Abstract
Objective
To identify relationships between idiopathic intracranial hypertension (IIH) and socioeconomic determinants of health, such as low-income status and proximity to healthy food.
Methods
This retrospective case–control study of adult female neuro-ophthalmology patients from one institution identified 223 women with and 4,783 women without IIH. Street addresses were geocoded and merged with US census data to obtain census tract–level information on income and food access. Choropleth maps visualized IIH clusters within certain neighborhoods. Logistic regression compared the proportion of patients with IIH from racial and ethnic minority backgrounds, low-income census tracts, and food deserts and swamps to controls without IIH.
Results
In our cohort, when adjusted for age, women with IIH were more likely to be Black (odds ratio [OR] 3.96, 95% confidence interval [CI] 2.98–5.25), Hispanic (OR 2.23, 95% CI 1.14–4.36), and live in low-income tracts (OR 2.24, 95% CI 1.71–2.95) or food swamps (OR 1.54, 95% CI 1.15–2.07). Patients with IIH were less likely to live in food deserts than controls (OR 0.61, 95% CI 0.45–0.83). The association between Black race and IIH remained significant even after adjusting for other variables.
Conclusion
IIH is more common among Black and Hispanic women than expected even when accounting for the demographics of a metropolitan city. Some of this relationship is driven by the association of obesity and IIH incidence with low income and proximity to unhealthy foods.
Idiopathic intracranial hypertension (IIH), also known as the primary pseudotumor cerebri syndrome, occurs most commonly in obese women of childbearing age.1 As obesity is the primary risk factor for IIH,2 socioeconomic and environmental factors related to obesity may provide context for understanding the incidence of IIH. Specifically, local food access has been shown to affect neighborhood-level obesity rates. A 2010 study found that a higher number of neighborhood supermarkets was associated with a lower obesity rate, whereas a higher amount of convenience stores and fast food restaurants was associated with higher levels of obesity.3 Food deserts (neighborhoods with low access to healthy food options) and food swamps (neighborhoods with a high density of high-calorie fast food and junk food options) are both risk factors for obesity, although the relationship with food swamps appears to be stronger.4,5 Food deserts and food swamps are both found more frequently in low-income communities, and a recent Centers for Disease Control and Prevention (CDC) review found that among women, higher incomes were associated with lower obesity prevalence.6 Both income and food access limitations disproportionately affect minority communities. As a result, obesity has been noted to be more common in the Black and Hispanic population,3 and the progression of IIH among African Americans appears to be more severe,7 although there are no known differences in terms of disease incidence. Although the causal link between obesity and IIH remains unclear, many different socioeconomic factors play a role in its incidence. We aimed to identify the relationships between certain socioeconomic and geographic factors and IIH incidence.
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
This study was approved by the University of Pennsylvania Institutional Review Board. For this retrospective analysis of existing data, the need for written informed consent was waived.
Study Methods
We performed a retrospective single-center cohort study of adult (age 18 or older) female patients with IIH as well as controls without IIH. Using electronic health record data, patients who had an initial outpatient visit with a neuro-ophthalmologist at the University of Pennsylvania between January 1, 2010 (when electronic medical records were first available) and July 1, 2018 were identified, and information regarding age, race (self-identified), ethnicity (self-identified), sex, insurance payer, and street address was obtained. Patients who had a diagnosis code for IIH (ICD-9 348.2, ICD-10 G93.2) or papilledema (ICD-9 377.00-01, ICD-10 H47.10–11) and order for a lumbar puncture were identified; their charts were reviewed to confirm that they met current diagnostic criteria for IIH,7 and data on weight and body mass index (BMI) at each health system encounter were collected. We limited our IIH cohort to women with no identifiable cause of increased intracranial pressure other than obesity. Controls without IIH were limited to women 50 years of age or younger in order to approximate IIH demographics. To ensure that the street address, weight, and BMI recorded at the initial neuro-ophthalmology visit were generally indicative of conditions at the time of disease onset, we also restricted the IIH cohort to newly diagnosed, incident cases and excluded patients who presented with a remote history of treated IIH.
