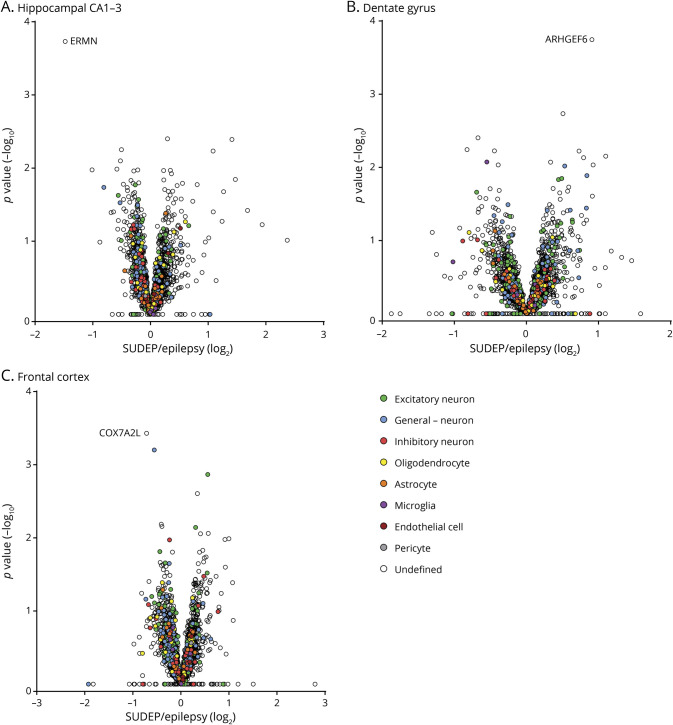
There were no significant differences in protein expression between patients with SUDEP and those with non-SUDEP epilepsy in any brain region (figure 3,A–C, Dryad figure e-1, A–C and table e-3, doi.org/10.5061/dryad.dfn2z3508). Furthermore, a correlation of LFQ values for all proteins showed the similarity in protein expression in comparisons of patients with SUDEP and non-SUDEP epilepsy in each brain region by a Pearson correlation (p < 0.0001) with the corresponding R2 values being ≥0.98 (Dryad, figure e-1). Brain cell type–specific annotation was evaluated in the 2,847 identified proteins, derived from previous methods,26 with 19.8% (564 of 2,847) proteins having an annotation while the remaining 80.2% did not and were more ubiquitously expressed or with unknown cell type. Most (78.2%, 502 of 564) annotated proteins were generally neuronal, with excitatory neuron proteins predominating (48.1%, 271 of 564) (figure 3, A–C, Dryad table e-3). Some proteins showed a trend for altered expression in patients with SUDEP (p < 0.01; Dryad tables e-1 and e-2), but these were not statistically significant at a 5% FDR. Several of these protein changes have been reported in epilepsy animal models and patients without epilepsy or include proteins encoded by genes in which mutations have been previously linked to epilepsy. Yet, none of the proteins trending for altered expression in this study (Dryad tables e-1 and 3-2) have been previously linked to SUDEP pathogenesis. ERMN had the strongest trend for difference in SUDEP with a 2.8-fold decrease in the hippocampal CA1-3 region when patients with SUDEP and patients with non-SUDEP epilepsy were compared by MS (Dryad figure e-2A). Furthermore, ERMN was detected in more patients with non-SUDEP epilepsy than patients with SUDEP by MS, indicating lower abundance of this protein in SUDEP. Validation of the quantitative MS findings with semiquantification of immunohistochemistry (Dryad figure e-2B) also showed a decrease of ERMN in patients with SUDEP with a 1.3-fold change but was not significant (Student unpaired t test, p = 0.4871). Because ERMN may play a role in myelinogenesis and myelin maintenance, we reviewed the mature oligodendrocyte marker myelin basic protein (MBP) but found no difference between patients with SUDEP and those with non-SUDEP epilepsy in the hippocampal CA1-3 region by MS (Dryad figure e-2C).
Figure 3. Proteomics Analyses in Hippocampus, Dentate Gyrus, and Frontal Cortex of Patients With SUDEP and Non-SUDEP Epilepsy.
