Abstract
Previous studies have only investigated age-related differences in emotional processing and encoding in response to, not in anticipation of, emotional stimuli. In the current study, we investigated age-related differences in the impact of emotional anticipation on affective responses and episodic memory for emotional images. Young and older adults were scanned while encoding negative and neutral images preceded by cues that were either valid or invalid predictors of image valence. Participants were asked to rate the emotional intensity of the images and to complete a recognition task. Using multivariate behavioral partial least squares (PLS) analysis, we found that greater anticipatory recruitment of the amygdala, ventromedial prefrontal cortex (vmPFC), and hippocampus in older adults predicted reduced memory for negative than neutral images and the opposite for young adults. Seed PLS analysis further showed that following negative cues older adults, but not young adults, exhibited greater activation of vmPFC, reduced activation of amygdala, and worse memory for negative compared with neutral images. To the best of our knowledge, this is the first study to provide evidence that the “positivity effect” seen in older adults’ memory performance may be related to the spontaneous emotional suppression of negative affect in anticipation of, not just in response to, negative stimuli.
Keywords: aging, fMRI, memory, spontaneous emotional regulation, uncertainty
Introduction
Though many cognitive abilities have been found to decline with age, emotional processing seems to improve with age. This has been described as both an age-related shift toward positive and away from negative information (Mather and Carstensen 2005). For example, eye-tracking studies have shown that older adults fixate more on neutral than negative images, whereas young adults show the opposite pattern (Mather and Carstensen 2003; Isaacowitz et al. 2006; Knight et al. 2007). In memory studies, older adults typically remember more positive and/or neutral than negative stimuli, whereas young adults remember more negative stimuli (for review, see Mather 2012). It could be argued that rather than showing an improvement in emotional processing, older adults show reduced sensitivity to negative affect. However, visual search studies have demonstrated that the ability to detect negative affect is spared with age (Mather and Knight 2006; Leclerc and Kensinger 2008). This “positivity effect” in older adults is best explained by the socioemotional selectivity theory (SST), which states: as individuals age and their perceived time left in life diminishes, they focus more on improving their quality of life by regulating their emotions and less on other goals, such as information seeking (Carstensen et al. 1999).
Consistent with SST, a number of studies have found that older adults are more likely to spontaneously downregulate negative affect than young adults. That is, when not given instructions to regulate, older adults spontaneously engage in emotional regulation strategies, whereas young adults have to be explicitly instructed to do so (for review, see Mather 2012). When older adults do spontaneously downregulate, self-reports suggest that they are more likely to engage in suppression than any other type of emotional regulation strategy (Nolen-Hoeksema and Aldao 2011; Eldesouky and English 2018). Emotional suppression is defined as inhibiting one’s outward emotional reaction (for review, see Richards 2004). During negative reappraisal, individuals try to reinterpret the emotional meaning of the stimulus. This elaborative processing during reappraisal tends to result in enhanced memory for the emotional stimulus, but this avoidance during emotional suppression tends to result in impaired memory (Dillon et al. 2007). Consistent with self-reports, emotional suppression seems to be the most likely explanation for the pattern of results we see in older adults outlined above, notably the negativity avoidance and impaired memory for negative events.
The “cognitive control model” extension of the SST posits that the ability to regulate emotion is dependent upon prefrontal cortical (PFC) function (Mather and Knight 2005). Older adults with higher cognitive control abilities, as determined with neuropsychological tests, are more likely to show the positivity effect than those with lower cognitive control abilities (Petrican et al. 2008). This theory is also supported by a number of neuroimaging studies that have found an increase in medial PFC (mPFC) activity for negative compared to neutral stimuli in older, but not young adults (for review, see Nashiro et al. 2012). Moreover, this increase in mPFC activity, particularly the ventromedial PFC (vmPFC), is coupled with a decrease in amygdala activity during viewing of negative images (Gunning-Dixon et al. 2003; Urry et al. 2006; St Jacques et al. 2010; Leclerc and Kensinger 2011; Sakaki et al. 2013). This inverse relationship is proposed to be reflective of successful emotional regulation, such that the increased vmPFC activity represents older adults’ employment of cognitive control strategies to reduce negative affect, which is mediated by amygdala activity (Leclerc and Kensinger 2011).
Previous studies have only assessed the impact of age on emotional processing and encoding in direct response to emotional stimuli. However, in the real world, we can often anticipate emotional events, such as a scheduled surgery or screeching tires before an accident, which allow us to prepare for and regulate our emotional reaction. This leads one to question if age-related differences in emotional processing and memory are due solely to changes in the recruitment of processes elicited by stimuli or also in those recruited in anticipation of these stimuli. The impact of emotional anticipation on selected attention, memory, and brain activity has been well-investigated in young adults (Mackiewicz et al. 2006; Nitschke et al. 2006; Galli et al. 2011; Grupe et al. 2013). As outlined above, even though aging is known to affect each of these aspects of emotional processing, virtually nothing is known about the impact of emotional anticipation on these processes in older adults. Emerging results from young adult studies suggest that the neural activity during the time period preceding stimulus presentation is also related to episodic memory performance. The majority of these studies have investigated “pre-stimulus subsequent memory effects” during encoding (Otten et al. 2006; Gruber and Otten 2010; Otten et al. 2010; Padovani et al. 2011; Galli et al. 2012; Cohen et al. 2015). Although the mechanisms underlying these effects are not entirely clear, existing evidence suggests that they are highly dependent on the same factors that influence encoding and retrieval effects like stimulus modality, emotional valence, type of encoding strategy implemented, reward incentives, and the format in which memory is tested. Consequently, pre-stimulus memory effects are thought to reflect, at least in part, preparatory mobilization of processes that contribute to memory performance (Adcock et al. 2006; Otten et al. 2006; Addante et al. 2015). It is possible that at least some previous evidence of age-related differences in emotional memory encoding may be more accurately characterized as on setting prior to stimulus presentation.
Typically, in emotional anticipation studies, young adults are given a cue (i.e., an X or O) that symbolizes the valence of the following image, thus allowing young adults to anticipate the upcoming image (Mackiewicz et al. 2006; Nitschke et al. 2006; Galli et al. 2011; Grupe et al. 2013). These studies found that the same brain regions involved in the presentation of negative images are also activated during the anticipation of negative images. These regions include a number of emotional processing and cognitive control regions: the amygdala, the anterior cingulate cortex (ACC), the mPFC, the dorsolateral PFC (DLPFC), the insula, and the hippocampus (Simmons et al. 2004; Mackiewicz et al. 2006; Nitschke et al. 2006). Interestingly, Mackiewicz et al. (2006) found that the anticipatory activity in the amygdala and hippocampus predicted successful memory encoding of negative stimuli above and beyond picture viewing activity, suggesting that emotional anticipation plays a crucial role in attention and cognitive processes that likely lead to elaborative encoding of the following stimuli in young adults.
However, there is often uncertainty regarding the emotional significance of future events. For example, one might be asked to unexpectedly meet with their boss and, given recent layoffs in the company, one may start to anticipate that they are getting laid off in that meeting. This uncertainty reduces one’s ability to effectively prepare an optimal response and potentially enhance anxiety (Grupe and Nitschke 2011). Generally, the effect of uncertainty is investigated by comparing certain negative events with uncertain negative events. The findings of these studies indicate that uncertain events are associated with stronger affective responses through self-reported mood, anxiety, and valence ratings (Nader and Balleine 2007; Bar-Anan et al. 2009; Grupe et al. 2011; Yoshida et al. 2013); physiological reactivity and hypervigilance (Grillon et al. 2004; Nelson et al. 2015); and greater activity in the amygdala, insula, ACC, and lateral PFC (Ploghaus et al. 2001; Sarinopoulos et al. 2010; Yoshida et al. 2013). In sum, these findings point to an uncertainty-dependent defensive state (i.e., threat-related vigilance) employed by young adults that results in enhanced attentional processing and emotional salience detection, likely aiming at threat elimination.
The current study investigated age-related differences in the impact of emotional uncertainty on the anticipatory processes engaged prior to encoding of naturalistic, negative, and neutral events. We manipulated participants’ emotional expectations using pre-stimulus cues in which a proportion invalidly indicated the valence of the impending image. To the best of our knowledge, this is the first functional magnetic resonance imaging (fMRI) study to investigate age-related differences in the impact of emotional anticipation on affective responses and memory for emotional events. We utilized behavioral partial least squares (PLS) analysis to determine the relationship between anticipatory neural activity, subjective emotional response ratings, and memory in young and older adults (McIntosh and Lobaugh 2004). Additionally, we utilized seed PLS to assess the functional connectivity among the regions revealed in our behavioral PLS. Based on previous findings, we hypothesize that older adults, but not young adults, will engage in spontaneous downregulation of negative affect following negative cues. Behaviorally, this will result in lower emotional intensity ratings and memory performance for negative valid than negative invalid events. Neurally, there should be evidence of an inverse coupling between the vmPFC and amygdala following negative, but not neutral cues, consistent with successful downregulation.
Materials and Methods
Participants
A total of 26 young adults and 22 older adults were initially recruited for this study. Four young adults’ data were not included in subsequent analyses due to three participants’ responses not being recorded as a result of technical issues with our experimental program and one who terminated the experiment early. Additionally, two older adults’ data were excluded due to excessive motion during scanning and an additional two participants did not complete the experimental task. These exclusions left a final sample of 22 young adults (13 females; age, 18–38 years; mean age = 22.68 years; standard deviation [SD] = 5.75 years) and 20 older adults (10 females; age, 60–76 years, mean age = 67.6 years; SD = 5.06 years). Older adults (mean education = 16.57, SD = 2.79) were slightly more educated than young adults (mean education = 14.90, SD = 2.54) [t(40) = 2.022, p = 0.050]. All participants were right handed, were native English speakers, and have normal or corrected to normal vision (using MRI-compatible glasses when necessary). Participants were excluded from the study if they reported any of the following conditions: epilepsy, Parkinson’s disease, a history of stroke or seizure, a clinical diagnosis of depression and/or anxiety, attention deficit disorder, multiple sclerosis, uncontrolled hyper- or hypo-tension, untreated diabetes, sickle cell anemia, smoking or other regular use of nicotine, use of beta blockers, alcoholism, and regular use of illegal drugs. Participants were given a battery of questionnaires including the Center for Epidemiological Studies—Depression (CES-D; Radloff 1977) and the Geriatric Depression Scale: Short Form (GDS; Yesavage 1988) to assess depressive symptomology, the Beck Anxiety Inventory Questionnaire (BAI; Beck et al. 1988) to assess anxiety symptoms, and the Intolerance of Uncertainty—12 Questionnaire (IUS-12; Carleton et al. 2007) to assess participants’ responses to uncertainty and ambiguous decisions due to the uncertainty induced in our design. Young adults scored significantly higher on all four questionnaires. Seven young adults surpassed the cut-off for clinical diagnosis on the CES-D and three on the GDS. Means and SDs of the scores for young and older adults are presented in Table 1. We collected these questionnaire data from participants between 27 and 36 months following their experimental participation. Six young adults and one older adult were not able to be reached to complete the battery of questionnaires. Although the questionnaires include both state and trait level assessments of mood, we cannot rule out the possibility that the results may have differed if the questionnaires were administered at the time of the experimental sessions. However, the data, namely the greater depression and anxiety levels for the young than older adults, are consistent with prior literature using these measures, as elaborated upon in the Discussion.
