Abstract
Background
Ustekinumab is currently approved globally in Crohn’s disease (CD) and psoriatic diseases. Recent phase 3 data demonstrate safety/efficacy in ulcerative colitis (UC). Crohn’s disease and UC phase 3 programs had similar study designs, facilitating integrated safety analyses.
Methods
Data from 6 ustekinumab phase 2/3 CD and UC studies were pooled, and safety was evaluated through 1 year. Patients received 1 placebo or ustekinumab (generally 130 mg or ~6 mg/kg) intravenous induction, then subcutaneous (90 mg) maintenance every 8/12 weeks. Analyses incorporated all patients who received ≥1 ustekinumab dose. Safety outcomes are presented as percentages of patients (induction) and as number of patients with events per 100 patient-years of follow-up (through 1 year). For key safety events, 95% confidence intervals (CIs) are provided, as appropriate. Hazard ratios with 95% CIs from time-to-event analyses for serious adverse events and serious infections were also performed.
Results
Through 1 year, 2574 patients received ustekinumab (1733 patient-years of follow-up). The number of patients with adverse events per 100 patient-years (placebo 165.99 [95% CI, 155.81–176.67] vs ustekinumab 118.32 [95% CI, 113.25–123.55]), serious AEs (27.50 [95% CI, 23.45–32.04] vs 21.23 [95% CI, 19.12–23.51]), infections (80.31 [95% CI, 73.28–87.84] vs 64.32 [95% CI, 60.60–68.21]), serious infections (5.53 [95% CI, 3.81–7.77] vs 5.02 [95% CI, 4.02–6.19]), and malignancies excluding nonmelanoma skin cancer (0.17 [95% CI, 0.00–0.93] vs 0.40 [95% CI, 0.16–0.83]) were similar between placebo and ustekinumab.
Conclusions
The safety profile of ustekinumab across the pooled inflammatory bowel disease population through 1 year was favorable and generally comparable to placebo. These data are consistent with the established safety profile of ustekinumab across indications.
ClinicalTrials.gov numbers
NCT00265122; NCT00771667; NCT01369329; NCT01369342; NCT01369355; NCT02407236.
Keywords: ustekinumab, inflammatory bowel disease, safety
INTRODUCTION
Ustekinumab is a human monoclonal antibody to interleukin (IL) 12/23p40, first approved to treat patients with moderate to severe psoriasis in 2009.1–3 Ustekinumab has also become a well-established therapy in Crohn’s disease (CD), after initial approval in 2016,4–6 and received initial approval for ulcerative colitis (UC) in September 2019. Total postmarketing exposure and experience across all indications over 10 years is cumulatively estimated to represent 1,375,000 patient-years (PYs).
Previous publications evaluating the safety of ustekinumab in psoriasis, psoriatic arthritis, CD, and—most recently—UC have consistently demonstrated a safety profile similar to placebo.7–11 Additionally, the Psoriasis Longitudinal Assessment and Registry (PSOLAR), focused on serious infections, malignancy, major adverse cardiovascular events (MACEs), and mortality, further supports this favorable safety profile, with 12,472 ustekinumab PYs of follow-up.12
Integration and analysis of the safety data for CD and UC for a combined inflammatory bowel disease (IBD) population provides more complete information for gastroenterologists, facilitating benefit/risk assessment for their IBD treatment choices. This increases the precision to detect safety signals compared with placebo for all events and increases identification of less frequent events (eg, serious adverse events [SAEs] or serious infections). Furthermore, because ustekinumab IBD indications uniquely employ intravenous (IV) induction, followed by subcutaneous (SC) maintenance dosing (90 mg every 8 or 12 weeks), these integrated safety analyses of all IBD phase 2/3 studies also provide important data examining the possibility that these higher doses or the IBD population might have a different safety profile than the psoriatic diseases.
METHODS
Trial Designs
Safety data in this analysis includes data from two phase 2 CD studies (C0379T07 [T07]13 and CERTIFI4), three phase 3 CD studies (two induction studies, UNITI-1 and UNITI-2, and one maintenance study, IM-UNITI5), and one phase 3 UC protocol (UNIFI,11 composed of separate induction and maintenance studies). Details of patient populations and treatment regimens for studies are presented in Supplemental Table S1 and have also been previously published.4, 5, 11, 13 All trials have been registered at Clinicaltrials.gov: NCT00265122, NCT00771667, NCT01369329, NCT01369342, NCT01369355, NCT02407236.
Randomization to study treatment was centrally performed across all studies. Stratification by investigative site was utilized across all IBD studies. Additional stratification attributes for each study are described. For CERTIFI induction, stratification included initial response to a tumor necrosis factor (TNF) antagonist (ie, response to first TNF antagonist if >1 agent was previously administered). For maintenance, stratification inclued induction dose and, for patients with an initial response to ustekinumab, remission status at 6 weeks. For UNITI, stratification included trial region and Crohn’s disease activity index (CDAI) score (≤300 or >300) in both induction studies and initial response to TNF antagonist therapy (yes or no) in UNITI-1. For IM-UNITI, stratification included ustekinumab dose during UNITI and remission status at week 0 of IM-UNITI. For UNIFI, stratification included trial region and biologic failure status (yes or no) in induction and ustekinumab dose during induction, remission status at week 0 of maintenance, and oral corticosteroid use (yes or no) at week 0 of maintenance.
In all studies, patients underwent radiographic, purified protein derivative and/or interferon-γ release assay screening for Mycobacterium tuberculosis (TB) at screening. Patients with latent TB could be enrolled with initiation of an established concomitant treatment protocol (eg, isoniazid). Concomitant biologic use was prohibited for all IBD studies, with protocol-specified prescreening washout periods.
Safety Outcomes
Adverse events (AEs) were systematically collected in each trial throughout the study duration until the last study visit (20 weeks after last dose of study agent) and were coded and classified according to the Medical Dictionary for Regulatory Activities (MedDRA), version 21.0.
Safety outcomes assessed include AEs, SAEs, infections, serious infections, AEs leading to study agent discontinuation, injection site reactions, AEs occurring during or within 1 hour of infusion, clinical laboratory parameters, and deaths. Infections were determined based on assessment by the investigator.
Other events of interest included malignancies, serious major adverse cardiac events (MACE; cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke), thromboembolic events (deep vein thrombosis [DVT]/pulmonary embolism [PE]), anaphylactic and serum sickness-like hypersensitivity reactions, opportunistic infections (OIs) including active TB, and serious neurological disorders.
To classify other infection events, the following categories were also analyzed: Clostridium difficile (MedDRA preferred terms [PTs] in the MedDRA higher-level term: clostridial infections); herpes zoster (PTs in the higher-level term: herpes viral infections that also contain the words “zoster” or “varicella”); gastrointestinal (GI)-related abscesses that include the subcategories of anal, rectal, and, perirectal infections (PTs: anal abscess, rectal abscess, perirectal abscess); abdominal and intestinal infections (PTs: abdominal abscess, abscess intestinal); and other abscesses (PTs: groin, vaginal, stoma site, vulval, pelvic, perineal, and genital abscess).
In CD as previously reported,7 all PTs possibly consistent with serious MACE were retrospectively adjudicated by an independent and blinded process performed by the Cleveland Clinic Coordinating Center for Clinical Research (http://c5research.clevelandclinic.org/Home.aspx). For UC, all PTs possibly consistent with serious MACE were adjudicated through clinical review by the sponsor. Analyses were based on adjudicated serious MACE in pooled CD and UC.
For hypersensitivity reactions, the terms anaphylactic reaction, anaphylactic shock, anaphylactoid reaction, anaphylactoid shock, type I hypersensitivity, and serum sickness or serum sickness-like reaction were included in the analysis.
Data Analysis
All patients who received ≥1 dose of ustekinumab (IV or SC) were included in analyses. Data from induction through week 8 and maintenance through week 44 were evaluated, totaling 1 year of treatment.
Safety outcomes from induction trials are presented as percentages of patients with events, whereas safety outcomes through 1 year are presented as the number of patients per 100 PYs (95% confidence interval [CIs]) of follow-up. The latter approach was taken to adjust for potential differences in exposure between ustekinumab and placebo groups (because placebo patients crossed over to receive ustekinumab). The 95% CIs are based on an exact method assuming the observed number of events follows a Poisson distribution.
Hazard ratios (ustekinumab:placebo) with 95% CIs from time-to-event analyses for SAEs and serious infections were based on Cox proportional hazards model, with treatment as the explanatory variable adjusting for age (continuous), gender, ethnicity, duration of disease (continuous variable defined from initial disease diagnosis to induction baseline visit in years), stratified by study (UC or CD), biologic failure status (yes or no), and clinical remission status at maintenance baseline (yes or no). Initial placebo-controlled period analyses only include phase 3 CD and UC induction studies because alternate IV doses were studied in phase 2 CD. Analyses through 1 year of exposure (induction through maintenance) are presented for UC, CD, and pooled IBD and incorporate all phase 2/3 IBD studies.
Adverse events were summarized by MedDRA, system organ class and PT. Patients were classified according to actual treatment received, including in the placebo group up until the time of administration of the first ustekinumab dose or, for patients rerandomized to placebo, starting at 16 weeks after ustekinumab induction (after >5 half-lives).
Rates of malignancies were compared with the general population, with adjustment for age, sex, and race using the external National Institutes of Health Surveillance, Epidemiology, and End Results (SEER) database (2015; https://seer.cancer.gov/resources; other than nonmelanoma skin cancer [NMSC] and cervical cancer in situ, which are not included in SEER). The expected number of patients with malignancies reported is based on SEER, adjusted for age, gender, and race. Standardized incidence ratios (SIRs) were calculated by dividing observed number of patients with a malignancy in the pooled IBD population by expected number of patients from SEER with a malignancy. Confidence intervals were calculated based on an exact method assuming that the observed number of events follows a Poisson distribution.
