Abstract
Objective: Traditional Chinese medicine has been increasingly used in the prevention and treatment of gastric cancer, especially in application of compound Chinese medicine. The aim of this study was to investigate the effect of Qi Ling decoction (QLD) on the invasion and metastasis of gastric cancer and its related signaling pathways at the cellular and molecular level in vitro, and explore the mechanism of QLD. Methods: Scratch assay, transwell assay, and adhesion experiments were used to study the effects of QLD and its compounds on gastric cancer. Western blot was employed to detect expression of the PI3K/Akt pathway after administration of QLD. Results: QLD can significantly inhibit the invasion, migration, and adhesion of gastric cancer cells in vitro. The main chemical components of QLD (diosgenin, catechins, and calycosin) can also inhibit the invasion, migration and adhesion of gastric cancer cells. Furthermore, QLD inhibits MMP-9 and affects gastric cancer cell metastasis through the PI3K/Akt pathway. Conclusion: QLD and its three main chemical components can inhibit the invasion, migration, and adhesion of gastric cancer cells, and the mechanism may be related to the PI3K/Akt pathway.
Keywords: Qi Ling decoction, gastric cancer cells, migration, PI3K/Akt pathway, MMP-9
Introduction
Gastric cancer has become the fifth most common cancer and third leading cause of cancer-related death worldwide. In recent years, its incidence rate has been on the rise [1].
At present, the main treatment methods of gastric cancer are surgery and chemotherapy. Although these methods have a curative effect, the high recurrence and metastasis rate of gastric cancer, gives many patients a poor prognosis [2]. In China, the recurrence rate of tumor one year after operation is 60%, and more than 80% of patients have died of tumor recurrence and metastasis in recent years [3].
Distant metastasis is often the main cause of treatment failure of malignant tumors. Tumor metastasis is a complex process, which refers to tumor cells shedding from the primary site and reaching other parts of body through lymphatic vessels, blood vessels, or body cavities [4]. In this process, tumor cells not only interact with each other, but also work with other cells and extracellular matrix in the blood. The adhesion, migration, invasion, signal transduction, and proliferation of tumor cells are all related to tumor metastatic potential. Notably, epithelial-mesenchymal transition (EMT) is often considered as the first step of cancer metastasis, so that cells lose their adhesion and polarity and are endowed with the ability of metastasis and invasion [5,6]. Cell adhesion also plays an important role in metastasis and invasion [7]. Migration refers to cancer cells reaching distant tissues or organs in various ways. Inhibiting the migration of cancer cells can make cancer cells stay in blood vessels and lymphatic vessels for a longer time, so that the immune system can kill them [8]. Tumor invasion refers to the invasion of tumor cells into adjacent tissues accompanied by the loss of a tissue barrier. It is one of the malignant behaviors of tumors, and it is also a necessary process for tumor cells to enter the metastasis from blood vessels, lymphatic vessels, and tissue spaces [9].
In view of the recurrence of cancer after surgery, more attention is being paid to traditional Chinese medicine (TCM). Nowadays, a large number of studies have shown that a variety of traditional Chinese medicines have the effect of anti-tumor metastasis. TCM combining with chemotherapy is better than modern medicine alone in preventing postoperative recurrence of gastric cancer [10]. According to the theory of TCM, blood stasis syndrome, qi stagnation syndrome, and dampness syndrome are the main syndromes of gastric cancer patients before operation, while qi deficiency syndrome, blood stasis syndrome, yin deficiency syndrome, and dampness syndrome are the main symptoms after operation [11]. Therefore, the TCM syndrome of gastric cancer patients after operation is still a “deficiency syndrome”, which is attributed to the spleen and stomach [12]. We believe that “Turbid Toxin” is the basis of gastric cancer, and that to resolve turbidity and detoxification is indispensable in the treatment of gastric cancer. Therefore, “essential empty and out solid” is the key pathogenesis of gastric cancer patients after operation, and the treatment should be based on reinforcement and elimination in combination.
