Abstract
Introduction
Tobacco is highly addictive, and after the development of dependence, it is difficult to quit smoking. Therefore, it is important to understand the factors that play a role in the initiation of smoking. The rewarding effects of nicotine play a role in the initiation of smoking and the goal of the present study was to determine the rewarding effects of nicotine in adolescent and adult male and female rats.
Methods
Male and female Wistar rats were prepared with intracranial self-stimulation (ICSS) electrodes between postnatal day (P) 23 and 33. They were then trained on the ICSS procedure and the effect of nicotine (0, 0.03, 0.1, 0.3 mg/kg) on the reward thresholds and response latencies was investigated during adolescence (P40–59) or adulthood (>P75).
Results
Nicotine lowered the brain reward thresholds of the adult and adolescent male and female rats. The nicotine-induced decrease in the reward thresholds was the same in the adult male and adult female rats. However, nicotine induced a greater decrease in the reward thresholds of the adolescent female rats than the adolescent male rats. Nicotine decreased the response latencies of all groups and there was no effect of age or sex.
Conclusions
Nicotine enhances reward function and psychomotor performance in adolescent and adult male and female rats. Adolescent female rats are more sensitive to the acute rewarding effects of nicotine than adolescent male rats. Therefore, the rewarding effects of nicotine might play a greater role in the initiation of smoking in adolescent females than in adolescent males.
Implications
The great majority of people start smoking during adolescence. The present studies suggest that during this period female rats are more sensitive to the acute rewarding effects of low and intermediate doses of nicotine than male rats. The rewarding properties of nicotine play a role in the initiation of smoking and establishing habitual smoking. Therefore, the present findings might explain why adolescent females are at a higher risk for becoming nicotine dependent than adolescent males.
Introduction
Tobacco is highly addictive, and after the development of dependence, it is difficult to quit smoking.1,2 To improve smoking rates, it is important to understand which factors contribute to the development of nicotine dependence. The age of onset of smoking is one of the factors that affects the development of nicotine dependence. People who start smoking during adolescence are more likely to become nicotine dependent than people who start smoking during adulthood.3,4 Furthermore, females who start smoking during adolescence are at an even greater risk for becoming nicotine dependent than males who start smoking during adolescence.5 Therefore, both the age of onset and sex affect the risk for becoming nicotine dependent later in life. It might be possible that adolescent females are at an increased risk of becoming nicotine dependent because they are more sensitive to the acute rewarding effects of nicotine. Animal studies have been conducted to investigate the effects of age and sex on the reinforcing properties of nicotine.
Previous studies compared nicotine self-administration between adult male and adult female rats.6–11 There are no differences in operant responding for nicotine between adult male and adult female rats when standard nicotine doses (0.03–0.06 mg/kg per infusion) are used and the rats are tested under fixed-ratio schedules.6–9 However, it has been reported that females have a higher level of nicotine intake during the acquisition phase and a higher breakpoint when tested under a progressive ratio schedule.7,10,11 These studies suggest that there are no differences in nicotine self-administration between adult male and adult female rats under standard nicotine self-administration conditions. However, females might acquire nicotine intake faster and possibly have a higher motivation to self-administer nicotine.
The effect of age and sex on the rewarding effects of nicotine has also been investigated with the conditioned place preference (CPP) procedure. In this test, nicotine is administered in the presence of distinct environmental stimuli, and depending on the dose, the animals may develop a preference or aversion for the environment in which they received the nicotine.12,13 It has been firmly established that low and intermediate doses of nicotine induce CPP in adult male rats,12 and adolescent male rats are somewhat more sensitive to the reward-enhancing effects of nicotine than adult rats.14 Place-conditioning studies have reported conflicting findings with regard to the role of sex in the rewarding effects of nicotine in adult rats.15,16 One CPP study reported that adult males are more sensitive to the rewarding effects of nicotine than adult females and another study reported that adult females are more sensitive.15,16 The CPP paradigm is an excellent test to investigate the rewarding effects of contextual cues associated with drug use.17 However, the test does not provide insight into the state of the reward system in the presence of the drug, and clear dose-dependent effects are often not observed with the place-conditioning procedure.18
We used the intracranial self-stimulation (ICSS) procedure to investigate the acute rewarding effects of nicotine in adolescent and adult male and female rats. The ICSS procedure provides insight into the acute rewarding effects of drugs as changes in the reward thresholds are determined in the presence of the drug. Nicotine and other widely abused drugs decrease the brain reward thresholds, which indicates that these drugs potentiate reward function.19,20 In the present studies, the response latencies were also assessed. The response latency is the time interval between the onset of the noncontingent electrical stimulus and the operant response of the rat. The response latency reflects psychomotor performance, and stimulants decrease the response latency and sedative drugs such as benzodiazepines increase the response latency.19,21
The goal of the present studies was to investigate the acute rewarding and psychomotor effects of nicotine in adolescent and adult male and female rats. To the best of our knowledge, this is the first study to test adolescent female rats in the ICSS procedure. Furthermore, the acute effects of nicotine on reward thresholds and response latencies have not been investigated in adolescent male, adolescent female, or adult female rats. The rats were prepared with electrodes between postnatal day (P) 23 and 33 and then trained on the ICSS procedure. The rats were considered adolescent between P40 and P60 and adult when they were older than P75.22,23 In the present studies, we investigated the effects of low and intermediate doses of nicotine (0.03, 0.1, 0.3 mg/kg) on the state of the brain reward system.19 In order to reduce the addictive properties of cigarettes, the US Food and Drug Administration plans to limit the amount of nicotine that is allowed in cigarettes.24,25 Therefore, it is important to gain insight into the acute rewarding properties of low and intermediate doses of nicotine in adolescent and adult male and female rats.