Next, de-identified street addresses were converted into US census tracts using the US Census Bureau's batch geocoding program.8 Using publicly available data from the US Department of Agriculture (USDA) Economic Research Service9 and the CDC Division of Nutrition, Physical Activity, and Obesity,10 we identified census tracts with low access to healthy food options (food deserts) or with a high proportion of unhealthy vs healthy food options (food swamps).5 The USDA defines a food desert as a census tract where a substantial number (at least 500 people) or proportion (at least 33%) of the population is more than 1 mile from the nearest supermarket, supercenter, or large grocery store for an urban area or more than 10 miles for a rural area. The US Census Bureau defines a census tract as urban if the geographic centroid of the tract is in an area with more than 2,500 people; all other tracts are rural. Using the CDC's Modified Retail Food Environment Index,10 we defined food swamps as census tracts where 10% or less of food retailers provided healthy food options. We also used publicly available 2010 US Census data to determine the total and minority population in each census tract and to identify low-income tracts (defined as census tracts where the poverty rate exceeds 20% or the tract's median family income is less than or equal to 80% of city-wide or state-wide median family income).
Statistical analyses were performed using STATA IC/15.1 (College Station, TX). Descriptive statistics for IIH and neuro-ophthalmology patients without IIH were summarized. We produced choropleth maps of the metropolitan Philadelphia area to visualize the geographic distribution of IIH and neuro-ophthalmology patients without IIH and used logistic regression to compare patients with IIH and controls adjusting for age, race, ethnicity, BMI, Medicaid coverage (as a proxy for socioeconomic status), and neighborhood characteristics (low-income census tracts, food desert, and food swamp census tracts). Among patients with IIH, we measured the association between race, ethnicity, income, neighborhood food access, and baseline weight/BMI using linear regression.
Data Availability
Raw data were generated at the University of Pennsylvania Health System. Personal and geographically de-identified data supporting the findings of this study are available from the corresponding author on request.
Results
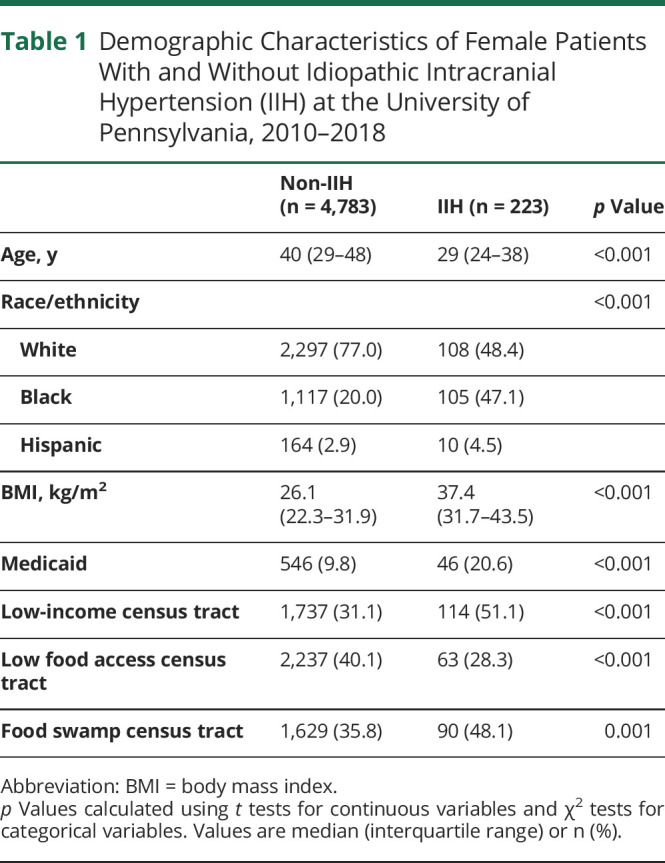
We included 223 women with IIH and 4,783 women without IIH from our tertiary neuro-ophthalmology clinics over an 8-year period (figure 1). Baseline demographic characteristics of patients with IIH and controls without IIH are summarized in table 1. Median age was 29 years for patients with IIH and 40 years for patients without IIH. Over half of patients with IIH were Black or Hispanic, compared to 22.9% of patients without IIH. A total of 51.1% of patients with IIH lived in low-income census tracts and 20.6% relied on Medicaid insurance.
