Abstract
Objective
To determine the association between electroencephalographic seizure (ES) and electroencephalographic status epilepticus (ESE) exposure and unfavorable neurobehavioral outcomes in critically ill children with acute encephalopathy.
Methods
This was a prospective cohort study of acutely encephalopathic critically ill children undergoing continuous EEG monitoring (CEEG). ES exposure was assessed as (1) no ES/ESE, (2) ES, or (3) ESE. Outcomes assessed at discharge included the Glasgow Outcome Scale–Extended Pediatric Version (GOS-E-Peds), Pediatric Cerebral Performance Category (PCPC), and mortality. Unfavorable outcome was defined as a reduction in GOS-E-Peds or PCPC score from preadmission to discharge. Stepwise selection was used to generate multivariate logistic regression models that assessed associations between ES exposure and outcomes while adjusting for multiple other variables.
Results
Among 719 consecutive critically ill patients, there was no evidence of ES in 535 patients (74.4%), ES occurred in 140 patients (19.5%), and ESE in 44 patients (6.1%). The final multivariable logistic regression analyses included ES exposure, age dichotomized at 1 year, acute encephalopathy category, initial EEG background category, comatose at CEEG initiation, and Pediatric Index of Mortality 2 score. There was an association between ESE and unfavorable GOS-E-Peds (odds ratio 2.21, 95% confidence interval 1.07–4.54) and PCPC (odds ratio 2.17, 95% confidence interval 1.05–4.51) but not mortality. There was no association between ES and unfavorable outcome or mortality.
Conclusions
Among acutely encephalopathic critically ill children, there was an association between ESE and unfavorable neurobehavioral outcomes, but no association between ESE and mortality. ES exposure was not associated with unfavorable neurobehavioral outcomes or mortality.
Electroencephalographic seizures (ES) occur in 10%–40% of critically ill children, and about one-third of patients with ES experience electroencephalographic status epilepticus (ESE).1-12 Because ES may be clinically undetectable, continuous EEG monitoring (CEEG) is increasingly used in the pediatric intensive care unit (PICU) based on the premise that ES identification and management may improve outcome.13-17 However, whether ES cause secondary brain injury or merely signify more severe brain injury is uncertain. There is mounting evidence of an association between high ES exposure and unfavorable outcomes,4-12,18 including evidence provided by studies that adjust for a small number of variables.4-6,9,10,12 In a large and contemporary cohort of consecutive critically ill children who underwent CEEG, we evaluated the association between ES exposure and short-term neurobehavioral outcomes using analyses that adjusted for numerous variables reflecting brain injury type, encephalopathy severity, and critical illness severity.
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
The Institutional Review Board approved the study with a waiver of written consent. The study was registered with clincialtrials.gov (NCT03419260). Data unrelated to outcomes have been published.19-21
Ethical Publication Statement
We confirm that this report is consistent with the journal's position on issues involved in ethical publication.
Intensive Care Unit CEEG Prospective Observational Study
This was a prospective observational study of consecutive critically ill children undergoing CEEG from April 2017 to February 2019 in the PICU. CEEG utilization was guided by an institutional pathway (figure) aligned with guidelines and consensus statements.13-15 We included patients undergoing CEEG with acute encephalopathy of any etiology. CEEG indications were encephalopathy with or without a prior clinical seizure or abnormal movements or vital sign fluctuations. We excluded neonates (<30 days from birthdate), children who underwent resective surgery for epilepsy, and children with refractory status epilepticus managed for more than 2 days at a different institution. We used Natus Neuroworks (Middleton, WI) with international 10–20 system electrode placement. The Electroencephalography Service interpreted the CEEG studies. The Critical Care Medicine and the Neurology Consultation Services led patient management. Patients did not routinely receive prophylactic antiseizure medications. For most patients with ES, clinicians administered antiseizure medications, which were most often levetiracetam and phenytoin.20,22
Figure. Summary of Intensive Care Unit (ICU) EEG Monitoring Pathway.
ECMO = extracorporeal membrane oxygenation; EMR = electronic medical record; PICU = pediatric intensive care unit.
Data Acquisition
Clinical and EEG data were collected using REDCap (Research Electronic Data Capture) prospectively. We categorized acute encephalopathy as (1) epilepsy-related (subcategorized as focal, generalized, mixed, or unknown); (2) acute structural (subcategorized as hypoxic-ischemic, hydrocephalus/shunt, traumatic brain injury, tumor/oncologic, stroke, inflammatory, CNS infection, and brain malformations); or (3) acute nonstructural (subcategorized as sepsis, metabolic, sedation without known neurologic problem, and toxin). Mental status was categorized as presence or absence of coma and baseline mental status. Patients were scored as comatose if clinician notes indicated “coma” or if a Glasgow Coma Scale score was listed as ≤8. Investigators determined whether each patient was worse than baseline mental status based on review of all available information; some patients initially considered to have acute encephalopathy by treating clinicians were later determined to be at baseline mental status. The Pediatric Index of Mortality 2 (PIM2) risk of mortality score was scored within 1 hour of contact with a critical care medicine fellow or attending physician.23 The Pediatric Risk of Mortality III (PRISM3) probability of death score was scored within 12 hours of admission.24
EEG studies were interpreted clinically by electroencephalographers on a critical care EEG service using a standardized EEG reporting template25 aligned with a critical care CEEG database26 that incorporated standardized terminology27 with documented good interrater reliability.28,29 For research purposes, a pediatric electroencephalographer (F.W.F.) reviewed each EEG tracing to assess the background during the initial 30 minutes and the timing of onset of epileptiform discharges. Concordant with prior studies,5,6,10,30 we categorized the EEG background as (1) normal/sleep, (2) slow-disorganized, (3) discontinuous, (4) burst-suppression, or (5) attenuated-featureless. The electroencephalographer then used the clinical reports to guide review of the CEEG tracings for seizure exposure in a targeted manner. If the report indicated there were ES, possible ES, periodic or rhythmic patterns, or other uncertain patterns, then the electroencephalographer performed targeted review of the conventional EEG tracing. ES exposure was categorized as (1) no ES/ESE, (2) ES, or (3) ESE. Consistent with prior studies1,5 and published definitions,31 we defined ES as an abnormal paroxysmal event that differed from the background lasting >10 seconds with a plausible electrographic field and temporal-spatial evolution in morphology, frequency, and amplitude. Consistent with prior studies,5,6 we defined ESE using standard nonconvulsive status epilepticus criteria31 as either a greater than 30-minute ES or recurrent ES totaling greater than 30 minutes within 1-hour epoch. EEG-only seizures had no clinical change identified by bedside caregivers or by electroencephalographers reviewing video.