Methods
Subjects
Adult male and female Wistar rats were purchased from Charles River Laboratories (Raleigh, NC) and housed with a rat of the same sex. The rats were housed in a climate-controlled vivarium on a reversed 12-hour light-dark cycle (light off at 8 am). The rats that were used for the ICSS studies were bred in our animal facilities. A female rat was housed with a male breeder and they were separated after at least 2 weeks if the female was visibly pregnant. If the female was not pregnant, then they remained together for another week. If this did not lead to a pregnancy, then the female was housed with another male. The females were housed with the pups until they were weaned at P21. After weaning, the rats were housed in same-sex pairs. Food and water were available ad libitum in the home cage. The animals were fed regular rodent chow (Teklad 2018, Envigo, Indianapolis, IN). The female breeders received a food with lower levels of natural phytoestrogens as these factors can have negative effects on reproductive health (Teklad 2019). The experimental protocols were approved by the University of Florida Institutional Animal Care and Use Committee.
Drugs
Nicotine was purchased from Sigma (N5260, Sigma-Aldrich, St. Louis, MO). Nicotine was dissolved in sterile saline (0.9% sodium chloride, pH not adjusted) and administered subcutaneously (sc) in a volume of 1 mL/kg body weight. Nicotine doses are expressed as base.
Experimental Design
Rats were bred in house and weaned at P21. A total of 53 rat pups from eight litters were used for this study (29 males and 24 females). We only used rats that had recovered well from the surgery and completed the ICSS training. The implantation of the electrodes started at P23 and most of the electrodes were implanted within several days. The rats were then trained on the ICSS procedure as described in our previous work.26,27 The nicotine injections started after the ICSS training was completed (>90% correct responses) and the reward thresholds and response latencies had been collected for at least 3 days. The effects of nicotine were investigated in adolescent rats (P40–59, n = 32) and adult (P75 and older, n = 20) rats.22 The main goal of the study was to compare the rewarding effects of nicotine in the adolescent and adult male and female rats. However, a subgroup of the adolescent male rats could be considered middle adolescent (P34–46). Therefore, a secondary analysis was conducted to determine if there was a difference in the response to nicotine between the middle and late adolescent male rats.23 It took longer to train the adolescent females than the adolescent males and only two female rats received nicotine during middle adolescence (Table 1). Therefore, the rewarding effects of nicotine were not compared between middle and late adolescent females. The doses of nicotine (0.03, 0.1, 0.3 mg/kg, sc) were based on a previous study in which we investigated the effects of nicotine on reward thresholds in adult male rats.19 All rats received all doses of nicotine on four consecutive days (different dose each day). Nicotine was administered according to a Latin square design 15 minutes prior to ICSS testing.
Table 1.
Age and Body Weight of the Adult and Adolescent Rats
| Group | Sex | Group size | Age at electrode implantation (days) | BW at electrode implantation (g) | Age at start of nicotine injections (days) | BW at start of nicotine injections (g) |
|---|---|---|---|---|---|---|
| Adult | Male | 8 | 26 ± 1 | 78 ± 3 | 92 ± 6 | 395 ± 19 |
| Female | 13 | 28 ± 1 | 80 ± 4 | 136 ± 24 | 285 ± 17** | |
| Adolescenta | Male | 21 | 27 ± 1 | 82 ± 4 | 49 ± 1 | 232 ± 13# |
| Female | 11 | 28 ± 1 | 79 ± 3 | 50 ± 1 | 175 ± 7*# | |
| Late adolescent | Male | 14 | 27 ± 1 | 82 ± 4 | 53 ± 1 | 266 ± 10# |
| Female | 9 | 28 ± 1 | 80 ± 4 | 52 ± 1 | 182 ± 7**# | |
| Middle adolescent | Male | 7 | 27 ± 1 | 81 ± 5 | 41 ± 0.3 | 162 ± 5# |
| Female | 2 | 27 ± 1 | 79 ± 0.4 | 41 ± 0.0 | 146 ± 6b |
Asterisks (*p < .01, **p < .001) indicate lower body weights compared to male rats of the same age. Pound signs (#p < .001) indicate lower body weights compared to adult rats of the same sex. Data are expressed as averages ± SEM. BW = body weights.
aBoth late and middle adolescent rats are included.
bA statistical comparison was not conducted because of the small group size.
