Abstract
Background
The 2016–2017 and 2017–2018 influenza seasons were notable for the high number of hospitalizations for influenza A(H3N2) despite vaccine and circulating strain match.
Methods
We evaluated vaccine effectiveness (VE) against hospitalization in the test-negative HAIVEN study. Nasal-throat swabs were tested by quantitative reverse transcription polymerase chain reaction (RT-PCR) for influenza and VE was determined based on odds of vaccination by generalized estimating equations. Vaccine-specific antibody was measured in a subset of enrollees.
Results
A total of 6129 adults were enrolled from 10 hospitals. Adjusted VE against A(H3N2) was 22.8% (95% confidence interval [CI], 8.3% to 35.0%), pooled across both years and 49.4% (95% CI, 34.3% to 61.1%) against B/Yamagata. In 2017–2018, the A(H3N2) VE point estimate for the cell-based vaccine was 43.0% (95% CI, −36.3% to 76.1%; 56 vaccine recipients) compared to 24.0% (95% CI, 3.9% to 39.9%) for egg-based vaccines. Among 643 with serology data, hemagglutinin antibodies against the egg-based A(H3N2) vaccine strain were increased in influenza-negative individuals.
Conclusions
Low VE for the A/Hong Kong/4801/2014 vaccine virus in both A(H3N2) seasons emphasizes concerns for continued changes in H3N2 antigenic epitopes, including changes that may impact glycosylation and ultimately reduce VE.
Keywords: adults, case-control study, hospitalization, influenza, vaccine, vaccine effectiveness
Across 2 years of a prospective, multicenter, test-negative study of influenza vaccine effectiveness in prevention of hospitalization, we found VE of 33.5% against any influenza-associated hospitalization and a low VE of 22.8% for the A/Hong Kong/4801/2014 vaccine strain.
The 2016–2017 and 2017–2018 US influenza seasons were characterized by use of the A/Hong Kong/4801/2014 (H3N2)-like vaccine virus coupled with a 2-year predominance of A(H3N2) viruses in the northern hemisphere. Additionally, 2017–2018 was notable for being the most severe influenza season since the 2009 A(H1N1) pandemic. During the US 2017–2018 season, it is estimated that 45 million people were infected, the highest since the 2009 pandemic, and 61 000 people died [1].
In both seasons, vaccine effectiveness (VE) was low against medically attended acute respiratory illness [1,2]. Factors that potentially reduce VE against influenza have included prior vaccination [3–5] and intraseason waning [6–8]. Specific to the 2016–2017 and 2017–2018 season, several studies have noted the presence of an egg-adaptation mutation in the egg-produced vaccine virus that may have precluded the ability of the vaccine to elicit a protective response against circulating viruses [9–11]. However, the degree to which this adaptation influenced observed effectiveness estimates remains unclear. These evaluations require the use of large surveillance systems with access to documented vaccination product information.
Our objective was to determine the effectiveness of influenza vaccine against hospitalization with laboratory-confirmed influenza during the 2016–2017 and 2017–2018 seasons using a test-negative study in a 10-hospital network called the US Hospital Adult Influenza Vaccine Effectiveness Network (HAIVEN) for yearly VE surveillance. The test-negative design compares influenza-positive patients to those negative using sensitive quantitative reverse transcription polymerase chain reaction (RT-PCR) testing [12]. Secondary analysis objectives included exploratory analyses by vaccine product and by prior vaccination.
METHODS
HAIVEN consists of 4 sites located in Texas (2 hospitals), Tennessee (3 hospitals), Pennsylvania (3 hospitals), and Michigan (2 hospitals). The methods have been described elsewhere [13]. Patients age ≥ 18 years hospitalized in the 2016–2017 and 2017–2018 seasons were selected for participation based on a standardized case definition indicating acute respiratory illness.
Upon obtaining informed consent from the participant, an enrollment interview collected data on demographics, health history, and vaccination. Throat and nasal swab specimens were collected for laboratory confirmation of influenza and respiratory syncytial virus (RSV) by RT-PCR using standard Centers for Disease Control and Prevention assays or multiplex PCR-based assays for clinical care.
Vaccination Status
Participants were considered vaccinated if they had documented or plausible self-reported vaccination (with inclusion of location of vaccine receipt) administered ≥ 14 days prior to illness onset [13]. Information was also collected regarding vaccination in the prior season using documented and self-reported data following similar methods to current-year vaccination.
Statistical Analyses
Patient characteristics were compared between influenza-positive and -negative individuals and vaccinated and unvaccinated individuals using a Pearson χ 2 test. Study data was analyzed in a test-negative design [12] with VE calculated as (1 − the adjusted odds ratio of the association between vaccination and infection) × 100%. Odds ratios were determined using generalized estimating equations models with a logit link and exchangeable correlation structure to account for individuals who were enrolled more than once (n = 60 individuals for 2016–2017; n = 113 individuals for 2017–2018). An independent correlation structure was used in subgroup analyses with small cluster and sample size (vaccination habit, prior vaccination, and study site-specific models). Adjusted models for overall, strain-specific, and product-type–specific estimates included terms for enrollment hospital, age (in groups of 18–49, 50–64, 65–74, ≥75 years), race, tertiles of calendar time from site-specific and influenza-type specific peaks, days from illness onset to specimen collection (categorized as 0–1, 2–4, 5–10 days), self-reported health (categorized as poor/fair vs good/very good/excellent) and self-reported hospitalizations in the year prior to enrollment. Logistic regression was used for age-group–specific models, which included these same covariates except for categorical age in the adjusted models and differences between age-specific strata were evaluated by an interaction term in the adjusted model. For analyses specific to vaccine product type, VE was evaluated among all enrollees with available documentation of vaccination (excluding those with self-reported data only), including documentation of product received. VE against RSV was also calculated as a negative control outcome, given that influenza vaccine is not expected to impact RSV [14–16]. This analysis compared RSV-positive to RSV-negative enrollees, restricting participants to those enrolled during times of RSV circulation and including the same adjustment factors as in the main influenza VE model.
For strain-specific analyses of VE against hospitalization, participants infected with other influenza strains were excluded, as were participants enrolled outside of periods of circulation of the strain under analysis. Stratified, adjusted models, using model variables above when applicable, were created by participant subgroup (Supplementary Figure 1). Two secondary analyses were a priori selected based on prior findings from HAIVEN and other literature: a comparison of product-specific VE [17–21] and an evaluation of VE by prior vaccination (ie, separately for individuals with single and 2-year vaccination compared to the unvaccinated group) [3, 4, 22]. A sensitivity analysis was performed for timing of sample collection (within 4 days versus more than 4 days) based on effects seen in prior studies [23–26].