Table 1.
Group characteristics
| Neuropsych scores | ||
|---|---|---|
| Measure | Young adults (n = 21) | Older adults (n = 19) |
| Letter fluency | 47.85 (12.07) | 50.63 (14.19) |
| List recall (immediate) | 10.62 (1.49) | 10.63 (1.54) |
| List recall (immediate, cued) | 10.71 (1.41) | 10.26 (1.37) |
| List recall (delayed) | 11.19 (1.21) | 11.05 (1.31) |
| List recall (delayed, cued) | 11.52 (.87) | 10.57 (1.57)* |
| List recognition | 11.91 (.30) | 11.95 (.22) |
| MAS digit span forward | 7.19 (1.29) | 7.21 (1.58) |
| MAS digit span backward | 5.09 (1.05) | 5.11 (1.41) |
| Trails A (in seconds) | 26.47 (7.55) | 32.55 (6.63)* |
| Trails B (in seconds) | 50.90 (16.29) | 72.02 (20.67)* |
| Visual recognition | 18.71 (1.23) | 16.58 (2.99)* |
| Delayed visual recognition | 18.52 (2.82) | 17.11 (1.97) |
| Visual reproduction | 8.95 (1.28) | 5.47 (2.22)* |
| Questionnaire scores | ||
| Measure | Young Adults (n = 16) | Older adults (n = 18) |
| CES-D | 11.88 (9.46) | 3.61 (4.72)* |
| GDS | 2.93 (2.99) | 0.63 (1.08)* |
| BAI | 10.69 (9.18) | 3.22 (3.13)* |
| IUS-12 | 25.81 (6.41) | 20.94 (5.82)* |
Note: SDs in parentheses. All neuropsychological test and questionnaire scores are reported as raw scores.
*Significantly lower scores than young (P < 0.05).
All participants were recruited from Georgia Tech or the surrounding Atlanta community. Participants were compensated $10 an hour plus an additional $5 for travel expenses. Any young adults who were enrolled in a psychology course at Georgia Tech were given the option to receive extra credit instead of monetary compensation. All participants signed consent forms approved by the Georgia Institute of Technology Institutional Review Board.
Neuropsychological Assessment
Immediately after the recognition session, the participants were given a series of neuropsychological tests to ensure that no individual differences in task performance were due to cognitive impairments. The testing contained the Wechsler Adult Intelligence Test (WAIS-R) (Wechsler 1981) digit symbol substitution and digit span forward and backward tasks, the Shipley Vocabulary Test (Shipley 1946), and the 64-card version of the Wisconsin Card Sorting Test (Lezak 1995). Any participant whose score fell two SDs below their age-adjusted mean score given their level of education was removed from the sample. One older adult who had recently completed the assessment for another experiment and one young adult who was already familiar with the test were excluded from testing. Means and SDs of the scores for each neuropsychological assessment for young and older adults are presented in Table 1.
Materials
Stimuli consisted of 480 colored images, 240 from the Nencki Affective Picture System (NAPS; Marchewka et al. 2013) and 240 from the International Affective Picture System (IAPS; Lang et al. 2008). We used both sets of images in order to achieve a sufficient number of highly arousing negative images. The images were selected to be the most unpleasant and arousing negative images based on published norms using the Self-Assessment Manikin Scale, each ranging from 1 to 9 (1 = very negative, 9 = very positive; 1 = relaxed, 9 = aroused). In total there were 240 negative images (mean valence = 3.11, mean arousal = 6.07) and 240 neutral images (mean valence = 6.21, mean arousal = 4.01). The two auditory cues were chosen were from the International Affective Digital Sounds (Bradley and Lang 2007). The negative cue was a 1-s clip of tires screeching (mean valence = 3.11, mean arousal = 6.07) and the neutral cue was a 1-s clip of a wind chime (mean valence = 6.10, mean arousal = 4.23).
Procedure
A practice session was administered before each task to familiarize the participants with the task and to ensure they could perform the task. Only the encoding blocks were scanned. Stimuli were counterbalanced across participants such that each picture was not in the same category (valid vs. invalid, old vs. new) across participants. There were 320 stimuli shown during encoding, as well as an additional 160 stimuli shown during retrieval.
The encoding task consisted of 5 blocks, with 80 trials in each block. Each block consisted of 64 regular trials (32 negative, 32 neutral), with 80% of each of the trials preceded by a valid cue and 20% by an invalid cue, and 16 catch trials (8 negative, 8 neutral), in which no stimulus followed the cue. The 80/20 valid/invalid ratio was used to ensure that participants did not distrust the cues (Wheeler et al. 2006). As seen in Figure 1A, each trial began with a central fixation cross for 300 ms. Next, an auditory cue indicated either the correct (valid) or incorrect (invalid) valence of the upcoming picture, followed by a fixation cross for a cue stimulus interval of 1000 ms. The stimulus was then presented, and the participant was asked to rate the emotional intensity of the image on a scale of 1–4 (1 being least intense and 4 being most intense). Participants were asked to make their responses on two button boxes, using only their index and middle fingers. They were asked to respond with their left middle finger as “1” for least intense, their left index finger as “2” for less intense, their right index finger as “3” for more intense, and their right middle finger as “4” for most intense. A fixation cross was then presented on the screen for a fixed 500-ms interval before the participant was asked to complete the arrows task. The arrows task maximizes design efficiency by pseudorandomly interspersing event trials with “active” baseline trials lasting between 2 and 6 s, jittered in increments of 2 s (Dale 1999). Every 2 s, an arrow appears on the screen and participants are asked to respond using the button box to indicate the direction of the arrow: “1” in response to a left pointing arrow and “2” for a right pointing arrow. Requiring participants to respond to the arrows kept them engaged in the task and minimized default mode network activity (Stark and Squire 2001). Catch trials differed from full trials in that no stimuli were presented following the cue. Because stimulus onset occurred at a short, fixed interval directly after cue onset, catch trials were used to separate cue activity from stimulus activity in blood-oxygen-level-dependent (BOLD) responses, as has been done in prior studies (Wheeler et al. 2006). Stimuli were presented in a pseudorandomized order in each block. Each block lasted 12 minutes, and a brief rest period was allowed between each block to prevent fatigue. Including the structural and resting state scans acquired, the encoding session lasted 1 hour and 15 minutes.
Figure 1.
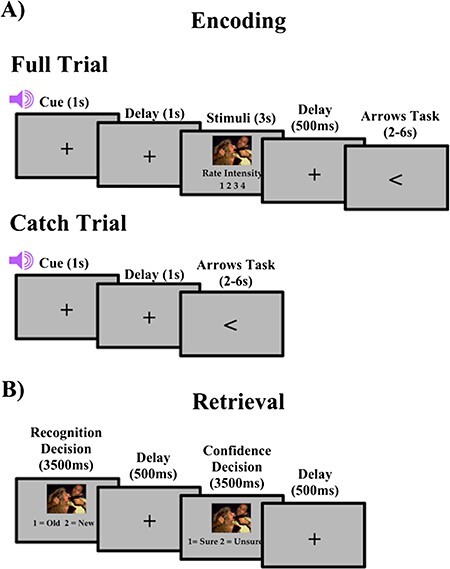
Task design for both encoding and retrieval. (A) Full and catch trial design for encoding. (B) Trial design for retrieval.
After the encoding task, participants immediately exited the scanner and began the recognition task. The recognition portion of the study was divided into 5 blocks with 96 trials, 64 old stimuli, and 32 new stimuli in each block. For each trial, participants were asked to answer two questions about the picture (Fig. 1B). First, participants viewed the picture for 3500 ms and indicated if the image was old by responding with “1” on a keypad or new by responding with “2” on a keypad. Second, participants viewed the image again for 3500 ms and made a confidence judgment, if they were sure about their recognition decision by responding with “1” or unsure by responding with “2”. A fixation cross was displayed in the center of the screen for 500 ms between the questions. Confidence judgments were used to separate strong recognition from guessing, with unsure responses being considered as guesses.
fMRI Acquisition
Scanning was performed on a 3-T Siemens TIM Trio system. Functional data were acquired using a gradient echo pulse sequence (37 transverse slices oriented along the anterior–posterior commissural axis with a 30-degree upward tilt to avoid the eyes, repetition time of 2 s, echo time of 30 ms, 3 × 3 × 3.5-mm voxels, 0.8-mm interslice gap). Five encoding blocks of 371 volumes each were acquired. The first two volumes of each block were discarded to allow for equilibration effects. A high-resolution T1-weighted magnetization-prepared rapid acquisition gradient echo (MPRAGE) image was collected for normalization.
fMRI Analyses
Preprocessing
Data were analyzed using SPM12 (SPM12, http://www.fil.ion.ucl.ac.uk/spm/software/spm12/). Images were corrected for differences in slice timing acquisition using the middle slice of each volume as the reference, spatially realigned and resliced with respect to the first volume of the first block. Each participant’s MPRAGE scan was coregistered to the mean echo planar imaging (EPI) image, produced from spatial realignment. Each coregistered structural scan was then segmented using the Diffeomorphic Anatomical Registration Through Exponentiated Lie algebra (DARTEL) SPM 12 toolbox (Ashburner 2007). DARTEL is a suite of tools fully integrated with SPM 12, which the SPM 12 manual recommends over optimized normalization, to achieve sharper nonlinear registration, for intersubject alignment. This method also achieves better localization of fMRI activations in Montreal Neurological Institute (MNI) space. This method has been used successfully in several previous studies with various healthy and neurological populations (Yassa and Stark 2009; Pereira et al. 2010). Briefly, the gray and white matter segmented images were used to create a study-specific template using the DARTEL toolbox and the flow fields containing the deformation parameters to this template for each subject were used to normalize each participant’s realigned and resliced EPIs to MNI space. Normalized EPI images were written to 3 × 3 × 3 mm and smoothed with an 8-mm full width at half-maximum isotropic Gaussian kernel.