Immunogenicity
Antibodies to ustekinumab were detected using a validated, drug-tolerant, electrochemiluminescence method (ECLIA) on the Meso Scale Discovery (MSD) platform in all phase 3 studies. In the phase 2 T07 and CERTIFI studies, an older nondrug-tolerant validated bridging enzyme immunoassay was used. Of note, though the same MSD assay was used in all phase 3 studies, a slightly more stringent cut point was employed in the UC studies, per updated regulatory guidelines. In all studies, a patient was considered positive for antibodies if treatment-emergent antibodies to ustekinumab were detected in the sample at any time, regardless of subsequent negative results.
RESULTS
Baseline Demographics
Through 1 year of follow-up across the pooled phase 2 and 3 IBD studies, a total of 2574 patients were treated with ustekinumab (825 patients in phase 3 UC studies and 1749 patients in phase 2/3 CD studies; Supplemental Figure S1), yielding a total of 1733 PYs of follow-up. In the phase 3 IBD population, median disease duration was 7.4 years. Disease duration was longer in CD (8.5 years) than UC patients (6.0 years; Table 1). The majority of CD patients had involvement of both ileum and colon (62.6%), and median baseline CDAI was 307.0. Patients with UC had a median Mayo score (range 0–12) of 9.0 at baseline. Baseline characteristics in the T07 and CERTIFI were generally similar to those in phase 3, except a slightly higher median age and weight in T07.
TABLE 1.
Disease Characteristics at Baseline (Week 0 of Induction; Phase 3 Studies Only)
| Ulcerative Colitis | Crohn’s Disease | Inflammatory Bowel Disease | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ustekinumab | Ustekinumab | Ustekinumab | ||||||||
| Placebo | 130 mg | 6 mg/kg | Combined | Placebo | 130 mg | 6 mg/kg | Combined | Placebo | ||
| Patients randomized | 319 | 320 | 322 | 642 | 470 | 467 | 472 | 939 | 789 | 1581 |
| Age (years) | ||||||||||
| Median | 40.0 | 42.0 | 41.0 | 41.5 | 37.0 | 37.0 | 36.0 | 39.0 | 39.0 | 38.0 |
| IQ range | (30.0; 51.0) | (31.0; 51.0) | (30.0; 52.0) | (30.0; 51.0) | (29.0; 47.0) | (28.0; 47.0) | (27.0; 46.0) | (27.0; 47.0) | (29.0; 49.0) | (29.0; 49.0) |
| Male | 197 (61.8) | 190 (59.4) | 195 (60.6) | 385 (60.0) | 222 (47.2) | 207 (44.3) | 198 (41.9) | 405 (43.1) | 419 (53.1) | 790 (50.0) |
| White | 248 (77.7) | 239 (74.7) | 243 (75.5) | 482 (75.1) | 399 (84.9) | 393 (84.2) | 399 (84.5) | 792 (84.3) | 647 (82.0) | 1274 (80.6) |
| Weight (kg) Mean (SD) | 72.9 (16.8) | 73.7 (16.8) | 73.0 (19.3) | 73.3 (18.1) | 72.7 (18.7) | 71.2 (19.5) | 70.4 (19.3) | 70.8 (19.4) | 72.8 (18.5) | 71.8 (18.9) |
| Current smokers | 20 (6.3) | 12 (3.8) | 15 (5.0) | 28 (4.4) | 117 (24.9)c | 119 (25.5) | 99 (21.0) | 218 (23.2) | 137 (17.4)c | 246 (15.6) |
| IBD disease duration (years) | ||||||||||
| N | 319 | 320 | 322 | 642 | 469 | 467 | 472 | 939 | 788 | 1581 |
| Mean (SD) | 8.0 (7.2) | 8.1 (7.2) | 8.2 (7.8) | 8.2 (7.5) | 11.4 (9.2) | 10.5 (8.5) | 10.9 (9.4) | 10.7 (9.0) | 10.0 (8.6) | 9.66 (8.5) |
| Median | 6.0 | 5.9 | 6.0 | 6.0 | 9.0 | 8.7 | 8.5 | 8.5 | 7.8 | 7.4 |
| IQ range | (2.7; 11.3) | (2.8; 11.4) | (2.7; 11.1) | (2.8; 11.2) | (4.4; 15.6) | (3.9; 14.8) | (3.8; 15.1) | (3.8; 14.8) | (3.5; 13.7) | (3.2; 13.5) |
| Extent of UC disease | ||||||||||
| N | 316 | 318 | 320 | 638 | 0 | 0 | 0 | 0 | 316 | 638 |
| Limited to left side of colon N (%) | 167 (52.8) | 183 (57.5) | 168 (52.5) | 351 (55.0) | - | - | - | - | 167 (52.8) | 351 (55.0) |
| Extensive | 149 (47.2) | 135 (42.5) | 152 (47.5) | 287 (45.0) | - | - | - | - | 149 (47.2) | 287 (45.0) |
| Involved GI areas | ||||||||||
| N | 0 | 0 | 0 | 0 | 468 | 466 | 472 | 938 | 468 | 938 |
| Ileum only N (%) | - | - | - | - | 75 (16.0) | 94 (20.2) | 87 (18.4) | 181 (19.3) | 75 (16.0) | 181 (19.3) |
| Colon only N (%) | - | - | - | - | 87 (18.6) | 82 (17.6) | 85 (18.0) | 167 (17.8) | 87 (18.6) | 167 (17.8) |
| Ileum and colon N (%) | - | - | - | - | 302 (64.5) | 288 (61.8) | 299 (63.3) | 587 (62.6) | 302 (64.5) | 587 (62.6) |
| Mayo score (0–12)a | ||||||||||
| N | 319 | 320 | 321 | 641 | 0 | 0 | 0 | 0 | 319 | 641 |
| Mean (SD) | 8.9 (1.6) | 8.9 (1.6) | 8.9 (1.5) | 8.9 (1.5) | - | - | - | - | 8.9 (1.6) | 8.9 (1.5) |
| Median | 9.0 | 9.0 | 9.0 | 9.0 | - | - | - | - | 9.0 | 9.0 |
| IQ range | (8.0; 10.0) | (8.0; 10.0) | (8.0; 10.0) | (8.0; 10.0) | - | - | - | - | (8.0; 10.0) | (8.0; 10.0) |
| CDAI scoreb | ||||||||||
| N | 0 | 0 | 0 | 0 | 470 | 467 | 472 | 939 | 470 | 939 |
| Mean (SD) | - | - | - | - | 311.7 (61.2) | 313.4 (62.0) | 315.6 (62.1) | 314.5 (62.0) | 311.7 (61.2) | 314.5 (62.0) |
| Median | - | - | - | - | 301.0 | 306.0 | 308.5 | 307.0 | 301.0 | 307.0 |
| IQ range | - | - | - | - | (261.0; 351.0) | (264.0; 352.0) | (267.0; 359.0) | (265.0; 354.0) | (261.0; 351.0) | (265.0; 354.0) |
Abbreviations: CDAI, Crohn’s Disease Activity Index; GI, gastrointestinal; IBD, inflammatory bowel disease; IQ, interquartile; SD, standard deviation; UC, ulcerative colitis.
Data presented as N (%) unless otherwise noted.
aMayo score is comprised of 4 parts: stool frequency, rectal bleeding, endoscopic findings and Physician’s Global Assessment, each scored from 0–3. Moderate-to-severe UC is defined as total score of 6–12 on Mayo score (range 0–12).
bCDAI has a possible range of 0 to 600 (with higher scores indicating more severe disease studies.). CDAI <150 is used as a marker of remission, >450 is a marker of severe disease.
cMissing data for one patient.
In the pooled IBD population at baseline, 47.1% (1388 of 2949) and 29.7% (877 of 2949) of patients were receiving concomitant corticosteroids and immunosuppressants, respectively (Supplemental Table S2). More than half (61.5%) of the pooled IBD population had a history of biological failure, 60.2% having failed at least 1 TNF antagonist, and 28.6% having failed ≥2 TNF antagonists; 30.4% of the pooled IBD population were biologic-naive. Of note, CERTIFI and UNITI-1 exclusively allowed previous TNF antagonist failures, whereas UNIFI included TNF antagonist and/or vedolizumab failures.
In the pooled IBD population, 2489 randomized patients received a single IV dose of ustekinumab; 1322 had been exposed to ustekinumab for ≥6 months and 871 for at least 1 year (Supplemental Figure S1).
Adverse Events
In induction, for the pooled phase 3 population, average duration of follow-up was similar between the placebo and ustekinumab groups (Table 2). Compared with placebo, similar proportions of ustekinumab-treated patients reported ≥1 AE (55.8% [438 of 785] vs 53.9% [853 of 1582], respectively), and these rates were not appreciably different between the two IV ustekinumab doses (Table 2). The most frequently reported AE was headache (6.5% placebo- and 6.7% ustekinumab-treated patients). Excluding IBD diseases under study, other frequently reported AEs (≥3% of patients in any treatment group) included nasopharyngitis, upper respiratory tract infection (URTI), nausea, abdominal pain, vomiting, arthralgia, pyrexia, and fatigue.
TABLE 2.
