Abstract
In a fast-changing world, polar ecosystems are threatened by climate variability. Understanding the roles of fine-scale processes, and linear and nonlinear effects of climate factors on the demography of polar species is crucial for anticipating the future state of these fragile ecosystems. While the effects of sea ice on polar marine top predators are increasingly being studied, little is known about the impacts of landfast ice (LFI) on this species community. Based on a unique 39-year time series of satellite imagery and in situ meteorological conditions and on the world's longest dataset of emperor penguin (Aptenodytes forsteri) breeding parameters, we studied the effects of fine-scale variability of LFI and weather conditions on this species' reproductive success. We found that longer distances to the LFI edge (i.e. foraging areas) negatively affected the overall breeding success but also the fledging success. Climate window analyses suggested that chick mortality was particularly sensitive to LFI variability between August and November. Snowfall in May also affected hatching success. Given the sensitivity of LFI to storms and changes in wind direction, important future repercussions on the breeding habitat of emperor penguins are to be expected in the context of climate change.
Keywords: emperor penguin, sea ice, breeding success, climate window analysis, nonlinear effect
1. Introduction
Polar ecosystems are subject to local and regionally contrasted sea ice trends as a result of climate change [1,2]. Given the complexity of these trends, which are tightly linked to the atmosphere and the ocean dynamics, there is an urgent need to measure and forecast how polar marine populations will respond to sea ice habitat changes [3,4]. Among the studies that have investigated the impacts of climate change and variability on population dynamics in the Southern Ocean [5,6], a thorough understanding of the fine-scale processes by which climate affects the population dynamics of polar organisms is still lacking, thereby preventing the scientific community from improving model projections to correctly assess the future states of polar populations and ecosystems. Given that population dynamics are driven by several demographic components whose sensitivities to climatic factors vary [7,8], it is important to investigate the links between climate and each demographic component. Determining the spatial and temporal scales at which climate variability affects biological parameters is also of prime importance [9]. Also crucial for improving projections, long-term multi-decadal biological series are required to detect nonlinear effects of climate on populations [10–13]. The obtention of such long time series is however often limited by logistical challenges associated with conducting long-term studies in these remote and extreme areas.
Many Antarctic marine top predators, such as seals and seabirds, are intricately linked to landfast ice (LFI), i.e. the narrow band of coastal, compact sea ice held in place by ice shelves and grounded icebergs [14], throughout their breeding period [15–17]. Therefore, LFI variability, such as extreme extent or early break up, can profoundly impact their breeding areas and breeding success [18–20]. However, functional relationships between LFI variability and demographic parameters of polar marine predators remain poorly known owing to the scarcity of biological datasets and the difficulty to characterize LFI variability over long time periods.
To improve our understanding of how polar species will respond to future climate changes, we explored the role LFI variability and in situ meteorological conditions have on the overall breeding success, but also the fledging and hatching success of a unique sea ice sentinel species [20], the emperor penguin (Aptenodytes forsteri). We used the longest historical time series of Antarctic LFI collected by the Advanced Very High Resolution Radiometer (AVHRR) and the Moderate-Resolution Imaging Spectroradiometer (MODIS), covering the years 1979–2017, i.e. since the inception of modern satellite monitoring. We also used the world's longest time series of emperor penguin breeding parameters, collected at Pointe Géologie, Adélie Land, since 1952. The novelty of this research, while relying on previous studies (e.g. [21–26]), lies in (i) assessing the climate effect on different components of the reproduction, (ii) using the longest time series available for LFI and emperor penguin reproduction, (iii) taking into account the relative contribution of fine-scale processes (local LFI and in situ meteorological conditions), (iv) exploring different time windows of these effects, and (v) testing nonlinear effects.
2. Material and methods
(a) . Landfast ice data
Three sources of satellite imagery were used to cover the 1979–2017 period and aggregate LFI data (see electronic supplementary material, figure S1 for examples):
-
(1)
1979–1991: visible (when available) or thermal infrared images from AVHRR's Global Area Coverage (GAC) mode (spatial resolution of 4 km px−1).
-
(2)
1992–1999: visible (when available) or thermal infrared images from the AVHRR Coastal Atlas of East Antarctica [27] (resolution of 1.1 km px−1).
-
(3)
2000–2017: LFI maps from Moderate-Resolution Imaging Spectroradiometer (MODIS) images (resolution of 1 km), classified by Fraser et al. [28].
