Abstract
Background
People with HIV are disproportionately coinfected with hepatitis C virus (HCV) and experience accelerated liver-related mortality. Direct-acting antivirals (DAAs) yield high sustained virologic response (SVR) rates, but uptake is suboptimal. This study characterizes the DAA-era HCV treatment cascade and barriers among US men and women with or at risk for HIV.
Methods
We constructed HCV treatment cascades using the Women’s Interagency HIV Study (women, 6 visits, 2015–2018, n = 2447) and Multicenter AIDS Cohort Study (men, 1 visit, 2015–2018, n = 2221). Cascades included treatment-eligible individuals (ie, HCV RNA-positive or reported DAAs). Surveys captured self-reported clinical (eg, CD4), patient (eg, missed visits), system (eg, appointment access), and financial/insurance barriers.
Results
Of 323/92 (women/men) treatment eligible, most had HIV (77%/70%); 69%/63% were black. HIV-positive women were more likely to attain cascade outcomes than HIV-negative women (39% vs 23% initiated, 21% vs 12% SVR); similar discrepancies were noted for men. Black men and substance users were treated less often. Women initiating treatment (vs not) reported fewer patient barriers (14%/33%). Among men not treated, clinical barriers were prevalent (53%).
Conclusions
HIV care may facilitate HCV treatment linkage and barrier navigation. HIV-negative individuals, black men, and substance users may need additional support.
Clinical trials registration
NCT00000797 (Women’s Interagency HIV Study); NCT00046280 (Multicenter AIDS Cohort Study).
Keywords: HIV, hepatitis C, linkage to care, direct-acting antivirals
People with HIV were more likely to receive HCV treatment than people without HIV. HIV care may facilitate HCV treatment linkage and barrier navigation. HIV-positive individuals, black men, and people who use alcohol and other drugs may need additional support.
Approximately 3.5 million people in the United States are chronically infected with hepatitis C virus (HCV), making it the most common chronic bloodborne infection and the leading cause of liver cirrhosis, hepatocellular carcinoma, and liver disease-related mortality in the United States [1–4]. Less than 2% of the general, noninstitutionalized population is living with HCV [1, 3]. In contrast, among people with human immunodeficiency virus (HIV), an estimated 8% of women and heterosexual men, 16% of men who have sex with men (MSM), and 83% of people who inject drugs are coinfected with HCV [5]. HCV is independently associated with an increase in liver-related and all-cause mortality among people with HIV [6].
The introduction of direct-acting antivirals (DAAs) in 2011 transformed treatment of chronic HCV in the United States. DAA-based regimens have demonstrated sustained virologic response (SVR) rates greater than 95% in clinical trials and are shorter in duration, more effective, and easier to tolerate than earlier interferon-based regimens [7–9]. HCV cure rates for DAA-based regimens are similar for people with and without HIV [10, 11]. The American Association for the Study of Liver Diseases (AASLD)/Infectious Diseases Society of America (IDSA) HCV guidance strongly recommends treatment for all people with chronic HCV, except for those with short life expectancy that cannot be remediated by HCV treatment, liver transplantation, or another directed therapy [12].
Successful HCV treatment requires that an individual is not only aware of their HCV infection, but also interested in HCV treatment and effectively engaged in the health care continuum (eg, referral, initiation). The vast majority of literature characterizing engagement in HCV care captures the period prior to widespread availability of DAAs [13]. A systematic review by Yehia and colleagues characterizing the HCV treatment cascade in the interferon-based regimen era found that among 3.5 million people with chronic HCV, 16% were prescribed treatment and 9% achieved SVR [13]. Although emerging work characterizing HCV treatment uptake early (pre-2015) in the DAA-era suggests the initiation of DAA-based HCV regimens among persons with HCV/HIV coinfection has increased, uptake remains suboptimal [10, 11, 14]. Notably, the majority of this literature is based on single clinic or health systems, and thus may not be generalizable to broader US contexts. This study characterizes the DAA-era HCV treatment cascade through the contemporary treatment era (2015–2018) among 2 multisite US cohorts of men and women with and without HIV and identifies clinical (eg, poor health, detectable HIV load), patient (eg, missed visits), system (eg, appointment access), and financial (eg, insurance) barriers to HCV treatment.
METHODS
Study Population and Data Collection
Our data sources included the Women’s Interagency HIV Study (WIHS) and the Multicenter AIDS Cohort Study (MACS), 2 longstanding US observational cohorts of persons with or at risk for HIV infection. The WIHS enrolled women at sites in Bronx and Brooklyn, NY; Washington, DC; Los Angeles and San Francisco, CA; and Chicago, IL in 1994–1995, 2001–2002, and 2011–2012, with additional women enrolled in 2013–2015 at sites in Chapel Hill, NC; Atlanta, GA; Miami, FL; Birmingham, AL; and Jackson, MS. The MACS enrolled MSM at sites in Baltimore, MD; Pittsburgh, PA; Chicago, IL; and Los Angeles, CA in 3 periods: pre-2001 (starting in 1984), 2001–2003, and 2010. In both cohorts, visits were conducted semiannually and included: (1) a structured interview to obtain self-reported information including medical history, medication use, and sociodemographic descriptors; (2) a physical examination; and (3) laboratory specimen collection. All participants provided written informed consent to participate and contribute their data to research studies. Detailed participant recruitment, retention, and characteristics have been previously described [15–17].
