Abstract
Current therapeutic options for renal diseases are limited, and the search for disease-specific treatments is ongoing. Nanobodies, single-domain antibodies with many advantages over conventional antibodies, provide flexible, easy-to-format biologicals with many possible applications. Here, we discuss the potential use of nanobodies for renal diseases.
Keywords: Nanobodies, Renal diseases, Conventional antibodies
Nanobody advantages and formats
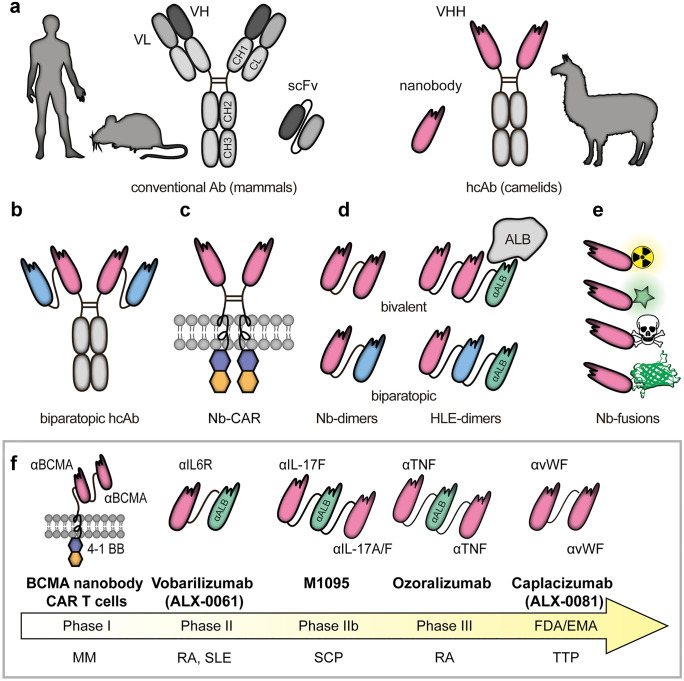
Nanobodies are derived from naturally occurring heavy chain antibodies. These unusual immunoglobulin molecules were first detected in the serum of a dromedary in the early 1990s (Hamers-Casterman et al. 1993). In addition to heterotetrameric conventional antibodies, llamas and other camelids (camels, dromedaries, alpacas, vicuñas, guanacos) produce homodimeric antibodies consisting of only two heavy chains (Fig. 1).
Fig. 1.
Structure, formats, and applications of VHHs. Conventional antibodies consist of two heavy and two light chains, and their antigen-binding region (paratope) is encoded by the variable domains of both chains (VH and VL). In case of camelid heavy chain antibodies, the antigen is recognized by the variable domain of the heavy chain (VHH) (A). Monomeric or dimeric (bivalent or biparatopic) nanobodies can be linked to the constant region (Fc) of any isotype to mediate different effector functions (e.g., complement dependent cytotoxicity (CDC) or antibody-dependent cellular cytotoxicity (ADCC)) (Schriewer et al. 2020) (B). VHHs can also be used as binding domains of chimeric antigen receptors (Nb-CAR) (Hambach et al. 2020) (C). Due to their modular structure, nanobodies can function as building blocks in multimeric constructs binding the same (multivalent) or different (multiparatopic) epitopes. The in vivo half-life of mono or multivalent nanobodies can be tuned, e.g., by genetic fusion to an albumin-specific nanobody (Tijink et al. 2008) (D). Monovalent VHHs can be conjugated chemically to radioisotopes (Huang et al. 2008) or fluorochromes (Fumey et al. 2017) and genetically to toxins (Mutter et al. 2018) and fluorescent proteins (Rothbauer et al. 2006) (E). Examples for nanobodies currently undergoing clinical trials include BCMA nanobody CAR T cells, Vobarilizumab, M1095, and Ozoralizumab, while Caplacizumab has already been FDA/EMA approved (F). scFv single-chain variable fragment, Ab antibody, hcAb heavy-chain antibody, ALB albumin, Nb nanobody, BCMA B cell maturation antigen, MM multiple myeloma, RA rheumatoid arthritis, SLE Systemic Lupus Erythematosus, SCP severe chronic psoriasis, TTP thrombotic thrombocytopenic purpura
The molecular basis for the generation of heavy chain antibodies in camelids can be explained by the missing CH1 domain of the heavy chain in two of the IgG-isotypes (Muyldermans et al. 1994) (Fig. 1A). Since the CH1 domain plays a major role in linking heavy and light chains, camelids can produce IgG isotypes consisting of only two heavy chains. In this case, antigen recognition is only performed by the variable domain of the heavy chain. Interestingly, camelids possess two subsets of variable domains that preferentially pair either with heavy chain or with conventional antibodies. The single variable domain of heavy chain antibodies (referred to as VHH or nanobody) is considered to be the smallest naturally occurring antigen-recognizing domain produced by the adaptive immune system (Muyldermans 2013).
Nanobodies have unique properties. With their long CDR3 regions, they can penetrate into functional cavities of proteins that cannot or only poorly be reached by the comparatively planar paratope of conventional antibodies (De Genst et al. 2006; Jahnichen et al. 2010; Maussang et al. 2013). Since functional clefts on proteins often correspond to the active site of an enzyme or the ligand binding pocket of a receptor, many nanobodies are excellent functional antagonists, an important property rarely attainable with conventional antibodies (Danquah et al. 2016).
Nanobodies have a number of other advantages over the variable domains of conventional antibodies, such as higher stability and solubility as well as better tissue penetration, reaching cell surface molecules in almost all major organs within minutes upon i.v. injection (Bannas et al. 2015; Bannas et al. 2014; Cheloha et al. 2020; Ingram et al. 2017; Rashidian et al. 2015). The high stability of VHHs is due to their structure consisting of two ß-folded sheets with a total of 9 ß-strands. The two ß-sheets are connected by a conserved canonical disulfide bridge connecting cysteine residues in framework (FR) 1 and 3. Some VHHs also have a second disulfide bridge linking the CDR3 with the CDR1 (lamas) or the CDR2 (camels) (Muyldermans et al. 1994).
Nanobodies are highly soluble and, in contrast to conventional VH domains, do not tend to aggregate due to hydrophilic amino acids in the FR2. The polarity of these amino acids promotes water solubility and also reduces the stickiness of the VHH domain. The corresponding FR2 region of the VH domain of conventional antibodies contains hydrophobic amino acids that mediate the association with the VL domain of the light chain (Wesolowski et al. 2009).
The modular structure of VHHs allows easy conversion into multivalent formats and easy linkage to functional groups or proteins (Fig. 1B–E). Importantly, the in vivo half-life of mono or multivalent nanobodies can be tuned, e.g., by genetic fusion to an albumin-specific nanobody (Tijink et al. 2008) (Fig. 1D). Similarly, genetic fusion to an engineered Fc-domain can endow nanobodies with potent effector functions, e.g., enhanced complement-dependent and antibody dependent cellular cytotoxicity (CDC, ADCC) (Schutze et al. 2018). These extraordinary features underline the high potential for the use of nanobodies in different therapeutic applications.
Nanobodies in clinical trials
The physicochemical advantages of nanobodies over monoclonal antibodies (mAbs) have spurred the development of therapeutic nanobodies, some of which have entered clinical trials (Jovcevska and Muyldermans 2020) (Fig. 1F). As of April 2021, 13 nanobody drugs are undergoing phase 1–3 trials, either for oncologic or inflammatory diseases. The first therapeutic nanobody, caplacizumab (ALX-0081, Ablynx), was approved in the European Union in 2018 and in the USA in 2019 for the treatment of adults with acquired thrombotic thrombocytopenic purpura (aTTP) in conjunction with plasma exchange and immunosuppression. Caplacizumab is a humanized, bivalent von Willebrand factor (vWF)-specific nanobody dimer, which inhibits the interaction between vWF multimers and platelets, thereby preventing thrombocytopenia, hemolytic anemia, and tissue ischemia (Scully et al. 2019). Another promising nanobody (vobarilizumab/ALX-0061, Ablynx), currently in a phase II trial for treatment of Rheumatoid Arthritis (RA) and Systemic Lupus Erythematosus (SLE), is directed against the Interleukin-6 Receptor (IL-6R) (Van Roy et al. 2015). It consists of two nanobodies: one directed against IL-6R in order to inhibit the pro-inflammatory activities of the IL-6 pathway and the other is designed to extend the half-life of the therapeutic by binding to human serum albumin (HSA). A third example, a trivalent, interleukin-17-specific nanobody (M1095, Avillion LLP) is currently under development for the treatment of Severe Psoriasis. M1095 comprises three sequence-optimized, monovalent nanobodies that recognize IL-17A, IL-17F, and HSA (for half-life extension). M1095 possesses humanized sequences to reduce immunogenicity. With three immunoglobulin domains, it is considerably smaller than conventional IgG antibodies with 12-Ig domains (40 kDa vs. 150 kDa) (Svecova et al. 2019).
