Abstract
Objective
To evaluate the comparative safety and efficacy of direct endovascular thrombectomy (dEVT) compared to bridging therapy (BT; IV tissue plasminogen activator + EVT) and to assess whether BT potential benefit relates to stroke severity, size, and initial presentation to EVT vs non-EVT center.
Methods
In a prospective multicenter cohort study of imaging selection for endovascular thrombectomy (Optimizing Patient Selection for Endovascular Treatment in Acute Ischemic Stroke [SELECT]), patients with anterior circulation large vessel occlusion (LVO) presenting to EVT-capable centers within 4.5 hours from last known well were stratified into BT vs dEVT. The primary outcome was 90-day functional independence (modified Rankin Scale [mRS] score 0–2). Secondary outcomes included a shift across 90-day mRS grades, mortality, and symptomatic intracranial hemorrhage. We also performed subgroup analyses according to initial presentation to EVT-capable center (direct vs transfer), stroke severity, and baseline infarct core volume.
Results
We identified 226 LVOs (54% men, mean age 65.6 ± 14.6 years, median NIH Stroke Scale [NIHSS] score 17, 28% received dEVT). Median time from arrival to groin puncture did not differ in patients with BT when presenting directly (dEVT 1.43 [interquartile range (IQR) 1.13–1.90] hours vs BT 1.58 [IQR 1.27–2.02] hours, p = 0.40) or transferred to EVT-capable centers (dEVT 1.17 [IQR 0.90–1.48] hours vs BT 1.27 [IQR 0.97–1.87] hours, p = 0.24). BT was associated with higher odds of 90-day functional independence (57% vs 44%, adjusted odds ratio [aOR] 2.02, 95% confidence interval [CI] 1.01–4.03, p = 0.046) and functional improvement (adjusted common OR 2.06, 95% CI 1.18–3.60, p = 0.011) and lower likelihood of 90-day mortality (11% vs 23%, aOR 0.20, 95% CI 0.07–0.58, p = 0.003). No differences in any other outcomes were detected. In subgroup analyses, patients with BT with baseline NIHSS scores <15 had higher functional independence likelihood compared to those with dEVT (aOR 4.87, 95% CI 1.56–15.18, p = 0.006); this association was not evident for patients with NIHSS scores ≥15 (aOR 1.05, 95% CI 0.40–2.74, p = 0.92). Similarly, functional outcomes improvements with BT were detected in patients with core volume strata (ischemic core <50 cm3: aOR 2.10, 95% CI 1.02–4.33, p = 0.044 vs ischemic core ≥50 cm3: aOR 0.41, 95% CI 0.01–16.02, p = 0.64) and transfer status (transferred: aOR 2.21, 95% CI 0.93–9.65, p = 0.29 vs direct to EVT center: aOR 1.84, 95% CI 0.80–4.23, p = 0.15).
Conclusions
BT appears to be associated with better clinical outcomes, especially with milder NIHSS scores, smaller presentation core volumes, and those who were “dripped and shipped.” We did not observe any potential benefit of BT in patients with more severe strokes.
Trial Registration Information
ClinicalTrials.gov Identifier: NCT02446587.
Classification of Evidence
This study provides Class III evidence that for patients with ischemic stroke from anterior circulation LVO within 4.5 hours from last known well, BT compared to dEVT leads to better 90-day functional outcomes.
Endovascular thrombectomy (EVT) is the current standard of care treatment for patients with acute ischemic stroke (AIS) with a proximal large vessel occlusion (LVO) in the anterior circulation1 because it has been robustly associated with both significant functional improvement and survival increase.2 Despite the fact that >80% of the participants in pivotal EVT trials received IV alteplase (tissue plasminogen activator [tPA]) before EVT3 and that current international recommendations advocate IV thrombolysis (IVT) before the initiation of EVT for all eligible patients with LVO (Class of Recommendation I, Level of Evidence IA),1,4 concerns have been raised regarding the utility of tPA pretreatment for patients with LVO who have been selected for EVT.5-7
The arguments in favor of direct EVT (dEVT) include the potential delay in EVT initiation with tPA pretreatment, the low overall recanalization rates with IV tPA before thrombectomy,8,9 increased thrombus fragility and migration with increased risk of distal emboli,10 increased risk for systemic and hemorrhagic complications with bridging therapy (BT; IVT plus EVT), and the increased costs of tPA administration.5,11,12The utility of IVT pretreatment has been further questioned after the publication of observational registry data suggesting better outcomes for patients with LVO presenting directly to an EVT-capable stroke center bypassing the interhospital transfers from primary stroke centers that can only initiate tPA administration.10,11
On the other hand, there are arguments in favor of IVT before EVT such as the potential for early reperfusion that was observed in 7% to 8% of patients in early-window EVT trials,8,9 thrombus softening and facilitation of successful reperfusion,13 the potential role of IV tPA in patients who do not achieve successful reperfusion with EVT, and the effect on distal residual occlusions after EVT.
In addition, the potential adjunctive benefit from IVT may not occur across all patients receiving EVT and rather would be specific to selected subgroups as related to stroke severity at the time of presentation and whether IVT is delivered at non-EVT center “drip and ship” or presenting directly to an EVT-capable center.
We aimed to investigate the comparative safety and efficacy of dEVT vs BT for patients with AIS with anterior circulation LVO presenting within 4.5 hours from last known well (LKW) in the Optimizing Patient Selection for Endovascular Treatment in Acute Ischemic Stroke (SELECT; NCT02446587) study. We also sought to assess whether the potential effect of BT was mediated by stroke severity, stroke size measured by ischemic core volume, and presentation status to the EVT-capable center (direct vs secondary transfer).
Methods
SELECT Trial Methods
The methods and results of SELECT cohort study have been published previously.14,15 Briefly, consecutive patients with AIS with anterior circulation LVO (internal carotid artery, M1 or M2 segments of middle cerebral artery), no or minimal prestroke deficit (modified Rankin Scale [mRS] score 0–1), and NIH Stroke Scale (NIHSS) score of ≥6 presenting to 9 US large-volume EVT centers from January 2016 to February 2018 were enrolled in the study. The initial enrollment window was up to 8 hours from LKW to groin puncture for patients with EVT and LKW to emergency room arrival for medical management only. This window was extended to up to 24 hours after results of the DWI or CTP Assessment With Clinical Mismatch in the Triage of Wake-Up and Late Presenting Strokes Undergoing Neurointervention With Trevo (DAWN) study were presented in May 2017. All patients received a unified prespecified imaging protocol with noncontrast CT, CT angiography, and CT perfusion (CTP) with core infarct and mismatch determination using RAPID software (iSchemaView, Menlo Park, CA). The prespecified favorable profiles on CT (Alberta Stroke Program Early CT Score [ASPECTS] ≥6) and CTP (1) core volume measured on cerebral blood flow (regional cerebral blood flow <30%) of <70 cm3 and (2) ratio between the critically hypoperfused tissue (Tmax > 6 seconds) and ischemic core (regional cerebral blood flow <30%) volume ≥1.2 with an absolute difference of ≥10 cm3) were provided to the site investigators, but the decision to proceed with thrombectomy vs medical management alone was left at the discretion of the treating physician. Final infarct volume was measured on magnetic resonance diffusion-weighted imaging sequences obtained after the procedure (up to 24–72 hours from stroke onset) using manual segmentation of the region of interest. If postprocedural MRI was not available, noncontrast CT was used to evaluate the final infarct size. An independent neuroimaging core laboratory blinded to clinical outcomes and enrollment site evaluated all imaging. Assessors blinded to treatment allocation and core laboratory imaging evaluations obtained mRS score assessment at the 90-day follow-up. Written informed consent was obtained from all patients or their legally authorized representatives before enrollment. The study aimed to evaluate different selection methodologies for endovascular therapy, to assess the correlation between the profiles on CT and CTP with the treatment decision and clinical outcomes after thrombectomy, to compare them against each other, and to identify which method provides the highest predictive ability in the selection of patients for EVT.
