Abstract
Background
The neutrophil fecal biomarkers, calprotectin (FCP) and lactoferrin (LCT), and peripheral blood neutrophil CD64 surface receptor (nCD64) are biomarkers for mucosal inflammation in inflammatory bowel disease (IBD). Although FCP has been evaluated as a biomarker for mucosal healing, cut points for LCT and nCD64 are less known. We aimed to identify the cut points for LCT and nCD64 that were associated with FCP remission, with a secondary aim to evaluate the relationship between biochemical outcomes and infliximab (IFX) trough concentrations.
Methods
We analyzed FCP, LCT, and nCD64 before and after IFX induction in a pediatric Crohn’s disease (CD) cohort study. Week-14 FCP biomarker remission was defined as FCP <250 µg/g, with clinical response defined as a weighted Pediatric Crohn’s Disease Activity Index <12.5 or Δ>17.5 improvement. Predictive outcomes were calculated by receiver operating characteristics (ROCs).
Results
Among 56 CD patients, ROC analysis identified an infusion 4 LCT <8.06 (area under the receiver operator characteristics [AUROC], 0.934, P < 0.001) and nCD64 <6.12 (AUROC, 0.76, P = 0.02) as the ideal cut points for week-14 FCP biomarker remission. End of induction IFX-trough of >9.4 µg/mL (AUROC, 0.799, P = 0.002) and >11.5 µg/mL (AUROC, 0.835, P = 0.003) were associated with a FCP <250 and FCP <100, respectively. We found patients achieving end of induction trough >5 µg/mL had a median FCP improvement (dose 1 to dose 4) of 90% compared with a median of 35% with levels <5 µg/mL (P = 0.024) with a similar median reduction in nCD64 (48% vs 20%, P = 0.031).
Conclusions
This study establishes cut points in neutrophil stool and blood biomarkers for both biochemical remission and therapeutic trough levels following induction therapy. Further studies that evaluate pharmacodynamic biomarker targets for endoscopic and histologic healing are warranted.
Keywords: biomarkers, fecal calprotectin, fecal lactoferrin, neutrophil CD64 expression, infliximab, pediatric Crohn’s disease
INTRODUCTION
Endoscopic evaluation remains the gold standard to monitor treatment response among patients with Crohn’s disease (CD).1 However, frequent reassessment of disease with endoscopy is associated with increased patient burden, risks, and cost. Although assessing patient-reported symptoms would be an ideal alternative method to monitor treatment response, the nonspecific and heterogeneous nature of CD symptoms has made it difficult to objectively compare symptoms alone with standard markers of disease activity.2–4
Surrogate noninvasive or minimally invasive biomarkers are warranted to address these needs and limitations and are an attractive alternative to circumvent the need for frequent invasive procedures, especially during induction when evaluation for primary nonresponse is so vital. The neutrophil-derived fecal biomarkers calprotectin (FCP) and lactoferrin (LCT) are now widely accepted as screening tools for new diagnosis of CD.5 Furthermore, a meta-analysis found that FCP and LCT were sensitive markers of active endoscopic inflammatory bowel disease (IBD) among multiple studies.6
Because FCP has shown the most robust correlation with endoscopic activity in clinical trials, it therefore is the most accepted fecal biomarker for mucosal healing in IBD.7 Moreover, the recently published PANTS study identified that lower FCP levels postinfliximab induction at week-14 were associated with higher infliximab (IFX) trough levels.8 Moreover, a recent post hoc analysis of the TAILORIX trial demonstrated that FCP is a responsive pharmacodynamic biomarker after IFX dose escalation.9 However, though calprotectin is an accepted biomarker,7 other noninvasive (neutrophil) fecal biomarkers (such as LCT) are sometimes preferred in real-world clinical practice due to local availability, cost differences, or third-party payor decisions.
