Abstract
Background
Tofacitinib is an oral, small molecule Janus kinase inhibitor for the treatment of ulcerative colitis (UC). We examined the effect of tofacitinib induction treatment on Inflammatory Bowel Disease Questionnaire (IBDQ) items in adults with moderate to severe UC.
Methods
Data were pooled from the randomized, 8‑week, double-blind, phase 3 OCTAVE Induction 1 and 2 studies. The IBDQ was self-administered by patients at baseline, week 4, and week 8, with higher scores indicating better health-related quality of life (HRQoL). Change from baseline in IBDQ items was analyzed for 10 mg of tofacitinib twice daily (BID) vs placebo using a linear mixed-effects model, with no multiplicity adjustment performed. Effect sizes were calculated. Subgroup analyses by tumor necrosis factor inhibitor (TNFi) experience were performed.
Results
Significant improvements (nominal P < 0.05) were observed in all IBDQ items with 10 mg of tofacitinib BID vs placebo at weeks 4 and 8. For the overall population, the largest treatment differences across all items were reported for “bowel movements been loose” at weeks 4 and 8, and “problem with rectal bleeding” at week 8 (mean treatment differences all 1.1; both in bowel symptoms domain). These items also showed the largest effect sizes. Treatment benefits were generally slightly numerically higher in TNFi-experienced vs TNFi-naïve patients.
Conclusions
Tofacitinib induction therapy improved all IBDQ items vs placebo in patients with UC, reflecting improvements in HRQoL, with greatest benefits reported in bowel symptoms domain items (Funded by Pfizer Inc; OCTAVE Induction 1 and OCTAVE Induction 2; ClinicalTrials.gov, NCT01465763 and NCT01458951, respectively).
Keywords: ulcerative colitis, tofacitinib, patient-reported outcomes
INTRODUCTION
Ulcerative colitis (UC) is a chronic inflammatory disease of the mucosa of the colon, with rectal bleeding and diarrhea the most common symptoms observed. In addition, patients can suffer from urgency, abdominal pain, incontinence, mucous discharge, night-time bowel movements, fatigue, fever, and weight loss.1
In addition to these bowel-related and systemic symptoms, patients with UC also report effects on their emotional functioning, including anxiety, depression, and fear, and their social functioning.2–5 Patients have reported anxiety relating to their UC symptoms controlling their lives, which consequently affects their health-related quality of life (HRQoL).2 Furthermore, a strong association among disease activity, disease-specific HRQoL, and psychological functioning has been shown in patients with UC.6 Therefore, UC therapies that improve clinical outcomes can also result in improvements in HRQoL measures.7, 8
Different instruments have been developed for the assessment of HRQoL in patients with inflammatory bowel disease (IBD), with the Inflammatory Bowel Disease Questionnaire (IBDQ) identified as the most widely used instrument with good reliability and validity.9 The IBDQ comprises 32 individual items that are grouped into 4 domains: bowel symptoms, systemic symptoms, emotional function, and social function.10, 11 However, the IBDQ is time-consuming to utilize; consequently, it is not routinely used in clinical practice.12
Tofacitinib is an oral, small molecule Janus kinase inhibitor for the treatment of UC. The efficacy and safety of tofacitinib has previously been shown in a phase 2 study, two phase 3 induction studies (OCTAVE Induction 1 and 2), a phase 3 maintenance study (OCTAVE Sustain), and an ongoing phase 3, open-label, long-term extension study.13–15 Furthermore, a previous publication has reported the findings for IBDQ total and domain scores for patients in the tofacitinib OCTAVE Induction 1 and 2 and OCTAVE Sustain phase 3 studies, with significant improvements reported with 10 mg of tofacitinib twice daily (BID) vs placebo in all 4 IBDQ domains at week 4 and week 8 in OCTAVE Induction 1 and 2 and with 5 and 10 mg of tofacitinib BID vs placebo in all 4 IBDQ domains at week 8, week 24, and week 52 in OCTAVE Sustain.16 However, this publication did not analyze the effect of tofacitinib on the individual IBDQ items within each domain.
The objective of the current analyses was to enrich understanding of the treatment effect of 10 mg of tofacitinib BID vs placebo on individual items of the IBDQ at weeks 4 and 8 by using data from the OCTAVE Induction 1 and 2 studies. To our knowledge, this is the first evaluation of the effect of any UC therapy on each individual IBDQ item.
MATERIALS AND METHODS
Study Design
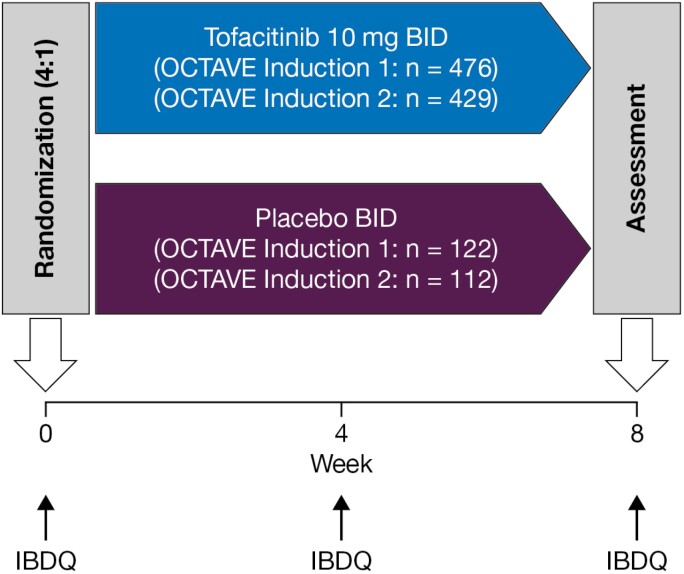
OCTAVE Induction 1 and 2 (NCT01465763 and NCT01458951) were identical 8-week, double-blind, placebo-controlled, multicenter, phase 3 induction studies in which patients were randomized 1:4 to receive placebo or 10 mg of tofacitinib BID (Fig. 1).14 There was a small number of patients who received 15 mg of tofacitinib BID before a protocol amendment who were excluded from these analyses.
FIGURE 1.
Study design of OCTAVE Induction 1 and 2. Final complete efficacy assessment at week 8. Treatment continued up to week 9; n, number of patients randomized in each treatment group.
Patients
Inclusion and exclusion criteria have been described previously.14 Briefly, patients (18 years and older) were required to have moderately to severely active disease (Mayo score 6–12, with a rectal bleeding subscore 1–3, and an endoscopic subscore of 2 or 3) and a confirmed UC diagnosis for at least 4 months. Treatment failure or intolerance to oral or intravenous glucocorticoids, azathioprine, mercaptopurine, infliximab, and/or adalimumab was a requirement for study entry. Exclusion criteria included clinical findings suggestive of Crohn’s disease, UC limited to the distal 15 cm of the colon, clinical signs of fulminant colitis, toxic megacolon, or indeterminate, microscopic, ischemic, or infectious colitis.
Concomitant oral 5-aminosalicylates and oral corticosteroids (at a maximum dose of 25 mg per day of prednisone or a prednisone equivalent) were permitted, provided that the dose remained stable throughout the induction studies, with a mandatory steroid tapering schedule to commence upon entry into the maintenance study. Tumor necrosis factor inhibitors (TNFi) were prohibited, with a washout period of 8 weeks required. Azathioprine, methotrexate, and 6-mercaptopurine were also prohibited, with a washout period of 2 weeks required.
