SUMMARY
The incidence of esophageal cancer has increased steadily in the last decades in the United States. The aim of this paper was to characterize disparities in esophageal cancer treatment in different racial and socioeconomic population groups and compare long-term survival among different treatment modalities. A retrospective analysis of the National Cancer Database was performed including adult patients (≥18 years old) with a diagnosis of resectable (stages I–III) esophageal cancer between 2004 and 2015. Multivariable logistic regression models were used to determine the odds of being offered no treatment at all and surgical treatment across race, primary insurance, travel distance, income, and education levels. Multivariable Cox proportional hazards models were used to compare 5-year survival rates across different treatment modalities. A total of 60,621 esophageal cancer patients were included. Black patients, uninsured patients, and patients living in areas with lower levels of education were more likely to be offered no treatment. Similarly, black race, female patients, nonprivately insured patients, and those living in areas with lower median residential income and lower education levels were associated with lower rates of surgery. Patients receiving surgical treatment, compared to both no treatment and definitive chemoradiation, had significant better long-term survival in stage I, II, and III esophageal cancer. In conclusion, underserved patients with esophageal cancer appear to have limited access to surgical care, and are, in fact, more likely to not be offered any treatment at all. Considering the survival benefits associated with surgical resection, greater public health efforts to reduce disparities in esophageal cancer are needed.
Keywords: disparity, esophageal cancer, surgery, survival
INTRODUCTION
The incidence of esophageal cancer, especially esophageal adenocarcinoma, has increased in the last decades in the United States and is expected to keep on increasing in the future.1 , 2 In 2015, there were approximately 47,000 people living with esophageal cancer, and in 2018 there will be an estimated of 17,290 new cases and 15,850 deaths related to this disease.3
Although esophagectomy is the mainstay treatment of esophageal cancer, it is considered one of the most demanding surgical procedures with significant associated morbidity and mortality.4 , 5 Better patient selection, enhancements in perioperative care, dedicated anesthetic teams, and high-dependency units have contributed to improved esophageal cancer outcomes; however, previous studies have suggested that certain populations are unable to obtain optimal esophageal cancer care.6 – 8
The aim of this study was to characterize disparities in esophageal cancer treatment in different racial and socioeconomic population groups, and compare long-term survival among different treatment modalities.
MATERIALS AND METHODS
A cohort of patients was identified using the National Cancer Database (NCDB). The NCDB is a national hospital-based cancer registry program implemented by the Commission on Cancer (COC) of the American College of Surgeons and the American Cancer Society. The database includes over 1500 hospitals and obtains nearly 70% of incident cancer cases in the United States. Data from the NCDB comes from cancer programs accredited by the COC, who are required to report incident cases to the NCDB.
The study cohort was restricted to adult patients (≥18 years old) with a diagnosis of nonmetastatic (stages I–III) esophageal cancer between January 1, 2004, and December 31, 2015. Stage at diagnosis was determined using the TNM staging system of the American Joint Commission of Cancer. This led to an initial cohort of 98,372 patients. Patients with missing TNM clinical stage (n = 27,337), missing treatment information (n = 2624), or with both missing (n = 2141) were excluded from the analysis. Next, if a patient had contraindicated treatments they were also excluded from the analysis (n = 5649). Chemoradiation was defined as receiving chemotherapy, radiation, or both during the course of treatment. Thus, the final analytic cohort consisted of 60,621 patients.
Statistical analysis
Basic descriptive analyses were used to present patient and cancer characteristics, stratified by treatment offered (no treatment, chemoradiation alone, surgery alone, and chemoradiation plus surgery).
Multivariable logistic regression models were generated to estimate the odds of being offered no treatment at all (compared to any treatment) and surgical treatment (compared to no surgery) across race, primary insurance, travel distance, median residential income in the patients’ ZIP code, and the proportion of adults with less than a high school education in the patients’ ZIP code. All analyses were stratified by clinical cancer stage by including interaction terms. Travel distance was categorized as quartiles. These models were both adjusted for clinical cancer stage, age, sex, year of diagnosis, and Charlson comorbidity score (categorized as 0, 1, 2, and 3 + as per NCDB coding). Age was modeled as a restricted cubic spline.
Kaplan–Meier curves and Cox proportional hazards models were used to obtain 5-year cumulative incidence of mortality, and assess the effects of receiving no treatment or definitive chemoradiation, compared to surgical intervention, stratified by clinical cancer stage. For all survival analyses, patients were classified using the treatment they actually received. Inverse probability of treatment weighting (IPTW) was used to balance the covariate distribution between the treatment groups contrasted in the models and generate standardized cumulative incidence estimates and survival plots.9 IPTW weights were constructed using a logistic regression model predicting probability of treatment group conditional on clinical cancer stage, age (modeled as a restricted cubic spline), sex, race, area-level income, area-level education, Charlson comorbidity score, insurance, distance to hospital, and year of diagnosis.