Nanobodies also have advantages compared to conventional single-chain variable fragments (scFv) in the design of chimeric antigen receptors (CAR) (Fig. 1A, C). To direct T cells against oncologic targets, they are genetically modified to express CARs consisting of an extracellular scFv or nanobody domain connected via a transmembrane domain to intracellular signaling modules derived from other cell surface signaling proteins (June et al. 2018). CAR T cell therapy is currently a rapidly developing approach for targeted therapy of specific cancer entities (Sermer and Brentjens 2019). It has proven successful in the treatment of hematological cancers but concerns remain about adverse effects. Furthermore, the efficiency to target solid tumors is still limited, and there is still a need for improved and safe alternatives (Xie et al. 2019). Nanobody CAR T cells may help to increase the local inflammatory response for improved immune recognition and enhance drug access to the tumor (Xie et al. 2019). Nanobody CAR T cells currently in active trials include BCMA nanobody CAR T cells against multiple myeloma, αPD1-MSLN-CAR T cells for colorectal and ovarian cancer, and CD19/CD20 bispecific CAR T cells against B-cell lymphoma (https://clinicaltrials.gov) (Jovcevska and Muyldermans 2020).
Nanobodies are presently also under investigation as potential therapeutics against SARS-CoV-2 infection (Dong et al. 2020; Konwarh 2020). Nanobodies are aimed against the receptor binding domain (RBD) of the spike protein of SARS-Cov-2 to prevent binding of the virus to the ACE2 receptor on host cells. Studies have identified two closely related nanobodies, H11-D4 and H11-H4, that bind RBD and block its interaction with ACE2. Nanobody-Fc fusion proteins showed neutralizing activity against SARS-CoV-2 and additive neutralization with the SARS-CoV-1/2 antibody CR3022 pointing to a possible therapeutic exploitation for the development of an inhalable drug as a prophylaxis against COVID-19 (Dong et al. 2020; Huo et al. 2020; Konwarh 2020).
The kidney’s role in nanobody applications
Due to their small size and modular structure, nanobodies offer advantages for various applications. However, with regard to renal retention, the small size of nanobodies in combination with the use of peptide tags also brings difficulties for some applications. Since the size of nanobodies is well below the filtration threshold of the glomerular membrane, nanobodies are rapidly excreted in the urine after their application (Bannas et al. 2015; Bannas et al. 2014; Debie et al. 2020). This is advantageous for molecular imaging of structures that are not close to the kidneys, as background signals decrease very rapidly and toxic side effects are minimized (Vaneycken et al. 2011). Depending on the format in which the nanobodies are applied, they may be retained in the kidney. For targeting structures close to the kidney, renal retention of nanobodies is disadvantageous because the strong signal in the kidney makes specific staining of adjacent tissue difficult (Bao et al. 2021). It has been shown that renal retention of nanobodies is primarily determined by the number of polar residues in the C-terminal amino acid tag. For a radiolabeled HER2-specific nanobody, application in the untagged format resulted in a decreased renal accumulation of almost 90% compared to a Myc-His-tagged format (D’Huyvetter et al. 2014).
Additional strategies have been developed to further reduce the accumulation of nanobodies in the kidney. Renal retention occurs through the endocytic apparatus of the proximal renal tubule. In this complex of different molecules, megalin is responsible for at least 40% of the total protein retention in the kidneys. Simultaneous injection of substances such as gelofusine, lysine, or monosodium glutamate can reduce renal reabsorption of nanobodies by inhibiting their binding to megalin (D’Huyvetter et al. 2014; Gainkam et al. 2011; Rousseau et al. 2018).
Nanobodies in kidney disease
Renal diseases comprise primary kidney disorders, such as diseases of the glomerulus (nephrotic syndrome or glomerulonephritis), the tubulointerstitium, or the reaction of the kidney against toxins (viral toxins, myoglobin), drugs (nonsteroidal anti-inflammatory drug), or diagnostic agents (contrast dyes, antibiotics), as well as infections and renal neoplasia (Basile et al. 2012). Additionally, kidney damage can result from many systemic diseases, such as heart failure, hypertension, and diabetes (Kazancioglu 2013). Apart from management of hypertension and treatment of the underlying conditions, therapeutic options are still limited. Patients with a declining renal function often progress to end stage renal disease (ESRD), which requires dialysis and renal transplantation (Krolewski et al. 2017). World-wide, patient numbers with chronic kidney disease and ESRD are on the rise, placing a huge burden on societies and health care systems (Hill et al. 2016; Szczech and Lazar 2004). Due to the limited treatment options and slow progress in recent decades for kidney-specific medications, the need for novel therapeutics remains high. Therefore, new avenues have to be explored to find novel drugs and biologics for the treatment of renal diseases. In many aspects of renal diseases, e.g., imaging, diagnostics and therapy, nanobodies might prove beneficial.
Glomerular diseases
The glomerular filter consists of 3 layers: fenestrated endothelium, glomerular basement membrane, and podocytes. The latter builds a complex interdigitated net around the capillary loops with the secondary foot processes forming the slit diaphragm, the smallest part of the glomerular filter. While large proteins are not able to pass the glomerular filter, antibodies targeting podocyte transmembrane proteins are known to cross the glomerular basement membrane (Akilesh et al. 2008). Thus, we presume that this is also true for the much smaller (half-life extended) nanobodies. Stabilization of the slit diaphragm has been implicated in protection against podocyte foot process effacement and proteinuria (Kawachi and Fukusumi 2020). Thus, enhancement of Neph1 - ZO-1 interaction was shown to protect from injury-induced renal damage (Sagar et al. 2017). Furthermore, several surface molecules on podocytes have been shown to be involved in glomerular diseases. Up-regulation of HB-EGF has been shown to lead to activation of EGFR, and rapid progressive glomerulonephritis (RPGN) can be attenuated by EGFR deletion in podocytes or by pharmacological blockade (Bollee et al. 2011). EGFR-specific nanobodies are well characterized for the treatment and detection of tumors and have been used in various formats (Roovers et al. 2011; Sharifi et al. 2021; Tintelnot et al. 2019). Additionally, it is conceivably that nanobody-mediated blockade of autoantibody binding to podocyte membrane proteins such as THSD7A and PLA2R might prove a useful therapeutic measure in treatment of membranous nephropathy (Beck et al. 2009; Tomas et al. 2014). Furthermore, blocking circulating molecules, such as suPAR, via nanobodies from inducing harm on kidney cells harbors therapeutic potential in glomerular diseases (Wei et al. 2011; Zeier and Reiser 2017). Besides podocytes, surface proteins of parietal epithelial cells surrounding the glomeruli, such as CD9, also represent potential novel nanobody targets, e.g., to treat or prevent glomerulosclerosis (Lazareth et al. 2019).
A hallmark of the glomerular damage is proteinuria caused by a leaky glomerular filter. Compromised filtration is bound to also affect nanobody half-life. This might require increasing the frequency of the nanobody administration during proteinuria. In contrast, with decreasing glomerular filtration rate (GFR) in kidney disease, accumulation of nanobodies in the kidney is a conceivable issue. Here, decreased blood flow and drainage by the lymphatic system might act as counteracting forces. The pharmacodynamics of nanobody treatments specifically for kidney diseases will have to be explored in future studies.
Acute kidney injury
Acute kidney injury (AKI) is reported in more than 50% of intensive care patients and involves clinical syndromes with acute renal dysfunction caused by sepsis, drug toxicity, and ischemia reperfusion injury (Gao et al. 2020; Ronco et al. 2019). Patients have a high mortality and risk to develop chronic kidney disease (CKD) (Coca et al. 2012; Hsu 2012). Several growth factors have been implicated in either attenuating or aggravating AKI. EGF administration and EFR activation appear to promote recovery of renal function after ischemia-reperfusion injury. However, their roles in fibrosis indicate that they may be unfit as targets unsuitable for long-term treatment (He et al. 2013; Humes et al. 1989; Tang et al. 2013). Of the multiple fibroblast growth factors with known roles in AKI, FGF-23 and its co-receptor Klotho have been implicated as potential predictive and prognostic biomarkers for AKI (Christov et al. 2019). As Klotho expression decreases with increasing kidney damage, stabilization of Klotho via nanobodies would represent a promising approach for individualized patient treatment (Ray et al. 2020). Likewise, bFGF and its receptor bFGFR2 have been shown to promote kidney recovery; thus, activation of bFGFR2 might have therapeutic potential (Villanueva et al. 2006; Villanueva et al. 2008). Tgfbr2 deletion in macrophages was shown to ameliorate AKI, indicating TgfβrII as a potential target to prevent tubulointerstitial fibrosis after severe ischemic renal injury (Chung et al. 2018). Another signaling axis, podocyte expression of VEGF, such as VEGF-121, and its signaling via VEGFR2 has been shown to be renoprotective in tubulointerstitial diseases, but not in diabetes (Facemire et al. 2009; Leonard et al. 2008). While homeostasis, milieu, isoform, site, and mode of action seem of vital importance for achieving beneficial effects, targeting this pathway with nanobodies is worth considering (Majumder and Advani 2017).