Inflammatory Bowel Disease Questionnaire
The IBDQ consists of 4 domains and 32 items. Items are rated on a 7-point scale, with 7 representing best function and 1 representing worst function, providing a total score ranging from 32 to 224; therefore, higher scores indicate better HRQoL.11 The bowel symptom domain consists of 10 items (total domain score range 10–70), the systemic symptom domain consists of 5 items (total domain score range 5–35), the emotional function domain consists of 12 items (total domain score range 12–84), and the social function domain consists of 5 items (total domain score range 5–35).11 The 32 IBDQ questions pertaining to each item are detailed in Supplementary Table 1. In OCTAVE Induction 1 and 2, the IBDQ was self-administered by patients at baseline, week 4, and week 8.
Statistical Analyses
Baseline demographics, baseline clinical characteristics, and IBDQ item scores at baseline, week 4, and week 8 were summarized descriptively.17
The change from baseline in IBDQ item scores at week 4 and week 8 were analyzed for 10 mg of tofacitinib BID vs placebo using the same linear mixed-effects (longitudinal) model,18 as previously used for the overall and domain IBDQ scores.16 Missing data were handled by the linear-mixed effects model and were not imputed otherwise. Treatment, study, prior treatment with TNFi, corticosteroid use at baseline, geographic region, week (categorical covariate), treatment-by-week interaction, and baseline score were fixed effects in the model, and patient was a random effect. Treatment comparisons at each time point were made within this model. This was an ad hoc analysis for exploratory purposes only. No adjustment was performed for multiplicity, with nominal P values reported.
Standardized effect sizes for each IBDQ item (to quantify the size of the difference between 10 mg of tofacitinib BID and placebo) were calculated as the difference in least squares means (LSMs) for 10 mg of tofacitinib BID vs placebo, divided by the standard deviation (SD) of baseline scores, where the SD was calculated using data from both treatment groups for all patients in both studies. Based initially on (standardized) effect sizes where 0.1 = trivial, 0.2 = small, 0.5 = medium, and 0.8 = large,19, 20 the midpoints between each of these values were used to create categorization intervals on effect size as adjectival descriptors across its entire possible range (in absolute value): 0 to 0.15, trivial; >0.15 to 0.35, small; >0.35 to 0.65, medium; and >0.65, large.
Analyses were performed overall by pooling data from the 2 studies. In addition, subgroup analyses were performed in TNFi-naïve and TNFi-experienced patients.
ETHICAL CONSIDERATIONS
Both studies were conducted in compliance with the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice Guidelines and were approved by the institutional review boards (IRBs) and/or independent ethics committees at each of the investigational centers participating in the studies or a central IRB. All patients provided written informed consent.
RESULTS
Patients
In the pooled OCTAVE Induction 1 and 2 studies, 234 patients were randomized to receive placebo, and 905 patients were randomized to receive 10 mg of tofacitinib BID. Overall, the majority of patients (age, mean [SD] 41.2 [13.9] years) were male (58.6%), white (80.1%), had never smoked (64.1%), had extensive/pancolitis UC (51.5%), and had received prior TNFi (54.3%), corticosteroid (90.3%), or immunosuppressant (74.0%) treatment. Baseline demographics and disease characteristics were generally similar between treatment arms for all patients, TNFi-naïve patients, and TNFi-experienced patients. Some numerical differences were observed when comparing patients with and without prior TNFi experience, including a numerically higher proportion of TNFi-experienced patients having extensive/pancolitis disease extent and previously receiving and failing immunosuppressant treatment compared with TNFi-naïve patients (Table 1).
TABLE 1.
Demographics and Baseline Disease Characteristics for Patients in the Pooled OCTAVE Induction 1 and 2 Studies (FAS)
| All Patients | TNFi-naïve Patients | TNFi-experienced Patients | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Placebo (N = 234) | Tofacitinib 10 mg BID (N = 905) | Overall (N = 1139) | Placebo (N = 104) | Tofacitinib 10 mg BID (N = 417) | Overall (N = 521) | Placebo (N = 130) | Tofacitinib 10 mg BID (N = 488) | Overall (N = 618) | |
| Age (years), mean (SD) | 41.1 (14.4) | 41.2 (13.8) | 41.2 (13.9) | 43.2 (13.9) | 41.1 (13.5) | 41.5 (13.6) | 39.4 (14.5) | 41.3 (14.1) | 40.9 (14.2) |
| Male, n (%) | 132 (56.4) | 536 (59.2) | 668 (58.6) | 64 (61.5) | 249 (59.7) | 313 (60.1) | 68 (52.3) | 287 (58.8) | 355 (57.4) |
| White, n (%) | 186 (79.5) | 726 (80.2) | 912 (80.1) | 83 (79.8) | 335 (80.3) | 418 (80.2) | 103 (79.2) | 391 (80.1) | 494 (79.9) |
| Never smoked, n (%) | 161 (68.8) | 569 (62.9) | 730 (64.1) | 75 (72.1) | 282 (67.6) | 357 (68.5) | 86 (66.2) | 287 (58.8) | 373 (60.4) |