Safety During Placebo-controlled Intravenous Induction Period (Week 0-Week 8; Phase 3 Studies Only)
| Ulcerative Colitis | Crohn’s Disease | Inflammatory Bowel Disease | ||||||
|---|---|---|---|---|---|---|---|---|
| Ustekinumab | Ustekinumab | Ustekinumab | ||||||
| Placebo | 130 mg | 6 mg/kg | Placebo | 130 mg | 6 mg/kg | Placebo | Combined | |
| Patients treated | 319 | 321 | 320 | 466 | 471 | 470 | 785 | 1582 |
| Average follow-up (weeks) | 7.96 | 8.11 | 8.16 | 8.18 | 8.22 | 8.16 | 8.09 | 8.17 |
| Number of Patients ≥1 N (%) | ||||||||
| Adverse events | 156 (48.9) | 133 (41.4) | 160 (50.0) | 282 (60.5) | 275 (58.4) | 285 (60.6) | 438 (55.8) | 853 (53.9) |
| 95% CI | (43.3, 54.5) | (36.0, 47.0) | (44.4, 55.6) | (55.9, 65.0) | (53.8, 62.9) | (56.1, 65.1) | (52.2, 59.3) | (51.4, 56.4) |
| Serious adverse events | 21 (6.6) | 12 (3.7) | 10 (3.1) | 28 (6.0) | 23 (4.9) | 25 (5.3) | 49 (6.2) | 70 (4.4) |
| 95% CI | (4.1, 9.9) | (2.0, 6.4) | (1.5, 5.7) | (4.0, 8.6) | (3.1, 7.2) | (3.5, 7.8) | (4.7, 8.2) | (3.5, 5.6) |
| Infections | 48 (15.0) | 53 (16.5) | 49 (15.3) | 108 (23.2) | 92 (19.5) | 111 (23.6) | 156 (19.9) | 305 (19.3) |
| 95% CI | (11.3, 19.5) | (12.6, 21.0) | (11.6, 19.7) | (19.4, 27.3) | (16.1, 23.4) | (19.9, 27.7) | (17.1, 22.8) | (17.4, 21.3) |
| Serious Infections | 4 (1.3) | 2 (0.6) | 1 (0.3) | 6 (1.3) | 7 (1.5) | 8 (1.7) | 10 (1.3) | 18 (1.1) |
| 95% CI | (0.3, 3.2) | (0.1, 2.2) | (0.0, 1.7) | (0.5, 2.8) | (0.6, 3.0) | (0.7, 3.3) | (0.6, 2.3) | (0.7, 1.8) |
| Number of patients with other infections of interesta N (%) | ||||||||
| Clostridium difficileb | 2 (0.6) | 1 (0.3) | 0 | 1 (0.2) | 1 (0.2) | 2 (0.4) | 3 (0.4) | 4 (0.3) |
| Herpes zosterc | 0 | 0 | 2 (0.6) | 1 (0.2) | 2 (0.4) | 0 | 1 (0.1) | 4 (0.3) |
| GI-related abscessesd | 1 (0.3) | 0 | 0 | 7 (1.5) | 7 (1.5) | 7 (1.5) | 8 (1.0) | 14 (0.9) |
| 95% CI | (0.0, 1.7) | - | - | (0.6, 3.1) | (0.6, 3.0) | (0.6, 3.0) | (0.4, 2.0) | (0.5, 1.5) |
| Anal, rectal, and perirectal | 1 (0.3) | 0 | 0 | 6 (1.3) | 3 (0.6) | 3 (0.6) | 7 (0.9) | 6 (0.4) |
| 95% CI | (0.0, 1.7) | - | - | (0.5, 2.8) | (0.1, 1.9) | (0.1, 1.9) | (0.4, 1.8) | (0.1, 0.8) |
| Abdominal and intestinal | 0 | 0 | 0 | 0 | 1 (0.2) | 1 (0.2) | 0 | 2 (0.1) |
| 95% CI | - | - | - | - | (0.0, 1.2) | (0.0, 1.2) | - | (0.0, 0.5) |
| Abscess, Other | 0 | 0 | 0 | 1 (0.2) | 3 (0.6) | 3 (0.6) | 1 (0.1) | 6 (0.4) |
| 95% CI | - | - | - | (0.0, 1.2) | (0.1, 1.9) | (0.1, 1.9) | (0.0, 0.7) | (0.1, 0.8) |
| Frequent adverse events (≥3% in any treatment group N (%) | ||||||||
| Nasopharyngitis | 9 (2.8) | 13 (4.0) | 18 (5.6) | 23 (4.9) | 22 (4.7) | 25 (5.3) | 32 (4.1) | 78 (4.9) |
| Upper respiratory tract infection | 4 (1.3) | 6 (1.9) | 4 (1.3) | 20 (4.3) | 17 (3.6) | 17 (3.6) | 24 (3.1) | 44 (2.8) |
| Nausea | 7 (2.2) | 8 (2.5) | 7 (2.2) | 22 (4.7) | 27 (5.7) | 25 (5.3) | 29 (3.7) | 67 (4.2) |
| Abdominal pain | 8 (2.5) | 8 (2.5) | 6 (1.9) | 20 (4.3) | 14 (3.0) | 24 (5.1) | 28 (3.6) | 52 (3.3) |
| Vomiting | 1 (0.3) | 3 (0.9) | 4 (1.3) | 12 (2.6) | 14 (3.0) | 20 (4.3) | 13 (1.7) | 41 (2.6) |
| Crohn’s disease | 0 | 0 | 0 | 35 (7.5) | 22 (4.7) | 14 (3.0) | 35 (4.5) | 36 (2.3) |
| Colitis Ulcerative | 18 (5.6) | 9 (2.8) | 7 (2.2) | 0 | 0 | 0 | 18 (2.3) | 16 (1.0) |
| Arthralgia | 3 (0.9) | 3 (0.9) | 6 (1.9) | 22 (4.7) | 36 (7.6) | 24 (5.1) | 25 (3.2) | 69(4.4) |
| Pyrexia | 6 (1.9) | 4 (1.2) | 6 (1.9) | 25 (5.4) | 21 (4.5) | 27 (5.7) | 31 (3.9) | 58 (3.7) |
| Fatigue | 5 (1.6) | 6 (1.9) | 8 (2.5) | 16 (3.4) | 9 (1.9) | 15 (3.2) | 21 (2.7) | 38 (2.4) |
| Headache | 14 (4.4) | 22 (6.9) | 13 (4.1) | 37 (7.9) | 40 (8.5) | 31 (6.6) | 51 (6.5) | 106 (6.7) |
Abbreviations: CI, confidence interval; GI, gastrointestinal.
aInfection as assessed by the investigator.
b Clostridium difficile included all preferred terms in the higher-level term of clostridial infections.
cHerpes zoster included preferred terms in the higher-level term of herpes viral infections that also contain the words “zoster” or “varicella.”
dGI abscesses include all preferred terms included in the following categories: anal, rectal, and perirectal: anal abscess, rectal abscess, perirectal abscess; abdominal and intestinal: abdominal abscess, abscess intestinal; abscess, other: groin abscess, vaginal abscess, stoma site abscess, vulval abscess, pelvic abscess, perineal abscess, genital abscess.
In the longitudinal analyses of the pooled IBD population (induction through maintenance up to 1 year), the average follow-up was 22.33 and 35.02 weeks for placebo- and combined ustekinumab-treated patients, respectively (Table 3). Adverse event rates through 1 year of treatment per 100 PYs of follow-up were 165.99 (95% CI, 155.81–176.67) on placebo and 118.32 (95% CI, 113.25–123.55) on ustekinumab. Of the 13 AEs occurring in at least 10 patients/100 PYs (Table 3), 3 were higher in combined ustekinumab group vs placebo (vomiting [7.21 vs 6.87], nasopharyngitis [18.11 vs 16.26], and headache [16.50 vs 16.43]), and the difference was not meaningful. In the randomized maintenance safety subset, similar trends were observed through week 44 in both dose regimens (ustekinumab 90 mg every 8 weeks and ustekinumab 90 mg every 12 weeks) compared with placebo (Table 4).
TABLE 3.