Study Population
We performed a prespecified subanalysis of SELECT including patients with LVO who arrived at the EVT-capable center within 4.5 hours from LKW. All patients who received EVT were included in this subanalysis. The study cohort was stratified based on IV tPA administration status into BT if they received IV tPA before thrombectomy (IVT + EVT) and dEVT if they did not receive IV tPA. SELECT trial inclusion criteria mandated that patients receive IV tPA only if they met the American Heart Association guidelines for IV tPA administration.15 SELECT was an intention-to-treat study; thus, patients who were taken for thrombectomy but demonstrated reperfusion on the first angiogram run were included in the EVT arm and in the BT group for this analysis.
Standard Protocol Approvals, Registrations, and Patient Consents
The study protocol for SELECT was approved at local institutional review boards for all sites, and the study was prospectively registered at ClinicalTrials.gov (NCT02446587). All participants or their legally authorized representatives provided written informed consent before enrollment in the study.
Interventions
All endovascular procedures were performed with the use of stent retrievers or other devices approved by the US Food and Drug Administration. Standard endovascular procedures, according to the practice of each site, were followed. Administration of tPA was decided on the basis of patient eligibility criteria if they met the American Heart Association guidelines and recommendations.1 The decision to proceed with dEVT instead of BT was at the discretion of the local investigators in a nonrandomized fashion after taking into account the absolute and relative contraindications for tPA administration.1
Outcomes
The primary efficacy outcome was the rate of functional independence, defined as mRS scores of 0 to 2 at 90 days after AIS onset. We also evaluated the following efficacy outcomes: the rate of patients with excellent functional outcomes at 90 days (defined as mRS scores of 0–1) and functional improvement at 90 days defined as a 1-point decrease across all mRS grades (shift analysis).
Safety outcomes included (1) the rates of symptomatic intracerebral hemorrhage (ICH) per European Cooperative Acute Stroke Study (ECASS) II and Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST) criteria, defined as worsening of the NIHSS score of ≥4 points accompanied by evidence of any ICH on follow-up imaging (ECASS II) or parenchymal hemorrhage type I or II (SITS-MOST)16,17; (2) the rates of asymptomatic ICH on follow-up neuroimaging; (3) the rates of neurologic worsening within 24 hours from symptom onset, defined as an increase in the NIHSS score of ≥4 points within 24 hours from hospital admission; and (4) the rates of all-cause 90-day mortality. Procedural and imaging outcomes included (1) the rates of successful reperfusion (modified Thrombolysis in Cerebral Ischemia [mTICI] score ≥2b) and rates of successful reperfusion after first pass; (2) the final infarct volume measured on follow-up MRI DWI or CT scans, when follow-up MRI was not available; and (3) the absolute infarct growth after comparing baseline ischemic core on CTP and follow-up MRIs.
Statistical Analysis
Continuous variables were presented as means with corresponding SDs or medians with corresponding interquartile ranges (IQRs). Dichotomous variables were presented with their absolute numbers and percentages. In case of continuous variables, baseline characteristics and outcomes between the 2 groups were compared with the use of t test if the variables had a normal distribution or Mann-Whitney U test if the variables had a nonnormal distribution and with the Pearson χ2 test if the all expected cell values were >5 or Fisher exact test if any expected cell values were <5 for categorical variables. The Shapiro-Wilk test was used to assess the normality of distribution. Time metrics, including time from arrival at EVT-capable center to groin puncture, were compared between the 2 groups. The likelihood of functional independence (mRS score 0–2) at 90 days according IVT pretreatment history was also assessed in univariable and multivariable binary logistic regression models, adjusting for the potential predefined confounders of age, NIHSS score at presentation, baseline ischemic core volume, serum glucose at presentation, location of the intracranial occlusion, transfer status, and time from symptom onset to arrival to EVT-capable center. The distribution of mRS scores (0–6 points) at 90 days between patients receiving dEVT or BT was assessed with the Cochran-Mantel-Haenszel test, as well as unadjusted and adjusted (for the same baseline variables used in the binary logistic regression models) ordinal logistic regression analyses (shift analyses). The unadjusted and adjusted odds ratios (ORs) and common ORs (cORs) with the corresponding 95% confidence intervals (95% CIs) were reported for all univariable and multivariable logistic regression analyses.
We further explored the effect of tPA pretreatment on the primary outcome of functional independence (mRS score 0–2) at 90 days in predefined subgroup analyses according to stroke severity using an admission NIHSS score of 15 as a cutoff and ischemic core volume (relative cerebral blood flow <30%) on admission using a cutoff of 50 cm3 on CTP. A sensitivity analysis using the cutoff of 17 for presentation NIHSS score was also performed. We also evaluated the effect of BT by the occlusion location at the time of presentation.
Finally, we performed further analyses on all patient baseline characteristics, outcomes of interest, and subgroup comparisons for patients who were admitted within 4.5 hours directly to EVT-capable comprehensive stroke care centers and those who presented initially to non-EVT centers and then were transferred to an EVT-capable centers drip-and-ship cases. In all analyses, we reported p values as 2 sided, and values of p < 0.05 were considered statistically significant for reported associations. Statistical significance for reported interactions was set at p < 0.1.
Data Availability
The individual patient data will not be made available. Analysis codes and outputs will be made available on reasonable request after review by the study steering and publication committees.
Results
Baseline Characteristics
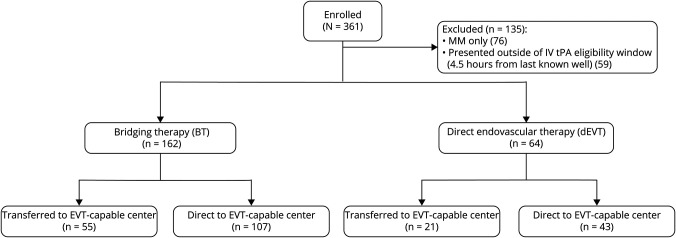
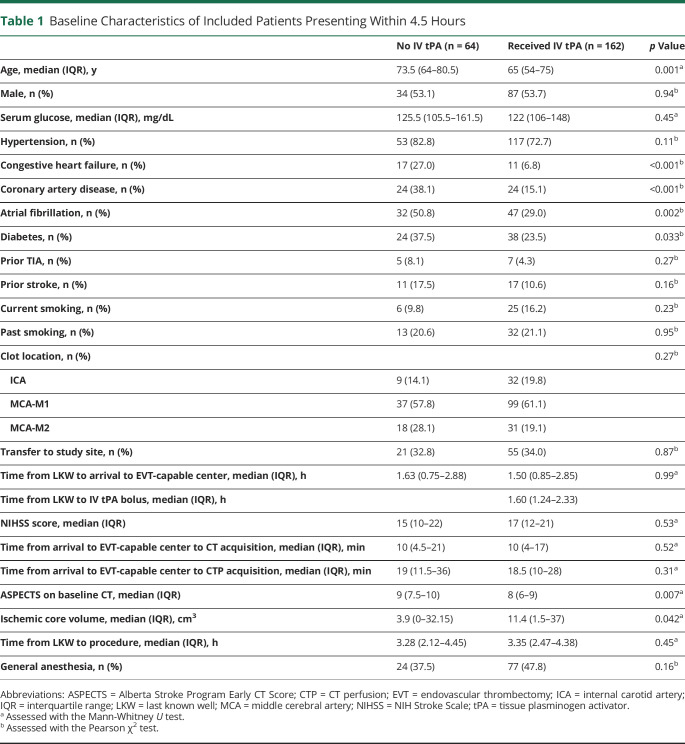
Overall, of 285 patients who received EVT, we identified a total of 226 patients with LVO (54% men, figure 1) fulfilling our prespecified inclusion criteria. Mean age was 65.6 ± 14.6 years, and median NIHSS score at presentation was 17 (IQR 12–21). Sixty-six percent (n = 150) patients presented directly to an EVT center, while 34% (76) were transfers. Median time from LKW to groin puncture was 3.3 (IQR 1.9–4.4) hours. Patients with BT (n = 162, 72%) were younger (p = 0.001) and had significantly lower prevalence of congestive heart failure (p < 0.001), coronary artery disease (p < 0.001), atrial fibrillation (p = 0.002), and diabetes mellitus (p = 0.033) compared to patients receiving dEVT (table 1). Median time from LKW to IV tPA bolus was 1.6 (IQR 1.2–2.3) hours. On baseline neuroimaging, patients receiving BT had lower median ASPECTS on admission CT (8 vs 9, p = 0.007) and larger ischemic core volumes median (11.4 [IQR 1.5–37] mL vs 3.9 [IQR 0–32.15] mL, p = 0.042) compared to patients receiving dEVT (table 1). The reasons for IV tPA ineligibility are listed in table e-1 (data available from Dryad, doi.org/10.5061/dryad.sxksn0323). Treatment with anticoagulation or a coagulopathy disorder and recent major surgery were the 2 main reasons for not receiving IV tPA. Three patients in the BT group demonstrated successful reperfusion on first angiogram run and did not receive further intervention. Primary occlusion was observed in the internal carotid artery in 41 (18%), in the middle cerebral artery M1 segment in 136 (60%), and in the middle cerebral artery M2 segment in 49 (22%) patients. Further results based on occlusion location are provided in the supplemental results.