Distances between the penguin colony location and the nearest landfast ice edge (LFIE) (i.e. proxy for access to the ocean) and landfast ice areas (LFIA) were extracted from the images.
(b) . Meteorological data
Meteorological data were obtained from the French weather station of Dumont D'Urville. Three parameters were used in this study: the number of days per month with (i) temperatures under −10°C, (ii) winds above 28 m s−1, and (iii) snowfall. We hypothesized that egg loss during incubation and chick mortality could be enhanced during cold and windy conditions caused by katabatic winds and winter storms [15]. Heat loss due to cold temperatures and strong winds, which could be enhanced by snowfall, may increase chick mortality.
(c) . Reproductive data
Data are similar to those used by Barbraud and co-workers [21,29], with updated estimates (electronic supplementary material). From count data, we estimated ‘breeding success' as the number of fledged chicks divided by the number of breeding pairs; ‘hatching success' as the number of breeding pairs minus the number of dead eggs divided by the number of breeding pairs; and ‘fledging success' as the number of fledged chicks divided by the number of breeding pairs minus the number of dead eggs. Breeding success was estimated over the period 1979–2017; hatching and fledging success over the period 1983–2017.
(d) . Climate window analysis
We performed a ‘climate window analysis' using the R package climwin, following the steps described in [30]. Climate window analyses determine, without any a priori hypothesis, the best climate window(s) (i.e. candidate models) that identify potential climate signals between biological and climate data. Two datasets were analysed: one that contained our monthly climate data, i.e. LFI or meteorological data covering the 1979–2017 period, and one that contained information on the response variable, i.e. breeding, hatching and fledging success. For each climate window, a model was computed. Akaike information criteria (AICs) were used for ranking and comparing different candidate climate windows, and then for assessing the best models, their uncertainty, explanatory power and applicability. Details on the analysis and outputs of the analysis are provided in the electronic supplementary material. The full dataset and codes can be found on the Dryad Digital Repository [31].
3. Results
(a) . Reproduction time series
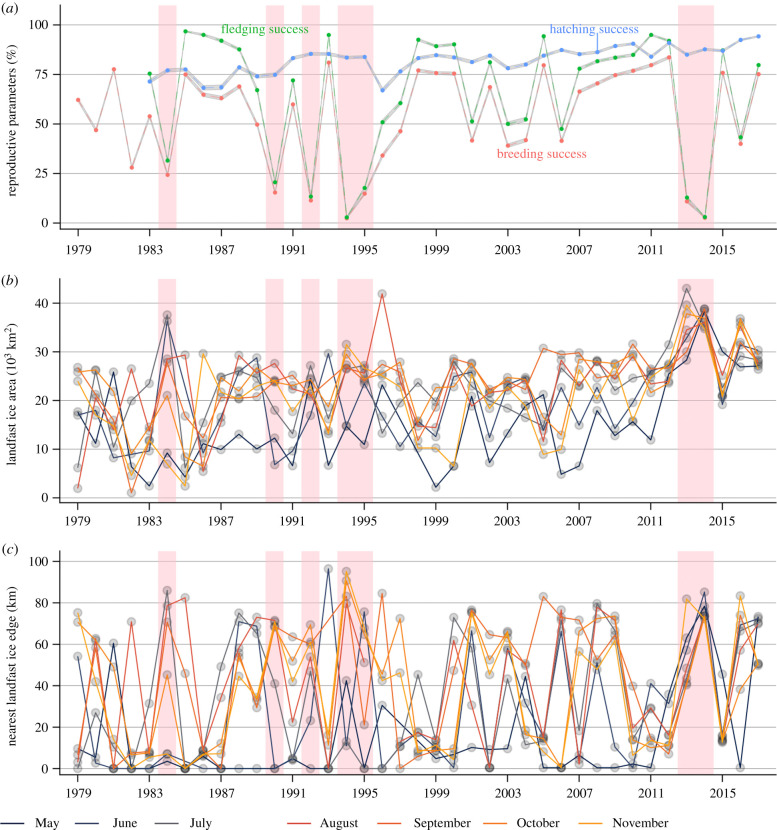
Hatching success was the most stable reproductive parameter (mean ± s.d. = 0.82 ± 0.07, CV = 8.3%), while fledging success (0.65 ± 0.30) and breeding success (0.53 ± 0.25) were more variable (CV = 46.4% and 46.5%, respectively; figure 1a). Hatching success increased during the study period (slope = 0.051 ± 0.007 (s.e.), p < 0.001), while fledging success (slope = −0.003 ± 0.052, p = 0.96) and breeding success (0.026 ± 0.040, p = 0.52) remained stable (figure 1a). Variations in both fledging and breeding successes seemed to covary with the LFIA, but even more so with the distance to the nearest LFIE (figure 1b,c).