Electrode Implantations
Rats were anesthetized with an isoflurane and oxygen vapor mixture (1%–3% isoflurane) and placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA). The top of the head was shaved and a 1-cm incision was made. After cleaning the skull, five small holes were drilled and four skull screws (0–80 × 1/16, Plastics One, Roanoke, VA) were implanted. The fifth hole was used for the electrode. The coordinates for the electrodes were based on a previous study in which we implanted electrodes in adolescent rats.28 The electrodes (11 mm in length, Plastics One) were implanted in the medial forebrain bundle with the incisor bar set 5 mm above the interaural line (adolescents: −0.5 anterior posterior, ±1.48 medial lateral, −8.3 dorsal ventral from dura). After at least 3 days of recovery, the rats were trained on a modified discrete-trial ICSS procedure.29,30
ICSS Procedure
The operant conditioning chambers were housed in sound-attenuating chambers (Med Associates, Georgia, VT). The operant conditioning chambers had a 5-cm wide metal response wheel that was centered on a sidewall and a photobeam detector recorded every 90° of rotation. Brain stimulation was delivered by constant current stimulators (Model 1200C, Stimtek, Acton, MA). The rats were initially trained to turn the wheel on a fixed-ratio 1 schedule of reinforcement. Each quarter turn of the wheel resulted in the delivery of a 0.5 s train of 0.1 ms cathodal square-wave pulses at a frequency of 100 Hz. After the acquisition of responding for stimulation on this fixed-ratio 1 schedule, defined as 100 reinforcements within 10 minutes, the rats were trained on a discrete-trial current-threshold procedure. The discrete-trial current-threshold procedure that was used was a modification of a task developed by Kornetsky and Esposito,31 and previously described in detail by Bruijnzeel and Markou.32,33 Each trial began with the delivery of a noncontingent electrical stimulus, followed by a 7.5-second response window during which the animal could respond to receive a second identical stimulus. A response during this 7.5-second response window was labeled a positive response, whereas the lack of a response was labeled a negative response. During the 2-second period immediately after a positive response, additional responses had no consequence. The intertrial interval, which followed either a positive response or the end of the response window, had an average duration of 10 seconds (ranging from 7.5 seconds to 12.5 seconds). Responses that occurred during the intertrial interval resulted in a further 12.5-second delay of the onset of the next trial. During training on the discrete-trial procedure, the duration of the intertrial interval and delay periods induced by time-out responses were gradually increased until animals performed consistently at standard test parameters. Training was completed when the animals responded correctly to more than 90% of the noncontingent electrical stimuli. It took 1–2 weeks of training for most rats to meet this response criterion. The rats were then tested on the current-threshold procedure in which stimulation intensities varied according to the classical psychophysical method of limits. A test session consisted of four alternating series of descending and ascending current intensities starting with a descending series. Blocks of three trials were presented to the rats at a given stimulation intensity, and the intensity was altered systematically between blocks of trials by 5-µA steps. The initial stimulus intensity was set 40 µA above the baseline current-threshold for each animal. All the rats were tested at least three times on the current threshold procedure before the start of the nicotine injections. The coefficient of variation of the thresholds and latencies on the 3 days prior to the nicotine injections is reported in Supplementary Table 1. Each test session typically lasted 30–40 minutes and provided two dependent variables for behavioral assessment (brain reward thresholds and response latencies). The brain reward threshold (microampere) was defined as the midpoint between stimulation intensities that supported responding and stimulation intensities that failed to support responding. The response latency(s) was defined as the time interval between the beginning of the noncontingent stimulus and a positive response. A decrease in reward thresholds is indicative of a potentiation of reward function.31 Drugs that have sedative effects or induce motor impairments increase the response latency and stimulants decrease the response latency.19,21 The nicotine injections started at least several days after the onset of the current-threshold procedure.
Statistics
The body weights of the adolescent and adult male and female rats were compared with a two-way analysis of variance (ANOVA) with age and sex as between-subject factors. The body weights of the middle adolescent and late adolescent rats were compared with a one-way ANOVA. The baseline brain reward thresholds and response latencies were also compared with one-way ANOVAs. The brain reward thresholds and response latencies were expressed as a percentage of the pretest day threshold or latency (Supplementary Table 1). The ICSS parameters were analyzed with two-way ANOVAs with age or sex as between-subject factors and nicotine dose as within-subject factor. When the ANOVA revealed significant effects, the Bonferroni post hoc test was conducted. The data were analyzed with SPSS Statistics 25 and GraphPad Prism 7 for Windows.
Results
The electrode implantations started at P23, and at the onset of the implantations, there were no differences in body weights between the groups (Table 1, Supplementary Figure 1A). On the day of the electrode implantations, the average age of the rats was 27.3 days and the average body weight was 80.2 g. On the first day of the nicotine injections, the body weights of the adult rats were higher than the body weight of the adolescent rats and the body weights of the female rats were lower than the body weight of the male rats (Sex F1,49 = 26.55, p < .0001; Age F1,49 = 71.59, p < .0001, Table 1, Supplementary Figure 1B). The late adolescent male rats had a higher body weight than the middle adolescent male rats on the day of the first nicotine injection (F1,19 = 48.231, p < .0001, Supplementary Figure 1C). Prior to the onset of the nicotine injections, the brain reward thresholds of the adult male rats were higher than those of the adult females and the adolescent males and females (F3,49 = 5.353, p < .01, Supplementary Table 1). The thresholds of the adult males were also higher than those of the late adolescent males and females (F4,46 = 4.088, p < .01). There was no difference between the baseline thresholds of the adult males and middle adolescent males. There were no differences in the response latencies between the adult and adolescent male and female groups.