Although well-defined cut points for fecal biomarkers such as FCP have been validated,6, 7 blood biomarkers may be preferred at times due to disease characteristics or patient preferences. Our group previously identified that the novel blood biomarker neutrophil CD64, which reflects the surface expression of FcγRI on circulating (activated) neutrophils is elevated in CD patients who are newly diagnosed and treatment naïve.10 Subsequently, we found that the neutrophil CD64 index and the neutrophil CD64 activity ratio (nCD64, 2 similar approaches to detect surface expression of CD64 by flow cytometry) were identified as correlates with endoscopic severity11, with elevations in neutrophil CD64 expression identified as a risk factor for treatment relapse in asymptomatic CD.12 Moreover, baseline (pretreatment) elevations in nCD64 were associated with a poor biochemical response to IFX (odds ratio 8.9, P = 0.011) in pediatric CD patients.13
Although all these biomarkers (FCP, LCT, and nCD64) seem to be correlated with endoscopic activity, limited studies have evaluated the utility of change in FCP, LCT, or nCD64 over time or their relationship with anti-TNF drug concentrations. However, as frequent endoscopic evaluations are invasive and there is a limited window of opportunity to objectively determine primary nonresponse, there is a critical need to establish minimally invasive pharmacodynamic biomarker targets to monitor and guide treatment over time. We hypothesized that improvement in the neutrophil fecal markers (FCP or LCT) and peripheral blood nCD64 during induction therapy would be associated with a higher likelihood of achieving target IFX concentrations. The aim was to identify cut points for LCT and nCD64 that were associated with week-14 FCP biomarker remission (WK14BR), with a secondary aim to evaluate the relationship between biochemical outcomes and IFX trough concentrations.
MATERIALS AND METHODS
Study Design
This is a post hoc analysis of a multicenter, observational cohort of pediatric Crohn’s disease patients who were induced with IFX and who provided longitudinal blood and stool biospecimens. For this study, only biospecimens of patients recruited at Cincinnati Children’s Hospital were included for analysis. Patients received standard-of-care (SOC) IFX infusions at 5 to 10 mg/kg at weeks 0, 2, and 6. Our institutional practice is to administer IFX as monotherapy. Blood and fecal samples were collected to measure pharmacokinetic and pharmacodynamic parameters at baseline (before infusion 1; IFX-1) and throughout the infliximab induction phase until the first postinduction (before infusion 4; IFX-4).
Fecal Biomarkers
To assess fecal biomarker response, we evaluated the commonly used neutrophil fecal markers, FCP and LCT. Fecal calprotectin was measured with the Bühlmann Calprotectin ELISA kit (Bühlmann laboratories AG, Schönenbuch, Switzerland). This assay has a lower detection limit of 30 µg/g. An FCP less than 250 µg/g was considered indicative of biomarker remission, as this level has previously been demonstrated to be the most robust levels associated with mucosal healing.6 An FCP less than 100 µg/g was used as a second FCP cut point, as this the second most robust marker associated with mucosal healing.6
Fecal lactoferrin was measured with the lactoferrin Scan ELISA kit (Techlab, Blacksburg, VA, USA). The manufacturer reports that an LCT level at or over 7.25 µg/mL (g) feces is elevated. As cut points of mucosal healing are less established with LCT, we evaluated LCT cut points in our cohort associated with the FCP cut points of less than 250 µg/g and less than 100 µg/g.
Peripheral-blood Biomarker nCD64
In addition to neutrophil-derived stool biomarkers, we also analyzed a blood biomarker that reflects circulating neutrophil activation. For this study, we used the Leuko64 assay kit (Trillium Diagnostics, Brewer, ME) to measure the whole blood neutrophil CD64 by quantitative flow cytometry on a FACSCalibur (BD Biosciences, San Jose, CA). This kit utilizes florescent beads and CD64 and CD163 antibodies. Neutrophil CD64 activation is reported as the neutrophil CD64 index. The neutrophil CD64 activity ratio, nCD64, is the ratio between the CD64 mean fluorescent intensity (MFI) of circulating granulocytes and CD64 MFI of circulating lymphocytes. As nCD64 is a novel method to detect neutrophil CD64 surface expression, it was only calculated in a subset of patients. Therefore, the nCD64 was extrapolated from the neutrophil CD64 index by creating a linear regression standard curve with R2 = 0.673 and y = 5.37x + 0.9147 based on 243 paired samples (unpublished).
Infliximab Assay
Infliximab serum concentrations were measured at trough with the electrochemiluminescence-based immunoassay (ECLIA) method by Esoterix (LabCorp specialty lab, Calabasas, CA). This drug tolerant assay detects IFX drug concentrations of ≥0.4 µg/mL and detects antibodies to infliximab (ATIs) ≥22 ng/mL.
