Abstract
Background
To assess the possible impact of antiretroviral therapy improvements, aging, and comorbidities, we examined trends in all-cause and cause-specific hospitalization rates among persons with HIV (PWH) from 2005 to 2015.
Methods
In 6 clinical cohorts, we followed PWH in care (≥1 outpatient CD4 count or HIV load [VL] every 12 months) and categorized ICD codes of primary discharge diagnoses using modified Clinical Classifications Software. Poisson regression estimated hospitalization rate ratios for calendar time trends, adjusted for demographics, HIV risk factor, and annually updated age, CD4, and VL.
Results
Among 28 057 patients (125 724 person-years), from 2005 to 2015, the median CD4 increased from 389 to 580 cells/µL and virologic suppression from 55% to 85% of patients. Unadjusted all-cause hospitalization rates decreased from 22.3 per 100 person-years in 2005 (95% confidence interval [CI], 20.6–24.1) to 13.0 in 2015 (95% CI, 12.2–14.0). Unadjusted rates decreased for almost all diagnostic categories. Adjusted rates decreased for all-cause, cardiovascular, and AIDS-defining conditions, increased for non-AIDS–defining infection, and were stable for most other categories.
Conclusions
Among PWH with increasing CD4 counts and viral suppression, unadjusted hospitalization rates decreased for all-cause and most cause-specific hospitalizations, despite the potential effects of aging, comorbidities, and cumulative exposure to HIV and antiretrovirals.
Keywords: HIV, hospitalization, cohort studies
From 2005 to 2015, in 6 US and Canadian clinical cohorts of persons with HIV with increasing age, viral suppression, and CD4 counts, unadjusted hospitalization rates decreased overall and for most causes.
Antiretroviral treatment (ART) for human immunodeficiency virus (HIV) infection has changed substantially over the last 2 decades. In the mid-2000s, the Food and Drug Administration approved the second-generation protease inhibitors (PIs) atazanavir and darunavir, an efavirenz-containing single-tablet regimen, and a new class of drugs, integrase strand transfer inhibitors (INSTIs) [1]. These new regimens provided persons with HIV (PWH) with safer and more effective ART options, generally with a lower pill burden. With evidence of the clinical benefit of earlier ART, HIV treatment guidelines recommended ART initiation at higher CD4 cell counts and eventually for all PWH [1]. Together, these changes have resulted in substantial improvements in viral suppression in the United States and Canada [2, 3]. Life expectancy for PWH in this region is now approaching that of the general population, and almost one-half of PWH are over 50 years of age [4–6].
Despite these improvements, PWH experience a high burden of severe non-AIDS comorbidities, such as cardiovascular, renal, and liver diseases and malignancies [7–10]. Cumulative exposure to more toxic, older antiretroviral drugs and to uncontrolled viremia leading to chronic inflammation may contribute to the incidence of comorbidities [11]. In addition, a high prevalence of at-risk behaviors, such as smoking and substance use, and clinical risk factors, such as hypertension and dyslipidemia, put PWH at risk of developing comorbid conditions [12, 13].
Hospitalizations provide an important clinical endpoint for examining morbidity trends among PWH. The introduction of combination ART in 1996 led to a sharp drop in hospitalization rates in the United States and Canada, particularly for AIDS-defining conditions [14, 15]. In the 2000s, some studies reported a continued decline in all-cause hospitalization rates, while others showed stable or increasing age-adjusted hospitalization rates for cardiovascular, pulmonary, and renal conditions [14–17]. More recent trends in hospitalization rates and causes among North American PWH have not been well-described, with studies limited to smaller geographic areas or populations such as veterans [18–20]. Older age and increasing prevalence of chronic conditions could contribute to more frequent hospitalizations, while expanded use of more potent ART might mitigate this risk. Identifying frequent causes of hospitalization may inform health care policy and clinical efforts to manage comorbidities in the outpatient setting. In this study, we examined trends in hospitalization rates between 2005 and 2015 among PWH in clinical care in the United States and Canada.
METHODS
Study Population and Follow-up
Data for this study come from the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD), a consortium of cohorts following PWH who are linked to care (≥2 visits in a 12-month period) [21]. We included 6 clinical cohorts (5 in the United States, 1 in Canada) that collected data on hospitalizations from electronic health records in their medical system for the period 2005–2015, including discharge diagnosis International Classification of Diseases (ICD) codes. Local Institutional Review Boards (IRBs) approved prospective data collection and the University of North Carolina IRB approved this secondary data analysis.
We included patients ≥18 years old in care between 2005 and 2015, defined as at least 1 outpatient CD4 count or HIV load (VL) measurement in that period. Patients contributed person-time from cohort entry or 1 January 2005, whichever occurred later, and until death or 31 December 2015, whichever occurred first. Person-time was censored at loss to follow-up (LTFU), defined as 12 months with no outpatient CD4 count or VL, but patients contributed additional person-time if they reentered HIV care, defined as an outpatient CD4 count or VL. Person-time was divided into calendar years for analysis, allowing patients to contribute partially to years when their person-time began or ended. Inpatient days were not counted as person-time at risk.
Study Measures
Annual hospitalization rates were calculated as the number of hospitalizations divided by the person-time at risk in each calendar year, for all-cause and cause-specific hospitalizations. Patients could contribute more than 1 hospitalization. Hospitalizations with same-day discharge were not counted as outcomes, because these are rare events and could not be distinguished from outpatient procedures (eg, endoscopy).
We assigned primary hospital discharge diagnoses into categories. We used Clinical Classifications Software (CCS; Agency for Healthcare Research and Quality) to categorize ICD, Ninth Revision, Clinical Modification (ICD-9-CM) codes [22]. We modified the CCS to classify AIDS-defining illnesses as a separate category and reassign all other infections from organ system categories into a non-AIDS–defining infection category [17]. Using a previously validated approach, if the primary diagnosis was an ICD-9-CM code for HIV infection or chronic hepatitis C virus infection, we assigned the next highest-ranked ICD-9-CM code as the primary diagnosis [23]. We converted ICD, Tenth Revision codes (11% of hospitalizations, including 9% in Canada and 2% in the United States) to ICD-9-CM using General Equivalence Mappings from the Centers for Medicare and Medicaid Services and manual review by a physician (S. A. B.). Hospitalizations missing discharge diagnosis (n = 206, <1%) were included in all-cause analyses only. To describe frequent diagnoses within each category, a physician (S. A. B.) grouped ICD-9-CM codes into clinically meaningful groups, as previously published [17].