| Body mass index (kg/ m2),a mean (SD) | 24.6 (4.7) | 24.9 (5.0) | 24.8 (4.9) | 24.9 (4.3) | 25.2 (5.2) | 25.2 (5.0) | 24.4 (5.0) | 24.6 (4.8) | 24.6 (4.9) |
| Disease extent,b n (%) | |||||||||
| Proctosigmoiditis | 35 (15.0) | 132 (14.6) | 167 (14.7) | 23 (22.3) | 69 (16.6) | 92 (17.7) | 12 (9.2) | 63 (12.9) | 75 (12.2) |
| Left-sided colitis | 76 (32.6) | 307 (34.0) | 383 (33.7) | 35 (34.0) | 160 (38.5) | 195 (37.6) | 41 (31.5) | 147 (30.2) | 188 (30.5) |
| Extensive/ pancolitis | 122 (52.4) | 463 (51.3) | 585 (51.5) | 45 (43.7) | 186 (44.7) | 231 (44.5) | 77 (59.2) | 277 (56.9) | 354 (57.4) |
| Proctitis | 0 (0.0) | 1 (0.1)c | 1 (0.1) | 0 (0.0) | 1 (0.2)c | 1 (0.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Extraintestinal manifestations present,d n (%) | 58 (24.9) | 249 (27.6) | 307 (27.0) | 15 (14.6) | 101 (24.3) | 116 (22.4) | 43 (33.1) | 148 (30.4) | 191 (31.0) |
| Mayo score,e mean (SD) | 9.0 (1.5) | 9.0 (1.4) | 9.0 (1.4) | 8.8 (1.4) | 8.8 (1.4) | 8.8 (1.4) | 9.1 (1.5) | 9.1 (1.4) | 9.1 (1.4) |
| Prior TNFi treatment, n (%) | 130 (55.6) | 488 (53.9) | 618 (54.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 130 (100.0) | 488 (100.0) | 618 (100.0) |
| Prior corticosteroid treatment, n (%) | 217 (92.7) | 812 (89.7) | 1029 (90.3) | 101 (97.1) | 375 (89.9) | 476 (91.4) | 116 (89.2) | 437 (89.5) | 553 (89.5) |
| Oral corticosteroid use at baseline, n (%) | 113 (48.3) | 412 (45.5) | 525 (46.1) | 44 (42.3) | 173 (41.5) | 217 (41.7) | 69 (53.1) | 239 (49.0) | 308 (49.8) |
| <15 mg/dayf | 35 (34.3) | 122 (32.4) | 157 (32.8) | 14 (33.3) | 49 (29.3) | 63 (30.1) | 21 (35.0) | 73 (34.9) | 94 (34.9) |
| ≥15 mg/dayf | 67 (65.7) | 254 (67.6) | 321 (67.2) | 28 (66.7) | 118 (70.7) | 146 (69.9) | 39 (65.0) | 136 (65.1) | 175 (65.1) |
| Prior immunosuppressant treatment, n (%) | 160 (68.4) | 683 (75.5) | 843 (74.0) | 57 (54.8) | 266 (63.8) | 323 (62.0) | 103 (79.2) | 417 (85.5) | 520 (84.1) |
| Prior immunosuppressant failure, n (%) | 158 (67.5) | 661 (73.0) | 819 (71.9) | 57 (54.8) | 263 (63.1) | 320 (61.4) | 101 (77.7) | 398 (81.6) | 499 (80.7) |
aAll patients: placebo, N = 233; 10 mg of tofacitinib BID, N = 905; TNFi-naïve patients: placebo, N = 104; 10 mg of tofacitinib BID, N = 417; TNFi-experienced patients: placebo, N = 129; 10 mg of tofacitinib BID, N = 488; bAll patients: placebo, N = 233; 10 mg of tofacitinib BID, N = 903; TNFi-naïve patients: placebo, N = 103; 10 mg of tofacitinib BID, N = 416; TNFi-experienced patients: placebo, N = 130; 10 mg of tofacitinib BID, N = 487; cOne patient with proctitis was enrolled as a protocol deviation; dAll patients: placebo, N = 233; 10 mg of tofacitinib BID, N = 902; TNFi-naïve patients: placebo, N = 103; 10 mg of tofacitinib BID, N = 415; TNFi-experienced patients: placebo, N = 130; 10 mg of tofacitinib BID, N = 487; eAll patients: placebo, N = 233; 10 mg of tofacitinib BID, N = 903; TNFi-naïve patients: placebo, N = 104; 10 mg of tofacitinib BID, N = 417; TNFi-experienced patients: placebo, N = 129; 10 mg of tofacitinib BID, N = 486; fAll patients: placebo, N = 102; 10 mg of tofacitinib BID, N = 376; TNFi-naïve patients: placebo, N = 42; 10 mg of tofacitinib BID, N = 167; TNFi experienced patients: placebo, N = 60; 10 mg of tofacitinib BID, N = 209; excludes patients who took budesonide or beclometasone.
Abbreviation: FAS, full analysis set.
Baseline Distribution of IBDQ Items
In the overall population in the pooled Induction 1 and 2 studies, the IBDQ items within each domain that had the lowest baseline scores and were impacted the most by disease were “bowel movements been loose” and “problem with rectal bleeding” (mean 2.3 in both treatment arms) for the bowel symptom domain; “getting good night’s sleep” (mean 3.1 and 3.0 for 10 mg of tofacitinib BID and placebo, respectively) for the systemic symptom domain; “felt relaxed/free of tension” (mean 2.9 and 2.8 for 10 mg of tofacitinib BID and placebo, respectively) for the emotional function domain; “difficulty doing leisure/sports” (mean 3.1 and 3.0 for 10 mg of tofacitinib BID and placebo, respectively) for the social function domain. The baseline distribution for each IBDQ item was generally similar between the 2 treatment groups. Furthermore, baseline IBDQ item scores were generally similar between the overall population and TNFi-naïve and TNFi-experienced patients (Table 2).
TABLE 2.
Mean Baseline Distribution of Each IBDQ Item in the Overall Population and by TNFi Experience (FAS, Observed Case)
| Overall Population | TNFi-naïve | TNFi-experienced | ||||
|---|---|---|---|---|---|---|
| Mean (SD) | Placebo (N = 234) | Tofacitinib 10 mg BID (N = 905) | Placebo (N = 104) | Tofacitinib 10 mg BID (N = 417) | Placebo (N = 130) | Tofacitinib 10 mg BID (N = 488) |
| Bowel symptoms | ||||||
| 1. Bowel movement frequency | 3.4 (1.6)a | 3.4 (1.6)b | 3.4 (1.5)c | 3.4 (1.5)d | 3.5 (1.7) | 3.4 (1.7)e |
| 5. Bowel movements been loose | 2.3 (1.4) | 2.3 (1.3)f | 2.3 (1.5) | 2.3 (1.3)g | 2.2 (1.4) | 2.3 (1.3)h |
| 9. Troubled by cramps | 3.8 (1.5) | 3.9 (1.7)f | 3.8 (1.5) | 3.9 (1.6)g | 3.8 (1.6) | 3.9 (1.7)h |