Figure 1. Flowchart Depicting Subject Identification.
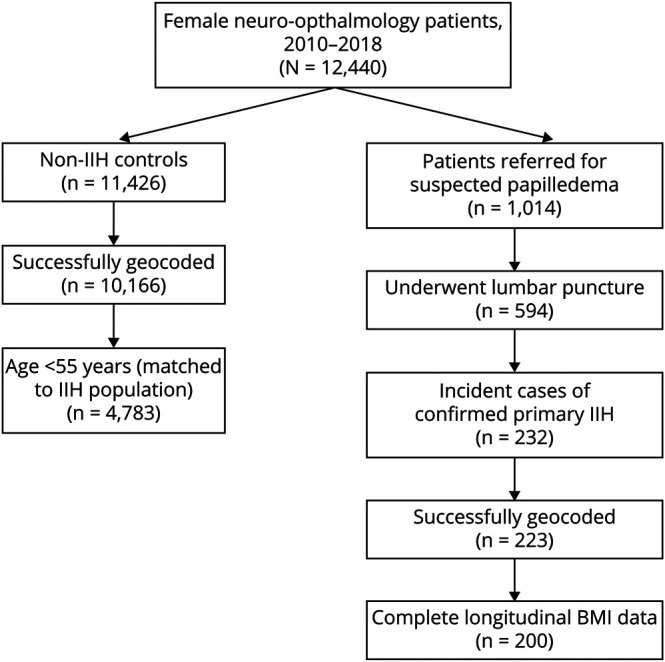
BMI = body mass index; IIH = idiopathic intracranial hypertension.
Table 1.
Demographic Characteristics of Female Patients With and Without Idiopathic Intracranial Hypertension (IIH) at the University of Pennsylvania, 2010–2018
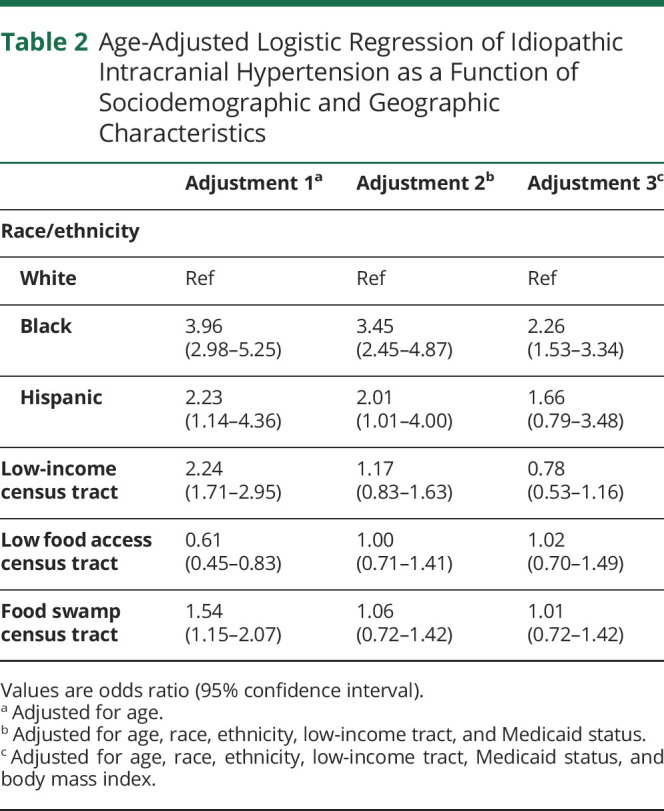
When adjusted for age, women with IIH were more likely to be Black (odds ratio [OR] 3.96, 95% confidence interval [CI] 2.98–5.25), Hispanic (OR 2.23, 95% CI 1.14–4.36), and live in low-income (OR 2.24, 95% CI 1.71–2.95) or food swamp census tracts (OR 1.54, 95% CI 1.15–2.07) (table 2). In contrast, patients with IIH were less likely to live in a food desert census tract than controls without IIH (OR 0.61, 95% CI 0.45–0.83). After adjusting additionally for race, ethnicity, and Medicaid status, patients with IIH were still more likely to be Black (OR 3.45, 95% CI 2.45–4.87) or Hispanic (OR 2.01; 95% CI 1.01–4.00), but