Covariates included NA-ACCORD cohort, gender, race/ethnicity, HIV risk factor, and annually updated age, CD4 count, and VL. Categories were created for age (<40, 40–49, 50–59, ≥60 years), CD4 count (<50, 50–200, 201–350, 351–500, >500 cells/µL), and VL (<400, ≥400 copies/mL, the highest lower limit of quantification [LLQ] of assays used during the study period). For each calendar year of analysis, we used the earliest CD4 count and VL measurement in that year. If none was available (9% of person-years), we used the earliest measurement in the last 6 months of the previous year or the first 6 months of the following year. Person-years still missing CD4 count or VL (3%) were excluded from adjusted analyses only. Analyses of hospitalizations in the pregnancy category were restricted to cisgender women aged <50 years.
Statistical Analysis
Unadjusted annual rates were plotted for all-cause hospitalizations and the 10 most frequent diagnostic categories. We then plotted rates standardized to the 2010 distribution of variables that changed substantially over the study period: age, CD4 count, and VL. We evaluated this approach by standardizing rates for all-cause hospitalizations and the most common diagnostic category to each covariate, and visually comparing standardized to unadjusted estimates. Standardizing for other variables had little to no effect on estimates (Supplementary Figure 1).
We used Poisson regression models to estimate unadjusted and adjusted incidence rate ratios (IRR) of hospitalization assessing linear calendar time trends, with generalized estimating equations with an independent correlation matrix to account for patients contributing more than 1 hospitalization. IRR were reported as a mean percentage change, for example, an IRR of 0.95 per 1-year increase was reported as a −5% annual rate change. Unadjusted models included only NA-ACCORD cohort as a covariate. Partially adjusted models also included age, CD4 count, and VL. Fully adjusted models included all covariates. P values were 2-sided, and .05 was considered statistically significant. We conducted 4 sets of sensitivity analyses: (1) using 6 or 18 months to define LTFU; (2) using restricted quadratic splines to adjust for age and CD4 count; (3) using negative binomial models to account for possible overdispersion; and (4) adjusting for annually updated lowest prior CD4 count. Analyses were conducted in SAS, version 9.4 (SAS Institute).
RESULTS
Study Sample
The study included 28 057 patients who were followed a median of 3.4 years (interquartile range [IQR], 1.6–7.0), contributing a total of 125 724 person-years of follow-up. Follow-up duration varied by NA-ACCORD enrollment year, with a median of 8.0 years per patient (IQR, 3.3–10.9) for patients enrolled before 2006, 4.8 (IQR, 2.0–7.0) for those enrolled 2006–2010, and 1.7 (IQR, 1.0–2.9) for those enrolled 2011–2015. The person-time of most patients was censored at study end (52%) or LTFU (42%) (Supplementary Figure 2). Annual LTFU was 8%–11%. Patients were 80% cisgender men, 41% White, 32% Black, and 52% men who have sex with men (Table 1). From 2005 to 2015, the median patient age increased from 43 (IQR, 38–50) to 49 years (IQR, 39–56), the median CD4 count increased from 389 (IQR, 242–578) to 580 cells/µL (IQR, 387–786), and the proportion of patients with VL<400 copies/mL increased from 55% to 85% (Table 1). Among patients whose VL was measured using an assay with an LLQ of 75 copies/mL or lower, the proportion with VL<75 copies/mL increased from 53% to 81%. In addition, the proportion of patients with injection drug use (IDU) as HIV risk factor decreased from 16% to 11%, and the proportion of patients who were White decreased from 47% to 41%.
Table 1.
Characteristics of 28 057 Patients in HIV Care Between 1 January 2005 and 31 December 2015 in 6 NA-ACCORD Cohorts, at Enrollment and Across Calendar Years
| Characteristic | All Patients at Enrolment (n = 28 057) | Patients in Care in 2005 (n = 9174) | Patients in Care in 2010 (n = 13 596) | Patients in Care in 2015 (n = 16 380) |
|---|---|---|---|---|
| Gender, No. (%) | ||||
| Cisgender men | 22 560 (80) | 7258 (79) | 10 775 (79) | 13 229 (81) |
| Cisgender women | 5339 (19) | 1879 (20) | 2757 (20) | 3062 (19) |
| Transgendera | 158 (1) | 37 (<1) | 64 (<1) | 89 (1) |
| Race/ethnicity, No. (%) | ||||
| Black, not Hispanic | 8947 (32) | 2802 (31) | 4297 (32) | 5109 (31) |
| White, not Hispanic | 11 526 (41) | 4305 (47) | 5830 (43) | 6786 (41) |
| Hispanic | 4611 (16) | 1365 (15) | 2174 (16) | 2805 (17) |
| Otherb | 2973 (11) | 702 (8) | 1295 (10) | 1680 (10) |
| HIV acquisition risk factor, No. (%) | ||||
| MSM | 14 622 (52) | 4591 (50) | 7008 (52) | 8899 (54) |
| IDU | 3706 (13) | 1458 (16) | 1877 (14) | 1826 (11) |
| Heterosexual or other | 9729 (35) | 3125 (34) | 4711 (35) | 5655 (35) |
| Age, y, median (IQR) | 41 (33–48) | 43 (38–50) | 46 (39–53) | 49 (39–56) |
| CD4 count, cells/µL, median (IQR)c | 387 (200–592) | 389 (242–578) | 475 (308–661) | 580 (387–786) |
| HIV load <400 copies/mL, No. (%)d | 9162 (37) | 4954 (55) | 9377 (73) | 13 131 (85) |
| HIV load <75 copies/mL, No. (%)d,e | 7543 (31) | 4142 (53) | 8533 (68) | 12 500 (81) |
Abbreviations: HIV, human immunodeficiency virus; IDU, injection drug use; MSM, men who have sex with men; NA-ACCORD, North American AIDS Cohort Collaboration on Research and Design.
aTransgender patients were identified either from locally collected data, or from having female sex and MSM as risk factor.
bIncludes 972 patients with missing race/ethnicity.
cCD4 count at enrollment was the closest measurement a year before to 30 days after enrollment date and was missing for 2538 patients. Time-updated CD4 count was the first measurement in a given calendar year, or, if unavailable, in the last 6 months of the previous year or first 6 months of the following year. Time-updated CD4 count was missing for 2510 (2%) patient-years.
dHIV load at enrollment was the closest measurement a year before to 30 days after enrollment date and was missing for 3612 patients. Time-updated HIV load was the first measurement in a given calendar year, or, if unavailable, in the last 6 months of the previous year or first 6 months of the following year. Time-updated viral load was missing for 2588 (2%) person-years.
eRestricted to patients with a viral load measurement from assays with a lower limit of quantification of 75 copies/mL or lower.