(A–C) Volcano plots indicate that there are no significantly different proteins in the hippocampal CA1-3 region, dentate gyrus, or frontal cortex of patients with sudden unexpected death in epilepsy (SUDEP) and non-SUDEP epilepsy as determined by a Student 2-tailed t test with permutation correction at a 5% false discovery rate. The top proteins with the lowest p values in each brain region are noted. Cell type–specific protein annotation is included, with the most predominant listed in decreasing order in the legend. Proteins annotated general—neuron have both excitatory and inhibitory neuron annotations.
Analysis of RNAseq and Small RNAseq in Patients With Low and High Risk of SUDEP
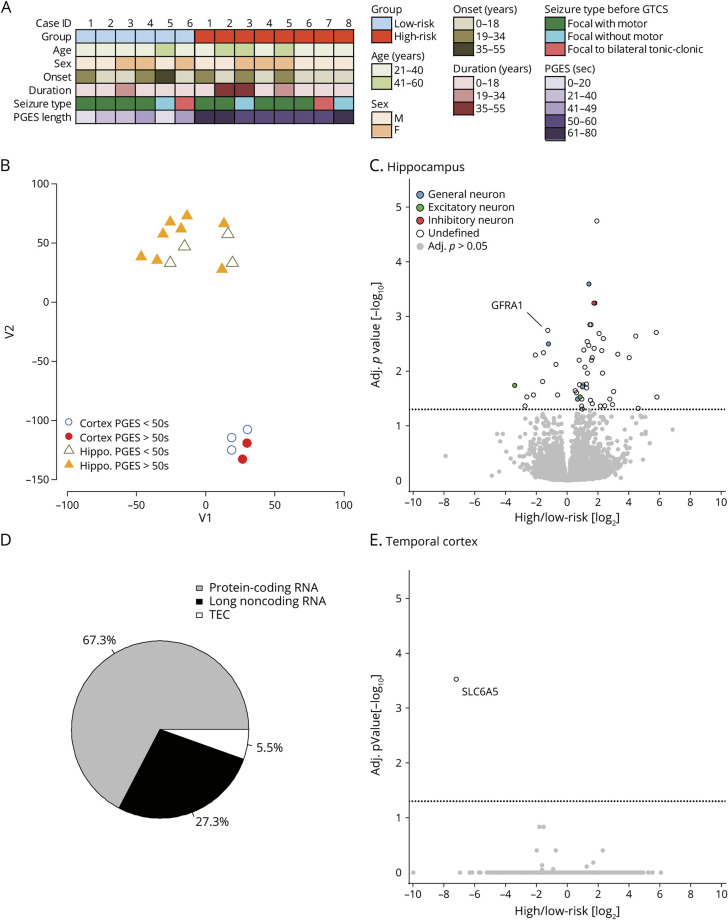
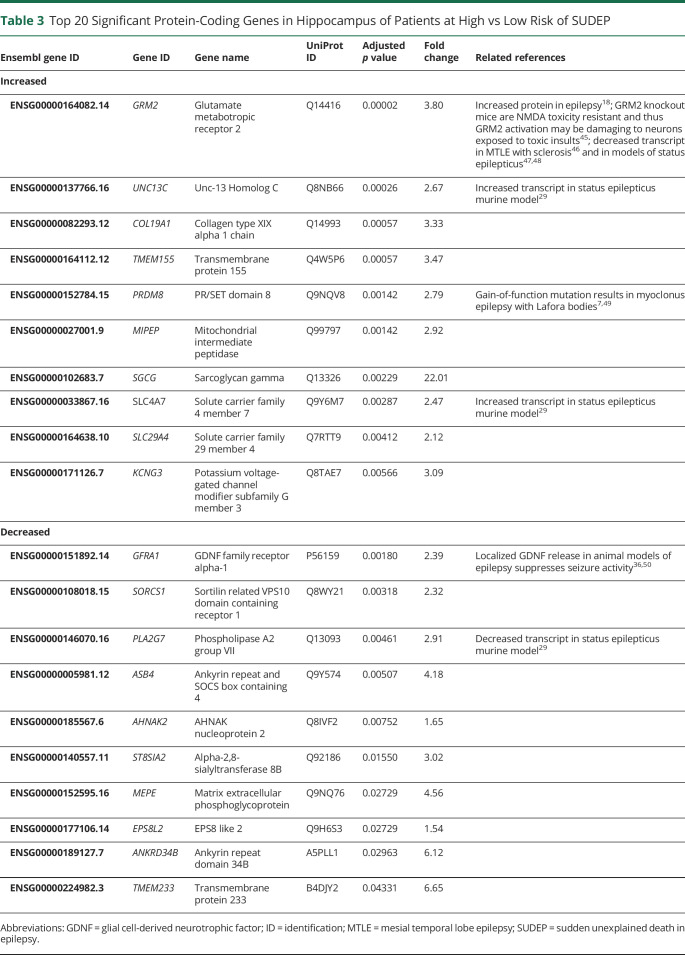
To determine whether there is a pathologic difference in patients with epilepsy of low (PGES <50 seconds, n = 6) and high (PGES ≥50 seconds, n = 8) risk of SUDEP, RNAseq and small RNAseq analyses were performed on resected surgical frozen hippocampal and temporal cortex tissue. Patient histories are summarized in table 2 and figure 4A. A t-distributed stochastic neighbor embedding plot revealed that anatomic region rather than PGES segregated patients (figure 4B). A differential expression analysis comparing the hippocampus of patients at low and high risk of SUDEP identified 55 differentially expressed genes: 11 were decreased and 44 were increased in patients at high risk for SUDEP (figure 4C and Dryad table e-4, doi.org/10.5061/dryad.dfn2z3508). Brain cell type–specific annotation was evaluated in the 55 differentially expressed genes in the hippocampus, derived from previous methods,26 with 14.5% (8 of 55) of genes having a cell type–specific annotation: 4 generally neuronal, 3 excitatory neuron, and 1 inhibitory neuron. The dominant transcripts for the differentially expressed genes in hippocampus were as follows: 37 protein-coding, 15 long noncoding RNAs (lncRNAs), and 3 awaiting confirmation (figure 4D). A Reactome pathway analysis on the 55 significant genes in the hippocampus did not reveal a significant association with any signaling pathways. Several of these genes have been associated with epilepsy human disease and have been studied in animal models; however, none of the genes in table 3 have been linked to SUDEP pathogenesis. The