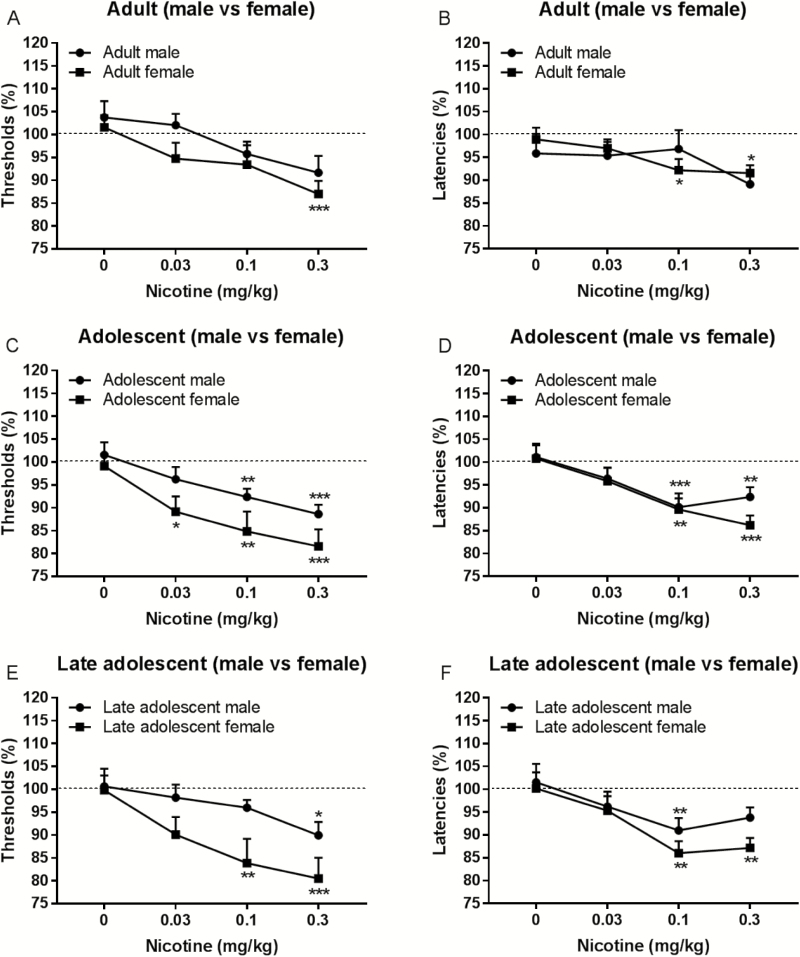
The administration of nicotine decreased the brain reward thresholds of the adult male and adult female rats and there was no effect of sex (Dose F3,57 = 6.141, p < .01, Figure 1A). Nicotine also lowered the brain reward thresholds of the adolescent rats and nicotine induced a larger decrease in the reward thresholds of the adolescent females than the adolescent males (Dose F3,90 = 14.16, p < .0001; sex F1,30 = 4.558, p < 0.05, Figure 1C). A similar effect was observed when the data from the late adolescent rats were analyzed. Nicotine decreased the brain reward thresholds of the late adolescent rats and the nicotine-induced decrease in the reward thresholds was larger in the females (Dose F3,63 = 9.968, p < .0001; Sex F1,21 = 4.501, p < .05, Figure 1E).
Figure 1.
Nicotine lowers the brain reward thresholds and response latencies of adult, adolescent, and late adolescent male and female rats. Figures depict the effect of nicotine on the reward thresholds and response latencies of the adult rats (A, B), adolescent rats (C, D), and late adolescent rats (E, F). Asterisks indicate a decrease in the reward thresholds and response latencies compared to rats of the same age or sex treated with vehicle. *p < .05, **p < .01, ***p < .001. Data are expressed as means ± SEM.
Nicotine lowered the brain reward thresholds of the adolescent male and adult male rats (Dose F3,81 = 8.661, p < .0001, Supplementary Figure 2A), and the middle adolescent male rats and late adolescent male rats (Dose F3,57 = 8.594, p < .0001, Supplementary Figure 2C), but there was no effect of age (adolescent vs. adult or early-adolescent vs. late adolescent). There was, however, a trend toward a larger nicotine-induced decrease in the reward thresholds of the adolescent female rats compared to the adult female rats (Dose F3,66 = 9.917, p < .0001; Age F1,22 = 2.925, p = .10, Supplementary Figure 2E). These findings indicate that nicotine decreases the brain reward thresholds and that nicotine induces a larger decrease in the reward thresholds of the adolescent female rats than the adolescent male rats.
Nicotine decreased the response latencies of the adult male and adult female rats (F3,57 = 4.486, p = .01, Figure 1B), the adolescent male end adolescent female rats (F3,90 = 13.54, p < .0001, Figure 1D), and the late adolescent male and late adolescent female rats (F3,63 = 11.51, p < .0001, Figure 1F). There was no effect of sex on the nicotine-induced decrease in the response latencies in the adult and adolescent rats.
Additional analyses were conducted to compare the latencies between the adult and adolescent rats for each sex. Nicotine decreased the response latencies of the adult male rats and the adolescent male rats (Dose F3,81 = 4.035, p < .05, Supplementary Figure 2B), and the middle and late adolescent male rats (Dose F3,57 = 7.213, p ≤ .001, Supplementary Figure 2D). In both analyses, there was no effect of age. Nicotine also decreased the latencies of the adult female and adolescent female rats and there was no effect of age (Dose F3,66 = 12.57, p < .0001, Supplementary Figure 2F). These findings indicate that nicotine decreases the response latencies of the adolescent and adult male and female rats and the effects of nicotine are not affected by the age or sex of the animals.
Discussion
The goal of the present studies was to investigate the rewarding and psychomotor effects of nicotine in adolescent and adult male and female rats. The studies showed that nicotine decreased the brain reward thresholds of all groups of rats. However, nicotine induced a larger decrease in the reward thresholds of the adolescent females than the adolescent males. The adolescent females were also slightly more sensitive to the rewarding effects of nicotine than the adult females (nonsignificant trend). Nicotine decreased the response latencies of all groups of rats and there was no effect of age or sex. Overall, these findings indicate that nicotine is more rewarding in adolescent female than in adolescent male rats.