Figure 1.
Times series of the emperor penguin reproductive parameters ((a), 95% confidence intervals in grey) and LFI conditions (LFIA (b); nearest distance to LFIE (c)) at Pointe Géologie, 1979–2017. Pink rectangles highlight years for which breeding success was below 25%.
(b) . Climate window analysis
Breeding success was higher for shorter distances to the LFIE between August and November (prandomization = 0.006; adjusted R2 = 0.4), while the LFIA did not have a significant influence (i.e. based on the randomization test; table 1 and figure 2a). The number of days per month with temperatures under −10°C, with winds above 28 m s−1, and with snowfall did not influence the breeding success (table 1). Neither the LFIA nor the number of days per month with winds above 28 m s−1 or temperature below −10°C had an influence on the hatching success (table 1). However, the hatching success appeared to be influenced by the number of days with snowfall in May (prandomization = 0.0003, adjusted R2 = 0.3; table 1 and figure 2c). This relationship was nonlinear, with hatching success increasing with the proportion of days with snowfall per month up to 37% and remaining stable or decreasing slightly for higher proportions. Finally, fledging success was higher for shorter distances to the LFIE in November (prandomization = 0.035, adjusted R2 = 0.5; table 1 and figure 2b), while the LFIA, and the number of days per month with temperature below −10°C, with winds above 28 m s−1, and with snowfall did not have a significant influence (table 1). Fledging success declined nonlinearly with the nearest distance to the LFIE, with an accelerated decline for distances greater than ca 50 km.
Table 1.
Summary of the climate window analysis. n.s., not significant.
| climate variables | biological variable | period considered | years | best climate window | p-value best modela | p-value after randomization | fit selected [alternative fit] | sign of the relation | R2 after randomization (k = 10) |
|---|---|---|---|---|---|---|---|---|---|
| nearest open water (LFIE) | breeding success | May–Nov | 1979–2017 | Aug– Nov | 1.48 × 10−5 | 0.006 | linear, AIC = −125.8826 [quadratic, AIC = −125.082] | – | 0.386 |
| hatching success | May–Aug | 1983–2017 | |||||||
| fledging success | May–Nov | 1983–2017 | Nov |
x = 0.500 x2 = 0.025 |
0.035 | quadratic, AIC = −107.642 [linear, AIC = −105.3069] |
– | 0.530 | |
| landfast ice area (136°–146°E) | breeding success | May–Nov | 1979–2017 | n.s. | 0.003 | 0.499 | n.s. | ||
| hatching success | May–Aug | 1983–2017 | n.s. | 0.007 | 0.715 | n.s. | |||
| fledging success | May–Nov | 1983–2017 | n.s. | 0.0001 | 0.266 | n.s. | |||
| no. days per month with temperatures under −10°C | breeding success | May–Nov | 1979–2017 | n.s. | n.s. | – | n.s. | ||
| hatching success | May–Aug | 1983–2017 | n.s. | n.s. | – | n.s. | |||
| fledging success | May–Nov | 1983–2017 | n.s. | n.s. | – | n.s. | |||
| no. days per month with winds above 28 m/s | breeding success | May–Nov | 1979–2017 | n.s. | n.s. | – | n.s. | ||
| hatching success | May–Aug | 1983–2017 | n.s. | n.s. | – | n.s. | |||
| fledging success | May–Nov | 1983–2017 | n.s. | n.s. | – | n.s. | |||
| no. days per month with snowfall | breeding success | May–Nov | 1979–2017 | n.s. | 0.044 | 0.926 | n.s. | ||
| hatching success | May–Aug | 1983–2017 | May |
x = 0.003 x2 = 0.017 |
0.0003 | quadratic, AIC = −198.8435 [linear, AIC = −194.5369] |
+ (bell shape) | 0.321 | |
| fledging success | May–Nov | 1983–2017 | n.s. | n.s. | – | n.s. |
aFor quadratic relationships, p-values for the linear and quadratic terms are given as x and x2, respectively.