the association between census tract characteristics and IIH was no longer statistically significant. The association between Black race and IIH remained significant even after adjusting for BMI (OR 2.261, 95% CI 1.53–3.34).
Table 2.
Age-Adjusted Logistic Regression of Idiopathic Intracranial Hypertension as a Function of Sociodemographic and Geographic Characteristics
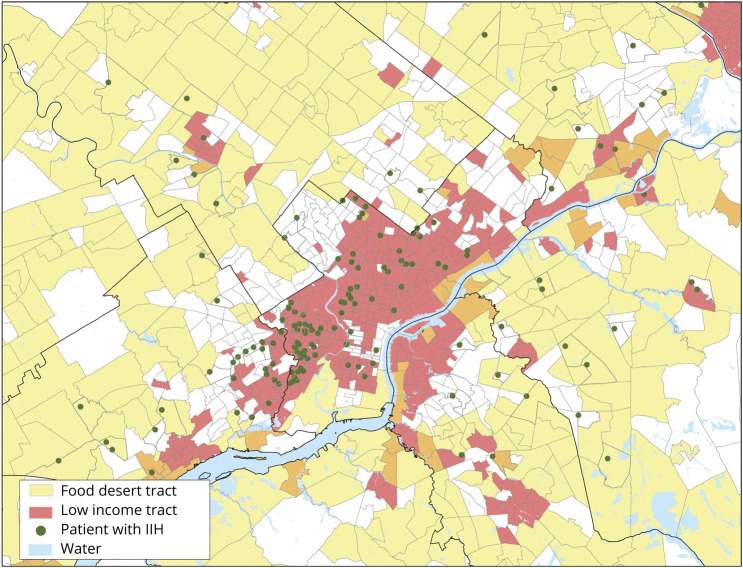
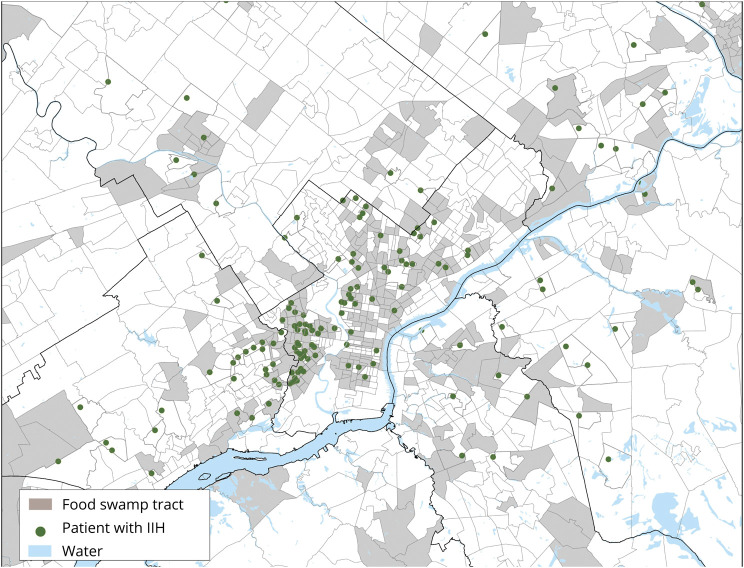
Figures 2 and 3 show the approximate geographic distribution of patients with IIH in metropolitan Philadelphia relative to census tract characteristics. Census tracts with one or more patients with IIH (denoted by a green dot randomly located within their respective boundaries and thus not indicative of exact geographic coordinates) were clustered primarily in West and North Philadelphia. These areas correspond to low-income and food swamp census tracts but not food desert census tracts, which are concentrated in outlying areas.