In the present study, we found that nicotine lowered the brain reward thresholds of the adult male rats. The highest dose of nicotine (0.3 mg/kg) lowered the brain reward thresholds by about 10% in the adult male rats. This is in line with former studies by us and others that showed that this dose of nicotine decreases the reward thresholds in adult males by about 10% and is the most rewarding dose of nicotine.19,34 The nicotine-induced decrease in the reward thresholds in the adult females was similar as in the adult males. This finding suggests that there are no differences in the rewarding effects of nicotine between adult males and adult females. On the basis of this observation, one would expect that there are no differences in nicotine self-administration in adult males and adult females. Indeed, previous studies have reported that adult male and adult female rats self-administer the same amount of nicotine under standard self-administration conditions (fixed-ratio schedule, 0.03–0.06 mg/kg per infusion).6–8 Place-conditioning studies have been conducted to compare the rewarding effects of nicotine in adult male and adult female rats. One study did not find significant CPP in adult females with low or intermediate doses of nicotine (0.2–0.8 mg/kg), and only a high dose of nicotine produced CPP in the females and aversion in males.15 This suggests that adult females are more sensitive to the rewarding effects of a high dose of nicotine and possibly less sensitive to the aversive effects of a high dose. Another CPP study did not find differences in the rewarding effect of nicotine between adult males and adult females.16 One specific dose of nicotine (0.4 mg/kg) induced CPP in the female rats but not in the adult male rats. There was, however, no significant difference between the adult male and adult female rats at this dose. Therefore, these CPP studies suggest that there is no or possibly only very small difference in the rewarding effects of nicotine between adult males and adult females but these groups might react differently to high and aversive doses of nicotine. Overall, the present ICSS study and the aforementioned CPP and self-administration studies would suggest that there are no differences in the rewarding effects of low and intermediate doses of nicotine between adult male and adult female rats.
The present results show that adolescent female rats are more sensitive to the rewarding effects of nicotine than the adolescent male rats. The highest dose of nicotine induced a 17% decrease in the brain reward thresholds of the adolescent females. Although this is a relatively large decrease in reward thresholds, it is not the maximum response that can be observed with the ICSS method. Larger decreases in the thresholds have been observed with more potent psychostimulants such as methamphetamine and the bath salt 3,4-methylenedioxypyrovalerone.20,35 We are not aware of any studies that have directly compared the acute rewarding effects of nicotine in adolescent male and adolescent female rats. However, in one CPP study both adolescent male and adolescent female rats received 0.03 mg/kg of nicotine prior to the training sessions.36 Nicotine induced a 400% increase in preference in the adolescent female rats and a 200% increase in the adolescent male rats. Although only one dose of nicotine was used and the groups were not directly compared, this observation suggests that adolescent females are more sensitive to the rewarding effects of nicotine than adolescent males. Several studies have compared intravenous nicotine self-administration between adolescent male and adolescent female rats. Two of these studies reported similar levels of nicotine self-administration in the adolescent male and adolescent female rats.11,37 One study reported a higher level of nicotine self-administration in adolescent males than adolescent females, but this effect was only observed during the first 2 weeks of nicotine self-administration.11 Therefore, the adolescent nicotine self-administration studies do not support that there is a difference in the reinforcing effects of nicotine between males and females. Nicotine intake has also been compared between adolescent male and adolescent female mice using a voluntary oral nicotine consumption procedure.38 The female mice consumed a larger amount of nicotine when intake was adjusted for body weights. Taken together, our ICSS study suggests that nicotine is more rewarding in adolescent female rats than in adolescent male rats. This is in line with CPP and oral self-administration studies that suggest that the adolescent females might be somewhat more sensitive to the rewarding effects of nicotine than adolescent males.
In the present study, it was not investigated if the increased sensitivity to the rewarding effects of nicotine in the adolescent females translates into a higher risk for becoming dependent. However, several studies suggest that adolescent females are particularly vulnerable for becoming nicotine dependent. Adolescent female rats have a higher level of nicotine self-administration than adult female rats.39 Interestingly, female rats that start with nicotine self-administration during adolescence have a higher level of nicotine intake in adulthood than females that start during adulthood. Rats with a high level of nicotine intake are more likely to become dependent40; therefore, female rats that start nicotine intake during adolescence might be more likely to become nicotine dependent. A similar pattern of results has been reported in humans. Lanza and Vasilenko5 reported that the rate of nicotine dependence in adulthood is related to the age of onset of regular smoking. People who start smoking during adolescence are at a higher risk for becoming nicotine dependent in adulthood than people who start smoking as adults. Interestingly, females who start smoking during adolescence are more likely to become nicotine dependent during adulthood than males who start smoking during adolescence.5 Overall, these findings indicate that nicotine is more rewarding in adolescent females than in adolescent males. People who experience their first cigarette as very pleasurable are more likely to become daily smokers than people who have an unpleasant first experience.4 Therefore, the fact that adolescent females experience nicotine as more rewarding than their male counterparts could play a role in their increased risk for becoming nicotine dependent.