Figure 2.
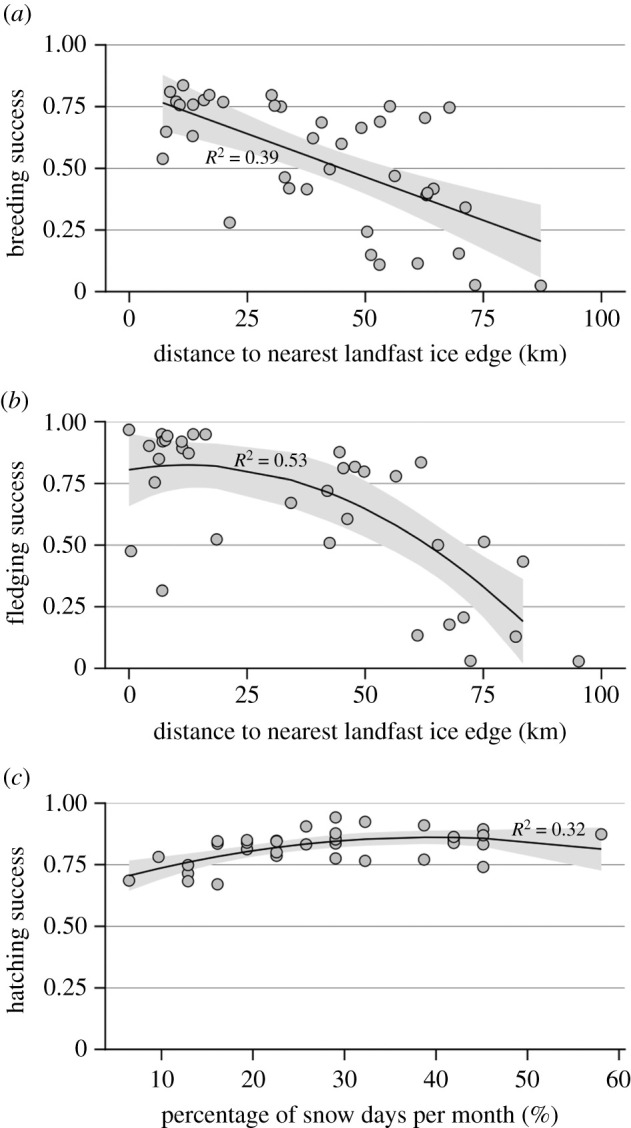
Relationships between emperor penguin reproductive parameters (breeding, fledging and hatching successes, (a–c), respectively) and climate variables from 1979 to 2017 at Pointe Géologie obtained from the climate window analysis. The best climate window was August to November for (a), November for (b) and May for (c).
4. Discussion
We showed that over 39 years, different components of the reproduction of an Antarctic seabird were affected by fine-scale LFI and in situ meteorological conditions at different times of its breeding season, and, importantly, these relationships were nonlinear.
Adult emperor penguins during the breeding season forage and hunt by diving at the edge of the LFI in cracks, flaw leads and polynyas [32]. Longer distances between the colony and foraging grounds accessed by the LFIE imply lower chick-feeding frequency, and thus lower chick growth, with negative consequences on fledging and breeding success. Using historical AVHRR and recent MODIS images, our study brings important and novel results. First, we identified that distance to nearest LFIE particularly affected fledging success in November (and the second-best model identified a window between August and November), indicating that chick mortality was the main cause of declining breeding success with increasing distance to LFIE. Second, this relationship was nonlinear, with over 50% chick mortality when the distance to LFIE exceeded ca 65 km. Nonlinearity could be detected by extending the time series from 8 years in a previous work [26] to nearly 40 years in our study. Third, we identified that the best climate window explaining the relationships between distance to LFIE (i.e. foraging grounds) and breeding success was between August and November, suggesting chicks were particularly sensitive to environmental variability during this period of high energetic demands for body growth [33,34].