Figure 2. Geographic Approximation of Patients With Idiopathic Intracranial Hypertension (IIH) in Low-Income and Food Desert Census Tracts in Metropolitan Philadelphia.
Green dots denote census tracts with 1 or more patients with IIH. Dots are randomly placed within corresponding census tract and are therefore approximations that do not indicate exact geographic coordinates.
Figure 3. Geographic Approximation of Patients With Idiopathic Intracranial Hypertension (IIH) in Food Swamp Census Tracts in Metropolitan Philadelphia.
Green dots denote census tracts with 1 or more patients with IIH. Dots are randomly placed within corresponding census tract and are therefore approximations that do not indicate exact geographic coordinates.
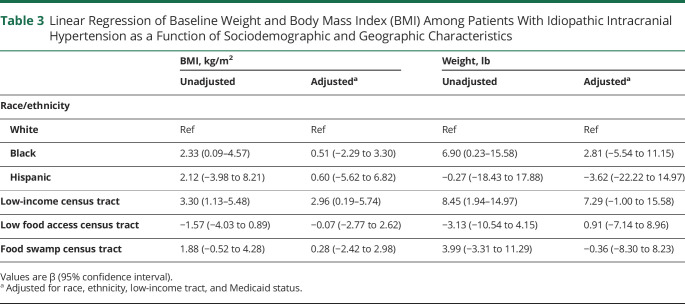
Among patients with IIH in our cohort, baseline BMI was higher in patients who were Black (mean difference 2.33 kg/m2, 95% CI 0.09–4.57) or lived in low-income census tracts (mean difference 3.30 kg/m2, 95% CI 1.13–5.48) than those who did not, but these did not remain significant after adjusting for Medicaid use and census tract characteristics (table 3).
Table 3.
Linear Regression of Baseline Weight and Body Mass Index (BMI) Among Patients With Idiopathic Intracranial Hypertension as a Function of Sociodemographic and Geographic Characteristics
Discussion
In this study of over 5,000 neuro-ophthalmology patients, we found that IIH was more common among Black and Hispanic women and those living in low-income and food swamp census tracts. While obesity is more prevalent in minority populations and is associated with both income and neighborhood characteristics, in this study, race appeared to be additionally independently associated with IIH even after adjusting for BMI, income, and food access.
Previous studies have reported that a significant proportion of patients with IIH come from minority backgrounds in the United States, but direct comparisons with the general population are lacking. In academic centers in Atlanta7 and Detroit,11 44%–65% of patients with IIH are Black, but this may be similar to or higher than the general population depending on whether the catchment area is primarily within city limits or whether it includes surrounding suburbs.2 Other population-based studies of IIH prevalence have been conducted in predominantly White populations in the United States and Europe.12 In our cohort, 51.6% of patients with IIH in Philadelphia were Black or Hispanic, but by using our non-IIH neuro-ophthalmology patient population as a control group that captures our actual sociodemographic catchment area, we are able to show that this is significantly higher than expected.