Hospitalization Characteristics
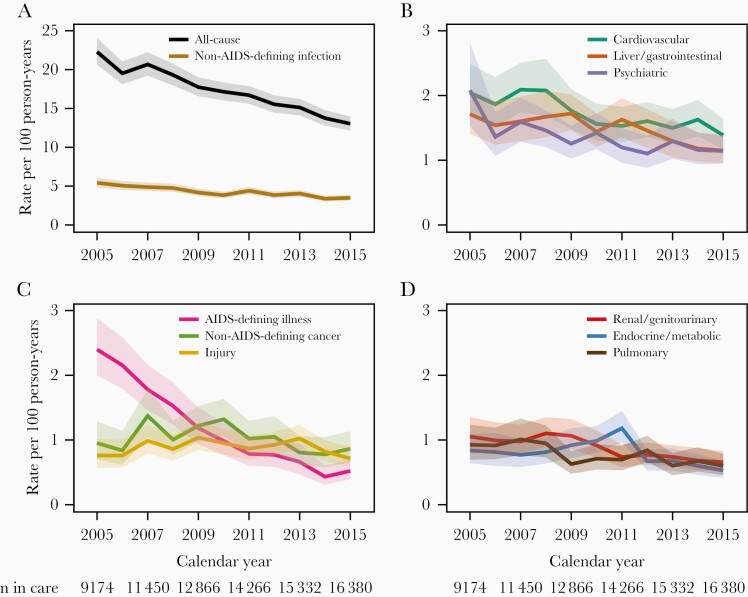
During the observed person-time, 7503 (27%) patients were hospitalized at least once. Among these, the median number of hospitalizations was 2 (IQR, 1–3), for a total of 21 230 hospitalizations. The median age of hospitalized patients was 44 years (IQR, 38–52) in 2005 and 52 years (IQR, 44–60) in 2015. The 10 most frequent diagnostic categories of hospital discharge diagnoses were non-AIDS–defining infection (25% of hospitalizations), cardiovascular (10%), liver/gastrointestinal (9%), psychiatric (8%), AIDS-defining illness (6%), neoplasm excluding AIDS-defining cancer (6%), injury/poisoning/complication of therapy (6%), renal/genitourinary (5%), endocrine/metabolic (5%), and pulmonary (4%) (Table 2). In the non-AIDS–defining infection category, the most frequent diagnoses were sepsis/bacteremia (23%), bacterial pneumonia (18%), and cellulitis/cutaneous abscess (12%). For the cardiovascular category, the most frequent diagnosis was congestive heart failure (21%); for the liver/gastrointestinal category, acute or chronic pancreatitis (16%); for the psychiatric category, major depressive disorder (20%); for the AIDS-defining illness category, Pneumocystis jirovecii pneumonia (18%); and for the non-AIDS–defining neoplasm category, non-Hodgkin lymphoma (24%) (Supplementary Table 1).
Table 2.
Distribution of Categories for Primary Hospital Discharge Diagnosis and Annual Percentage Change in Hospitalization Rates by Diagnostic Category, Among 28 057 Patients in HIV Care in NA-ACCORD, 2005–2015
| Diagnostic Categorya | No. (%) | Unadjustedb | Partially Adjustedc | Fully Adjustedd | |||
|---|---|---|---|---|---|---|---|
| Annual % Change (95% CI) | P | Annual % Change (95% CI) | P | Annual % Change (95% CI) | P | ||
| All-cause | 21 230 (100) | −4 (−5 to −4) | <.01 | −1 (−2 to 0) | <.01 | −1 (−2 to 0) | <.05 |
| Non-AIDS–defining infection | 5274 (25) | −3 (−5 to −2) | <.01 | 1 (0 to 2) | .06 | 2 (0 to 3) | <.05 |
| Cardiovascular | 2132 (10) | −3 (−5 to −1) | <.01 | −4 (−6 to −2) | <.01 | −4 (−6 to −2) | <.01 |
| Liver/gastrointestinal | 1841 (9) | −3 (−5 to −1) | <.01 | −1 (−3 to 2) | .55 | −1 (−3 to 2) | .62 |
| Psychiatric | 1675 (8) | −4 (−7 to −1) | <.01 | 0 (−3 to 3) | .93 | 1 (−2 to 4) | .53 |
| AIDS-defining illness | 1373 (6) | −15 (−18 to −13) | <.01 | −7 (−10 to −5) | <.01 | −7 (−10 to −4) | <.01 |
| Neoplasm excluding AIDS-defining cancer | 1270 (6) | −2 (−5 to 0) | .11 | −1 (−4 to 2) | .51 | −1 (−4 to 2) | .52 |
| Injury/poisoning/complication of therapy | 1112 (6) | 0 (−2 to 2) | .89 | 1 (−2 to 3) | .62 | 1 (−1 to 3) | .42 |
| Renal/genitourinary | 1078 (5) | −4 (−6 to −1) | <.01 | −1 (−4 to 1) | .37 | −1 (−4 to 1) | .27 |
| Endocrine/metabolic | 992 (5) | −2 (−5 to 0) | .08 | 0 (−3 to 3) | .82 | 0 (−3 to 3) | .91 |
| Pulmonary | 953 (4) | −4 (−7 to −1) | <.05 | −1 (−4 to 2) | .48 | −1 (−4 to 2) | .58 |
| Musculoskeletal | 710 (3) | 1 (−2 to 4) | .41 | −1 (−4 to 2) | .39 | −1 (−3 to 2) | .62 |
| Symptoms | 620 (3) | −7 (−10 to −4) | <.01 | −3 (−6 to 0) | .07 | −3 (−6 to 1) | .11 |
| Pregnancye | 569 (3) | −3 (−6 to 0) | <.05 | −2 (−5 to 2) | .29 | −2 (−5 to 1) | .25 |
| Neurological | 504 (2) | −4 (−7 to −1) | <.01 | −1 (−5 to 2) | .52 | −1 (−4 to 2) | .54 |
| Hematological | 453 (2) | −11 (−14 to −7) | <.01 | −7 (−10 to −3) | <.01 | −7 (−10 to −3) | <.01 |
| Dermatological | 84 (<1) | −9 (−15 to −2) | <.01 | −2 (−8 to 6) | .68 | −1 (−8 to 7) | .85 |
| Congenital | 24 (<1) | 18 (5 to 32) | <.01 | 18 (3 to 35) | <.05 | f | |
| Other | 360 (2) | −11 (−14 to −7) | <.01 | −2 (−7 to 2) | .29 | −2 (−7 to 2) | .26 |
Abbreviations: AIDS, acquired immunodeficiency syndrome; CI, confidence interval; HIV, human immunodeficiency virus; ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification; NA-ACCORD, North American AIDS Cohort Collaboration on Research and Design.
aDiagnostic categories are ordered by frequency. ICD-9-CM codes for primary discharge diagnoses were categorized using modified Clinical Classifications Software. Discharge diagnosis was missing for 206 hospitalizations.