Serology
Sera from clinical blood specimens taken during hospital admission were collected and tested from both seasons by the University of Michigan site’s laboratory for characterization of preinfection antibody levels. We have previously demonstrated that similar specimens from HAIVEN participants in previous seasons were collected soon enough after symptom onset such that antibodies to the infecting strain were not increased [27]. Specimens from both years were tested by hemagglutination inhibition (HAI) assay for antibodies against egg-based vaccine antigens, including ether-split egg-based vaccine antigen (Sanofi Pasteur) representing the A/Hong Kong (H3N2) vaccine strain. Specimens from 2017–2018 were tested by HAI against ether-split vaccine antigen (Sanofi Pasteur) representing the A/Singapore (H3N2) vaccine for 2018–2019 to evaluate the presence of cross-reactive antibodies to the subsequent year’s vaccine. Antineuraminidase antibody was measured using methods as previously described [9] by neuraminidase inhibition assay (NAI). NAI targets included the representative neuraminidases from A/Hong Kong (H3N2) (both study years) and A/Singapore (H3N2) (2017–2018 only). Logistic regression models were used to determine the independent reduction in odds of infection associated with HAI or NAI holding the other constant.
RESULTS
A total of 7398 subjects were enrolled (3290 in 2016–2017 and 4108 in 2017–2018), and 1269 were excluded (Supplementary Table 1) for a final analysis sample of 6129. Overall, 21.1% (n = 1291) of participants were 75 years of age or older and 22.6% (n = 1388) of participants were 65 to 74 years of age (Table 1). Age distribution varied by site and season (Supplementary Table 2A–D). The most commonly reported race and ethnicity was white, non-Hispanic (n = 3979, 65.0%) and race varied by site. High-risk conditions were present in 98% (n = 2554) of participants in the first analysis year and 95.3% (n = 3359) in the second year (Supplementary Table 3). Half of participants (n = 3065) self-reported poor or fair health, 2809 enrollees (45.8%) had a Charlson Comorbidity Index greater than 3, and 2364 enrollees (39.2%) self-reported 3 or more markers of frailty. Overall, 3790 (61.8%) participants reported being hospitalized at least once in the past year. Over one-fifth of participants (n = 1343; 21.9%) reported being hospitalized 4 or more times during the year prior to study enrollment. Vaccination uptake at the 4 sites ranged from 70.2% (468 of 667, Michigan site) to 74.4% (656 of 882, Pennsylvania site) in the first year of analysis and was lower in the second year, ranging from 64.5% (747 of 1158, Texas site) to 69.4% (409 of 589, Tennessee site) (Supplementary Table 3).
Table 1.
Overall Hospitalized Adult Influenza Vaccine Effectiveness Network Study Population, Combined Years (N = 6129)a
| Characteristic | Overall | Influenza Positive | Influenza Negative | P Value | Vaccinated | Not Vaccinated | P Value | Received Cell-Based Vaccine | |
|---|---|---|---|---|---|---|---|---|---|
| 2016–2017 | 2017–2018 | ||||||||
| Season | |||||||||
| 2016–2017 | 2605 (42.5) | 463 (17.8) | 2142 (82.2) | <.001 | 1886 (72.4) | 719 (27.6) | <.001 | 9 (0.5) | -- |
| 2017–2018 | 3524 (57.5) | 910 (25.8) | 2614 (74.2) | 2350 (66.7) | 1174 (33.3) | -- | 66 (2.8) | ||
| Study site | |||||||||
| Michigan | 1610 (26.3) | 412 (25.6) | 1198 (74.4) | .002 | 1089 (67.6) | 521 (32.4) | .002 | 2 (0.4) | 31 (5.0) |
| Pennsylvania | 1716 (28.0) | 385 (22.4) | 1331 (77.6) | 1229 (71.6) | 487 (28.4) | 1 (0.2) | 12 (2.1) | ||
| Tennessee | 1194 (19.5) | 246 (20.6) | 948 (79.4) | 851 (71.3) | 343 (28.7) | 6 (1.4) | 15 (3.7) | ||
| Texas | 1609 (26.3) | 330 (20.5) | 1279 (79.5) | 1067 (66.3) | 542 (33.7) | 0 (0.0) | 8 (1.1) | ||
| Sex | |||||||||
| Female | 3467 (56.6) | 777 (22.4) | 2690 (77.6) | .984 | 2406 (69.4) | 1061 (30.6) | .584 | 6 (0.6) | 34 (2.5) |
| Male | 2662 (43.4) | 596 (22.4) | 2066 (77.6) | 1830 (68.7) | 832 (31.3) | 3 (0.4) | 32 (3.2) | ||
| Age group, y | |||||||||
| 18–49 | 1376 (22.5) | 274 (19.9) | 1102 (80.1) | <.001 | 759 (55.2) | 617 (44.8) | <.001 | 2 (0.6) | 15 (3.7) |
| 50–64 | 2074 (33.8) | 415 (20.0) | 1659 (80.0) | 1323 (63.8) | 751 (36.2) | 5 (0.8) | 33 (4.7) | ||
| 65–74 | 1388 (22.6) | 317 (22.8) | 1071 (77.2) | 1043 (75.1) | 345 (24.9) | 0 (0.0) | 6 (1.0) | ||
| 75+ | 1291 (21.1) | 367 (28.4) | 924 (71.6) | 1111 (86.1) | 180 (13.9) | 2 (0.4) | 12 (1.8) | ||
| Race and ethnicity | |||||||||
| White, non-Hispanic | 3979 (65.0) | 858 (21.6) | 3121 (78.4) | .21 | 2908 (73.1) | 1071 (26.9) | <.001 | 5 (0.4) | 39 (2.5) |
| Black, non-Hispanic | 1723 (28.2) | 411 (23.9) | 1312 (76.1) | 1061 (61.6) | 662 (38.4) | 3 (0.7) | 25 (3.9) | ||
| Other race, non-Hispanic | 183 (3.0) | 43 (23.5) | 140 (76.5) | 117 (63.9) | 66 (36.1) | 0 (0.0) | 1 (1.6) | ||
| Hispanic, any race | 235 (3.8) | 58 (24.7) | 177 (75.3) | 147 (62.6) | 88 (37.4) | 1 (2.0) | 1 (1.0) | ||
| Vaccinated in prior season | |||||||||
| Yes | 4614 (76.2) | 956 (20.7) | 3658 (79.3) | <.001 | 3921 (85.0) | 693 (15.0) | <.001 | 8 (0.5) | 58 (2.7) |
| No | 1438 (23.8) | 395 (27.5) | 1043 (72.5) | 275 (19.1) | 1163 (80.9) | 1 (0.8) | 6 (4.1) | ||
| High risk in past year | |||||||||
| Yes | 5913 (96.5) | 1304 (22.1) | 4609 (77.9) | <.001 | 4130 (69.8) | 1783 (30.2) | <.001 | 7 (0.4) | 59 (2.6) |