Figure 1. Flow Diagram of SELECT Participants Included in the Analysis.
EVT = endovascular thrombectomy; MM = medical management; SELECT = Optimizing Patient Selection for Endovascular Treatment in Acute Ischemic Stroke.
Table 1.
Baseline Characteristics of Included Patients Presenting Within 4.5 Hours
Time Metrics for dEVT vs BT
No statistically significant difference (p = 0.99) was observed in median time from LKW to arrival to EVT-capable center for patients who received BT (1.5 [IQR 0.9–2.9] hours) and dEVT (1.6 [IQR 0.8–2.9] hours). The median times from arrival to EVT-capable center to groin puncture did not differ between the 2 groups (BT 1.6 [IQR 1.1–2.0] hours vs dEVT 1.3 [IQR 1.1–1.8] hours, p = 0.21). The overall times from LKW to groin puncture (including transfer times) also were similar between patients who received IV tPA (median 3.35 [IQR 2.47–4.38] hours) and patients who did not receive IV tPA (median 3.28 [IQR 2.12–4.45] hours, p = 0.45).
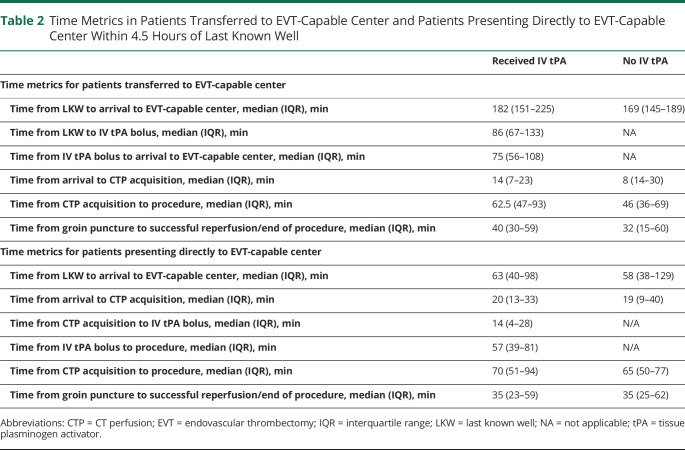
An analysis of patients who presented directly (n = 150) to EVT-capable centers within 4.5 hours of LKW demonstrated that patients with dEVT (n = 43) presented at 1.0 (IQR 0.6–2.2) hours from LKW, whereas patients who received BT (n = 107) presented at 1.1 (IQR 0.7–1.6) hours from LKW. Median time from arrival at EVT-capable center to groin puncture did not differ between the 2 groups (dEVT 1.4 [IQR 1.1–1.9] hours vs BT 1.6 [IQR 1.3–2.0] hours, p = 0.40). Fifty-three (50%) patients received a thrombectomy procedure within <1 hour of IV tPA bolus. Table 2 describes the various time metrics for patients who presented directly to EVT-capable centers within 4.5 hours of stroke onset and received EVT.
Table 2.
Time Metrics in Patients Transferred to EVT-Capable Center and Patients Presenting Directly to EVT-Capable Center Within 4.5 Hours of Last Known Well
Similarly, evaluating patients who were transferred (n = 76) to EVT-capable centers within 4.5 hours of LKW demonstrated that those receiving dEVT (n = 21) presented at 2.8 (IQR 2.4–3.2) hours, whereas those receiving BT (n = 55) presented at 3.0 (IQR 2.5–3.8) hours. Median time from arrival at EVT-capable center to groin puncture did not differ between the 2 groups (dEVT 1.2 [IQR 0.9–1.5] hours vs BT 1.3 [IQR 1.0–1.9] hours, p = 0.24; table 2). Four (7%) of the 55 transferred patients in the BT group received IV tPA after arriving at the EVT-capable center, while 51 (93%) were thrombolyzed at the non-EVT center before transfer.
Outcomes of dEVT vs BT
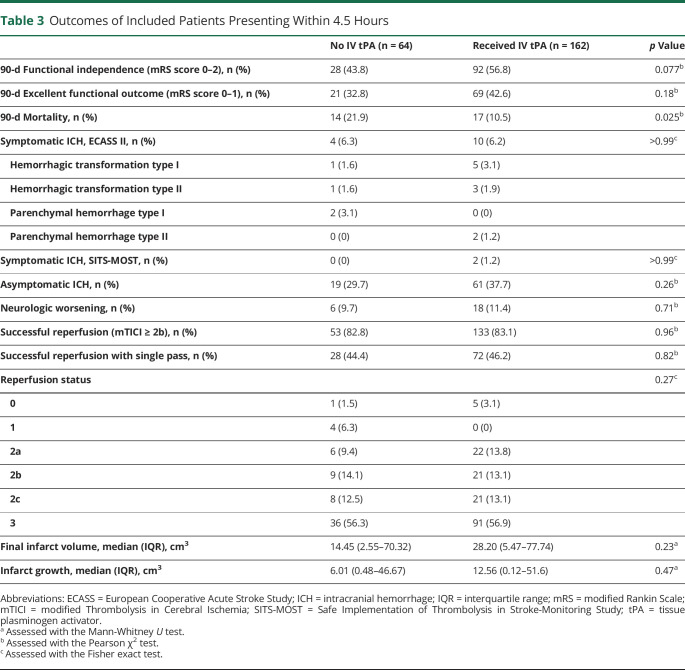
Table 3 and figure 2 show the comparisons of clinical and imaging outcomes between the 2 groups. No statistically significant difference in 90-day functional independence between BT group and dEVT group (BT 56.8% vs dEVT 43.8%, OR 1.69, 95% CI 0.94–3.03, p = 0.077). In addition, the distribution of mRS scores at 90 days was lower with a shift toward better functional outcomes (p = 0.046 by Cochran-Mantel-Haenszel test) in the BT group (figure 3) that corresponded to a cOR of 1.66 (95% CI 0.99–2.76, p = 0.053) for 90-day functional improvement on unadjusted ordinal logistic regression analyses. When these associations were adjusted for potential confounders, IV-tPA administration before EVT was independently associated with higher likelihood of both functional independence (adjusted OR [aOR] 2.02, 95% CI 1.01–4.03, p = 0.046) and a shift towards better functional outcomes (adjusted cOR 2.06, 95% CI 1.18–3.60, p = 0.011).
Table 3.
Outcomes of Included Patients Presenting Within 4.5 Hours
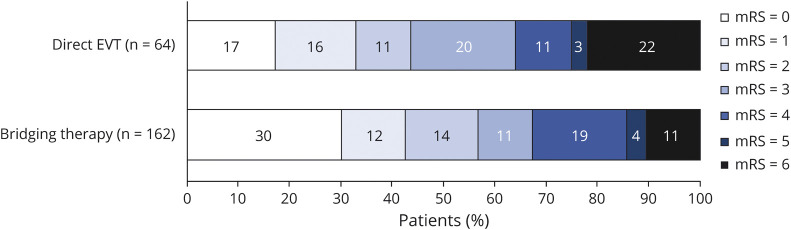
Figure 2. Distribution of the mRS Scores at 90 Days According to History of IV tPA Pretreatment in Patients Presenting Within 4.5 Hours From Stroke Onset.
Distribution of modified Rankin Scale (mRS) scores between the 2 groups was compared by use of the Cochran-Mantel-Haenszel test, with patients treated with bridging therapy demonstrating significantly better functional outcomes at the 90-day follow-up (p = 0.046). EVT = endovascular thrombectomy; tPA = tissue plasminogen activator.