In the present study, we also investigated the effect of nicotine on the response latencies. The administration of nicotine decreased the response latencies in all groups to a similar degree. Nicotine is a mild psychostimulant and it was therefore expected to improve psychomotor performance. Previous ICSS studies have also shown that nicotine decreases the response latencies.19,41 The present study showed that there is no effect of sex or age on the nicotine-induced decrease in the response latencies. We only investigated the effects of low and intermediate doses of nicotine. The pharmacological effects of nicotine typically follow a U-shaped dose-response curve, and in a previous study with adult male rats, we showed that an intermediate dose of nicotine (0.3 mg/kg) decreased the response latencies and a higher dose of nicotine (0.6 mg/kg) was without effect.19 In the present study, nicotine induced a slightly larger decrease in the latencies of the adolescent females than the adolescent males and the difference in the response latencies between these groups became larger with increasing doses of nicotine. Therefore, it cannot be ruled out that larger differences in the response latencies between the adolescent male and adolescent females might have been detected with higher doses of nicotine. This is supported by studies with adolescent rats in which the locomotor response to nicotine was investigated. Nicotine induced a larger increase in locomotor activity in adolescent female rats than in the adolescent male rats and this effect was largest with the highest doses of nicotine (0.5 and 1 mg/kg).42
There is some evidence that the rewarding properties of nicotine differ for early, middle, and late adolescent rats. Low doses of nicotine induce CPP in early adolescent mice, but not in middle adolescent, late adolescent, or adult mice.43 Furthermore, early adolescent mice prefer a nicotine solution over water but middle and late adolescent mice do not prefer the nicotine solution over water.44 In both studies, there were no differences in the rewarding effects of nicotine between the middle and late adolescent mice.43,44 That is in line with the present study in which the ANOVA analysis did not reveal a significant difference in the rewarding effects of nicotine between the middle and late adolescent rats. In the present study, we were not able to compare the rewarding effects of nicotine between the middle and late adolescent female rats. We trained 24 female rats on the ICSS procedure, but only two of the female rats met the response requirement during middle adolescence. In contrast, seven out of 29 males met the response requirements during middle adolescence. The adolescent females were somewhat more difficult to train than the adolescent males. This might have been due to the fact that the adolescent female rats are more active compared to the adolescent male rats.42 However, it should be noted that in both groups, only a relatively small number of animals met the response requirements during middle adolescence. The rat ICSS procedure might have to be modified in order to be able to better investigate the rewarding properties of drugs in early and middle adolescent male and female rats.
In the present study, the baseline brain reward thresholds of the adult male rats were higher than those of the adult females and the adolescent males and females. We believe that this did not affect the response to nicotine. Nicotine decreased the brain reward thresholds of the adult male rats by 12%. We found a similar, 14%, nicotine-induced decrease in brain reward thresholds in adult male rats in a previous study.19 In the previous study, the baseline brain reward thresholds were much lower than in the present study (185 vs. 91 μA). This suggests that the baseline reward threshold has little or no effect on the nicotine-induced changes in the reward thresholds. Furthermore, in the present study, the latencies of the adult male rats did not differ from the latencies of the adult females and the adolescent males and females. This indicates that the adult males performed as well on the ICSS test procedure as the other groups of rats. In our study, we did not compare plasma and brain nicotine levels between the adolescent and adult rats. However, there is evidence that the age of the rats affects plasma and brain nicotine levels.45 Plasma and brain nicotine levels are lower in adolescent male rats (P40) than in adult male rats (P90), 30 minutes after systemic nicotine administration (0.8 mg/kg nicotine base, sc).45 In the present studies, we observed a trend toward a larger nicotine-induced decrease in the reward thresholds of the adolescent female rats compared to the adult female rats. It cannot be ruled out that the differential sensitivity of the adolescent and adult female rats was due to differences in nicotine metabolism.
The present study provides insight into the dose–effects of nicotine in adolescent and adult male and female rats on brain reward function. Our study showed that adolescent females are most sensitive to the acute rewarding effects of nicotine. There has been a strong interest in reducing the nicotine content of tobacco to decrease the addictive properties of cigarettes.46,47 Preclinical studies have focused on determining the threshold dose below which nicotine has no reinforcing properties.9,48,49 However, so far most of these studies have been conducted with adult rats. The present finding suggest that adolescent females are very sensitive to the rewarding effects of nicotine, and most people start smoking during adolescence.50 Therefore, in future animal studies, adolescent females should be included to be able to provide scientific guidance regarding the effectiveness of nicotine reduction policies in people with different age and sex. In the present study, we investigated the effects of low and intermediate doses of nicotine on the reward thresholds. Higher doses of nicotine are aversive, and the aversive aspects of nicotine might deter some people from continuing to experiment with cigarettes.19,51 Therefore, future studies may also explore the aversive aspects of nicotine in adolescent and adult male and female rats.
In conclusion, our study shows that adolescent females are more sensitive to the acute rewarding effects of nicotine than adolescent males. This increased sensitivity to the acute rewarding effects of nicotine in adolescent females could potentially increase the risk for becoming nicotine dependent.
Funding
This work was supported by an FDA Center for Tobacco Products (CTP) grant (DA042530 to AB). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Food and Drug Administration. ST was supported by a visiting fellowship from Chinese Scholarship Council (201608430251).
Declaration of Interests
None declared.