Multivariate PLS Analysis
Multivariate behavioral PLS was used to analyze the fMRI data to identify whole-brain patterns of task-related activity at encoding that correlate with subsequent memory accuracy and/or subjective emotional intensity ratings in young and older adults (McIntosh et al. 2004). We chose this approach because PLS is a powerful data-driven method that identifies distributed patterns of activated voxels that differ across experimental conditions and/or relate to a specific behavioral measure without using pre-specified contrasts and does not require that the assumptions of normality, independence of observations, and linearity for general linear models be met (Van Roon et al. 2014).
Cue Period Behavioral PLS
As our primary interest was to investigate regions recruited during the anticipation of emotional events that correlate with subsequent emotional memory accuracy and subjective emotional intensity ratings, we only included catch trails (i.e., trials in which the participant was only presented a cue, but no stimulus) in this behavioral PLS. There were two conditions, negative catch trials and neutral catch trials, and two groups, young adults and older adults. fMRI data for these catch trials were stored in a data matrix that was organized by condition and stacked across participants, within age group, and age groups were stacked above one another as follows: young-negative catch trial, young-neutral catch trial, old-negative catch trial, and old-neutral catch trial. fMRI data for each event onset (time lag = 0) as well as the ensuing seven volumes (2 s × 7 = 14 sec) following each event onset were included in the data matrix. We then created two behavioral vectors that were stacked in the same order as our fMRI data matrix. The first vector included each individual’s memory accuracy benefit for negative compared with neutral events calculated as d’ for negative minus d’ for neutral events. The second vector included each individual’s intensity rating difference between negative and neutral events. We included difference scores in order to investigate how anticipatory neural activity relates to proportionally better memory performance due to emotional valence. Because participants did not know if a picture was valid or invalid until stimulus presentation, we only investigated valence effects in this analysis and, thus, our behavioral measures were collapsed across cue validity. We could have separated valid and invalid for our behavioral measures; however, the analysis would be less powerful due to lower trial counts. Further, our valid and invalid d’ [r(42) = 0.940, p < 0.001] and intensity rating [r(42) = 0.897, p < 0.001] estimates are correlated; thus when we ran the PLS with just valid d’ and intensity rating measures, we got similar results. The two behavioral vectors were cross correlated with the fMRI data. Singular value decomposition of the resulting cross-correlation matrix was conducted to yield a set of orthogonal latent variables (LVs). Each LV contains 1) a singular value, which reflects the amount of covariance accounted for by the LV, and 2) a singular image, showing a pattern of whole-brain activity that is symmetrically related to 3) a correlation profile, which depicts how subjects’ memory accuracy and intensity ratings related to the pattern of brain activity observed in the singular image. The singular image includes brain saliences, which are numerical weights assigned to each voxel at each TR/time lag included in the data matrix. Brain saliences can be negative or positive. Brain regions with positive voxel saliences are positively related to the correlations profile, and those with negative voxel saliences are negatively related to the correlational profile. Thus, the pattern of whole-brain activity identified by the singular image is symmetrically related to the correlation profile.
The significance of LVs was assessed through permutation tests on the singular values using an alpha of p < 0.05 and 1000 permutations. The permutation test involved sampling each subject’s behavioral measure and event-related activity without replacement to randomly reassign the behavior–brain activity associations. For each permuted iteration, a PLS was recalculated, and the probability that the permuted singular values exceeded the observed singular value for a given LV was used to assess significance at p < 0.05 (McIntosh et al. 2004). The permutation method used met the exchangeability criterion as described in McIntosh and Lobaugh (2004). The standard error for each singular image was calculated by conducting 500 bootstraps to sample subjects with replacement while maintaining the experimental and group order. The ratio of the original brain salience to the bootstrap standard error (bootstrap ratio [BSR]) was used to identify maximal reliable patterns of positive and negative brain saliences represented by the singular image. In the current study, BSR significance was set to ±3.28, which is equivalent to p < 0.001, with a minimum cluster size of 10. In order to determine the subset of time lags that maximally represented the correlation profiles of LVs, we computed temporal brain scores for each condition in each significant LV (see McIntosh et al. 2004 for further details). Using these temporal brain scores, we identified the peak time lags in this B-PLS to be 2–6 s (4–12 s post-event onset). All activations reported for this analysis will only be within these lags.
Cue Period Seed PLS
Seed PLS was conducted to assess the functional connectivity of regions identified in our behavioral PLS. Specifically, we wanted to assess the functional connectivity between the vmPFC and the amygdala to determine if older adults were engaging in spontaneous emotional regulation following the presentation of the cue, to a greater extent than young adults. We identified one seed voxel in the right vmPFC [x = 18, y = 66, z = −3 mm] and extracted its mean baseline corrected activity values, with 0 neighborhood voxels, for each subject. This seed was selected for three reasons: 1) it was reliably activated during our behavioral PLS, 2) it is well evidenced that this region is recruited during emotional processing and regulation (for review, Nashiro et al. 2012), and 3) although there are other vmPFC clusters from our behavioral PLS, this seed voxel was in a cluster that did not extend beyond the vmPFC. Based on the results of our previous analysis described above, which indicated that memory accuracy but not intensity ratings was indicative of emotional regulation, we additionally included memory accuracy (d’ for negative minus d’ for neutral events) as a behavioral vector in this PLS to determine the relationship of memory accuracy with vmPFC activity. Seed PLS was conducted identically to the behavioral PLS described above except that, instead of using only behavioral measures, we used each subject’s BOLD signal value from the seed voxel averaged across lags 2–6 and memory accuracy as vectors of interest.
Results
For all behavioral statistical tests, the Huynh–Feldt correction was applied where appropriate and is reflected in the P values and the error terms.
Neuropsychological Assessment Results
Means and SDs for the neuropsychological assessment scores are shown in Table 1 . All participants scored within 2 SDs of age-adjusted normative averages for all the neuropsychological tests. Older adults had significantly worse performance than young adults on several tests: cued/delayed list recall, trails A, trails B, visual recognition, delayed visual recognition, and visual reproduction (t’s > 2.071, p’s < 0.045]. There were no other significant group differences (t’s < 1.372, p’s > 0.178].
Depression and Anxiety Questionnaire Results
Means and SDs for the questionnaire scores are shown in Table 1. Young adults had significantly higher scores than older adults on the measures of depression, CES-D [t(32) = 3.277, p = 0.003] and GDS [t(32) = 3.041, p = 0.005], and on the measures of anxiety and uncertainty, BAI [t(32) = 3.247, p = 0.003] and the IUS-12 [t(32) = 2.320, p = 0.027]. One older adult outlier was removed from the analysis because they were at least 4 SDs above the mean for each questionnaire. Removing them from our fMRI analyses did not affect the results.
To determine if depression or anxiety symptoms contributed to differences in memory performance and emotional intensity ratings between negative and neutral images and invalid and valid images for each age group, we ran Pearson correlations between the scores on the questionnaires with difference scores of negative–neutral memory accuracy and emotional intensity ratings and difference scores of invalid–valid memory accuracy and emotional intensity ratings. For young adults, questionnaire scores did not correlate with negative–neutral memory (r’s < 0.329, p’s < 0.861) and negative–neutral emotional intensity ratings (r’s < 0.331, p’s < 0.853) and invalid–valid memory (r’s < 0.261, p’s < 0.749) and invalid–valid emotional intensity ratings (r’s < 0.422, p’s < 0.916). For older adults, questionnaire scores did not correlate with negative–neutral memory (r’s < 0.048, p’s < 0.861) and negative–neutral emotional intensity ratings (r’s < 0.051, p’s < 0.853) and invalid–valid memory (r’s < 0.087, p’s < 0.749) or invalid–valid emotional intensity ratings (r’s < 0.029, p’s < 0.916).
Behavioral Results
Emotional Intensity Ratings
We created a weighted emotional intensity score by giving a 4 to highly intense emotional ratings, a 3 to moderately intense emotional ratings, a 2 to less intense emotional ratings, and a 1 to least intense emotional ratings. We then averaged all the rating scores for each stimulus category for each participant. The emotional intensity data is displayed in Table 2.
Table 2.
Mean emotional intensity ratings across valence, cue, and age
| Young adults | Older adults | ||
|---|---|---|---|
| Neutral | Valid | 1.84 (0.51) | 1.93 (0.61) |
| Invalid | 1.82 (0.45) | 1.89 (0.55) | |
| Negative | Valid | 2.76 (0.43) | 2.83 (0.46) |
| Invalid | 2.72 (0.43) | 2.63 (0.52) |
Note: SDs in parentheses.
These data were submitted to a 2 age (young, old) × 2 valence (negative, neutral) × 2 cue (valid, invalid) analysis of variance (ANOVA). There was a main effect of valence [F(1,40) = 148.493, p < 0.0001, η2 = 0.788], a main effect of cue [F(1,40) = 7.164, p = 0.011, η2 = 0.152], and a valence × cue interaction [F(1,40) = 5.146, p = 0.029, η2 = 0.114]; all other effects were not significant (Fs < 1, ps > 0.777, η2s < 0.002). Follow-up ANOVAs for each separate valence category revealed that the main effect of cue was reliable for negative images [F(1,41) = 8.775, p = 0.005, η2 = 0.176] but not for neutral images [F(1,41) < 1, p = 0.400, η2 = 0.017]. Both young and older adults rated negative valid images as more intense than negative invalid images [t(41) = 2.962, p = 0.005].
Memory Accuracy
Percentage of hits, misses, and false alarms (FAs) for each of the stimulus categories and groups are shown in Table 3. Only high-confidence responses were included in the analyses.
Table 3.
Mean proportion of hits, misses, false alarms, and correct rejections for young and older adults for each valence, validity, and confidence category
| Old images | ||||||||
|---|---|---|---|---|---|---|---|---|
| Young adults | ||||||||
| Neutral | Negative | |||||||
| Valid | Invalid | Valid | Invalid | |||||
| Hits | Misses | Hits | Misses | Hits | Misses | Hits | Misses | |
| High confidence | 0.67 (0.16) | 0.12 (0.13) | 0.70 (0.16) | 0.13 (0.12) | 0.73 (0.13) | 0.10 (0.07) | 0.74 (0.14) | 0.09 (0.07) |
| Low confidence | 0.11 (0.07) | 0.10 (0.08) | 0.08 (0.05) | 0.09 (0.07) | 0.08 (0.05) | 0.09 (0.07) | 0.08 (0.05) | 0.09 (0.05) |
| Older adults | ||||||||
| Neutral | Negative | |||||||
| Valid | Invalid | Valid | Invalid | |||||
| Hits | Misses | Hits | Misses | Hits | Misses | Hits | Misses | |
| High confidence | 0.67 (0.20) | 0.14 (0.12) | 0.67 (0.18) | 0.14 (0.12) | 0.68 (0.17) | 0.12 (0.09) | 0.64 (0.17) | 0.17 (0.19) |
| Low confidence | 0.09 (0.10) | 0.10 (0.07) | 0.08 (0.10) | 0.11 (0.06) | 0.10 (0.09) | 0.10 (0.07) | 0.09 (0.10) | 0.10 (0.07) |
| New images | ||||||||
| Young adults | ||||||||
| Neutral | Negative | |||||||
| CRs | FAs | CRs | FAs | |||||
| High confidence | 0.67 (0.16) | 0.06 (0.07) | 0.62 (0.15) | 0.08 (0.07) | ||||
| Low confidence | 0.18 (0.12) | 0.09 (0.06) | 0.20 (0.13) | 0.10 (0.05) | ||||
| Older adults | ||||||||
| Neutral | Negative | |||||||
| CRs | FAs | CRs | FAs | |||||
| High Confidence | 0.57 (0.22) | 0.10 (0.06) | 0.44 (0.22) | 0.17 (0.08) | ||||
| Low Confidence | 0.24 (0.16) | 0.09 (0.07) | 0.24 (0.15) | 0.15 (0.10) | ||||
Note: FAs, false alarms; CRs, correct rejections; SDs in parentheses.