Obesity is the primary risk factor for IIH, and there are also data revealing that obesity is more prevalent in minority populations in the United States,4 so it is possible that the association between race/ethnicity and IIH is confounded by obesity. However, in our study, patients with IIH were still more than twice as likely to be Black than neuro-ophthalmology patients without IIH even after adjusting for BMI, and among patients with IIH, neither baseline weight/BMI nor weight loss was significantly associated with race after adjusting for confounders. Thus, racial differences in IIH prevalence do not appear to be explained solely by obesity. In light of other studies that have found more acutely presenting symptoms, higher CSF opening pressure, and more severe papilledema and vision loss in Black patients with IIH compared to White patients with IIH,7 our results provide further support for racial disparities that can be seen in IIH. Although at least some of this relationship is driven by the association of obesity and IIH incidence with low income and food swamps, it does not fully explain the observed differences, and other systemic health disparities and inequities are likely involved.
The relationship between race/ethnicity, obesity, and IIH is further complicated by complex socioeconomic determinants of health. Specifically, low-income census tracts and those with limited food access are associated with an increased risk of obesity, both of which disproportionately affect minorities.4-6 Calorie-dense foods (such as those served in fast food restaurants) are less expensive than healthy alternatives,4,5 and reliance on these food sources in the setting of income limitation is associated with obesity. The term food desert is used to identify areas that have limited food access. Food swamps are described as areas with a higher ratio of calorie-dense and unhealthy foods compared to healthy foods and have recently been identified as a better predictor of obesity rates than food deserts.5 We found significant associations between low-income and food swamp census tracts and IIH prevalence, which appear to be mediated primarily through their association with obesity. Given the relatively compact dimensions of central Philadelphia and that the definition of a food desert in an urban setting only requires a supermarket to be within 1 mile of the population, most inner-city census tracts were effectively disqualified from being a food desert. This would explain why in our group, patients with IIH were more likely to live in food swamps than food deserts.
There are a few limitations to this study. Given the proximity of our center to certain low-income neighborhoods in Philadelphia, it is possible that our sample of patients with IIH may not be representative of all patients with IIH in Philadelphia, with patients from higher-income areas pursing care elsewhere in the city. However, our use of a control group from the same health care system suggests that this is not the case, as neuro-ophthalmology patients without IIH are not concentrated within the same neighborhoods. Similarly, it is possible that the socioeconomic background of our controls without IIH may not be representative of all patients without IIH in the area. Because the controls in our study were required to have been seen by a neuro-ophthalmologist, both to confirm the presence or absence of papilledema and account for geographical referral patterns, they may not be representative of the general population due to their underlying reason for referral, and it is possible that non-IIH referrals to neuro-ophthalmology, such as multiple sclerosis, myasthenia gravis, or concussion, may be more prevalent among White patients, which would skew comparisons made between patients with IIH and their controls without IIH. However, the only way to truly sample appropriate controls would be to have a large cohort of patients throughout the entire community who undergo surveillance fundus photography to screen for papilledema regardless of symptoms, which would not be feasible.
We demonstrate that in our cohort, IIH is more common than expected among Black and Hispanic women, even when accounting for the demographics of a metropolitan city. At least some of this relationship is driven by the association of obesity and IIH incidence with low income and food swamps. Given the many variables associated with the disease process, these findings, in some part, can likely be attributed to systemic health disparities and inequity, suggestive more towards a multifactorial basis rather than simply race or ethnicity. Further studies are warranted to better elucidate the causal link between race, ethnicity, access to healthy foods, exposure to unhealthy foods, and the incidence of IIH.
Glossary
- BMI
body mass index
- CDC
Centers for Disease Control and Prevention
- CI
confidence interval
- ICD-9
International Classification of Diseases–9
- ICD-10
International Classification of Diseases–10
- IIH
idiopathic intracranial hypertension
- OR
odds ratio
- USDA
US Department of Agriculture
Appendix. Authors
Footnotes
CME Course: NPub.org/cmelist
Study Funding
The study is funded by the NIH (NINDS T32 NS061779-10).