bEstimates, 95% CI, and P values for each category were obtained from separate Poisson regression models including only calendar year as a linear variable and NA-ACCORD cohort, with generalized estimating equations with an independent correlation matrix to account for patients contributing more than 1 hospitalization to the analysis.
cEstimates, 95% CI, and P values for each category were obtained from separate Poisson regression models with generalized estimating equations with an independent correlation matrix. Models include calendar year as a linear variable, NA-ACCORD cohort, and annually updated age, CD4 count, and HIV load. Categories were created for age (<40, 40–49, 50–59, ≥60 years) and CD4 count (<50, 50–200, 201–350, 351–500, >500 cells/µL). HIV load was dichotomized as <400 or ≥400 copies/mL. For each calendar year of analysis, we used the earliest CD4 count and VL measurement in that year. If none was available, we used the earliest measurement in the last 6 months of the previous year or the first 6 months of the following year.
dEstimates, 95% CI, and P values for each category were obtained from separate Poisson regression models with generalized estimating equations. Models include calendar year as a linear variable, NA-ACCORD cohort, gender, race/ethnicity, HIV risk group, and annually-updated age, CD4 count, and HIV load, using aforementioned parametrization.
eModeling analyses are restricted to cisgender women younger than 50 years.
fThere were too few hospitalizations in this category to estimate a fully adjusted trend.
Hospitalization Rates Over Time
From 2005 to 2015, the unadjusted all-cause hospitalization rate per 100 person-years decreased from 22.3 (95% confidence interval [CI], 20.6–24.1) to 13.0 (95% CI, 12.2–14.0), with a mean change of −4% per year (95% CI, −5% to −4%; P < .01) (Figure 1A and Table 2). Unadjusted hospitalization rates decreased over the study period for each of the 10 most common diagnostic categories except non-AIDS–defining neoplasm and injury (Figure 1A–1D). Hospitalizations for AIDS-defining illness had the greatest decrease, with an annual change of −15% (95% CI, −18% to −13%; P < .01), reaching 0.5 hospitalizations per 100 person-years in 2015 (95% CI, 0.4–0.7). Among less common diagnostic categories, rates were stable for musculoskeletal and pregnancy hospitalizations, increased for congenital hospitalizations, and decreased for remaining categories (Table 2). In 2015, the highest hospitalization rates per 100 person-years were those for non-AIDS–defining infection with 3.5 (95% CI, 3.1–3.9), cardiovascular with 1.4 (95% CI, 1.2–1.6), psychiatric with 1.1 (95% CI, 0.9–1.4), liver/gastrointestinal with 1.1 (95% CI, 1.0–1.4), and neoplasm excluding AIDS-defining cancer with 0.9 (95% CI, 0.7–1.1) (Supplementary Table 2).
Figure 1.
A–D, Unadjusted annual all-cause and cause-specific hospitalization rates with 95% confidence bands, among 28 057 patients in HIV care in the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD), 2005–2015. Shown are the 10 most common diagnostic categories ordered by frequency.
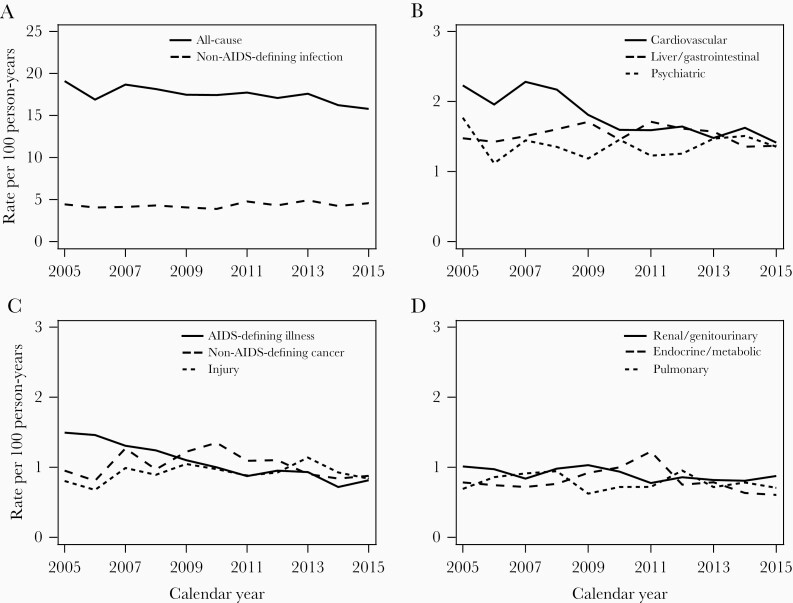
After standardizing rates for the 10 most frequent diagnostic categories to the 2010 age, CD4 count, and VL distribution (Figure 2), we observed decreases in hospitalization rates for all-cause, cardiovascular, and AIDS-defining conditions, and rates for other causes appeared stable. Similar to these visual trends, in partially adjusted models (Table 2), hospitalization rates decreased over time for all-cause (annual change −1% [95% CI, −2% to 0%]; P < .01), cardiovascular (−4% [95% CI, −6% to −2%]; P < .01), and AIDS-defining conditions (−7% [95% CI, −10% to −5%]; P < .01). Partially adjusted trends were stable for most other diagnostic categories. In fully adjusted models, most calendar time trend estimates were similar to partially adjusted estimates (Table 2). However, in fully adjusted analyses, hospitalization rates for non-AIDS–defining infections increased 2% annually (95% CI, 0%–3%; P < .05). For most diagnostic categories, higher hospitalization rates were associated with older age, lower CD4 counts, and detectable VL (Supplementary Table 3). Estimates in all sensitivity analyses were similar to the main findings (Supplementary Tables 4–8).
Figure 2.
A–D, Annual all-cause and cause-specific hospitalization rates, among 28 057 patients in HIV care in the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD), 2005–2015, standardized to age, CD4 count, and HIV load distribution of person-years in 2010. Standardization strata were defined according to the following categories: age <40, 40–49, 50–59, and ≥60 years; CD4 count <50, 50–200, 201–350, 351–500, and >500 cells/μL; HIV load <400 and ≥400 copies/mL. Shown are the 10 most common diagnostic categories ordered by frequency.
Discussion
In this study of PWH in care from 2005 to 2015, despite a 6-year median age increase, unadjusted all-cause hospitalization rates decreased from 22 to 13 hospitalizations per 100 person-years. Unadjusted rates decreased for most diagnostic categories as well, with AIDS-defining conditions decreasing to the greatest extent. Hospitalizations for non-AIDS–defining infections accounted for 25% of all hospitalizations and mostly comprised diagnoses of sepsis/bacteremia, bacterial pneumonia, and cellulitis/cutaneous abscess. In fully adjusted analyses, rates decreased over time for all-cause, cardiovascular, and AIDS-defining conditions, increased for non-AIDS–defining infections, and remained stable for other categories.