We analyzed high-confidence item memory performance using signal detection theory [z (hit rate) − z (FA rate)]. The d’ data were submitted to a 2 age (young, old) × 2 valence (negative, neutral) × 2 cue (valid, invalid) ANOVA. These data are presented in Figure 2. There was a main effect of valence [F(1,40) = 14.170, p = 0.001, η2 = 0.262], a main effect of age [F(1,40) = 21.764, p < 0.001, η2 = 0.352], a valence × age interaction [F(1,40) = 8.423, p = 0.006, η2 = 0.174], and a cue × age interaction [F(1,40) = 5.988, p = 0.019, η2 = 0.130]; all other effects were not significant (Fs < 1, p’s > 0.311, η2s < 0.017). Follow-up ANOVAs for each age group showed that the main effect of valence was reliable for older adults only. Older adults remembered more neutral than negative images [F(1,19) = 26.889, p < 0.001, η2 = 0.586], whereas young adults remembered a similar number of neutral and negative images [F(1,21) < 1, p = 0.573, η2 = 0.015]. The main effect of cue was reliable for young adults only. Young adults remembered more invalid than valid images [F(1,21) = 5.179, p = 0.033, η2 = 0.198], whereas older adults remembered a similar number of invalid and valid images [F(1,19) = 1.327, p = 0.264, η2 = 0.065].
Figure 2.
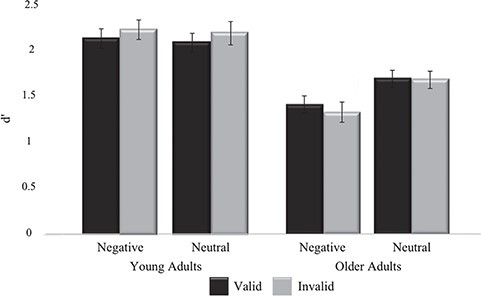
High confidence memory accuracy (d’) for young and older adults for each stimulus category. Error bars represent the standard error of the mean.
Memory Bias
To determine if older adults do have a disproportionately higher FA rate for negative than neutral stimuli compared to young adults, we submitted the high-confidence negative and neutral hit rate and high-confidence negative and neutral FA rate data to a 2 age (young, old) × 2 valence (negative, neutral) × 2 memory (hit rate, FA rate) ANOVA. There was a main effect of valence [F(1,40) = 21.709, p < 0.001, η2 = 0.352], a main effect of memory [F(1,40) = 770.810, p < 0.001, η2 = 0.951], a memory × age interaction [F(1,40) = 5.708, p = 0.022, η2 = 0.125], a valence × memory interaction [F(1,40) = 5.819, p = 0.021, η2 = 0.127], and a valence × memory × age interaction [F(1,40) = 17.905, p < 0.001, η2 = 0.309]; all other effects were not significant (Fs < 1, p’s < 0.519, η2s < 0.010). Follow-up ANOVAs for each age group revealed that the valence × memory interaction was significant in older adults [F(1,19) = 15.720, p = 0.001, η2 = 0.453) but not young adults [F(1,21) = 2.510, p = 0.128, η2 = 0.107]. Paired t-tests showed that older adults had a higher FA rate for negative compared to neutral images [t(19) = 4.161, p = 0.001] but had no difference in hit rate for negative compared to neutral images [t(19) = 0.526, p = 0.605].
Since we confirmed that older adults do have a disproportionately higher FA rate for negative than neutral stimuli compared to young adults, we calculated response bias using criterion estimates (c). Using signal detection theory, c was calculated as [(((−z (hit rate)) + z (false alarm rate))/2)] for both negative and neutral images. The c data were submitted to a 2 age (young, old) × valence (negative, neutral) ANOVA. There was a main effect of valence [F(1,40) = 14.170, p = 0.001, η2 = 0.262], a main effect of age [F(1,40) = 21.764, p < 0.001, η2 = 0.352], a valence × age interaction [F(1,40) = 8.423, p = 0.006, η2 = 0.174]. Follow-up ANOVAs for each age group revealed that the main effect of valence was reliable for older adults only. Older adults were significantly more biased toward selecting old for negative than neutral images [F(1,19) = 26.889, p < 0.001, η2 = 0.586], whereas young adults showed no bias for negative compared with neutral images [F(1,21) < 1, p = 0.573, η2 = 0.015].
Correlation between Memory Accuracy and Emotional Intensity Ratings
We hypothesized that if older adults were engaging in spontaneous downregulation, then intensity ratings would decrease with memory performance following negative, but not neutral cues. However, recent research has determined that when older adults are not given instructions on how to regulate, they are more likely to engage in emotional suppression than any other type of emotional regulation strategy (Nolen-Hoeksema and Aldao 2011; Eldesouky and English 2018). As emotional suppression has been found to impair memory performance but has no effect on intensity ratings (Gross 2002), we wanted to determine if both intensity ratings and memory accuracy were indicative of regulation in the present study. In order to determine this, we ran Pearson correlations between intensity ratings and memory accuracy for both valence categories. Results indicate that intensity ratings and memory accuracy were not correlated for young adults for both negative [r(22) = −0.290, p = 0.191] and neutral images [r(22) = 0.004, p = 0.986] nor for older adults for both negative [r(20) = −.057, p = 0.811] and neutral images [r(20) = −.093, p = 0.696].
fMRI Results
Cue Period Behavioral PLS
The behavioral PLS analysis identified one significant LV. This LV, which accounted for 31.49% of the total cross-block covariance (p < 0.001), is presented in Figure 3A, and the local maxima for this LV are presented in Table 4. Only negative salience brain regions from this LV withstood our spatial extent cutoff of a minimum of 10 clusters and BSR threshold of ±3.28, meaning all negative bars in the correlation profile represent positive relationships with the regions and all positive bars represent negative relationships with the regions. The PLS correlation profile in Figure 3B demonstrates that, following negative cues, activity in these negative salience regions was positively correlated with negative intensity ratings and with negative, compared with neutral, memory accuracy, relative to neutral intensity and accuracy, respectively, for young adults. In contrast, in older adults’ activity in these same regions was negatively correlated with memory accuracy for older adults. Following neutral cues, activity in these negative salience regions was positively correlated with negative intensity ratings for both young and older adults. These regions included bilateral amygdala, bilateral ACC, bilateral dorsomedial PFC, right vmPFC, bilateral insula, and the left hippocampus.
Figure 3.
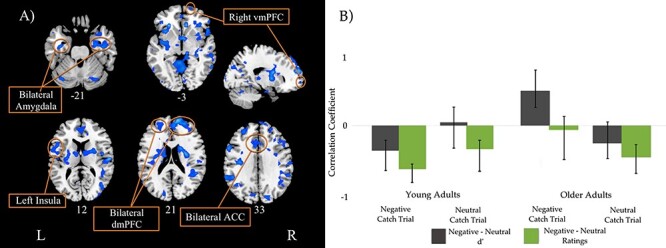
Singular image and correlation profile for the behavioral PLS LV. (A) Singular image for the behavioral PLS LV. Only negative salience regions (in blue) withstood our spatial extent cutoff (minimum of 10 clusters), threshold BSR of ±3.28, P < 0.001. (B) Brain–behavior correlation profile for LV. The correlation profile indicates following negative cues; these negative salience regions are positively correlated with greater negative intensity ratings and with greater negative memory accuracy for young adults, but worse negative memory accuracy for older adults. Following neutral cues, these negative salience regions are positively correlated with greater negative intensity ratings for both young and older adults. Error bars represent 95% confidence.
Table 4.
Behavioral PLS LV brain regions in which task-related activity showed age effects and differentially related to memory accuracy and ratings
| Temporal lag | BSR | Spatial extent | MNI coordinates | HEM | Gyral location | Brodmann area | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Negative salience regions | ||||||||
| 3 | −5.7118 | 28 | −36 | −9 | −27 | Left | Amygdala | |
| 4, 6 | −4.4107 | 51 | 24 | −3 | −27 | Right | Amygdala | |
| 3 | −4.0841 | 15 | 18 | 66 | −3 | Right | Medial frontal gyrus (vmPFC) d | 10 |
| 3 | −4.8688 | 15 | 3 | 39 | −21 | Right | Medial frontal gyrus (vmPFC) | 10/11 |
| 3, 5, 6 | −6.07 | 438 | −42 | 3 | −12 | Left | Insula a | 13 |
| 2, 4 | −5.3362 | 145 | 33 | 30 | −6 | Right | Insulaa | 13 |
| 2, 3, 4, 5, 6 | −5.356 | 253 | −15 | −87 | 24 | Left | Cuneus | 17/18 |
| 3, 5 | −4.7151 | 38 | −12 | −51 | 0 | Left | Lingual gyrus | 19 |
| 2, 4, 5 | −5.6808 | 238 | 42 | −69 | 15 | Right | Middle occipital gyrus | 19/39 |
| 3 | −4.4994 | 107 | 60 | −15 | 33 | Right | Postcentral gyrus | 2/3 |
| 2 | −5.0489 | 15 | 15 | 36 | 12 | Right | Anterior cingulate | 24/32 |
| 6 | −4.1266 | 35 | 0 | 3 | −6 | Left | Anterior cingulate | 25 |
| 2, 6 | −4.5913 | 40 | −6 | −15 | 45 | Left | Cingulate gyrus | 31 |
| 2, 3 | −4.7605 | 45 | 21 | −66 | 21 | Right | Posterior cingulate | 31 |
| 3, 5 | −5.2796 | 111 | −12 | 24 | 30 | Left | Cingulate gyrus (dACC) | 24/32 |
| 2 | −4.3596 | 51 | 3 | 21 | 33 | Right | Cingulate gyrus (dACC) | 32 |
| 3, 5, 6 | −5.4848 | 119 | 57 | −69 | 3 | Right | Middle temporal gyrus | 21/37 |
| 2, 3, 5, 6 | −6.6655 | 225 | −3 | 42 | 60 | Left | Superior frontal gyrus (premotor cortex) | 6 |
| 2, 3, 5, 6 | −7.7168 | 27 | 45 | 12 | 54 | Right | Middle frontal gyrus (premotor cortex) | 6 |
| 3, 4 | −5.392 | 15 | 15 | −57 | 42 | Right | Precuneus | 7 |
| 3, 4 | −7.2164 | 553 | −27 | 42 | 45 | Left | Middle frontal gyrusc | 8/9 |
| 2, 4 | −5.6301 | 92 | −21 | 54 | 24 | Left | Superior frontal gyrus (dmPFC) | 9/10 |
| 2, 3, 4, 5, 6 | −7.1361 | 521 | 12 | 60 | 21 | Right | Superior frontal gyrus (dmPFC) | 9/10 |
| 4 | −6.7549 | 1904 | −30 | −24 | −12 | Left | Hippocampusb | |
| 2, 6 | −5.0098 | 67 | −30 | −9 | 9 | Left | Putamen | |
| 2, 3, 5 | −7.0149 | 2781 | 24 | 6 | 6 | Right | Putamen | |
Note: HEM = hemisphere. bolded regions are shown in Figure 3.
aExtends into inferior frontal gyrus.
bExtends into parahippocampus.
cExtends into superior frontal gyrus.
dCan also be referred to as the orbitofrontal cortex.