| 13. Troubled by pain | 3.8 (1.5) | 3.9 (1.6)f | 3.8 (1.4) | 4.0 (1.6)g | 3.8 (1.6) | 3.9 (1.6)h |
| 17. Problems with passing gas | 3.9 (1.6) | 3.8 (1.7)f | 3.9 (1.5) | 3.8 (1.7)g | 3.9 (1.7) | 3.9 (1.7)h |
| 20. Troubled by bloating | 3.9 (1.6) | 3.9 (1.6)f | 4.0 (1.5) | 3.8 (1.6)g | 3.9 (1.6) | 4.0 (1.7)h |
| 22. Problem with rectal bleeding | 2.3 (1.3) | 2.3 (1.3)i | 2.3 (1.3) | 2.3 (1.4)g | 2.2 (1.3) | 2.4 (1.3)j |
| 24. Feeling of having to go | 3.6 (1.5) | 3.5 (1.4)i | 3.4 (1.4) | 3.4 (1.4)g | 3.7 (1.5) | 3.6 (1.4)j |
| 26. Accidental soiling underpants | 4.9 (1.7) | 4.7 (1.6)k | 4.8 (1.7) | 4.8 (1.7)l | 5.0 (1.6) | 4.6 (1.6)m |
| 29. Troubled by nausea | 5.2 (1.6) | 5.2 (1.6)f | 5.3 (1.7) | 5.2 (1.6)g | 5.1 (1.6) | 5.2 (1.5)h |
| Systemic symptoms | ||||||
| 2. Feeling fatigued or tired | 3.2 (1.4) | 3.2 (1.4)f | 3.2 (1.5) | 3.2 (1.4)g | 3.2 (1.3) | 3.2 (1.4)h |
| 6. How much energy have you had | 3.4 (1.2) | 3.4 (1.3)f | 3.4 (1.2) | 3.4 (1.3)g | 3.4 (1.2) | 3.5 (1.2)h |
| 10. Felt generally unwell | 3.4 (1.5) | 3.5 (1.5)i | 3.3 (1.5) | 3.5 (1.5)g | 3.4 (1.5) | 3.5 (1.6)j |
| 14. Getting good night’s sleep | 3.0 (1.7) | 3.1 (1.7)f | 3.1 (1.6) | 3.3 (1.8)g | 2.9 (1.8) | 2.9 (1.6)h |
| 18. Problem maintaining weight | 4.6 (1.9)n | 4.5 (2.0)i | 4.6 (2.0) | 4.4 (2.0)g | 4.6 (1.9)o | 4.6 (2.0)j |
| Emotional function | ||||||
| 3. Felt frustrated/impatient/restless | 3.8 (1.5) | 3.9 (1.5)f | 3.7 (1.5) | 3.8 (1.5)g | 4.0 (1.6) | 4.0 (1.5)h |
| 7. Worry about needing surgery | 4.7 (1.8) | 4.7 (1.8)f | 4.8 (1.9) | 4.7 (1.8)g | 4.6 (1.8) | 4.6 (1.7)h |
| 11. Fear of not finding a washroom | 3.2 (1.7) | 3.3 (1.7)f | 3.2 (1.7) | 3.5 (1.7)g | 3.2 (1.7) | 3.2 (1.7)h |
| 15. Felt depressed/discouraged | 4.1 (1.6) | 4.2 (1.6)f | 3.9 (1.6) | 4.1 (1.6)g | 4.3 (1.6) | 4.2 (1.6)h |
| 19. Felt worried or anxious | 3.8 (1.7) | 3.7 (1.6)f | 3.4 (1.6) | 3.7 (1.7)g | 4.1 (1.7) | 3.7 (1.6)h |
| 21. Felt relaxed/free of tension | 2.8 (1.2) | 2.9 (1.3)i | 2.7 (1.1) | 2.7 (1.3)g | 2.9 (1.2) | 3.0 (1.3)j |
| 23. Felt embarrassed | 3.7 (1.8) | 3.7 (1.7)i | 3.7 (1.8) | 3.6 (1.7)g | 3.7 (1.7) | 3.8 (1.6)j |
| 25. Felt tearful or upset | 4.8 (1.6) | 4.8 (1.6)p | 4.5 (1.7) | 4.7 (1.7)l | 5.0 (1.5) | 5.0 (1.6)j |
| 27. Felt angry | 4.3 (1.7) | 4.3 (1.7)i | 4.4 (1.8) | 4.4 (1.7)g | 4.2 (1.6) | 4.3 (1.7)j |
| 30. Felt irritable | 4.1 (1.6) | 4.2 (1.5)f | 4.2 (1.7) | 4.2 (1.5)g | 4.0 (1.5) | 4.1 (1.5)h |
| 31. Lack of understanding from others | 4.8 (1.7) | 4.9 (1.6)f | 4.8 (1.9) | 4.8 (1.6)g | 4.8 (1.6) | 4.9 (1.6)h |
| 32. Satisfied with personal life | 3.5 (1.3)n | 3.5 (1.3)f | 3.5 (1.3)q | 3.4 (1.3)g | 3.5 (1.4) | 3.6 (1.4)h |
| Social function | ||||||
| 4. Unable to attend school/work | 4.3 (1.9)r | 4.2 (2.0)p | 4.2 (2.0) | 4.3 (1.9)g | 4.4 (1.9)s | 4.2 (2.1)m |
| 8. Delay/cancel social engagements | 4.1 (1.8) | 4.1 (1.8)i | 4.0 (1.7) | 4.1 (1.8)l | 4.1 (1.8) | 4.0 (1.8)h |
| 12. Difficulty doing leisure/sports | 3.0 (1.6) | 3.1 (1.7)f | 3.1 (1.6) | 3.1 (1.7)g | 2.9 (1.6) | 3.1 (1.6)h |
| 16. Avoid attending events | 3.9 (2.1) | 3.8 (2.1)i | 4.0 (2.0) | 3.9 (2.1)l | 3.8 (2.2) | 3.7 (2.0)h |
| 28. Limited sexual activity | 3.9 (2.2)t | 3.8 (2.1)u | 4.1 (2.2)q | 3.7 (2.1)v | 3.8 (2.2)s | 3.8 (2.1)w |
aN = 229; bN = 884; cN = 99; dN = 400; eN = 484; fN = 902; gN = 415; hN = 487; iN = 901; jN = 486; kN = 899; lN = 414; mN = 485; nN = 233; oN = 129; pN = 900; qN = 103; rN = 232; sN = 128; tN = 231; uN = 895; vN = 412; wN = 483.
Abbreviations: FAS, full analysis set.
Change from Baseline in IBDQ Items
In the overall population in the pooled Induction 1 and 2 studies, significant differences (nominal P value < 0.05) in the changes from baseline for 10 mg of tofacitinib BID vs placebo were observed in all IBDQ items at week 4 and week 8. Within each domain, the largest numerical differences for 10 mg of tofacitinib BID vs placebo in the overall population were reported for “bowel movements been loose” at week 4 (difference 1.1; 95% confidence interval [CI], 0.8–1.3) and week 8 (difference 1.1; 95% CI, 0.9–1.4; both P < 0.0001), and “problem with rectal bleeding” at week 8 (difference 1.1; 95% CI, 0.9–1.4; P < 0.0001) for the bowel symptoms domain. These were also reported for “getting a good night’s sleep” at week 4 (difference 0.8; 95% CI, 0.6–1.0) and week 8 (difference 0.9; 95% CI, 0.7–1.1; both P < 0.0001) for the systemic symptoms domain. They were also reported for “fear of not finding a washroom” at week 4 (difference 0.6; 95% CI, 0.3–0.8) and week 8 (difference 0.8; 95% CI, 0.6–1.0; both P < 0.0001), “felt embarrassed” at week 4 (difference 0.6; 95% CI, 0.4–0.9; P < 0.0001), and “felt angry” at week 4 (difference 0.6; 95% CI, 0.4–0.8; P < 0.0001) for the emotional function domain; They were also reported for “avoid attending events” at week 4 (difference 0.8; 95% CI, 0.5–1.0) and week 8 (difference 1.0; 95% CI, 0.7–1.2; both P < 0.0001) and “difficulty doing leisure/sports” at week 8 (difference 1.0; 95% CI, 0.8–1.2; P < 0.0001) for the social function domain (Table 3).
TABLE 3.