| No | 216 (3.5) | 69 (31.9) | 147 (68.1) | 106 (49.1) | 110 (50.9) | 2 (8.7) | 7 (8.4) | ||
| Self-reported health | |||||||||
| Poor | 961 (15.7) | 155 (16.1) | 806 (83.9) | <.001 | 679 (70.7) | 282 (29.3) | <.001 | 3 (1.0) | 8 (2.1) |
| Fair | 2104 (34.3) | 442 (21.0) | 1662 (79.0) | 1529 (72.7) | 575 (27.3) | 2 (0.3) | 24 (2.9) | ||
| Good | 1918 (31.3) | 480 (25.0) | 1438 (75.0) | 1322 (68.9) | 596 (31.1) | 2 (0.4) | 23 (3.0) | ||
| Very good | 828 (13.5) | 211 (25.5) | 617 (74.5) | 518 (62.6) | 310 (37.4) | 2 (0.9) | 8 (2.8) | ||
| Excellent | 318 (5.2) | 85 (26.7) | 233 (73.3) | 188 (59.1) | 130 (40.9) | 0 (0.0) | 3 (3.3) | ||
| Charlson comorbidity index | |||||||||
| 0 | 504 (8.2) | 159 (31.5) | 345 (68.5) | <.001 | 272 (54.0) | 232 (46.0) | <.001 | 1 (0.9) | 10 (6.3) |
| 1 | 1080 (17.6) | 264 (24.4) | 816 (75.6) | 602 (55.7) | 478 (44.3) | 0 (0.0) | 8 (2.5) | ||
| 2–3 | 1736 (28.3) | 382 (22.0) | 1354 (78.0) | 1194 (68.8) | 542 (31.2) | 2 (0.4) | 22 (3.3) | ||
| 4+ | 2809 (45.8) | 568 (20.2) | 2241 (79.8) | 2168 (77.2) | 641 (22.8) | 6 (0.6) | 26 (2.1) | ||
| Frailty level | |||||||||
| 0 | 1096 (18.2) | 313 (28.6) | 783 (71.4) | <.001 | 696 (63.5) | 400 (36.5) | <.001 | 1 (0.3) | 13 (3.4) |
| 1 | 1323 (21.9) | 294 (22.2) | 1029 (77.8) | 886 (67.0) | 437 (33.0) | 2 (0.5) | 17 (3.5) | ||
| 2 | 1253 (20.8) | 263 (21.0) | 990 (79.0) | 873 (69.7) | 380 (30.3) | 3 (0.8) | 14 (2.8) | ||
| 3 | 1086 (18.0) | 240 (22.1) | 846 (77.9) | 771 (71.0) | 315 (29.0) | 1 (0.3) | 10 (2.3) | ||
| 4 | 912 (15.1) | 178 (19.5) | 734 (80.5) | 681 (74.7) | 231 (25.3) | 2 (0.7) | 7 (1.9) | ||
| 5 | 366 (6.1) | 62 (16.9) | 304 (83.1) | 266 (72.7) | 100 (27.3) | 0 (0.0) | 4 (3.0) | ||
| Days from onset to specimen collection | |||||||||
| 0–2 | 2253 (36.8) | 533 (23.7) | 1720 (76.3) | <.001 | 1599 (71.0) | 654 (29.0) | .081 | 3 (0.4) | 20 (2.3) |
| 3–4 | 1734 (28.3) | 425 (24.5) | 1309 (75.5) | 1175 (67.8) | 559 (32.2) | 4 (0.8) | 16 (2.3) | ||
| 5–7 | 1481 (24.2) | 306 (20.7) | 1175 (79.3) | 1020 (68.9) | 461 (31.1) | 2 (0.5) | 19 (3.3) | ||
| 8–10 | 661 (10.8) | 109 (16.5) | 552 (83.5) | 442 (66.9) | 219 (33.1) | 0 (0.0) | 11 (5.2) | ||
| Self-reported number of hospitalizations in past year | |||||||||
| 0 | 2339 (38.2) | 679 (29.0) | 1660 (71.0) | <.001 | 1469 (62.8) | 870 (37.2) | <.001 | 3 (0.5) | 21 (2.5) |
| 1 | 1153 (18.8) | 264 (22.9) | 889 (77.1) | 790 (68.5) | 363 (31.5) | 2 (0.5) | 15 (3.6) | ||
| 2 | 772 (12.6) | 151 (19.6) | 621 (80.4) | 550 (71.2) | 222 (28.8) | 1 (0.4) | 5 (1.7) | ||
| 3 | 522 (8.5) | 105 (20.1) | 417 (79.9) | 395 (75.7) | 127 (24.3) | 1 (0.6) | 7 (3.1) | ||
| 4+ | 1343 (21.9) | 174 (13.0) | 1169 (87.0) | 1032 (76.8) | 311 (23.2) | 2 (0.4) | 18 (3.2) |
Data are n (%). Row percents are displayed for all columns except the Overall.
aSixty participants had 2–4 enrollments (giving a total of 69 additional enrollments) in 2016–2017; 113 participants had 2–6 enrollments (giving a total of 134 additional enrollments) in 2017–2018.
Influenza Infection
Influenza A(H3N2) strains from the vaccine-like clade 3c.2a predominated nationally during both the 2016–2017 and 2017–2018 seasons [28, 29]. Influenza was identified in 17.8% (463 of 2605) of participants during the 2016–2017 season but in 25.8% (910 of 3524) of participants during the 2017–2018 season. Percent of participants with influenza ranged from 13.7% (62 out of 451, Texas site) to 21.1% (141 out of 667, Michigan site) in the 2016–2017 season and ranged from 23.1% (268 out of 1158, Texas site) to 28.7% (271 out of 943, Michigan site) in the 2017–2018 season (Supplementary Table 3). Influenza detection increased with age in both years. The majority of influenza detections (61.1%) were influenza A(H3N2) (839 of 1373 total detections) followed by 21.6% influenza B/Yamagata (n = 296) (Table 2).
Table 2.
Overall and Strain-Specific, Vaccine Effectiveness Against Hospitalization With Laboratory-Confirmed Influenza, Hospitalized Adult Influenza Vaccine Effectiveness Network 2016–2018 (N = 6129)
| Outcome | Year | Vaccinateda Positives, n/Total (%) | Unvaccinated Positives, n/Total (%) | Unadjusted VE (95% CI) | Adjustedb VE (95% CI) |
|---|---|---|---|---|---|
| Influenza, all | 2016–2017 | 302/1886 (16.0) | 161/719 (22.4) | 33.4 (17.5 to 46.3) | 30.6 (12.4 to 45.0) |
| 2017–2018 | 557/2350 (23.7) | 353/1174 (30.1) | 26.6 (14.2 to 37.3) | 34.2 (21.9 to 44.6) | |
| 2016–2018 (2 y) | 859/4236 (20.3) | 514/1893 (27.2) | 31.0 (21.7 to 39.2) | 33.5 (23.6 to 42.0) | |
| A/H3N2 | 2016–2017 | 214/1658 (12.9) | 95/596 (15.9) | 21.4 (−2.2 to 39.5) | 18.0 (−9.2 to 38.3) |
| 2017–2018 | 343/2044 (16.8) | 187/970 (19.3) | 14.9 (−3.7 to 30.1) | 23.8 (5.3 to 38.7) | |
| 2016–2018 (2 y) | 557/3702 (15.0) | 282/1566 (18.0) | 18.8 (5.0 to 30.6) | 22.8 (8.3 to 35.0) | |
| A/H1N1 pdm09 | 2016–2017 | 7/391 (1.8) | 4/150 (2.7) | … | … |
| 2017–2018 | 48/1567 (3.1) | 56/729 (7.7) | 61.92 (43.4 to 74.4) | 56.1 (33.1 to 71.2)c | |
| 2016–2018 (2 y) | 55/1958 (2.8) | 60/879 (6.8) | 60.5 (42.5 to 72.8) | 53.7 (31.4 to 68.7)c | |
| B/Yamagata | 2016–2017 | 56/1356 (4.1) | 39/502 (7.8) | 45.0 (15.1 to 64.3) | 47.9 (16.8 to 67.3) |
| 2017–2018 | 119/1615 (7.4) | 82/738 (11.1) | 36.2 (14.1 to 52.5) | 50.2 (31.4 to 63.8) | |
| 2016–2018 (2 y) | 175/2971 (5.9) | 121/1240 (9.8) | 42.0 (26.1 to 54.4) | 49.4 (34.3 to 61.1) |
Abbreviations: CI, confidence interval; VE, vaccine effectiveness.