Reproduction has been monitored at extremely few other emperor penguin colonies. Surprisingly, no relationship was found between LFI and breeding success of emperor penguins at Taylor Glacier colony [35]. Although this may depict the complex interactions between environment and penguin foraging behaviour and their consequences for breeding performances, Robertson et al. [35] used distance to LFIE in April and September, and our time windows analysis indicated that these months did not represent the full critical period for fledging and breeding success. Nevertheless, this highlights the need to monitor multiple sites in order to understand how sea ice variability, and especially LFI, is affecting the global emperor penguin population.
Our study supports previous findings that it is crucial to consider both fine-scale climate processes and fine-scale temporal windows when investigating the relationships between climate variability and demographic traits [9,36]. Despite the diversity of studies that have investigated the effect of climate change on polar species, there is a strong need to account for the factors that control population dynamics at local/regional scales in order to understand how they may modulate the effects of large-scale environmental variations on long-term population trend [13]. For example, Olivier et al. [37] compared the influence of environmental factors on the breeding success of snow petrels (Pagodroma nivea) at Casey station with the colony of Adélie Land, and showed that despite similarities in the biological processes controlling snow petrel breeding success, the correlation of large-scale environmental factors with breeding success differed substantially between the two colonies, likely owing to the effects of the environmental factors at the local/regional scale.
LFI variability may have important indirect effects that we did not consider in this study. For example, LFI break-ups could contribute to the phytoplankton seeding process (e.g. [38–40]) and may drive a phytoplankton bloom associated with trophic cascades. This could in turn benefit emperor penguins through bottom-up processes with a temporal lag depending on the timing within the breeding period. In the Arctic, longer temporal lags between sea ice melting and phytoplankton bloom resulted in rapidly decreasing breeding performance for little auks (Alle alle) and Brünnich's guillemots (Uria lomvia) [41]. Thus, considering local- to regional-scale phenology in the development of potential phytoplankton blooms in response to LFI variability may help understand climate-driven environmental impacts on seabirds.
During breeding, individual emperor penguins do not use a fixed nest site as do other penguins. Therefore, the colony is mobile during the breeding season and can move several hundred metres or even a few kilometres. Therefore, the selection of nest site (and experience to nest site) is not relevant for this species. However, there might be selection for sites where colonies are situated, as these sites are generally occupied for long time periods (several decades at least), as our results suggest a strong selection pressure from environmental factors such as LFIE. Nevertheless, the environmental factors affecting colony site selection have not been investigated and quantified to date.
Finally, none of the meteorological variables, except snow-falls for the hatching success, had an influence on reproductive parameters. The positive relationship between the number of days with snowfall in May and the hatching success may be associated with the hydration of males during their long fasting period of about four months. We speculate that important snowfalls in May allow males to supplement their water intake by eating snow, thus decreasing dehydration potentially leading to the abandonment of the egg before it hatches. Indeed, field observations during winter indicate that male emperor penguins eat snow throughout the incubation period ([42]; C.B. 2021, personal observation).
Our results bring new insights on the proximate mechanisms through which a poorly known polar habitat feature, LFI, affects demographic parameters of polar top predators. We note that, although we might be able to better predict the future state of polar populations once such fine-scale processes are fully understood, population projections based on sea ice models (e.g. [43]) remain hampered by the fact that these models project sea ice extent but do not provide information on LFI dynamics yet. Important future repercussions on the breeding habitat of emperor penguins and ultimately their persistence are to be expected in the context of climate change [2], given the sensitivity of LFI to storms and changes in wind direction [44], as well as the recently observed strong and opposed LFI trends in adjacent regions [45]. Given the demographic sensitivity of emperor penguins associated with postglacial warming leading to a major southward expansion [46], major shifts such as decline or extinction of emperor penguin populations are expected under anthropogenic climate change.
Acknowledgements
We warmly thank all the wintering fieldworkers for conducting counts and monitoring at Pointe Géologie since 1979, R. Massom for all the initial discussions, D. Iles for methodological advice and M. LaRue for very rich emperor penguin discussions.
Ethics
All animals in this study were treated in accordance with the project number IPEV 109 and the IPEV Ethics Committee.
Data accessibility
Data and codes supporting the paper are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.cnp5hqc47 [31].