We assessed outcome at PICU discharge. The primary outcome measure was the Glasgow Outcome Scale–Extended Pediatric Version (GOS-E-Peds), which categorizes global functional outcome using 8 levels, and it has high criterion-related validity, discriminant validity, and sensitivity to injury severity.32 It is recommended as a core global outcome measure for pediatric traumatic brain injury research.33 Secondary outcome measures were mortality and Pediatric Cerebral Performance Category (PCPC) score, which categorizes functional impairment using 6 levels.34 Preadmission GOS-E-Peds and PCPC scores were estimated based on information provided by parents/guardians at the time of admission or prior medical visits. Because GOS-E-Peds was designed to assess outcome, several questions were modified to assign preadmission scores. For example, “child able to resume” was changed to “child able to perform.” Unfavorable GOS-E-Peds and PCPC outcomes were defined as a ≥1 point reduction from preadmission to discharge, consistent with prior studies.35,36
Statistical Analyses
We used RStudio Team (Boston, MA) for statistical analyses. Summary statistics are reported. Univariate analyses were performed for potential predictors of each outcome using the 2-sample t test, Wilcoxon rank sum test, Pearson χ2, or Fisher exact test. Variables that had a p value <0.1 that were not strongly correlated with each other, assessed by Phi or Cramer V statistic, were included in subsequent multivariable logistic regression models. A stepwise selection approach determined the final models, and the goodness-of-fit of the final models was evaluated using the Hosmer-Lemeshow statistic. An odds ratio (OR) with 95% confidence interval (CI) presented the association of each variable with the binary outcome. In addition, we assessed interactions between the primary exposure variable (ES exposure) and other covariates. Sensitivity analyses assessed the robustness of the final models when we exchanged variables that were correlated with each other. The median length of PICU stay was assessed among the 3 ES exposure groups using the Kruskal-Wallis test, and the Dunn test was used to perform post hoc pairwise comparison.
We performed 4 subgroup analyses. First, we analyzed patients who were neurobehaviorally normal prior to admission (GOS-E-Peds level 1) using ordinal logistic regression in which outcome was assessed as 3 GOS-E-Peds categories (normal–mild, moderate, severe–death) rather than dichotomized (unchanged or worsened) as in the primary analyses. The Brant test was used to verify the proportional odds assumption in ordinal logistic regression. The other subgroup analyses used logistic regression. The analyses excluded (1) patients with epilepsy as the acute encephalopathy category, (2) patients with an epileptic encephalopathy diagnosis, and (3) patients who were initially diagnosed with acute encephalopathy by treating clinicians at CEEG initiation but later considered to be at their mental status baseline upon more detailed review of the medical history.
Data Availability
We will make the underlying data available to investigators with appropriate data transfer and institutional review board approval.
Results
We previously described the clinical and EEG characteristics of the cohort.19-21 The median CEEG duration was 23 hours (interquartile range [IQR] 14, 42). Among 719 consecutive critically ill patients, there was no evidence of ES in 535 patients (74.4%), ES occurred in 140 patients (19.5%), and ESE in 44 patients (6.1%). ESE was categorized as a longer than 30-minute seizure in 14 patients (32%) and recurrent seizures totaling longer than 30 minutes within a 1-hour epoch in 30 patients (68%). ES were categorized as EEG-only, all clinically evident, and a mixture in 76 (41%), 57 (31%), and 51 (28%) patients, respectively. The acute encephalopathy category was structural in 350 patients (49%), epilepsy-related in 213 patients (30%), and acute nonstructural in 156 patients (22%). The most common acute structural diagnoses (multiple permitted) were hypoxic-ischemic brain injury (129 [37%]), hydrocephalus/shunt (68 [19%]), traumatic brain injury (57 [16%]), tumor/oncologic (56 [16%]), stroke (45 [13%]), inflammatory (29 [8%]), CNS infection (19 [5%]), and malformations (19 [5%]). The epilepsy subtypes were focal (103 [48%]), mixed (45 [21%]), generalized (27 [13%]), and unknown (38 [18%]). The most common acute nonstructural diagnoses (multiple permitted) were sepsis (43 [28%]), metabolic (32 [21%]), sedation without known neurologic problem (27 [17%]), and toxin (9 [6%]).