most significantly decreased protein-coding gene in the high-risk SUDEP patients, GFRA1, was validated by real-time quantitative PCR (table 3 and Dryad figure S3). In accordance with the RNAseq analysis, GFRA1 was decreased 1.7-fold in the patients at high risk for SUDEP (Mann-Whitney U test, p = 0.0121). In the temporal cortex, 1 protein-coding gene (SLC6A5) with an undefined cell type annotation was significantly decreased in the patients at high risk for SUDEP, within this small group of patients (figure 4E and Dryad table e-5). No genes were differentially expressed in the small RNAseq analyses in the hippocampus and temporal cortex (Dryad tables e-6 and e-7).
Figure 4. RNAseq in Hippocampus and Temporal Cortex With Low- and High-Risk SUDEP, as Determined by PGES.
(A) Patient history is summarized for patients at low and high risk for sudden unexpected death in epilepsy (SUDEP). (B) The t-distributed stochastic neighbor embedding plot of RNA sequencing (RNAseq) data shows separation by brain region rather than SUDEP risk status. (C) Volcano plot shows the results of differential expression analysis of the hippocampus from patients at low risk (n = 4) and high risk (n = 8) of SUDEP. Eleven genes were decreased and 44 genes were increased in hippocampus of patients at high risk of SUDEP. The Wald test identified differentially expressed genes using a Benjamini-Hochberg–adjusted value of p < 0.05 for significance. Cell type–specific gene annotation is included, with the most predominant listed in decreasing order in the legend. Genes annotated general—neuron have both excitatory and inhibitory neuron annotations. (D) Biotypes of differentially expressed genes are depicted in the hippocampus for patients at high risk for SUDEP compared to those at low risk of SUDEP. Of the 55 differentially expressed genes, 67.3% were protein-coding genes, 27.3% were long noncoding RNAs, and 5.5% are yet to be experimentally confirmed (TEC). (E) Volcano plot shows the results of differential expression analysis in the temporal cortex from patients at low risk (n = 2) and high risk (n = 3) of SUDEP. One gene was decreased and no genes were increased in the temporal cortex. The Wald test identified differentially expressed genes using a Benjamini-Hochberg–adjusted value of p < 0.05 for significance.
Table 3.