Additionally, in order to account for the fact that treatment was not randomized and that we are only able to observe received treatments (as opposed to scheduled or planned treatment plans), a sensitivity analysis was performed. The effect of receiving no treatment or definitive chemoradiation, compared to surgical intervention, was performed among patients who survived at least 12 months after diagnosis. This allowed us to account for potential immortal-time bias (i.e. bias introduced when time zero, eligibility, and treatment assignment are misaligned) and be more confident that included patients who underwent their entire treatment plan.10
A P-value < 0.05 was considered significant for all the statistical methods.
All analyses were performed using SAS software version 9.4 (SAS Inc., Cary, NC).
RESULTS
A total of 60,621 esophageal cancer patients were included; 4063 (7%) were offered no treatment, 25,977 (43%) were offered definitive chemotherapy and/or radiation (further referred to as definitive chemoradiation), 7430 (12%) were offered surgery only, and 23,151 (38%) were offered chemotherapy and/or radiation plus surgery (further referred to as chemoradiation plus surgery). Of note, 2958 (5%) of the patients who were offered surgery did not receive it, either due to patient refusal or due to death before scheduled surgery. Similarly, 4584 (8%) patients who were offered chemotherapy and/or radiation did not receive it, either due to patient refusal or due to death before planned treatment. Patient characteristics, stratified by treatment type, are described in Table 1.
Table 1.
Distribution of patient characteristics among nonmetastatic adult esophageal cancer cases, stratified by type of treatment offered
| No treatment 4063 (7%) | Definitive chemoradiation 25,977 (43%) | Chemoradiation plus surgery 23,151 (38%) | Surgery only 7430 (12%) | |
|---|---|---|---|---|
| Stage, n (%) | ||||
| I | 1897 (47) | 3340 (13) | 2877 (12) | 5656 (76) |
| II | 875 (22) | 9168 (35) | 9360 (40) | 1373 (18) |
| III | 1291 (32) | 13,469 (52) | 10,914 (47) | 401 (5) |
| Age, years, median (IQR) | 73 (63–81) | 69 (60–77) | 63 (57–70) | 67 (60–74) |
| Sex, n (%) | ||||
| Male | 2907 (72) | 19,264 (74) | 19,269 (83) | 5966 (80) |
| Female | 1156 (28) | 6713 (26) | 3883 (17) | 1464 (20) |
| Charlson–Deyo score, n (%) | ||||
| 0 | 2880 | 19,070 | 17,318 | 5273 |
| 1 | 824 | 5078 | 4658 | 1640 |
| 2 | 257 | 1327 | 901 | 371 |
| 3+ | 102 | 502 | 274 | 146 |
| Race, n (%) | ||||
| White | 3399 (86) | 21,537 (84) | 21,338 (93) | 6871 (94) |
| Black | 461 (12) | 3462 (13) | 1098 (5) | 285 (4) |
| Other | 113 (3) | 769 (3) | 493 (2) | 135 (2) |
| Primary insurance, n (%) | ||||
| Private | 942 (24) | 6724 (27) | 10,561 (47) | 2528 |
| Medicaid | 234 (6) | 2102 (8) | 1264 (6) | 310 |
| Medicare | 2575 (66) | 15,206 (61) | 9913 (45) | 4121 |
| Uninsured | 155 (4) | 979 (4) | 505 (2) | 86 |
| Travel distance, miles, n (%) | ||||
| <5 miles | 1219 (31) | 8263 (32) | 4582 (20) | 1030 (14) |
| 5–11.9 miles | 990 (25) | 7014 (27) | 5167 (23) | 1338 (18) |
| 12–31.9 miles | 835 (21) | 6194 (24) | 6072 (27) | 1743 (24) |
| ≥32 miles | 934 (23) | 4079 (16) | 7039 (31) | 3206 (44) |
| Median residential income†, n (%) | ||||
| Less than $38,000 | 805 (20) | 5346 (21) | 3332 (15) | 1091 (15) |
| $38,000–$47,999 | 966 (24) | 6280 (25) | 5580 (24) | 1741 (24) |
| $48,000–$62,999 | 1071 (27) | 6637 (26) | 6468 (28) | 2051 (28) |
| $63,000 or more | 1130 (28) | 7273 (28) | 7465 (33) | 2432 (33) |
| Education, % without high school degree‡, n (%) | ||||
| <7% | 804 (2) | 5508 (22) | 5840 (26) | 1934 (26) |
| 7%–12.9% | 1264 (32) | 8330 (33) | 8319 (36) | 2644 (36) |
| 13%–20.9% | 1128 (28) | 6902 (27) | 5819 (25) | 1810 (25) |
| ≥21% | 780 (20) | 4817 (19) | 2879 (13) | 930 (13) |
† Median residential household income of each patient's ZIP code was estimated using the 2012 American Community Survey.
‡Proportion of adults in patient's ZIP code who did not complete high school, measured in the 2012 American Community Survey.