Inflammatory kidney disease
Promising advances have also been made with mAbs in inflammatory renal diseases, for which the standard protocols have for decades been based only on corticosteroids and non-specific immunosuppressants with heavy side effects for the patients (Santoro et al. 2015). Important mAbs for the treatment of inflammatory kidney disorders include rituximab (used in membranous glomerulonephritis, steroid-resistant nephrotic syndromes and anti-neutrophil cytoplasmic antibodies (ANCA)-associated vasculitis, membranoproliferative glomerulonephritis (MPGN)), eculizumab (used in typical hemolytic uremic syndrome, C3 nephropathy and MPGN), and fresolimumab (focal segmental glomerulosclerosis and kidney cancer) (Santoro et al. 2015). In one recent approach, anti-inflammatory nanobodies have targeted the adenosine 5′-triphosphate (ATP)–gated P2X7 ion channel (Danquah et al. 2016), (Menzel et al. 2018). P2X7 is expressed on macrophages and CD4-positive T cell subpopulations. Upon sensing ATP released as a danger signal from cells during infection and sterile inflammation, P2X7 initiates a proinflammatory signaling cascade resulting in the release of the pro inflammatory cytokines IL-1β and IL-18. Treatment with the P2X7-blocking nanobody 13A7 ameliorated disease in mouse models of glomerulonephritis and contact dermatitis, while treatment with the P2X7-agonistic nanobody 14D5 aggravated glomerulonephritis (Danquah et al. 2016). As P2X7 constitutes a trimeric ion channel, both nanobodies had increased potency as dimers or Fc fusion proteins. Furthermore, Dano1, a nanobody generated against human P2X7, inhibited the release of IL-1β from endotoxin-treated human blood cells 1000-fold more potently than the small-molecule drugs against P2X7 currently in development (Danquah et al. 2016).
While anti-TNF treatment was so far not effective for maintenance of remission in ANCA-patients (Wegener’s Granulomatosis Etanercept Trial Research 2005), its receptors TNFR1 and TNFR2 might be potential targets for nanobodies in inflammatory kidney disease (Speeckaert et al. 2012). Targeted blockade of either TNFR1 or TNFR2 might be effective by preserving other proinflammatory and immunosuppressive functions (Ernandez and Mayadas 2009). So far, several nanobodies have already been developed against various chemokines, potentially enabling effective and specific treatment of renal inflammatory diseases by inhibiting chemokine receptor activation and chemotaxis (Blanchetot et al. 2013).
In autoimmune diseases affecting the kidney, such as membranous nephropathy or SLE, targeting autoantibody-producing plasma cells is a promising therapeutic strategy (Hofmann et al. 2018). Among others, proteasome inhibitor bortezomib (Neubert et al. 2008; Tasaki et al. 2019) and CD20-specific monoclonal antibody rituximab (Uematsu-Uchida et al. 2019) have been successfully used. Here, using nanobodies in a heavy-chain format to induce antibody-dependent cellular cytotoxicity is a promising and specific targeting strategy to deplete autoantibody-producing cells. Several nanobodies targeting B cells and plasma cell have been produced so far for anti-tumorigenic purposes, but their effectiveness for depletion of autoantibodies in the kidney has yet to be investigated (Schriewer et al. 2020; Zhao et al. 2018).
Renal cell carcinoma
In recent years, many mAbs have been approved for the treatment of cancers (Chiavenna et al. 2017). Due to their high specificity towards malignant cells, mAbs have distinct advantages over conservative therapies, such as chemotherapy, radiotherapy and surgery, and can act in synergy with these therapies. For example, bevazizumab (Avastin), an antiangiogenic VEGF-A-specific mAb, was approved in 2004 for the treatment of colon cancer. Avastin is also indicated for the treatment of metastatic renal cell carcinoma in combination with interferon alpha. Renal cell carcinoma is the most common type of kidney cancer in adults and has a poor prognosis in stage III and IV (Siegel et al. 2017; Znaor et al. 2015). In a phase III trial, bevazizumab treatment of the highly vascularized tumor led to a reduction in the risk of disease progression by 37% but no overall survival benefit (Escudier et al. 2007; Rini et al. 2010). Anti-angiogenic therapy has since been combined with immunotherapy for better results (Garcia et al. 2020).
As nanobodies are able to adopt all the different targeting strategies of mAbs, such as direct targeting of cancer cells, cytotoxic moiety delivery, or modification of the host immune response, they have the potential to offer additional benefits. For instance, monomeric anti-Her2 nanobodies show a faster accumulation and more homogenous distribution in the tumor within minutes after injection in the mouse model than mAbs (Debie et al. 2020). Nanobodies could also be utilized in concert with the immune system to target cancer cells. One potential target is CD38, an ecto-enzyme that is highly expressed on the surface of malignant plasma cells in multiple myeloma (Lin et al. 2004; Mesguich et al. 2016). Here, nanobody-based CD38-specific hcAbs are able to induce antibody-dependent cellular cytotoxicity in tumor cancer cell lines and inhibit tumor growth in a mouse xenograft model, warranting further clinical development as therapeutics for multiple myeloma and other hematological malignancies (Schriewer et al. 2020). Furthermore, nanobodies can be conjugated to deliver drugs to the target cell as demonstrated by tetrameric anti-EGFR nanobodies (7D12) conjugated to tetravalent platinum Pt(iv) prodrugs (e.g., cisplatin) to generate a nanobody–drug conjugate (NDC) (Wu et al. 2020). In a mouse model, this NDC showed high targetability, high antitumor efficacy, and low systemic side-effects (Wu et al. 2020). Thus, nanobodies with their many possibilities for formatting, linking, and targeted drug-therapy may have potential for novel approaches for the diagnosis and treatment of renal cancer.
Renal replacement therapy
Patients progressing to ESRD require renal replacement therapy, either dialysis or renal transplantation, as a last resort. Major issues with renal transplantation involve activation of the complement system, e.g., due to ischemia-reperfusion or antibody-mediated rejection. The C5-specific mAb eculizumab (Legendre et al. 2013) has been used in kidney transplantation to prevent delayed graft function, in antibody-mediated rejection, atypical hemolytic uremic syndrome (aHUS) recurrence, and anti-phospholipid syndrome (Legendre et al. 2017). While trials did not show efficacy in delayed graft function, a study did show a decrease in antibody-mediated rejection (Stegall et al. 2011). However, there was no long-term benefit of eculizumab-mediated C5 blockage in regard to transplant glomerulopathy and microvascular inflammation after 2 years (Cornell et al. 2015) and an early benefit is still debated. Likewise, C1 esterase inhibitors have been used in renal transplantation with variable results (Bhalla et al. 2020; Huang et al. 2020b; Montgomery et al. 2016). Nanobodies against members of the complement cascade, such as C5 and C4b, have shown promising selective inhibition (Yatime et al. 2018; Zarantonell et al. 2020). Complement-inhibiting nanobodies, perhaps in a half-life extended format, may have the potential for synergistic and/or more cost-effective treatment in renal replacement therapy. Furthermore, plasma cell depletion has also been used to counteract antibody-mediated graft rejection (Kwun et al. 2019; Tasaki et al. 2019), indicating a potential therapeutic strategy by use of nanobodies as discussed above.
In a dialysis setting, a recent proof-of-concept study showed that nanobodies are suitable immunosorbents to specifically purify toxic substances from the blood. In this specific example, a highly selective immunosorbent was prepared using a β2-microtubulin- (β2M-) specific nanobody to prospectively purify β2M from the blood of patients with dialysis-related amyloidosis (DRA). The accumulation of β2M is a serious side effect in patients with end-stage kidney disease, indicating that selective extracorporeal removal of β2M is a promising method to delay the onset and progression of DRA (Huang et al. 2020a).
Imaging and diagnostics
Due to their small size and excellent tissue penetration properties, nanobodies have been successfully used for in vivo imaging. Optical imaging is achieved by combining nanobodies with near-infrared (700–900 nm) fluorophores, such as AlexaFluor680, Cy7, or quantum dots (Qd800) (Fatehi et al. 2014; Zheng et al. 2019). This was used, for example, in an experimental mouse models as intraoperative fluorescence imaging to increase the sensitivity of peritoneal tumor implant debulking surgery (Debie et al. 2018). Comparisons of nanobodies, heavy-chain antibodies, and conventional antibodies labeled with AlexaFluor680 showed that monovalent nanobodies had the best signal-to-noise ratio due to the lowest background staining, while the heavy-chain antibody showed the highest labelling efficiency (Bannas et al. 2014).
Deeper tissue penetration can be achieved by combining radionuclide-based techniques such as SPECT (single-photon emission computed tomography; e.g., 99mTc or 111In) (Gomes et al. 2011) and PET (Positron emission tomography, e.g., 18F, 64Cu, 68Ga, or 89Zr) (Ametamey et al. 2008; Chakravarty et al. 2014) with nanobodies, which has been successfully used in mouse models of cancer or atherosclerosis (Balhuizen et al. 2017; Jailkhani et al. 2019; Keyaerts et al. 2016). In a Phase I study of 68Ga-labelled HER2 nanobody for PET/CT in breast carcinoma, the treatment showed no adverse reactions and a fast blood clearance of 90% within 1 h with a radiation dose comparable to other routinely used PET tracers (Keyaerts et al. 2016). Experimental studies are also focusing on imaging of inflammatory diseases with 99mTc, such as nuclear imaging of atherosclerotic lesions or tumor-associated macrophages (Broisat et al. 2012; Movahedi et al. 2012; Schoonooghe et al. 2012).