Clinical Disease Activity
Clinical disease activity was measured with the weighted Pediatric Crohn’s Disease Activity Index (wPCDAI), which has demonstrated to perform comparable to the original PCDAI and its other modified versions.14 Clinical response was defined by a wPCDAI score less than 12.5 at infusion 4 or more than 17.5 point reduction in wPCDAI between baseline and infusion 4 per prior publications.14 Sustained clinical remission was defined as clinical remission (wPCDAI <12.5) from postinduction (infusion 4) up to 6 months. Corticosteroid use beyond the induction phase was considered a treatment failure for all outcomes.
Outcomes
The primary outcome was end of induction LCT and nCD64 associated with WK14BR (defined as FCP <250 µg/g). The key secondary outcome was IFX-4 levels and the percentage change in the neutrophil biomarkers (baseline to end of induction).
Ethical Considerations
This study was approved by the Cincinnati Children’s Hospital Medical Center (CCHMC) institutional review board (IRB). All participants provided consent before study enrollment.
Statistical Analysis
Differences in linear biomarker levels between nominal outcome variables were compared using the nonparametric Mann-Whitney U test. The Kruskal-Wallis test and the Dunn post-test of multiple comparisons were used to compare the differences in biomarker outcomes with IFX-4 levels. Data were presented as medians with interquartile range (IQR). Area under the receiver operator characteristics (AUROCs) and Youden-J statistics were calculated with R version 3.6.1 (R Foundation for Statistical Computing, R Core team 2019, Vienna Austria) and the “cutpointr” package. The ROC graphs were illustrated with IBM SPSS Statistics version 20.0 (IBM, Armonk, NY). Correlations between the different biomarkers were calculated by Spearman correlation. A P value <0.05 was considered statistically significant.
RESULTS
Among the 56 pediatric patients who submitted stool samples, 89% (50 of 56) submitted stool at infusion 1 and 93% (52 of 56) at infusion 4. Eighty-two percent (46 of 52) had paired fecal samples from infusion 1 and infusion 4. Sixty-six percent were male, and the mean age was 11.4 years (SD, 4.1; Table 1). The median time from diagnosis to IFX start was 2 months (IQR, 0–15.5). The majority of patients (88%) had ileocolonic disease with a predominately (91%) inflammatory (B1) phenotype.
TABLE 1.
Baseline Patient Demographics and Dose Regimens
| Patient Demographics | N = 56a | Abnormal Baseline Level Among Paired Samples |
|---|---|---|
| Age at diagnosis (yrs) | 11.4 (SD 4.1) | |
| Sex (male) | 66% | |
| Disease duration at induction (months) | 2 (0, 15.5) | |
| Paris classification | ||
| A1b (10 to <17 y) | 32 (57%) | |
| L1; L2; L3 | 2; 5; 49 (88%) | |
| B1; B2; B3 | 51 (91%); 3; 2 | |
| FCP (n = 50) | 2612 (1563.6, 3733.0) | 98% (45/46) |
| LCT (n = 50) | 62.7 (33.1, 93.0) | 98% (45/46) |
| nCD64 (n = 53) | 7.8 (5.3, 10.8) | 67% (30/45) |
| CRP (n = 43) | 1.00 (0.33, 2.10) | 79% (30/38) |
| Dose regimens | ||
| Induction doses (mg/kg) | 5.8 (5.1, 6.9) | |
| Steroid use at baseline | 27 (48%) | |
| IMM use | 1 (2%) |
aData presented as n (%), median (IQR) or mean (SD)
The median induction dose was 5.8 mg/kg (IQR, 5.1–6.9). Forty-eight percent of the patients were co-induced with prednisone (at baseline), with 2 patients remaining on prednisone at infusion 4. One patient was on an immunomodulator (methotrexate) at baseline but was discontinued after the second IFX infusion.
At baseline, 98% (45 of 46) had an elevated FCP, with a median FCP of 2612 µg/g (IQR, 1563.6–3733.0) and a median FCP of 596.6 µg/g (IQR, 211.6–2675.8) at infusion 4. We found that approximately one third of patients (16 of 46) had FCP <250 µg/g, and 22% (10 of 46) were <100 µg/g at infusion 4. Of note, 63% (10 of 16) of the biochemical remitters (FCP <250 µg/g) also achieved clinical remission (wPCDAI <12.5) at infusion 4. Additional baseline biomarker values are listed in Table 1. The median percentage change in LCT and nCD64 from baseline to infusion 4 was 66% and 29%, respectively.