Eligibility
For the analyses described herein, all women enrolled in the WIHS and men enrolled in the MACS, whether living with or at risk for HIV, were eligible if (1) their most recent study-indicated HCV testing was antibody positive and RNA positive (ie, active infection) or they stated having ever taken DAA medication for HCV and (2) they attended and reported on their HCV treatment experiences between April 2015 and March 2018 for women and October 2015 and March 2018 for men. Self-report of DAA medication use included all classes (NS3/4A protease inhibitors, NS5B nucleoside polymerase inhibitors, NS5B nonnucleoside polymerase inhibitors, and NS5A protein inhibitors) [9].
Hepatitis C Treatment Cascade and Barriers to HCV Treatment
Study questionnaires captured participant experiences with HCV treatment, specifically, whether they: (1) were interested, (2) talked to a provider, (3) were referred, (4) were recommended, (5) initiated, (6) completed, or (7) were told that treatment was successful (cure/SVR). Participants who reported “no” to items 2–6 (eg, did not talk to a provider) were asked to select all that applied from a prespecified list of reasons regarding why they had not achieved this step, including an open-ended “other” write-in option. Participants responding “no” to a question were not asked subsequent cascade questions (eg, a participant responding he/she had not talked to a provider was not asked about treatment referral). Reasons, including “other” write-ins, were categorized into 4 mutually exclusive domains: (1) clinical (eg, competing health issues, HIV viral load or CD4 count did not meet treatment criteria due to clinical indicators [eg, genotype or laboratory abnormalities]), (2) system (eg, waiting for appointments or prescriptions, laboratory testing, no health care provider), (3) patient (eg, missed visits, side effect or efficacy concerns, perception that treatment was not necessary, substance use, nonadherent to other medications), and (4) financial (eg, insurance denials or delays).
Women enrolled in the WIHS could complete the staff-administered survey at each of 6 study visits, allowing the progression of women through the HCV treatment cascade to be tracked longitudinally. Men enrolled in the MACS were asked about their experiences once during the study period using a staff-administered survey. Questionnaires are publicly available through the MACS/WIHS Combined Cohort Study website (https://statepi.jhsph.edu/mwccs/data-collection-forms/).
We constructed a cross-sectional HCV cascade utilizing data from each woman’s first semiannual visit during the study period (per study eligibility criteria) and longitudinal cascades reflecting women’s HCV treatment experiences over time. Repeated data collection in the WIHS resulted in potentially discrepant information between visits (eg, a woman may have reported having been cured at 1 visit and report having not completed treatment at the next visit). In such cases, data were individually reviewed by A. E. (author and analyst) and assigned the most likely true value(s). Progression in the cascade was assigned conservatively (eg, a woman who may have either been cured or did not complete treatment was assigned the latter). We evaluated the robustness of findings by comparing available data sources (reported DAA medication use, SVR confirmatory testing).
Analysis
We stratified HCV treatment cascades within each cohort by HIV status. The χ 2 and Fisher exact test were used to compare proportions, with continuous variables compared using the nonparametric Wilcoxon rank sum test. Nonparametric estimates of the survival function were obtained using the product-limit (Kaplan-Meier) method in order to quantify the median progression time through HCV treatment cascade steps, with 95% confidence intervals for median survival times calculated using the log-log transformation of the survival function. Analyses were conducted using SAS version 9.4 (SAS Institute).
RESULTS
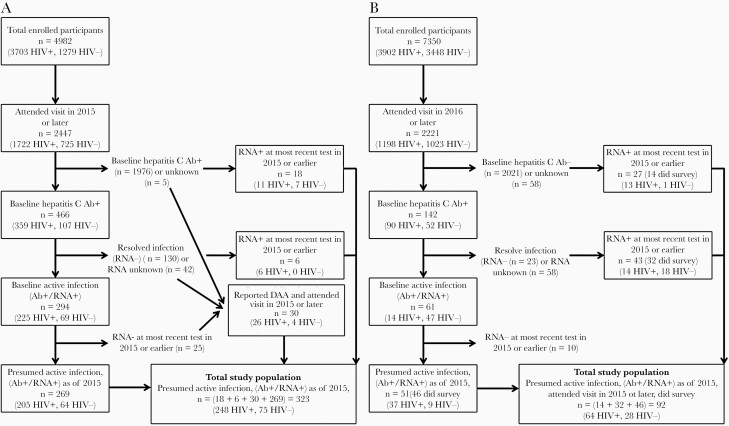
Of the 2447 women attending a WIHS visit in 2015 or later, 323 (13%) met the inclusion criteria: 30 who self-reported DAA medication use, plus 293 who were HCV RNA positive at their most recent HCV test (Figure 1A). Among 2221 men attending a visit 2016 forward, 92 (4%) met the inclusion criteria; half of men (n = 46, 50%) were HCV antibody positive/RNA positive at MACS enrollment with the other 46 RNA positive at their most recent HCV test. None of the RNA-negative men reported pre-2015 DAA medication use (Figure 1B). Women and men were comparable with respect to advanced liver fibrosis, defined as an aspartate aminotransferase to platelet ratio index (APRI) of at least 1.5 or a Fibrosis-4 (FIB-4) of at least 3.25 (18% vs 12%).