Top 20 Significant Protein-Coding Genes in Hippocampus of Patients at High vs Low Risk of SUDEP
Comparison of SUDEP Proteome to High-Risk SUDEP Transcriptome
Comparing the 37 differentially expressed protein-coding genes in the RNAseq analyses to the proteomics analyses, only 4 (GRM2, ERC2, CRTC1, AHNAK2) were detected in the proteomics analyses. Two (GRM2, ERC2) were detected in most patients in the hippocampal CA1-3 region but showed no trend in differential expression for patients with SUDEP compared to those with non-SUDEP epilepsy in the proteome. Additional analysis on the fold change of proteins in the hippocampus with a value of p < 0.05 (before the FDR at 5%, n = 83 proteins) that match RNA gene identifications (n = 83 gene identifications) do not show a significant correlation (p = 0.3510, R2 = 0.01075, Pearson correlation).
Discussion
Our study compared patients with SUDEP or at high risk for SUDEP to controls with epilepsy and revealed no differentially expressed proteins in the hippocampus and frontal cortex and limited transcriptomic changes in the hippocampus and temporal cortex. Thus, the proteome in SUDEP and transcriptome in high-risk SUDEP largely reflects those in other patients with epilepsy, consistent with the diverse spectrum of syndromes and severities associated with SUDEP.2 In the hippocampus, the few differentially expressed genes identified in high-risk SUDEP included a high proportion of lncRNAs (15 of 55, 27%). Given that we detect robust proteome18 and transcriptome17 differences in the hippocampus and cortex with similar group sizes for patients with epilepsy and controls without epilepsy, our data in this study suggest that these brain regions are not especially or uniquely affected in SUDEP.
To validate the label-free quantitative MS findings, immunohistochemistry was used to confirm changes in ERMN expression because this protein had the strongest trend for difference in SUDEP. Immunohistochemistry results corroborated a trend in a decreased fold change of ERMN in the hippocampal CA1-3 region of patients with SUDEP compared to those with non-SUDEP epilepsy, although this similarly was not significant. Furthermore, ERMN was not significantly altered in the current RNAseq study or in our previous proteomics analyses between patients with non-SUDEP epilepsy and controls.18 However, in our previous RNAseq study between patients with MTLE and controls without epilepsy, ERMN was decreased17 and is reportedly decreased in a murine model of status epilepticus.29 Expressed by oligodendrocytes, ERMN regulates cytoskeleton arrangement during myelinogenesis and myelin sheath maintenance.30 Myelin damage may occur after prolonged seizures, and its loss may promote further seizure activity.31 We found that the mature oligodendrocyte marker MBP is decreased in patients with epilepsy compared with controls without epilepsy,18 and it is decreased in the hippocampus of an animal model of epilepsy.32 However, we found no further decrease in MBP expression in patients with SUDEP or those at high risk of SUDEP compared to controls in this study, nor was MBP different in our recent RNAseq analysis between patients with MTLE and controls without epilepsy.17 Overall, ERMN is significantly decreased in those with surgical MTLE vs controls without epilepsy at the transcriptomic level17 and trending to decrease in protein expression of SUDEP vs non-SUDEP epilepsy, indicating that ERMN may be decreased in response to the elevated seizure activity that may be seen in refractory epilepsy that requires surgery and in some patients with SUDEP. The effect on myelination, as measured by MBP, is apparent only in these patients for protein expression rather than gene expression in patients with epilepsy vs controls without epilepsy with no further decrease in SUDEP. Thus, further investigation should assess the potential role of ERMN in epilepsy and SUDEP and whether reduced ERMN may reflect the severity of pathology resulting from seizure burden in some patients with SUDEP.