Cut Points Associated With Biomarker Remission
The AUROC for infusion 4 LCT for distinguishing biochemical remission (WK14BR, defined by FCP of <250 μg/g) was 0.934 (95% CI, 0.85–1.00; P < 0.001; Figure, Supplementary Data Content 1A). An LCT level of 8.06 µg/g had the highest sensitivity (89%) and specificity (98%) for WK14BR (Table 2). The AUROC for LCT associated with a more stringent level of biomarker quiescence (FCP <100 μg/g) demonstrated an even stronger association of 0.972 (95% CI, 0.94–1.00; P < 0.0010.001; Figure, Supplementary Data Content 1B). An LCT level of 6.02 µg/g had the highest sensitivity (100%) and specificity (95%) associated with this FCP <100 µg/g remission range (Table 2).
TABLE 2.
Area Under the Receiver Operating Characteristics (AUROC) for Calprotectin Biomarker Remission and Cut Points for Lactoferrin at Week-14
| AUROC | FCP Biomarker Remission | Optimal LCT Cut point | Sensitivity | Specificity | PPV | NPV | P |
|---|---|---|---|---|---|---|---|
| 0.934 (0.848–1.000) | <250 µg/g | 8.06 µg/g | 89% | 98% | 98% | 89% | < 0.001 |
| 0.972 (0.943–1.000) | <100 µg/g | 6.02 µg/g | 100% | 95% | 100% | 69% | < 0.001 |
Estimates presented as AUROC (95% confidence interval). Abbreviations: PPV, positive predictive value; NPV, negative predictive value.
Although the infusion 4 nCD64 discriminatory performance for WK14BR was not statistically significant with an optimal cut point of 6.12 (AUROC, 0.67; 95% CI, 0.50–0.84; P = 0.07), we performed a secondary analysis to test nCD64 in discriminating WK14BR at infusion 4 in those patients with a baseline nCD64 elevation (n = 30; 67% of the cohort). We found that at the same cut point (6.12), the test was 71% sensitive and 92% specific for WK14BR (AUROC, 0.76; 95% CI, 0.59–0.94; P = 0.02).
A similar overall accuracy was observed for those with a baseline elevated CRP, as we found CRP <0.47 mg/dL was 45% sensitive and 100% specific for WK14BR (AUROC, 0.72; 95% CI, 0.58–0.86; P = 0.056).
Biomarkers and Target Infliximab Levels
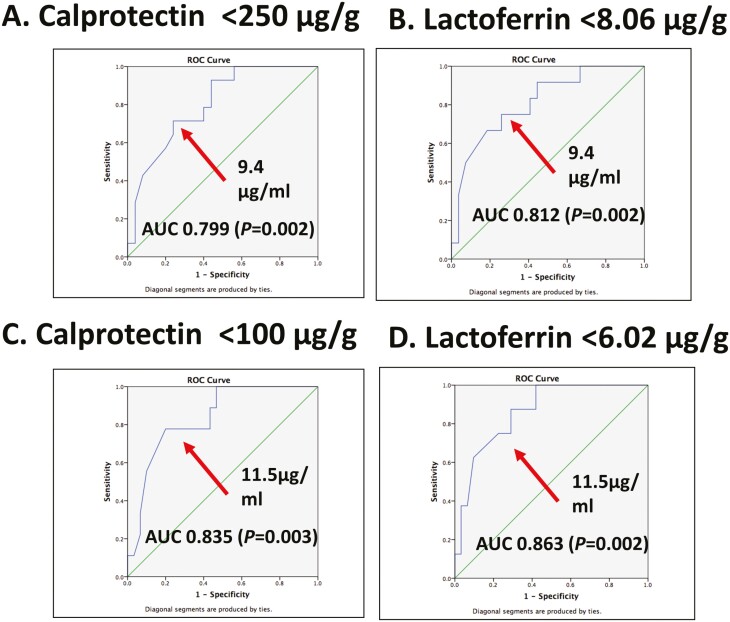
Next, we evaluated the relationship of the fecal and blood biomarkers with end of induction IFX trough levels. As expected, elevations in fecal biomarkers were significantly associated with reductions in IFX concentrations (Fig. 1). In contrast, the association between blood biomarkers overall and IFX concentrations was not statistically significant (Figure, Supplementary Data Content 2). For fecal biomarkers, patients in WK14BR (FCP <250 µg/g; n = 16) had a median 4 IFX level of 13 µg/mL (IQR, 7.7–14 µg/mL), while patients who did not achieve biomarker remission (FCP>250 µg/g; n = 30), had a median IFX level of 4.9 µg/mL (IQR, 1.9–9.8 µg/mL; P = 0.008). An infusion 4 IFX level of ≥9.4 µg/mL was associated with WK14BR and LCT <8.06 µg/g, with an AUROC of 0.799 (P = 0.002) and AUROC of 0.812 (P = 0.002; Fig. 2A and 2B), respectively. Fecal biomarkers with biomarker quiescence FCP <100 µg/g and LCT <6.02 µg/g predicted an infusion 4 IFX level of ≥11.5 µg/mL, with an AUROC of 0.835 (P = 0.003) and AUROC of 0.863 (P = 0.002), respectively (Fig. 2C and 2D).