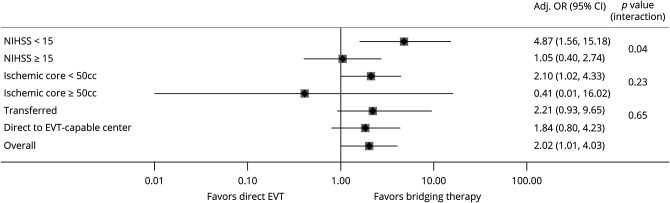
Figure 3. Subgroup Analyses of the Probability of Functional Independence (mRS Score 0–2) at 90 Days According to the History of IV tPA Pretreatment.
Bridging therapy was associated with a significantly higher odds of functional independence in patients with NIH Stroke Scale (NIHSS) score <15 and ischemic core <50 cm3, whereas no significant difference in functional independence was observed in patients with NIHSS score ≥15 and ischemic core ≥50 cm3. Effect of tissue plasminogen activator (tPA) was more pronounced in patients who were transferred to the endovascular thrombectomy (EVT)–capable center compared to patients who presented directly. CI = confidence interval; OR = odds ratio.
We also observed lower mortality rates at 90 days in patients treated with BT compared to dEVT (10.5% vs 21.9%, OR 0.42, 95% CI 0.19–0.91, p = 0.025) with reduced 3-month mortality odds with IV tPA administration (aOR 0.20, 95% CI 0.07–0.58, p = 0.003) in a multivariable analysis. No other differences between the 2 groups were detected with regard to the remaining safety outcomes, including symptomatic (ECASS II: 6.2% in BT vs 6.3% in dEVT, OR 0.98, 95% CI 0.30–3.27, p > 0.99; SITS-MOST: 1.2% in BT vs 0% in dEVT) and asymptomatic (37.7% in BT vs 29.7% in dEVT, OR 1.43, 95% CI 0.77–2.67, p = 0.26) ICH.
Procedural outcomes for EVT did not differ between patients who received and did not receive BT, with rates of successful reperfusion mTICI ≥2b (BT 133 (83.1%) vs dEVT 53 (82.8%), OR 1.02, 95% CI 0.47–2.20, p = 0.96) and successful reperfusion achieved with first pass of stent retriever (BT 72 [47.2%] vs dEVT 28 [44.4%], OR 1.11, 95% CI 0.58–2.10, p = 0.82) similar in both groups. The rates of TICI 2b (BT 13.1% vs dEVT 14.1%), TICI 2c (BT 13.1% vs dEVT 12.5%), and TICI 3 (BT 56.9% vs dEVT 56.2%) were also similar between the 2 groups.
Final infarct volume (BT 28.20 [5.47, 77.74] mL vs dEVT 14.45 [2.55, 70.32] mL, p = 0.23) and infarct growth (BT 12.56 [0.12, 51.6] mL vs dEVT 6.01 [0.48, 46.67] mL, p = 0.47) also were not statistically significantly different between the 2 groups.
Outcomes Based on Presentation Stroke Severity
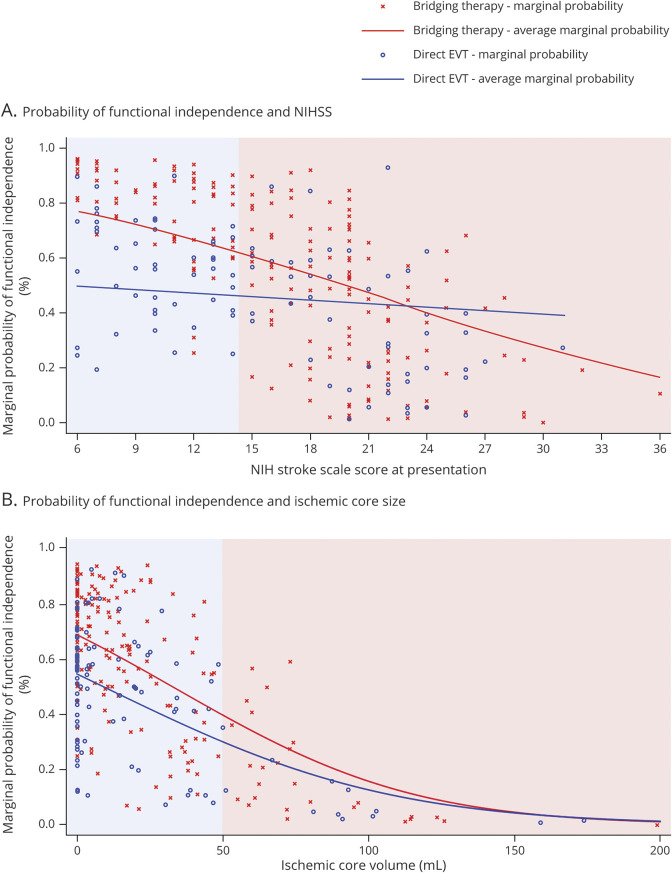
In the predefined subgroup analyses (figure 3), patients presenting with baseline NIHSS scores <15 points treated with BT had significantly higher rates and likelihood of 90-day functional independence (BT 83% vs dEVT 50%, aOR 4.87, 95% CI 1.56–15.18, p = 0.006; table e-2, doi.org/10.5061/dryad.sxksn0323); this association was not evident in patients presenting with baseline NIHSS scores ≥15 points (BT 41.2% vs dEVT 38.2%, aOR 1.05, 95% CI 0.40–2.74, p = 0.92; table e-3). An interaction on the treatment effect according to baseline stroke severity was also uncovered (p for interaction = 0.04). Figure 4A illustrates the higher likelihood of achieving functional independence in patients receiving BT with NIHSS score <15 compared to patients receiving dEVT, which decreases as NIHSS score increases.
Figure 4. Average Marginal Probability for Functional Independence in Patients Receiving Bridging Therapy vs Direct EVT as Associated With NIHSS and Ischemic Core at Presentation.
(A) Graphical representation of the association of the marginal probability for functional independence (modified Rankin Scale [mRS] score 0–2) according to NIH Stroke Scale (NIHSS) score at presentation, stratified by the history of IV alteplase administration before endovascular thrombectomy (EVT). In patients with NIHSS score <15 (indicated by the blue area), average marginal probabilities are significantly higher in patients receiving bridging therapy (BT), whereas in patients with NIHSS score ≥15 (indicated by the red area), the difference between average marginal probabilities decreases and then inverts so that the average marginal probabilities for direct EVT is higher than for BT. (B) Graphical representation of the association of the marginal probability for functional independence (mRS score 0–2) according to baseline ischemic core volume, stratified by the history of IV alteplase administration before EVT. In patients with ischemic core <50 mL (indicated by the blue area), average marginal probabilities are significantly higher in patients receiving BT, whereas in patients with ischemic core ≥50 mL (indicated by the red area), the difference between average marginal probabilities decreases and marginal probabilities in both groups become almost similar as the ischemic core size increases.
Similarly, patients treated with BT also demonstrated lower rates of mortality (BT 0% vs dEVT 13%, p = 0.011) in those with baseline NIHSS score <15. The rates of mortality were numerically lower in patients with NIHSS score ≥15 (BT 17% vs dEVT 29%, OR 0.47, 95% CI 0.19–1.18, p = 0.11), but the difference did not reach statistical significance. The rates of symptomatic ICH and neurologic worsening were similar across treatment arms in both NIHSS score strata.
A sensitivity analysis using the cutoff of 17 also demonstrated similar results with better functional independence (BT 78% vs dEVT 54%, aOR 2.64, 95% CI 1.04–6.37, p = 0.042) and reduced mortality (BT 1.1% vs dEVT 13.5%, p = 0.009) with NIHSS score ≤17 and no statistically significant difference in functional independence (BT 33% vs dEVT 30%, aOR 1.07, 95% CI 0.34–3.37, p = 0.91) and mortality (BT 21.1% vs dEVT 33.3%, p = 0.21) with NIHSS score >17. An interaction term between NIHSS score strata (≤17 vs > 17) and IVT on functional independence demonstrated a value of p = 0.008.