Mean Change From Baseline for Each IBDQ Item in the Overall Population (FAS, Observed Case)
| Week 4 | Week 8 | |||
|---|---|---|---|---|
| Placebo (N = 234) | Tofacitinib 10 mg BID (N = 905) | Placebo (N = 234) | Tofacitinib 10 mg BID (N = 905) | |
| Bowel symptoms | ||||
| 1. Bowel movement frequency, LSM (SE) | 1.1 (0.1)a | 2.1 (0.1)b | 1.3 (0.1)c | 2.2 (0.1)d |
| Difference vs placebo (95% CI); P value | 1.0 (0.7, 1.3); P < 0.0001 | 0.9 (0.6, 1.2); P < 0.0001 | ||
| 5. Bowel movements been loose, LSM (SE) | 0.7 (0.1)e | 1.8 (0.1)f | 1.0 (0.1)g | 2.1 (0.1)h |
| Difference vs placebo (95% CI); P value | 1.1 (0.8, 1.3); P < 0.0001 | 1.1 (0.9, 1.4); P < 0.0001 | ||
| 9. Troubled by cramps, LSM (SE) | 0.6 (0.1)e | 1.3 (0.0)f | 0.8 (0.1)g | 1.4 (0.1)h |
| Difference vs placebo (95% CI); P value | 0.7 (0.5, 0.9); P < 0.0001 | 0.6 (0.4, 0.8); P < 0.0001 | ||
| 13. Troubled by pain, LSM (SE) | 0.6 (0.1)e | 1.3 (0.0)f | 0.7 (0.1)g | 1.4 (0.0)h |
| Difference vs placebo (95% CI); P value | 0.6 (0.4, 0.8); P < 0.0001 | 0.7 (0.5, 0.9); P < 0.0001 | ||
| 17. Problems with passing gas, LSM (SE) | 0.5 (0.1)e | 0.9 (0.1)f | 0.7 (0.1)g | 1.1 (0.1)h |
| Difference vs placebo (95% CI); P value | 0.3 (0.1, 0.5); P = 0.0014 | 0.4 (0.2, 0.6); P = 0.0001 | ||
| 20. Troubled by bloating, LSM (SE) | 0.6 (0.1)e | 0.9 (0.0)f | 0.6 (0.1)g | 1.0 (0.0)h |
| Difference vs placebo (95% CI); P value | 0.3 (0.1, 0.4); P = 0.0095 | 0.4 (0.2, 0.6); P < 0.0001 | ||
| 22. Problem with rectal bleeding, LSM (SE) | 1.3 (0.1)e | 2.3 (0.1)i | 1.6 (0.1)g | 2.7 (0.1)j |
| Difference vs placebo (95% CI); P value | 1.0 (0.7, 1.3); P < 0.0001 | 1.1 (0.9, 1.4); P < 0.0001 | ||
| 24. Feeling of having to go, LSM (SE) | 0.9 (0.1)e | 1.5 (0.1)i | 1.1 (0.1)g | 1.7 (0.1)j |
| Difference vs placebo (95% CI); P value | 0.5 (0.3, 0.7); P < 0.0001 | 0.7 (0.5, 0.9); P < 0.0001 | ||
| 26. Accidental soiling underpants, LSM (SE) | 0.6 (0.1)e | 1.1 (0.0)k | 0.6 (0.1)g | 1.1 (0.0)l |
| Difference vs placebo (95% CI); P value | 0.4 (0.2, 0.6); P < 0.0001 | 0.5 (0.4, 0.7); P < 0.0001 | ||
| 29. Troubled by nausea, LSM (SE) | 0.4 (0.1)e | 0.7 (0.0)f | 0.5 (0.1)g | 0.7 (0.0)h |
| Difference vs placebo (95% CI); P value | 0.3 (0.1, 0.5); P = 0.0004 | 0.3 (0.1, 0.4); P = 0.0012 | ||
| Systemic symptoms | ||||
| 2. Feeling fatigued or tired, LSM (SE) | 0.7 (0.1)e | 1.1 (0.1)f | 0.8 (0.1)g | 1.4 (0.1)h |
| Difference vs placebo (95% CI); P value | 0.5 (0.3, 0.7); P < 0.0001 | 0.6 (0.4, 0.8); P < 0.0001 | ||
| 6. How much energy have you had, LSM (SE) | 0.4 (0.1)e | 0.9 (0.0)f | 0.5 (0.1)m | 1.0 (0.0)h |
| Difference vs placebo (95% CI); P value | 0.4 (0.3, 0.6); P < 0.0001 | 0.5 (0.4, 0.7); P < 0.0001 | ||
| 10. Felt generally unwell, LSM (SE) | 0.9 (0.1)e | 1.4 (0.1)n | 1.0 (0.1)g | 1.6 (0.1)j |
| Difference vs placebo (95% CI); P value | 0.5 (0.3, 0.7); P < 0.0001 | 0.6 (0.4, 0.8); P < 0.0001 | ||
| 14. Getting good night’s sleep, LSM (SE) | 0.7 (0.1)e | 1.5 (0.1)f | 0.8 (0.1)g | 1.7 (0.1)h |
| Difference vs placebo (95% CI); P value | 0.8 (0.6, 1.0); P < 0.0001 | 0.9 (0.7, 1.1); P < 0.0001 | ||
| 18. Problem maintaining weight, LSM (SE) | 0.3 (0.1)o | 0.6 (0.1)n | 0.4 (0.1)m | 0.7 (0.1)j |
| Difference vs placebo (95% CI); P value | 0.3 (0.1, 0.5); P = 0.0071 | 0.3 (0.0, 0.5); P = 0.0215 | ||
| Emotional function | ||||
| 3. Felt frustrated/impatient/restless, LSM (SE) | 0.5 (0.1)e | 0.9 (0.0)f | 0.5 (0.1)g | 1.0 (0.0)h |
| Difference vs placebo (95% CI); P value | 0.4 (0.2, 0.6); P = 0.0002 | 0.4 (0.2, 0.6); P < 0.0001 | ||
| 7. Worry about needing surgery, LSM (SE) | 0.6 (0.1)e | 1.0 (0.0)f | 0.5 (0.1)g | 1.0 (0.0)h |
| Difference vs placebo (95% CI); P value | 0.4 (0.2, 0.6); P < 0.0001 | 0.5 (0.3, 0.7); P < 0.0001 | ||
| 11. Fear of not finding a washroom, LSM (SE) | 0.9 (0.1)e | 1.4 (0.1)f | 0.9 (0.1)g | 1.7 (0.1)h |
| Difference vs placebo (95% CI); P value | 0.6 (0.3, 0.8); P < 0.0001 | 0.8 (0.6, 1.0); P < 0.0001 | ||
| 15. Felt depressed/discouraged, LSM (SE) | 0.6 (0.1)e | 1.0 (0.0)f | 0.6 (0.1)g | 1.1 (0.0)h |
| Difference vs placebo (95% CI); P value | 0.5 (0.3, 0.7); P < 0.0001 | 0.5 (0.3, 0.7); P < 0.0001 | ||
| 19. Felt worried or anxious, LSM (SE) | 0.8 (0.1)e | 1.2 (0.1)f | 0.9 (0.1)g | 1.4 (0.1)h |
| Difference vs placebo (95% CI); P value | 0.3 (0.1, 0.5); P = 0.0009 | 0.5 (0.2, 0.7); P < 0.0001 | ||
| 21. Felt relaxed/free of tension, LSM (SE) | 0.6 (0.1)e | 1.0 (0.1)i | 0.7 (0.1)g | 1.2 (0.1)j |
| Difference vs placebo (95% CI); P value | 0.3 (0.1, 0.5); P = 0.0009 | 0.5 (0.3, 0.7); P < 0.0001 | ||
| 23. Felt embarrassed, LSM (SE) | 0.7 (0.1)e | 1.4 (0.1)i | 0.9 (0.1)g | 1.6 (0.1)j |
| Difference vs placebo (95% CI); P value | 0.6 (0.4, 0.9); P < 0.0001 | 0.7 (0.5, 0.9); P < 0.0001 | ||
| 25. Felt tearful or upset, LSM (SE) | 0.5 (0.1)e | 0.8 (0.0)p | 0.5 (0.1)g | 0.8 (0.0)q |
| Difference vs placebo (95% CI); P value | 0.3 (0.1, 0.5); P = 0.0004 | 0.3 (0.1, 0.5); P = 0.0008 | ||
| 27. Felt angry, LSM (SE) | 0.4 (0.1)e | 1.1 (0.0)i | 0.5 (0.1)g | 1.1 (0.1)j |
| Difference vs placebo (95% CI); P value | 0.6 (0.4, 0.8); P < 0.0001 | 0.6 (0.4, 0.8); P < 0.0001 | ||
| 30. Felt irritable, LSM (SE) | 0.4 (0.1)e | 0.8 (0.0)f | 0.6 (0.1)g | 1.0 (0.0)h |
| Difference vs placebo (95% CI); P value | 0.4 (0.2, 0.6); P < 0.0001 | 0.4 (0.2, 0.6); P < 0.0001 | ||
| 31. Lack of understanding from others, LSM (SE) | 0.4 (0.1)e | 0.6 (0.0)f | 0.4 (0.1)g | 0.6 (0.0)h |
| Difference vs placebo (95% CI); P value | 0.2 (0.1, 0.4); P = 0.0098 | 0.2 (0.1, 0.4); P = 0.0099 | ||
| 32. Satisfied with personal life, LSM (SE) | 0.4 (0.1)r | 0.7 (0.0)f | 0.6 (0.1)m | 0.9 (0.0)h |
| Difference vs placebo (95% CI); P value | 0.4 (0.2, 0.5); P < 0.0001 | 0.3 (0.2, 0.5); P = 0.0001 | ||
| Social function | ||||
| 4. Unable to attend school/work, LSM (SE) | 0.7 (0.1)o | 1.1 (0.1)i | 0.7 (0.1)s | 1.4 (0.1)q |
| Difference vs placebo (95% CI); P value | 0.4 (0.2, 0.6); P = 0.0007 | 0.7 (0.5, 0.9); P < 0.0001 | ||
| 8. Delay/cancel social engagements, LSM (SE) | 0.8 (0.1)e | 1.4 (0.1)n | 0.8 (0.1)g | 1.5 (0.1)j |
| Difference vs placebo (95% CI); P value | 0.6 (0.4, 0.8); P < 0.0001 | 0.8 (0.5, 1.0); P < 0.0001 | ||
| 12. Difficulty doing leisure/sports, LSM (SE) | 0.9 (0.1)e | 1.6 (0.1)n | 1.0 (0.1)g | 2.0 (0.1)h |
| Difference vs placebo (95% CI); P value | 0.7 (0.5, 0.9); P < 0.0001 | 1.0 (0.8, 1.2); P < 0.0001 | ||
| 16. Avoid attending events, LSM (SE) | 0.5 (0.1)e | 1.3 (0.1)n | 0.5 (0.1)g | 1.5 (0.1)j |
| Difference vs placebo (95% CI); P value | 0.8 (0.5, 1.0); P < 0.0001 | 1.0 (0.7, 1.2); P < 0.0001 | ||
| 28. Limited sexual activity, LSM (SE) | 0.5 (0.1)t | 1.1 (0.1)u | 0.7 (0.1)v | 1.4 (0.1)w |
| Difference vs placebo (95% CI); P value | 0.7 (0.4, 0.9); P < 0.0001 | 0.7 (0.4, 0.9); P < 0.0001 | ||
aN = 227; bN = 862; cN = 213; dN = 832; eN = 231; fN = 881; gN = 218; hN = 849; iN = 879; jN = 848; kN = 877; lN = 846; mN = 217; nN = 880; oN = 229; pN = 878; qN = 847; rN = 230; sN = 216; tN = 228; uN = 872; vN = 215; wN = 839. Abbreviations: FAS, full analysis set.