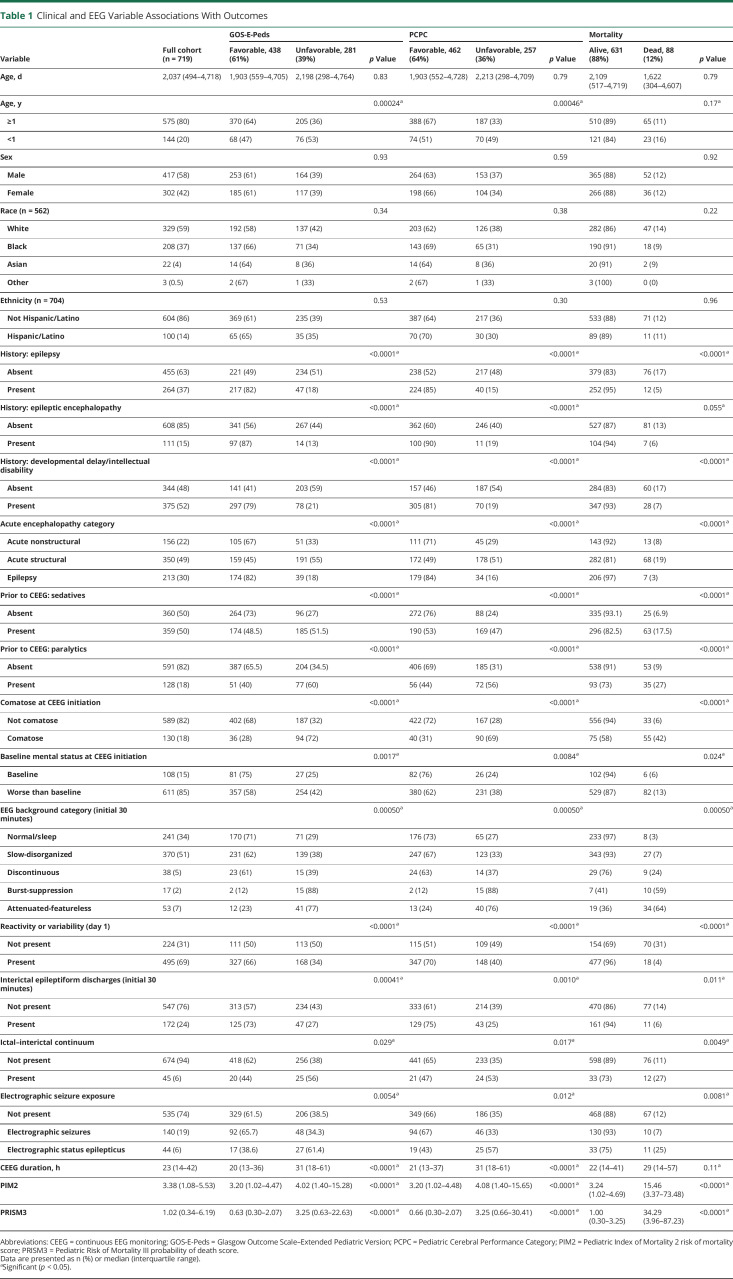
Table 1 presents univariate analyses of risk factors for each outcome (unfavorable GOS-E-Peds, unfavorable PCPC, and mortality). ES exposure category was associated with all 3 outcomes. Age was significantly associated with unfavorable GOS-E-Peds and unfavorable PCPC but not mortality when analyzed categorically with the cut point at 1 year. Age was not associated with any of the 3 outcomes when analyzed as a continuous variable, indicating a potential nonlinear relationship with the likelihood of unfavorable GOS-E-Peds or unfavorable PCPC. Past medical histories of epilepsy, epileptic encephalopathy, and developmental delay/intellectual disability were associated with all 3 outcomes. Clinical variables associated with outcomes included acute encephalopathy category, sedatives prior to CEEG initiation, paralytics prior to CEEG initiation, comatose mental status at CEEG initiation, worse than baseline mental status at CEEG initiation, PIM2 score, and PRISM3 score. EEG variables associated with outcomes included EEG background category, reactivity or variability on the first day of CEEG, presence of interictal epileptiform discharges, presence of ictal–interictal continuum patterns, and CEEG duration. Multiple variables associated with outcomes in univariate analyses were correlated with each other, including (1) acute encephalopathy category and other medical history variables including prior epilepsy, developmental delay or intellectual disability, and epileptic encephalopathy (all p < 0.01); (2) mental status assessed as comatose or worse than baseline (p < 0.01); (3) PIM2 score and PRISM3 score (p < 0.01); and (4) initial EEG background category and reactivity or variability on the first day of CEEG, presence of interictal epileptiform discharges, and the presence of ictal–interictal continuum patterns (all p < 0.01).
Table 1.