IQR, interquartile range.
A non-negligible number of patients were excluded from the analysis due to missing stage or missing stage data (n = 27,337). To consider the potential for selection bias, the characteristics of the patients with missing stage were assessed. Patients with missing stage data did not differ substantively from those included in the analysis with respect to any of the socioeconomic disparity variables considered for the primary analysis, including sex, race, income, distance to facility, insurance, and education (Appendix Table A1).
No treatment versus any treatment
After adjustment, black patients, compared to white patients, were more likely to be offered no treatment when diagnosed as stage I (OR 1.42, 95% CI 1.17, 1.72), stage II (OR 1.33, 95% CI 1.04, 1.69), and stage III (OR 1.34, 95% CI 1.11, 1.63). Female patients were more likely to be offered no treatment when diagnosed at stage III (OR 1.47, 95% CI 1.29, 1.67). Uninsured patients, compared to patients with private insurance, were also more likely to be offered no treatment when diagnosed as stage I (OR 1.77, 95% CI 1.25, 2.50), stage II (OR 2.58, 95% CI 1.75, 3.80), and stage III (OR 2.14, 95% CI 1.61, 2.83). Similarly, Medicare and Medicaid patients had higher odds of being offered no treatment when diagnosed as stage II and stage III. Patients living in areas where ≥21% of adults did not complete high school were consistently more likely to be offered no treatment in stage I, II, and III. Minimal differences in treatment offered were seen across median household income and travel distance after adjustment (Table 2).
Table 2.
Adjusted odds ratios of being offered no treatment, compared to any treatment, across racial and socioeconomic population groups among nonmetastatic adult esophageal cancer cases, stratified by clinical cancer stage
| Stage I | Stage II | Stage III | |
|---|---|---|---|
| OR (95% CI)† | OR (95% CI)† | OR (95% CI)† | |
| Race | |||
| White | REF | REF | REF |
| Black | 1.42 (1.17, 1.72) | 1.33 (1.04, 1.69) | 1.34 (1.11, 1.63) |
| Other | 0.90 (0.60, 1.35) | 1.14 (0.73, 1.77) | 1.60 (1.20, 2.14) |
| Sex | |||
| Male | REF | REF | REF |
| Female | 1.08 (0.96, 1.22) | 1.14 (0.97, 1.33) | 1.47 (1.29, 1.67) |
| Primary insurance | |||
| Private | REF | REF | REF |
| Medicaid | 0.95 (0.72, 1.25) | 1.47 (1.06, 2.04) | 1.46 (1.15, 1.86) |
| Medicare | 0.91 (0.80, 1.04) | 1.19 (0.99, 1.43) | 1.20 (1.03, 1.40) |
| Uninsured | 1.77 (1.25, 2.50) | 2.58 (1.75, 3.80) | 2.14 (1.61, 2.83) |
| Travel distance, miles | |||
| <5 miles | REF | REF | REF |
| 5–11.9 miles | 0.92 (0.80, 1.06) | 0.91 (0.75, 1.10) | 0.86 (0.73, 1.02) |
| 12–31.9 miles | 0.77 (0.66, 0.90) | 0.72 (0.59, 0.89) | 0.91 (0.77, 1.07) |
| ≥32 miles | 0.61 (0.53, 0.71) | 1.09 (0.90, 1.33) | 1.10 (0.93, 1.30) |
| Median household income‡ | |||
| ≥$63,000 | REF | REF | REF |
| $48,000–$62,999 | 1.03 (0.89, 1.20) | 0.87 (0.71, 1.08) | 1.02 (0.85, 1.22) |
| $38,000–$47,999 | 0.88 (0.74, 1.05) | 0.77 (0.61, 0.98) | 1.01 (0.83, 1.23) |
| <$38,000 | 0.89 (0.72, 1.10) | 0.73 (0.55, 0.97) | 0.97 (0.77, 1.22) |
| Education, % without high school degree§ | |||
| <7% | REF | REF | REF |
| 7–12.9% | 1.09 (0.93, 1.28) | 1.22 (0.98, 1.52) | 1.18 (0.98, 1.43) |
| 13%–20.9% | 1.49 (1.24, 1.79) | 1.55 (1.20, 2.00) | 1.35 (1.09, 1.68) |
| ≥21% | 1.68 (1.35, 2.09) | 2.07 (1.54, 2.79) | 1.62 (1.27, 2.07) |
†Adjusted for clinical cancer stage, year of diagnosis, age (modeled as a restricted cubic spline), sex, Charlson–Deyo score, race, primary insurance, travel distance to hospital, median household income, and education; interaction terms between clinical cancer stage and race, sex, primary insurance, travel distance, median household income, and education were used to get stage-stratified estimates.‡Median residential household income of each patient's ZIP code was estimated using the 2012 American Community Survey.
§Proportion of adults in patient's ZIP code who did not complete high school, measured in the 2012 American Community Survey.