Figure 1.
Active Hepatitis C infection among (A) Women’s Interagency HIV Study (n = 323) and (B) Multicenter AIDS Cohort Study (n = 92) participants, 2015–2018. Abbreviations: AB, antibody; DAA, directly acting antiviral; HIV, human immunodeficiency virus.
The median age in eligible women and men were 56 (interquartile range [IQR], 51–60) and 58 (IQR, 55–63) years, respectively. The majority were living with HIV (77% women, 70% men; Table 1). Most women (69%) and men (63%) were black non-Hispanic. Both groups were low income, with 78% of women having annual household earnings ≤$18 000 and 63% of men earning <$20 000 per year. Men were almost universally insured (99%); 90% of women reported having insurance.
Table 1.
Characteristics of 415 US Women and Men (at First Visit 2015–2018) Who Were HCV RNA Positive or Had Initiated a Direct-Acting Antiviral for HCV
| Characteristic | WIHS (n = 323) | MACS (n = 92) |
|---|---|---|
| Demographics | ||
| Living with HIV | 248 (77) | 64 (70) |
| Age, y, median (IQR) | 56 (51–60) | 58 (55–63) |
| Race/ethnicity | ||
| Black, non-Hispanic | 223 (69) | 58 (63) |
| Hispanic white, black, other | 55 (17) | 11 (12) |
| White non-Hispanic, Asian/Pacific islander, native American/Alaskan, other | 45 (14) | 23 (25) |
| Less than high school education | 128 (40) | 18 (20) |
| Low annual incomea | 252 (78) | 58 (67) |
| Employed | 55 (17) | 22 (24) |
| Health insurance | 292 (90) | 91 (99) |
| Medicaid | 245 (84) | 46 (51) |
| Private | 32 (11) | 17 (19) |
| Medicare | 8 (3) | 20 (22) |
| Other | 7 (2) | 8 (9) |
| Behavioral | ||
| Injection drug use, past 6 mo | 12 (4) | 9 (10) |
| Noninjection substance use, past 6 mob | 102 (32) | 54 (59) |
| Alcohol use, past 6 moc | ||
| Abstainer | 192 (59) | 38 (41) |
| Low/moderate | 87 (27) | 31 (34) |
| Moderate/heavy | 12 (4) | 13 (14) |
| Heavy/binge | 32 (10) | 10 (11) |
| Advanced liver fibrosis, APRI of at least 1.5 or FIB-4 of at least 3.25 | 57 (18) | 11 (12) |
Data are n (%) unless otherwise noted.
Abbreviations: APRI, aspartate aminotransferase to platelet ratio index; IQR, interquartile range; MACS, Multicenter AIDS Cohort Study; WIHS, Women’s Interagency Cohort Study.
a≤$18 000 for household in women, and <$20 000 in men. Income data missing for 8 men.
bIn women, noninjection substance use included crack, cocaine, heroin, marijuana, hallucinogens, club drugs, and methamphetamines. In men, noninjection substance use included hash/marijuana, poppers, crack, other street drugs, or snorted or swallowed or put in anus (“booty bumped”) speed/ methamphetamines/ ice/ heroin/ speedball/ other cocaine.
cIn women, low/moderate drinking was defined as 1–7 drinks/week, moderate/heavy as 7–12 drinks/week, and heavy/binge as 13 or more drinks/week. In men, low/moderate drinking was defined as 1–2 drinks/day or 3–4 drinks/day no more than once a month, moderate/heavy as 3–4 drinks/day more than once a month or 5 or more drinks/day less than once a month. Binge drinking was defined as 5 or more drinks/day at least once a month.