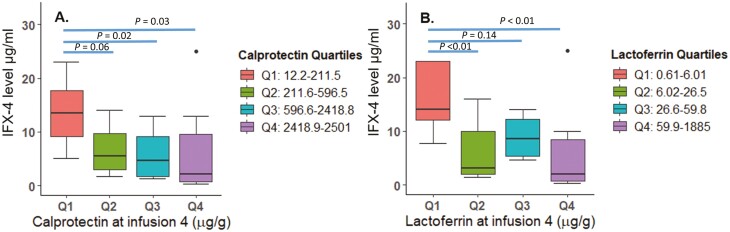
FIGURE 1.
Fecal biomarkers (A) fecal calprotectin and (B) fecal lactoferrin at infusion 4 are associated with therapeutic IFX concentrations.
FIGURE 2.
Levels of biochemical remission for fecal calprotectin (A) <250 µg/g, (B) <100 µg/g, (C) fecal lactoferrin <8.6 µg/g, and <6.02 µg/g associated with infliximab drug concentration cut points.
We found a 90% (IQR, 55%–98%) median decrease in FCP from baseline to IFX-4 for patients with an IFX-4 level of ≥5 μg/mL compared with a 35% (IQR, –41 to 84%) median decrease among patients <5 μg/mL at IFX-4 (P = 0.024; Figure, Supplementary Data Content 3). There was no difference in the median percentage change in LCT during induction (77 vs 45%).
For the blood biomarkers, patients who had an IFX-4 target of 5–10 µg/mL decreased their nCD64 by 48% (IQR, 27%–58%) from baseline to infusion 4, whereas patients outside this range had only a 20% (IQR, –6% to 36%, P = 0.031) reduction in nCD64. We did not find any difference in CRP percentage change in patients with target IFX levels (66% vs 61%). Of note, infusion-4 IFX levels were also not associated with changes in CRP.
Response in Biomarkers is Associated with Clinical Response
Clinical responders at infusion 4 had a median decrease in FCP from baseline to postinduction of 81.3% (IQR, 47%–94%), whereas clinical nonresponders had a median increase in FCP by 30% (IQR, –99% to 66%; P < 0.01).
For blood biomarkers, clinical responders at infusion 4 had a median decrease in nCD64 of 37% (IQR, 19%–49%), whereas clinical nonresponders had a median decrease of 9% (IQR, –21% to 23%; P = 0.07). Clinical responders had a median decrease in CRP of 77% (56%–91%) ,and nonresponders had a median increase of 50% (IQR, –123 to 53; P < 0.01).
Stool Biomarker Levels Predict Sustained Clinical Remission
Fecal calprotectin of 378.09 µg/g at infusion 4 predicted a postinduction sustained clinical remission up to 6 months with a sensitivity of 58% and a specificity of 71% (AUROC, 0.708, ;95% CI, 0.564–0.853; P = 0.01; Figure, Supplementary Data Content 4; Table, Supplementary Data Content 5). An LCT of 25.00 µg/g at infusion 4 predicted a postinduction sustained clinical remission up to 6 months with a sensitivity of 62% and a specificity of 68% (AUROC, 0.667; 95% CI, 0.516–0.818; P = 0.04).
Of note, CRP <0.29 at infusion 4 predicted 6-month sustained clinical remission in a similar fashion with a sensitivity of 86% and specificity of 55% (AUROC, 0.725; 95% CI, 0.589–0.861; P = 0.01). However, nCD64 was not predictive of clinical remission outcomes.