Furthermore, treatment benefits were numerically higher in TNFi-experienced patients vs TNFi-naïve patients in many IBDQ items, with differences more marked in some IBDQ items including “bowel movement frequency,” “getting a good night’s sleep,” “fear of not finding a washroom,” and “difficulty doing leisure/sports” (Supplementary Tables 2 and 3).
Effect Sizes of IBDQ Items
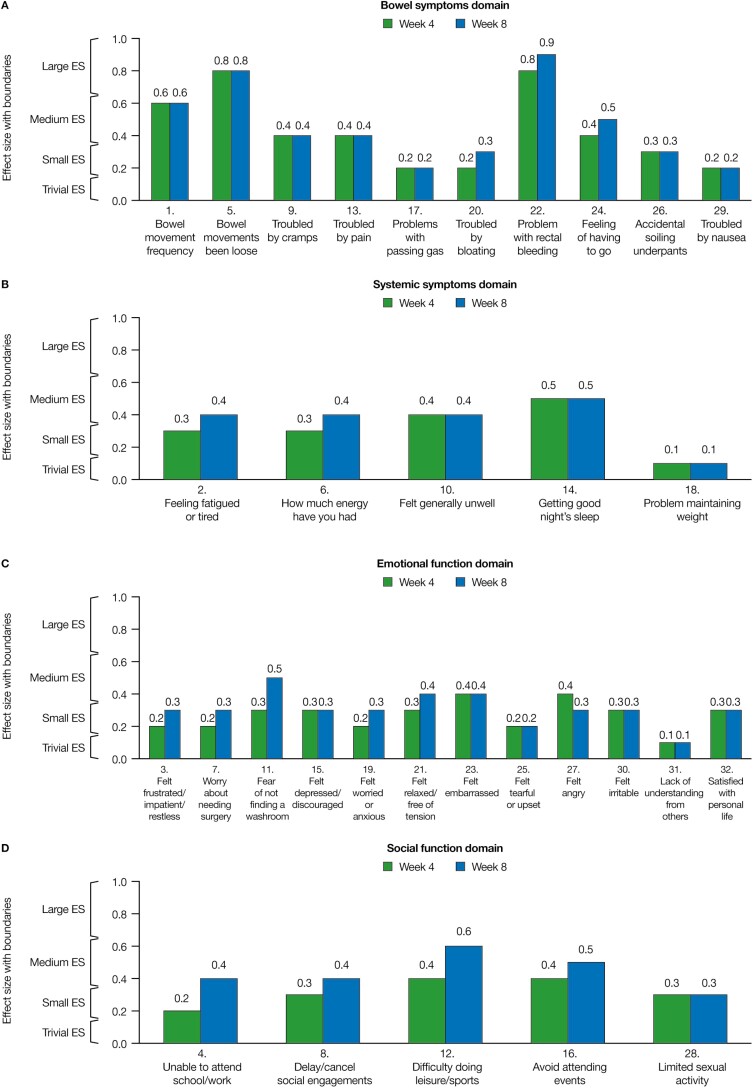
For the overall population, large effect sizes (>0.65) were observed for 2 IBDQ items: “bowel movements been loose” and “problem with rectal bleeding” at weeks 4 and 8 (both within the bowel symptom domain). Small or medium effect sizes (>0.15–0.65) were observed for all other IBDQ items, with the exception of “problem with maintaining weight” (systemic symptom domain) and “lack of understanding from others” (emotional function domain) at weeks 4 and 8, both of which had trivial effect sizes (0–0.15; Fig. 2A–D).
FIGURE 2.
Effect sizes of 10 mg of tofacitinib BID vs placebo for the change from baseline for each IBDQ item in the overall population (FAS, observed case). Effect sizes are the difference in least squares means for 10 mg of tofacitinib BID vs placebo, divided by the standard deviation (SD) of baseline scores (where the SD was calculated using data from both treatment groups in both studies); 0.1 = trivial, 0.2 = small, 0.5 = medium, and 0.8 = large. Effect size categorization intervals: 0–0.15, trivial; >0.15–0.35, small; >0.35–0.65, medium; and >0.65, large. Abbreviations: ES effect sizes.