Clinical and EEG Variable Associations With Outcomes
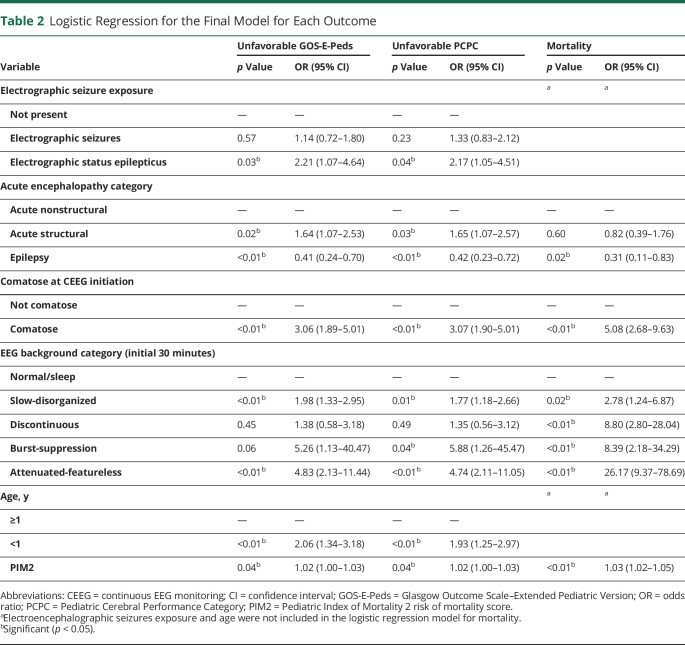
For each of the 3 binary outcomes, we performed multivariable logistic regression analyses that included ES exposure, age dichotomized at 1 year, acute encephalopathy category, initial EEG background category, comatose at CEEG initiation, and PIM2 score. Table 2 summarizes the results of the final regression models for each outcome determined by the stepwise selection approach. All 6 variables were selected into the final model for unfavorable GOS-E-Peds and unfavorable PCPC, suggesting that the association between ES exposure and the 2 outcomes remained significant after adjusting for the other 5 variables. There was an association between ESE and unfavorable GOS-E-Peds (OR 2.21, 95% CI 1.07-4.54) and PCPC (OR 2.17, 95% CI 1.05-4.51) but not mortality. There was no association between ES and unfavorable outcome or mortality. Four variables were selected into the final model for mortality, and there was no evidence of significant association with age or ES exposure. The Hosmer-Lemeshow statistics of goodness-of-fit for each final model suggested a good model fitting of the data (all p < 0.2). There were no significant interactions between the primary exposure variable (seizure exposure) and the other covariates for any of the 3 outcomes.
Table 2.
Logistic Regression for the Final Model for Each Outcome
We performed sensitivity analyses for each outcome using alternative predictor variables. First, we replaced the 5-level EEG background variable with a dichotomized variable (normal/sleep/slow-disorganized vs discontinuous/burst-suppression/attenuated-featureless). Second, we exchanged the PIM2 and PRISM3 scores. Third, we replaced comatose state with worse than baseline mental status at CEEG initiation. These models yielded the same conclusions as the main analyses for each outcome.
Patients in the main analysis were neurobehaviorally normal or abnormal prior to PICU admission. We performed a subanalysis that included 339 neurobehaviorally normal patients. Outcome was defined as good (GOS-E-Peds 1–2) in 183 patients (54%), moderate (GOS-E-Peds 3–4) in 59 patients (17%), and severe or death (GOS-E-Peds 4–8) in 97 patients (29%). Using this 3-level outcome, seizure exposure was significantly associated with outcome (p = 0.004). In univariate analysis, patients with ESE were at higher risk of having an unfavorable GOS-E-Peds outcome (OR, 3.11; 95% CI, 1.42–6.80) compared to those without ES. Patients with ES were not at higher risk of having an unfavorable GOS-E-Peds outcome (OR, 1.06; 95% CI, 0.61–1.86) compared to those without ES. After controlling for the same covariates as the primary analysis, we observed similar results as in the unadjusted model: patients with ESE were at higher risk of having an unfavorable GOS-E-Peds outcome (OR, 3.45; 95% CI, 1.45–8.38), while patients with ES were not (OR, 1.51; 95% CI, 0.79–2.86), as compared to patients with no seizures.
We performed several other subgroup analyses using logistic regression models that controlled for the same covariates as the primary analysis to compare patients with ES and ESE to patients with no seizures. First, we included only the 506 patients with acute structural or acute nonstructural acute encephalopathy categories and excluded patients with epilepsy as the acute encephalopathy category. Patients with ESE were at higher risk of having an unfavorable outcome (OR, 2.50; 95% CI, 1.03–6.05) while patients with ES were not at higher risk of having an unfavorable outcome (OR, 0.92; 95% CI, 0.52–1.64). Second, we included only the 608 patients without epileptic encephalopathy and found that patients with ESE were at higher risk of having an unfavorable outcome (OR, 2.31; 95% CI, 1.06, 5.07), while patients with ES were not at higher risk of having an unfavorable outcome (OR, 1.48; 95% CI, 0.89, 2.48). Third, we included only the 611 patients with mental status worse than baseline and excluded 108 patients initially considered to have acute encephalopathy but later determined to be at their baseline mental status. Patients with ESE were at higher risk of having an unfavorable outcome (OR, 2.19; 95% CI, 1.04–4.60), while patients with ES were not at higher risk of having an unfavorable outcome (OR, 1.16; 95% CI, 0.70–1.92).
The median length of stay in the PICU was 4.9 days (IQR 1.7–12.8), 6.9 days (IQR 3.2–17.0), and 13.8 days (IQR 6.5–27.2) for the no ES, ES, and ESE groups, respectively (p < 0.0001). These durations were significantly different between any 2 ES exposure groups by the post hoc pairwise comparison (all p < 0.01).
Discussion
This large and prospective observational study of consecutive acutely encephalopathic critically ill children undergoing CEEG indicated that children experiencing ESE were at higher risk of unfavorable neurobehavioral outcomes (GOS-E-Peds and PCPC) after adjusting for numerous variables including age, acute encephalopathy category, encephalopathy severity (initial EEG background category and comatose state at CEEG initiation), and critical illness severity (PIM2 score). ESE occurred in 6% of patients, and given that ESE was independently associated with unfavorable outcomes, management strategies to avoid ESE could benefit these patients. Conversely, children with ES were not at higher risk of unfavorable neurobehavioral outcomes. Thus, aggressive treatment of patients with low ES exposure may not improve outcomes, except by avoiding potential progression to ESE. Neither ES nor ESE was associated with mortality. A prior study similarly reported no association between ES and mortality,4 while other studies have shown associations between ES and mortality.5,9,10,18 The current study included risk of mortality score in the model, which may explain this difference.