After adjusting for changes in age, CD4 count, and viral load over time, decreases in unadjusted hospitalization rates were attenuated or no longer present. The substantial improvements in immunologic and virologic status in our study sample might be partly attributed to the use of safer, more potent ART. After adjusting for these clinical characteristics as well as demographics, we still observed rate decreases for all-cause, cardiovascular, and AIDS-defining conditions, indicating other factors may also be contributing to decreasing hospitalization rates. For example, increased awareness of cardiovascular, renal, and metabolic comorbidities among PWH, along with better evidence for treating and managing these conditions in the context of ART, might have led to more aggressive comorbidity management, and fewer cardiovascular hospitalizations [24, 25]. Among patients with cardiovascular risk, decreasing use of agents with cardiovascular toxicity, such as lopinavir/ritonavir and, potentially, abacavir, might have contributed to reducing hospitalizations, though any impact would likely be small considering the estimated cumulative effects of these agents [26]. It is also possible that prevention efforts among PWH led to decreases in smoking over the study period, but smoking was not captured in this study [27]. Finally, earlier HIV diagnosis and ART initiation could have led to decreased AIDS and non-AIDS morbidity. However, adjusting for lowest known CD4 count, a marker of prior disease progression, did not appreciably affect our estimates.
The decrease in unadjusted all-cause hospitalization rates observed in this study through 2015 extends prior large HIV cohort studies showing rate decreases during the mid- to late 2000s and is consistent with trends from more recent work among persons with HIV in the southeastern United States and Italy [14, 16, 17, 19, 28]. The 2015 all-cause hospitalization rate in our study is similar to the Italian estimate but substantially lower than rates observed in North Carolina, New York City, and a multisite US study in similar time periods (22, 37, and 20 hospitalizations per 100 person-years, respectively) [18, 19, 29]. However, the New York City study estimated population-based rates among all PWH rather than our estimates based on patients receiving HIV clinical care. These discrepancies might also be attributable to population differences such as demographics, socioeconomic status, and insurance coverage.
Unlike prior studies, we did not find an increase in hospitalization rates for cardiovascular, renal/genitourinary, or pulmonary conditions [14, 15, 17]. It is possible that our study population had a different prevalence of smoking or comorbidities. Older, more toxic ART regimens might have also contributed to hospitalizations in those studies. Our findings do extend earlier reports of decreasing rates for AIDS-defining conditions and most other diagnostic categories [14, 15, 17]. Previous studies in the United States and Canada, mostly with data prior to 2010, have also shown that non-AIDS–defining infections made up a large proportion of discharge diagnoses among hospitalized PWH [17, 29, 30].
In both the United States and Canada, hospitalization rates in the general population, based on national inpatient data and population estimates, remained relatively stable during our study period [31, 32]. Similarly, a study of adults aged 19–64 years in 7 US states reported stable hospitalization rates between 2012 and 2015, irrespective of Medicaid expansion [33]. Among Medicare beneficiaries, there was a small decrease in hospitalization rates from 2004 to 2017 of 1.4% annually [34]. In the Veterans Aging Cohort Study, hospitalization rates decreased for the period 2005–2011 among persons with HIV but were stable for those without HIV [20]. While it is possible that some factors that led to hospitalization rate decreases among Medicare recipients also affected rates among PWH, overall this evidence suggests that factors external to HIV care are unlikely to have contributed substantially to the trends we observed in this study.
The large proportion of hospitalizations due to non-AIDS–defining infections and the small rate increase for this category in fully adjusted analyses are important findings. Possible explanations including immune dysfunction, comorbidities, and risk factors such as IDU. Although median CD4 counts and viral suppression rates improved in our overall study sample, patients with poorly controlled HIV or persistently low CD4 counts remain at risk of developing infections [35–37]. Patients with immunosenescence, including altered T-cell subsets, might also be at higher infection risk [38]. Another possible contributor to hospitalizations for sepsis/bacteremia and cellulitis/cutaneous abscess is IDU, with 13% of our sample reporting a history of IDU. While we did not have data on current IDU, opioid and methamphetamine epidemics in this region might have contributed to IDU during the study period [39]. Counseling and resources should continue to be provided to patients to support harm reduction practices and treatment for substance use disorders. In addition, chronic obstructive pulmonary disease (COPD), tobacco smoking, high alcohol intake, and opioid use, even when medically appropriate, can play a role in incidence and severity of bacterial pneumonia and may be contributing to hospitalizations [40, 41]. Close to one-half of PWH in care in the United States were current smokers in 2009 [12]. COPD prevalence is substantial among PWH, with several US estimates as high as 15%–25% [42]. Finally, diabetes mellitus and obesity are common among North American PWH and are also risk factors for infections [8, 43]. Infection prevention and treatment, including vaccination against influenza and pneumonia, should be emphasized in HIV care. Future studies should further examine drivers of infection-related hospitalizations among PWH.
The findings of this study have several important implications for the clinical management of PWH. Earlier data had raised concerns that hospitalization rates would increase due to cumulative end-organ damage from long-term HIV infection, antiretroviral exposure, or both [17]. Our results suggest that, even in aging cohorts, improvements in viral suppression and immune recovery have contributed to reductions in hospitalization for both AIDS-defining illness and non-AIDS comorbidities. To reduce hospitalization burden further among PWH, continued efforts should aim to support uninterrupted care engagement and ART adherence, by addressing social and clinical barriers such as homelessness, food insecurity, lack of transport to clinic, poor insurance coverage, substance use, and other mental health disorders. Secondly, while we did not examine what specific health care patients received in the ambulatory setting, clinical efforts targeting both HIV and comorbidities may become increasingly important to minimize the need for inpatient care.
The strengths of this study include data on both HIV history and hospitalizations, over 11 years from 5 cohorts across the United States and 1 cohort in Canada. In addition, the period of analysis spans several recent important changes in HIV management, namely the expansion of ART to all PWH and the uptake of more potent, less toxic, and more convenient ART regimens. However, we did not examine some important factors that might affect hospitalization risk, such as comorbidities, obesity, vaccination status, smoking, and other substance use. Hospitalization rates in our study could be slightly underestimated due to hospitalizations occurring at external sites, although a previous analysis showed that these missing events are unlikely to affect calendar time trends [44]. Another potential limitation is that we used 400 copies/mL as the threshold of viral suppression to accommodate the different LLQs of assays in this study. This approach could have overestimated viral suppression and failed to capture low-level viremia, which is associated with morbidity and mortality [45]. We did not treat mortality as a competing risk in our analyses, as mortality rates are less than 5 deaths per 100 person-years in this region for PWH engaged in care [46].