Treatment Through 1 Year: Patients with Safety Events Per 100 Patient-Years of Follow-Upa
| Ulcerative Colitis | Crohn’s Disease | Inflammatory Bowel Disease | ||||
|---|---|---|---|---|---|---|
| Placebo | Ustekinumab | Placebo | Ustekinumab | Placebo | Ustekinumab | |
| Patients Treated | 446 | 825 | 943 | 1749 | 1389 | 2574 |
| Average follow-up (weeks) | 29.14 | 39.53 | 19.11 | 32.89 | 22.33 | 35.02 |
| Patient-years of follow-up | 250 | 627 | 347 | 1106 | 596 | 1733 |
| Key safety events, Rate (N) | ||||||
| Adverse events | 121.65 (304) | 95.03 (596) | 197.97 (686) | 131.52 (1455) | 165.99 (990) | 118.32 (2051) |
| 95% CI | (108.36, 136.12) | (87.55, 102.97) | (183.43, 213.36) | (124.85, 138.46) | (155.81, 176.67) | (113.25, 123.55) |
| Serious adverse events | 18.41 (46) | 12.91 (81) | 34.05 (118) | 25.94 (287) | 27.50 (164) | 21.23 (368) |
| 95% CI | (13.48, 24.55) | (10.26, 16.05) | (28.19, 40.78) | (23.03, 29.12) | (23.45, 32.04) | (19.12, 23.51) |
| Infectionsb | 59.23 (148) | 49.75 (312) | 95.52 (331) | 72.59 (803) | 80.31 (479) | 64.32 (1115) |
| 95% CI | (50.07, 69.57) | (44.38, 55.58) | (85.51, 106.39) | (67.65, 77.78) | (73.28, 87.84) | (60.60, 68.21) |
| Serious infections | 4.00 (10) | 3.19 (20) | 6.64 (23) | 6.06 (67) | 5.53 (33) | 5.02 (87) |
| 95% CI | (1.92, 7.36) | (1.95, 4.92) | (4.21, 9.96) | (4.69, 7.69) | (3.81, 7.77) | (4.02, 6.19) |
| Serious MACEc | 0.80 (2) | 0.16 (1) | 0.00 (0) | 0.09 (1) | 0.34 (2) | 0.12 (2) |
| 95% CI | (0.10, 2.89) | (0.00, 0.89) | (0.00, 0.86) | (0.00, 0.50) | (0.04, 1.21) | (0.01, 0.42) |
| Discontinuation due to adverse event | 13.21 (33) | 4.15 (26) | 13.56 (47) | 9.76 (108) | 13.41 (80) | 7.73 (134) |
| 95% CI | (9.09, 18.55) | (2.71, 6.07) | (9.97, 18.04) | (8.01, 11.79) | (10.64, 16.69) | (6.48, 9.16) |
| Death | 0.00 (0) | 0.32 (2) | 0.00 (0) | 0.00 (0) | 0.00 (0) | 0.12 (2) |
| 95% CI | (0.00, 1.20) | (0.04, 1.15) | (0.00, 0.86) | (0.00, 0.27) | (0.00, 0.50) | (0.01, 0.42) |
| Malignancies (excluding NMSC) | 0.40 (1) | 0.64 (4) | 0.00 (0) | 0.27 (3) | 0.17 (1) | 0.40 (7) |
| 95 % CI | (0.01, 2.23) | (0.17, 1.63) | (0.00, 0.86) | (0.06, 0.79) | (0.00, 0.93) | (0.16, 0.83) |
| Infections of Interest, Rate (N) | ||||||
| Clostridium difficile infectiond | 2.00 (5) | 0.64 (4) | 2.31 (8) | 1.08 (12) | 2.18 (13) | 0.92 (16) |
| Herpes Zostere | 1.20 (3) | 1.12 (7) | 1.44 (5) | 0.99 (11) | 1.34 (8) | 1.04 (18) |
| GI-related abscessesf | 0.80 (2) | 0.16 (1) | 8.37 (29) | 4.61 (51) | 5.20 (31) | 3.00 (52) |
| 95% CI | (0.10, 2.89) | (0.00, 0.89) | (5.60, 12.02) | (3.43, 6.06) | (3.53, 7.38) | (2.24, 3.93) |
| Anal, rectal, and perirectal | 0.80 (2) | 0.16 (1) | 7.21(25) | 2.98 (33) | 4.53 (27) | 1.96 (34) |
| 95% CI | (0.10, 2.89) | (0.00, 0.89) | (4.67, 10.65) | (2.05, 4.19) | (2.98, 6.59) | (1.36, 2.74) |
| Abdominal and intestinal | 0.00 (0) | 0.00 (0) | 0.87 (3) | 0.45 (5) | 0.50 (3) | 0.29 (5) |
| 95% CI | (0.00, 1.20) | (0.00, 0.48) | (0.18, 2.53) | (0.15, 1.05) | (0.10, 1.47) | (0.09, 0.67) |
| Abscess, Other | 0.00 (0) | 0.00 (0) | 0.29 (1) | 1.18 (13) | 0.17 (1) | 0.75 (13) |
| 95% CI | (0.00, 1.20) | (0.00, 0.48) | (0.01, 1.61) | (0.63, 2.01) | (0.00, 0.93) | (0.40, 1.28) |
| ≥10 Adverse events occurring in any treatment group | ||||||
| Nasopharyngitis | 17.21 | 17.86 | 15.58 | 18.26 | 16.26 | 18.11 |
| Upper respiratory tract infection | 6.00 | 6.70 | 15.30 | 14.01 | 11.40 | 11.36 |
| Nausea | 5.60 | 5.74 | 18.76 | 15.46 | 13.25 | 11.94 |
| Crohn’s disease | 0.00 | 0.00 | 37.81 | 23.59 | 21.96 | 15.06 |
| Abdominal pain | 6.00 | 5.10 | 20.78 | 15.19 | 14.59 | 11.54 |
| Diarrhea | 1.20 | 3.51 | 10.10 | 5.88 | 6.37 | 5.02 |
| ≥10 Adverse events occurring in any treatment group | ||||||
| Vomiting | 2.40 | 2.55 | 10.10 | 9.85 | 6.87 | 7.21 |
| Ulcerative Colitis | 36.82 | 15.15 | 0.00 | 0.00 | 15.43 | 5.48 |
| Arthralgia | 9.20 | 8.45 | 22.51 | 21.15 | 16.93 | 16.56 |
| Headache | 9.20 | 11.00 | 21.64 | 19.62 | 16.43 | 16.50 |
| Fatigue | 4.80 | 4.46 | 10.10 | 8.23 | 7.88 | 6.86 |
| Pyrexia | 6.80 | 3.99 | 17.03 | 12.29 | 12.74 | 9.29 |
| Anemia | 11.20 | 7.81 | 7.21 | 4.07 | 8.89 | 5.42 |
Abbreviations: CI, confidence interval; NMSC, non-melanoma skin cancer.
aUlcerative Colitis: CNTO1275UCO3001; Crohn’s disease: C0379T07 (only placebo-controlled IV population), C0743T26, CNTO1275CRD3001 and CNTO1275CRD3002.
bInfection as assessed by the investigator.
cMajor adverse cardiovasulcar events (MACE) events in Crohn’s disease studies were externally adjudicated, and in ulcerative colitis, studies were identified through clinical review by the sponsor.
d Clostridium difficile included all preferred terms in the higher-level term of clostridial infections.
eHerpes zoster included preferred terms in the higher-level term of herpes viral infections that also contain the words zoster or varicella.
fGI abscesses include all preferred terms included in the following categories: anal, rectal, and perirectal: anal abscess, rectal abscess, perirectal abscess; abdominal and intestinal: abdominal abscess, abscess intestinal; abscess, other: groin abscess, vaginal abscess, stoma site abscess, vulval abscess, pelvic abscess, perineal abscess, genital abscess.
TABLE 4.
Safety During Randomized Maintenance Period: Patients With Safety Events Per 100 Patient-Years of Follow-Up (Maintenance Week 0 Through Week 44; Phase 3 Studies Onlya)
| Ulcerative Colitis | Crohn’s Diseasec | Inflammatory Bowel Diseasec | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Ustekinumab | Ustekinumab | Ustekinumab | |||||||
| Placebo SCb | 90 mg SC 12qw | 90 mg SC 8qw | Placebo SCb | 90 mg SC 12qw | 90 mg SC 8qw | Placebo SCb | 90 mg SC 12qw | 90 mg SC 8qw | |
| Patients Treated | 175 | 172 | 176 | 133 | 132 | 131 | 308 | 304 | 307 |
| Average follow-up (weeks) | 42.30 | 41.80 | 42.20 | 31.96 | 36.73 | 35.21 | 37.84 | 39.60 | 39.22 |
| Patient-years of follow-up | 142 | 138 | 143 | 82 | 93 | 89 | 224 | 231 | 232 |
| Key Safety Events, Rate (N) | |||||||||
| Adverse events | 96.94 (138) | 86.07 (119) | 95.22 (136) | 135.77 (111) | 113.70 (106) | 120.65 (107) | 111.10 (249) | 97.20 (225) | 104.96 (243) |
| Serious adverse events | 11.94 (17) | 9.40 (13) | 10.50 (15) | 24.46 (20) | 17.16 (16) | 14.66 (13) | 16.51 (37) | 12.53 (29) | 12.09 (28) |
| Infectionsd | 56.90 (81) | 41.95 (58) | 60.21 (86) | 80.73 (66) | 66.50 (62) | 71.03 (63) | 65.59 (147) | 51.84 (120) | 64.36 (149) |
| Serious infectionsd | 2.81 (4) | 4.34 (6) | 2.10 (3) | 3.67 (3) | 7.51 (7) | 3.38 (3) | 3.12 (7) | 5.62 (13) | 2.59 (6) |
| Discontinuation due to adverse event | 14.05 (20) | 6.51 (9) | 3.50 (5) | 12.23 (10) | 10.73 (10) | 4.51 (4) | 13.39 (30) | 8.21 (19) | 3.89 (9) |
| Death | 0.00 (0) | 0.00 (0) | 0.00 (0) | 0.00 (0) | 0.00 (0) | 0.00 (0) | 0.00 (0) | 0.00 (0) | 0.00 (0) |
| Malignancies (excluding NMSC) | 0.00 (0) | 0.72 (1) | 0.70 (1) | 0.00 (0) | 0.00 (0) | 0.00 (0) | 0.00 (0) | 0.43 (1) | 0.43 (1) |
| ≥10 Adverse events occurring in any treatment group | |||||||||
| Nasopharyngitis | 19.67 | 22.42 | 18.20 | 12.23 | 18.23 | 15.79 | 16.96 | 20.74 | 17.28 |
| Upper respiratory tract infection | 5.62 | 3.62 | 11.20 | 25.69 | 9.65 | 14.66 | 12.94 | 6.05 | 12.53 |
| Abdominal pain | 2.81 | 4.34 | 5.60 | 20.79 | 12.87 | 12.40 | 9.37 | 7.78 | 8.21 |
| Ulcerative Colitis | 35.12 | 13.74 | 12.60 | 0.00 | 0.00 | 0.00 | 22.31 | 8.21 | 7.77 |
| Crohn’s disease | 0.00 | 0.00 | 0.00 | 23.24 | 17.16 | 18.04 | 8.48 | 6.91 | 6.91 |
| Diarrhea | 1.40 | 3.62 | 4.90 | 8.56 | 11.80 | 5.64 | 4.02 | 6.91 | 5.18 |
| Nausea | 2.81 | 2.89 | 4.20 | 11.01 | 10.73 | 4.51 | 5.80 | 6.05 | 4.32 |
| Vomiting | 4.21 | 0.72 | 1.40 | 11.01 | 5.36 | 4.51 | 6.69 | 2.59 | 2.59 |
| Arthralgia | 10.54 | 10.85 | 5.60 | 23.24 | 23.60 | 20.30 | 15.17 | 15.98 | 11.23 |
| Pyrexia | 4.92 | 0.72 | 6.30 | 13.45 | 11.80 | 10.15 | 8.03 | 5.18 | 7.77 |
| Headache | 4.92 | 7.96 | 12.60 | 18.35 | 16.09 | 16.91 | 9.82 | 11.23 | 14.25 |
Abbreviations: SC, subcutaneous; NMSC, non-melanoma skin cancer.
aUlcerative Colitis: CNTO1275UCO3001; Crohn’s disease: CNTO1275CRD3003.
bPatients who were in clinical response to ustekinumab IV induction dosing and were randomized to placebo SC on entry into this maintenance study.
cIncludes data up to the time of meeting loss of response criteria for patients who had a dose adjustment in Crohn’s disease.
dInfection as assessed by the investigator.