Going even further, the diagnostic potential of nanobodies can be linked to therapeutic properties. For instance, nanobody-photosensitizer conjugates, such as IRDye700DX, are able to induce cell toxicity upon treatment with near-infrared light called photo dynamic therapy and trigger immune responses (Beltran Hernandez et al. 2020; De Groof et al. 2019). Furthermore, novel ultrasound molecular probes using nanobody-coupled lipid nanobubbles for enhanced ultrasound imaging of renal cell carcinoma or prostate cancer are potential carriers of therapeutics, which can be released through an ultrasound-targeted nanobubble destruction technique for local accumulation of the drug (Fan et al. 2015; Yu et al. 2020). Many more applications for the kidney are imaginable in terms of imaging and diagnostics, e.g., analysis of renal fibrosis, perfusion, and filtration.
Summary and conclusion
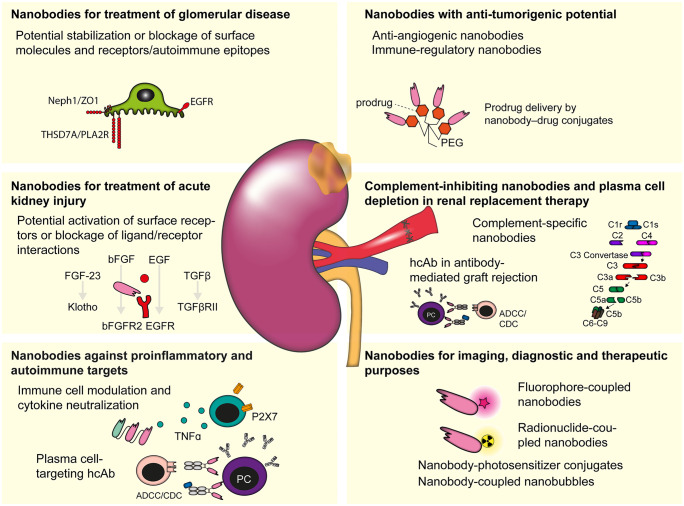
Camelid VHH domains have been tailored for expression as heavy chain antibodies throughout 50 million years of evolutionary history. The resulting biochemical properties distinguish them from human VH domains: small size, excellent solubility, good tissue penetration, and rapid elimination via the kidney combined with the possibility of targeted conjugation of dyes make nanobodies particularly interesting for use in imaging applications. Moreover, numerous clinical and preclinical studies have already demonstrated that nanobodies can be used as molecular building blocks for highly efficient drugs in various therapeutic applications. The approval of the first therapeutic nanobody (caplacizumab) is expected to pave the way for further nanobody-based products in immunotherapy. Due to the limited treatment options, there is a huge unmet need in nephrology for novel and specific therapies. Thus, nephrology could profit immensely from tailored nanobody applications, e.g., by stabilizing the filtration apparatus, by neutralizing pro-inflammatory cytokines, complement components, and purinergic receptors, by blocking the binding of fibrosis-promoting growth factors, by blocking the binding of autoantibodies, or for the extracorporal removal of nephrotoxic proteins from the blood (Fig. 2). In the future, enhanced disease stratification will lead to a better understanding of disease entities and increasingly individualized medicine which make utilization of nanobodies excellent for the development of novel innovative treatments for kidney diseases and beyond.
Fig. 2.
Potential applications for nanobodies in kidney disease. PEG polyethylene glycol, PC plasma cell, CDC complement-dependent cytotoxicity, ADCC antibody-dependent cellular cytotoxicity
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by the German Research Foundation (DFG): CRC 1192 (to N.W. and F.K.N).
Declarations
Conflict of interest
F.K.-N. is a co-inventor on patent applications on CD38-specific and P2X7-specific nanobodies.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Akilesh S, Huber TB, Wu H, Wang G, Hartleben B, Kopp JB, Miner JH, Roopenian DC, Unanue ER, Shaw AS. Podocytes use FcRn to clear IgG from the glomerular basement membrane. Proc Natl Acad Sci U S A. 2008;105:967–972. doi: 10.1073/pnas.0711515105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ametamey SM, Honer M, Schubiger PA. Molecular imaging with PET. Chem Rev. 2008;108:1501–1516. doi: 10.1021/cr0782426. [DOI] [PubMed] [Google Scholar]
- Balhuizen A, Massa S, Mathijs I, Turatsinze JV, De Vos J, Demine S, Xavier C, Villate O, Millard I, Egrise D, Capito C, Scharfmann R, In't Veld P, Marchetti P, Muyldermans S, Goldman S, Lahoutte T, Bouwens L, Eizirik DL, Devoogdt N. A nanobody-based tracer targeting DPP6 for non-invasive imaging of human pancreatic endocrine cells. Sci Rep. 2017;7:15130. doi: 10.1038/s41598-017-15417-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannas P, Lenz A, Kunick V, Well L, Fumey W, Rissiek B, Haag F, Schmid J, Schutze K, Eichhoff A, Trepel M, Adam G, Ittrich H, Koch-Nolte F. Molecular imaging of tumors with nanobodies and antibodies: timing and dosage are crucial factors for improved in vivo detection. Contrast Media Mol Imaging. 2015;10:367–378. doi: 10.1002/cmmi.1637. [DOI] [PubMed] [Google Scholar]
- Bannas P, Well L, Lenz A, Rissiek B, Haag F, Schmid J, Hochgrafe K, Trepel M, Adam G, Ittrich H, Koch-Nolte F. In vivo near-infrared fluorescence targeting of T cells: comparison of nanobodies and conventional monoclonal antibodies. Contrast Media Mol Imaging. 2014;9:135–142. doi: 10.1002/cmmi.1548. [DOI] [PubMed] [Google Scholar]
- Bao G, Tang M, Zhao J, Zhu X. Nanobody: a promising toolkit for molecular imaging and disease therapy. EJNMMI Res. 2021;11:6. doi: 10.1186/s13550-021-00750-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile DP, Anderson MD, Sutton TA. Pathophysiology of acute kidney injury. Compr Physiol. 2012;2:1303–1353. doi: 10.1002/cphy.c110041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck LH, Jr, Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, Klein JB, Salant DJ. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361:11–21. doi: 10.1056/NEJMoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltran Hernandez I, Angelier ML, Del Buono D'Ondes T, Di Maggio A, Yu Y, Oliveira S (2020) The potential of nanobody-targeted photodynamic therapy to trigger immune responses. Cancers (Basel) 12 [DOI] [PMC free article] [PubMed]
- Bhalla A, Alachkar N, Alasfar S. Complement-based therapy in the management of antibody-mediated rejection. Adv Chronic Kidney Dis. 2020;27:138–148. doi: 10.1053/j.ackd.2019.12.002. [DOI] [PubMed] [Google Scholar]
- Blanchetot C, Verzijl D, Mujic-Delic A, Bosch L, Rem L, Leurs R, Verrips CT, Saunders M, de Haard H, Smit MJ. Neutralizing nanobodies targeting diverse chemokines effectively inhibit chemokine function. J Biol Chem. 2013;288:25173–25182. doi: 10.1074/jbc.M113.467969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollee G, Flamant M, Schordan S, Fligny C, Rumpel E, Milon M, Schordan E, Sabaa N, Vandermeersch S, Galaup A, Rodenas A, Casal I, Sunnarborg SW, Salant DJ, Kopp JB, Threadgill DW, Quaggin SE, Dussaule JC, Germain S, Mesnard L, Endlich K, Boucheix C, Belenfant X, Callard P, Endlich N, Tharaux PL. Epidermal growth factor receptor promotes glomerular injury and renal failure in rapidly progressive crescentic glomerulonephritis. Nat Med. 2011;17:1242–1250. doi: 10.1038/nm.2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broisat A, Hernot S, Toczek J, De Vos J, Riou LM, Martin S, Ahmadi M, Thielens N, Wernery U, Caveliers V, Muyldermans S, Lahoutte T, Fagret D, Ghezzi C, Devoogdt N. Nanobodies targeting mouse/human VCAM1 for the nuclear imaging of atherosclerotic lesions. Circ Res. 2012;110:927–937. doi: 10.1161/CIRCRESAHA.112.265140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarty R, Goel S, Cai W. Nanobody: the “magic bullet” for molecular imaging? Theranostics. 2014;4:386–398. doi: 10.7150/thno.8006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheloha RW, Harmand TJ, Wijne C, Schwartz TU, Ploegh HL. Exploring cellular biochemistry with nanobodies. J Biol Chem. 2020;295:15307–15327. doi: 10.1074/jbc.REV120.012960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiavenna SM, Jaworski JP, Vendrell A. State of the art in anti-cancer mAbs. J Biomed Sci. 2017;24:15. doi: 10.1186/s12929-016-0311-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christov M, Neyra JA, Gupta S, Leaf DE. Fibroblast Growth Factor 23 and Klotho in AKI. Semin Nephrol. 2019;39:57–75. doi: 10.1016/j.semnephrol.2018.10.005. [DOI] [PubMed] [Google Scholar]