Outcomes Based on Presentation Ischemic Core Size
Patients presenting with baseline ischemic core volume of <50 cm3 treated with BT had significantly higher rates and likelihood of 90-day functional independence (BT 61.9% vs dEVT 46.4%, aOR 2.10, 95% CI 1.02–4.33, p = 0.044) compared to patients receiving dEVT (table e-4, doi.org/10.5061/dryad.sxksn0323); this association was not evident in patients presenting with ischemic core volume of ≥50 cm3 (BT 26% vs dEVT 25%, aOR 0.41, 95% CI 0.01–16.02, p = 0.64; table e-5, doi.org/10.5061/dryad.sxksn0323). However, the interaction term on the treatment effect according to the baseline ischemic core volume was not significant (p for interaction = 0.23). An almost inverse linear association between the baseline ischemic core volume and the likelihood of good functional outcome at 90 days was uncovered for both patients treated with dEVT and those treated with BT. Figure 4B illustrates the higher likelihood of achieving functional independence at 90 days in patients receiving BT with small core infarcts compared to those receiving dEVT with average marginal probabilities decreasing in both groups as core infarcts increase. Significantly fewer deaths were observed in patients with ischemic core volume of <50 cm3 treated with BT (BT 5% vs dEVT 18%, OR 0.24, 95% CI 0.09–0.68, p = 0.004), while mortality rates were similar in patients with ischemic core volume of ≥50 cm3 (BT 43% vs dEVT 50%, OR 0.77, 95% CI 0.15–3.86, p > 0.99). No differences in the rates of symptomatic ICH (BT 4% vs dEVT 5%, OR 0.80, 95% CI 0.19–3.30, p = 0.72) and neurologic worsening (BT 7% vs dEVT 9%, OR 0.78, 95% CI 0.25–2.39, p = 0.66) were observed in patients with ischemic core <50 cm3. Similarly, in patients with ischemic core ≥50 cm3, the rates of neurologic worsening (BT 36% vs dEVT 13%, OR 4.0, 95% CI 0.41–38.65, p = 0.37) and symptomatic hemorrhage (BT 17% vs dEVT 13%, OR 1.47, 95% CI 0.14–15.55, p > 0.99) did not differ significantly.
Outcomes Based on Presentation Status: Direct vs Transfer
In patients who presented directly within 4.5 hours to EVT-capable centers (n = 150, 29% treated with dEVT), rates of excellent outcomes (BT 49 [46%] vs dEVT 19 [44%], OR 1.06, 95% CI 0.52–2.17, p = 0.86) did not differ between BT and dEVT. Furthermore, there were no significant differences in functional independence (BT 66 [62%] vs dEVT 22 [51%], OR 1.54, 95% CI 0.75–3.14, p = 0.24) and lower mortality (BT 10 [9%] vs dEVT 8 [19%], OR 0.45, 95% CI 0.16–1.23, p = 0.11) in patients receiving BT (table e-6 and figure e-1a, doi.org/10.5061/dryad.sxksn0323). In adjusted multivariable logistic regression analyses, there was no association between BT and 90-day functional independence (aOR 1.84, 95% CI 0.80–4.23, p = 0.15) and 90-day functional improvement (cOR 1.41, 95% CI 0.76–2.65, p = 0.28; and adjusted cOR 1.80, 95% CI 0.91–3.55, p = 0.089). We did not observe a significant interaction of BT in patients presenting directly to the EVT-capable center after stratification for baseline stroke severity (p for interaction = 0.39) or infarct core volume (p for interaction = 0.41; figure e-2).
Analyzing patients who were transferred to an EVT-capable center within 4.5 hours of LKW (n = 76) showed that the rates of excellent outcomes were significantly higher in patients receiving BT (20 [36%] vs dEVT 2 [10%], OR 5.43, 95% CI 1.14–25.76, p = 0.024) but with no difference in functional independence (BT 26 [47%] vs dEVT 6 [29%], OR 2.24, 95% CI 0.76–6.63, p = 0.14) and mortality rates (BT 7 [13%] vs dEVT 6 [29%], OR 0.36, 95% CI 0.11–1.25, p = 0.10). However, there was an overall shift toward better functional outcomes with BT (adjusted cOR 4.51 [95% CI 1.44–14.15], p = 0.010) (table e-7 and figure e-1bdoi.org/10.5061/dryad.sxksn0323). Logistic regression models, however, did not show significant improvement in functional independence with BT in adjusted (aOR 2.21 [95% CI 0.50–9.65], p = 0.29) analyses. Subgroup analyses demonstrated a significant interaction of BT before the transfer of patients to the EVT center after stratification for baseline stroke severity (p = 0.014).
Discussion
This prespecified subanalysis of the SELECT cohort study14,15 showed that IV tPA administration before EVT in patients with AIS with anterior circulation LVOs may be associated with increased likelihood of functional independence and functional improvement at 90 days, while it is also related to a decrease in the odds of 90-day mortality. We observed no difference in other efficacy or safety outcomes, including the risk of symptomatic or asymptomatic ICH and neurologic worsening. In addition, IVT was not associated with delays in EVT because the median times from hospital arrival to groin puncture were similar in the 2 groups. Furthermore, we detected an interaction that may modify the beneficial effect of BT compared to dEVT in patients with LVO. More specifically, BT appears to be more effective in patients with LVO with mild or moderate baseline stroke severity (NIHSS scores <15 points) who were transfers to EVT centers and those with smaller infarct core volume.
Prior observational studies attempted to assess the adjunctive benefit of BT on EVT outcomes with mixed results. Some demonstrated better outcomes with IV tPA,18-23 whereas others showed no improvement in functional independence or mortality rates.3,13,24 However, many of these studies represented single-center data,13,18-20,23,24 small sample sizes (<100,)13,18,21,23,24 or retrospective study designs.18-23
Our results are in accordance with a recent systematic review and meta-analysis suggesting that BT is independently related to a higher likelihood of 3-month good functional outcome without any evidence for safety concerns, including the risk of symptomatic ICH.25 However, this was not a patient-level meta-analysis with adjustments limited only to the study level. Because the rates of successful reperfusion with first or multiple passes were similar between the 2 groups, the beneficial effect of IVT pretreatment on clinical outcomes may be related to improvement in collateral circulation because of dissolution of distal microthrombi and reduction of the likelihood of infarction in new (previously unaffected territory) complicating EVT.5,7 Our findings do not support previous results suggesting that pretreatment with IVT is associated with increased risk of symptomatic ICH and time delays in the onset of EVT.5
Our results suggested a modulation of potential IVT effect by stroke severity. These findings may be related to increased baseline NIHSS scores being indicative of high clot burden, which is in turn associated with reduced drug permeability and low probability of successful recanalization after IVT.26-28 Recent reports underscored that the length of LVO is inversely associated with the likelihood of tPA-induced recanalization and good functional outcomes in patients receiving IV tPA only.29,30 However, the relationship of IVT effect with stroke severity and clot length is not well established in patients undergoing EVT. This finding has potential implications for in-field triage, suggesting that patients with milder stroke might be the best candidates for transport to the nearest IV tPA centers, while those with more severe strokes should be taken directly to EVT-capable centers. This is supported by our finding that transferred patients with less severe strokes were more likely to benefit from BT compared to those with more severe strokes. The in-field severity-based paramedic triage scales (Rapid Arterial Occlusion Evaluation, Los Angeles Motor Scale, Ambulance Clinical Triage for Acute Stroke Treatment, etc) have been demonstrated to have reduced sensitivity to identify patients with LVO but milder strokes. While not definitive, the data demonstrating benefit for IV tPA in milder strokes and LVO may help balance the need for timely EVT intervention vs bridging with IV tPA administration as well as preventing overtriage to EVT centers using these triage scores.
Our study identified that patients with mild to moderate stroke severity (NIHSS score <15) at presentation derive significant benefit from BT, with limited if any benefit observed in patients with more severe strokes. While our study population excluded patients with minor stroke, these patients were evaluated in a recent multicenter retrospective cohort study.31 They identified an adjunctive benefit of thrombectomy in these group of patients over IVT. These results suggest a significant role of IVT in patients with minor strokes and an LVO who may benefit from adjunctive reperfusion therapies. Further randomized data are required to definitively identify the optimal treatment strategies in these patients with minor strokes due to LVOs.