During the placebo-controlled periods, 6.2% (95% CI, 4.7–8.2) of patients in the placebo group experienced ≥1 SAE vs 4.4% (95% CI, 3.5–5.6) in the ustekinumab group. Rates were similar between ustekinumab doses (Table 2). Per 100 PYs of follow-up, SAE rates through 1 year of treatment were 27.50 (95% CI, 23.45–32.04) in the placebo and 21.23 (95% CI, 19.12–23.51) in the ustekinumab group (Table 3). The hazard ratio of time to first SAE through 1 year was 0.69 (95% CI, 0.43–1.11). Serious adverse events occurring in >3 patients in the ustekinumab group (ie, rate of ≥0.2) included CD, UC, abdominal pain, anal abscess, pneumonia, anemia, small intestinal obstruction, gastroenteritis, and nephrolithiasis; only the latter 3 occurred at numerically slightly higher rates in the combined ustekinumab vs placebo groups (small intestine obstruction (1.21 vs 0.84), gastroenteritis (0.52 vs 0.00), and nephrolithiasis (0.40 vs 0.34; Table 5).
TABLE 5.
Patients With Serious Adverse Events (SAEs) and Serious Infections Per 100 Patient Years of Follow-up Through 1 Yeara
| Ulcerative Colitis | Crohn’s Disease | Inflammatory Bowel Disease | ||||
|---|---|---|---|---|---|---|
| Placebo | Ustekinumab | Placebo | Ustekinumab | Placebo | Ustekinumab | |
| Patients treated | 446 | 825 | 943 | 1749 | 1389 | 2574 |
| Average duration of follow-up (weeks) | 29.14 | 39.53 | 19.11 | 32.89 | 22.33 | 35.02 |
| Average duration of treatment (weeks) | 20.28 | 28.82 | 9.87 | 18.80 | 13.46 | 22.01 |
| Total patient-years of follow-up | 250 | 627 | 347 | 1106 | 596 | 1733 |
| SAEs (≥0.2 in Ustekinumab-Treatment Groups) | ||||||
| Number of patients with SAEs | 46 | 81 | 118 | 287 | 164 | 368 |
| Number of patients with SAEs per 100 patient-years of follow-up | 18.41 | 12.91 | 34.05 | 25.94 | 27.50 | 21.23 |
| System-organ class/preferred term | ||||||
| Crohn’s disease | 0.00 | 0.00 | 13.28 | 10.21 | 7.71 | 6.52 |
| Ulcerative colitis | 8.80 | 4.30 | 0.00 | 0.00 | 3.69 | 1.56 |
| Small intestinal obstruction | 0.00 | 0.00 | 1.44 | 1.90 | 0.84 | 1.21 |
| Abdominal pain | 0.40 | 0.16 | 0.58 | 0.63 | 0.50 | 0.46 |
| Anal abscess | 0.80 | 0.00 | 2.31 | 0.99 | 1.68 | 0.63 |
| Pneumonia | 0.00 | 0.48 | 0.87 | 0.36 | 0.50 | 0.40 |
| Gastroenteritis | 0.00 | 0.64 | 0.00 | 0.45 | 0.00 | 0.52 |
| Nephrolithiasis | 0.00 | 0.48 | 0.58 | 0.36 | 0.34 | 0.40 |
| Anemia | 0.40 | 0.32 | 0.58 | 0.18 | 0.50 | 0.23 |
| Serious infections (≥0.1 in Ustekinumab Treatment Groups) | ||||||
| Number of patients with serious infectionsb | 10 | 20 | 23 | 67 | 33 | 87 |
| Number of patients with serious infectionsb per 100 patient-years of follow-up | 4.00 | 3.19 | 6.64 | 6.06 | 5.53 | 5.02 |
| System-organ class/preferred term | ||||||
| Pneumonia | 0.00 | 0.48 | 0.87 | 0.36 | 0.50 | 0.40 |
| Anal abscess | 0.80 | 0.00 | 2.02 | 0.90 | 1.51 | 0.58 |
| Gastroenteritis | 0.00 | 0.48 | 0.00 | 0.45 | 0.00 | 0.46 |
| Gastroenteritis viral | 0.00 | 0.00 | 0.00 | 0.36 | 0.00 | 0.23 |
| Pyelonephritis | 0.00 | 0.32 | 0.00 | 0.09 | 0.00 | 0.17 |
| Abdominal abscess | 0.00 | 0.00 | 0.58 | 0.18 | 0.34 | 0.12 |
| Cytomegalovirus colitis | 0.00 | 0.32 | 0.00 | 0.00 | 0.00 | 0.12 |
| Perirectal abscess | 0.00 | 0.00 | 0.29 | 0.18 | 0.17 | 0.12 |
| Cholecystitis | 0.00 | 0.00 | 0.00 | 0.18 | 0.00 | 0.12 |
aUlcerative Colitis: CNTO1275UCO3001; Crohn’s disease: C0379T07 (only placebo-controlled IV population), C0743T26, CNTO1275CRD3001 and CNTO1275CRD3002.
bInfection as assessed by the investigator
No association between ustekinumab therapy and changes in laboratory parameters was observed (data on file).
Infections
During the placebo-controlled period, infections occurred in similar proportions of patients in placebo and combined phase 3 ustekinumab groups (19.9% [95% CI, 17.1–22.8] vs 19.3% [95% CI, 17.4–21.3], respectively; Table 2). Through 1 year of treatment in the pooled IBD population, rates of patients with any infection (per 100 PYs) was not higher in the ustekinumab (64.32 [95% CI, 60.60–68.21]) than the placebo group (80.31 [95% CI, 73.28–87.84]; Table 3). The most frequently reported infections were nasopharyngitis and URTI. Rates of other infections such as herpes zoster and Clostridium difficile were similar between treatment groups in both induction period (Table 2) and through 1 year (Table 3).
The number of patients with serious infections per 100 PYs was 5.53 (95% CI, 3.81–7.77) in the placebo and 5.02 (95% CI, 4.02–6.19) in the ustekinumab groups (Table 4.) The hazard ratio of time to first serious infection through 1 year was 1.03 (95% CI, 0.39–2.72). Serious infections occurring in >1 patient in the ustekinumab group (ie, a rate of ≥0.1) included pneumonia, anal abscess, gastroenteritis, viral gastroenteritis, pyelonephritis, abdominal abscess, cytomegalovirus (CMV) colitis, perirectal abscess, and cholecystitis (Table 5).
Although rates between placebo and ustekinumab are similar by indication and overall across IBD, the overall rate of serious infections in CD was numerically higher than in UC. Crohn’s disease–specific manifestation of anal abscess occurred at a rate of 2.02 and 0.90 patients per 100 PYs of follow-up in placebo and ustekinumab patients, respectively (Table 5). Additionally, overall rates of GI abscesses in the induction period and through 1 year were generally similar between treatment groups, occurring in 8 (1.0%) in placebo patients and 14 (0.9%) ustekinumab patients (Table 2) and were not higher on ustekinumab when adjusted for PYs (5.20 in placebo and 3.00 in ustekinumab patients; Table 3).
As previously published,5 1 case of active TB was reported in a 32-year-old Hungarian man in IM-UNITI who received one IV ustekinumab dose of 130 mg 10 months before onset. The patient experienced flu-like symptoms and yellowish sputum; chest radiograph and computed tomography scan results were consistent with pulmonary TB. Empiric treatment with triple anti-TB therapy resolved the symptoms. The only other active TB case through 1 year was a patient in UNIFI maintenance with pulmonary TB from Korea who received placebo (never received ustekinumab).
Through 1 year, 12 patients reported OIs in the pooled IBD population (10 ustekinumab [0.58/100PYs], 2 placebo [0.34/100 PYs]). Nine of 12 patients were receiving concomitant immunosuppressants (including corticosteroids). These events were esophageal candidiasis (all nonserious in 3 ustekinumab- and 2 placebo-treated CD patients), CMV colitis in 2 (0.32/100 PYs) ustekinumab-treated UC patients (both diagnosed ≥4 months after discontinuation of ustekinumab due to lack of efficacy/worsening UC and receiving concomitant immunosuppressants [1 receiving steroids, 1 receiving steroids/adalimumab]). Other OIs occurring in 1 patient each (ustekinumab group) were Legionella pneumonia, Listeria meningitis, disseminated histoplasmosis, cryptosporidiosis infection, and concurrent ophthalmic and oral herpes simplex.