Cue Period Seed PLS
The seed PLS analysis identified one significant LV. This LV, which accounted for 28.22% of the total cross-block covariance (p = 0.001), is presented in Figure 4A, and local maxima of selected regions for this LV are presented in Table 5 . Only negative salience brain regions from this LV withstood our spatial extent cut-off of a minimum of 10 clusters and BSR threshold of ±3.28, meaning all negative bars in the correlation profile represent positive relationships with the regions and all positive bars represent negative relationships with the regions. The PLS correlation profile in Figure 4B demonstrates that, for young adults, activity in this set of regions is positively correlated with activity in the right vmPFC seed following negative and neutral cues and additionally with negative relative to neutral memory accuracy following negative cues. For older adults, activity in this set of regions is negatively correlated with activity in the right vmPFC seed as well as negative relative to neutral memory accuracy following negative cues. These regions included bilateral anterior cingulate, right amygdala, bilateral insula and bilateral ventrolateral PFC.
Figure 4.
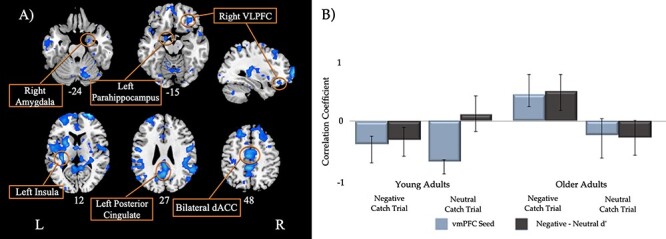
Singular image and correlation profile for the Seed PLS LV. (A) Singular image for the seed PLS LV. Only negative salience regions (in blue) withstood our spatial extent cutoff (minimum of 10 clusters), threshold BSR of ±3.28, p < 0.001. (B) Brain–behavior correlation profile for LV. The correlation profile indicates following negative cues; these negative salience regions are positively correlated with activity in the right vmPFC seed and negative relative to neutral memory accuracy for young adults but are negatively correlated with activity in the right vmPFC seed and negative relative to neutral memory accuracy for older adults. Following neutral cues, activity in these negative salience regions is positively correlated with activity in the right vmPFC seed. Error bars represent 95% confidence.
Table 5.
Regions functionally connected to the right vmPFC and differentially related to memory accuracy following negative and neutral cues for both young and older adults
| Temporal lag | BSR | Spatial extent | MNI coordinates | HEM | Gyral location | Brodmann area | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Negative salience regions | ||||||||
| 4 | −5.175 | 10 | 21 | −3 | −24 | Right | Amygdala | |
| 2, 5 | −6.218 | 227 | −33 | 63 | 15 | Left | Superior frontal gyrusa | 9/10 |
| 2, 3, 4, 5 | −9.7633 | 934 | 27 | 66 | 0 | Right | Superior frontal gyrusa | 10 |
| 4, 5 | −7.0219 | 1618 | −33 | −27 | 9 | Left | Insula | 13 |
| 4 | −6.1168 | 831 | 45 | −27 | 24 | Right | Insula | 13 |
| 6 | −6.092 | 32 | −27 | −96 | 12 | Left | Middle occipital gyrus | 18/19 |
| 6 | −4.3786 | 16 | 33 | −96 | 0 | Right | Inferior occipital gyrus | 18 |
| 5 | −5.6946 | 84 | −3 | −3 | 48 | Left | Cingulate gyrus (dACC) | 24 |
| 2, 3 | −6.7313 | 1160 | 6 | 0 | 42 | Right | Cingulate gyrus (dACC) | 24 |
| 5 | −4.9929 | 19 | −15 | −3 | −15 | Left | Parahippocampal gyrus | 28/34 |
| 5, 6 | −6.0352 | 44 | −42 | −18 | 66 | Left | Postcentral gyrus | 3 |
| 2, 3 | −6.1053 | 224 | 0 | −57 | 27 | Left | Posterior cingulate | 30/31 |
| 3, 4 | −5.1565 | 81 | −45 | 18 | −18 | Left | Inferior frontal gyrus (VLPFC) | 47 |
| 3, 5 | −5.3838 | 40 | 33 | 36 | −15 | Right | Inferior frontal gyrus (VLPFC) | 47 |
Discussion
The current study used event-related fMRI to investigate how anticipation affects episodic memory and the affective response associated with naturalistic emotional events in young and older adults. Emotional anticipation was induced by presenting participants with a cue that indicated if a negative or a neutral image was to follow, thereby allowing participants to engage in anticipatory processes. We additionally investigated the impact of emotional uncertainty on these anticipatory processes by violating participants’ expectations about the impending emotional stimuli. That is, a proportion of our cues invalidly indicated the valence of the impending image. We were able to isolate these anticipatory processes from the stimulus processes through our use of catch trials as described in the Method section. Utilizing PLS, we found that aging alters the manner in which brain regions supporting emotional processing are recruited in anticipation of emotional experiences. Consistent with previous findings, older but not young adults showed worse memory for negative compared to neutral events. Our findings suggest that older adults recruit this set of regions to engage in spontaneous regulation following negative, but not neutral cues, whereas young adults may recruit the same regions to engage in threat-related vigilance following both cues. This was further supported by the results of our seed PLS analysis in which older adults who recruited the vmPFC to a greater extent recruited the amygdala less and had worse negative memory performance following negative cues, indicative of downregulation of negative affect. The interpretation of these findings and their possible implications are discussed below.
Behavioral Results
Behaviorally, young adults remembered a similar number of negative and neutral images while older adults showed reduced memory for negative than neutral images. This was somewhat unexpected given that young adults typically remember more negative than neutral items (for review, see Talmi et al. 2013). There are a couple of possible explanations for this discrepancy. First, we tested participants’ memory relatively shortly after encoding (10–60 min.). The “modulation hypothesis of emotional memory” states that the emotional arousal of negative images induces the secretion of stress hormones that enhances activity in both the amygdala and medial temporal lobe, thereby modulating memory consolidation (Dolcos et al. 2004; McGaugh 2004), thus greater memory for negative images should only emerge after a more substantial delay (Cahill and McGaugh 1998). It is likely that if we had delayed our recognition test to 48 hours or more, we would have seen this negative memory benefit. Another nonmutually exclusive explanation for this finding is that our validity manipulation resulted in encoding to take place under divided attention. Typically, the memory benefit for negative images is under conditions of full attention to the stimuli (for review, see Talmi et al. 2013). Here, young adults were likely focusing, at least partially, on the cues to potentially determine which images were invalid versus valid, resulting in only a subset of their attention being allocated to stimulus valence (Schmidt and Saari 2007; Talmi and McGarry 2012). Consistent with this explanation is our finding that young adults remember more invalid than valid images, whereas older adults showed no difference. This likely reflects young adults but not older adults’ sensitivity to the emotional uncertainty induced in our task.
In support of our hypothesis that older adults would spontaneously downregulate negative affect following cues, older adults remembered more neutral than negative images. This is consistent with the SST. Compared with young adults, older adults have been found to focus more on their emotional regulation goals, a consequence of which is attenuated processing of negative information (Mather and Carstensen 2005; Carstensen et al. 1999). Further supporting the SST is our finding that older adults, compared with young adults, were biased to respond with “old” for negative compared with neutral images. This suggests that emotional regulation impairs the specificity of older adults’ memories of the negative images, in which they likely rely on familiarity rather than recollection for these negative images at retrieval. Previous research has investigated the role of recollection and familiarity in emotional memory and has found that memory for negative stimuli is disproportionately dependent on recollection when compared with memory for neutral stimuli (Comblain et al. 2004; Aupee 2007). Comblain et al. (2004) presented young and older adults with negative, positive, and neutral images and tested their memory for these images in a remember–know paradigm 1 week later. They found that both young and older adults report more remember responses than know responses for negative and positive than neutral images; however, older adults reported significantly less remember responses than young adults for both negative and positive images, but not neutral images. These findings suggest that the ability to rely on recollection for emotional memory is weakened with age, likely due to older adult’s engagement of emotional regulation processes that impair their memory. The imaging data discussed below support this, such that older adults who engage in emotional suppression following negative cues not only suppress their emotional response (as evidenced by their decreased amygdala activity) but also suppress activity in visual association areas, likely contributing to their impaired memory for negative images. Unfortunately, little research has been conducted on the effect of emotional regulation processes on familiarity and recognition processes. Future work should aim to determine this effect by scanning retrieval processes.
An extension of the SST, the cognitive control model, hypothesizes that emotion regulation is dependent upon cognitive control resources (Mather and Knight 2005). Consistent with both the SST and the cognitive control model was the lack of a difference in memory performance between valid and invalid images for older adults. It is possible that the 80/20 validity manipulation was insufficiently distracting to draw older adults’ attention away from emotion regulation, which they prioritize, if permitted by task demands. Other studies have found that when older adults have a challenging secondary task to perform, such as a concurrent auditory listening task while encoding emotional images, they perform similarly to the young, remembering more negative than neutral or positive images (Mather and Knight 2005; Knight et al. 2007).