Our findings may not be generalizable to all PWH in the United States and Canada. In 2010, our study sample included higher proportions of patients who were White (43% vs 33%), cisgender men (79% vs 75%), and younger than 45 years (50% vs 45%), compared to PWH in the United States that year [47]. In addition, PWH who are not engaged in care are likely at higher risk of hospitalization. Future studies should examine hospitalization rates among patients who are not in care and across subpopulations of PWH, for example demographic and geographic differences in trends. Finally, our data extend through 2015, and hospitalization rates should continue to be assessed as PWH age and HIV management evolves.
In conclusion, unadjusted rates of hospitalization among PWH in care decreased for all-cause and most diagnostic categories between 2005 and 2015 in the United States and Canada, largely driven by improvements in viral suppression and CD4 cell counts, and in spite of increasing patient age. After adjustment for demographics and HIV clinical characteristics, rates were still decreasing for all-cause, cardiovascular, and AIDS-defining conditions, likely due to better awareness and evidence for managing comorbidities among PWH. Future efforts to reduce hospitalizations among PWH should focus on addressing barriers to care engagement and on managing risk factors for non-AIDS morbidity, particularly infections.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
NA-ACCORD Collaborating Cohorts and Representatives. AIDS Clinical Trials Group Longitudinal Linked Randomized Trials: Constance A. Benson and Ronald J. Bosch; AIDS Link to the IntraVenous Experience: Gregory D. Kirk; Fenway Health HIV Cohort: Kenneth H. Mayer and Chris Grasso; HAART Observational Medical Evaluation and Research: Robert S. Hogg, P. Richard Harrigan, Julio SG Montaner, Benita Yip, Julia Zhu, Kate Salters, and Karyn Gabler; HIV Outpatient Study: Kate Buchacz and Jun Li; HIV Research Network: Kelly A. Gebo and Richard D. Moore; Johns Hopkins HIV Clinical Cohort: Richard D. Moore; John T. Carey Special Immunology Unit Patient Care and Research Database, Case Western Reserve University: Benigno Rodriguez; Kaiser Permanente Mid-Atlantic States: Michael A. Horberg; Kaiser Permanente Northern California: Michael J. Silverberg; Longitudinal Study of Ocular Complications of AIDS: Jennifer E. Thorne; MACS/WIHS Combined Cohort Study: Todd Brown, Phyllis Tien, and Gypsyamber D’Souza; Multicenter Hemophilia Cohort Study–II: Charles Rabkin; Montreal Chest Institute Immunodeficiency Service Cohort: Marina B. Klein; Ontario HIV Treatment Network Cohort Study: Abigail Kroch, Ann Burchell, Adrian Betts, and Joanne Lindsay; Retrovirus Research Center, Bayamon Puerto Rico: Robert F. Hunter-Mellado and Angel M. Mayor; Southern Alberta Clinic Cohort: M. John Gill; Study of the Consequences of the Protease Inhibitor Era: Jeffrey N. Martin; Study to Understand the Natural History of HIV/AIDS in the Era of Effective Therapy: Jun Li and John T. Brooks; University of Alabama at Birmingham 1917 Clinic Cohort: Michael S. Saag, Michael J. Mugavero, and James Willig; University of California at San Diego: William C. Mathews; University of North Carolina at Chapel Hill HIV Clinic Cohort: Joseph J. Eron and Sonia Napravnik; University of Washington HIV Cohort: Mari M. Kitahata and Heidi M. Crane; Vanderbilt Comprehensive Care Clinic HIV Cohort: Timothy R. Sterling, David Haas, Peter Rebeiro, and Megan Turner; Veterans Aging Cohort Study: Janet Tate, Robert Dubrow, and David Fiellin.
NA-ACCORD Study Administration: Executive Committee: Richard D. Moore, Keri N. Althoff, Stephen J. Gange, Mari M. Kitahata, Michael S. Saag, Michael A. Horberg, Marina B. Klein, Rosemary G. McKaig, and Aimee M. Freeman. Administrative Core: Richard D. Moore, Keri N. Althoff, and Aimee M. Freeman. Data Management Core: Mari M. Kitahata, Stephen E. Van Rompaey, Heidi M. Crane, Liz Morton, Justin McReynolds, and William B. Lober. Epidemiology and Biostatistics Core: Stephen J. Gange, Keri N. Althoff, Jennifer S. Lee, Bin You, Brenna Hogan, Jinbing Zhang, Jerry Jing, Elizabeth Humes, Lucas Gerace, and Sally Coburn.
Disclaimer. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Centers for Disease Control and Prevention.
Financial support. This work was supported by National Institutes of Health (grant numbers U01AI069918, F31AI124794, F31DA037788, G12MD007583, K01AI093197, K01AI131895, K23EY013707, K24AI065298, K24AI118591, K24DA000432, KL2TR000421, N01CP01004, N02CP055504, N02CP91027, P30AI027757, P30AI027763, P30AI027767, P30AI036219, P30AI050409, P30AI050410, P30AI094189, P30AI110527, P30MH62246, R01AA016893, R01DA011602, R01DA012568, R01 AG053100, R24AI067039, U01AA013566, U01AA020790, U01AI038855, U01AI038858, U01AI068634, U01AI068636, U01AI069432, U01AI069434, U01DA03629, U01DA036935, U10EY008057, U10EY008052, U10EY008067, U01HL146192, U01HL146193, U01HL146194, U01HL146201, U01HL146202, U01HL146203, U01HL146204, U01HL146205, U01HL146208, U01HL146240, U01HL146241, U01HL146242, U01HL146245, U01HL146333, U24AA020794, U54MD007587, UL1RR024131, UL1TR000004, UL1TR000083, Z01CP010214, and Z01CP010176); Centers for Disease Control and Prevention (contract numbers CDC-200-2006-18797 and CDC-200-2015-63931); Agency for Healthcare Research and Quality (contract number 90047713); Health Resources and Services Administration (contract number 90051652); Canadian Institutes of Health Research (grant numbers CBR-86906, CBR-94036, HCP-97105, and TGF-96118); Ontario Ministry of Health and Long Term Care; and the Government of Alberta, Canada. Additional support was provided by the National Institute of Allergy and Infectious Diseases (NIAID), National Cancer Institute, National Heart, Lung, and Blood Institute, Eunice Kennedy Shriver National Institute Of Child Health and Human Development, National Human Genome Research Institute, National Institute for Mental Health, National Institute on Drug Abuse (NIDA), National Institute on Aging, National Institute of Dental and Craniofacial Research, National Institute of Neurological Disorders and Stroke, National Institute of Nursing Research, National Institute on Alcohol Abuse and Alcoholism, National Institute on Deafness and Other Communication Disorders, and National Institute of Diabetes and Digestive and Kidney Diseases. T. D. M. has received training support from the NIAID (grant number T32AI007001) and the NIDA (grant number T32DA007250).