HCV Treatment Cascade Among Women Enrolled in the WIHS
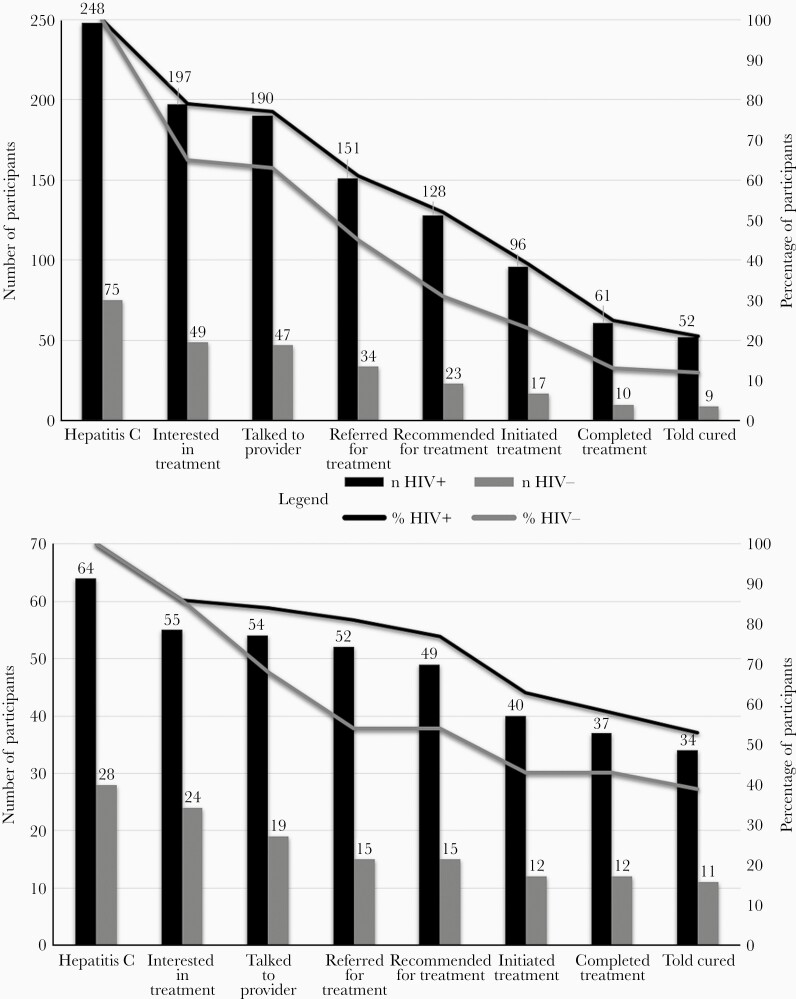
Using data from women’s first RNA-positive semiannual visit during the study period, women with HIV more successfully attained cascade outcomes than women without HIV, despite similar treatment interest: (1) 52% versus 31% were recommended for treatment (P = .001), (2) 39% versus 23% initiated treatment (P = .011), and (3) 21% versus 12% achieved SVR (P = .08) (Figure 2A). Cascades reflecting progression longitudinally revealed similar trends among women with and without HIV: (1) 82% versus 61% recommended for treatment (P < .001), (2) 69% versus 43% initiated treatment (P < .001), and (3) 63% versus 37% achieved SVR (P < .001).
Figure 2.
Hepatitis C treatment cascade, ranging from HCV treatment interest to cure, among women and men with and without HIV infection enrolled in (A) the Women’s Interagency HIV Study (n = 323) and (B) men enrolled in the Multicenter AIDS Cohort Study (n = 92), 2015–2018.
Regardless of HIV status, women with annual incomes ≤$18 000 (P = .001) and women who injected drugs (P = .033) or used other illicit substances (P < .001) in the past 6 months were less likely to initiate treatment. Employed women were more likely to initiate treatment (P = .009). There were no differences (P > .05) in treatment initiation by age, race/ethnicity, education, health insurance, alcohol use, or advanced liver fibrosis.
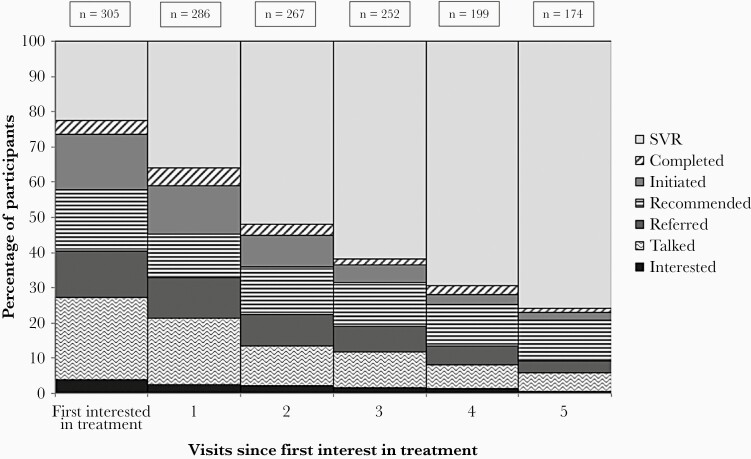
Women attended a median of 5 visits (IQR, 5–6) during the study period. Nearly 25% (n = 305) reported having already been cured and another 20% had either initiated or completed treatment at the first data collection time point (Figure 3). Increasing proportions of women attained later cascade outcomes over time, with over three-quarters of women having attained SVR approximately 3 years after first expressing interest in treatment. Substantial proportions of women who were interested in treatment but had not yet started (n = 176) failed to initiate treatment in the subsequent 1 year (n = 129, 73%), 2 years (n = 79, 45%), and 3 years (n = 36, 20%). Failure to progress along the treatment cascade is also reflected by the median number of semiannual visits needed to progress from a given step in the cascade to a later step (Supplementary Figure 1). It took roughly 18 months from reporting having talked with a provider to reporting having attained a later step in the cascade (ie, treatment referral, recommendation, initiation, completion, or cure) (Supplementary Figure 1B). Similarly, it took roughly 1 year to report having been cured following treatment completion (Supplementary Figure 1F), indicating delays in seeing a provider and obtaining confirmatory testing following treatment completion.
Figure 3.
Hepatitis C treatment cascade progression over time among 305 women with confirmed hepatitis C virus infection enrolled in the Women’s Interagency HIV Study expressing interest in treatment, 2015–2018. Abbreviations: SVR, sustained virological response.