Authors' contributions
S.L., S.J. and C.B.: study design. A.D.F., C.S., K.D. and C.B.: data acquisition. A.D.F., M.S., F.L.M., I.H., E.D., S.J. and S.L.: data analysis and processing. S.L., C.B. and F.L.M.: writing. S.L., C.B. and S.J.: interpretation of the results. All authors edited and revised the manuscript. All authors approved the final version of the manuscript and agree to be held accountable for the content therein.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by ExPé and IPEV program 109, WHOI's postdoctoral scholar award, NSF awards 1744794 and 1643901, the AAD, the Australian Government's Antarctic Science Collaboration Initiative Program and the Australian Research Council's Special Research Initiative for Antarctic Gateway Partnership (Project ID SR140300001). This study is a contribution to the Project SENSEI funded by Foundation BNP Paribas.
References
- 1.Parkinson CL, Cavalieri DJ. 2012. Antarctic sea ice variability and trends, 1979–2010. Cryosphere 6, 871-880. ( 10.5194/tc-6-871-2012) [DOI] [Google Scholar]
- 2.Meredith M, et al. 2019. Polar regions. In IPCC Special Report on the Ocean and Cryosphere in a Changing Climate (eds H-O Pörtner et al.), ch. 3. IPCC. See https://www.ipcc.ch/srocc/.
- 3.Post E, et al. 2009. Ecological dynamics across the Arctic associated with recent climate change. Science 325, 1355-1358. ( 10.1126/science.1173113) [DOI] [PubMed] [Google Scholar]
- 4.Massom RA, Stammerjohn SE. 2010. Antarctic sea ice change and variability—physical and ecological implications. Polar Sci. 4, 149-186. ( 10.1016/j.polar.2010.05.001) [DOI] [Google Scholar]
- 5.Constable AJ, et al. 2014. Climate change and Southern Ocean ecosystems I: how changes in physical habitats directly affect marine biota. Glob. Change Biol. 20, 3004-3025. ( 10.1111/gcb.12623) [DOI] [PubMed] [Google Scholar]
- 6.Bestley S, et al. 2020. Marine ecosystem assessment for the Southern Ocean: birds and marine mammals in a changing climate. Front. Ecol. Evol. 8, 338. ( 10.3389/fevo.2020.566936) [DOI] [Google Scholar]
- 7.Pardo D, Jenouvrier S, Weimerskirch H, Barbraud C. 2017. Effect of extreme sea surface temperature events on the demography of an age-structured albatross population. Phil. Trans. R. Soc. B 372, 20160143. ( 10.1098/rstb.2016.0143) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pacoureau N, Delord K, Jenouvrier S, Barbraud C. 2019. Demographic and population responses of an apex predator to climate and its prey: a long-term study of South Polar skuas. Ecol. Monogr. 89, e01388. ( 10.1002/ecm.1388) [DOI] [Google Scholar]
- 9.Van De Pol M, Cockburn A. 2011. Identifying the critical climatic time window that affects trait expression. Am. Nat. 177, 698-707. ( 10.1086/659101) [DOI] [PubMed] [Google Scholar]
- 10.Bjørnstad ON, Grenfell BT. 2001. Noisy clockwork: time series analysis of population fluctuations in animals. Science 293, 638-643. ( 10.1126/science.1062226) [DOI] [PubMed] [Google Scholar]
- 11.Mysterud A, Stenseth NC, Yoccoz NG, Langvatn R, Steinheim G. 2001. Nonlinear effects of large-scale climatic variability on wild and domestic herbivores. Nature 410, 1096-1099. ( 10.1038/35074099) [DOI] [PubMed] [Google Scholar]
- 12.Clark TJ. 2020. Nonlinear population dynamics are ubiquitous in animals. Nat. Ecol. Evol. 4, 75-81. ( 10.1038/s41559-019-1052-6) [DOI] [PubMed] [Google Scholar]