- Chung S, Overstreet JM, Li Y, Wang Y, Niu A, Wang S, Fan X, Sasaki K, Jin GN, Khodo SN, Gewin L, Zhang MZ, Harris RC (2018) TGF-beta promotes fibrosis after severe acute kidney injury by enhancing renal macrophage infiltration. JCI Insight 3 [DOI] [PMC free article] [PubMed]
- Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81:442–448. doi: 10.1038/ki.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell LD, Schinstock CA, Gandhi MJ, Kremers WK, Stegall MD. Positive crossmatch kidney transplant recipients treated with eculizumab: outcomes beyond 1 year. Am J Transplant. 2015;15:1293–1302. doi: 10.1111/ajt.13168. [DOI] [PubMed] [Google Scholar]
- D'Huyvetter M, Vincke C, Xavier C, Aerts A, Impens N, Baatout S, De Raeve H, Muyldermans S, Caveliers V, Devoogdt N, Lahoutte T. Targeted radionuclide therapy with A 177Lu-labeled anti-HER2 nanobody. Theranostics. 2014;4:708–720. doi: 10.7150/thno.8156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danquah W, Meyer-Schwesinger C, Rissiek B, Pinto C, Serracant-Prat A, Amadi M, Iacenda D, Knop JH, Hammel A, Bergmann P, Schwarz N, Assuncao J, Rotthier W, Haag F, Tolosa E, Bannas P, Boue-Grabot E, Magnus T, Laeremans T, Stortelers C, Koch-Nolte F (2016) Nanobodies that block gating of the P2X7 ion channel ameliorate inflammation. Sci Transl Med 8:366ra162 [DOI] [PubMed]
- De Genst E, Silence K, Decanniere K, Conrath K, Loris R, Kinne J, Muyldermans S, Wyns L. Molecular basis for the preferential cleft recognition by dromedary heavy-chain antibodies. Proc Natl Acad Sci U S A. 2006;103:4586–4591. doi: 10.1073/pnas.0505379103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groof TWM, Mashayekhi V, Fan TS, Bergkamp ND, Sastre Torano J, van Senten JR, Heukers R, Smit MJ, Oliveira S. Nanobody-targeted photodynamic therapy selectively kills viral GPCR-expressing glioblastoma cells. Mol Pharm. 2019;16:3145–3156. doi: 10.1021/acs.molpharmaceut.9b00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debie P, Lafont C, Defrise M, Hansen I, van Willigen DM, van Leeuwen FWB, Gijsbers R, D'Huyvetter M, Devoogdt N, Lahoutte T, Mollard P, Hernot S. Size and affinity kinetics of nanobodies influence targeting and penetration of solid tumours. J Control Release. 2020;317:34–42. doi: 10.1016/j.jconrel.2019.11.014. [DOI] [PubMed] [Google Scholar]
- Debie P, Vanhoeij M, Poortmans N, Puttemans J, Gillis K, Devoogdt N, Lahoutte T, Hernot S. Improved debulking of peritoneal tumor implants by near-infrared fluorescent nanobody image guidance in an experimental mouse model. Mol Imaging Biol. 2018;20:361–367. doi: 10.1007/s11307-017-1134-2. [DOI] [PubMed] [Google Scholar]
- Dong J, Huang B, Jia Z, Wang B, Gallolu Kankanamalage S, Titong A, Liu Y. Development of multi-specific humanized llama antibodies blocking SARS-CoV-2/ACE2 interaction with high affinity and avidity. Emerg Microbes Infect. 2020;9:1034–1036. doi: 10.1080/22221751.2020.1768806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernandez T, Mayadas TN. Immunoregulatory role of TNFalpha in inflammatory kidney diseases. Kidney Int. 2009;76:262–276. doi: 10.1038/ki.2009.142. [DOI] [PubMed] [Google Scholar]
- Escudier B, Pluzanska A, Koralewski P, Ravaud A, Bracarda S, Szczylik C, Chevreau C, Filipek M, Melichar B, Bajetta E, Gorbunova V, Bay JO, Bodrogi I, Jagiello-Gruszfeld A, Moore N, investigators AT, Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet. 2007;370:2103–2111. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- Facemire CS, Nixon AB, Griffiths R, Hurwitz H, Coffman TM. Vascular endothelial growth factor receptor 2 controls blood pressure by regulating nitric oxide synthase expression. Hypertension. 2009;54:652–658. doi: 10.1161/HYPERTENSIONAHA.109.129973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Wang L, Guo Y, Tu Z, Li L, Tong H, Xu Y, Li R, Fang K. Ultrasonic nanobubbles carrying anti-PSMA nanobody: construction and application in prostate cancer-targeted imaging. PLoS One. 2015;10:e0127419. doi: 10.1371/journal.pone.0127419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatehi D, Baral TN, Abulrob A. In vivo imaging of brain cancer using epidermal growth factor single domain antibody bioconjugated to near-infrared quantum dots. J Nanosci Nanotechnol. 2014;14:5355–5362. doi: 10.1166/jnn.2014.9076. [DOI] [PubMed] [Google Scholar]
- Fumey W, Koenigsdorf J, Kunick V, Menzel S, Schutze K, Unger M, Schriewer L, Haag F, Adam G, Oberle A, Binder M, Fliegert R, Guse A, Zhao YJ, Cheung Lee H, Malavasi F, Goldbaum F, van Hegelsom R, Stortelers C, Bannas P, Koch-Nolte F. Nanobodies effectively modulate the enzymatic activity of CD38 and allow specific imaging of CD38(+) tumors in mouse models in vivo. Sci Rep. 2017;7:14289. doi: 10.1038/s41598-017-14112-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainkam LO, Caveliers V, Devoogdt N, Vanhove C, Xavier C, Boerman O, Muyldermans S, Bossuyt A, Lahoutte T. Localization, mechanism and reduction of renal retention of technetium-99m labeled epidermal growth factor receptor-specific nanobody in mice. Contrast Media Mol Imaging. 2011;6:85–92. doi: 10.1002/cmmi.408. [DOI] [PubMed] [Google Scholar]
- Gao L, Zhong X, Jin J, Li J, Meng XM. Potential targeted therapy and diagnosis based on novel insight into growth factors, receptors, and downstream effectors in acute kidney injury and acute kidney injury-chronic kidney disease progression. Signal Transduct Target Ther. 2020;5:9. doi: 10.1038/s41392-020-0106-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia J, Hurwitz HI, Sandler AB, Miles D, Coleman RL, Deurloo R, Chinot OL. Bevacizumab (Avastin(R)) in cancer treatment: a review of 15 years of clinical experience and future outlook. Cancer Treat Rev. 2020;86:102017. doi: 10.1016/j.ctrv.2020.102017. [DOI] [PubMed] [Google Scholar]
- Gomes CM, Abrunhosa AJ, Ramos P, Pauwels EK. Molecular imaging with SPECT as a tool for drug development. Adv Drug Deliv Rev. 2011;63:547–554. doi: 10.1016/j.addr.2010.09.015. [DOI] [PubMed] [Google Scholar]
- Hambach J, Riecken K, Cichutek S, Schutze K, Albrecht B, Petry K, Rockendorf JL, Baum N, Kroger N, Hansen T, Schuch G, Haag F, Adam G, Fehse B, Bannas P, Koch-Nolte F (2020) Targeting CD38-expressing multiple myeloma and burkitt lymphoma cells in vitro with nanobody-based chimeric antigen receptors (Nb-CARs). Cells 9 [DOI] [PMC free article] [PubMed]
- Hamers-Casterman C, Atarhouch T, Muyldermans S, Robinson G, Hamers C, Songa EB, Bendahman N, Hamers R. Naturally occurring antibodies devoid of light chains. Nature. 1993;363:446–448. doi: 10.1038/363446a0. [DOI] [PubMed] [Google Scholar]
- He S, Liu N, Bayliss G, Zhuang S. EGFR activity is required for renal tubular cell dedifferentiation and proliferation in a murine model of folic acid-induced acute kidney injury. Am J Physiol Renal Physiol. 2013;304:F356–366. doi: 10.1152/ajprenal.00553.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill NR, Fatoba ST, Oke JL, Hirst JA, O'Callaghan CA, Lasserson DS, Hobbs FD. Global prevalence of chronic kidney disease—a systematic review and meta-analysis. PLoS One. 2016;11:e0158765. doi: 10.1371/journal.pone.0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann K, Clauder AK, Manz RA. Targeting B cells and plasma cells in autoimmune diseases. Front Immunol. 2018;9:835. doi: 10.3389/fimmu.2018.00835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu CY. Yes, AKI truly leads to CKD. J Am Soc Nephrol. 2012;23:967–969. doi: 10.1681/ASN.2012030222. [DOI] [PubMed] [Google Scholar]