Various structural and personal factors were stated as reasons for not achieving cascade steps. At the initial (baseline) visit, 10% of women reported clinical, 7% of women reported patient, 4% of women reported system, and 6% of women reported financial barriers. Over time, women who ultimately initiated treatment, as compared to women who did not initiate treatment were: (1) less likely to report patient barriers (14% vs 33%, P = .002) and (2) more likely to report systems barriers (34% vs 17%, P = .005). There were no differences in reporting of clinical (32% vs 35%, P = .60) or financial (26% vs 24%, P = .73) barriers for women who initiated treatment, compared to those who did not.
HCV Treatment Cascade Among Men Enrolled in the MACS
Despite similar treatment interest, men with HIV more successfully attained cascade outcomes than men without HIV: (1) 77% versus 54% were recommended for treatment (P = .027), (2) 63% versus 43% initiated treatment (P = .08), and (3) 53% versus 39% achieved SVR (P = .22) (Figure 2B). Regardless of HIV status, black men (P = .04) and men using illicit substances in the past 6 months (P = .05) were less likely to initiate treatment. There were no differences (P > .05) in treatment initiation by age, education, employment, health insurance, injection drug use, alcohol use, or advanced liver fibrosis.
Among men who reported not initiating treatment, clinical barriers were reported more commonly (53%) than patient (1%), system (2%), and financial (2%) barriers. Cross-sectional data collection precluded the comparison of barriers between men who did and did not initiate treatment.
Validation of Cascades
To validate the self-reported cascade outcomes and evaluate their robustness vis-à-vis other data sources, we first examined overlap with medication use separately ascertained at visits. Of the 202 women reporting HCV treatment, all but 18% (n = 37) reported DAA medication use. Approximately two-thirds of initiators who did not report DAA medication use reported having attained SVR (n = 25).
Among men, 25% (n = 13) of the 52 who reported having initiated treatment did not report DAA medication use. Eleven of 13 initiators without documented DAA medication reported SVR.
In the WIHS only, we compared self-reported cascade outcomes to HCV RNA results. Of 184 women reporting SVR, 151 (82%) had follow-up RNA testing. Of these, 147 (97%) had virologic evidence of cure, defined as an RNA-negative result at their most recent test.
DISCUSSION
In our cohorts, people with HIV were more likely than HIV-seronegative individuals to receive treatment for HCV and attain SVR. It is possible that having HIV infection facilitates connection to HCV treatment through one or more pathways. First, engagement in HIV care may facilitate linkage both to health care systems and providers. Linkage to HCV care providers has been identified as a major barrier to HCV treatment initiation [14, 18]. Infectious disease providers may have greater knowledge of and willingness to treat HCV or refer to HCV care, in part due to the higher prevalence and greater morbidity of HCV among people with HIV [19, 20]. This approach would streamline timely connection to HCV-specific care and enhance the likelihood of treatment initiation [21, 22]. In contrast, people with HCV monoinfection may need to identify and link to another provider in order to access HCV-related care. Secondly, HIV accelerates the progression of liver disease, liver failure, and liver-related mortality [23, 24]. Although current AASLD/IDSA HCV guidance strongly recommends treatment for all people with chronic HCV, it is likely that the more aggressive progression of liver disease among people with HIV/HCV coinfection promotes greater urgency for HCV treatment initiation and may facilitate eligibility for reimbursement of treatment costs [23–25].
Regardless of HIV status, participants cited multiple barriers to cascade progression. Although DAA treatment regimens during the study period were typically 12 weeks in duration, one-fifth of women had not progressed through the cascade 3 years after expressing interest in HCV treatment. Women who initiated treatment, as compared to women who did not, were more likely to report system-level barriers (eg, waiting for available appointments, laboratory results). Given that it took women a median of 18 months from reporting having talked with a provider to reporting having attained a later step in the cascade, the additional burden of system barriers among women initiating treatment likely reflects: (1) administrative challenges associated with cascade progression and (2) these women’s persistence and ability to effectively navigate the health care system. Participants with HIV may be more adept at navigating these barriers or have access to additional case management support as a function of their HIV [14]. These findings highlight the substantial administrative burden associated with cascade progression and support calls for streamlined, integrated approaches as a way to optimize treatment and related outcomes.
Comparing the cohorts descriptively, we found that HCV treatment initiation and barriers to treatment varied by sex, regardless of HIV status. Men were nearly twice as likely to have initiated treatment for HCV as women, when comparing baseline progression through the cascade at a single baseline visit. Data collection spanned the same time period for both men and women, so it is unlikely that temporal changes in the availability of DAAs explains this disparity. In addition, women and men did not vary with respect to advanced fibrosis. Men overwhelmingly reported clinical barriers to treatment (but few participant, system, or financial barriers), whereas women tended to report barriers across all domains. Past work has not revealed differences in HCV treatment uptake between men and women [26, 27]. Men enrolled in the MACS reported higher incomes and more formal education than women enrolled in WIHS. It is likely that the observed difference in treatment initiation reflects socioeconomic disparities in access to HCV treatment, rather than sex differences [24, 27]. Cost has been identified as a major barrier to HCV treatment [24, 25, 27–31]. Insurance coverage is a primary facilitator of treatment access, although findings of research exploring relationships between insurance type and HCV treatment uptake have been mixed [25, 29–31].