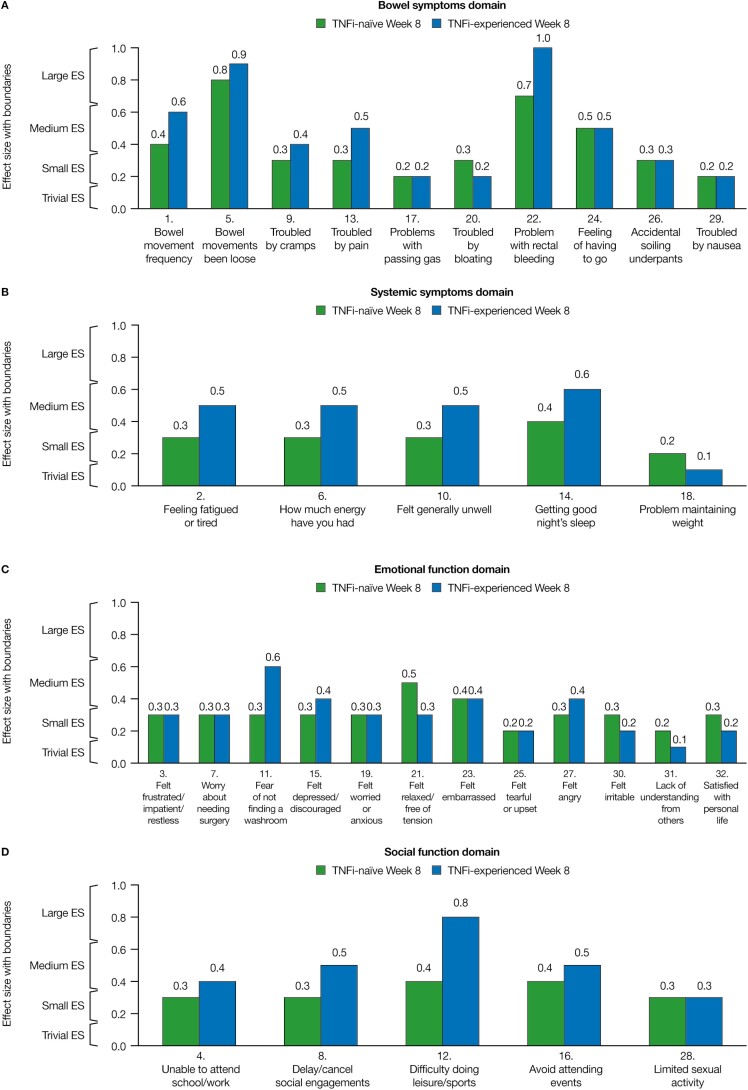
Similarly, large effect sizes were observed for “bowel movements been loose” and “problem with rectal bleeding” at weeks 4 and 8 for TNFi-naïve and TNFi-experienced patients. Additionally, large effect sizes were reported for “bowel movement frequency” at week 4 (bowel symptoms domain) and “difficulty doing leisure/sports” at week 8 (social function domain) in TNFi-experienced patients. In the TNFi subgroups, all other IBDQ items had small or medium effect sizes, with the exception of “unable to attend school/work” (social function domain), “lack of understanding from others,” and “felt tearful/upset” (emotional function domain) at week 4 in TNFi-naïve patients and “trouble with bloating” (bowel symptom domain) at week 4 and “problem maintaining weight” and “lack of understanding from others” at weeks 4 and 8 in TNFi-experienced patients, which all had trivial effect sizes (Fig. 3A–D and Supplementary Fig. 1A–D).
FIGURE 3.
Effect sizes of 10 mg of tofacitinib BID vs placebo for the change from baseline for each IBDQ item in TNFi-naïve and TNFi-experienced patients at week 8 (FAS, observed case). Effect sizes are the difference in least squares means for 10 mg of tofacitinib BID vs placebo, divided by the standard deviation (SD) of baseline scores (where the SD was calculated using data from both treatment groups in both studies); 0.1, trivial; 0.2, small; 0.5, medium; and 0.8, large. Effect size categorization intervals: 0–0.15, trivial; >0.15–0.35, small; >0.35–0.65, medium; and >0.65, large. Abbreviations: ES, effect sizes; FAS, full analysis set.
DISCUSSION
This pooled post hoc analysis assessed the effect of 10 mg of tofacitinib BID compared with placebo on individual items of the IBDQ. Because the IBDQ has a strong correlation with disease severity, baseline IBDQ scores are valuable in capturing the burden of disease.21 In addition, the IBDQ has shown validity and reliability in clinical trials, highlighting that improvements in IBDQ scores reflect improved HRQoL.21 Furthermore, IBDQ total scores could potentially be used as a predictor of remission and response in patients with UC, independent of Mayo score–based assessments.22 Our findings may assist with understanding the effects of UC treatment on IBD-specific HRQoL by highlighting which aspects are most likely to be improved. Furthermore, investigating treatment effects on individual items of the IBDQ in clinical trials may allow study findings to be more relatable to both community physicians and patients. Having a comprehensive understanding of the effect of different UC therapies on IBD-specific aspects of patients’ lives, as determined by analysis of individual IBDQ items, may enable physicians to better tailor treatment to the needs of individual patients in clinical practice.
In the overall population from the pooled OCTAVE Induction studies, significant improvements were observed in each individual IBDQ item with 10 mg of tofacitinib BID vs placebo at week 4 and week 8, which has not been analyzed previously in tofacitinib clinical trials. Significant improvements have previously been reported with 10 mg of tofacitinib BID vs placebo in all 4 IBDQ domains at week 4 and week 8 in the OCTAVE Induction 1 and 2 studies.16 Furthermore, significant improvements were reported with 5 mg and 10 mg of tofacitinib BID vs placebo in all 4 IBDQ domains at week 8, week 24, and week 52 in the OCTAVE Sustain maintenance study,16 indicating that the benefit afforded by tofacitinib therapy is durable over this time period. In addition, the results are consistent with the OCTAVE Induction 1 and 2 efficacy analyses, in which the proportion of patients achieving clinical and endoscopic outcomes were significantly improved with induction therapy of 10 mg of tofacitinib BID vs placebo.14 Effect sizes were generally unchanged between week 4 and week 8 for many of the IBDQ items, indicating that most of the treatment effects with tofacitinib were observed early as patients’ inflammatory burden and symptoms improved, with effects then plateauing.
The finding that treatment benefits with 10 mg of tofacitinib BID vs placebo were slightly numerically higher in TNFi-experienced vs TNFi-naïve patients for many IBDQ items is of clinical relevance because TNFi-experienced patients are commonly considered to be more difficult to treat in clinical practice.23 Previously reported differences in the LSM change from baseline in IBDQ total scores for 10 mg of tofacitinib BID vs placebo at week 8 in OCTAVE Induction 1 and 2 were 15.8 for TNFi-experienced patients and 11.3 for TNFi-naïve patients.16 Baseline IBDQ item level scores were similar between both TNFi subgroups, and therefore, the numerically higher, placebo-adjusted treatment benefits reported in TNFi-experienced patients for some IBDQ items were not due to these patients having worse HRQoL at baseline but were instead due to the lower placebo response rates that were observed in the TNFi-experienced subpopulation compared with the TNFi-naïve subpopulation.
A limitation of this analysis was that the IBDQ was performed in randomized controlled trials, and therefore, future studies should focus on how these findings extrapolate to the general UC patient population. Although moderate correlations have been reported between IBDQ total scores and Mayo scores from OCTAVE Induction week 8 to OCTAVE Sustain week 52,22 it is unclear how the improvements in individual IBDQ items relate to changes in Mayo scores and with the inflammatory burden of disease. In addition, it needs to be determined how these IBDQ item findings can be applied within clinical practice. Furthermore, this was an ad hoc analysis with no multiplicity adjustment, as the OCTAVE Induction studies were not originally designed to test the individual IBDQ items within each domain. In the subgroup analysis, the high placebo response rate observed in the TNFi-naïve patient population limited the interpretation of these data. Analyses of IBDQ item scores by clinical and endoscopic outcomes would provide a worthwhile extension of the current research.
CONCLUSIONS
In conclusion, significant improvements (nominal P value < 0.05 without multiplicity adjustment) were observed in all bowel-related symptoms, systemic symptoms, emotional functioning, and social functioning IBDQ items with 10 mg of tofacitinib BID vs placebo at both week 4 and week 8, highlighting the broad impact of tofacitinib on all aspects of HRQoL. In the overall population, the greatest numerical improvements and largest effect sizes across all items were reported for “bowel movements been loose” and “problem with rectal bleeding,” which are both components of the bowel symptom domain and were the most affected at baseline.