aAll denominators exclude other influenza types and any enrollments occurring outside of influenza type-specific periods for each site.
bAdjusted VE models are adjusted for enrollment hospital, age (18–49, 50–64, 65–74, ≥75 y), race, tertiles (for 1 y) or 6 quantiles (for 2 y) of calendar time including a site-specific and influenza-type specific peak for each year, days from illness onset to specimen collection (0–1, 2–4, 5–10 d), self-reported health (poor/fair vs good/very good/excellent), and self-reported past year hospitalizations.
cVE could not be calculated due to small numbers in 2016–2017. Adjusted VE models for A/H1N1 are adjusted for site instead of enrollment hospital due to small case numbers per hospital.
Overall Influenza Vaccine Effectiveness
In an adjusted model pooled across all influenza strains and both years, VE against any influenza-associated hospitalization was 33.5% (95% confidence interval [CI], 23.6%–42.0%; Table 2). Effectiveness against A(H3N2) hospitalization was lower (22.8%; 95% CI, 8.3%–35.0%) than against B/Yamagata (49.4%; 95% CI, 34.3%–61.1%). The point estimate for the VE for each influenza type was higher in 2017–2018 than in 2016–2017, however CIs overlapped. Effectiveness in 2016–2017 against A(H3N2)-associated hospitalization had a CI including the null (18.0%; 95% CI, −9.2% to 38.3%). A total of 328 of 2605 (12.6%) and 422 of 3524 (12.0%) individuals required intensive care unit (ICU) admission in 2016–2017 and 2017–2018, respectively. Among this subgroup of individuals, VE comparing ICU patients with and without A(H3N2) was 23.7% (95% CI, −75.4% to 66.8%; 36 total A(H3N2) infections) for 2016–2017 and 27.0% (95% CI, −56.3% to 65.9%; 41 total A/H3N2) infections) for 2017–2018. VE against RSV, the negative control, was −28.1% (95% CI, −85.7% to 11.7%) in 2016–2017 and −8.1% (95% CI, −52.1% to 23.2%) in 2017–2018, confirming that positive VE was not observed against a virus with no biologic association to the vaccine. The negative control estimate was lowest for the 18–49 years age group −199.7% (95% CI, −579.0% to −32.2%) in 2016–2017 and −169.7% (95% CI, −469.7% to −27.7%) in 2017–2018.
Effect of Vaccine Type
Analyses of cell-based vaccines were performed for the 2017–2018 season only, given the small numbers in the cell-based vaccine group in 2016–2017 (n = 9). In 2017–2018, 66 individuals (2.8% of all vaccinated participants in that season) received a non-egg–based type of vaccine product, including 65 Flucelvax (Sequiris) and 1 Flublok Quadrivalent (Sanofi) vaccines. The individual receiving Flublok was excluded from comparative analyses. In addition, we excluded 608 individuals who had unknown vaccine type, leaving 2915 individuals. Overall, the point estimate for the cell-based VE against A(H3N2) was higher than for egg-based vaccines, but CIs overlapped (cell based, 43.0% [95% CI, −36.3% to 76.1%] vs egg based, 24.0% [95% CI, 3.9% to 39.9%]); the difference in VE was not statistically significant (P = .52) (Table 3). Point estimates against B/Yamagata were similar (cell based, 51.5% [95% CI, −77.0% to 86.7%)] vs egg based, 50.6% [95% CI, 30.2% to 65.1%]) with P = .98 for the difference in VE. In a model restricting the egg-based vaccine group to only those receiving standard-dose vaccine, excluding high-dose recipients, the egg-based VE estimate was 26.6% (95% CI, 5.1% to 43.3%) comparable to the estimate against A(H3N2), combining standard and high dose recipients.
Table 3.
Overall and Strain-Specific, Vaccine Effectiveness of Flucelvax Versus Egg-Based Vaccines Against Hospitalization With Laboratory-Confirmed Influenza, Hospitalized Adult Influenza Vaccine Effectiveness Network 2017–2018 (N = 2915)
| Outcome | Comparison Groups (1 vs 2) | Group 1a Positives, n/Total (%) | Group 2a Positives, n/Total (%) | Unadjusted VE (95% CI) | Adjusted VE (95% CI) | |
|---|---|---|---|---|---|---|
| 2017–2018 | Influenza, all | Flucelvax vs unvaccinated | 14/65 (21.5) | 353/1174 (30.1) | 35.6 (−18.4 to 64.9) | 40.1 (−15.2 to 68.8)b |
| Egg-based vs unvaccinated | 399/1676 (23.8) | 353/1174 (30.1) | 26.1 (12.5 to 37.5) | 34.5 (21.1 to 45.7)b | ||
| Flucelvax vs egg-based | 14/65 (21.5) | 399/1676 (23.8) | 12.8 (−59.8 to 52.4) | 8.5 (−75.9 to 52.3)b | ||
| A/H3N2 | Flucelvax vs unvaccinated | 7/56 (12.5) | 187/970 (19.3) | 39.9 (−35.4 to 73.3) | 43.0 (−36.3 to 76.1)c | |
| Egg-based vs unvaccinated | 248/1459 (17.0) | 187/970 (19.3) | 13.5 (−6.8 to 29.9) | 24.0 (3.9 to 39.9)c | ||
| Flucelvax vs egg-based | 7/56 (12.5) | 248/1459 (17.0) | 30.5 (−55.8 to 69.0) | 24.9 (−78.8 to 68.5)c | ||
| B/Yamagata | Flucelvax vs unvaccinated | 3/43 (7.0) | 82/738 (11.1) | 39.9 (−99.1 to 81.9) | 51.5 (−77.0 to 86.7)d | |
| Egg-based vs unvaccinated | 83/1135 (7.3) | 82/738 (11.1) | 36.6 (12.6 to 54.0) | 50.6 (30.2 to 65.1)d | ||
| Flucelvax vs egg-based | 3/43 (7.0) | 83/1135 (7.3) | 5.2 (−213.5 to 71.4) | 1.8 (−254.0 to 72.8)d |
Abbreviations: CI, confidence interval; VE, vaccine effectiveness.
aAll denominators exclude other influenza types, any enrollments occurring outside of influenza type-specific periods, and unknown vaccine types for each site.
b P values calculated for adjusted VE for all influenza types were: .13 for Flucelvax vs unvaccinated, <.001 for egg-based vs unvaccinated, and .79 for Flucelvax vs egg-based.
c P values calculated for adjusted VE for A/H3N2 were: .21 for Flucelvax vs unvaccinated, .02 for egg-based vs unvaccinated, and .52 for Flucelvax vs egg-based.
d P values calculated for adjusted VE for B/Yamagata were: .27 for Flucelvax vs unvaccinated, <.001 for egg-based vs unvaccinated, and .98 for Flucelvax vs egg-based.