Hypersensitivity Reactions
No serious anaphylactic reactions or serum sickness-like reactions to ustekinumab were observed. As previously reported,7 2 CD patients displayed signs/symptoms of hypersensitivity temporally associated with treatment (1 [0.1%] with throat tightness/shortness of breath/flushing after first and only SC ustekinumab administration and 1 [0.8%] with chest discomfort/flushing/urticaria/fever after initial IV ustekinumab dose).14 Symptoms resolved within 1 hour after oral corticosteroid/antihistamine treatment.
Injection-site Reactions
In the phase 3 population, 26 of 7154 (0.4%) SC injections were reported to have injection-site reactions (ISRs) in the placebo group and 51 of 7055 (0.7%) in the ustekinumab group, corresponding to 1.7% of patients in the placebo and 2.6% of patients in the ustekinumab group (data not shown). The most common ISR was injection site erythema (1.6% for ustekinumab vs 0.9% for placebo), an identified adverse drug reaction for ustekinumab that has not been associated with the small (<4%) number of patients who exhibit antibodies to ustekinumab.
Malignancies
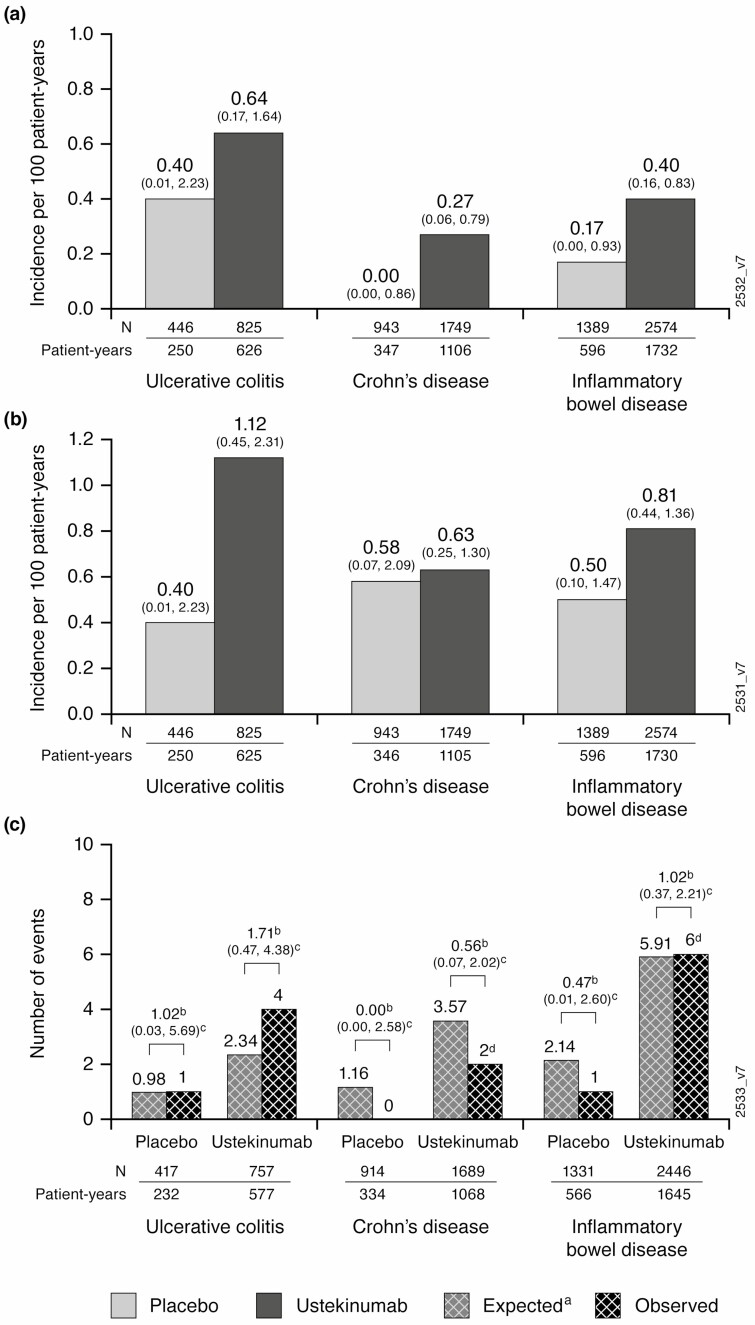
Through 1 year of treatment in the pooled IBD population, rates of malignancies other than NMSC per 100 PYs were low and similar between placebo and ustekinumab patients (0.17 [95% CI, 0.00–0.93]; and 0.40 [95% CI, 0.16–0.83], respectively; Fig. 1A). Rates were similar for the placebo and ustekinumab groups for malignancies including NMSCs, (0.50 [95% CI, 0.10–1.47]; and 0.81 [95% CI, 0.44–1.36], respectively; Fig. 1B). Rates of patients with 1 or more NMSC were also low and comparable between treatment groups, 0.34 (95% CI, 0.04–1.21) for the placebo and 0.40 (95% CI, 0.16–0.83) for ustekinumab groups. Overall, 4 basal cell carcinomas (BCCs) and 3 squamous cell carcinomas (SCCs) were reported in the IBD population.
FIGURE 1.
All malignances excluding NMSC (a), malignancies including NMSC (b), and malignancies compared with National Institutes of Health Surveillance, Epidemiology, and End Results (SEER) database (c). aOne malignancy was not included as patient’s race was unknown. Abbreviation: NMSC, non-melanoma skin cancer.
Across the pooled IBD population, no cases of lymphoma were reported through 1 year. Details on the reported malignancies other than NMSC are presented in Table 6. Most patients with malignancies were treated with at least 1 other biologic before ustekinumab. Only 1 patient (plasma cell myeloma) received any prior immunomodulator therapy. Additionally, half of the patients only received 2 doses of ustekinumab (one IV and one SC), and the longest exposure was 3 doses (one IV and two SC).
TABLE 6.
Reported Malignancies Through 1 Year (Excluding NMSC)
| Malignacy (PT) | Age/ Sex | IBD Duration (years) | Relevant Risk Factors | Smoking Status | Study Treatment | Last Study Agent Dose (study day) | Malignancy Diagnosis (study day) | Prior IBD Therapy |
|---|---|---|---|---|---|---|---|---|
| Ulcerative colitis | ||||||||
| Prostate Cancer | 61/M | 1 | Family history of prostate cancer | Yes-Past smoker | Ustekinumab 130 mg IV; 1 dose of 90 mg SC | 56 | 69 | Steroids, IFX, 6-MP |
| Rectal adenocarcinoma | 32/M | 6 | Ulcerative colitis | No | Ustekinumab 130 mg IV; 1 dose of 90 mg SC | 56 | 159 | Steroids, ADA, IFX, GOL, 6-MP |
| Colon cancer | 48/F | 28 | None | No | Ustekinumab 6 mg/kg IV; 1 dose of 90 mg SC | 58 | 59 | IFX, steroids |
| Papillary renal cell carcinoma | 70/M | 33 | hypertension, diuretic use | Yes-Past smoker | Ustekinumab 130 mg IV; 1 dose of 90 mg SC | 56 | 92 | Steroids |
| Testis cancer | 26/M | 1 | None | No | Placebo | 285 | 302 | Steroids |
| Crohn’s disease | ||||||||
| Adenocarcinoma of small intestine & incidental carcinoid tumor | 68/M | 4 | Family history of cancer | Yes, Past smoker | Placebo, ustekinumab 130 mg IV, 2 doses of 90 mg | 196 | 255 | IFX, steroids |
| Plasma cell myeloma (multiple myeloma) | 57/M | 34 | Monoclonal IgG kappa gammopathy of undetermined significance | No | Ustekinumab 6 mg/ kg IV | 1 | 199 | IFX, ADA, steroids, IMMa |
| Prostate cancer | 53/M | 30 | Elevated prostate-specific antigen levels before randomization | Not reported | Ustekinumab induction IV 4.3 mg/kg | 1 | 64 | steroids, 5-ASA |
Abbreviations: 5-ASA, 5-aminosalicylates; 6-MP, 6-mercaptopurine; ADA, adalimumab; F, female; GOL, golimumab; IFX, infliximab; IV, intravenous; NMSC, non-melanoma skin cancer; PT, preferred term Y, yes; M, male; N, no.
aIMM is azathioprine and/or 6-MP and/or methotrexate
For CD and UC combined, SIR of the observed number of patients with malignancies in the pooled IBD population compared with the expected number from SEER was 0.47 (95% CI, 0.01–2.60) in the ustekinumab and 1.02 (95% CI, 0.37–2.21) in the placebo groups. Within UC and CD, results were generally similar (Fig. 1C). Data interpretation is limited by small numbers of events and relatively short duration of exposure.
Major Adverse Cardiovascular Events and Deep Vein Thrombosis/Pulmonary Embolism
Incidence of adjudicated serious MACE per 100 PYs was low through 1 year, with 2 patients each in the placebo- (0.34) and ustekinumab-treated (0.12) pooled IBD population (Table 3).” instead of “Incidence of adjudicated serious MACE per 100 PYs was low through 1 year, with 1 patient each in the placebo- (0.34) and ustekinumab-treated (0.12) pooled IBD population (Table 4). Adjudicated UC events were 1 nonfatal myocardial infarction (perioperative cardiac arrest) and 1 nonfatal stroke in the placebo group. Additionally, there was 1 acute myocardial infarction diagnosed in a patient who experienced respiratory failure with prolonged hypoxemia postanesthesia. This patient ultimately died from sequelae of acute respiratory distress syndrome, categorized as cardiovascular death for purpose of this analysis. In CD, there was one adjudicated nonfatal stroke in a ustekinumab-treated patient.