Treatment benefits were slightly numerically higher for many IBDQ items in TNFi-experienced patients compared with TNFi-naïve patients. Because this analysis focused on individual IBDQ items, the magnitude of the improvements of individual symptoms and functioning has now been defined, providing an informative and useful perspective on which components of the IBDQ domains are the most improved after tofacitinib induction therapy, which may help to facilitate patient-physician dialogue. This allows the findings from the OCTAVE Induction studies to be more accessible to those who are not overly familiar with the IBDQ, such as community physicians and patients, and may assist with the development of treatment paradigms.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank the patients, investigators, and trial teams who were involved in the tofacitinib OCTAVE Induction studies. These studies were sponsored by Pfizer Inc. Medical writing support, under the guidance of the authors, was provided by Pauline Craig, PhD, CMC Connect, and McCann Health Medical Communications and was funded by Pfizer Inc., New York, New York, USA, in accordance with Good Publication Practice (GPP3) guidelines (Ann Intern Med. 2015;163:461–64).
Author Contribution: The study concept and design were developed by MCD and JP in collaboration with the sponsor, Pfizer Inc. MCD and JP were involved in the recruitment of study patients. All authors were involved in the analysis and/or interpretation of the data and were involved in drafting the manuscript and critically evaluating it for important intellectual content. All authors had full access to the data in the study and had final responsibility for the decision to submit for publication.
Presented at: Data in this manuscript were presented at the American College of Gastroenterology Annual Meeting & Postgraduate Course, ACG 2019, October 25–30, 2019, San Antonio, Texas, USA, and United European Gastroenterology week, UEGW 2019, October 19–23, 2019, Barcelona, Spain.
Supported by: These studies were sponsored by Pfizer Inc.
Conflicts of Interest: MCD has received consulting fees from AbbVie, Bristol-Myers Squibb, Celgene, Gilead, Janssen, Pfizer Inc., Takeda, and UCB. MD, HF, AGB, JCC, AJT, and LS are employees and stockholders of Pfizer Inc. EM is a former employee and stockholder of Pfizer Inc. JP has received consulting fees from AbbVie, Arena, Boehringer Ingelheim, Celgene, Ferring, Genentech-Roche, Glenmark, GSK, Immunic, Janssen, MSD, Nestlé, Oppilan, Pfizer Inc., Progenity, Shire, Takeda, Theravance, and TiGenix; research grants from AbbVie and MSD; speaker fees from AbbVie, Biogen, Janssen, MSD, Pfizer Inc., and Takeda.
DATA SHARING STATEMENT
Upon request and subject to certain criteria, conditions, and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual de-identified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines, and medical devices (i) for indications that have been approved in the US and/or EU or (ii) in programs that have been terminated (ie, development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions and for which an exception does not apply via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
REFERENCES
- 1. Ungaro R, Mehandru S, Allen PB, et al. Ulcerative colitis. Lancet. 2017;389:1756–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Waljee AK, Joyce JC, Wren PA, et al. Patient reported symptoms during an ulcerative colitis flare: a Qualitative Focus Group Study. Eur J Gastroenterol Hepatol. 2009;21:558–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wolfe BJ, Sirois FM. Beyond standard quality of life measures: the subjective experiences of living with inflammatory bowel disease. Qual Life Res. 2008;17:877–886. [DOI] [PubMed] [Google Scholar]
- 4. Sammut J, Scerri J, Xuereb RB. The lived experience of adults with ulcerative colitis. J Clin Nurs. 2015;24:2659–2667. [DOI] [PubMed] [Google Scholar]
- 5. McCormick JB, Hammer RR, Farrell RM, et al. Experiences of patients with chronic gastrointestinal conditions: in their own words. Health Qual Life Outcomes. 2012;10:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Graff LA, Walker JR, Lix L, et al. The relationship of inflammatory bowel disease type and activity to psychological functioning and quality of life. Clin Gastroenterol Hepatol. 2006;4:1491–1501. [DOI] [PubMed] [Google Scholar]
- 7. Feagan BG, Reinisch W, Rutgeerts P, et al. The effects of infliximab therapy on health-related quality of life in ulcerative colitis patients. Am J Gastroenterol. 2007;102:794–802. [DOI] [PubMed] [Google Scholar]
- 8. Panés J, Su C, Bushmakin AG, et al. Randomized trial of tofacitinib in active ulcerative colitis: analysis of efficacy based on patient-reported outcomes. BMC Gastroenterol. 2015;15:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen XL, Zhong LH, Wen Y, et al. Inflammatory bowel disease-specific health-related quality of life instruments: a systematic review of measurement properties. Health Qual Life Outcomes. 2017;15:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guyatt GH, Deyo RA, Charlson M, et al. Responsiveness and validity in health status measurement: a clarification. J Clin Epidemiol. 1989;42:403–408. [DOI] [PubMed] [Google Scholar]
- 11. Guyatt G, Mitchell A, Irvine EJ, et al. A new measure of health status for clinical trials in inflammatory bowel disease. Gastroenterology. 1989;96:804–810. [PubMed] [Google Scholar]
- 12. Walsh AJ, Bryant RV, Travis SP. Current best practice for disease activity assessment in IBD. Nat Rev Gastroenterol Hepatol. 2016;13:567–579. [DOI] [PubMed] [Google Scholar]
- 13. Sandborn WJ, Ghosh S, Panes J, et al. ; Study A3921063 Investigators . Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med. 2012;367:616–624. [DOI] [PubMed] [Google Scholar]
- 14. Sandborn WJ, Su C, Sands BE, et al. ; OCTAVE Induction 1, OCTAVE Induction 2, and OCTAVE Sustain Investigators . Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2017;376: 1723–1736. [DOI] [PubMed] [Google Scholar]
- 15. Bloom S, Lichtenstein GR, Loftus EV, et al. PTU-001 Tofacitinib, an oral jak inhibitor, in the treatment of ulcerative colitis: open-label, long-term extension study (abstract). Gut. 2018;67(Suppl 1):PTU-001. [Google Scholar]
- 16. Panés J, Vermeire S, Lindsay JO, et al. Tofacitinib in patients with ulcerative colitis: health-related quality of life in phase 3 randomised controlled induction and maintenance studies. J Crohns Colitis. 2018;12:145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van Belle G, Fisher LD, Heagerty PJ, et al. Biostatistics: A Methodology for the Health Sciences. 2nd ed. New York, NY: John Wiley & Sons; 2004. [Google Scholar]
- 18. Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. 2nd ed. Hoboken, NJ: Wiley-Blackwell; 2011. [Google Scholar]
- 19. Cappelleri JC, Bushmakin AG. Interpretation of patient-reported outcomes. Stat Methods Med Res. 2014;23:460–483. [DOI] [PubMed] [Google Scholar]
- 20. Cohen J Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 21. Irvine EJ. Development and subsequent refinement of the inflammatory bowel disease questionnaire: a quality-of-life instrument for adult patients with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 1999;28:S23–S27. [DOI] [PubMed] [Google Scholar]
- 22. Dubinsky MC, Bressler B, Armuzzi A, et al. P685 Improvement in patient-reported Inflammatory Bowel Disease Questionnaire outcomes, and relationship with disease activity, in tofacitinib-treated patients with ulcerative colitis: data from the OCTAVE clinical trials (abstract). J Crohns Colitis. 2019;13(Suppl 1):P685. [Google Scholar]
- 23. Vickers AD, Ainsworth C, Mody R, et al. Systematic review with network meta-analysis: comparative efficacy of biologics in the treatment of moderately to severely active ulcerative colitis. PLoS One. 2016;11:e0165435. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.