Surgery versus no surgery
After adjustment, black patients, compared to white patients, were less likely to be offered surgery when diagnosed as stage I (OR 0.39, 95% CI 0.33, 0.46), stage II (OR 0.47, 95% CI 0.42, 0.53), and stage III (OR 0.39, 95% CI 0.35, 0.43). Female patients were also less likely to be offered surgical treatment when diagnosed as stage I (OR 0.77, 95% CI 0.70, 0.85), stage II (OR 0.72, 95% CI 0.67, 0.77), and stage III (OR 0.70, 95% CI 0.65, 0.75). Uninsured patients, compared to those with private insurance, were less likely to be offered a surgical intervention in stage I (OR 0.36, 95% CI 0.27, 0.47), stage II (OR 0.43, 95% CI 0.36, 0.52), and stage III (OR 0.36, 95% CI 0.31, 0.42). Similarly, Medicare and Medicaid patients were less likely to be offered surgical treatment, compared to patients with private insurance. Travel distance had an inverse association with the odds of undergoing esophagectomy, with patients traveling farther distances being more likely to be offered surgery, across all clinical cancer stages. Patients from areas in the lowest quartile of median household income, compared to patients residing in the highest quartile, were significantly less likely to receive surgical treatment in stages I, II, and III. Patients from areas with the highest proportion of citizens without a high school diploma (≥21%), compared to those from areas with the lowest proportion (<7%), were also less likely to receive surgical treatment in stages I, II, and III (Table 3).
Table 3.
Adjusted odds ratios of being offered surgery (with or without chemoradiation), compared to no treatment or definitive chemoradiation across racial and socioeconomic population groups, among nonmetastatic adult esophageal cancer cases, stratified by clinical cancer stage
| Stage I | Stage II | Stage III | |
|---|---|---|---|
| OR (95% CI)† | OR (95% CI)† | OR (95% CI)† | |
| Race | |||
| White | REF | REF | REF |
| Black | 0.39 (0.33, 0.46) | 0.47 (0.42, 0.53) | 0.39 (0.35, 0.43) |
| Other | 1.03 (0.77, 1.38) | 0.80 (0.65, 0.97) | 0.58 (0.49, 0.69) |
| Sex | |||
| Male | REF | REF | REF |
| Female | 0.77 (0.70, 0.85) | 0.72 (0.67, 0.77) | 0.70 (0.65, 0.75) |
| Primary insurance | |||
| Private | REF | REF | REF |
| Medicaid | 0.62 (0.51, 0.75) | 0.53 (0.46, 0.61) | 0.48 (0.43, 0.54) |
| Medicare | 0.85 (0.77, 0.94) | 0.67 (0.62, 0.72) | 0.72 (0.67, 0.77) |
| Uninsured | 0.36 (0.27, 0.47) | 0.43 (0.36, 0.52) | 0.36 (0.31, 0.42) |
| Travel distance, miles | |||
| <5 miles | REF | REF | REF |
| 5–11.9 miles | 1.26 (1.13, 1.41) | 1.14 (1.05, 1.24) | 1.13 (1.05, 1.23) |
| 12–31.9 miles | 1.60 (1.42, 1.79) | 1.45 (1.33, 1.58) | 1.39 (1.28, 1.50) |
| ≥32 miles | 4.01 (3.57, 4.51) | 2.80 (2.56, 3.07) | 2.45 (2.26, 2.66) |
| Median household income ‡ | |||
| ≥$63,000 | REF | REF | REF |
| $48,000–$62,999 | 0.93 (0.83, 1.05) | 0.89 (0.81, 0.97) | 0.95 (0.87, 1.02) |
| $38,000–$47,999 | 0.82 (0.72, 0.94) | 0.83 (0.75, 0.92) | 0.90 (0.83, 0.99) |
| <$38,000 | 0.80 (0.68, 0.94) | 0.70 (0.61, 0.79) | 0.83 (0.74, 0.93) |
| Education, % without high school degree§ | |||
| <7% | REF | REF | REF |
| 7%–12.9% | 0.84 (0.74, 0.94) | 0.92 (0.84, 1.01) | 0.87 (0.81, 0.94) |
| 13%–20.9% | 0.67 (0.58, 0.77) | 0.84 (0.75, 0.93) | 0.77 (0.71, 0.85) |
| ≥21% | 0.54 (0.46, 0.64) | 0.76 (0.66, 0.86) | 0.61 (0.54, 0.69) |
†Adjusted for clinical cancer stage, year of diagnosis, age (modeled as a restricted cubic spline), sex, Charlson–Deyo score, race, primary insurance, travel distance to hospital, median household income, and education; interaction terms between clinical cancer stage and race, sex, primary insurance, travel distance, median household income, and education were used to get stage-stratified estimates.
‡ Median residential household income of each patient's ZIP code was estimated using the 2012 American Community Survey.
§Proportion of adults in patient's ZIP code who did not complete high school, measured in the 2012 American Community Survey.