The RNAseq and small RNAseq analyses showed moderate changes in the hippocampus and minimal differences in the temporal cortex in patients with MTLE at high risk compared to low risk for SUDEP. Fifteen of 55 differentially expressed genes in the hippocampus were lncRNAs. LncRNAs are an understudied transcriptomic component implicated in many neurologic disorders,33 but few studies have been done regarding their role in epilepsy or SUDEP.34 Among the protein-coding genes differentially expressed in the hippocampus, GFRA1 was the most decreased. GDNF binds to GFRA1 and plays a role in neuronal survival and differentiation, including that of GABAergic interneurons.35 Localized release of GDNF in the hippocampus of an animal model of epilepsy suppresses seizure activity.36 Thus, decreased GFRA1 may reflect a change in cell survival or result in reduced GDNF-mediated seizure suppression in patients at high risk for SUDEP. Of the top 20 differentially expressed genes (table 3), sarcoglycan gamma (SGCG) had the largest change at a 22.0-fold increase (adjusted p = 0.0023) in the patients at high risk for SUDEP. SGCG is expressed in the cerebrovascular system and may localize to vascular smooth muscle cells, potentially involved in membrane contractility, stabilization, and signaling in the associated dystrophin complex affecting neurovascular coupling.37 Its neural role is unknown, but aberrant cerebrovascular organization occurs in MTLE.38 Additional studies are needed to determine how the altered levels of some protein-coding genes and lncRNAs we identified may affect mechanisms related to SUDEP risk.
Protein expression in the brain has rarely been studied in human SUDEP. Hippocampal HSP70-positive neurons are reportedly increased in postmortem patients with SUDEP compared to patients with non-SUDEP epilepsy but are similar to patients with surgical epilepsy, suggesting this is likely related to antemortem neuronal injury perhaps due to a terminal seizure in patients with SUDEP.39 HSP70 expression was similar in both the proteomic and RNAseq analyses among our patients. Another immunohistochemistry study found few differences in the hippocampus, amygdala, and medulla of postmortem SUDEP compared to non-SUDEP epilepsy and controls without epilepsy with minimal significant changes reported for several markers of inflammation (CD163, HLA-DR, GFAP), compromised blood-brain barrier (immunoglobulin G, albumin), and hypoxia-inducible factor-1α, a transcriptional regulator of cellular responses to hypoxia.12 We found increased GFAP in the hippocampus of 3 patients with epilepsy (3 of 26, 11.5%); 2 had gliosis independently of SUDEP status. GFAP was not increased in most patients with non-SUDEP epilepsy compared to controls without epilepsy,18 but it was increased in the hippocampus of 1 (1 of 14, 7.1%) patient with epilepsy with hippocampal gliosis. Increased GFAP occurs in some patients with epilepsy and after prolonged seizures in rodent models.40 Furthermore, GFAP was not altered in patients with MTLE with high risk of SUDEP in the current RNAseq analysis, but this gene was significantly increased in the hippocampus of patients with MTLE compared to controls without epilepsy.17
Our study had some limitations. The LCM-derived label-free quantitative MS allows detection of localized protein changes that would not be possible with bulk homogenate; however, this technique detects a lower quantity of membrane proteins that are relatively insoluble with this method. Thus, we may not detect differential expression of some membrane proteins, although downstream signaling pathways reflecting their functional activity may be identified. Additional limitations include the heterogeneity of epilepsies, seizure types, and neuropathology due to available patients, further reinforcing the importance of banking various brain tissue samples from patients with SUDEP. Our study was powered to identify proteomic differences across the representative SUDEP group rather than epilepsy subgroups. Potential pathogenic gene variants were not assessed in our patients. Our proteomics analyses were based on NASR referrals, skewed by major referral sources: the San Diego Medical Examiner Office (mainly White and Hispanic patients at low socioeconomic levels) and direct referrals (mainly White patients at high socioeconomic levels). For the RNAseq analyses, surgical patients had treatment-resistant MTLE. PGES duration as a biomarker of SUDEP risk has not been validated and can vary within the same patient for different seizures, and the number of video EEG-recorded GTCS in each patient was limited.4,41,42 Thus, group differences may reflect sampling bias. Furthermore, the number of patients used for the RNAseq temporal cortex analyses was low. Last, further investigation is needed in brain regions implicated in SUDEP, including the brainstem, because it modulates autonomic functions, and it has been suggested that seizure-induced postictal depression of arousal, respiratory, and cardiac function may occur in SUDEP.43,44
In contrast to the robust differences we found in proteomic and RNAseq analyses between patients with epilepsy and those without epilepsy,17,18 no differences were detected in the proteomic analyses of autopsy tissue from patients with SUDEP and those with non-SUDEP epilepsy, and limited transcriptomic differences were seen in comparisons of surgical tissue from patients at low and high risk for SUDEP in the brain regions analyzed, consistent with the diverse epilepsy syndromes and comorbid conditions associated with SUDEP and indicating that epilepsy subtypes and additional brain regions should be examined further.