Potential conflicts of interest. K. N. A. serves as a consultant to the All of Us study (National Institutes of Health) and on the scientific advisory board for TrioHealth, outside the scope of the work. J. A. C. has received honoraria from Integritas Communications (Gilead educational grant) and Vindico Medical Education (ViiV education grant). H. M. C. has received grants from ViiV and served on the advisory board of Bristol Myers Squibb. J. J. E. has received grants and personal fees from ViiV, Janssen, and Gilead, and personal fees from Merck. M. J. G. has received honoraria for membership in ad hoc national HIV advisory committee meetings for Merck, Gilead, and ViiV. V. C. M. has served as a consultant or received research support from Lilly, Gilead, ViiV, and Bayer. M. J. S. has received grants from Gilead. D. V. D. has served on the advisory boards of Allergan, Achaogen, Qpex, Shionogi, Sanofi-Pasteur, T2 Biosystems, NeuMedicine, Roche, MedImmune, Astellas, and Merck. D. A. W. has served on the advisory boards of Gilead, Merck, ViiV, and Janssen, and has received grants from Gilead, ViiV, and Merck.
All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
North American AIDS Cohort Collaboration on Research and Design of IeDEA:
Constance A Benson, Ronald J Bosch, Gregory D Kirk, Kenneth H Mayer, Chris Grasso, Robert S Hogg, P Richard Harrigan, Julio S G Montaner, Benita Yip, Julia Zhu, Kate Salters, Karyn Gabler, Kate Buchacz, Jun Li, Kelly A Gebo, Richard D Moore, Richard D Moore, John T Carey, Benigno Rodriguez, Michael A Horberg, Michael J Silverberg, Jennifer E Thorne, Todd Brown, Phyllis Tien, Gypsyamber D’Souza, Charles Rabkin, Marina B Klein, Abigail Kroch, Ann Burchell, Adrian Betts, Joanne Lindsay, Robert F Hunter-Mellado, Angel M Mayor, M John Gill, Jeffrey N Martin, Jun Li, John T Brooks, Michael S Saag, Michael J Mugavero, James Willig, William C Mathews, Joseph J Eron, Sonia Napravnik, Mari M Kitahata, Heidi M Crane, Timothy R Sterling, David Haas, Peter Rebeiro, Megan Turner, Janet Tate, Robert Dubrow, David Fiellin, Richard D Moore, Keri N Althoff, Stephen J Gange, Mari M Kitahata, Michael S Saag, Michael A Horberg, Marina B Klein, Rosemary G McKaig, Aimee M Freeman, Richard D Moore, Keri N Althoff, Aimee M Freeman, Mari M Kitahata, Stephen E Van Rompaey, Heidi M Crane, Liz Morton, Justin McReynolds, William B Lober, Stephen J Gange, Keri N Althoff, Jennifer S Lee, Bin You, Brenna Hogan, Jinbing Zhang, Jerry Jing, Elizabeth Humes, Lucas Gerace, and Sally Coburn
References
- 1. Thompson MA, Aberg JA, Hoy JF, et al. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society-USA panel. JAMA 2012; 308:387–402. [DOI] [PubMed] [Google Scholar]
- 2. Hanna DB, Buchacz K, Gebo KA, et al. Trends and disparities in antiretroviral therapy initiation and virologic suppression among newly treatment-eligible HIV-infected individuals in North America, 2001–2009. Clin Infect Dis 2013; 56:1174–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nance RM, Delaney JAC, Simoni JM, et al. HIV viral suppression trends over time among HIV-infected patients receiving care in the United States, 1997 to 2015: a cohort study. Ann Intern Med 2018; 169:376–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Samji H, Cescon A, Hogg RS, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One 2013; 8:e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention. HIV among people aged 50 and over. https://www.cdc.gov/hiv/group/age/olderamericans/index.html. Accessed 12 June 2019.
- 6. Marcus JL, Leyden WA, Alexeeff SE, et al. Comparison of Overall and Comorbidity-Free Life Expectancy Between Insured Adults With and Without HIV Infection, 2000-2016. JAMA Netw Open 2020;3:e207954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Drozd DR, Kitahata MM, Althoff KN, et al. Increased risk of myocardial infarction in HIV-infected individuals in North America compared with the general population. J Acquir Immune Defic Syndr 2017; 75:568–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Abraham AG, Althoff KN, Jing Y, et al. End-stage renal disease among HIV-infected adults in North America. Clin Infect Dis 2015; 60:941–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Klein MB, Althoff KN, Jing Y, et al. Risk of end-stage liver disease in HIV-viral hepatitis coinfected persons in North America from the early to modern antiretroviral therapy eras. Clin Infect Dis 2016; 63:1160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Silverberg MJ, Lau B, Achenbach CJ, et al. Cumulative incidence of cancer among persons with HIV in North America: a cohort study. Ann Intern Med 2015; 163:507–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet 2013; 382:1525–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mdodo R, Frazier EL, Dube SR, et al. Cigarette smoking prevalence among adults with HIV compared with the general adult population in the United States: cross-sectional surveys. Ann Intern Med 2015; 162:335–44. [DOI] [PubMed] [Google Scholar]
- 13. Wong C, Gange SJ, Moore RD, et al. ; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) . Multimorbidity among persons living with human immunodeficiency virus in the United States. Clin Infect Dis 2018; 66:1230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Buchacz K, Baker RK, Moorman AC, et al. Rates of hospitalizations and associated diagnoses in a large multisite cohort of HIV patients in the United States, 1994–2005. AIDS 2008; 22:1345–54. [DOI] [PubMed] [Google Scholar]
- 15. Krentz HB, Dean S, Gill MJ. Longitudinal assessment (1995–2003) of hospitalizations of HIV-infected patients within a geographical population in Canada. HIV Med 2006; 7:457–66. [DOI] [PubMed] [Google Scholar]
- 16. Yehia BR, Fleishman JA, Hicks PL, Ridore M, Moore RD, Gebo KA. Inpatient health services utilization among HIV-infected adult patients in care 2002–2007. J Acquir Immune Defic Syndr 2010; 53:397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Berry SA, Fleishman JA, Moore RD, Gebo KA. Trends in reasons for hospitalization in a multisite United States cohort of persons living with HIV, 2001–2008. J Acquir Immune Defic Syndr 2012; 59:368–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lazar R, Kersanske L, Xia Q, Daskalakis D, Braunstein SL. Hospitalization rates among people with HIV/AIDS in New York City, 2013. Clin Infect Dis 2017; 65:469–76. [DOI] [PubMed] [Google Scholar]
- 19. Davy-Mendez T, Napravnik S, Wohl DA, et al. Hospitalization rates and outcomes among persons living with HIV in the Southeastern United States, 1996–2016. Clin Infect Dis 2020; 71:1616–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rentsch C, Tate JP, Akgun KM, et al. Alcohol-related diagnoses and all-cause hospitalization among HIV-infected and uninfected patients: a longitudinal analysis of United States veterans from 1997 to 2011. AIDS Behav 2016; 20:555–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gange SJ, Kitahata MM, Saag MS, et al. Cohort profile: the North American AIDS cohort collaboration on research and design (NA-ACCORD). Int J Epidemiol 2007; 36:294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Elixhauser A, Steiner C, Palmer L. Clinical classifications software (CCS) for ICD-9-CM. https://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp. Accessed 31 March 2020.