Through 1 year in the pooled IBD population, DVT and/or PE events were reported in 2 placebo patients (0.34 per 100 PYs, [95% CI, 0.04–1.21]) and 13 ustekinumab patients (0.75 per 100 PYs, [95% CI, 0.40–1.28]), with 16 events of DVT and/or PE. One patient with UC who was on ustekinumab therapy reported both DVT and PE events. A total of 4 IBD patients reported only PEs, 2 receiving placebo and 2 receiving ustekinumab; and 10 patients (all ustekinumab-treated) reported only DVT. In CD, 11 patients reported DVT and/or PE events, 2 in the placebo and 9 in the ustekinumab groups.
Neurologic Events
No cases of progressive multifocal leukoencephalopathy or reversible posterior leukoencephalopathy were reported. A nonserious case of progression of multiple sclerosis was reported in a UC patient (ustekinumab) with a history of relapsing-remitting multiple sclerosis. A case of possible demyelination was reported in a patient with CD (nonresponder who received 130 mg ustekinumab by IV and 90 mg SC 8 weeks later); magnetic resonance imaging findings were consistent with microvascular disease and previous small vessel insults.
Deaths
Through 1 year of follow-up, 2 deaths were reported in UC patients (1 patient who died of acute respiratory distress syndrome, described previously, and 1 due to esophageal varices hemorrhage), both previously reported in UNIFI.11
Immunogenicity
Through 1 year, for IBD patients who received IV and SC ustekinumab, antibodies to ustekinumab were found in 3.6% of patients. Rates were numerically lower among CD patients than UC patients (2.9% vs 4.6%), as anticipated due to the more stringent cut point used in UC studies and described previously. Overall incidence of antibodies to ustekinumab was low in CERTIFI and T07, 0.7% and 0%, respectively, as previously reported.4, 13
DISCUSSION
This is the first published integrated IBD safety analysis for ustekinumab, representing 2574 IBD patients (1733 PYs). Overall, ustekinumab exposure was not associated with an increase in safety events, supporting a favorable benefit-risk profile with single initial dosing up to 6 mg/kg IV followed by 90 mg SC every 8/12 weeks. These results are consistent with other integrated analyses, including cross-indications through 1 year,7 5-year integrated psoriasis analysis,2 and safety data through 1 year in UC.11 Additionally, results from this analysis further support the absence of a laboratory monitoring requirement during ustekinumab treatment.
Overall, the integrated IBD safety profile was comparable to placebo during induction and through 1 year of follow-up (including the randomized maintenance subset) in the occurrence of AEs, SAEs, and infections (including serious infections and other infections of interest) with no clinically meaningful differences between doses or dose regimens (90 mg every 8 or 12 weeks). Results of time-to-event analysis adjusted by baseline characteristics for SAEs and serious infections do not suggest any increased risks for ustekinumab and are similar to the results adjusted by 100 PYs inasmuch as baseline characteristics in these randomized studies were generally well-balanced among treatment groups.
Through 1 year, a small number of OIs were reported in placebo and ustekinumab patients. Of note, CMV colitis was diagnosed in 2 ustekinumab-treated UC patients. Although detection of CMV seems to be more common in UC compared with CD, its clinical relevance has been widely debated. Ultimately, it is unclear whether CMV in UC truly represents active infection requiring antiviral therapy15, 16 or is simply detected by highly sensitive assays in the context of severe inflammation and often oral immunosuppressants/steroids (both of these cases). Given the low number of events, individual case descriptions, and concomitant immunosuppressant use, there is no clear increased risk of OIs with ustekinumab, which is consistent with previously published data on redundancies in the immune system.17
With both 5 years of follow-up in psoriasis8 and pooled safety analysis (before UC data availability), no safety signals for malignancy were identified with ustekinumab.2, 7, 12, 18 In this IBD population, no increased malignancy risk was identified when compared with SEER (SIR, 1.02, [95% CI, 0.37–2.21] for ustekinumab), including within individual indications of CD or UC. SEER provides a real-world evidence comparison for clinical trial data. These rates are also similar to those observed in a Swiss IBD population (SIR, 0.93, [95% CI, 0.72–1.18]).19 A small number of NMSCs were reported in this data set and comparable between ustekinumab and placebo. A reversal of BCC:SCC ratio (4:1),20 a marker of immunosuppression impact, was not observed. Though these and previously reported findings are reassuring, longer-term longitudinal data and larger (eg, real-world observational) studies are ongoing to confirm current findings of no increased malignancy risk with IL-12/23 inhibition.
As reported in the literature, overall IBD patients have a two- to three-fold increased risk of venous thromboembolism,21–23 with a reported absolute risk of 0.26 per 100 PYs.24 This risk was higher in patients with a disease flare (0.90 per 100 PYs) vs those with chronic activity (0.54 per 100 PYs) and lower for those in remission (0.14 per 100 PYs).24 In patients with active IBD, additional risk factors including corticosteroid use, oral contraceptive use, IBD flare, and surgery confer an even higher risk of thromboembolic events compared with the overall IBD population.23, 24 This is relevant when evaluating risk in patients enrolled in IBD trials, as these generally require patients to have moderately to severely active disease. As presented in these analyses, the overall incidence of DVT/PE was low through 1 year, similar to placebo, and not higher than what is expected in this population.
Rates of antibodies to ustekinumab were low through 1 year, with a numerically higher rate in UC vs CD patients. Of note, a change in the ustekinumab assay specificity cut point was implemented for the UC studies to comply with updated regulatory guidelines. This change resulted in a higher false-positive rate for UC compared with CD (1.6% vs 0.8% respectively; data on file). Thus, this slight difference between rates was expected but not clinically meaningful.
There are several limitations to this study. In a lifetime disease, 1 year of treatment is relatively short; longer-term data will be needed to further support these findings. This may limit comparisons, especially for long latency events like malignancies or certain infections. Although the data contained in this article are only from clinical trials, limitations on interpretation may differ from outcomes observed in the real-world.
This integrated analysis provides additional robust safety data for the use of ustekinumab in patients with IBD. Overall, the safety profile of ustekinumab was favorable and generally comparable to placebo. These data are consistent with the established safety profile of ustekinumab across all approved indications and support a favorable benefit-risk profile of ustekinumab treatment in patients with IBD.
Supplementary Material
Author Contribution: EO, CG, CM, CO, WS, BF, BS, SG, and SD were involved in data collection, data analysis, interpretation of results, drafting the manuscript, and revising critically for content. YZ, SV, IT, and TB were involved in data analysis, interpretation of results, drafting of the manuscript, and revising critically for content. All authors approved the final manuscript for submission.
Supported by: This study was supported by Janssen Research & Development, LLC.
Presented at: Some of the data displayed in this article were presented at United European Gastroenterology Week 2019 in Barcelona, Spain, and American College of Gastroenterology 2019 Meeting in San Antonio, Texas.