Effect of Prior Vaccination
Prior vaccination was evaluated in the 2017–2018 season, finding that over three-quarters of the study population in this year had documentation or a self-report of influenza vaccination in the previous year, thus receiving 2 consecutive vaccines containing the A/Hong Kong (H3N2) vaccine strain. Previous-year vaccine receipt was common among individuals with vaccination in the current year (n = 1739, 87.3% in 2016–2017; n = 2182, 83.3% in 2017–2018). VE against influenza A(H3N2) hospitalization in 2017–2018 was higher among the subgroup of individuals vaccinated in 2016–2017 (30.0%; 95% CI, 5.5% to 48.2%) than among those not vaccinated previously (5.7%; 95% CI, −63.7 to 45.7); however, the sample size for those unvaccinated in 2016–2017 was very small (Supplementary Figure 1) and the P value for the interaction was 0.34.
Timing of Sample Collection
Although enrollment was permitted for up to 10 days after the onset of symptoms, most samples were collected within 7 days (n = 5468, 89.2%) and 3987 specimens (65.1%) were collected within 4 days. While influenza detection rate was lower among individuals enrolled ≥ 4 days after illness onset compared to within 4 days of onset (P = .04 in 2016–2017 and P = .004 in 2017–2018), the difference in VE was not statistically significant between individuals enrolled within 4 days after onset compared to individuals enrolled more than 4 days after onset (35.1% vs 21.4% in 2016–2017; 39.9% vs 21.3% in 2017–2018; P values for interaction, respectively, P = .42 in 2016–2017 and P = .13 in 2017–2018).
Serology
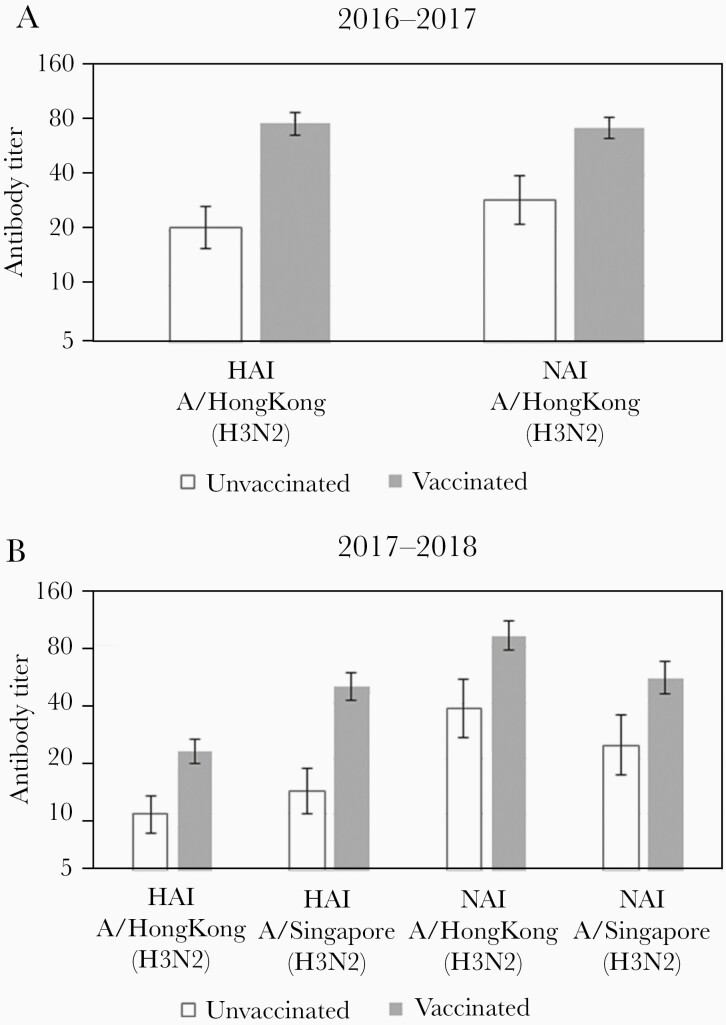
Serum specimens collected upon hospital admission were obtained from 643 enrollees from 1 hospital within the Michigan site during the 2016–2017 (n = 379) and the 2017–2018 (n = 264) seasons (Supplementary Table 6). One hundred specimens (15.6%) were from individuals with influenza A (H3N2) and 498 specimens (77.4%) were from vaccinated individuals (Supplementary Table 6). HAI and NAI titers against A/Hong Kong (H3N2), representing titers against the egg-adapted vaccine strain in both seasons, were significantly higher among vaccinated individuals in both years (Figure 1). Similarly, vaccinated individuals in 2017–2018 had higher HAI and NAI titers that were cross-reactive against A/Singapore (H3N2), which was selected as the vaccine strain for the following year. In the 2016–2017 season, increasing levels of the vaccine-like A/Hong Kong (H3N2) HAI antibody were found to significantly reduce the odds of infection per log2 increase in titer (odds ratio [OR], 0.76; 95% CI, .63–.91), but NAI antibodies against A/Hong Kong (H3N2) were not associated with protection (OR, 0.99; 95% CI, .82–1.19). The point estimates were similar in 2017–2018 for both HAI (OR, 0.80; 95% CI, .64–1.00) and NAI (OR, 1.02; 95% CI, .86–1.22) antibodies against vaccine-like A/Hong Kong (H3N2), but neither were statistically significant.
Figure 1.
Antibody titers by hemagglutination inhibition (HAI) and neuraminidase inhibition (NAI) against vaccine antigens in 2016–2017 (A) and 2017–2018 (B). The A/Hong Kong/4801/2014 (A/Hong Kong) strain was included in both the 2016–2017 and 2017–2018 influenza vaccines; the A/Singapore/INFIMH-16–0019/2016 strain was subsequently selected as the H3N2 strain included in the 2018–2019 vaccine. Error bars represent 95% confidence intervals.
DISCUSSION
In this analysis of 2 years of a prospective, multicenter, test-negative study of influenza VE in prevention of hospitalization, we found VE of 33.5% against any influenza-associated hospitalization and a low VE of 22.8% for the A/Hong Kong/4801/2014 vaccine strain. VE was similarly low across adult age groups and levels of frailty and chronic comorbidities (Supplementary Figure 1). These estimates are similar to the Ambulatory US Flu VE Network results for A(H3N2) in the 2016–2017 and 2017–2018 seasons (33% [2] and 22% [1], respectively) and the Canadian Sentinel Practitioner Surveillance Network results (42% [30] and 17% [31]).