5-year survival
After standardizing, the 5-year survival rate among patients with clinical cancer stage I disease was 34.1% with no treatment, 21.2% with definitive chemoradiation, and 54.2% with surgery (with or without chemoradiation) (Fig. 1a). Among patients with clinical cancer stage II disease, the 5-year survival rate was 9.8% with no treatment, 17.8% with definitive chemoradiation, and 35.3% with surgery (with or without chemoradiation) (Fig. 1b). Finally, the 5-year survival rate in patients with clinical cancer stage III was 9.9% with no treatment, 15.6% with definitive chemoradiation, and 33.2% with surgery (with or without chemoradiation) (Fig. 1c).
Fig. 1.
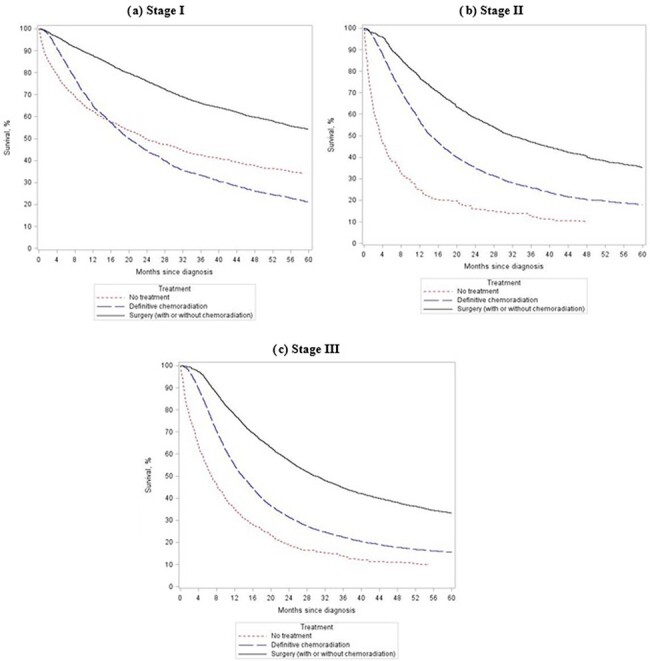
Standardized 5-year survival among nonmetastatic adult esophageal cancer cases, stratified by received treatment and across clinical cancer stage.
As compared to patients undergoing surgery (with or without chemoradiation), both patients receiving no treatment and those receiving definitive chemoradiation were significantly more likely to die when diagnosed with clinical cancer stage I, II, and III (Table 4). Even after restricting to patients who survived at least 12 months after diagnosis, patients undergoing surgery had significantly higher 5-year survival rates across all clinical cancer stages (Appendix Table A2).
Table 4.
Crude and standardized effect of no treatment and definitive chemoradiation, compared to surgery, on 5-year mortality among nonmetastatic adult esophageal cancer cases, stratified by stage
| Crude | Standardized† | |||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| STAGE I | ||||
| No treatment | 3.67 (3.41, 3.95) | <0.0001 | 2.43 (2.19, 2.69) | <0.0001 |
| Definitive chemoradiation | 3.82 (3.59, 4.07) | <0.0001 | 3.19 (2.95, 3.44) | <0.0001 |
| Surgery (with or without chemoradiation) | REF | – | REF | – |
| STAGE II | ||||
| No treatment | 3.96 (3.65, 4.30) | <0.0001 | 2.66 (2.29, 3.07) | <0.0001 |
| Definitive chemoradiation | 2.15 (2.07, 2.24) | <0.0001 | 1.80 (1.71, 1.89) | <0.0001 |
| Surgery (with or without chemoradiation) | REF | – | REF | – |
| STAGE III | ||||
| No treatment | 5.03 (4.70, 5.38) | <0.0001 | 3.66 (3.24, 4.13) | <0.0001 |
| Definitive chemoradiation | 2.15 (2.08, 2.23) | <0.0001 | 1.92 (1.83, 2.02) | <0.0001 |
| Surgery (with or without chemoradiation) | REF | – | REF | – |
†Standardized across clinical cancer stage, year of diagnosis, age (modeled as a restricted cubic spline), sex, Charlson-Deyo score, race, primary insurance, travel distance to hospital, median household income, and education
CI, confidence interval; HR, hazard ratio.
DISCUSSION
The objective of this study was to characterize potential disparities in esophageal cancer treatment and survival. We found that (a) black patients, uninsured patients, patients with government insurance, and patients from areas with lower levels of education were more likely to be offered no treatment at all; (b) the use of surgery was affected by gender, race, and socioeconomic factors; and (c) patients receiving surgical treatment had significantly better long-term survival.