DISCUSSION
In this prospective observational cohort, we tracked fecal and blood biomarkers throughout IFX induction and found an association between changes in FCP, nCD64, and target IFX trough concentrations in CD patients completing IFX induction. Moreover, we identified novel cut points and changes over time in FCP, LCT, and nCD64.
Although endoscopic evaluation is the gold standard to assess for mucosal healing, FCP is often used as the surrogate marker of mucosal healing. Despite some variation in the literature, FCP levels of <250 µg/g and <100 µg/g are the most accepted cut points that were associated with mucosal healing in IBD, with a paucity of articles evaluating percentage change.6, 15 Much less evidence exists regarding the other neutrophil-derived stool biomarker LCT.6 However, as there are discrepancies of stool biomarkers in clinical practice, it is vital to establish cut points to have the ability to compare these stool markers. Although endoscopy was not performed, we identified that a cut point of LCT 8.06 µg/g was associated with a calprotectin of <250 µg/g, and a cut point of LCT 6.02 µg/g was associated with a calprotectin of <100 µg/g. This is similar to the cut points of <7.05 µg/g or <7.25 µg/g that have been identified previously in a meta-analysis and are suggested by the manufacturer as normal.6
One other pediatric study recently evaluated several fecal biomarkers with both endoscopic and radiologic disease activity with MR enterography.16 This study compared FCP with S100A12 (FA12), tumor pyruvate kinase isoenzyme type M2 (FM2PK), and osteoprotegerin (FOPG)—but not with lactoferrin. Those authors found that FCP had a better correlation with endoscopic disease activity as defined by the simple endoscopic severity index for CD (SES-CD). However, that study only evaluated these tests at one time point, with a clear need to further test longitudinal changes.
Though biomarkers are incorporated into some clinical disease activity scores, they generally do not correlate well with disease activity scores, such as variants of the PCDAI.14 We found that wPCDAI (which has been described to best correlate with endoscopic disease activity compared with other versions of the PCDAI) was poorly correlated with any of the blood biomarkers (data not shown). This finding was consistent with the literature.4 Alternatively, we found that blood biomarkers CRP and nCD64 were more strongly correlated with stool biomarkers than clinical disease activity (data not shown). In adults, CRP has been found to be correlated with endoscopic disease activity,6 and an early decrease in CRP has been associated with endoscopic response to IFX.9 In our study, we did not find a change in CRP to be associated with IFX response. However, we did find a strong correlation between CRP and FCP at IFX-4.
As CD is a heterogeneous disease, biomarker accuracy for clinical disease activity may be patient-specific. Prior studies identified that there are no differences in CRP levels among IBD patients with certain TNF-α gene polymorphisms.17 In fact, when we conducted a secondary analysis among patients who had elevated CRP at baseline (79% of the cohort), we identified that a CRP <0.47 at infusion 4 was associated with FCP levels in the biochemical remission range <250 µg/g.
Another novel blood biomarker that our group previously described to be associated with disease activity, neutrophil CD64 expression, has been demonstrated to correlate with endoscopic activity.10, 11 In addition, it has also been identified as a predictive marker for treatment relapse in asymptomatic CD during maintenance therapy.10, 12 Our current study identified that nCD64, which is the ratio of CD64 MFI on granulocytes to CD64 MFI on lymphocytes, was the blood biomarker with the strongest correlation with LCT. Even though we were not able to define a cut point for nCD64 that was associated with FCP remission for the overall sample, this study newly identified that an nCD64 cut point of 6.12 postinduction therapy was associated with FCP in the remission range (<250 µg/g among patients with an elevated nCD64 at baseline (67% of the cohort). This emphasizes the need for additional nontraditional blood biomarkers besides traditional markers such as CRP to guide the management of CD. This study underscores that not all patients are generating CRP during intestinal inflammation. Moreover, there is an urgent need for blood biomarkers that guide therapy. In this study, we showed that patients who were able to achieve target IFX drug concentration levels between 5 and 10 µg/mL at IFX-4 had a 48% decrease of their baseline nCD64. In contrast, changes in CRP in the entire cohort or sensitivity analysis were not associated with target IFX levels.
A small study of 15 CD patients previously evaluated the role of FCP and LCT during anti-TNF therapy and found that stool biomarkers were correlated with macroscopic disease measured by the Crohn’s Disease Endoscopic Index of Severity (CDEIS).18 However, that study did not report any IFX drug concentrations.