Past work has consistently found that people of color are less likely to initiate HCV treatment [11, 31]. Women in our sample were almost universally insured, which may explain why we did not detect differences by race [28]. However, black men were less likely to initiate HCV treatment than other men in the MACS, despite high rates of health insurance. A defining feature of the MACS is that all of the participants are MSM. Black MSM experience notable disparities in access to health care attributable to structural inequities and experiences of stigma, medical mistrust, and racism [32]. Layered HCV-related stigma may further hinder engagement in care [33]. The rise in HCV infections among MSM has received attention in recent years [34]. Addressing added barriers that black MSM face engaging in HCV care may increase initiation of HCV treatment and prevention of new HCV infections in this key population.
Similar to past research, we found that people reporting substance use were less likely to initiate treatment [19, 20]. Substance use may act as a multilevel barrier to HCV treatment initiation. On a patient level, individuals using substances may face challenges effectively linking to and engaging in care [10]. On a financial level, lack of insurance or coverage exclusions prohibiting the approval of HCV therapies for individuals using substances may delay or prevent treatment initiation. Barua and colleagues found that 88% of state Medicaid plans had coverage restrictions for people who use drugs, with 50% requiring a period of abstinence prior to treatment approval [25]. On a system level, providers may be reluctant to initiate treatment among individuals whom they perceive unlikely to adhere to and complete treatment [19, 20]. However, this perception is counter to systematic reviews supporting high HCV treatment adherence, low discontinuation, and low rates of HCV reinfection among people who inject drugs [35]. There is a substantial burden of HCV among people using substances, especially those who inject drugs [5]. DAA-based treatment of HCV is both cost-effective and successful among people who use drugs [35]. Removing insurance barriers and engaging drug users in HCV treatment should remain a priority area, to mitigate HCV transmission and HCV-related morbidity and mortality.
Our findings are subject to limitations. Cascades reflect participant-reported data and may be subject to recall or social desirability bias. However, our sensitivity analyses suggest that self-reported measures correspond well with laboratory indicators. Many participants did not progress through the HCV treatment cascade. It is possible that among those that were not treated, there were clinical indications against treatment. The broad AASLD/IDSA treatment guidelines, however, make it unlikely that a large proportion of the cohort was contraindicated for DAAs [12]. Unfortunately, the nature of the data does not allow us to assess these distinctions. Although our data include both women and men, participants were enrolled in distinct cohorts, precluding comparisons by sex. Despite these limitations, our study has several strengths. This study utilized high-quality data from 2 long-standing US multisite cohorts; cohorts were designed to reflect sociodemographics of people with HIV in the United States [15, 16]. Participants agree to long-term follow-up and as a result may not be representative of all people with HCV or HIV. The availability of only cross-sectional MACS data precluded our ability to assess cascade progression among men longitudinally. However, the cross-sectional and longitudinal nature of analyses among the WIHS participants demonstrate that cascade progression and related barriers shift over time, highlighting the importance of assessing HCV treatment experiences longitudinally. In addition, our study allowed us to examine within-cohort differences by HIV status.
Collectively, our findings support past work demonstrating higher levels of HCV treatment initiation in the DAA era [10, 11, 13, 14]. This work also highlights the substantial time and challenge associated with progressing from being interested in receiving HCV treatment to treatment initiation and, ultimately, SVR. In our cohorts, people with HIV were more likely than seronegative individuals to receive HCV treatment and attain SVR. HIV-related care may facilitate treatment linkage and navigation of barriers. Subpopulations of individuals with HCV may require additional supports to successfully achieve treatment outcomes, including black MSM, people of lower socioeconomic status, and substance users. Effective linkage to care and initiation of HCV treatment is critical to reaching AASLD/IDSA HCV treatment guidelines, preventing new HCV infections, and reducing HCV-related morbidity and mortality.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors acknowledge and thank Multicenter AIDS Cohort Study/Women’s Interagency HIV Study (MACS/WIHS) Combined Cohort Study participants and staff for their time and dedication. Thank you to Magdalena Pankowska for her assistance in creating Figure 3.
Disclaimer . The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health .