Overall, evaluations in recent years have found VE to be consistently lower against A(H3N2) compared to A(H1N1)pdm09 or influenza B [32–34]. VE against hospitalization in the HAIVEN study was higher in 2015–2016 (51%) against A(H1N1)pdm09 [13]. In some years, most notably in 2014–2015, low VE against A(H3N2) has been attributed to difficulties with matching the vaccine strain to the clade of the subsequently circulating virus [35]. However, our resulting VE in these 2 seasons is unexpectedly low even when considering the degree of antigenic match. Antigenic studies indicate that most circulating viruses in the United States during those years were similar to the A/Hong Kong/4801/2014 strain used in the vaccine [36], yet a VE below 25% was observed in both years. This pattern, found even in years when the vaccine strain was antigenically similar, prompts concern for additional, continued changes in A(H3N2).
In recent years, mutations resulting from egg adaptation that change glycosylation have modified antigenic sites available for antihemagglutinin antibody binding such that the vaccine strain may elicit antibodies to sites that are unavailable in circulating viruses. The A/Hong Kong/4801/2014 strain acquires a specific egg-adaptation mutation during propagation in eggs, altering antigenic sites (T160K) [11]. The seed virus for the cell-based vaccine included in our analysis was propagated without the use of eggs [17], avoiding this change. We have previously demonstrated that vaccinated individuals enrolled in HAIVEN (2017–2018 season) had antibodies specific to the egg-propagated A/Hong Kong/4801/2014 virus and antibody titers against cell-grown A/Hong Kong/4801/2014 and other A(H3N2) 3c.2a2 and 3c.2a1 viruses were lower [9]. The 2016–2017 and 2017–2018 US vaccine evaluation landscape included the increasing use of quadrivalent influenza vaccines grown in cell systems, in contrast to growth in eggs, although initially produced with seed stock originating in eggs. By the 2017–2018 season, production of cell-based vaccines began using cell-based seed stocks for the H3N2 strain [17], and use of these vaccines became more common in the United States. Our findings are consistent with modest increases in VE for cell-based vaccines observed in studies in hospitalized adults [18, 19], across a large health plan [20], and in a Department of Defense sentinel surveillance study [37]. Complimenting these findings is an immunogenicity study where cell-based vaccine elicited an antibody response slightly higher than egg-based vaccine but lower than high-dose or recombinant products [21]. In our product-type specific analyses for the 2017–2018 season, the point estimate for the VE for the cell-based vaccine was higher than for egg-based vaccines, but this difference was not statistically significant and this estimate (43%) is at the low end of the range expected from a well-matched vaccine. Our estimates for egg-based vaccines were similar when excluding those receiving high-dose vaccine. Recently, a dedicated analysis of high- versus standard-dose vaccination in HAIVEN participants in the 2015–2017 seasons has found the increase in VE for high-dose to be moderate, and not statistically significant, in seasons with low to moderate overall VE [38]. While the egg adaptation is one explanation proposed for the low VE observed in 2017–2018, other factors impacting VE, including prior vaccination and the role of neuraminidase antibodies, cannot be ruled out. Ongoing observational studies and randomized trials of egg- and non-egg–based influenza vaccine products will shed light on these potential hypotheses of the impact of egg adaptation going forward, and more mechanistic studies are needed.
In both seasons, increasing HAI, but not NAI, antibody against egg-adapted A/Hong Kong (H3N2) vaccine strain was associated with protection. This appears to be inconsistent with low VE against influenza A(H3N2) in these 2 seasons and it is possible this association is affected by unmeasured antibody with better specificity to the circulating virus. We have demonstrated in a smaller subset of this population that antibody titers against cell-grown viruses, although present only at low levels, were better correlates of protection [9]. Interestingly, antibody titers against both the hemagglutinin and neuraminidase of A/Singapore (H3N2) were higher among individuals vaccinated with A/Hong Kong (H3N2) relative to unvaccinated individuals. This suggests cross-reactivity between these antigens. For hemagglutinin, the cross-reactivity is not surprising; A/Singapore (H3N2) and A/Hong Kong (H3N2) are antigenically similar with both expected to have the same egg-related mutation demonstrated to drive antibody response. However, neuraminidase cross-reactivity between the 2 strains was unexpected given that A(H3N2) viruses predominant in 2017–2018 were reassortants with hemagglutinin and neuraminidase segments from separate 3c.2A subclades [39]. NAI titers did not protect against infection, a finding that may be related to issues with the quality of the antibody [40] or with continued antigenic change of the neuraminidase [39]. Increased antigenic characterization of the neuraminidase of circulating viruses is potentially needed to understand future patterns of circulation and severity, and to aid in vaccine strain selection.
Limitations of this study include its small sample size for evaluation of product-specific effects, given the low uptake of newer vaccine products observed in 2016–2017 and 2017–2018. This study is also limited by challenges inherent in observational studies of VE, particularly those conditional upon hospital admission. The majority of adults of all ages in HAIVEN have chronic health conditions, a population not fully captured in ambulatory-based studies with differing VE estimates [1, 2, 41, 42]. While presenting an opportunity to study this important and high-risk population, the potential for bias arising from differential vaccination and hospital admission patterns as they impact the test-negative design is an area for further attention. Analyses of the association between vaccination and a negative control outcome (RSV) resulted in a strongly negative point estimate. As VE for the prevention of RSV using influenza vaccine should be zero, this largely negative VE raises concern for bias in the age stratified estimate for 18–49 year olds, leading to an underestimation of VE in that age group. While we have previously found minimal bias in the test-negative design as applied to hospitalized adults using testing of other respiratory viruses as a control [43], more work to understand the 18 to 49-year-old population is needed, particularly in years with low influenza VE. In this analysis, we also evaluated the potential for bias in our estimates when including individuals for whom sample collection occurred >4 days after symptom onset, potentially raising the possibility of false-negative results. While this was not statistically significant, our finding of reduced VE and reduced overall detection rate in this group should prompt further attention to the importance of timing of sample collection in studies of respiratory virus hospitalization.
In summary, we found that VE for prevention of A(H3N2) hospitalization was under 25% in both years where the A/Hong Kong/4801/2014 vaccine strain was used. Following the 2017–2018 season, the A(H3N2) component of the vaccine was updated to A/Singapore/INFIMH-16–0019/2016 A(H3N2)-like virus and since has been updated for the 2019–2020 season. Active surveillance of influenza VE against hospitalization is a key component in understanding prevention of severe morbidity. Given challenges with VE against A(H3N2) infection, and increasing variety of vaccine products, efforts to monitor VE with prospective collection of viruses and product-specific vaccine documentation is critical to inform seasonal vaccination strategies from year to year. Longer-term vaccine development plans support testing of future universal influenza vaccines with longer-lasting, broader protection; however, these products are likely to be years away. Until then, ongoing investment is needed to monitor changes in A(H3N2) strains and their impact on VE against severe disease, coupled with continued, appropriate use of antivirals in hospitalized adults [44].