In regard to our intensity ratings, we found that, for both young and older adults, negative images, regardless of validity, induced a greater affective response than did neutral images. Interestingly, we found that both young and older adults rated negative valid images as more intense than negative invalid images. This is consistent with young adults engaging in threat-related vigilance due to their increased sensitivity to our cue-validity manipulation. This is inconsistent, however, with the idea that spontaneous downregulation during anticipation of negative events would manifest as reduced intensity ratings for valid than invalid stimuli. This discrepancy between affective ratings and memory has some precedent in the existing literature, however. Older adults are more likely to engage in emotional suppression than any other type of emotional regulation strategy when not given instructions on how to regulate (Nolen-Hoeksema and Aldao 2011; Eldesouky and English 2018). Previous evidence suggests that emotional suppression instructions generally result in impaired memory but has no effect on the subjective affective response (Gross 2002). That is, affective response ratings for emotional stimuli are similar under passive viewing and directed emotional suppression instructions across age. Researchers have speculated that, during emotional suppression, young adults are focused on controlling their outward expression of negative affect (i.e., facial expression and vocal responses) at the expense of deep encoding of the emotional stimuli (for review, see Richards 2004; Dillon et al. 2007). In the current study, older adults may not have engaged in elaborative processing of the negative stimuli, at the expense of their memory. The imaging data discussed below support this. Further evidence supporting the notion that arousal ratings are not indicative of regulation in the current study is our finding that, for both young and older adults, there was no correlation between intensity ratings and memory accuracy for negative or neutral images.
Consistent with our behavioral results that reflect young adults’ increased sensitivity to the emotional uncertainty induced in our task, young adults were significantly more anxious and more intolerant of uncertainty than older adults. Previous research has shown that young adults with heightened anxiety show excessive anticipatory responses under conditions of emotional uncertainty (for review, Grupe and Nitschke 2013). Older adults, on the other hand, had none to very little mood disturbances and showed the typical emotional regulation pattern expected by the SST (Carstensen et al. 1999). Older adults who show mood disturbances tend to show mood-related biases such that when older adults are induced into a sad mood they remember and attend to more negative than positive and neutral images (for review, see Knight and Durbin 2015). Additionally, anxious older adults tend to show attentional and memory biases toward threatening information (for review, see Knight and Durbin 2015) but we found no evidence that older adults were sensitive to the emotional uncertainty induced in our task. Further, our results on the questionnaires are consistent with epidemiology studies that have found that anxiety and depression are more prevalent in young than older adults (Hasin et al. 2005). Even though our questionnaires were administered after our experiment, the data are consistent with the pattern of results found in our study. However, we cannot rule out that the possibility that the results may have differed if the questionnaires were administered at the time of the experiment.
Older Adults Engage in Spontaneous Downregulation
It is important to note before we discuss our results that the behavioral PLS analyses are capturing the between-subject differences in the relationship between memory performance, emotional intensity ratings, and brain activity. These analyses are not sensitive to within-person trial-to-trial variability. Our behavioral PLS revealed a set of regions in which activity during negative versus neutral catch trials was differentially correlated with memory accuracy and emotional intensity ratings for negative relative to neutral images in young adults, compared with older adults. The regions identified in this analysis included areas associated with emotional processing (i.e., the amygdala, ACC, and insula) (Davis 1992; Davis and Whalen 2001), memory encoding (i.e., the hippocampus) (for review, see Yonelinas 2013), and self-referential processing (i.e., the vmPFC and dmPFC) (for further discussion, see Etkin et al. 2011). In young adults, increased cue-related (anticipatory) activity in these regions was related to subsequent increases in intensity ratings and to greater subsequent memory accuracy for negative, compared with neutral images. In contrast, in older adults, activation of these same brain regions was negatively associated with subsequent memory accuracy for negative, compared with neutral stimuli. This suggests that older adults may have engaged in spontaneous downregulation of negative affect when they anticipated an impending negative image, which in turn negatively impacted their ability to effectively encode mnemonic stimuli. This interpretation is corroborated by the fact that the set of regions identified by this analysis has also been identified in studies assessing automatic/spontaneous downregulation, namely emotional suppression and by prior findings showing that older adults rely more on the medial PFC for emotional suppression, whereas reappraisal has been found to rely more on the lateral PFC (for further discussion see, Phillips et al. 2003; Gyurak et al. 2011). Together, our imaging and behavioral results add to the growing body of literature suggesting that older adults, to a greater extent than young adults, engage in spontaneous emotional suppression that results in impaired negative memory accuracy. Most notably though, this is the first study, to the best of our knowledge, to provide evidence that older adults engage in spontaneous emotional suppression in anticipation, not just presentation, of negative events.
While behavioral PLS reveals the relationship between brain regions and behavioral measures, it does not reveal the relationship between the brain regions. We were particularly interested in the functional connectivity between the vmPFC and the amygdala for a few reasons. The vmPFC has been repeatedly indicated in emotional processing (Bush et al. 2000; Taylor et al. 2003; Ochsner et al. 2004), instructed downregulation of negative affect (for review, Ochsner 2008) and self-relevant processing (for review, Wagner et al. 2012). Neuroimaging evidence has shown that it is functionally connected with the amygdala (Urry et al. 2006; St Jacques et al. 2010; Sakaki et al. 2013). Furthermore, research in rodents and human lesion studies shows that the vmPFC has a direct anatomical connection to the amygdala. In rodents, direct high-frequency stimulation to the infralimbic cortex, the homolog to the vmPFC in humans, results in reduced responsiveness of neurons in the amygdala (Quirk et al. 2003). Humans with bilateral vmPFC lesions show greater amygdala responses to negative images as well as elevated resting-state amygdala functional connectivity (Motzkin et al. 2015). Collectively, these results suggest that connectivity between the vmPFC and amygdala may underlie older adults’ anticipatory downregulation of negative affect. Using seed PLS, we found that older adults who activated the vmPFC to a greater degree exhibited less activity in the amygdala, the ACC, the hippocampus, the insula, and visual association cortex, and had worse subsequent memory for negative, compared with neutral, images following negative cues. By contrast, young adults who recruited the vmPFC to a greater extent showed positive connectivity in this same network and better subsequent memory for negative relative to neutral images following negative cues. Previous perception and memory neuroimaging studies using naturalistic emotional events, like those used here, have demonstrated that older adults, to a greater extent than young, show a negative coupling between vmPFC and amygdala activity (Gunning-Dixon et al. 2003; St Jacques et al. 2010; Leclerc and Kensinger 2011; Roalf et al. 2011). This negative connectivity pattern has been posited to represent older adults’ greater focus on emotional regulation goals that results in a top-down of the vmPFC on the amygdala.
To confirm that the relationship between the vmPFC and the set of regions in the current study indicated a top-down emotion regulation process and could not be attributed to other neural processes, we conducted a separate seed PLS using the insula as the seed. The insula was chosen as our seed for two reasons: 1) it was reliably activated in our behavioral PLS and 2) it is well evidenced that this region is recruited during emotional processing (Augustine 1996; Phelps et al. 2001; Critchley et al. 2002). If the pattern of vmPFC seed connectivity is reflective of top-down suppression of negative affect contributing to reduced negative memory in older adults, as we propose, the same pattern of results should not be observed in the insula seed PLS. These data are presented in our supplementary materials. As expected, we found the opposite relationship in the insula seed PLS, such that older adults who activated the insula more had worse subsequent memory for negative, compared with neutral images following negative cues and activated the amygdala, the ACC and visual association cortex more. Thus, the results of this PLS confirm that the negative coupling between the vmPFC and the set of regions, specifically the amygdala, is indicative of emotional regulation. The current results extend previous findings by showing that older adults who employ vmPFC-mediated emotional suppression strategies to reduce negative affect do so proactively, during anticipation of negative events. Notably, the anticorrelation between the vmPFC, the hippocampus, and visual association cortex areas further suggests that anticipatory emotion regulation engagement contributes to older adults’ impoverished negative memory accuracy through emotional suppression of episodic encoding mechanisms.
These findings are consistent with other findings of age-related differences in motivated anticipation tasks that have young and older adults anticipate monetary gains (i.e., positive experiences) and monetary losses (i.e., negative experiences) (Samanez-Larkin et al. 2007; Nielsen et al. 2008). These studies typically find that young and older adults do not differ in gain anticipation but do in loss anticipation. Older adults typically have less self-reported negative arousal and less activation of the insula, caudate, and ACC than young adults, suggesting that older adults are engaging in some type of anticipatory emotional regulation to reduce their negative affect following loss cues but not gain cues. Interestingly, the findings of the current study and the motivated anticipation studies previously described are in contrast to other findings of age-related differences in motivated anticipation tasks such as goal maintenance tasks that require young and older adults to update and maintain goal information from contextual cues before deciding about a probe. Such studies typically find that older adults, compared with young adults, are less likely to engage in proactive control processes and more likely to engage in reactive control processes (Paxton et al. 2008). Paxton et al. (2008) suggest that older adults have difficulty initiating and maintaining a goal in order to engage in a proactive control strategy. They hypothesize that this is due to older adults having reduced neural resources relative to young adults, which inhibits their ability to process the cue proactively and maintain the cue information throughout the trial. However, we believe that the present results suggest that older adults’ ability to use a proactive strategy depends on their priority of the goal the task requires. Such that, as discussed in the SST, older adults prioritize emotional goals more than information-seeking goals and thus in emotional regulation paradigms, such as the one in the current study, older adults are able proactively process the cue as it is more in line with their goals.
It is important to mention that we cannot rule out the possibility that at least some of the results may be attributed to subjects, particularly older adults, averting their gaze to impending negative images. Previous eye-tracking studies have found that older adults, but not young adults, look away from negative stimuli, which they speculate may have resulted in their worse memory for negative stimuli (Isaacowitz et al. 2006; Knight et al. 2007). However, we do not believe that eye movements can fully account for the present pattern of results. First, if older adults were looking away when presented with negative cues, we would predict that their memory would be worse for neutral invalid (i.e., negatively cued) than neutral valid images and better for negative invalid (i.e., neutral cued) than negative valid images. Instead, we found that validity had no impact on memory performance for older adults. Further, it is unclear how the relationship we observed between activity in emotional processing regions and memory performance could be explained by older adults looking away from the screen when presented with a negative cue. Future fMRI studies incorporating eye tracking may prove useful in determining the presence and impact of eye movements on the neural processes of emotional regulation and its relationship with memory.
Young Adults May Engage in Threat-Related Vigilance
The set of emotional processing regions recruited during spontaneous downregulation by older adults was differently recruited by young adults. Specifically, young adults who exhibited greater activity in these regions had subsequent increases in intensity ratings and greater subsequent memory accuracy for negative, compared with neutral images following both negative and neutral cues. This pattern of results is most consistent with a threat-monitoring strategy in which young adults were constantly preparing for negative affect, regardless of the cue. As discussed above, young adults were particularly sensitive to the cue-validity manipulation, having better memory for invalid than valid images. We tentatively suggest that this induced a sense of unpredictability, which has been shown previously to result in a threat-monitoring state in young adults (Grillon et al. 2004; Bar-Anan et al. 2009). This sense of unpredictability results in stronger affective responses (Nader and Balleine 2007; Bar-Anan et al. 2009; Grupe and Nitschke 2011; Yoshida et al. 2013) and similar engagement of emotional processing regions during negative and neutral events (Baas et al. 2006; Shackman et al. 2011; Nelson et al. 2015). Thus, our finding of greater activity in the amygdala, ACC, and insula resulting in a stronger affective response following both negative and neutral cues is consistent with previous threat-monitoring research.