Although this study does not establish a causative link between seizures and brain injury, it adds to growing evidence that critically ill children with high ES exposure are at increased risk for adverse outcomes4-12,18 given several methodologic strengths. First, the data were collected over a relatively short timeframe from a large and contemporary cohort. Consecutive patients were included, thereby avoiding enrollment bias, given a waiver for consent. Patient management was guided by a well-established clinical pathway (figure). Second, a pediatric electroencephalographer performed research-based review of EEG reports and tracings using standardized terminology27 with good interrater reliability28 and commonly used definitions for seizures1,5,31 and background classification categories.5,6,10,30 The study enabled efficient but accurate and standardized EEG interpretation through a hybrid approach in which the electroencephalographer reviewed EEG reports generated using standardized forms and terminology and also verified key EEG features (including ES and ESE occurrence) for any patients for whom reports noted ES or other potentially uncertain findings. Third, we incorporated critical illness severity instruments (PIM2 and PRISM3), which are accepted and standardized metrics in pediatric critical care research.23,24 Fourth, the outcome measures (GOS-E-Peds and PCPC) are standardized and accepted in pediatric critical care research.32,34 These are core outcome measures suggested for research regarding pediatric traumatic brain injury,33 which is a clinically relevant condition with available recommendations. Fifth, the large cohort permitted evaluation of many predictor variables. Those included in the final model reflect age, brain injury type, degree of brain injury (as assessed using comatose mental status and EEG background category), and standard measures of critical illness severity such that the analyses might better assess associations between seizure exposure and outcomes. Sixth, multiple subanalyses yielded the same conclusions as the main analysis, including subanalyses that excluded patients who were neurobehaviorally abnormal prior to PICU admission and patients with complex conditions that might have affected the findings (baseline mental status, epilepsy, and epileptic encephalopathy).
The effect of seizure exposure on outcome has been evaluated in prior smaller studies in critically ill children using multivariate analyses adjusting for variables reflecting demographic variables, acute encephalopathy etiology, and illness severity.4-6,9,10,12 Among 204 comatose patients, more ES and longer ES duration were associated with unfavorable outcome (vegetative state, nonambulatory, or severe learning difficulties) independent of age, etiology, mortality and coma scores, and EEG background. Children who had more than 139 seizures, a sum of ES totaling more than 759 minutes, or an individual ES lasting more than 360 minutes all had unfavorable outcome at 1 month.12 Among 200 consecutive acutely encephalopathic patients, ESE, but not ES, was associated with mortality and worsened PCPC score at discharge after adjusting for age, acute neurologic disorder, EEG background, and prior neurodevelopmental condition.5 A multicenter retrospective study of 550 patients indicated that ESE was associated with in-hospital mortality after adjusting for neurologic diagnosis and EEG background category. ES was not associated with mortality.10 Among 60 patients who were previously normal at PICU admission and then admitted with acute encephalopathy evaluated at a median follow-up of 2.7 years, ESE was associated with unfavorable GOS-E-Peds, lower pediatric quality of life scores, and an increased risk of epilepsy after adjusting for EEG background, acute neurologic disorder, age, and length of stay. ES was not associated with worse outcomes.6 Among 414 children, the presence of ESE was associated with in-hospital mortality after adjusting for sex and age.9 Moreover, a study of 154 children assessed at a mean follow-up of 4 years indicated patients with refractory status epilepticus were less likely to return to their previous neurologic status by discharge and more likely to develop new neurologic deficits and epilepsy than patients with aborted status epilepticus.18 A study of 259 neonates and children demonstrated that across all etiologic categories, an hourly seizure burden exceeding 20% (12 minutes per hour) was associated with a significant increase in the probability and magnitude of neurologic decline.4 For every 1% increase in the hourly seizure burden, the odds of neurologic decline rose by 1.13. This indicated that when seizure exposure was assessed as a continuous variable, ES burden below that of ESE could be associated with unfavorable outcome. This may explain why prior studies that assessed ES exposure categorically (no ES, ES, or ESE) indicated that only ESE is associated with unfavorable outcomes. Furthermore, it suggests that management to prevent ESE could be a useful strategy for improving outcome. Although the documented associations between ESE and unfavorable outcome do not prove causation, they may suggest a plausible link between seizure exposure and adverse neurobehavioral outcome.