- Huang C, Ren J, Ji F, Muyldermans S, Jia L. Nanobody-based high-performance immunosorbent for selective beta 2-microglobulin purification from blood. Acta Biomater. 2020;107:232–241. doi: 10.1016/j.actbio.2020.02.028. [DOI] [PubMed] [Google Scholar]
- Huang E, Vo A, Choi J, Ammerman N, Lim K, Sethi S, Kim I, Kumar S, Najjar R, Peng A, Jordan SC. Three-year outcomes of a randomized, double-blind, placebo-controlled study assessing safety and efficacy of C1 esterase inhibitor for prevention of delayed graft function in deceased donor kidney transplant recipients. Clin J Am Soc Nephrol. 2020;15:109–116. doi: 10.2215/CJN.04840419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Gainkam LO, Caveliers V, Vanhove C, Keyaerts M, De Baetselier P, Bossuyt A, Revets H, Lahoutte T. SPECT imaging with 99mTc-labeled EGFR-specific nanobody for in vivo monitoring of EGFR expression. Mol Imaging Biol. 2008;10:167–175. doi: 10.1007/s11307-008-0133-8. [DOI] [PubMed] [Google Scholar]
- Humes HD, Cieslinski DA, Coimbra TM, Messana JM, Galvao C. Epidermal growth factor enhances renal tubule cell regeneration and repair and accelerates the recovery of renal function in postischemic acute renal failure. J Clin Invest. 1989;84:1757–1761. doi: 10.1172/JCI114359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo J, Le Bas A, Ruza RR, Duyvesteyn HME, Mikolajek H, Malinauskas T, Tan TK, Rijal P, Dumoux M, Ward PN, Ren J, Zhou D, Harrison PJ, Weckener M, Clare DK, Vogirala VK, Radecke J, Moynie L, Zhao Y, Gilbert-Jaramillo J, Knight ML, Tree JA, Buttigieg KR, Coombes N, Elmore MJ, Carroll MW, Carrique L, Shah PNM, James W, Townsend AR, Stuart DI, Owens RJ, Naismith JH. Neutralizing nanobodies bind SARS-CoV-2 spike RBD and block interaction with ACE2. Nat Struct Mol Biol. 2020;27:846–854. doi: 10.1038/s41594-020-0469-6. [DOI] [PubMed] [Google Scholar]
- Ingram JR, Dougan M, Rashidian M, Knoll M, Keliher EJ, Garrett S, Garforth S, Blomberg OS, Espinosa C, Bhan A, Almo SC, Weissleder R, Lodish H, Dougan SK, Ploegh HL. PD-L1 is an activation-independent marker of brown adipocytes. Nat Commun. 2017;8:647. doi: 10.1038/s41467-017-00799-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnichen S, Blanchetot C, Maussang D, Gonzalez-Pajuelo M, Chow KY, Bosch L, De Vrieze S, Serruys B, Ulrichts H, Vandevelde W, Saunders M, De Haard HJ, Schols D, Leurs R, Vanlandschoot P, Verrips T, Smit MJ. CXCR4 nanobodies (VHH-based single variable domains) potently inhibit chemotaxis and HIV-1 replication and mobilize stem cells. Proc Natl Acad Sci U S A. 2010;107:20565–20570. doi: 10.1073/pnas.1012865107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jailkhani N, Ingram JR, Rashidian M, Rickelt S, Tian C, Mak H, Jiang Z, Ploegh HL, Hynes RO. Noninvasive imaging of tumor progression, metastasis, and fibrosis using a nanobody targeting the extracellular matrix. Proc Natl Acad Sci U S A. 2019;116:14181–14190. doi: 10.1073/pnas.1817442116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovcevska I, Muyldermans S. The therapeutic potential of nanobodies. BioDrugs. 2020;34:11–26. doi: 10.1007/s40259-019-00392-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June CH, O'Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science. 2018;359:1361–1365. doi: 10.1126/science.aar6711. [DOI] [PubMed] [Google Scholar]
- Kawachi H, Fukusumi Y. New insight into podocyte slit diaphragm, a therapeutic target of proteinuria. Clin Exp Nephrol. 2020;24:193–204. doi: 10.1007/s10157-020-01854-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazancioglu R. (2013) Risk factors for chronic kidney disease: an update. Kidney Int Suppl. 2011;3:368–371. doi: 10.1038/kisup.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyaerts M, Xavier C, Heemskerk J, Devoogdt N, Everaert H, Ackaert C, Vanhoeij M, Duhoux FP, Gevaert T, Simon P, Schallier D, Fontaine C, Vaneycken I, Vanhove C, De Greve J, Lamote J, Caveliers V, Lahoutte T. Phase I Study of 68Ga-HER2-nanobody for PET/CT assessment of HER2 expression in breast carcinoma. J Nucl Med. 2016;57:27–33. doi: 10.2967/jnumed.115.162024. [DOI] [PubMed] [Google Scholar]
- Konwarh R. Nanobodies: prospects of expanding the gamut of neutralizing antibodies against the novel coronavirus, SARS-CoV-2. Front Immunol. 2020;11:1531. doi: 10.3389/fimmu.2020.01531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krolewski AS, Skupien J, Rossing P, Warram JH. Fast renal decline to end-stage renal disease: an unrecognized feature of nephropathy in diabetes. Kidney Int. 2017;91:1300–1311. doi: 10.1016/j.kint.2016.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwun J, Matignon M, Manook M, Guendouz S, Audard V, Kheav D, Poullot E, Gautreau C, Ezekian B, Bodez D, Damy T, Faivre L, Menouch D, Yoon J, Park J, Belhadj K, Chen D, Bilewski AM, Yi JS, Collins B, Stegall M, Farris AB, Knechtle S, Grimbert P. Daratumumab in prospects of expanding the gamut of neutralizing antibodies against the novel coronavirus. J Am Soc Nephrol. 2019;30:1206–1219. doi: 10.1681/ASN.2018121254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazareth H, Henique C, Lenoir O, Puelles VG, Flamant M, Bollee G, Fligny C, Camus M, Guyonnet L, Millien C, Gaillard F, Chipont A, Robin B, Fabrega S, Dhaun N, Camerer E, Kretz O, Grahammer F, Braun F, Huber TB, Nochy D, Mandet C, Bruneval P, Mesnard L, Thervet E, Karras A, Le Naour F, Rubinstein E, Boucheix C, Alexandrou A, Moeller MJ, Bouzigues C, Tharaux PL. The tetraspanin CD9 controls migration and proliferation of parietal epithelial cells and glomerular disease progression. Nat Commun. 2019;10:3303. doi: 10.1038/s41467-019-11013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre C, Sberro-Soussan R, Zuber J, Fremeaux-Bacchi V. The role of complement inhibition in kidney transplantation. Br Med Bull. 2017;124:5–17. doi: 10.1093/bmb/ldx037. [DOI] [PubMed] [Google Scholar]
- Legendre CM, Licht C, Muus P, Greenbaum LA, Babu S, Bedrosian C, Bingham C, Cohen DJ, Delmas Y, Douglas K, Eitner F, Feldkamp T, Fouque D, Furman RR, Gaber O, Herthelius M, Hourmant M, Karpman D, Lebranchu Y, Mariat C, Menne J, Moulin B, Nurnberger J, Ogawa M, Remuzzi G, Richard T, Sberro-Soussan R, Severino B, Sheerin NS, Trivelli A, Zimmerhackl LB, Goodship T, Loirat C. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med. 2013;368:2169–2181. doi: 10.1056/NEJMoa1208981. [DOI] [PubMed] [Google Scholar]
- Leonard EC, Friedrich JL, Basile DP. VEGF-121 preserves renal microvessel structure and ameliorates secondary renal disease following acute kidney injury. Am J Physiol Renal Physiol. 2008;295:F1648–1657. doi: 10.1152/ajprenal.00099.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P, Owens R, Tricot G, Wilson CS. Flow cytometric immunophenotypic analysis of 306 cases of multiple myeloma. Am J Clin Pathol. 2004;121:482–488. doi: 10.1309/74R4TB90BUWH27JX. [DOI] [PubMed] [Google Scholar]
- Majumder S, Advani A. VEGF and the diabetic kidney: more than too much of a good thing. J Diabetes Complications. 2017;31:273–279. doi: 10.1016/j.jdiacomp.2016.10.020. [DOI] [PubMed] [Google Scholar]