Supplementary Material
References
- 1. Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction. 2004;99(1):29–38. [DOI] [PubMed] [Google Scholar]
- 2. Bruijnzeel AW. Tobacco addiction and the dysregulation of brain stress systems. Neurosci Biobehav Rev. 2012;36(5):1418–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kendler KS, Myers J, Damaj MI, Chen X. Early smoking onset and risk for subsequent nicotine dependence: A monozygotic co-twin control study. Am J Psychiatry. 2013;170(4):408–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hu MC, Davies M, Kandel DB. Epidemiology and correlates of daily smoking and nicotine dependence among young adults in the United States. Am J Public Health. 2006;96(2):299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lanza ST, Vasilenko SA. New methods shed light on age of onset as a risk factor for nicotine dependence. Addict Behav. 2015;50:161–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Feltenstein MW, Ghee SM, See RE. Nicotine self-administration and reinstatement of nicotine-seeking in male and female rats. Drug Alcohol Depend. 2012;121(3):240–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Donny EC, Caggiula AR, Rowell PP, et al. Nicotine self-administration in rats: estrous cycle effects, sex differences and nicotinic receptor binding. Psychopharmacology (Berl). 2000;151(4):392–405. [DOI] [PubMed] [Google Scholar]
- 8. Chaudhri N, Caggiula AR, Donny EC, et al. Sex differences in the contribution of nicotine and nonpharmacological stimuli to nicotine self-administration in rats. Psychopharmacology (Berl). 2005;180(2):258–266. [DOI] [PubMed] [Google Scholar]
- 9. Grebenstein P, Burroughs D, Zhang Y, LeSage MG. Sex differences in nicotine self-administration in rats during progressive unit dose reduction: implications for nicotine regulation policy. Pharmacol Biochem Behav. 2013;114-115:70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Park MK, Belluzzi JD, Han SH, Cao J, Leslie FM. Age, sex and early environment contribute to individual differences in nicotine/acetaldehyde-induced behavioral and endocrine responses in rats. Pharmacol Biochem Behav. 2007;86(2):297–305. [DOI] [PubMed] [Google Scholar]
- 11. Chen H, Matta SG, Sharp BM. Acquisition of nicotine self-administration in adolescent rats given prolonged access to the drug. Neuropsychopharmacology. 2007;32(3):700–709. [DOI] [PubMed] [Google Scholar]
- 12. Le Foll B, Goldberg SR. Nicotine induces conditioned place preferences over a large range of doses in rats. Psychopharmacology (Berl). 2005;178(4):481–492. [DOI] [PubMed] [Google Scholar]
- 13. Fudala PJ, Teoh KW, Iwamoto ET. Pharmacologic characterization of nicotine-induced conditioned place preference. Pharmacol Biochem Behav. 1985;22(2):237–241. [DOI] [PubMed] [Google Scholar]
- 14. Vastola BJ, Douglas LA, Varlinskaya EI, Spear LP. Nicotine-induced conditioned place preference in adolescent and adult rats. Physiol Behav. 2002;77(1):107–114. [DOI] [PubMed] [Google Scholar]
- 15. Torres OV, Natividad LA, Tejeda HA, Van Weelden SA, O’Dell LE. Female rats display dose-dependent differences to the rewarding and aversive effects of nicotine in an age-, hormone-, and sex-dependent manner. Psychopharmacology (Berl). 2009;206(2):303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lenoir M, Starosciak AK, Ledon J, et al. Sex differences in conditioned nicotine reward are age-specific. Pharmacol Biochem Behav. 2015;132:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward?Psychopharmacology (Berl). 2000;153(1):31–43. [DOI] [PubMed] [Google Scholar]
- 18. Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol. 1998;56(6):613–672. [DOI] [PubMed] [Google Scholar]
- 19. Igari M, Alexander JC, Ji Y, Qi X, Papke RL, Bruijnzeel AW. Varenicline and cytisine diminish the dysphoric-like state associated with spontaneous nicotine withdrawal in rats. Neuropsychopharmacology. 2014;39(2):455–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sambo DO, Lin M, Owens A, et al. The sigma-1 receptor modulates methamphetamine dysregulation of dopamine neurotransmission. Nat Commun. 2017;8(1):2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liebman JM. Anxiety, anxiolytics and brain stimulation reinforcement. Neurosci Biobehav Rev. 1985;9(1):75–86. [DOI] [PubMed] [Google Scholar]
- 22. Jankord R, Solomon MB, Albertz J, Flak JN, Zhang R, Herman JP. Stress vulnerability during adolescent development in rats. Endocrinology. 2011;152(2):629–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tirelli E, Laviola G, Adriani W. Ontogenesis of behavioral sensitization and conditioned place preference induced by psychostimulants in laboratory rodents. Neurosci Biobehav Rev. 2003;27(1–2):163–178. [DOI] [PubMed] [Google Scholar]
- 24. Perkins KA. FDA policy on setting maximum nicotine content in cigarettes. Nicotine & Tobacco Research. 2018, 1–2. [DOI] [PubMed] [Google Scholar]
- 25. Smith TT, Rupprecht LE, Denlinger-Apte RL, et al. Animal research on nicotine reduction: current evidence and research gaps. Nicotine & Tobacco Research. 2017;19(9):1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marcinkiewcz CA, Prado MM, Isaac SK, Marshall A, Rylkova D, Bruijnzeel AW. Corticotropin-releasing factor within the central nucleus of the amygdala and the nucleus accumbens shell mediates the negative affective state of nicotine withdrawal in rats. Neuropsychopharmacology. 2009;34(7):1743–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Qi X, Guzhva L, Yang Z, et al. Overexpression of CRF in the BNST diminishes dysphoria but not anxiety-like behavior in nicotine withdrawing rats. Eur Neuropsychopharmacol. 2016;26(9):1378–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. O’Dell LE, Bruijnzeel AW, Smith RT, et al. Diminished nicotine withdrawal in adolescent rats: implications for vulnerability to addiction. Psychopharmacology (Berl). 2006;186(4):612–619. [DOI] [PubMed] [Google Scholar]