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
HAIVEN study investigators. University of Michigan: Lois E. Lamerato, Adam Lauring, and Ryan E. Malosh. Baylor Scott and White: Kempapura Murthy, Tresa McNeal, Kevin Chang, Heath White, Alejandro Arroliga, Laurel Kilpatrick, Meredith Wimberly, Victor Escobedo, JoAnn Nichols, Lydia Clipper, Chandni Raiyani, Wencong Chen, Anne Robertson, Arundhati Rao, Robert Fader, Kimberly Walker, and Marcus Volz. University of Pittsburgh Medical Center and/or University of Pittsburgh: Mary Patricia Nowalk, Kailey Hughes, Sean Saul, Lori Stiefel, Michael Susick, Balasubramani K. Goundappa, Charles Rinaldo, John Williams, Monika Johnson, Julie Gealey, Heather Eng, and Melissa Saul.
Acknowledgments. The authors thank the following individuals for their contribution to this work. Centers for Disease Control and Prevention: Emily Smith. University of Michigan and Henry Ford Health System: EJ McSpadden, Hannah Segaloff, Rachel Truscon, Emileigh Johnson, Amy Callear, Anne Kaniclides, Richard Evans, Nishat Islam, Michelle Groesbeck, Andrew Miller, Evelina Kutyma, Chasity Moore, Kaitlyn Digna, Elizabeth Alleman, Sarah Bauer, Marlisa Granderson, Kimberly Berke, Mackenzie Smith, Amanda Cyrus, Alana Johnson, and Jayla Jackson. Vanderbilt University Medical Center: Dayna Wyatt, Stephanie Longmire, Seibert Tregoning, Erin Zipperer, Gina Smith, Judith McCarty, Lisa Davis, Reiner Venegas, Hollie Horton, Alina Simion, Jessica Freeman, Judy McCarthy, and Bethany Alicie. Baylor Scott and White: Crystal Hodges, Ineshia Jackson, Deborah Furze, Martha Zayed, Melissa Zdroik, Todd Crumbaker, Iosefo, Chooihoong Choo, Mary Kylberg, Hania Wehbe-Janek, Madhava Beeram, Natalie Settele, Jennifer Thomas, Jaime Walkowiak, Adelfa Alcozer, Evangeline Knight, Jeremy Ray, Renee Day, Deborah Price, Jennifer Fox, and Robert Probe. University of Pittsburgh: Theresa Sax and Arlene Bullotta.
Disclaimer . The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Financial Support. This work was supported by the Centers for Disease Control and Prevention (cooperative agreement IP15-002; grant numbers U01IP000979 to the Tennessee site, U01IP000974 to the Michigan site, U01IP000972 to the Texas site, and U01IP000969 to the Pennsylvania site); and supported in part at the Pennsylvania site by the National Institutes of Health Clinical and Translational Science Award program (grant number UL1TR001857).
Potential conflicts of interest. E. T. M. has received personal fees from Pfizer and grant funding from Merck. D. M. has received personal fees from Seqirus, Pfizer, Sanofi Pasteur, and GlaxoSmithKline, and grant funding from Pfizer. R. Z. has received grant funding from Sanofi Pasteur and Merck. A. S. M. has received personal fees from Sanofi Pasteur and Seqirus. H. K. T. has served on a Data Safety Monitoring Board for Seqirus. J. F. reports nonfinancial support from the Institute for Influenza Epidemiology, funded in part by Sanofi Pasteur. All other authors report no potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
HAIVEN Study Investigators:
Lois E Lamerato, Adam Lauring, Ryan E Malosh, Kempapura Murthy, Tresa McNeal, Kevin Chang, Heath White, Alejandro Arroliga, Laurel Kilpatrick, Meredith Wimberly, Victor Escobedo, JoAnn Nichols, Lydia Clipper, Chandni Raiyani, Wencong Chen, Anne Robertson, Arundhati Rao, Robert Fader, Kimberly Walker, Marcus Volz, Kailey Hughes, Sean Saul, Lori Stiefel, Michael Susick, Balasubramani K Goundappa, Charles Rinaldo, John Williams, Monika Johnson, Julie Gealey, Heather Eng, and Melissa Saul
References
- 1. Rolfes MA, Flannery B, Chung JR, et al. . Effects of influenza vaccination in the United States during the 2017–2018 influenza season. Clin Infect Dis 2019; 69:1845–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Flannery B, Chung JR, Monto AS, et al. . Influenza vaccine effectiveness in the United States during the 2016–2017 season. Clin Infect Dis 2019; 68:1798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ohmit SE, Thompson MG, Petrie JG, et al. . Influenza vaccine effectiveness in the 2011–2012 season: protection against each circulating virus and the effect of prior vaccination on estimates. Clin Infect Dis 2014; 58:319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McLean HQ, Thompson MG, Sundaram ME, et al. . Impact of repeated vaccination on vaccine effectiveness against influenza A(H3N2) and B during 8 seasons. Clin Infect Dis 2014; 59:1375–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Skowronski DM, Chambers C, Sabaiduc S, et al. . A perfect storm: impact of genomic variation and serial vaccination on low influenza vaccine effectiveness during the 2014–2015 season. Clin Infect Dis 2016; 63:21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zelner J, Petrie JG, Trangucci R, Martin ET, Monto AS. Effects of sequential influenza A(H1N1)pdm09 vaccination on antibody waning. J Infect Dis 2019; 220:12–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ferdinands JM, Fry AM, Reynolds S, et al. . Intraseason waning of influenza vaccine protection: evidence from the US Influenza Vaccine Effectiveness Network, 2011–12 through 2014–15. Clin Infect Dis 2017; 64:544–50. [DOI] [PubMed] [Google Scholar]
- 8. Ray GT, Lewis N, Klein NP, et al. . Intraseason waning of influenza vaccine effectiveness. Clin Infect Dis 2019; 68:1623–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Levine MZ, Martin ET, Petrie JG, et al. . Antibodies against egg- and cell-grown influenza A(H3N2) viruses in adults hospitalized during the 2017–2018 influenza season. J Infect Dis 2019; 219:1904–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wu NC, Zost SJ, Thompson AJ, et al. . A structural explanation for the low effectiveness of the seasonal influenza H3N2 vaccine. PLoS Pathog 2017; 13:e1006682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zost SJ, Parkhouse K, Gumina ME, et al. . Contemporary H3N2 influenza viruses have a glycosylation site that alters binding of antibodies elicited by egg-adapted vaccine strains. Proc Natl Acad Sci U S A 2017; 114:12578–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jackson ML, Nelson JC. The test-negative design for estimating influenza vaccine effectiveness. Vaccine 2013; 31:2165–8. [DOI] [PubMed] [Google Scholar]
- 13. Ferdinands JM, Gaglani M, Martin ET, et al. . Prevention of influenza hospitalization among adults in the United States, 2015–2016: results from the US Hospitalized Adult Influenza Vaccine Effectiveness Network (HAIVEN). J Infect Dis 2019; 220:1265–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lipsitch M, Tchetgen Tchetgen E, Cohen T. Negative controls: a tool for detecting confounding and bias in observational studies. Epidemiology 2010; 21:383–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tchetgen Tchetgen E. The control outcome calibration approach for causal inference with unobserved confounding. Am J Epidemiol 2014; 179:633–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Arnold BF, Ercumen A, Benjamin-Chung J, Colford JM Jr. Brief report: negative controls to detect selection bias and measurement bias in epidemiologic studies. Epidemiology 2016; 27:637–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Barr IG, Donis RO, Katz JM, et al. . Cell culture-derived influenza vaccines in the severe 2017–2018 epidemic season: a step towards improved influenza vaccine effectiveness. NPJ Vaccines 2018; 3:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Izurieta HS, Chillarige Y, Kelman J, et al. . Relative effectiveness of cell-cultured and egg-based influenza vaccines among elderly persons in the United States, 2017–2018. J Infect Dis 2019; 220:1255–64. [DOI] [PubMed] [Google Scholar]