Financial support. This work was supported by the National Institutes of Health (NIH). Data were collected by the MACS/WIHS Combined Cohort Study (MWCCS). MWCCS sites, principal investigators (NIH grant numbers): Atlanta CRS, Ighovwerha Ofotokun, Anandi Sheth, and Gina Wingood, (U01-HL146241-01); Baltimore CRS, Todd Brown and Joseph Margolick, (U01-HL146201-01); Bronx CRS, Kathryn Anastos and Anjali Sharma, (U01-HL146204-01); Brooklyn CRS, Deborah Gustafson and Tracey Wilson, (U01-HL146202-01); Data Analysis and Coordination Center, Gypsyamber D’Souza, Stephen Gange, and Elizabeth Golub, (U01-HL146193-01); Chicago-Cook County CRS, Mardge Cohen and Audrey French, (U01-HL146245-01); Chicago-Northwestern CRS, Steven Wolinsky, (U01-HL146240-01); Connie Wofsy Women’s HIV Study, Northern California CRS, Bradley Aouizerat and Phyllis Tien, (U01-HL146242-01); Los Angeles CRS, Roger Detels and Otoniel Martinez-Maza, (U01-HL146333-01); Metropolitan Washington CRS, Seble Kassaye and Daniel Merenstein, (U01-HL146205-01); Miami CRS, Maria Alcaide, Margaret Fischl, and Deborah Jones, (U01-HL146203-01); Pittsburgh CRS, Jeremy Martinson and Charles Rinaldo, (U01-HL146208-01); UAB-MS CRS, Mirjam-Colette Kempf and Deborah Konkle-Parker, (U01-HL146192-01); UNC CRS, Adaora Adimora, (U01-HL146194-01).
The MWCCS is supported by National Heart, Lung, and Blood Institute, with cofunding from Eunice Kennedy Shriver National Institute Of Child Health and Human Development, National Human Genome Research Institute, National Institute On Aging, National Institute Of Dental & Craniofacial Research, National Institute Of Allergy And Infectious Diseases, National Institute Of Neurological Disorders And Stroke, National Institute Of Mental Health, National Institute On Drug Abuse, National Institute Of Nursing Research, National Cancer Institute, National Institute on Alcohol Abuse and Alcoholism, National Institute on Deafness and Other Communication Disorders, National Institute of Diabetes and Digestive and Kidney Diseases.
MWCCS data collection is supported by NIH (grant numbers UL1-TR000004 to UCSF CTSA, P30-AI-050409 to Atlanta CFAR, P30-AI-050410 to UNC CFAR, and P30-AI-027767 to UAB CFAR). Additional support was provided by NIH (grant numbers U01-AI-103390 and 7 K01 DA046307 to D. F. H.; P30-AI-094189 to E. C. D. Johns Hopkins University CFAR; and UL1TR001881 to M. D. W. Lundquist Institute, CTSI).
Potential conflicts of interest. A. A. A. reports consultancies with Merck, Gilead, and Viiv, and research funding from Gilead. P. T. reports research funding from Merck. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: 10th International AIDS Society Conference on HIV Science in Mexico City, Mexico, 21–24 July 2019 (abstract TUPDB0105).
References
- 1. Ditah I, Ditah F, Devaki P, et al. . The changing epidemiology of hepatitis C virus infection in the United States: National Health and Nutrition Examination Survey 2001 through 2010. J Hepatol 2014; 60:691–8. [DOI] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention. Viral hepatitis surveillance—United States, 2016. Atlanta, Georgia: Division of Viral Hepatitis and National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, 2018:75. [Google Scholar]
- 3. Hall EW, Rosenberg ES, Sullivan PS. Estimates of state-level chronic hepatitis C virus infection, stratified by race and sex, United States, 2010. BMC Infect Dis 2018; 18:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ly KN, Hughes EM, Jiles RB, Holmberg SD. Rising mortality associated with hepatitis C virus in the United States, 2003–2013. Clin Infect Dis 2016; 62:1287–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Platt L, Easterbrook P, Gower E, et al. . Prevalence and burden of HCV co-infection in people living with HIV: a global systematic review and meta-analysis. Lancet Infect Dis 2016; 16:797–808. [DOI] [PubMed] [Google Scholar]
- 6. Thornton AC, Jose S, Bhagani S, et al. ; UK Collaborative HIV cohort (UK CHIC) Steering Committee . Hepatitis B, hepatitis C, and mortality among HIV-positive individuals. AIDS 2017; 31:2525–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen TY, Jain MK. Treatment of hepatitis C in HIV-infected patients: moving towards an era of all oral regimens. AIDS Patient Care STDS 2015; 29:329–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kohli A, Shaffer A, Sherman A, Kottilil S. Treatment of hepatitis C: a systematic review. JAMA 2014; 312:631–40. [DOI] [PubMed] [Google Scholar]
- 9. Jakobsen JC, Nielsen EE, Feinberg J, et al. . Direct-acting antivirals for chronic hepatitis C. Cochrane Database Syst Rev 2017; ( 9):CD012143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cachay ER, Wyles D, Hill L, et al. . The impact of direct-acting antivirals in the hepatitis C-sustained viral response in human immunodeficiency virus-infected patients with ongoing barriers to care. Open Forum Infect Dis 2015; 2:ofv168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Collins LF, Chan A, Zheng J, et al. . Direct-acting antivirals improve access to care and cure for patients with HIV and chronic HCV infection. Open Forum Infect Dis 2018; 5:ofx264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. American Association for the Study of Liver Diseases (AASLD) and The Infectious Diseases Society of America (IDSA). HCV guidance: recommendations for testing, managing, and treating Hepatitis C. http://hcvguidelines.org/. Accessed 7 November 2020. [Google Scholar]