Animal models have elucidated mechanisms by which seizures could induce secondary brain injury and affect outcomes. Early-life recurrent seizures yield molecular changes that enhance neocortical excitability,37 which may prime the brain for future epilepsy and cellular changes that alter hippocampal synaptic connectivity, which may adversely affect learning and memory.38 Further, studies in humans have demonstrated physiologic, metabolic, and structural changes associated with seizures. Magnetic resonance spectroscopy indicated that neonatal seizures result in elevated cerebral metabolic demands above supply.39 Among adults with traumatic brain injury, intracranial pressure and lactate/pyruvate ratios were higher during seizures.40 Further, among adults with traumatic brain injury, hippocampal atrophy was identified ipsilateral to seizures.41 Among adults with subarachnoid hemorrhage, intracortical seizures were associated with alterations in systemic physiology42 and a proinflammatory state.43
The finding that ESE occurs in 6% of patients and is associated with unfavorable outcomes indicates that aggressive efforts to identify and manage ESE could improve outcomes in some patients. Several studies have demonstrated that strategies to reduce ESE exposure are feasible and potentially beneficial. First, incorporation of EEG data into clinical management of neonates was associated with decreased seizure exposure,44,45 and implementation of an EEG-based pathway was associated with a reduction in the proportion of neonates who progressed from seizures to status epilepticus.46 Second, a study suggested that CEEG initiation delays were associated with increased mortality,9 suggesting prompt CEEG implementation could be beneficial. Third, data derived from the initial 472 patients included in this cohort indicated that ES management with 1–2 standard antiseizure medications was often effective with minimal risk of adverse effects.20 Fourth, pathway-driven management of ES in critically ill children was associated with more rapid antiseizure medication administration after ES onset and a greater chance of ES resolution following administration of the first antiseizure medication.47 Increasing data that ESE exposure is independently associated with unfavorable outcomes should motivate additional efforts to develop evidence-based ESE management strategies and evaluate whether these approaches improve outcomes. Conversely, data indicating that low ES exposure is not associated with worse outcomes suggests that aggressive treatment of ES might not affect outcomes. Therefore, efforts aimed at identifying alternative modifiable risk factors for unfavorable outcome may be required to drive development of novel neuroprotective strategies.
The finding that children with ES were not at higher risk of unfavorable neurobehavioral outcomes or mortality indicates that identification and management of brief and self-limited ES may not be necessary. This would represent is a substantial departure from current practice in which most clinicians aim to terminate all ES.48 Aggressive management of patients with ES may be reserved for patients at risk for escalating from ES to ESE. Future studies are needed to determine the incidence of escalation of ES to ESE, and to identify risk factors for progression. Further, quantitative EEG approaches that reliably identify ESE may be beneficial even if they fail to detect brief ES, thereby resetting the goals of these approaches and potentially making these techniques more clinically useful.
Several variables other than ESE were associated with unfavorable neurobehavioral outcomes. More severe EEG background categories (burst-suppression or attenuated-featureless) were associated with unfavorable outcome, as has been reported for prior heterogeneous cohorts of critically ill children5 and homogeneous cohorts such as children after cardiac arrest.36,49 Similarly, patients who were comatose at CEEG initiation also had unfavorable outcomes. Both EEG background category and comatose state may reflect encephalopathy severity. Patients with epilepsy as an acute encephalopathy category had a lower risk for unfavorable outcome than patients with acute structural or nonstructural categories.
This study has limitations. First, although the cohort was large, it was conducted at one center. Multicenter studies would increase generalizability. Second, scoring was performed by 1 electroencephalographer using clinical reports to guide targeted review of the EEG tracing. This approach was selected to balance standardization and accuracy with study feasibility. Subsequent studies could benefit from multirater review of predefined epochs of the EEG tracing. Third, since CEEG duration was clinically determined, some patients scored as having no ES may have experienced ES after CEEG was discontinued, thereby yielding misclassification of ES exposure category. Fourth, we dichotomized seizure burden as ES and ESE. The optimal method for quantifying seizure burden has not been delineated. Most likely, the seizure burden sufficient to induce secondary brain injury varies based on age, severity, and etiology of acute encephalopathy, as well as seizure volume of distribution. Fifth, we only assessed short-term outcome, and future studies including long-term neuropsychological assessments would be informative. Sixth, we evaluated the change in estimated preadmission to discharge outcome scores as the primary outcome. This permitted inclusion of patients who were neurobehaviorally abnormal at admission, making the study more generalizable. However, there could be differential effect of ES exposure on patients with varying preexisting neurobehavioral abnormalities. Similar to the primary analysis, a subanalysis including only patients who were neurobehaviorally normal prior to admission that assessed outcome as 3 levels (normal–mild, moderate, severe–death) indicated ESE, but not ES, were significantly associated with unfavorable outcome. Seventh, our clinical practice involved aggressive ES and ESE management, yet only patients with ESE had worse outcome. This could imply that efforts to identify and mitigate ES do not improve outcome. Current ES treatment is variable and guided by limited data,16,20,22 and optimized evidence-based management strategies might yield improved outcomes.
Overall, the current study indicates that ESE occurred in 6% of patients and was independently associated with unfavorable outcomes even after adjusting for brain injury type and severity of encephalopathy and critical illness. Although the association does not establish causality, it suggests that mitigating ESE may constitute a viable strategy for neuroprotection in some patients. Because ES was not associated with unfavorable outcomes, aggressive management of patients with low ES exposure may not improve outcome. It is possible that ES management prevented progression to ESE in some patients, but the proportion of patients who would progress to ESE is unknown. Future studies are needed with larger and multicenter cohorts, more standardized ESE management approaches, and long-term assessment of more comprehensive neurobehavioral outcomes.
Acknowledgment
Staff of the Children's Hospital of Philadelphia Critical Care Center for Evidence and Outcomes abstracted and coded the Virtual Pediatric Systems data. Virtual Pediatric Systems provided PRISM3 and PIM2 data.
Glossary
- CEEG
continuous EEG monitoring
- CI
confidence interval
- ES
electroencephalographic seizures
- ESE
electroencephalographic status epilepticus
- GOS-E-Peds
Glasgow Outcome Scale–Extended Pediatric Version
- OR
odds ratio
- PICU
pediatric intensive care unit
- PCPC
Pediatric Cerebral Performance Category
- PIM2
Pediatric Index of Mortality 2
- PRISM3
Pediatric Risk of Mortality III
Appendix. Authors
Footnotes
CME Course: NPub.org/cmelist
Editorial, page 1019
Study Funding
NIH K02NS096058, the Wolfson Family Foundation, and NIH U54-HD086984.