Disclosure
V.L. Brahma, J. Snow, and V. Tam report no financial disclosures. A.G. Ross receives honoraria and consulting fees from Noveome Biotherapeutics, Inc.; grant support from NIH grant EY030163, F. M. Kirby Foundation, the RWJ Harold Amos Faculty Development Award, and the American Glaucoma Society; and an unrestricted grant to the Department of Ophthalmology by Research to Prevent Blindness. M.A. Tamhankar receives royalty from UpToDate, Inc, and has an unrestricted grant to the Department of Ophthalmology by Research to Prevent Blindness. K.S. Shindler receives grant support from NIH grant EY019014, Research to Prevent Blindness Physician Scientist Award, the F. M. Kirby Foundation, and Mallinckrodt Pharmaceuticals; honoraria and consulting fees from Noveome Biotherapeutics; royalties from UpToDate, Inc.; fees for expert testimony and opinions in medical-legal cases; US patent pending 16/607,834; and an unrestricted grant to the Department of Ophthalmology by Research to Prevent Blindness. R.A. Avery has an unrestricted grant to the Department of Ophthalmology by Research to Prevent Blindness. G.T. Liu receives royalties for Liu, Volpe, and Galetta's Neuro-ophthalmology: Diagnosis and Management. A.G. Hamedani receives grant support from the NIH (NINDS T32 NS061779-10 [source of funding for this paper]) and the Parkinson Study Group and speaking honoraria from Northwell Health. Go to Neurology.org/N for full disclosures.
References
- 1.Friedman DI, Liu GT, Digre KB. Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology 2013;81(13):1159-1165. [DOI] [PubMed] [Google Scholar]
- 2.Kilgore KP, Lee MS, Leavitt JA, et al. Re-evaluating the incidence of idiopathic intracranial hypertension in an era of increasing obesity. Ophthalmology 2017;124(5):697-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bodor JN, Rice JC, Farley TA, Swalm CM, Rose D. The association between obesity and urban food environments. J Urban Health 2010;87(5):771-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghosh-Dastidar B, Cohen D, Hunter G, et al. Distance to store, food prices, and obesity in urban food deserts. Am J Prev Med 2014;47(5):587-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooksey-Stowers K, Schwartz MB, Brownell KD. Food swamps predict obesity rates better than food deserts in the United States. Int J Environ Res Public Health 2017;14(11):1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogden C, Fakhouri T, Carroll M, et al. Prevalence of obesity among adults, by household income and education: United States, 2011-2014. Morb Mortal Wkly Rep 2017;66(50):1369-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruce BB, Preechawat P, Newman NJ, Lynn MJ, Biousse V. Racial differences in idiopathic intracranial hypertension. Neurology 2008;70(11);861-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Welcome to Geocoder. Accessed October 3, 2019. geocoding.geo.census.gov/geocoder/geographies/addressbatch?form.
- 9.USDA ERS: Data Access and Documentation Downloads. Accessed October 3, 2019ers.usda.gov/data-products/food-environment-atlas/data-access-and-documentation-downloads/. [Google Scholar]
- 10.CDC. CDC Nutrition Resources & Publications. Centers for Disease Control and Prevention; 2018. Accessed October 3, 2019. cdc.gov/nutrition/resources-publications/index.html. [Google Scholar]
- 11.Galvin JA, Van Stavern GP. Clinical characterization of idiopathic intracranial hypertension at the Detroit Medical Center. J Neurol Sci 2004;223(2):157-160. [DOI] [PubMed] [Google Scholar]
- 12.McCluskey G, Doherty-Allan R, McCarron P, et al. Meta-analysis and systematic review of population-based epidemiological studies in idiopathic intracranial hypertension. Eur J Neurol 2018;25(10):1218-1227. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data were generated at the University of Pennsylvania Health System. Personal and geographically de-identified data supporting the findings of this study are available from the corresponding author on request.