- 23. Gebo KA, Diener-West M, Moore RD. Hospitalization rates in an urban cohort after the introduction of highly active antiretroviral therapy. J Acquir Immune Defic Syndr 2001; 27:143–52. [DOI] [PubMed] [Google Scholar]
- 24. Lucas GM, Ross MJ, Stock PG, et al. Clinical practice guideline for the management of chronic kidney disease in patients infected with HIV: 2014 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2014; 59:e96–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Aberg JA, Gallant JE, Ghanem KG, Emmanuel P, Zingman BS, Horberg MA. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2014; 58:1–10. [DOI] [PubMed] [Google Scholar]
- 26. Worm SW, Sabin C, Weber R, et al. Risk of myocardial infarction in patients with HIV infection exposed to specific individual antiretroviral drugs from the 3 major drug classes: the data collection on adverse events of anti-HIV drugs (D:A:D) study. J Infect Dis 2010; 201:318–30. [DOI] [PubMed] [Google Scholar]
- 27. Frazier EL, Sutton MY, Brooks JT, Shouse RL, Weiser J. Trends in cigarette smoking among adults with HIV compared with the general adult population, United States—2009–2014. Prev Med 2018; 111:231–4. [DOI] [PubMed] [Google Scholar]
- 28. Bellino S, Borghetti A, Lombardi F, et al. Trends of hospitalisations rates in a cohort of HIV-infected persons followed in an Italian hospital from 1998 to 2016. Epidemiol Infect 2019; 147:e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fleming J, Berry SA, Moore RD, et al. U.S. hospitalization rates and reasons stratified by age among persons with HIV 2014–15. AIDS Care 2020; 32:1353–62. [DOI] [PubMed] [Google Scholar]
- 30. Jaworsky D, Phillips P, Cui Z, et al. Trends in discharges from the HIV/AIDS ward at a tertiary Canadian Hospital from 2005 to 2014. AIDS Care 2018; 30:1099–106. [DOI] [PubMed] [Google Scholar]
- 31. Healthcare Cost and Utilization Project. HCUP fast stats—trends in inpatient stays. https://www.hcup-us.ahrq.gov/faststats/NationalTrendsServlet. Accessed 12 August 2019.
- 32. Canadian Institute for Health Information. Inpatient hospitalizations: volumes, length of stay and standardized rates. https://apps.cihi.ca/mstrapp/asp/Main.aspx. Accessed 8 April 2020.
- 33. Admon AJ, Valley TS, Ayanian JZ, Iwashyna TJ, Cooke CR, Tipirneni R. Trends in hospital utilization after Medicaid expansion. Med Care 2019; 57:312–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wadhera RK, Wang Y, Figueroa JF, Dominici F, Yeh RW, Joynt Maddox KE. Mortality and hospitalizations for dually enrolled and nondually enrolled Medicare beneficiaries aged 65 years or older, 2004 to 2017. JAMA 2020; 323:961–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hemmige V, McNulty M, Silverman E, David MZ. Predictors of skin and soft tissue infections in HIV-infected outpatients in the community-associated methicillin-resistant Staphylococcus aureus era. Eur J Clin Microbiol Infect Dis 2015; 34:339–47. [DOI] [PubMed] [Google Scholar]
- 36. Kohli R, Lo Y, Homel P, et al. ; HER Study Group . Bacterial pneumonia, HIV therapy, and disease progression among HIV-infected women in the HIV epidemiologic research (HER) study. Clin Infect Dis 2006; 43:90–8. [DOI] [PubMed] [Google Scholar]
- 37. Larsen MV, Harboe ZB, Ladelund S, et al. Major but differential decline in the incidence of Staphylococcus aureus bacteraemia in HIV-infected individuals from 1995 to 2007: a nationwide cohort study. HIV Med 2012; 13:45–53. [DOI] [PubMed] [Google Scholar]
- 38. Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med 2011; 62:141–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Warner M, Trinidad JP, Bastian BA, Minino AM, Hedegaard H. Drugs most frequently involved in drug overdose deaths: United States, 2010–2014. Natl Vital Stat Rep 2016; 65:1–15. [PubMed] [Google Scholar]
- 40. Attia EF, McGinnis KA, Feemster LC, et al. Association of COPD with risk for pulmonary infections requiring hospitalization in HIV-infected veterans. J Acquir Immune Defic Syndr 2015; 70:280–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Edelman EJ, Gordon KS, Crothers K, et al. Association of prescribed opioids with increased risk of community-acquired pneumonia among patients with and without HIV. JAMA Intern Med 2019; 179:297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Drummond MB, Kunisaki KM, Huang L. Obstructive lung diseases in HIV: a clinical review and identification of key future research needs. Semin Respir Crit Care Med 2016; 37:277–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Koethe JR, Jenkins CA, Lau B, et al. ; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) . Rising obesity prevalence and weight gain among adults starting antiretroviral therapy in the United States and Canada. AIDS Res Hum Retroviruses 2016; 32:50–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Davy-Mendez T, Napravnik S, Zakharova O, Wohl DA, Farel CE, Eron JJ.Estimating bias in hospitalization rates due to missing hospitalization data. In: Society for Epidemiologic Research Annual Meeting, 19–22 June 2018: Baltimore, MD, abstract 49604.
- 45. Elvstam O, Marrone G, Medstrand P, et al. All-cause mortality and serious non-AIDS events in adults with low-level HIV viremia during combination antiretroviral therapy: results from a Swedish Nationwide Observational Study [published online ahead of print 9 April 2020]. Clin Infect Dis doi: 10.1093/cid/ciaa413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Anderegg N, Johnson LF, Zaniewski E, et al. ; IeDEA, MeSH consortia . All-cause mortality in HIV-positive adults starting combination antiretroviral therapy: correcting for loss to follow-up. AIDS 2017; 31(suppl 1):31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Centers for Disease Control and Prevention. Diagnoses of HIV infection in the United States and dependent areas, 2012. https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-2012-vol-24.pdf. Accessed 22 September 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.