While functional independence was significantly improved by BT in patients with smaller baseline ischemic core, the rates were similar in patients who did and did not receive tPA if the baseline ischemic core was >50 cm3. Our findings are consistent with prior reports assessing the relationship between IV tPA and stroke size and suggesting lower recanalization rates and worse outcomes in patients with lower ASPECTS.32 A recent post hoc analysis from the Highly Effective Reperfusion Evaluated in Multiple Endovascular Stroke Trials (HERMES) collaboration uncovered no effect modification of EVT by baseline ischemic core volume, as quantified by ASPECTS.33 Similarly, another study describing the analysis based on the ischemic core volume measured by perfusion or DWI found EVT to be effective in patients with up to 125 to 150 cm3 of infarct core volume.34 While these studies examined the effect of EVT compared to no EVT, our study examined the potential effect of BT in patients who received EVT and found it to be effective only in patients with smaller ischemic core.
We used the cutoffs of ischemic core size of 50 cm3 and NIHSS score of ≥15 for the subgroup analysis. Ischemic core size of ≥50 cm3 has been one of the standardized definitions, used in Solitaire™ With the Intention for Thrombectomy as Primary Endovascular Treatment Trial (SWIFT PRIME)9 and SELECT large core analysis.14 It has also been the definition for enrollment for ongoing SELECT 235 randomized clinical trial assessing the efficacy and safety of EVT in patients with large core strokes. In addition, because stroke severity remains a vital clinical variable that clinicians rely heavily on while making treatment decisions for AISs, we aimed to identify a cutoff that clinically determines moderately severe vs severe strokes. Within LVOs, a severity of 15 is a reasonable clinical cutoff for that strata. We further conducted a sensitivity analysis with an NIHSS score cutoff of 17 because this cutoff was the median in prior thrombectomy randomized controlled trials (RCTs)3 and was assessed in recent trials assessing BT,36 with findings similar to the NIHSS score cutoff of 15 that we used in the SELECT cohort.
The modulation of IVT effect on EVT outcomes by stroke severity and initial infarct size may be clinically relevant because several RCTs are assessing BT vs direct thrombectomy. Because the thrombolysis potential treatment effect appears to be driven by patients with milder strokes and smaller to moderate infarcts, our results suggest that such studies should be powered to detect a differential treatment effect based on baseline stroke severity.
Prior data suggested shorter times from stroke onset to thrombectomy in patients receiving direct thrombectomy.24 Our data did not show IV tPA administration to be associated with delays in time metrics because both patients presenting directly to EVT-capable centers and those who were transferred have similar times from LKW and EVT arrival to the initiation of thrombectomy. Our results are consistent with recent large registry results suggesting no delay with IV tPA in both transfer and direct patients.37
Finally, we identified no improvement with BT in the outcomes of patients who presented directly to an EVT-capable stroke center. On the other hand, we observed a higher likelihood of better outcomes with BT in transferred patients. It should be noted that in half of the patients directly presenting to EVT-capable centers, the IV tPA infusion was not complete at the time of the beginning of EVT procedure (time from tPA bolus to groin puncture <60 minutes), which may have affected the overall efficacy of IV tPA in those patients. It is plausible that more time afforded for the IV tPA to work in transferred patients may have resulted in better outcomes compared to transferred patients who did not receive IV tPA. This finding also highlights the importance of swift tPA delivery, regardless of the setting, because earlier onset-to-treatment times are associated with faster and more frequent tPA-induced recanalization, with earlier onset-to-recanalization time finally being the key determinant for improved functional recovery.38 This finding is also important because direct access to EVT in the United States is limited to only one-fifth of the population.39 Thus, until more effective in-field triage algorithms are available, most patients receiving EVT will continue to be seen and treated with tPA at the nearest non-EVT stroke centers first.
Randomized trials are ongoing to evaluate the role of dEVT vs BT- in IV tPA–eligible patients.40,41
Recently, 3 randomized trials evaluating the role of dEVT vs BT in EVT-eligible patients who presented directly to EVT-capable centers were published. Direct Intraarterial Thrombectomy in Order to Revascularize Acute Ischemic Stroke Patients with Large Vessel Occlusion Efficiently in Chinese Tertiary Hospitals: a Multicenter Randomized Clinical Trial (DIRECT-MT)42 and Direct Endovascular Thrombectomy vs Combined IVT and Endovascular Thrombectomy for Patients With Acute Large Vessel Occlusion in the Anterior Circulation (DEVT)43 found noninferiority of dEVT compared to BT with IV tPA, whereas Direct Mechanical Thrombectomy in Acute LVO Stroke (SKIP)36 failed to achieve noninferiority of the dEVT approach. All 3 trials set up generous noninferiority margins: of 20% effect size in DIRECT-MT42; 26% effect size in SKIP,36 and 10% absolute clinical effect (43% vs 33% functional independence) in DEVT.43 In DIRECT-MT, 27% of the eligible study population declined to participate and 10% of the study population did not receive thrombectomy. Furthermore, in line with our findings, the trial reported that 87% of the enrolled patients did not completing their IV tPA infusion before the start of EVT. SKIP and DEVT did not report the proportions but specified beginning of EVT as soon as possible, before completion of IVT. In the SKIP trial, the times from randomization to the initiation of thrombectomy were 20 and 22 minutes in the EVT alone and bridging groups, respectively, while randomization to IV tPA time was at a mean of 14 minutes, leaving a mean of 8 minutes from the initiation of IV tPA to groin puncture. These represent very short times, which are inadequate for IVT completion, which plausibly reduces the potential benefit with BT. The SKIP trial also used a lower-dose thrombolysis regimen (0.6 mg/kg instead of the standard 0.9 mg/kg dose of IV alteplase). These considerations have been described in detail in a recent commentary on the DIRECT-MT and SKIP trials.44
The most common scenario in practice is for patients to present initially to the closest center with the capability to deliver thrombolysis and then transfer to an EVT center, allowing the alteplase time to work. All aforementioned trials included only patients who presented directly to EVT centers, thus excluding patients who are most likely to benefit from BT. In contrast, 34% of our study cohort includes patients transferred to EVT-capable centers, and our finding that drip-and-ship patients were more likely to benefit from BT may be due to the fact they had time for thrombolysis to deliver its potential effect. These trials also lacked evaluation using advanced perfusion imaging. In addition, in the SELECT cohort, we evaluated perfusion imaging parameters, which were not available in the aforementioned trials, and found that in patients with large ischemic core (≥50 mL), BT was not associated with improved functional outcomes. Our results provide an insight into the potential subgroups of patients who may benefit from IVT before EVT. Specifically, we found that those with mild to moderate strokes and those small to moderate infarct size are more likely to have adjunctive benefit from BT. These findings highlight that trials assessing BT potential benefit may show significance only if they were enriched with selected subpopulations. Furthermore, our results supported that the adjunctive benefit of IV tPA are more likely in transferred patients compared to those presenting directly to a thrombectomy-capable center. This finding is particularly relevant because the decision to bridge or not bridge with tPA is often made in the non-EVT center before it is even certain that the patient will be receiving EVT. To accurately assess the true advantage of BT vs direct thrombectomy will require a randomized intention-to-treat analysis of BT vs no BT in patients with LVO who meet both tPA and EVT treatment criteria presenting to both non-EVT and EVT hospitals.
Our analysis based on clot location did not show significant improvement in functional independence with BT, nor did we find a significant interaction of BT with clot location. However, our analysis may have been underpowered because of the small number of patients with M2 occlusions (n = 49) in our dataset. This does not preclude more distal locations to be potential targets for IVT because we report the highest unadjusted improvement in functional independence of 24% with BT in patients with M2 occlusion.