- 13.Iles DT, Lynch H, Ji R, Barbraud C, Delord K, Jenouvrier S. 2020. Sea ice predicts long-term trends in Adélie penguin population growth, but not annual fluctuations: results from a range-wide multiscale analysis. Glob. Change Biol. 26, 3788-3798. ( 10.1111/gcb.15085) [DOI] [PubMed] [Google Scholar]
- 14.Fraser AD, Massom RA, Michael KJ, Galton-Fenzi BK, Lieser JL. 2012. East Antarctic landfast sea ice distribution and variability, 2000–08. J. Clim. 25, 1137-1156. ( 10.1175/JCLI-D-10-05032.1) [DOI] [Google Scholar]
- 15.Jouventin P. 1975. Mortality parameters in emperor penguins Aptenodytes forsteri. In The biology of penguins (ed. Stonehouse B), pp. 435-446. London, UK: Macmillan. ( 10.1007/978-1-349-02270-0_19) [DOI] [Google Scholar]
- 16.Ainley D. 2002. The Adélie penguin: bellwether of climate change. New York, NY: Columbia University Press. [Google Scholar]
- 17.Larue MA, et al. 2019. Physical and ecological factors explain the distribution of Ross Sea Weddell seals during the breeding season. Mar. Ecol. Prog. Ser. 612, 193-208. ( 10.3354/meps12877) [DOI] [Google Scholar]
- 18.Barbraud C, Delord K, Weimerskirch H. 2015. Extreme ecological response of a seabird community to unprecedented sea ice cover. R. Soc. Open Sci. 2, 140456. ( 10.1098/rsos.140456) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fretwell PT, Trathan PN. 2019. Emperors on thin ice: three years of breeding failure at Halley Bay. Antarct. Sci. 31, 133-138. ( 10.1017/S0954102019000099) [DOI] [Google Scholar]
- 20.Boersma PD. 2008. Penguins as marine sentinels. BioScience 58, 597-607. ( 10.1641/B580707) [DOI] [Google Scholar]
- 21.Barbraud C, Weimerskirch H. 2001. Emperor penguins and climate change. Nature 411, 183-186. ( 10.1038/35075554) [DOI] [PubMed] [Google Scholar]
- 22.Jenouvrier S, Barbraud C, Weimerskirch H. 2005. Long-term contrasted responses to climate of two Antarctic seabirds species. Ecology 86, 2889-2903. ( 10.1890/05-0514) [DOI] [Google Scholar]
- 23.Jenouvrier S, Weimerskirch H, Barbraud C, Park Y-H, Cazelles B. 2005. Evidence of a shift in the cyclicity of Antarctic seabird dynamics linked to climate. Proc. R. Soc. B 272, 887-895. ( 10.1098/rspb.2004.2978) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jenouvrier S, Caswell H, Barbraud C, Holland M, Strø EJ, Weimerskirch H. 2009. Demographic models and IPCC climate projections predict the decline of an emperor penguin population. Proc. Natl Acad. Sci. USA 106, 1844-1847. ( 10.1073/pnas.0806638106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jenouvrier S, Holland M, Stroeve J, Barbraud C, Weimerskirch H, Serreze M, Caswell H. 2012. Effects of climate change on an emperor penguin population: analysis of coupled demographic and climate models. Glob. Change Biol. 18, 2756-2770. ( 10.1111/j.1365-2486.2012.02744.x) [DOI] [PubMed] [Google Scholar]
- 26.Massom R, Hill K, Barbraud C, Adams N, Ancel A, Emmerson L, Pook M. 2009. Fast ice distribution in Adélie Land, East Antarctica: interannual variability and implications for emperor penguins Aptenodytes forsteri. Mar. Ecol. Prog. Ser. 374, 243-257. ( 10.3354/meps07734) [DOI] [Google Scholar]
- 27.Michael KJ, Hill K. 2003. Sea ice atlas: East Antarctica: AVHRR imagery 1992–1999. Hobart, Tasmania: Antarctic CRC.