- Maussang D, Mujic-Delic A, Descamps FJ, Stortelers C, Vanlandschoot P, Stigter-van Walsum M, Vischer HF, van Roy M, Vosjan M, Gonzalez-Pajuelo M, van Dongen GA, Merchiers P, van Rompaey P, Smit MJ. Llama-derived single variable domains (nanobodies) directed against chemokine receptor CXCR7 reduce head and neck cancer cell growth in vivo. J Biol Chem. 2013;288:29562–29572. doi: 10.1074/jbc.M113.498436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzel S, Schwarz N, Haag F, Koch-Nolte F. Nanobody-based biologics for modulating purinergic signaling in inflammation and immunity. Front Pharmacol. 2018;9:266. doi: 10.3389/fphar.2018.00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesguich C, Zanotti-Fregonara P, Hindie E. New perspectives offered by nuclear medicine for the imaging and therapy of multiple myeloma. Theranostics. 2016;6:287–290. doi: 10.7150/thno.14400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery RA, Orandi BJ, Racusen L, Jackson AM, Garonzik-Wang JM, Shah T, Woodle ES, Sommerer C, Fitts D, Rockich K, Zhang P, Uknis ME. Plasma-derived C1 esterase inhibitor for acute antibody-mediated rejection following kidney transplantation: results of a randomized double-blind placebo-controlled pilot study. Am J Transplant. 2016;16:3468–3478. doi: 10.1111/ajt.13871. [DOI] [PubMed] [Google Scholar]
- Movahedi K, Schoonooghe S, Laoui D, Houbracken I, Waelput W, Breckpot K, Bouwens L, Lahoutte T, De Baetselier P, Raes G, Devoogdt N, Van Ginderachter JA. Nanobody-based targeting of the macrophage mannose receptor for effective in vivo imaging of tumor-associated macrophages. Cancer Res. 2012;72:4165–4177. doi: 10.1158/0008-5472.CAN-11-2994. [DOI] [PubMed] [Google Scholar]
- Mutter NL, Soskine M, Huang G, Albuquerque IS, Bernardes GJL, Maglia G. Modular pore-forming immunotoxins with caged cytotoxicity tailored by directed evolution. ACS Chem Biol. 2018;13:3153–3160. doi: 10.1021/acschembio.8b00720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muyldermans S. Nanobodies: natural single-domain antibodies. Annu Rev Biochem. 2013;82:775–797. doi: 10.1146/annurev-biochem-063011-092449. [DOI] [PubMed] [Google Scholar]
- Muyldermans S, Atarhouch T, Saldanha J, Barbosa JA, Hamers R. Sequence and structure of VH domain from naturally occurring camel heavy chain immunoglobulins lacking light chains. Protein Eng. 1994;7:1129–1135. doi: 10.1093/protein/7.9.1129. [DOI] [PubMed] [Google Scholar]
- Neubert K, Meister S, Moser K, Weisel F, Maseda D, Amann K, Wiethe C, Winkler TH, Kalden JR, Manz RA, Voll RE. The proteasome inhibitor bortezomib depletes plasma cells and protects mice with lupus-like disease from nephritis. Nat Med. 2008;14:748–755. doi: 10.1038/nm1763. [DOI] [PubMed] [Google Scholar]
- Rashidian M, Keliher EJ, Bilate AM, Duarte JN, Wojtkiewicz GR, Jacobsen JT, Cragnolini J, Swee LK, Victora GD, Weissleder R, Ploegh HL. Noninvasive imaging of immune responses. Proc Natl Acad Sci U S A. 2015;112:6146–6151. doi: 10.1073/pnas.1502609112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray SK, Masarkar N, Mukherjee S (2020) Implications of klotho protein for managing kidney disease—an emerging role in therapeutics and molecular medicine. Curr Mol Med [DOI] [PubMed]
- Rini BI, Halabi S, Rosenberg JE, Stadler WM, Vaena DA, Archer L, Atkins JN, Picus J, Czaykowski P, Dutcher J, Small EJ. Phase III trial of bevacizumab plus interferon alfa versus interferon alfa monotherapy in patients with metastatic renal cell carcinoma: final results of CALGB 90206. J Clin Oncol. 2010;28:2137–2143. doi: 10.1200/JCO.2009.26.5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronco C, Bellomo R, Kellum JA. Acute kidney injury. Lancet. 2019;394:1949–1964. doi: 10.1016/S0140-6736(19)32563-2. [DOI] [PubMed] [Google Scholar]
- Roovers RC, Vosjan MJ, Laeremans T, el Khoulati R, de Bruin RC, Ferguson KM, Verkleij AJ, van Dongen GA, van Bergen en Henegouwen PM, A biparatopic anti-EGFR nanobody efficiently inhibits solid tumour growth. Int J Cancer. 2011;129:2013–2024. doi: 10.1002/ijc.26145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbauer U, Zolghadr K, Tillib S, Nowak D, Schermelleh L, Gahl A, Backmann N, Conrath K, Muyldermans S, Cardoso MC, Leonhardt H. Targeting and tracing antigens in live cells with fluorescent nanobodies. Nat Methods. 2006;3:887–889. doi: 10.1038/nmeth953. [DOI] [PubMed] [Google Scholar]
- Rousseau E, Lau J, Kuo HT, Zhang Z, Merkens H, Hundal-Jabal N, Colpo N, Lin KS, Benard F. Monosodium glutamate reduces (68)Ga-PSMA-11 uptake in salivary glands and kidneys in a preclinical prostate cancer model. J Nucl Med. 2018;59:1865–1868. doi: 10.2967/jnumed.118.215350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagar A, Arif E, Solanki AK, Srivastava P, Janech MG, Kim SH, Lipschutz JH, Kwon SH, Ashish Nihalani D. Targeting Neph1 and ZO-1 protein-protein interaction in podocytes prevents podocyte injury and preserves glomerular filtration function. Sci Rep. 2017;7:12047. doi: 10.1038/s41598-017-12134-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro D, Pellicano V, Visconti L, Trifiro G, Cernaro V, Buemi M. Monoclonal antibodies for renal diseases: current concepts and ongoing treatments. Expert Opin Biol Ther. 2015;15:1119–1143. doi: 10.1517/14712598.2015.1045870. [DOI] [PubMed] [Google Scholar]
- Schoonooghe S, Laoui D, Van Ginderachter JA, Devoogdt N, Lahoutte T, De Baetselier P, Raes G. Novel applications of nanobodies for in vivo bio-imaging of inflamed tissues in inflammatory diseases and cancer. Immunobiology. 2012;217:1266–1272. doi: 10.1016/j.imbio.2012.07.009. [DOI] [PubMed] [Google Scholar]
- Schriewer L, Schutze K, Petry K, Hambach J, Fumey W, Koenigsdorf J, Baum N, Menzel S, Rissiek B, Riecken K, Fehse B, Rockendorf JL, Schmid J, Albrecht B, Pinnschmidt H, Ayuk F, Kroger N, Binder M, Schuch G, Hansen T, Haag F, Adam G, Koch-Nolte F, Bannas P. Nanobody-based CD38-specific heavy chain antibodies induce killing of multiple myeloma and other hematological malignancies. Theranostics. 2020;10:2645–2658. doi: 10.7150/thno.38533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutze K, Petry K, Hambach J, Schuster N, Fumey W, Schriewer L, Rockendorf J, Menzel S, Albrecht B, Haag F, Stortelers C, Bannas P, Koch-Nolte F. CD38-specific biparatopic heavy chain antibodies display potent complement-dependent cytotoxicity against multiple myeloma cells. Front Immunol. 2018;9:2553. doi: 10.3389/fimmu.2018.02553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scully M, Cataland SR, Peyvandi F, Coppo P, Knobl P, Kremer Hovinga JA, Metjian A, de la Rubia J, Pavenski K, Callewaert F, Biswas D, De Winter H, Zeldin RK, Investigators H. Caplacizumab treatment for acquired thrombotic thrombocytopenic purpura. N Engl J Med. 2019;380:335–346. doi: 10.1056/NEJMoa1806311. [DOI] [PubMed] [Google Scholar]
- Sermer D, Brentjens R. CAR T-cell therapy: Full speed ahead. Hematol Oncol. 2019;37(Suppl 1):95–100. doi: 10.1002/hon.2591. [DOI] [PubMed] [Google Scholar]
- Sharifi J, Khirehgesh MR, Safari F, Akbari B. EGFR and anti-EGFR nanobodies: review and update. J Drug Target. 2021;29:387–402. doi: 10.1080/1061186X.2020.1853756. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- Speeckaert MM, Speeckaert R, Laute M, Vanholder R, Delanghe JR. Tumor necrosis factor receptors: biology and therapeutic potential in kidney diseases. Am J Nephrol. 2012;36:261–270. doi: 10.1159/000342333. [DOI] [PubMed] [Google Scholar]
- Stegall MD, Diwan T, Raghavaiah S, Cornell LD, Burns J, Dean PG, Cosio FG, Gandhi MJ, Kremers W, Gloor JM. Terminal complement inhibition decreases antibody-mediated rejection in sensitized renal transplant recipients. Am J Transplant. 2011;11:2405–2413. doi: 10.1111/j.1600-6143.2011.03757.x. [DOI] [PubMed] [Google Scholar]
- Svecova D, Lubell MW, Casset-Semanaz F, Mackenzie H, Grenningloh R, Krueger JG. A randomized, double-blind, placebo-controlled phase 1 study of multiple ascending doses of subcutaneous M1095, an anti-interleukin 17A/F nanobody, in moderate-to-severe psoriasis. J Am Acad Dermatol. 2019;81:196–203. doi: 10.1016/j.jaad.2019.03.056. [DOI] [PubMed] [Google Scholar]