- 13. Yehia BR, Schranz AJ, Umscheid CA, Lo Re V 3rd. The treatment cascade for chronic hepatitis C virus infection in the United States: a systematic review and meta-analysis. PLoS One 2014; 9:e101554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Roberson JL, Lagasca AM, Kan VL. Comparison of the hepatitis C continua of care between hepatitis C virus/HIV coinfected and hepatitis C virus mono-infected patients in two treatment eras during 2008–2015. AIDS Res Hum Retroviruses 2018; 34:148–55. [DOI] [PubMed] [Google Scholar]
- 15. Adimora AA, Ramirez C, Benning L, et al. . Cohort profile: the women’s interagency HIV study (WIHS). Int J Epidemiol 2018; 47:393–4i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Detels R, Jacobson L, Margolick J, et al. . The multicenter AIDS Cohort Study, 1983 to …. Public Health 2012; 126:196–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hessol NA, Weber KM, Holman S, et al. . Retention and attendance of women enrolled in a large prospective study of HIV-1 in the United States. J Womens Health (Larchmt) 2009; 18:1627–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zuckerman A, Douglas A, Nwosu S, Choi L, Chastain C. Increasing success and evolving barriers in the hepatitis C cascade of care during the direct acting antiviral era. PLoS One 2018; 13:e0199174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Adams LM, Balderson B, Packett BJ 2nd. Meeting the challenge: hepatitis C virus and HIV care experiences among HIV specialty providers. AIDS Patient Care STDS 2018; 32:314–20. [DOI] [PubMed] [Google Scholar]
- 20. Rogal SS, McCarthy R, Reid A, et al. . Primary care and hepatology provider-perceived barriers to and facilitators of hepatitis C treatment candidacy and adherence. Dig Dis Sci 2017; 62:1933–43. [DOI] [PubMed] [Google Scholar]
- 21. Cachay ER, Hill L, Torriani F, et al. . Predictors of missed hepatitis C intake appointments and failure to establish hepatitis C care among patients living with HIV. Open Forum Infect Dis 2018; 5:ofy173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Allyn PR, O’Malley SM, Ferguson J, Tseng CH, Chew KW, Bhattacharya D. Attitudes and potential barriers towards hepatitis C treatment in patients with and without HIV coinfection. Int J STD AIDS 2018; 29:334–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kaspar MB, Sterling RK. Mechanisms of liver disease in patients infected with HIV. BMJ Open Gastroenterol 2017; 4:e000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen YC, Thio CL, Cox AL, Ruhs S, Kamangar F, Wiberg KJ. Trends in hepatitis C treatment initiation among HIV/hepatitis C virus-coinfected men engaged in primary care in a multisite community health centre in Maryland: a retrospective cohort study. BMJ Open 2019; 9:e027411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Barua S, Greenwald R, Grebely J, Dore GJ, Swan T, Taylor LE. Restrictions for Medicaid reimbursement of sofosbuvir for the treatment of hepatitis C virus infection in the United States. Ann Intern Med 2015; 163:215–23. [DOI] [PubMed] [Google Scholar]
- 26. Kanwal F, Kramer JR, El-Serag HB, et al. . Race and gender differences in the use of direct acting antiviral agents for hepatitis C virus. Clin Infect Dis 2016; 63:291–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Spradling PR, Xing J, Rupp LB, et al. . Uptake of and factors associated with direct-acting antiviral therapy among patients in the chronic hepatitis cohort study, 2014 to 2015. J Clin Gastroenterol 2018; 52:641–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. DeBose-Scarlett A, Balise R, Kwon D, et al. . Obstacles to successful treatment of hepatitis C in uninsured patients from a minority population. J Transl Med 2018; 16:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gowda C, Lott S, Grigorian M, et al. . Absolute insurer denial of direct-acting antiviral therapy for hepatitis C: a National Specialty Pharmacy Cohort Study. Open Forum Infect Dis 2018; 5:ofy076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lo Re V 3rd, Gowda C, Urick PN, et al. . Disparities in absolute denial of modern hepatitis C therapy by type of insurance. Clin Gastroenterol Hepatol 2016; 14:1035–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Marcus JL, Hurley LB, Chamberland S, et al. . Disparities in initiation of direct-acting antiviral agents for hepatitis C virus infection in an insured population. Public Health Rep 2018; 133:452–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Eaton LA, Earnshaw VA, Maksut JL, Thorson KR, Watson RJ, Bauermeister JA. Experiences of stigma and health care engagement among black MSM newly diagnosed with HIV/STI. J Behav Med 2018; 41:458–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dowsett LE, Coward S, Lorenzetti DL, MacKean G, Clement F. Living with hepatitis C virus: a systematic review and narrative synthesis of qualitative literature. Can J Gastroenterol Hepatol 2017; 2017:3268650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Salazar-Vizcaya L, Kouyos RD, Zahnd C, et al. ; Swiss HIV Cohort Study . Hepatitis C virus transmission among human immunodeficiency virus-infected men who have sex with men: modeling the effect of behavioral and treatment interventions. Hepatology 2016; 64:1856–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Aspinall EJ, Corson S, Doyle JS, et al. . Treatment of hepatitis C virus infection among people who are actively injecting drugs: a systematic review and meta-analysis. Clin Infect Dis 2013; 57 (suppl 2):S80–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.