Our study has several limitations. Patients were not randomized to BT vs dEVT, and there is a risk for potential unmeasured confounders that cannot be incorporated in multivariable models, including a risk for selection bias because all treatment decisions were made by the treating physicians at the participating institutions. Only 1 patient who did not receive IV tPA was actually eligible for IV tPA, which may create potential selection bias compared to RCTs evaluating IV tPA adjunctive benefit when only tPA-eligible patients are randomized. The 2 groups, however, had largely similar baseline characteristics. Another limitation is the relatively small sample size of some of our subgroups, resulting in low statistical power to uncover significant differences. Especially for subgroup analyses, complex associations can confound analysis of clinical outcomes. Finally, while SELECT adopted an intention-to-treat paradigm for enrolled patients, there is a possibility that patients achieving successful recanalization after IV-tPA administration and before the initiation of EVT were excluded from enrollment in SELECT. Because it has previously been estimated that ≈1 in 10 patients with AIS with LVO achieves successful reperfusion after tPA infusion that obviates the need for further endovascular reperfusion therapies,34 this additional advantage of tPA pretreatment becomes very relevant, particularly for patients transferred from non-EVT to EVT-capable centers to receive thrombectomy. With Tenecteplase versus Alteplase before Endovascular Therapy for Ischemic Stroke demonstrating improved recanalization rates while using IV tenecteplase, the effect observed can even be larger, especially in countries that have deferred to the tenecteplase-based management strategies for acute strokes.45 In addition, a recent meta-analysis of available RCTs reported that patients with confirmed LVO receiving tenecteplase had higher odds of mRS scores 0 to 2 (OR 2.06, 95% CI 1.15–3.69), successful recanalization (OR 3.05, 95% CI 1.73–5.40), and functional improvement defined as 1-point decrease across all mRS grades (cOR 1.84, 95% CI 1.18–2.87) at 3 months compared with patients with confirmed LVO receiving alteplase.46
We found that BT may be associated with more favorable 90-day functional outcomes, without safety concerns in patients with AIS with anterior circulation LVO, especially in patients with milder strokes, those with smaller initial infarcts, and those who were dripped and shipped. Ongoing RCTs comparing dEVT to BT in tPA-eligible patients with AIS with LVO will provide more definitive data. Our findings shed light on how those studies might be optimally designed and interpreted. For now, it is appropriate to follow current guidelines that recommend IVT pretreatment for all eligible patients.
Glossary
- AIS
acute ischemic stroke
- aOR
adjusted OR
- ASPECTS
Alberta Stroke Program Early CT Score
- BT
bridging therapy
- CI
confidence interval
- cOR
common OR
- CTP
CT perfusion
- DAWN
DWI or CTP Assessment With Clinical Mismatch in the Triage of Wake-Up and Late Presenting Strokes Undergoing Neurointervention With Trevo
- dEVT
direct EVT
- ECASS
European Cooperative Acute Stroke Study
- EVT
endovascular thrombectomy
- HERMES
Highly Effective Reperfusion Evaluated in Multiple Endovascular Stroke Trials
- ICH
intracerebral hemorrhage
- IQR
interquartile range
- IVT
IV thrombolysis
- LKW
last known well
- LVO
large vessel occlusion
- mRS
modified Rankin Scale
- mTICI
modified Thrombolysis in Cerebral Ischemia
- NIHSS
NIH Stroke Scale
- OR
odds ratio
- RCT
randomized controlled trial
- SELECT
Optimizing Patient Selection for Endovascular Treatment in Acute Ischemic Stroke
- SITS-MOST
Safe Implementation of Thrombolysis in Stroke-Monitoring Study
- SWIFT PRIME
Solitaire™ With the Intention for Thrombectomy as Primary Endovascular Treatment Trial
- tPA
tissue plasminogen activator
Appendix 1. Authors
Appendix 2. Coinvestigators

Footnotes
Class of Evidence: NPub.org/coe
Podcast: NPub.org/9isdp3
Study Funding
The study was supported by an unrestricted grant from Stryker Neurovascular to UT McGovern Medical School.
Disclosure
A. Sarraj reports serving as the principal investigator of the SELECT and SELECT 2 trials through a grant from Stryker Neurovascular to University of Texas McGovern–Houston; as a consultant, speaker bureau member, and advisory board member for Stryker; and as a site principal investigator for the TREVO Registry and Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke 3 (DEFUSE 3) trials. J. Grotta reports grants from the Patient-Centered Outcomes Research Institute, NIH, Genentech, and CSL Behring and is a consultant for Frazier Ltd. G.W. Albers has an ownership interest in iSchemaView and is a consultant or on advisory boards for iSchemaView and Genentech. A.E. Hassan is a consultant, speaker bureau member, and proctor for Medtronic and Microvention and a consultant and speaker bureau member for Stryker, Penumbra, GE Healthcare, Balt, and Genentech. S. Blackburn reports a grant from the NIH. C. Sitton has served as the core laboratory for the SELECT trial. M. Abraham is a consultant for Stryker Neurovascular and Penumbra Inc. W. Hicks is a member of speaker bureau for BMS/Pfizer and Portola. R. Gupta is the principal investigator of the ASSIST Registry sponsored by Stryker Neurovascular, of RECCLAIM II sponsored by Zoll Therapeutics, for the Tiger Study sponsored by RAPID medical and is a member of the clinical endpoint committee for MIND Trial sponsored by Penumbra. No other relevant disclosures are made. Go to Neurology.org/N for full disclosures.
References
- 1.Powers WJ, Rabinstein AA, Ackerson T, et al. . Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke. Stroke. 2019;50(12):e344-e418. [DOI] [PubMed] [Google Scholar]
- 2.Katsanos AH, Malhotra K, Goyal N, et al. . Mortality risk in acute ischemic stroke patients with large vessel occlusion treated with mechanical thrombectomy. J Am Heart Assoc. 2019;8(21):e014425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goyal M, Menon BK, van Zwam WH, et al. . Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723-1731. [DOI] [PubMed] [Google Scholar]
- 4.Wahlgren N, Moreira T, Michel P, et al. . Mechanical thrombectomy in acute ischemic stroke: consensus statement by ESO-Karolinska Stroke Update 2014/2015, supported by ESO, ESMINT, ESNR and EAN. Int J Stroke. 2016;11(1):134-147. [DOI] [PubMed] [Google Scholar]
- 5.Fischer U, Kaesmacher J, Mendes Pereira V, et al. . Direct mechanical thrombectomy versus combined intravenous and mechanical thrombectomy in large-artery anterior circulation stroke: a topical review. Stroke. 2017;48(10):2912-2918. [DOI] [PubMed] [Google Scholar]
- 6.Fischer U, Kaesmacher J, Molina CA, Selim MH, Alexandrov AV, Tsivgoulis G. Primary thrombectomy in tPA (tissue-type plasminogen activator) eligible stroke patients with proximal intracranial occlusions. Stroke. 2018;49(1):265-269. [DOI] [PubMed] [Google Scholar]
- 7.Katsanos AH, Tsivgoulis G. Is intravenous thrombolysis still necessary in patients who undergo mechanical thrombectomy? Curr Opin Neurol. 2019;32(1):3-12. [DOI] [PubMed] [Google Scholar]
- 8.Berkhemer OA, Fransen PSS, Beumer D, et al. . A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372(1):11-20. [DOI] [PubMed] [Google Scholar]
- 9.Saver JL, Goyal M, Bonafe A, et al. . Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372(24):2285-2295. [DOI] [PubMed] [Google Scholar]
- 10.Kaesmacher J, Boeckh-Behrens T, Simon S, et al. . Risk of thrombus fragmentation during endovascular stroke treatment. AJNR Am J Neuroradiol. 2017;38(5):991-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsivgoulis G, Katsanos AH, Mavridis D, Magoufis G, Arthur A, Alexandrov AV. Mechanical thrombectomy improves functional outcomes independent of pretreatment with intravenous thrombolysis. Stroke. 2016;47(6):1661-1664. [DOI] [PubMed] [Google Scholar]
- 12.Hassan AE, Kotta H, Garza L, et al. . Pre-thrombectomy intravenous thrombolytics are associated with increased hospital bills without improved outcomes compared with mechanical thrombectomy alone. J Neurointerv Surg. 2019;11(12):1187-1190. [DOI] [PubMed] [Google Scholar]