Esophageal cancer is often an aggressive disease, with around 50% of the patients presenting with potentially resectable disease. While esophagectomy remains the primary treatment for early stage tumors, patients with locally advanced disease often require multimodality therapy (i.e. esophagectomy + chemoradiation). Unfortunately, some esophageal cancer patients still do not receive any treatment at all in the United States. For instance, a study using the Surveillance, Epidemiology and End Results (SEER) Medicare-linked database with a cohort of 5072 esophageal cancer patients showed that 34.7% of patients received no treatment of any kind, and patients who left their cancer untreated tended to be nonwhite and had lower median income and lower education.11 Consistent with these results, we found that black patients, uninsured patients, patients with government insurance, and patients with lower levels of education were more likely to be offered no treatment at all. Lineback et al.12 conducted interviews and surveys among patients with esophageal cancer and found that communication difficulties, lack of understanding of treatment, and financial troubles were the main barriers to accessing optimal care in the lower socioeconomic status groups. Lack of physician expertise, nihilistic attitudes towards esophageal cancer, and mistrust in the healthcare system may also play an important role. All these obstacles should be carefully considered when developing strategies to address disparities in esophageal cancer care and improve outcomes in vulnerable populations.
Underutilization of esophagectomy in certain groups of patients has also been previously reported.13 – 15 Paulson et al. 13 found a significant underuse of esophagectomy for stages I, II, and III across all patient groups; however, in non-white and low socioeconomic patient cohorts, the underuse was even more pronounced. Steyerberg et al. 15 also reported that black patients were half as likely to undergo esophageal cancer surgery, compared to white patients. Moreover, they found that not only were black patients significantly less likely to be seen by a surgeon (70% vs. 78%), but that even among patients seen by a surgeon, black patients were significantly less likely to be offered surgical intervention (35% vs. 59%). Interestingly, we found that female patients were less likely to be offered surgical treatment when diagnosed as stage I, stage II, and stage III. Previous studies analyzing gender disparities in esophageal cancer outcomes have mostly focused on epidemiology, tumor biology, and survival outcomes.16 – 18 Based on our results, further research is needed to understand the potential underutilization of surgical treatment in female patients.
Our analysis also showed that the use of surgery was intimately affected by racial and socioeconomic factors, suggesting that improvements in referral practices are needed to ensure optimal care across all esophageal cancer patients, irrespective of race, insurance, or income status. In fact, Dubecz and colleagues19 reported that 44% of estimated esophageal cancer patients underwent esophagectomy, yet, by comparison, more than 60% of the patients seen at specialized referral centers underwent surgical resection. A main concern with referring patients to specialized centers is that it may increase disparities, particularly in patients who cannot afford to travel. Interestingly, we recently described that a process of spontaneous centralization of esophageal cancer surgery is occurring in the United States, and its benefits were reached by black patients, patients with government insurance, and patients with low household income.20 In this study, we found that patients traveling longer distances were significantly more likely to receive surgical treatment. Speicher et al.21 also reported that esophageal cancer patients who travel longer distances to high-volume centers were more likely to undergo esophagectomy and had better outcomes than patients who stay close to home at low-volume centers. Therefore, we strongly believe that enhanced referral practices will ultimately increase the use of surgery to treat esophageal cancer.
Our analysis also found that there were significant survival benefits for performing surgery in clinical stage I, II, and III esophageal cancer patients. Interestingly, the use of esophagectomy in patients with locally advanced disease has been previously challenged.22 – 24 Despite this, we found significantly better outcomes in patients with regional disease who underwent surgery, compared to those receiving definitive chemotherapy and/or radiation. Our results further highlight the importance of increasing access to surgical care in potentially resectable esophageal cancer patients.
This study has several limitations. First, the NCDB is not a population-based registry, but rather identifies patients from 1500 commission-accredited cancer programs, which potentially limits the generalizability of our findings. For instance, the possibility exists that hospitals reporting data to the NCDB may provide characteristically higher quality care than those not included. In addition, coding errors or different coding practices can occur among the different participant centers. Second, endoscopic therapies were not included in our analysis (this may explain, for example, the relatively good survival of patients with stage I disease receiving no treatment). Third, education and income are not patient-level measurements, but rather ZIP-code-level covariates obtained from the 2012 American Community Survey. Finally, it is likely that some immortal person-time bias exists in our survival analysis, as treatment was not randomized, only received treatments were assessed, and diagnosis and exposure time do not line up. However, even when we restricted to patients who survived for at least 12 months after diagnosis (in order to remove the time bias), significant differences in survival across the treatment groups were seen.
In conclusion, traditionally underserved patients with esophageal cancer appear to have limited access to surgical care, and are, in fact, more likely to not be offered any treatment at all. Considering the significant survival benefits associated with surgical resection, greater public health efforts to reduce disparities in esophageal cancer are needed.
APPENDIX
Table A1.