Disclosure
F.W. Fung, Z. Wang, D.S. Parikh, M. Jacobwitz, L. Vala, M. Donnelly, and A.A. Topjian report no disclosures. N.S. Abend has received royalties from Demos Publishing, consulting income from the Epilepsy Foundation, and research funding from the Wolfson Family Foundation, NIH (National Institute of Neurological Disorders and Stroke), PCORI, and UCB Pharma. Go to Neurology.org/N for full disclosures.
References
- 1.Abend NS, Gutierrez-Colina AM, Topjian AA, et al. Nonconvulsive seizures are common in critically ill children. Neurology. 2011;76:1071-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams K, Jarrar R, Buchhalter J. Continuous video-EEG monitoring in pediatric intensive care units. Epilepsia. 2011;52:1130-1136. [DOI] [PubMed] [Google Scholar]
- 3.McCoy B, Sharma R, Ochi A, et al. Predictors of nonconvulsive seizures among critically ill children. Epilepsia. 2011;52:1973-1978. [DOI] [PubMed] [Google Scholar]
- 4.Payne ET, Zhao XY, Frndova H, et al. Seizure burden is independently associated with short term outcome in critically ill children. Brain. 2014;137:1429-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Topjian AA, Gutierrez-Colina AM, Sanchez SM, et al. Electrographic status epilepticus is associated with mortality and worse short-term outcome in critically ill children. Crit Care Med. 2013;41:215-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wagenman KL, Blake TP, Sanchez SM, et al. Electrographic status epilepticus and long-term outcome in critically ill children. Neurology. 2014;82:396-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gwer S, Idro R, Fegan G, et al. Continuous EEG monitoring in Kenyan children with non-traumatic coma. Arch Dis Child. 2012;97:343-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schreiber JM, Zelleke T, Gaillard WD, Kaulas H, Dean N, Carpenter JL. Continuous video EEG for patients with acute encephalopathy in a pediatric intensive care unit. Neurocrit Care. 2012;17:31-38. [DOI] [PubMed] [Google Scholar]
- 9.Sanchez Fernandez I, Sansevere AJ, Guerriero RM, et al. Time to electroencephalography is independently associated with outcome in critically ill neonates and children. Epilepsia. 2017;58:420-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abend NS, Arndt DH, Carpenter JL, et al. Electrographic seizures in pediatric ICU patients: cohort study of risk factors and mortality. Neurology. 2013;81:383-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greiner HM, Holland K, Leach JL, Horn PS, Hershey AD, Rose DF. Nonconvulsive status epilepticus: the encephalopathic pediatric patient. Pediatrics. 2012;129:e748-e755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirkham FJ, Wade AM, McElduff F, et al. Seizures in 204 comatose children: incidence and outcome. Intens Care Med. 2012;38:853-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brophy GM, Bell R, Claassen J, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17:3-23. [DOI] [PubMed] [Google Scholar]
- 14.Herman ST, Abend NS, Bleck TP, et al. Consensus statement on continuous EEG in critically ill adults and children, part I: indications. J Clin Neurophysiol. 2015;32:87-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herman ST, Abend NS, Bleck TP, et al. Consensus statement on continuous EEG in critically ill adults and children, part II: personnel, technical specifications, and clinical practice. J Clin Neurophysiol. 2015;32:96-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abend NS, Dlugos DJ, Hahn CD, Hirsch LJ, Herman ST. Use of EEG monitoring and management of non-convulsive seizures in critically ill patients: a survey of neurologists. Neurocrit Care. 2010;12:382-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanchez SM, Carpenter J, Chapman KE, et al. Pediatric ICU EEG monitoring: current resources and practice in the United States and Canada. J Clin Neurophysiol. 2013;30:156-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lambrechtsen FA, Buchhalter JR. Aborted and refractory status epilepticus in children: a comparative analysis. Epilepsia. 2008;49:615-625. [DOI] [PubMed] [Google Scholar]
- 19.Fung FW, Jacobwitz M, Parikh DS, et al. Development of a model to predict electroencephalographic seizures in critically ill children. Epilepsia. 2020;61:498-508. [DOI] [PubMed] [Google Scholar]
- 20.Fung FW, Jacobwitz M, Vala L, et al. Electroencephalographic seizures in critically ill children: management and adverse events. Epilepsia. 2019;60:2095-2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fung FW, Fan J, Vala L, et al. EEG monitoring duration to identify electroencephalographic seizures in critically ill children. Neurology. 2020;95:e1599-e1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abend NS, Sanchez SM, Berg RA, Dlugos DJ, Topjian AA. Treatment of electrographic seizures and status epilepticus in critically ill children: a single center experience. Seizure. 2013;22:467-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slater A, Shann F, Pearson G; Paediatric Index of Mortality Study Group. PIM2: a revised version of the Paediatric Index of Mortality. Intens Care Med. 2003;29:278-285. [DOI] [PubMed] [Google Scholar]
- 24.Pollack MM, Patel KM, Ruttimann UE. PRISM III: an updated pediatric risk of mortality score. Crit Care Med. 1996;24:743-752. [DOI] [PubMed] [Google Scholar]