It is important to note here the discrepancy between our behavioral and PLS findings for young adults in which memory accuracy and emotional intensity ratings were not behaviorally correlated yet they shared a similar relationship with activity in the regions mentioned above. The intensity ratings were measured at the same time as our imaging data (i.e., during encoding), whereas memory performance was measured following the collection of imaging data. As memory performance is a function of encoding, consolidation, and retrieval processes, it is possible that this dissociation arises from a discrepancy in the latter two processes. Unfortunately, we cannot address this discrepancy in the current study as we only collected imaging data at encoding. Future studies should investigate the role retrieval and consolidation neural processes have in emotional anticipation.
One last point to consider is our use of auditory cues to indicate the valence of the impending stimuli. In previous emotion anticipation studies, visually presented letter cues, (i.e., X or O) or different neutral tones, have been used to cue impending emotional or neutral events in young adults (Mackiewicz et al. 2006; Nitschke et al. 2006; Sarinopoulos et al. 2010; Grupe et al. 2013). We initially piloted our study using these kinds of cues and found that older adults had greater difficulty mapping associations between these cues and valence categories than young adults. We instead opted to use negative and neutral cues depicting typical naturalistic sounds: screeching tires and wind chimes, respectively, which differed in arousal and valence, similarly to the stimuli they predicted. Piloting showed that young and older adults were equally able to remember the association between these cues and the stimulus valence they predicted. These cues possess ecological validity in their association with neutral and negative emotional events, and this may have contributed to the ability of both young and older adults to map these associations. It should also be noted that the use of auditory, rather than visual, cues reduced the possibility that any anticipatory visual perceptual neural activity could be explained by perceptual processing of the cues. Because cue-related neural activity was predictive of intensity and memory performance indices associated with the stimuli, we believe that the current results support our assertion that cue-related neural activity reflects the mobilization of emotion- and memory-related network activity in anticipation of impending emotional events.
Additionally, it is important to mention that, because we used emotionally valanced auditory cues, it is possible that older adults were more distracted by the negative screeching tires cue than the neutral tone cue and this in turn affected their encoding processes rather than motivated emotional regulation having an effect on their encoding processes. Unfortunately, we cannot be certain if the anticipatory regulation, and its subsequent effect on memory, is a proactive-motivational process or a reactive-distractive process in the current study. However, we favor the proactive regulation account for a few reasons. For one, the pattern of brain activity we observed is consistent with previous findings in which cues were not emotionally salient. In an emotional memory study in young adults, Mackiewicz et al. (2006) used visual cues (i.e., X and O) to indicate the valence of the upcoming stimulus and found that amygdala and hippocampus activity during the anticipatory period was related to subsequent memory performance of the negative images. We observed a similar pattern following the auditory cues in young adults in our study. Second, we found in our older adults that the inverse relationship between the vmPFC and amygdala, a well-established neutral pattern of activity that is associated with emotional regulation (Gunning-Dixon et al. 2003; St Jacques et al. 2010; Leclerc and Kensinger 2011; Roalf et al. 2011) was also predictive of subsequent memory accuracy. If older adults were just being distracted by the auditory cues, we do not believe that we would see activity consistent with regulation and see this relationship between that activity and subsequent memory performance. Third, the negative and neutral auditory cues were repeated 200 times each across the experiment while the pictures were trial unique. It seems quite likely that any emotional response to the cues, per se, would habituate to a degree over time. Nonetheless, the possibility remains that reactive emotional responses to the auditory cues may contribute to the patterns of neural activity and memory performance. However, this may be less important to disentangle because in the real world we often cannot disentangle negative sounds from negative events. For example, we may hear screeching tires and then get into a car accident. The screeching tires cannot be separated from the accident itself and are still apart of how we react to and encode the event. It seems that proactively regulating an event can be a mixture of both a reaction to the cue and a motivation to downregulate the upcoming event.
Conclusion
The current study provides evidence that older adults, but not young adults, spontaneously engage in downregulation of negative affect, namely emotional suppression during the anticipation of negative events. These novel results show that a contributor to the “positivity effect” in aging is anticipatory emotional suppression that leads to impaired memory for negative relative to neutral events. Future work should investigate how spontaneous emotional suppression of negative emotion in older adults contributes to the positivity effect in real-world situations. One question that remains unanswered is when spontaneous emotional suppression manifests in the adult lifespan. Future studies should include middle-aged adults to determine when this age-related shift to spontaneous suppression occurs.
Supplementary Material
Funding
Ruth L. Kirschstein National Research Service Award (NRSA) Institutional Research Training Grant (T32AG000175).
Notes
We would like to thank our research participants and research assistants for their time and contribution to the study.
References
- Adcock RA, Thangavel A, Whitfield-Gabrieli S, Knutson B, Gabrieli JD. 2006. Reward-motivated learning: mesolimbic activation precedes memory formation. Neuron. 50:507–517. [DOI] [PubMed] [Google Scholar]
- Addante RJ, de Chastelaine M, Rugg MD. 2015. Pre-stimulus neural activity predicts successful encoding of inter-item associations. Neuroimage. 105:21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J. 2007. A fast diffeomorphic image registration algorithm. Neuroimage. 38:95–113. [DOI] [PubMed] [Google Scholar]
- Augustine JR. 1996. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev. 22:229–244. [DOI] [PubMed] [Google Scholar]
- Aupee AM. 2007. A detrimental effect of emotion on picture recollection. Scand J Psychol. 48:7–11. [DOI] [PubMed] [Google Scholar]
- Baas JM, Milstein J, Donlevy M, Grillon C. 2006. Brainstem correlates of defensive states in humans. Biol Psychiatry. 59:588–593. [DOI] [PubMed] [Google Scholar]
- Bar-Anan Y, Wilson TD, Gilbert DT. 2009. The feeling of uncertainty intensifies affective reactions. Emotion. 9:123–127. [DOI] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. 1988. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 56:893–897. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. The international affective digitized sounds (2nd edition; IADS-2): affective ratings of sounds and instruction manual. Technical report B-3. Gainesville, Fl: University of Florida; 2007. [Google Scholar]
- Bush G, Luu P, Posner MI. 2000. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 4:215–222. [DOI] [PubMed] [Google Scholar]
- Cahill L, McGaugh JL. 1998. Mechanisms of emotional arousal and lasting declarative memory. Trends Neurosci. 21:294–299. [DOI] [PubMed] [Google Scholar]
- Carleton RN, Norton MA, Asmundson GJ. 2007. Fearing the unknown: a short version of the intolerance of uncertainty scale. J Anxiety Disord. 21:105–117. [DOI] [PubMed] [Google Scholar]
- Carstensen LL, Isaacowitz DM, Charles ST. 1999. Taking time seriously. A theory of socioemotional selectivity. Am Psychol. 54:165–181. [DOI] [PubMed] [Google Scholar]
- Cohen N, Pell L, Edelson MG, Ben-Yakov A, Pine A, Dudai Y. 2015. Peri-encoding predictors of memory encoding and consolidation. Neurosci Biobehav Rev. 50:128–142. [DOI] [PubMed] [Google Scholar]
- Comblain C, D'Argembeau A, Van der Linden M, Aldenhoff L. 2004. The effect of ageing on the recollection of emotional and neutral pictures. Memory. 12:673–684. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Dolan RJ. 2002. Fear conditioning in humans: the influence of awareness and autonomic arousal on functional neuroanatomy. Neuron. 33:653–663. [DOI] [PubMed] [Google Scholar]
- Dale AM. 1999. Optimal experimental design for event-related fMRI. Hum Brain Mapp. 8:109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. 1992. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 15:353–375. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. 2001. The amygdala: vigilance and emotion. Mol Psychiatry. 6:13–34. [DOI] [PubMed] [Google Scholar]
- Dillon DG, Ritchey M, Johnson BD, LaBar KS. 2007. Dissociable effects of conscious emotion regulation strategies on explicit and implicit memory. Emotion. 7:354–365. [DOI] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. 2004. Interaction between the amygdala and the medial temporal lobe memory system predicts better memory for emotional events. Neuron. 42:855–863. [DOI] [PubMed] [Google Scholar]
- Eldesouky L, English T. 2018. Another year older, another year wiser? Emotion regulation strategy selection and flexibility across adulthood. Psychol Aging. 33(4):572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. 2011. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 15:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli G, Choy TL, Otten LJ. 2012. Prestimulus brain activity predicts primacy in list learning. Cogn Neurosci. 3:160–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli G, Wolpe N, Otten LJ. 2011. Sex differences in the use of anticipatory brain activity to encode emotional events. J Neurosci. 31:12364–12370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Baas JP, Lissek S, Smith K, Milstein J. 2004. Anxious responses to predictable and unpredictable aversive events. Behav Neurosci. 118:916–924. [DOI] [PubMed] [Google Scholar]
- Gross JJ. 2002. Emotion regulation: affective, cognitive, and social consequences. Psychophysiology. 39:281–291. [DOI] [PubMed] [Google Scholar]
- Gruber MJ, Otten LJ. 2010. Voluntary control over prestimulus activity related to encoding. J Neurosci. 30:9793–9800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupe DW, Nitschke JB. 2011. Uncertainty is associated with biased expectancies and heightened responses to aversion. Emotion. 11:413–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupe DW, Nitschke JB. 2013. Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nat Rev Neurosci. 14:488–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupe DW, Oathes DJ, Nitschke JB. 2013. Dissecting the anticipation of aversion reveals dissociable neural networks. Cereb Cortex. 23:1874–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Gur RC, Perkins AC, Schroeder L, Turner T, Turetsky BI, Chan RM, Loughead JW, Alsop DC, Maldjian J et al. 2003. Age-related differences in brain activation during emotional face processing. Neurobiol Aging. 24:285–295. [DOI] [PubMed] [Google Scholar]