Moreover, we found that patients with infusion-4 fecal biomarker levels in the lowest quartile had higher IFX-4 levels than patients with fecal biomarkers in higher quartiles. This was similar to recent data from the PANTS study; however, patients in the lowest FCP quartile in our cohort had even higher IFX levels (>10 µg/mL vs <10 µg/mL) than reported in the PANTS study.8 The difference in the numbers could be related to difference in IFX concentration detection assays used.19, 20 In addition to what was found in the PANTS study, we demonstrated that patients who had an infusion-4 level of ≥5 µg/mL had a 90% decrease of FCP from baseline to infusion 4 by, whereas patients with an IFX< 5 µg/mL had only a 35% decrease after induction.
Our study also had several limitations. Although our study was a prospective observational study with longitudinal sampling, we had a relatively small sample size (n = 56). Additionally, we used well-described fecal inflammatory marker levels that reflect mucosal healing, but we did not perform follow-up endoscopic evaluations at the end of induction (which is not standard of care) and therefore could not evaluate endoscopic healing as an outcome. Also, 48% of patients were on corticosteroids at time of induction; although this could have skewed part of the analysis, this is likely a reflection of the disease severity of these patients. Moreover, when we repeated the PK-analysis for patients without steroids, similar infusion-4 cut points were found (data not shown). Interestingly, though there was a strong relationship between higher IFX infusion-4 levels with lower FCP and LCT levels, this inverse trend was less pronounced among higher fecal levels, particularly for LCT. This could be a reflection of differences in the exact mechanism that each test measures in combination with heterogeneity in disease in CD. Alternatively, it could be that lactoferrin is particularly less sensitive in distinguishing severity among patients with higher inflammatory burden. It was reassuring, however, that there was a strong relationship between distinguishing values consistent with healing vs values reflecting inflammation. Despite these limitations, the methods of this observational cohort are robust and describe several novel findings related to fecal and blood biomarkers in association with IFX-levels.
CONCLUSION
This longitudinal, prospective, observational cohort study of IFX induction therapy among pediatric CD patients identified LCT and nCD64 cut points for week-14 FCP remission, along with establishing pharmacodynamic percentage changes for postinduction target levels. This study identified that an infusion-4 IFX level of ≥9.4 µg/mL were associated with fecal biomarker levels of remission at an FCP of <250 µg/g or LCT of 8.06 µg/g. A higher infusion-4 IFX level of ≥11.5 µg/mL was associated with a more stringent FCP level of <100 µg/g or LCT of 6.02 µg/g. These data also support the need for routine pharmacokinetic monitoring with personalized pharmacodynamic biomarkers. Further research exploring these biomarkers as pharmacodynamic targets in PK-PD models is warranted.
Supplementary Material
Supported by: This project was supported in part by National Institutes of Health (NIH) P30 DK078392 (Clinical Component of the Digestive Diseases Research Core Center in Cincinnati). This work was supported in part by NIH (K23DK105229 [PM], R03DK118314 [PM], T32DK007727 [RJC)], and a Crohn’s and Colitis Foundation PROKIIDS grant (PM). Research Electronic Data Capture (REDCap) electronic data capture tools hosted at Cincinnati Children’s Hospital Medical Center were supported in part by the NIH (NIH/NCATS UL1 TR000445).