- 29. Markou A, Koob GF. Construct validity of a self-stimulation threshold paradigm: effects of reward and performance manipulations. Physiol Behav. 1992;51(1):111–119. [DOI] [PubMed] [Google Scholar]
- 30. Bruijnzeel AW, Prado M, Isaac S. Corticotropin-releasing factor-1 receptor activation mediates nicotine withdrawal-induced deficit in brain reward function and stress-induced relapse. Biol Psychiatry. 2009;66(2):110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kornetsky C, Esposito RU. Euphorigenic drugs: effects on the reward pathways of the brain. Fed Proc. 1979;38(11):2473–2476. [PubMed] [Google Scholar]
- 32. Bruijnzeel AW, Markou A. Characterization of the effects of bupropion on the reinforcing properties of nicotine and food in rats. Synapse. 2003;50(1):20–28. [DOI] [PubMed] [Google Scholar]
- 33. Bruijnzeel AW, Markou A. Adaptations in cholinergic transmission in the ventral tegmental area associated with the affective signs of nicotine withdrawal in rats. Neuropharmacology. 2004;47(4):572–579. [DOI] [PubMed] [Google Scholar]
- 34. Harris AC, Tally L, Muelken P, et al. Effects of nicotine and minor tobacco alkaloids on intracranial-self-stimulation in rats. Drug Alcohol Depend. 2015;153:330–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Colon-Perez LM, Pino JA, Saha K, et al. Functional connectivity, behavioral and dopaminergic alterations 24 hours following acute exposure to synthetic bath salt drug methylenedioxypyrovalerone. Neuropharmacology. 2018;137:178–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Natarajan R, Wright JW, Harding JW. Nicotine-induced conditioned place preference in adolescent rats. Pharmacol Biochem Behav. 2011;99(3):519–523. [DOI] [PubMed] [Google Scholar]
- 37. Schassburger RL, Pitzer EM, Smith TT, et al. Adolescent rats self-administer less nicotine than adults at low doses. Nicotine Tob Res. 2016;18(9):1861–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Klein LC, Stine MM, Vandenbergh DJ, Whetzel CA, Kamens HM. Sex differences in voluntary oral nicotine consumption by adolescent mice: a dose-response experiment. Pharmacol Biochem Behav. 2004;78(1):13–25. [DOI] [PubMed] [Google Scholar]
- 39. Levin ED, Rezvani AH, Montoya D, Rose JE, Swartzwelder HS. Adolescent-onset nicotine self-administration modeled in female rats. Psychopharmacology (Berl.). 2003;169(2):141–149. [DOI] [PubMed] [Google Scholar]
- 40. O’Dell LE, Chen SA, Smith RT, et al. Extended access to nicotine self-administration leads to dependence: circadian measures, withdrawal measures, and extinction behavior in rats. J Pharmacol Exp Ther. 2007;320(1):180–193. [DOI] [PubMed] [Google Scholar]
- 41. Davis JM, Svendsgaard DJ. U-shaped dose-response curves: their occurrence and implications for risk assessment. J Toxicol Environ Health. 1990;30(2):71–83. [DOI] [PubMed] [Google Scholar]
- 42. Elliott BM, Faraday MM, Phillips JM, Grunberg NE. Effects of nicotine on elevated plus maze and locomotor activity in male and female adolescent and adult rats. Pharmacol Biochem Behav. 2004;77(1):21–28. [DOI] [PubMed] [Google Scholar]
- 43. Kota D, Robinson SE, Imad Damaj M. Enhanced nicotine reward in adulthood after exposure to nicotine during early adolescence in mice. Biochem Pharmacol. 2009;78(7):873–879. [DOI] [PubMed] [Google Scholar]
- 44. Adriani W, Macrì S, Pacifici R, Laviola G. Peculiar vulnerability to nicotine oral self-administration in mice during early adolescence. Neuropsychopharmacology. 2002;27(2):212–224. [DOI] [PubMed] [Google Scholar]
- 45. Vieira-Brock PL, Andrenyak DM, Nielsen SM, Fleckenstein AE, Wilkins DG. Age-related differences in the disposition of nicotine and metabolites in rat brain and plasma. Nicotine Tob Res. 2013;15(11):1839–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Benowitz NL, Henningfield JE. Establishing a nicotine threshold for addiction. The implications for tobacco regulation. N Engl J Med. 1994;331(2):123–125. [DOI] [PubMed] [Google Scholar]
- 47. Bevins RA, Barrett ST, Huynh YW, Thompson BM, Kwan DA, Murray JE. Experimental analysis of behavior and tobacco regulatory research on nicotine reduction. J Exp Anal Behav. 2018;110(1):1–10. [DOI] [PubMed] [Google Scholar]
- 48. Smith TT, Schassburger RL, Buffalari DM, Sved AF, Donny EC. Low-dose nicotine self-administration is reduced in adult male rats naïve to high doses of nicotine: implications for nicotine product standards. Exp Clin Psychopharmacol. 2014;22(5):453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Smith TT, Rupprecht LE, Sved AF, Donny EC. Characterizing the relationship between increases in the cost of nicotine and decreases in nicotine content in adult male rats: implications for tobacco regulation. Psychopharmacology (Berl). 2016;233(23–24):3953–3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Klein H, Sterk CE, Elifson KW. Initial smoking experiences and current smoking behaviors and perceptions among current smokers. J Addict. 2013;2013:491797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Silverstein B, Feld S, Kozlowski LT. The availability of low-nicotine cigarettes as a cause of cigarette smoking among teenage females. J Health Soc Behav. 1980;21(4):383–388. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.