Comparison of characteristics of patients excluded due to missing stage with the final study sample
| Analysis sample (N = 60, 621) | Missing stage (N = 24, 867) | |
|---|---|---|
| Treatment | 4063 (7) | 5292 (21) |
| Definitive chemoradiation | 25,977 (43) | 8738 (35) |
| Chemoradiation plus surgery | 23,151 (38) | 5980 (24) |
| Surgery only | 7430 (12) | 4857 (20) |
| Race | ||
| White | 53,145 (88) | 21,482 (86) |
| Black | 5306 (9) | 2479 (10) |
| Other | 1510 (2) | 595 (2) |
| Age, years, median (IQR) | 66 (59–75) | 68 (59–78) |
| Sex | ||
| Male | 47,405 (78) | 18,752 (75) |
| Female | 13,216 (22) | 6115 (25) |
| Charlson–Deyo score, n (%) | ||
| 0 | 44,541 (73) | 17,944 (72) |
| 1 | 12,200 (20) | 5011 (20) |
| 2 | 2856 (5) | 1385 (6) |
| 3+ | 1024 (2) | 527 (2) |
| Primary insurance, n (%) | ||
| Private | 20,755 (34) | 7809 (31) |
| Medicaid | 3910 (6) | 1471 (6) |
| Medicare | 31,815 (52) | 13,846 (56) |
| Uninsured | 1725 (3) | 796 (3) |
| Travel distance, miles, n (%) | ||
| <5 miles | 15,094 (25) | 7303 (29) |
| 5–11.9 miles | 14,509 (24) | 6182 (25) |
| 12–31.9 miles | 14,844 (24) | 5555 (22) |
| ≥32 miles | 15,258 (25) | 5264 (21) |
| Median residential income†, n (%) | ||
| Less than $38,000 | 10,574 (17) | 4681 (19) |
| $38,000–$47,999 | 14,567 (24) | 6081 (24) |
| $48,000–$62,999 | 16,227 (27) | 6622 (27) |
| $63,000 or more | 18,300 (30) | 6900 (28) |
| Education, % without high school degree‡, n (%) | ||
| <7% | 14,086 (23) | 5275 (21) |
| 7–12.9% | 20,557 (34) | 8183 (33) |
| 13%-20.9% | 15,659 (26) | 6569 (26) |
| ≥21% | 9406 (16) | 4282 (17) |
† Median residential household income of each patient's ZIP code was estimated using the 2012 American Community Survey.
‡Proportion of adults in patient's ZIP code who did not complete high school, measured in the 2012 American Community Survey.
Table A2.
Standardized effect of no treatment and definitive chemoradiation, compared to surgery, on 5-year mortality among nonmetastatic adult esophageal cancer cases who survived ≥12 months after diagnosis, stratified by stage
| HR (95% CI)† | P-value | |
|---|---|---|
| STAGE I | ||
| No treatment | 1.52 (1.33, 1.74) | <0.0001 |
| Definitive chemoradiation | 3.04 (2.75, 3.37) | <0.0001 |
| Surgery (with or without chemoradiation) | REF | – |
| STAGE II | ||
| No treatment | 1.41 (1.13, 1.75) | 0.0021 |
| Definitive chemoradiation | 1.57 (1.47, 1.67) | <0.0001 |
| Surgery (with or without chemoradiation) | REF | – |
| STAGE III | ||
| No treatment | 1.75 (1.45, 2.12) | <0.0001 |
| Definitive chemoradiation | 1.58 (1.49, 1.67) | <0.0001 |
| Surgery (with or without chemoradiation) | REF | – |
† Standardized across clinical cancer stage, year of diagnosis, age (modeled as a restricted cubic spline), sex, Charlson–Deyo score, race, primary insurance, travel distance to hospital, median household income, and education.
CI, confidence interval; HR, hazard ratio.
Specific author contributions: Designing the study: Francisco Schlottmann, Charles Gaber, Paula D. Strassle, Fernando A.M. Herbella, Daniela Molena, Marco G. Patti; Collecting, analyzing, and interpreting the data: Francisco Schlottmann, Charles Gaber, Paula D. Strassle, Fernando A.M. Herbella, Daniela Molena, Marco G. Patti; Writing the report: Francisco Schlottmann, Charles Gaber, Paula D. Strassle, Fernando A.M. Herbella, Daniela Molena, Marco G. Patti; Making the decision to submit for publication: Francisco Schlottmann, Charles Gaber, Paula D. Strassle, Fernando A.M. Herbella, Daniela Molena, Marco G. Patti.
Conflicts of interest: The authors have no conflict of interest.