- 19. Bruxvoort KJ, Luo Y, Ackerson B, et al. . Comparison of vaccine effectiveness against influenza hospitalization of cell-based and egg-based influenza vaccines, 2017–2018. Vaccine 2019; 37:5807–11. [DOI] [PubMed] [Google Scholar]
- 20. Klein NP, Fireman B, Goddard K, et al. . LB15. Vaccine effectiveness of Flucelvax relative to inactivated influenza vaccine during the 2017–18 influenza season in Northern California. Open Forum Infect Dis 2018; 5(Suppl 1):S764. [Google Scholar]
- 21. Gouma S, Zost SJ, Parkhouse K, et al. . Comparison of human H3N2 antibody responses elicited by egg-based, cell-based, and recombinant protein-based influenza vaccines during the 2017–2018 season. Clin Infect Dis 2019; 71:1447–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Monto AS, Malosh RE, Petrie JG, Martin ET. The doctrine of original antigenic sin: separating good from evil. J Infect Dis 2017; 215:1782–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Petrie JG, Ohmit SE, Cheng CK, et al. . Influenza vaccine effectiveness against antigenically drifted influenza higher than expected in hospitalized adults: 2014–2015. Clin Infect Dis 2016; 63:1017–25. [DOI] [PubMed] [Google Scholar]
- 24. Cheng AC, Kotsimbos T, Kelly HA, et al. . Effectiveness of H1N1/09 monovalent and trivalent influenza vaccines against hospitalization with laboratory-confirmed H1N1/09 influenza in Australia: a test-negative case control study. Vaccine 2011; 29:7320–5. [DOI] [PubMed] [Google Scholar]
- 25. Savulescu C, Jiménez-Jorge S, de Mateo S, et al. . Using surveillance data to estimate pandemic vaccine effectiveness against laboratory confirmed influenza A(H1N1)2009 infection: two case-control studies, Spain, season 2009–2010. BMC Public Health 2011; 11:899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kissling E, Valenciano M, Cohen JM, et al. . I-MOVE multi-centre case control study 2010-11: overall and stratified estimates of influenza vaccine effectiveness in Europe. PLoS One 2011; 6:e27622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Petrie JG, Martin ET, Truscon R, et al. . Evaluation of correlates of protection against influenza A(H3N2) and A(H1N1)pdm09 infection: Applications to the hospitalized patient population. Vaccine 2019; 37:1284–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Blanton L, Mustaquim D, Alabi N, et al. . Update: influenza activity—United States, October 2, 2016–February 4, 2017. MMWR Recomm Rep 2017; 66:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Garten R, Blanton L, Elal AIA, et al. . Update: influenza activity in the United States during the 2017–18 season and composition of the 2018–19 influenza vaccine. Morb Mortal Wkly Rep 2018; 67:634–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Skowronski DM, Chambers C, Sabaiduc S, et al. . Interim estimates of 2016/17 vaccine effectiveness against influenza A(H3N2), Canada, January 2017. Euro Surveill 2017; 22:30460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Skowronski DM, Chambers C, Serres GD, et al. . Early season co-circulation of influenza A(H3N2) and B(Yamagata): interim estimates of 2017/18 vaccine effectiveness, Canada, January 2018. Euro Surveill 2018; 23:18-00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Belongia EA, McLean HQ. Influenza vaccine effectiveness: defining the H3N2 problem. Clin Infect Dis 2019; 69:1817–23. [DOI] [PubMed] [Google Scholar]
- 33. Treanor JJ, Talbot HK, Ohmit SE, et al. ; US Flu-VE Network . Effectiveness of seasonal influenza vaccines in the United States during a season with circulation of all three vaccine strains. Clin Infect Dis 2012; 55:951–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pebody RG, Warburton F, Andrews N, et al. . Uptake and effectiveness of influenza vaccine in those aged 65 years and older in the United Kingdom, influenza seasons 2010/11 to 2016/17. Euro Surveill 2018; 23:1800092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Flannery B, Zimmerman RK, Gubareva LV, et al. . Enhanced genetic characterization of influenza A(H3N2) viruses and vaccine effectiveness by genetic group, 2014–2015. J Infect Dis 2016; 214:1010–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. World Health Organization. Recommended composition of influenza virus vaccines for use in the 2018–2019 northern hemisphere influenza season, 2018. http://www.who.int/influenza/vaccines/virus/recommendations/2018_19_north/en/. Accessed 20 June 2018.
- 37. DeMarcus L, Shoubaki L, Federinko S. Comparing influenza vaccine effectiveness between cell-derived and egg-derived vaccines, 2017–2018 influenza season. Vaccine 2019; 37:4015–21. [DOI] [PubMed] [Google Scholar]
- 38. Doyle JD, Beacham L, Martin ET, et al. . Relative and absolute effectiveness of high-dose and standard-dose influenza vaccine against influenza-related hospitalization among older adults—United States, 2015–2017 [published online ahead of print 18 February 2020]. Clin Infect Dis doi: 10.1093/cid/ciaa160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Potter BI, Kondor R, Hadfield J, et al. . Evolution and rapid spread of a reassortant A(H3N2) virus that predominated the 2017–2018 influenza season. Virus Evol 2019; 5:vez046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chen Y-Q, Wohlbold TJ, Zheng N-Y, et al. . influenza infection in humans induces broadly cross-reactive and protective neuraminidase-reactive antibodies. Cell 2018; 173:417–29.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stein Y, Mandelboim M, Sefty H, et al. . Seasonal influenza vaccine effectiveness in preventing laboratory-confirmed influenza in primary care in Israel, 2016–2017 season: insights into novel age-specific analysis. Clin Infect Dis 2018; 66:1383–91. [DOI] [PubMed] [Google Scholar]
- 42. Kissling E, Rondy M, Team I-M-M study . Early 2016/17 vaccine effectiveness estimates against influenza A(H3N2): I-MOVE multicentre case control studies at primary care and hospital levels in Europe. Euro Surveill 2017; 22:30464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Segaloff HE, Cheng B, Miller AV, et al. . Influenza vaccine effectiveness in the inpatient setting; evaluation of potential bias in the test-negative design by use of alternate control groups. Am J Epidemiol 2019; 189: 250–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Uyeki TM, Bernstein HH, Bradley JS, et al. . Clinical practice guidelines by the Infectious Diseases Society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenza A. Clin Infect Dis 2019; 68:895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.