REFERENCES
- 1. Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol. 2015;110:1324–1338. [DOI] [PubMed] [Google Scholar]
- 2. Colombel JF, Panaccione R, Bossuyt P, et al. Effect of tight control management on Crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet. 2018;390:2779–2789. [DOI] [PubMed] [Google Scholar]
- 3. Carman N, Tomalty D, Church PC, et al. ; Canadian Children Inflammatory Bowel Disease Network: A Joint Partnership of Canadian Institutes of Health Research and the Children with Intestinal and Liver Disorders Foundation . Clinical disease activity and endoscopic severity correlate poorly in children newly diagnosed with Crohn’s disease. Gastrointest Endosc. 2019;89:364–372. [DOI] [PubMed] [Google Scholar]
- 4. Turner D, Levine A, Walters TD, et al. Which PCDAI version best reflects intestinal inflammation in pediatric Crohn disease? J Pediatr Gastroenterol Nutr. 2017;64:254–260. [DOI] [PubMed] [Google Scholar]
- 5. Holtman GA, Lisman-van Leeuwen Y, van Rheenen PF, et al. Evaluation of point-of-care test calprotectin and lactoferrin for inflammatory bowel disease among children with chronic gastrointestinal symptoms. Fam Pract. 2017;34:400–406. [DOI] [PubMed] [Google Scholar]
- 6. Mosli MH, Zou G, Garg SK, et al. C-reactive protein, fecal calprotectin, and stool lactoferrin for detection of endoscopic activity in symptomatic inflammatory bowel disease patients: a systematic review and meta-analysis. Am J Gastroenterol. 2015;110:802–819; quiz 820. [DOI] [PubMed] [Google Scholar]
- 7. Reinisch W, Panaccione R, Bossuyt P, et al. Association of biomarker cutoffs and endoscopic outcomes in Crohn’s disease: a post hoc analysis from the CALM Study. Inflamm Bowel Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kennedy NA, Heap GA, Green HD, et al. ; UK Inflammatory Bowel Disease Pharmacogenetics Study Group . Predictors of anti-TNF treatment failure in anti-TNF-naive patients with active luminal Crohn’s disease: a prospective, multicentre, cohort study. Lancet Gastroenterol Hepatol. 2019;4:341–353. [DOI] [PubMed] [Google Scholar]
- 9. Dreesen E, Baert F, Laharie D, et al. Monitoring a combination of calprotectin and infliximab identifies patients with mucosal healing of Crohn’s disease. Clin Gastroenterol Hepatol. 2020;18:637–646.e11. [DOI] [PubMed] [Google Scholar]
- 10. Minar P, Haberman Y, Jurickova I, et al. Utility of neutrophil Fcγ receptor I (CD64) index as a biomarker for mucosal inflammation in pediatric Crohn’s disease. Inflamm Bowel Dis. 2014;20:1037–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Minar P, Jackson K, Tsai YT, et al. Validation of neutrophil CD64 blood biomarkers to detect mucosal inflammation in pediatric Crohn’s disease. Inflamm Bowel Dis. 2017;24:198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Minar P, Jackson K, Tsai YT, et al. A low neutrophil CD64 index is associated with sustained remission during infliximab maintenance therapy. Inflamm Bowel Dis. 2016;22:2641–2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Minar P, Lehn C, Tsai YT, et al. Elevated pretreatment plasma oncostatin M is associated with poor biochemical response to infliximab. Crohns Colitis 360. 2019;1:otz026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Turner D, Griffiths AM, Walters TD, et al. Mathematical weighting of the pediatric Crohn’s disease activity index (PCDAI) and comparison with its other short versions. Inflamm Bowel Dis. 2012;18:55–62. [DOI] [PubMed] [Google Scholar]
- 15. Zubin G, Peter L. Predicting endoscopic Crohn’s disease activity before and after induction therapy in children: a comprehensive assessment of PCDAI, CRP, and fecal calprotectin. Inflamm Bowel Dis. 2015;21:1386–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Leach ST, Day AS, Messenger R, et al. ; ImageKids study group . Fecal markers of inflammation and disease activity in pediatric Crohn disease: results from the imagekids study. J Pediatr Gastroenterol Nutr. 2020;70:580–585. [DOI] [PubMed] [Google Scholar]
- 17. Vatay A, Bene L, Kovács A, et al. Relationship between the tumor necrosis factor alpha polymorphism and the serum C-reactive protein levels in inflammatory bowel disease. Immunogenetics. 2003;55:247–252. [DOI] [PubMed] [Google Scholar]
- 18. Sipponen T, Savilahti E, Kärkkäinen P, et al. Fecal calprotectin, lactoferrin, and endoscopic disease activity in monitoring anti-TNF-alpha therapy for Crohn’s disease. Inflamm Bowel Dis. 2008;14:1392–1398. [DOI] [PubMed] [Google Scholar]
- 19. Clarke WT, Papamichael K, Vande Casteele N, et al. Infliximab and adalimumab concentrations may vary between the enzyme-linked immunosorbent assay and the homogeneous mobility shift assay in patients with inflammatory bowel disease: a prospective cross-sectional observational study. Inflamm Bowel Dis. 2019;25:e143–e145. [DOI] [PubMed] [Google Scholar]
- 20. Marini JC, Sendecki J, Cornillie F, et al. Comparisons of serum infliximab and antibodies-to-infliximab tests used in inflammatory bowel disease clinical trials of Remicade®. Aaps J. 2017;19:161–171. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.