- Szczech LA, Lazar IL (2004) Projecting the United States ESRD population: issues regarding treatment of patients with ESRD. Kidney Int Suppl S3-7 [DOI] [PubMed]
- Tang J, Liu N, Tolbert E, Ponnusamy M, Ma L, Gong R, Bayliss G, Yan H, Zhuang S. Sustained activation of EGFR triggers renal fibrogenesis after acute kidney injury. Am J Pathol. 2013;183:160–172. doi: 10.1016/j.ajpath.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasaki M, Saito K, Nakagawa Y, Ikeda M, Imai N, Ito Y, Sudo M, Ikezumi Y, Yamada T, Hasegawa H, Kobayashi T, Miura K, Narita I, Takahashi K, Tomita Y. Bortezomib Eliminates Plasma Cells From a Renal Graft in Plasma Cell-Rich Acute Rejection. Transplant Proc. 2019;51:1732–1738. doi: 10.1016/j.transproceed.2019.02.038. [DOI] [PubMed] [Google Scholar]
- Tijink BM, Laeremans T, Budde M, Stigter-van Walsum M, Dreier T, de Haard HJ, Leemans CR, van Dongen GA. Improved tumor targeting of anti-epidermal growth factor receptor Nanobodies through albumin binding: taking advantage of modular Nanobody technology. Mol Cancer Ther. 2008;7:2288–2297. doi: 10.1158/1535-7163.MCT-07-2384. [DOI] [PubMed] [Google Scholar]
- Tintelnot J, Baum N, Schultheiss C, Braig F, Trentmann M, Finter J, Fumey W, Bannas P, Fehse B, Riecken K, Schuetze K, Bokemeyer C, Rosner T, Valerius T, Peipp M, Koch-Nolte F, Binder M. Nanobody targeting of epidermal growth factor receptor (EGFR) ectodomain variants overcomes resistance to therapeutic EGFR antibodies. Mol Cancer Ther. 2019;18:823–833. doi: 10.1158/1535-7163.MCT-18-0849. [DOI] [PubMed] [Google Scholar]
- Tomas NM, Beck LH, Jr, Meyer-Schwesinger C, Seitz-Polski B, Ma H, Zahner G, Dolla G, Hoxha E, Helmchen U, Dabert-Gay AS, Debayle D, Merchant M, Klein J, Salant DJ, Stahl RAK, Lambeau G. Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med. 2014;371:2277–2287. doi: 10.1056/NEJMoa1409354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uematsu-Uchida M, Ohira T, Tomita S, Satonaka H, Tojo A, Ishimitsu T. Rituximab in treatment of anti-GBM antibody glomerulonephritis: a case report and literature review. Medicine (Baltimore) 2019;98:e17801. doi: 10.1097/MD.0000000000017801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Roy M, Ververken C, Beirnaert E, Hoefman S, Kolkman J, Vierboom M, Breedveld E, t Hart B, Poelmans S, Bontinck L, Hemeryck A, Jacobs S, Baumeister J, Ulrichts H, The preclinical pharmacology of the high affinity anti-IL-6R Nanobody(R) ALX-0061 supports its clinical development in rheumatoid arthritis. Arthritis Res Ther. 2015;17:135. doi: 10.1186/s13075-015-0651-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaneycken I, D'Huyvetter M, Hernot S, De Vos J, Xavier C, Devoogdt N, Caveliers V, Lahoutte T. Immuno-imaging using nanobodies. Curr Opin Biotechnol. 2011;22:877–881. doi: 10.1016/j.copbio.2011.06.009. [DOI] [PubMed] [Google Scholar]
- Villanueva S, Cespedes C, Gonzalez A, Vio CP. bFGF induces an earlier expression of nephrogenic proteins after ischemic acute renal failure. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1677–1687. doi: 10.1152/ajpregu.00023.2006. [DOI] [PubMed] [Google Scholar]
- Villanueva S, Cespedes C, Gonzalez AA, Roessler E, Vio CP. Inhibition of bFGF-receptor type 2 increases kidney damage and suppresses nephrogenic protein expression after ischemic acute renal failure. Am J Physiol Regul Integr Comp Physiol. 2008;294:R819–828. doi: 10.1152/ajpregu.00273.2007. [DOI] [PubMed] [Google Scholar]
- Wegener’s Granulomatosis Etanercept Trial Research G Etanercept plus standard therapy for Wegener’s granulomatosis. N Engl J Med. 2005;352:351–361. doi: 10.1056/NEJMoa041884. [DOI] [PubMed] [Google Scholar]
- Wei C, El Hindi S, Li J, Fornoni A, Goes N, Sageshima J, Maiguel D, Karumanchi SA, Yap HK, Saleem M, Zhang Q, Nikolic B, Chaudhuri A, Daftarian P, Salido E, Torres A, Salifu M, Sarwal MM, Schaefer F, Morath C, Schwenger V, Zeier M, Gupta V, Roth D, Rastaldi MP, Burke G, Ruiz P, Reiser J. Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat Med. 2011;17:952–960. doi: 10.1038/nm.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesolowski J, Alzogaray V, Reyelt J, Unger M, Juarez K, Urrutia M, Cauerhff A, Danquah W, Rissiek B, Scheuplein F, Schwarz N, Adriouch S, Boyer O, Seman M, Licea A, Serreze DV, Goldbaum FA, Haag F, Koch-Nolte F. Single domain antibodies: promising experimental and therapeutic tools in infection and immunity. Med Microbiol Immunol. 2009;198:157–174. doi: 10.1007/s00430-009-0116-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Liu M, Huang H, Sheng Y, Xiao H, Liu Y. Clustered nanobody-drug conjugates for targeted cancer therapy. Chem Commun (Camb) 2020;56:9344–9347. doi: 10.1039/D0CC03396K. [DOI] [PubMed] [Google Scholar]
- Xie YJ, Dougan M, Jailkhani N, Ingram J, Fang T, Kummer L, Momin N, Pishesha N, Rickelt S, Hynes RO, Ploegh H. Nanobody-based CAR T cells that target the tumor microenvironment inhibit the growth of solid tumors in immunocompetent mice. Proc Natl Acad Sci U S A. 2019;116:7624–7631. doi: 10.1073/pnas.1817147116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatime L, Merle NS, Hansen AG, Friis NA, Ostergaard JA, Bjerre M, Roumenina LT, Thiel S, Kristensen P, Andersen GR. A single-domain antibody targeting complement component C5 acts as a selective inhibitor of the terminal pathway of the complement system and thus functionally mimicks the C-terminal domain of the Staphylococcus aureus SSL7 Protein. Front Immunol. 2018;9:2822. doi: 10.3389/fimmu.2018.02822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Hu M, Li Z, Dan X, Zhu L, Guo Y, Liu Q, Lan W, Jiang J, Wang L. Anti-G250 nanobody-functionalized nanobubbles targeting renal cell carcinoma cells for ultrasound molecular imaging. Nanotechnology. 2020;31:205101. doi: 10.1088/1361-6528/ab7040. [DOI] [PubMed] [Google Scholar]
- Zarantonello A, Presumey J, Simoni L, Yalcin E, Fox R, Hansen A, Olesen HG, Thiel S, Johnson MB, Stevens B, Laursen NS, Carroll MC, Andersen GR. An ultrahigh-affinity complement C4b-specific nanobody inhibits in vivo assembly of the classical pathway proconvertase. J Immunol. 2020;205:1678–1694. doi: 10.4049/jimmunol.2000528. [DOI] [PubMed] [Google Scholar]
- Zeier M, Reiser J. suPAR and chronic kidney disease-a podocyte story. Pflugers Arch. 2017;469:1017–1020. doi: 10.1007/s00424-017-2026-7. [DOI] [PubMed] [Google Scholar]
- Zhao WH, Liu J, Wang BY, Chen YX, Cao XM, Yang Y, Zhang YL, Wang FX, Zhang PY, Lei B, Gu LF, Wang JL, Yang N, Zhang R, Zhang H, Shen Y, Bai J, Xu Y, Wang XG, Zhang RL, Wei LL, Li ZF, Li ZZ, Geng Y, He Q, Zhuang QC, Fan XH, He AL, Zhang WG. A phase 1, open-label study of LCAR-B38M, a chimeric antigen receptor T cell therapy directed against B cell maturation antigen, in patients with relapsed or refractory multiple myeloma. J Hematol Oncol. 2018;11:141. doi: 10.1186/s13045-018-0681-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng F, Luo S, Ouyang Z, Zhou J, Mo H, Schoonooghe S, Muyldermans S, De Baetselier P, Raes G, Wen Y (2019) NIRF-molecular imaging with synovial macrophages-targeting Vsig4 nanobody for disease monitoring in a mouse model of arthritis. Int J Mol Sci 20 [DOI] [PMC free article] [PubMed]
- Znaor A, Lortet-Tieulent J, Laversanne M, Jemal A, Bray F. International variations and trends in renal cell carcinoma incidence and mortality. Eur Urol. 2015;67:519–530. doi: 10.1016/j.eururo.2014.10.002. [DOI] [PubMed] [Google Scholar]