- 25.Witzman S, Massey SL, Kessler S, et al. Acceptability of standardized EEG reporting in an electronic health record. J Clin Neurophysiol. 2020;37:455-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee JW, LaRoche S, Choi H, et al. Development and feasibility testing of a critical care EEG monitoring database for standardized clinical reporting and multicenter collaborative research. J Clin Neurophysiol. 2016;33:133-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirsch LJ, LaRoche SM, Gaspard N, et al. American Clinical Neurophysiology Society's standardized critical care EEG terminology: 2012 version. J Clin Neurophysiol. 2013;30:1-27. [DOI] [PubMed] [Google Scholar]
- 28.Abend NS, Gutierrez-Colina A, Zhao H, et al. Interobserver reproducibility of electroencephalogram interpretation in critically ill children. J Clin Neurophysiol. 2011;28:15-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abend NS, Massey SL, Fitzgerald M, et al. Interrater agreement of EEG interpretation after pediatric cardiac arrest using standardized critical care EEG terminology. J Clin Neurophysiol. 2017;34:534-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang A, Arndt DH, Berg RA, et al. Development and validation of a seizure prediction model in critically ill children. Seizure. 2015;25:104-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beniczky S, Hirsch LJ, Kaplan PW, et al. Unified EEG terminology and criteria for nonconvulsive status epilepticus. Epilepsia. 2013;54(suppl 6):28-29. [DOI] [PubMed] [Google Scholar]
- 32.Beers SR, Wisniewski SR, Garcia-Filion P, et al. Validity of a pediatric version of the Glasgow Outcome Scale-Extended. J Neurotrauma. 2012;29:1126-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCauley SR, Wilde EA, Anderson VA, et al. Recommendations for the use of common outcome measures in pediatric traumatic brain injury research. J Neurotrauma. 2012;29:678-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fiser DH, Long N, Roberson PK, Hefley G, Zolten K, Brodie-Fowler M. Relationship of pediatric overall performance category and pediatric cerebral performance category scores at pediatric intensive care unit discharge with outcome measures collected at hospital discharge and 1- and 6-month follow-up assessments. Crit Care Med. 2000;28:2616-2620. [DOI] [PubMed] [Google Scholar]
- 35.Topjian AA, French B, Sutton RM, et al. Early postresuscitation hypotension is associated with increased mortality following pediatric cardiac arrest. Crit Care Med. 2014;42:1518-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Topjian AA, Sanchez SM, Shults J, Berg RA, Dlugos DJ, Abend NS. Early electroencephalographic background features predict outcomes in children resuscitated from cardiac arrest. Pediatr Crit Care Med. 2016;17:547-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Isaeva E, Isaev D, Savrasova A, Khazipov R, Holmes GL. Recurrent neonatal seizures result in long-term increases in neuronal network excitability in the rat neocortex. Eur J Neurosci. 2010;31:1446-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karnam HB, Zhou JL, Huang LT, Zhao Q, Shatskikh T, Holmes GL. Early life seizures cause long-standing impairment of the hippocampal map. Exp Neurol. 2009;217:378-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Younkin DP, Delivoria-Papadopoulos M, Maris J, Donlon E, Clancy R, Chance B. Cerebral metabolic effects of neonatal seizures measured with in vivo 31P NMR spectroscopy. Ann Neurol. 1986;20:513-519. [DOI] [PubMed] [Google Scholar]
- 40.Vespa PM, Miller C, McArthur D, et al. Nonconvulsive electrographic seizures after traumatic brain injury result in a delayed, prolonged increase in intracranial pressure and metabolic crisis. Crit Care Med. 2007;35:2830-2836. [PMC free article] [PubMed] [Google Scholar]
- 41.Vespa PM, McArthur DL, Xu Y, et al. Nonconvulsive seizures after traumatic brain injury are associated with hippocampal atrophy. Neurology. 2010;75:792-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Claassen J, Perotte A, Albers D, et al. Nonconvulsive seizures after subarachnoid hemorrhage: multimodal detection and outcomes. Ann Neurol. 2013;74:53-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Claassen J, Albers D, Schmidt JM, et al. Nonconvulsive seizures in subarachnoid hemorrhage link inflammation and outcome. Ann Neurol. 2014;75:771-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Rooij LG, Toet MC, van Huffelen AC, et al. Effect of treatment of subclinical neonatal seizures detected with aEEG: randomized, controlled trial. Pediatrics. 2010;125:e358-e366. [DOI] [PubMed] [Google Scholar]
- 45.Srinivasakumar P, Zempel J, Trivedi S, et al. Treating EEG seizures in hypoxic ischemic encephalopathy: a randomized controlled trial. Pediatrics. 2015;136:e1302-e1309. [DOI] [PubMed] [Google Scholar]
- 46.Harris ML, Malloy KM, Lawson SN, Rose RS, Buss WF, Mietzsch U. Standardized treatment of neonatal status epilepticus improves outcome. J Child Neurol. 2016;31:1546-1554. [DOI] [PubMed] [Google Scholar]
- 47.Williams RP, Banwell B, Berg RA, et al. Impact of an ICU EEG monitoring pathway on timeliness of therapeutic intervention and electrographic seizure termination. Epilepsia. 2016;57:786-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abend NS, Dlugos DJ, Hahn CD, Hirsch LJ, Herman ST. Use of EEG Monitoring and management of non-convulsive seizures in critically ill patients: a survey of neurologists. Neurocrit Care. 2010;12(3):382-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fung FW, Topjian AA, Xiao R, Abend NS. Early EEG features for outcome prediction after cardiac arrest in children. J Clin Neurophysiol. 2019;36:349-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
We will make the underlying data available to investigators with appropriate data transfer and institutional review board approval.