Acknowledgment
The authors thank the participating families and clinicians for their involvement with the NASR.
Glossary
- ERMN
Ermin
- FCD
focal cortical dysplasia
- FDR
false discovery rate
- FFPE
formalin-fixed paraffin-embedded
- GDNF
glial cell-derived neurotrophic factor
- GTCS
generalized tonic-clonic seizure
- LC
liquid chromatography
- LCM
laser capture microdissection
- LFQ
label-free quantification
- lncRNA
long noncoding RNA
- MBP
myelin basic protein
- MS
mass spectrometry
- MTLE
mesial temporal lobe epilepsy
- NASR
North American SUDEP Registry
- PCA
principal component analysis
- PGES
postictal generalized EEG suppression
- RNAseq
RNA-sequencing
- SUDEP
sudden unexpected death in epilepsy
Appendix. Authors
Study Funding
National Institute of Neurological Disorders and Stroke UO1 NS090415 05 Center for SUDEP Research: The Neuropathology of SUDEP, Finding A Cure for Epilepsy and Seizures (FACES), National Institute of Aging P30AG066512, European Union's Seventh Framework Program (FP7/2007–2013) under grant agreement 602102 (EPITARGET; E.A.v.V., E.A.), and the Top Sector Life Sciences & Health via a PPP Allowance made available to the Dutch Epilepsy Foundation to stimulate public-private partnerships (E.A.v.V., E.A.). This work was supported by funding from the Bluesand Foundation to E.D. and Philippe Chatrier Foundation to G.P. The proteomics work was in part supported by the NYU School of Medicine and a shared instrumentation grant from the NIH, 1S10OD010582-01A1, for the purchase of an Orbitrap Fusion Lumos.
Disclosure
The authors declare that they have no competing interests. Go to Neurology.org/N for full disclosures.
References
- 1.Devinsky O, Hesdorffer DC, Thurman DJ, Lhatoo S, Richerson G. Sudden unexpected death in epilepsy: epidemiology, mechanisms, and prevention. Lancet Neurol. 2016;15(10):1075-1088. [DOI] [PubMed] [Google Scholar]
- 2.Verducci C, Hussain F, Donner E, et al. SUDEP in the North American SUDEP Registry: the full spectrum of epilepsies. Neurology. 2019;93(3):e227-e236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thom M, Michalak Z, Wright G, et al. Audit of practice in sudden unexpected death in epilepsy (SUDEP) post mortems and neuropathological findings. Neuropathol Appl Neurobiol. 2016;42(5):463-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang JY, Rabiei AH, Myint L, Nei M. Equivocal significance of post-ictal generalized EEG suppression as a marker of SUDEP risk. Seizure. 2017;48:28-32. [DOI] [PubMed] [Google Scholar]
- 5.Myers CT, Mefford HC. Advancing epilepsy genetics in the genomic era. Genome Med. 2015;7:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedman D, Kannan K, Faustin A, et al. Cardiac arrhythmia and neuroexcitability gene variants in resected brain tissue from patients with sudden unexpected death in epilepsy (SUDEP). NPJ Genom Med. 2018;3:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang J, Lin ZJ, Liu L, et al. Epilepsy-associated genes. Seizure. 2017;44:11-20. [DOI] [PubMed] [Google Scholar]
- 8.Zhang H, Zhao H, Zeng C, et al. Optogenetic activation of 5-HT neurons in the dorsal raphe suppresses seizure-induced respiratory arrest and produces anticonvulsant effect in the DBA/1 mouse SUDEP model. Neurobiol Dis. 2018;110:47-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang P, Zhang L, Li Y, et al. Quantitative proteomic analysis to identify differentially expressed proteins in myocardium of epilepsy using iTRAQ coupled with nano-LC-MS/MS. J Proteome Res. 2018;17(1):305-314. [DOI] [PubMed] [Google Scholar]
- 10.Kalume F, Westenbroek RE, Cheah CS, et al. Sudden unexpected death in a mouse model of Dravet syndrome. J Clin Invest. 2013;123(4):1798-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thom M, Boldrini M, Bundock E, Sheppard MN, Devinsky O. Review: the past, present and future challenges in epilepsy-related and sudden deaths and biobanking. Neuropathol Appl Neurobiol 2018;44(1):32-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michalak Z, Obari D, Ellis M, Thom M, Sisodiya SM. Neuropathology of SUDEP: role of inflammation, blood-brain barrier impairment, and hypoxia. Neurology. 2017;88(6):551-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aronica E, Mühlebner A. Neuropathology of epilepsy. Handb Clin Neurol. 2017;145:193-216. [DOI] [PubMed] [Google Scholar]