- 28.Fraser AD, Massom RA, Ohshima KI, Willmes S, Kappes PJ, Cartwright J, Porter-Smith R. 2020. High-resolution mapping of circum-Antarctic landfast sea ice distribution, 2000–2018. Earth Syst. Sci. Data 12, 2987-2999. ( 10.5194/essd-12-2987-2020) [DOI] [Google Scholar]
- 29.Barbraud C, Gavrilo M, Mizin Y, Weimerskirch H. 2011. Comparison of emperor penguin declines between Pointe Géologie and Haswell Island over the past 50 years. Antarct. Sci. 23, 461-468. ( 10.1017/S0954102011000356) [DOI] [Google Scholar]
- 30.Bailey LD, Van De Pol M. 2016. climwin: An R toolbox for climate window analysis. PLoS ONE 11, e0167980. ( 10.1371/journal.pone.0167980) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Labrousse S, et al. 2021. Data from: Landfast ice: a major driver of reproductive success in a polar seabird. Dryad Digital Repository. ( 10.5061/dryad.cnp5hqc47) [DOI] [PMC free article] [PubMed]
- 32.Labrousse S, et al. 2019. Dynamic fine-scale sea icescape shapes adult emperor penguin foraging habitat in East Antarctica. Geophys. Res. Lett. 46, 11 206-11 218. ( 10.1029/2019GL084347) [DOI] [Google Scholar]
- 33.Prevost J. 1961. Ecologie du manchot empereur. In Expéditions polaires françaises, vol. 222, pp. 1-204. Paris, France: Hermann Press. [In French.] [Google Scholar]
- 34.Mougin J-L. 1966. Observations écologiques à la colonie de manchots empereurs de Pointe Géologie (Terre Adélie) en 1964. Oiseau Rev. Française Ornithol. 36, 167-226. [In French.] [Google Scholar]
- 35.Robertson G, Wienecke B, Emmerson L, Fraser AD. 2014. Long-term trends in the population size and breeding success of emperor penguins at the Taylor Glacier Colony, Antarctica. Polar Biol. 37, 251-259. ( 10.1007/s00300-013-1428-z) [DOI] [Google Scholar]
- 36.Barreau E, Ropert-Coudert Y, Delord K, Barbraud C, Kato-Ropert A. 2019. Scale matters: sea ice and breeding success of Adélie penguins. Polar Biol. 42, 1405-1410. ( 10.1007/s00300-019-02531-2) [DOI] [Google Scholar]
- 37.Olivier F, Franeker JA, Creuwels JCS, Woehler EJ. 2005. Variations of snow petrel breeding success in relation to sea-ice extent: detecting local response to large-scale processes? Polar Biol. 28, 687-699. ( 10.1007/s00300-005-0734-5) [DOI] [Google Scholar]
- 38.McMinn A. 1996. Preliminary investigation of the contribution of fast-ice algae to the spring phytoplankton bloom in Ellis Fjord, eastern Antarctica. Polar Biol. 16, 301-307. ( 10.1007/s003000050057) [DOI] [Google Scholar]
- 39.Riaux-Gobin C, Poulin M, Dieckmann G, Labrune C, Vétion G. 2011. Spring phytoplankton onset after the ice break-up and sea-ice signature (Adélie Land, East Antarctica). Polar Res. 30, 5910. ( 10.3402/polar.v30i0.5910) [DOI] [Google Scholar]
- 40.Mangoni O, Saggiomo M, Modigh M, Catalano G, Zingone A, Saggiomo V. 2009. The role of platelet ice microalgae in seeding phytoplankton blooms in Terra Nova Bay (Ross Sea, Antarctica): a mesocosm experiment. Polar Biol. 32, 311-323. ( 10.1007/s00300-008-0507-z) [DOI] [Google Scholar]
- 41.Ramírez F, Tarroux A, Hovinen J, Navarro J, Afán I, Forero MG, Descamps S. 2017. Sea ice phenology and primary productivity pulses shape breeding success in Arctic seabirds. Scient. Rep. 7, 4500. ( 10.1038/s41598-017-04775-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Le Maho Y. 1977. The emperor penguin: a strategy to live and breed in the cold: morphology, physiology, ecology, and behavior distinguish the polar emperor penguin from other penguin species, particularly from its close relative, the king penguin. Am. Sci. 65, 680-693. [Google Scholar]
- 43.Jenouvrier S, et al. 2019. The Paris Agreement objectives will likely halt future declines of emperor penguins. Glob. Change Biol. 26, 1170-1184. ( 10.1111/gcb.14864) [DOI] [PubMed] [Google Scholar]
- 44.Zhai MX, Zhao TC, Hui FM. 2019. Anomalous extensive landfast sea ice in the vicinity of Inexpressible Island, Antarctica. Adv. Polar Sci. 30, 406-411. ( 10.13679/j.advps.2018.0044) [DOI] [Google Scholar]
- 45.Fraser AD, et al. 2021. 18 year record of circum-Antarctic landfast sea ice distribution allows detailed baseline characterisation, reveals trends and variability. Cryosphere Discuss. ( 10.5194/tc-2021-121) [DOI] [Google Scholar]
- 46.Cole TL, et al. 2019. Receding ice drove parallel expansions in Southern Ocean penguins. Proc. Natl Acad. Sci. USA 116, 26690. ( 10.1073/pnas.1904048116) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and codes supporting the paper are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.cnp5hqc47 [31].