References
- 1. Hur C Miller M Kong C Y et al. Trends in esophageal adenocarcinoma incidence and mortality.Cancer 2013;119:1149–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arnold M Laversanne M Brown L M Devesa S S Bray F.Predicting the future burden of esophageal cancer by histological subtype: international trends in incidence up to 2030.Am J Gastroenterol 2017;112:1247–55. [DOI] [PubMed] [Google Scholar]
- 3. National Cancer Institute .Surveillance, Epidemiology, and End Results Program. Cancer stat facts: esophageal cancer.Available at:https://seer.cancer.gov/statfacts/html/esoph.html.Accessed 6/29/2018. [Google Scholar]
- 4. Sauvanet A Mariette C Thomas P et al. Mortality and morbidity after resection for adenocarcinoma of the gastroesophageal junction: predictive factors.J Am Coll Surg 2005;201:253–62. [DOI] [PubMed] [Google Scholar]
- 5. Kassis E S Kosinski A S Ross P Jr Koppes K E Donahue J M Daniel V C.Predictors of anastomotic leak after esophagectomy: an analysis of the society of thoracic surgeons general thoracic database.Ann Thorac Surg 2013;96:1919–26. [DOI] [PubMed] [Google Scholar]
- 6. Baquet C R Commiskey P Mack K Meltzer S Mishra S I.Esophageal cancer epidemiology in blacks and whites: racial and gender disparities in incidence, mortality, survival rates and histology.J Natl Med Assoc 2005;97:1471–8. [PMC free article] [PubMed] [Google Scholar]
- 7. Revels S L Morris A M Reddy R M Akateh C Wong S L.Racial disparities in esophageal cancer outcomes.Ann Surg Oncol 2013;20:1136–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Taylor L J Greenberg C C Lidor A O Leverson G E Maloney J D Macke R A.Utilization of surgical treatment for local and locoregional esophageal cancer: analysis of the National Cancer Data Base.Cancer 2017;123:410–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cole S R Hernan M A.Adjusted survival curves with inverse probability weights.Comput Methods Programs Biomed 2004;75:45–49. [DOI] [PubMed] [Google Scholar]
- 10. Hernan M A Sauer B C Hernandez-Diaz S Platt R Shrier I.Specifying a target trial prevents immortal time bias and other self-inflicted injuries in observational analyses.J Clin Epidemiol 2016;79:70–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Molena D Stem M Blackford A L Lidor A O.Esophageal cancer treatment is underutilized among elderly patients in the USA.J Gastrointest Surg 2017;21:126–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lineback C M Mervak C M Revels S L Kemp M T Reddy R M.Barriers to accessing optimal esophageal cancer care for socioeconomically disadvantaged patients.Ann Thorac Surg 2017;103:416–21. [DOI] [PubMed] [Google Scholar]
- 13. Paulson E C Ra J Armstrong K Wirtalla C Spitz F Kelz R R.Underuse of esophagectomy as treatment for resectable esophageal cancer.Arch Surg 2008;143:1198–203. [DOI] [PubMed] [Google Scholar]
- 14. Taioli E Wolf A S Camacho-Rivera M et al. Racial disparities in esophageal cancer survival after surgery.J Surg Oncol 2016;113:659–64. [DOI] [PubMed] [Google Scholar]
- 15. Steyerberg E W Earle C C Neville B A Weeks J C.Racial differences in surgical evaluation, treatment, and outcome of locoregional esophageal cancer: a population-based analysis of elderly patients.JCO 2005;23:510–7. [DOI] [PubMed] [Google Scholar]
- 16. Tran P N Taylor T H Klempner S J Zell J A.The impact of gender, race, socioeconomic status, and treatment on outcomes in esophageal cancer: a population-based analysis.J Carcinog 2017;16:3.doi: 10.4103/jcar.JCar_4_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rowse P G Jaroszewski D E Thomas M et al. Sex disparities after induction chemoradiotherapy and esophagogastrectomy for esophageal cancer.Ann Thorac Surg 2017;104:1147–52. [DOI] [PubMed] [Google Scholar]
- 18. Litle V R Rice T W.The esophagus: do sex and gender matter? Semin Thorac Cardiovasc Surg 2011;23:131–6. [DOI] [PubMed] [Google Scholar]
- 19. Dubecz A Sepesi B Salvador R et al. Surgical resection for locoregional esophageal cancer is underutilized in the United States.J Am Coll Surg 2010;211:754–61. [DOI] [PubMed] [Google Scholar]
- 20. Schlottmann F Strassle P D Charles A G Patti M G.Esophageal cancer surgery: spontaneous centralization in the US contributed to reduce mortality without causing health disparities.Ann Surg Oncol 2018;25:1580–7. [DOI] [PubMed] [Google Scholar]
- 21. Speicher P J Englum B R Ganapathi A M et al. Traveling to a high-volume center is associated with improved survival for patients with esophageal cancer.Ann Surg 2017;265:743–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stahl M Stuschke M Lehmann N et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus.JCO 2005;23:2310–7. [DOI] [PubMed] [Google Scholar]
- 23. Bedenne L Michel P Bouché O et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102.JCO 2007;25:1160–8. [DOI] [PubMed] [Google Scholar]
- 24. Vellayappan B A Soon Y Y Ku G Y Leong C N Lu J J Tey J C.Chemoradiotherapy versus chemoradiotherapy plus surgery for esophageal cancer.Cochrane Database Syst Rev 2017;8:CD010511. [DOI] [PMC free article] [PubMed] [Google Scholar]