- 14.Mendonça CF, Kuras M, Nogueira FCS, et al. Proteomic signatures of brain regions affected by tau pathology in early and late stages of Alzheimer's disease. Neurobiol Dis. 2019;130:104509. [DOI] [PubMed] [Google Scholar]
- 15.Johnson ECB, Dammer EB, Duong DM, et al. Deep proteomic network analysis of Alzheimer's disease brain reveals alterations in RNA binding proteins and RNA splicing associated with disease. Mol Neurodegener. 2018;13(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu J, Patassini S, Rustogi N, et al. Regional protein expression in human Alzheimer's brain correlates with disease severity. Commun Biol. 2019;2:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mills JD, van Vliet EA, Chen BJ, et al. Coding and non-coding transcriptome of mesial temporal lobe epilepsy: critical role of small non-coding RNAs. Neurobiol Dis. 2020;134:104612. [DOI] [PubMed] [Google Scholar]
- 18.Pires G, Leitner D, Drummond E, et al. Proteomic differences in the hippocampus and cortex of epilepsy brain tissue. Brain Communications. 2021;doi: 10.1093/braincomms/fcab021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kinney HC, Poduri AH, Cryan JB, et al. Hippocampal formation maldevelopment and sudden unexpected death across the pediatric age spectrum. J Neuropathol Exp Neurol. 2016;75(10):981-997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drummond ES, Nayak S, Ueberheide B, Wisniewski T. Proteomic analysis of neurons microdissected from formalin-fixed, paraffin-embedded Alzheimer's disease brain tissue. Sci Rep. 2015;5:15456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drummond E, Nayak S, Ueberheide B, Wisniewski T. Localized proteomics of individual neurons isolated from formalin-fixed, paraffin-embedded tissue sections using laser capture microdissection. Acta Neuropathol. 2017:289-301. [Google Scholar]
- 22.Drummond E, Pires G, MacMurray C, et al. Phosphorylated tau interactome in the human Alzheimer's disease brain. Brain. 2020;143:2803-2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cox J, Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol. 2008;26(12):1367-1372. [DOI] [PubMed] [Google Scholar]
- 24.Cox J, Hein MY, Luber CA, Paron I, Nagaraj N, Mann M. Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ. Mol Cel Proteomics. 2014;13(9):2513-2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tyanova S, Temu T, Sinitcyn P, et al. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat Methods. 2016;13(9):731-740. [DOI] [PubMed] [Google Scholar]
- 26.Lake BB, Chen S, Sos BC, et al. Integrative single-cell analysis of transcriptional and epigenetic states in the human adult brain. Nat Biotechnol. 2018;36(1):70-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drummond E, Nayak S, Faustin A, et al. Proteomic differences in amyloid plaques in rapidly progressive and sporadic Alzheimer's disease. Acta Neuropathol. 2017;133(6):933-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ye J, Coulouris G, Zaretskaya I, Cutcutache I, Rozen S, Madden TL. Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics. 2012;13:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hansen KF, Sakamoto K, Pelz C, Impey S, Obrietan K. Profiling status epilepticus-induced changes in hippocampal RNA expression using high-throughput RNA sequencing. Sci Rep. 2014;4:6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brockschnieder D, Sabanay H, Riethmacher D, Peles E. Ermin, a myelinating oligodendrocyte-specific protein that regulates cell morphology. J Neurosci. 2006;26(3):757-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gibson EM, Geraghty AC, Monje M. Bad wrap: myelin and myelin plasticity in health and disease. Dev Neurobiol. 2018;78(2):123-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ye Y, Xiong J, Hu J, et al. Altered hippocampal myelinated fiber integrity in a lithium-pilocarpine model of temporal lobe epilepsy: a histopathological and stereological investigation. Brain Res. 2013;1522:76-87. [DOI] [PubMed] [Google Scholar]