Conflicts of Interest: WS reports research grants from Atlantic Healthcare Limited, Amgen, Genentech, Gilead Sciences, Abbvie, Janssen, Takeda, Lilly, Celgene/Receptos,Pfizer, Prometheus Laboratories (now Prometheus Biosciences); consulting fees from Abbvie, Allergan, Amgen, Arena Pharmaceuticals, Avexegen Therapeutics, BeiGene, Boehringer Ingelheim, Celgene, Celltrion, Conatus, Cosmo, Escalier Biosciences, Ferring, Forbion, Genentech, Gilead Sciences, Gossamer Bio, Incyte, Janssen, Kyowa Kirin Pharmaceutical Research, Landos Biopharma, Lilly, Oppilan Pharma, Otsuka, Pfizer, Progenity, Prometheus Biosciences (merger of Precision IBD and Prometheus Laboratories), Reistone, Ritter Pharmaceuticals, Robarts Clinical Trials (owned by Health Academic Research Trust, HART), Series Therapeutics, Shire, Sienna Biopharmaceuticals, Sigmoid Biotechnologies, Sterna Biologicals, Sublimity Therapeutics, Takeda, Theravance Biopharma, Tigenix, Tillotts Pharma, UCB Pharma, Ventyx Biosciences, Vimalan Biosciences, Vivelix Pharmaceuticals; and stock or stock options from BeiGene, Escalier Biosciences, Gossamer Bio, Oppilan Pharma, Prometheus Biosciences (merger of Precision IBD and Prometheus Laboratories), Progenity, Ritter Pharmaceuticals, Ventyx Biosciences, Vimalan Biosciences. Spouse: Opthotech, consultant, stock options; Progenity, consultant, stock; Oppilan Pharma, employee, stock options; Escalier Biosciences, employee, stock options; Prometheus Biosciences (merger of Precision IBD and Prometheus Laboratories), employee, stock options; Ventyx Biosciences, employee, stock options; Vimalan Biosciences, employee, stock options. BF has received grant/research support from AbbVie Inc., Amgen Inc., AstraZeneca/MedImmune Ltd., Atlantic Pharmaceuticals Ltd., Boehringer-Ingelheim, Celgene Corporation, Celltech, Genentech Inc/Hoffmann-La Roche Ltd., Gilead Sciences Inc., GlaxoSmithKline (GSK), Janssen Research & Development LLC., Pfizer Inc., Receptos Inc./Celgene International, Sanofi, Santarus Inc., Takeda Development Center Americas Inc., Tillotts Pharma AG, UCB; a consultant for Abbott/AbbVie, AdMIRx Inc., Akebia Therapeutics, Allergan, Amgen, Applied Molecular Transport Inc., Aptevo Therapeutics, Asta Pharma, Astra Zeneca, Atlantic Pharma, Avir Pharma, Biogen Idec, BioMx Israel, Boehringer-Ingelheim, Boston Pharmaceuticals, Bristol-Myers Squibb, Calypso Biotech, Celgene, Elan/Biogen, EnGene, Ferring Pharma, Roche/Genentech, Galapagos, Galen/Atlantica, GiCare Pharma, Gilead, Gossamer Pharma, GSK, Inception IBD Inc, Intact Therapeutics, JnJ/Janssen, Kyowa Kakko Kirin Co Ltd., Lexicon, Lilly, Lycera BioTech, Merck, Mesoblast Pharma, Millennium, Nestles, Nextbiotix, Novonordisk, ParImmune, Parvus Therapeutics Inc., Pfizer, Prometheus Therapeutics and Diagnostics, Progenity, Protagonist, Qu Biologics, Rebiotix, Receptos, Salix Pharma, Shire, Sienna Biologics, Sigmoid Pharma, Sterna Biologicals, Synergy Pharma Inc., Takeda, Teva Pharma, TiGenix, Tillotts, UCB Pharma, Vertex Pharma, Vivelix Pharma, VHsquared Ltd., Zyngenia; is a member of the speakers bureau for Abbott/AbbVie, JnJ/Janssen, Lilly, Takeda, Tillotts, UCB Pharma; is a member of the scientific advisory board for Abbott/AbbVie, Allergan, Amgen, Astra Zeneca, Atlantic Pharma, Avaxia Biologics Inc., Boehringer-Ingelheim, Bristol-Myers Squibb, Celgene, Centocor Inc., Elan/Biogen, Galapagos, Genentech/Roche, JnJ/Janssen, Merck, Nestles, Novartis, Novonordisk, Pfizer, Prometheus Laboratories, Protagonist, Salix Pharma, Sterna Biologicals, Takeda, Teva, TiGenix, Tillotts Pharma AG, UCB Pharma; and is a senior scientific officer for Robarts Clinical Trials Inc. SD has received consulting fees from AbbVie, Astra Zeneca, MSD, Takeda Millennium, Salix Pharmaceuticals, and Pfizer. CO, CM, YZ, SV, and IT are employees of Janssen Research & Development, LLC and own stock/stock options. EO, TB, and CG are employees of Janssen Scientific Affairs, LLC and own stock/stock options. BS has received grant support, personal fees, and nonfinancial support from Janssen during the conduct of this study and personal fees from AbbVie, Akros Pharma, Allergan, Amgen, Arena Pharmaceuticals, AstraZeneca, Boehringer Ingelheim, EnGene, Forward Pharma, Bristol-Myers Squibb, Immune Pharmaceuticals, Ironwood Pharmaceuticals, Lycera, Lyndra, Receptos, Shire, Synergy Pharmaceuticals, Target PharmaSolutions, Theravance Biopharma R&D, TiGenix, Topivert and Salix; grants, personal fees, and nonfinancial support from Celgene, Pfizer and Takeda; and personal fees and nonfinancial support from 4D Pharma, Capella Bioscience, F. Hoffman-La Roche, Ferring, Gilead, Lilly, MedImmune, Oppilan Pharmaceuticals, Otsuka, Palatin Technologies, Prometheus Laboratories, Protagonist Therapeutics, Rheos Medicines, Seres Therapeutics, Vivelix Pharmaceuticals and UCB, all outside of the submitted work. SG is a member of steering committees of Janssen, Abbvie, Boehringer Ingelheim, Gilead, Celgene, BMS; has received speaker honorarium from Abbvie, Takeda, Janssen, Ferring; and served on advisory committees of Janssen, Takeda, Abbvie, Eli Lilly.
ACKNOWLEDGMENTS
The authors would like to thank Yanlin Wang of Janssen Research & Development, LLC for assistance with statistical programming. Writing assistance was provided by Kirsten Schuck Gross of Janssen Scientific Affairs, LLC.
REFERENCES
- 1. Kimball AB, Gordon KB, Fakharzadeh S, et al. . Long-term efficacy of ustekinumab in patients with moderate-to-severe psoriasis: results from the PHOENIX 1 trial through up to 3 years. Br J Dermatol. 2012;166:861–872. [DOI] [PubMed] [Google Scholar]
- 2. Kimball AB, Papp KA, Wasfi Y, et al. ; PHOENIX 1 Investigators . Long-term efficacy of ustekinumab in patients with moderate-to-severe psoriasis treated for up to 5 years in the PHOENIX 1 study. J Eur Acad Dermatol Venereol. 2013;27:1535–1545. [DOI] [PubMed] [Google Scholar]
- 3. Leonardi CL, Kimball AB, Papp KA, et al. ; PHOENIX 1 study investigators . Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet. 2008;371: 1665–1674. [DOI] [PubMed] [Google Scholar]
- 4. Sandborn WJ, Gasink C, Gao LL, et al. ; CERTIFI Study Group . Ustekinumab induction and maintenance therapy in refractory Crohn’s disease. N Engl J Med. 2012;367:1519–1528. [DOI] [PubMed] [Google Scholar]
- 5. Feagan BG, Sandborn WJ, Gasink C, et al. ; UNITI–IM-UNITI Study Group . Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2016;375:1946–1960. [DOI] [PubMed] [Google Scholar]
- 6. Sandborn WJ, Rutgeerts P, Gasink C, et al. . Long-term efficacy and safety of ustekinumab for Crohn’s disease through the second year of therapy. Aliment Pharmacol Ther. 2018;48:65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ghosh S, Gensler LS, Yang Z, et al. . Ustekinumab safety in psoriasis, psoriatic arthritis, and Crohn’s disease: an integrated analysis of phase II/III clinical development programs. Drug Saf. 2019;42:751–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Papp KA, Griffiths CE, Gordon K, et al. ; PHOENIX 1 Investigators; PHOENIX 2 Investigators; ACCEPT Investigators . Long-term safety of ustekinumab in patients with moderate-to-severe psoriasis: final results from 5 years of follow-up. Br J Dermatol. 2013;168:844–854. [DOI] [PubMed] [Google Scholar]
- 9. Ritchlin C, Rahman P, Kavanaugh A, et al. ; PSUMMIT 2 Study Group . Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial. Ann Rheum Dis. 2014;73:990–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McInnes IB, Kavanaugh A, Gottlieb AB, et al. ; PSUMMIT 1 Study Group . Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet. 2013;382:780–789. [DOI] [PubMed] [Google Scholar]
- 11. Sands BE, Sandborn WJ, Panaccione R, et al. ; UNIFI Study Group . Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2019;381:1201–1214. [DOI] [PubMed] [Google Scholar]
- 12. Papp K, Gottlieb AB, Naldi L, et al. . Safety surveillance for ustekinumab and other psoriasis treatments from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Drugs Dermatol. 2015;14:706–714. [PubMed] [Google Scholar]
- 13. Sandborn WJ, Feagan BG, Fedorak RN, et al. ; Ustekinumab Crohn’s Disease Study Group . A randomized trial of Ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with moderate-to-severe Crohn’s disease. Gastroenterology. 2008;135:1130–1141. [DOI] [PubMed] [Google Scholar]
- 14. STELARA [package insert]. Horsham, PA: Janssen Biotech, Inc.; 2018. [Google Scholar]
- 15. Al-Zafiri R, Gologan A, Galiatsatos P, Szilagyi A. Cytomegalovirus complicating inflammatory bowel disease: a 10-year experience in a community-based, university-affiliated hospital. Gastroenterol Hepatol (NY). 2012;8:230–239. [PMC free article] [PubMed] [Google Scholar]
- 16. Domènech E, Vega R, Ojanguren I, et al. . Cytomegalovirus infection in ulcerative colitis: a prospective, comparative study on prevalence and diagnostic strategy. Inflamm Bowel Dis. 2008;14:1373–1379. [DOI] [PubMed] [Google Scholar]
- 17. Nembrini C, Abel B, Kopf M, Marsland BJ. Strong TCR signaling, TLR ligands, and cytokine redundancies ensure robust development of type 1 effector T cells. J Immunol. 2006;176:7180–7188. [DOI] [PubMed] [Google Scholar]
- 18. Fiorentino D, Ho V, Lebwohl MG, et al. . Risk of malignancy with systemic psoriasis treatment in the Psoriasis Longitudinal Assessment Registry. J Am Acad Dermatol. 2017;77:845–854.e5. [DOI] [PubMed] [Google Scholar]
- 19. Scharl S, Barthel C, Rossel J-B, et al. . Malignancies in inflammatory bowel disease: frequency, incidence and risk factors-results from the Swiss IBD Cohort Study. Am J Gastroenterol. 2019;114:116–126. [DOI] [PubMed] [Google Scholar]
- 20. Rubin AI, Chen EH, Ratner D. Basal-cell carcinoma. N Engl J Med. 2005;353:2262–2269. [DOI] [PubMed] [Google Scholar]
- 21. Nguyen GC, Bernstein CN, Bitton A, et al. . Consensus statements on the risk, prevention, and treatment of venous thromboembolism in inflammatory bowel disease: Canadian Association of Gastroenterology. Gastroenterology. 2014;146:835–848.e6. [DOI] [PubMed] [Google Scholar]
- 22. Zezos P, Kouklakis G, Saibil F. Inflammatory bowel disease and thromboembolism. World J Gastroenterol. 2014;20:13863–13878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Papa A, Gerardi V, Marzo M, et al. . Venous thromboembolism in patients with inflammatory bowel disease: focus on prevention and treatment. World J Gastroenterol. 2014;20:3173–3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grainge MJ, West J, Card TR. Venous thromboembolism during active disease and remission in inflammatory bowel disease: a cohort study. Lancet. 2010;375:657–663. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.