- 13.Leker RR, Pikis S, Gomori JM, Cohen JE. Is bridging necessary? A pilot study of bridging versus primary Stentriever-based endovascular reperfusion in large anterior circulation strokes. J Stroke Cerebrovasc Dis. 2015;24(6):1163-1167. [DOI] [PubMed] [Google Scholar]
- 14.Sarraj A, Hassan AE, Savitz S, et al. . Outcomes of endovascular thrombectomy vs medical management alone in patients with large ischemic cores: a secondary analysis of the Optimizing Patient's Selection for Endovascular Treatment in Acute Ischemic Stroke (SELECT) study. JAMA Neurol. 2019;76(10):1147-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarraj A, Hassan AE, Grotta J, et al. . Optimizing Patient Selection for Endovascular Treatment in Acute Ischemic Stroke (SELECT): a prospective, multicenter cohort study of imaging selection. Ann Neurol. 2020;87(3):419-433. [DOI] [PubMed] [Google Scholar]
- 16.Wahlgren N, Ahmed N, Davalos A, et al. . Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet. 2007;369(9558):275-282. [DOI] [PubMed] [Google Scholar]
- 17.Hacke W, Kaste M, Fieschi C, et al. . Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II): Second European-Australasian Acute Stroke Study Investigators. Lancet. 1998;352(9136):1245-1251. [DOI] [PubMed] [Google Scholar]
- 18.Rai AT, Boo S, Buseman C, et al. . Intravenous thrombolysis before endovascular therapy for large vessel strokes can lead to significantly higher hospital costs without improving outcomes. J Neurointerv Surg. 2018;10(1):17-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sallustio F, Koch G, Alemseged F, et al. . Effect of mechanical thrombectomy alone or in combination with intravenous thrombolysis for acute ischemic stroke. J Neurol. 2018;265(12):2875-2880. [DOI] [PubMed] [Google Scholar]
- 20.Guimaraes Rocha M, Carvalho A, Rodrigues M, et al. . Primary thrombectomy versus combined mechanical thrombectomy and intravenous thrombolysis in large vessel occlusion acute ischemic stroke. J Stroke Cerebrovasc Dis. 2019;28(3):627-631. [DOI] [PubMed] [Google Scholar]
- 21.Bourcier R, Alexandre PL, Eugene F, et al. . Is bridging therapy still required in stroke due to carotid artery terminus occlusions? J Neurointerv Surg. 2018;10(7):625-628. [DOI] [PubMed] [Google Scholar]
- 22.Bellwald S, Weber R, Dobrocky T, et al. . Direct mechanical intervention versus bridging therapy in stroke patients eligible for intravenous thrombolysis: a pooled analysis of 2 registries. Stroke. 2017;48(12):3282-3288. [DOI] [PubMed] [Google Scholar]
- 23.Choi JH, Im SH, Lee KJ, Koo JS, Kim BS, Shin YS. Comparison of outcomes after mechanical thrombectomy alone or combined with intravenous thrombolysis and mechanical thrombectomy for patients with acute ischemic stroke due to large vessel occlusion. World Neurosurg. 2018;114:e165-e172. [DOI] [PubMed] [Google Scholar]
- 24.Broeg-Morvay A, Mordasini P, Bernasconi C, et al. . Direct mechanical intervention versus combined intravenous and mechanical intervention in large artery anterior circulation stroke: a matched-pairs analysis. Stroke. 2016;47(4):1037-1044. [DOI] [PubMed] [Google Scholar]
- 25.Katsanos AH, Malhotra K, Goyal N, et al. . Intravenous thrombolysis prior to mechanical thrombectomy in large vessel occlusions. Ann Neurol. 2019;86(3):395-406. [DOI] [PubMed] [Google Scholar]
- 26.Tandberg Askevold E, Naess H, Thomassen L. Predictors for recanalization after intravenous thrombolysis in acute ischemic stroke. J Stroke Cerebrovasc Dis. 2007;16(1):21-24. [DOI] [PubMed] [Google Scholar]
- 27.Meyne JK, Zimmermann PR, Rohr A, et al. . Thrombectomy vs. systemic thrombolysis in acute embolic stroke with high clot burden: a retrospective analysis. Rofo. 2015;187(7):555-560. [DOI] [PubMed] [Google Scholar]
- 28.Bilgic AB, Gocmen R, Arsava EM, Topcuoglu MA. The effect of clot volume and permeability on response to intravenous tissue plasminogen activator in acute ischemic stroke. J Stroke Cerebrovasc Dis. 2020;29(2):104541. [DOI] [PubMed] [Google Scholar]
- 29.Rohan V, Baxa J, Tupy R, et al. . Length of occlusion predicts recanalization and outcome after intravenous thrombolysis in middle cerebral artery stroke. Stroke. 2014;45(7):2010-2017. [DOI] [PubMed] [Google Scholar]
- 30.Seners P, Delepierre J, Turc G, et al. . Thrombus length predicts lack of post-thrombolysis early recanalization in minor stroke with large vessel occlusion. Stroke. 2019;50(3):761-764. [DOI] [PubMed] [Google Scholar]
- 31.Seners P, Perrin C, Lapergue B, et al. . Bridging therapy or IV thrombolysis in minor stroke with large vessel occlusion. Ann Neurol. 2020;88(1):160-169. [DOI] [PubMed] [Google Scholar]
- 32.Tsivgoulis G, Saqqur M, Sharma VK, Lao AY, Hoover SL, Alexandrov AV. Association of pretreatment ASPECTS scores with tPA-induced arterial recanalization in acute middle cerebral artery occlusion. J Neuroimaging. 2008;18(1):56-61. [DOI] [PubMed] [Google Scholar]
- 33.Roman LS, Menon BK, Blasco J, et al. . Imaging features and safety and efficacy of endovascular stroke treatment: a meta-analysis of individual patient-level data. Lancet Neurol. 2018;17(10):895-904. [DOI] [PubMed] [Google Scholar]
- 34.Campbell BCV, Majoie CBLM, Albers GW, et al. . Penumbral imaging and functional outcome in patients with anterior circulation ischaemic stroke treated with endovascular thrombectomy versus medical therapy: a meta-analysis of individual patient-level data. Lancet Neurol. 2019;18(1):46-55. [DOI] [PubMed] [Google Scholar]
- 35.SELECT 2: A Randomized Controlled Trial to Optimize Patient's Selection for Endovascular Treatment in Acute Ischemic Stroke. Available at: clinicaltrials.gov/ct2/show/NCT03876457. Accessed May 29, 2020. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki K, Matsumaru Y, Takeuchi M, et al. . Effect of mechanical thrombectomy without vs with intravenous thrombolysis on functional outcome among patients with acute ischemic stroke: the SKIP randomized clinical trial. JAMA. 2021;325(3):244-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Froehler MT, Saver JL, Zaidat OO, et al. . Interhospital transfer before thrombectomy is associated with delayed treatment and worse outcome in the STRATIS registry (Systematic Evaluation of Patients Treated With Neurothrombectomy Devices for Acute Ischemic Stroke). Circulation. 2017;136(24):2311-2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsivgoulis G, Saqqur M, Sharma VK, et al. . Timing of recanalization and functional recovery in acute ischemic stroke. J Stroke. 2020;22(1):130-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sarraj A, Savitz S, Pujara D, et al. . Endovascular thrombectomy for acute ischemic strokes. Stroke. 2020;51(4):1207-1217. [DOI] [PubMed] [Google Scholar]
- 40.MR CLEAN-NOIV. Available at: mrclean-noiv.nl/. Accessed April 24, 2020.
- 41.The SWIFT DIRECT Trial. Available at: swift-direct.ch/the-swift-direct-trial/. Accessed April 24, 2020. [Google Scholar]
- 42.Yang P, Zhang Y, Zhang L, et al. . Endovascular thrombectomy with or without intravenous alteplase in acute stroke. N Engl J Med. 2020;382(21):1981-1993. [DOI] [PubMed] [Google Scholar]
- 43.Zi W, Qiu Z, Li F, et al. . Effect of endovascular treatment alone vs intravenous alteplase plus endovascular treatment on functional independence in patients with acute ischemic stroke: the DEVT randomized clinical trial. JAMA. 2021;325(3):234-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nogueira RG, Tsivgoulis G. Large vessel occlusion strokes after the DIRECT-MT and SKIP trials. Stroke. 2020;51(10):3182-3186. [DOI] [PubMed] [Google Scholar]
- 45.Campbell BCV, Mitchell PJ, Churilov L, et al. . Tenecteplase versus alteplase before thrombectomy for ischemic stroke. N Engl J Med. 2018;378(17):1573-1582. [DOI] [PubMed] [Google Scholar]
- 46.Katsanos AH, Safouris A, Sarraj A, et al. . Intravenous thrombolysis with tenecteplase in patients with large vessel occlusions. Stroke. 2021;52(1):308-312. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The individual patient data will not be made available. Analysis codes and outputs will be made available on reasonable request after review by the study steering and publication committees.