- 33.Li L, Zhuang Y, Zhao X, Li X. Long non-coding RNA in neuronal development and neurological disorders. Front Genet. 2018;9:744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Villa C, Lavitrano M, Combi R. Long non-coding RNAs and related molecular pathways in the pathogenesis of epilepsy. Int J Mol Sci. 2019;20(19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Canty AJ, Dietze J, Harvey M, Enomoto H, Milbrandt J, Ibáñez CF. Regionalized loss of parvalbumin interneurons in the cerebral cortex of mice with deficits in GFRalpha1 signaling. J Neurosci. 2009;29(34):10695-10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kanter-Schlifke I, Fjord-Larsen L, Kusk P, Angehagen M, Wahlberg L, Kokaia M. GDNF released from encapsulated cells suppresses seizure activity in the epileptic hippocampus. Exp Neurol. 2009;216(2):413-419. [DOI] [PubMed] [Google Scholar]
- 37.Boulay AC, Saubaméa B, Cisternino S, et al. The Sarcoglycan complex is expressed in the cerebrovascular system and is specifically regulated by astroglial Cx30 channels. Front Cel Neurosci. 2015;9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guelfi S, Botia JA, Thom M, et al. Transcriptomic and genetic analyses reveal potential causal drivers for intractable partial epilepsy. Brain. 2019;142(6):1616-1630. [DOI] [PubMed] [Google Scholar]
- 39.Thom M, Seetah S, Sisodiya S, Koepp M, Scaravilli F. Sudden and unexpected death in epilepsy (SUDEP): evidence of acute neuronal injury using HSP-70 and c-Jun immunohistochemistry. Neuropathol Appl Neurobiol. 2003;29(2):132-143. [DOI] [PubMed] [Google Scholar]
- 40.Robel S. Astroglial scarring and seizures: a cell biological perspective on epilepsy. Neuroscientist. 2017;23(2):152-168. [DOI] [PubMed] [Google Scholar]
- 41.Rajakulendran S, Nashef L. Postictal generalized EEG suppression and SUDEP: a review. J Clin Neurophysiol. 2015;32(1):14-20. [DOI] [PubMed] [Google Scholar]
- 42.Lamberts RJ, Gaitatzis A, Sander JW, Elger CE, Surges R, Thijs RD. Postictal generalized EEG suppression: an inconsistent finding in people with multiple seizures. Neurology. 2013;81(14):1252-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mueller SG, Nei M, Bateman LM, et al. Brainstem network disruption: a pathway to sudden unexplained death in epilepsy?. Hum Brain Mapp. 2018;39(12):4820-4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patodia S, Somani A, O'Hare M, et al. The ventrolateral medulla and medullary raphe in sudden unexpected death in epilepsy. Brain. 2018;141(6):1719-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Corti C, Battaglia G, Molinaro G, et al. The use of knock-out mice unravels distinct roles for mGlu2 and mGlu3 metabotropic glutamate receptors in mechanisms of neurodegeneration/neuroprotection. J Neurosci. 2007;27(31):8297-8308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Griffin NG, Wang Y, Hulette CM, et al. Differential gene expression in dentate granule cells in mesial temporal lobe epilepsy with and without hippocampal sclerosis. Epilepsia. 2016;57(3):376-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aronica EM, Gorter JA, Paupard MC, Grooms SY, Bennett MV, Zukin RS. Status epilepticus-induced alterations in metabotropic glutamate receptor expression in young and adult rats. J Neurosci. 1997;17(21):8588-8595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pacheco Otalora LF, Couoh J, Shigamoto R, Zarei MM, Garrido Sanabria ER. Abnormal mGluR2/3 expression in the perforant path termination zones and mossy fibers of chronically epileptic rats. Brain Res. 2006;1098(1):170-185. [DOI] [PubMed] [Google Scholar]
- 49.Turnbull J, Girard JM, Lohi H, et al. Early-onset Lafora body disease. Brain. 2012;135(pt 9):2684-2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martin D, Miller G, Rosendahl M, Russell DA. Potent inhibitory effects of glial derived neurotrophic factor against kainic acid mediated seizures in the rat. Brain Res. 1995;683(2):172-178. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data needed to evaluate the conclusions in the paper are present in the paper and on Dryad at doi.org/10.5061/dryad.dfn2z3508. Additional data related to this article may be requested from the authors.