- Gyurak A, Gross JJ, Etkin A. 2011. Explicit and implicit emotion regulation: a dual-process framework. Cognit Emot. 25:400–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Goodwin RD, Stinson FS, Grant BF. 2005. Epidemiology of major depressive disorder: results from the National Epidemiologic Survey on Alcoholism and Related Conditions. Arch Gen Psychiatry. 62:1097–1106. [DOI] [PubMed] [Google Scholar]
- Isaacowitz DM, Wadlinger HA, Goren D, Wilson HR. 2006. Selective preference in visual fixation away from negative images in old age? An eye-tracking study. Psychol Aging. 21:40–48. [DOI] [PubMed] [Google Scholar]
- Knight BG, Durbin K. 2015. Aging and the effects of emotion on cognition: implications for psychological interventions for depression and anxiety. Psych J. 4:11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight M, Seymour TL, Gaunt JT, Baker C, Nesmith K, Mather M. 2007. Aging and goal-directed emotional attention: distraction reverses emotional biases. Emotion. 7:705–714. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): affective ratings of pictures and instruction manual. Technical Report A-8. Gainesville, FL: University of Florida; 2008. [Google Scholar]
- Leclerc CM, Kensinger EA. 2008. Effects of age on detection of emotional information. Psychol Aging. 23:209–215. [DOI] [PubMed] [Google Scholar]
- Leclerc CM, Kensinger EA. 2011. Neural processing of emotional pictures and words: a comparison of young and older adults. Dev Neuropsychol. 36:519–538. [DOI] [PubMed] [Google Scholar]
- Lezak MD. 1995. Neuropsychological assessment. 3rd ed. New York, p. 1026. [Google Scholar]
- Mackiewicz KL, Sarinopoulos I, Cleven KL, Nitschke JB. 2006. The effect of anticipation and the specificity of sex differences for amygdala and hippocampus function in emotional memory. Proc Natl Acad Sci U S A. 103:14200–14205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchewka A, Zurawski L, Jednorog K, Grabowska A. 2013. The Nencki Affective Picture System (NAPS): introduction to a novel, standardized, wide range, high-quality, realistic picture database. Behav Res Methods. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M. 2012. The emotion paradox in the aging brain. Ann N Y Acad Sci. 1251:33–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Carstensen LL. 2003. Aging and attentional biases for emotional faces. Psychol Sci. 14:409–415. [DOI] [PubMed] [Google Scholar]
- Mather M, Carstensen LL. 2005. Aging and motivated cognition: the positivity effect in attention and memory. Trends Cogn Sci. 9:496–502. [DOI] [PubMed] [Google Scholar]
- Mather M, Knight M. 2005. Goal-directed memory: the role of cognitive control in older adults' emotional memory. Psychol Aging. 20:554–570. [DOI] [PubMed] [Google Scholar]
- Mather M, Knight MR. 2006. Angry faces get noticed quickly: threat detection is not impaired among older adults. J Gerontol B Psychol Sci Soc Sci. 61:54–57. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. 2004. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 27:1–28. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Chau WK, Protzner AB. 2004. Spatiotemporal analysis of event-related fMRI data using partial least squares. Neuroimage. 23:764–775. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Lobaugh NJ. 2004. Partial least squares analysis of neuroimaging data: applications and advances. Neuroimage. 23(Suppl 1):S250–S263. [DOI] [PubMed] [Google Scholar]
- Motzkin JC, Philippi CL, Wolf RC, Baskaya MK, Koenigs M. 2015. Ventromedial prefrontal cortex is critical for the regulation of amygdala activity in humans. Biol Psychiatry. 77:276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader K, Balleine B. 2007. Ambiguity and anxiety: when a glass half full is empty. Nat Neurosci. 10:807–808. [DOI] [PubMed] [Google Scholar]
- Nashiro K, Sakaki M, Mather M. 2012. Age differences in brain activity during emotion processing: reflections of age-related decline or increased emotion regulation? Gerontology. 58:156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BD, Hodges A, Hajcak G, Shankman SA. 2015. Anxiety sensitivity and the anticipation of predictable and unpredictable threat: evidence from the startle response and event-related potentials. J Anxiety Disord. 33:62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen L, Knutson B, Carstensen LL. 2008. Affect dynamics, affective forecasting, and aging. Emotion. 8:318–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitschke JB, Sarinopoulos I, Mackiewicz KL, Schaefer HS, Davidson RJ. 2006. Functional neuroanatomy of aversion and its anticipation. Neuroimage. 29:106–116. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Aldao A. 2011. Gender and age differences in emotion regulation strategies and their relationship to depressive symptoms. Pers Individ Dif. 51:704. [Google Scholar]
- Ochsner KN. 2008. The social-emotional processing stream: five core constructs and their translational potential for schizophrenia and beyond. Biol Psychiatry. 64:48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Knierim K, Ludlow DH, Hanelin J, Ramachandran T, Glover G, Mackey SC. 2004. Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. J Cogn Neurosci. 16:1746–1772. [DOI] [PubMed] [Google Scholar]
- Otten LJ, Quayle AH, Akram S, Ditewig TA, Rugg MD. 2006. Brain activity before an event predicts later recollection. Nat Neurosci. 9:489–491. [DOI] [PubMed] [Google Scholar]
- Otten LJ, Quayle AH, Puvaneswaran B. 2010. Prestimulus subsequent memory effects for auditory and visual events. J Cogn Neurosci. 22:1212–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padovani T, Koenig T, Brandeis D, Perrig WJ. 2011. Different brain activities predict retrieval success during emotional and semantic encoding. J Cogn Neurosci. 23:4008–4021. [DOI] [PubMed] [Google Scholar]
- Paxton JL, Barch DM, Racine CA, Braver TS. 2008. Cognitive control, goal maintenance, and prefrontal function in healthy aging. Cereb Cortex. 18:1010–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira JM, Xiong L, Acosta-Cabronero J, Pengas G, Williams GB, Nestor PJ. 2010. Registration accuracy for VBM studies varies according to region and degenerative disease grouping. Neuroimage. 49:2205–2215. [DOI] [PubMed] [Google Scholar]
- Petrican R, Moscovitch M, Schimmack U. 2008. Cognitive resources, valence, and memory retrieval of emotional events in older adults. Psychol Aging. 23:585–594. [DOI] [PubMed] [Google Scholar]
- Phelps EA, O'Connor KJ, Gatenby JC, Gore JC, Grillon C, Davis M. 2001. Activation of the left amygdala to a cognitive representation of fear. Nat Neurosci. 4:437–441. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. 2003. Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biol Psychiatry. 54:504–514. [DOI] [PubMed] [Google Scholar]
- Ploghaus A, Narain C, Beckmann CF, Clare S, Bantick S, Wise R, Matthews PM, Rawlins JN, Tracey I. 2001. Exacerbation of pain by anxiety is associated with activity in a hippocampal network. J Neurosci. 21:9896–9903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Pare D. 2003. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. 23:8800–8807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. 1977. The CES-D Scale: a self report depression scale for research in the general population. Appl Psychol Measur. 1:385–401. [Google Scholar]
- Richards JM. 2004. The cognitive consequences of concealing feelings. Curr Dir Psychol Sci. 13:131–134. [Google Scholar]
- Roalf DR, Pruis TA, Stevens AA, Janowsky JS. 2011. More is less: emotion induced prefrontal cortex activity habituates in aging. Neurobiol Aging. 32:1634–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaki M, Nga L, Mather M. 2013. Amygdala functional connectivity with medial prefrontal cortex at rest predicts the positivity effect in older adults' memory. J Cogn Neurosci. 25:1206–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanez-Larkin GR, Gibbs SE, Khanna K, Nielsen L, Carstensen LL, Knutson B. 2007. Anticipation of monetary gain but not loss in healthy older adults. Nat Neurosci. 10:787–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarinopoulos I, Grupe DW, Mackiewicz KL, Herrington JD, Lor M, Steege EE, Nitschke JB. 2010. Uncertainty during anticipation modulates neural responses to aversion in human insula and amygdala. Cereb Cortex. 20:929–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt SR, Saari B. 2007. The emotional memory effect: differential processing or item distinctiveness? Mem Cognit. 35:1905–1916. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Maxwell JS, McMenamin BW, Greischar LL, Davidson RJ. 2011. Stress potentiates early and attenuates late stages of visual processing. J Neurosci. 31:1156–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley W. 1946. Institute of living scale. Los Angeles: Western Psychological Services. [Google Scholar]
- Simmons A, Matthews SC, Stein MB, Paulus MP. 2004. Anticipation of emotionally aversive visual stimuli activates right insula. Neuroreport. 15:2261–2265. [DOI] [PubMed] [Google Scholar]
- St Jacques P, Dolcos F, Cabeza R. 2010. Effects of aging on functional connectivity of the amygdala during negative evaluation: a network analysis of fMRI data. Neurobiol Aging. 31:315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark CE, Squire LR. 2001. When zero is not zero: the problem of ambiguous baseline conditions in fMRI. Proc Natl Acad Sci U S A. 98:12760–12766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmi D, McGarry LM. 2012. Accounting for immediate emotional memory enhancement. J Mem Lang. 66:93–108. [Google Scholar]
- Talmi D, Ziegler M, Hawksworth J, Lalani S, Herman CP, Moscovitch M. 2013. Emotional stimuli exert parallel effects on attention and memory. Cognit Emot. 27:530–538. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Phan KL, Decker LR, Liberzon I. 2003. Subjective rating of emotionally salient stimuli modulates neural activity. Neuroimage. 18:650–659. [DOI] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Jackson CA, Frye CJ, Greischar LL, Alexander AL et al. 2006. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J Neurosci. 26:4415–4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Roon P, Zakizadeh J, Chartier S. 2014. Partial least squares tutorial for analyzing neuroimaging data. Tutorials quant. Methods Psychol. 10:200–215. [Google Scholar]
- Wagner DD, Haxby JV, Heatherton TF. 2012. The representation of self and person knowledge in the medial prefrontal cortex. Wiley Interdiscip Rev Cogn Sci. 3:451–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. 1981. Manual for the Wechsler Adult Intelligence Scale Revised. New York: Psychological Corporation. [Google Scholar]
- Wheeler ME, Shulman GL, Buckner RL, Miezin FM, Velanova K, Petersen SE. 2006. Evidence for separate perceptual reactivation and search processes during remembering. Cereb Cortex. 16:949–959. [DOI] [PubMed] [Google Scholar]
- Yassa MA, Stark CE. 2009. A quantitative evaluation of cross-participant registration techniques for MRI studies of the medial temporal lobe. Neuroimage. 44:319–327. [DOI] [PubMed] [Google Scholar]
- Yesavage JA. 1988. Geriatric Depression Scale. Psychopharmacol Bull. 24:709–711. [PubMed] [Google Scholar]
- Yonelinas AP. 2013. The hippocampus supports high-resolution binding in the service of perception, working memory and long-term memory. Behav Brain Res. 254:34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida W, Seymour B, Koltzenburg M, Dolan RJ. 2013. Uncertainty increases pain: evidence for a novel mechanism of pain modulation involving the periaqueductal gray. J Neurosci. 33:5638–5646. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