Qi Ling decoction (QLD) is a traditional Chinese medicine, consisting of Astragalus, Smilacisglabrae, Atractylodesmacrocephalae, Scutellariabarbata, Salvia chinensis, Benth, Eupolyphagasteleophaga, Agrimonia, and Forsythia suspense. QLD can effectively reduce the recurrence rate of gastric cancer and enhance the quality of life of gastric cancer patients after operation. In addition, many studies have shown that the eight herbs in QLD have an anti-tumor effect. Astragalus extract, astragalus polysaccharide, and astragaloside in Astragalus membranaceus have been proven to induce apoptosis of gastric cancer cells and have a good therapeutic effect on gastric cancer [13-15]. Smilax glabra has also been found to have anti-tumor and anti-gastric ulcer effects. Atractylodesmacrocephala can promote apoptosis of gastric cancer SGC-7901 cells by up regulating Bax and deregulating bcl-2 [16]. Kan et al. found that Scutellariabarbara extract could prevent tumor growth by downregulating Treg cells and regulating Th1/Th17 immune response in nude mice model [17]. Salvia chinensis Benth could inhibit proliferation and migration of cells by inhibiting the expression of IL-8 in human gastric cancer MGC-803 cells [18]. The ethanol extract of Eupolyphagasteleophaga could repress the proliferation of various tumor cells in vitro, and the protein extract of woodlouse worm also plays a role in vivo [19,20]. The extracts of Agrimonia and Agrimoniapilosa had an inhibitory effect on tumor cells in vitro, and the mechanism may be connected with cytotoxicity [21]. Ethanol extracts from Forsythia suspensa leaves and fruits have anti-tumor activity effects on human esophageal cancer cells [22].
However, for QLD, it is not clear how it works against recurrence and metastasis of gastric cancer. Further research is needed to reveal its mechanism of anti recurrence and metastasis, and further explore its role in the clinical treatment of gastric cancer. Therefore, we aimed to investigate the effects of QLD and its active ingredients on the migration, invasion, and adhesion of gastric cancer cells, and the related signaling pathways at the cellular and molecular level in vitro.
Materials and methods
Cell culture
Human gastric cancer cells BGC-823 and SGC-7901 were maintained in 1640 (Hyclone, SEMER science, USA) complete medium supplemented with 10% fetal bovine serum at 37°C in 5% CO2 atmosphere.
MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay
Cell proliferation was detected by MTT assay. MTT analysis data reflect the activity and quantity of living cells by detecting the activities of mitochondrial metabolic enzymes. Methods: the cells (1×105 cells/well) were inoculated into 96-well plates for 24 hours. MTT (5 mg/mL) reagent was added before the specified time. 4 hours later, the medium was taken out and replaced by 150 μL/well DMSO. The cells were shaken vigorously at room temperature. The absorbance was measured at 490 nm (OD490).
Flow cytometry
To detect the percentage of living cells, cells were digested and collected. Next, cells were incubated with Apoptosis Positive Control Solution for 30 min at 4°C, washed twice, then resuspended in PBS. The Binding Buffer and Annexin V-FITC (Multi Science (Lianke) Biotech, Hangzhou, China) were added. The experiments were repeated independently at least three times.
Immunofluorescence staining
After digestion, the cells were inoculated into the Coverglass Bottom Dishs (Solarbio, Beijing, China). 4 hours later, the cells were fixed with paraformaldehyde (4%) and Triton X-100 (0.5%), then blocked with blocking buffer (5% bovine serum albumin) for 1 hour, incubated overnight at 4°C, and then cultured for 2 hours with the secondary antibody (1:1000, Cambridge, UK Abcam). The images of each well were taken in three different areas, and the same area was taken at each point in time.
Scratch assay
The cells were cultured and scratched in wells with a sterile plastic wand. After scratch, cells were washed with PBS 3 times. Scratch images were taken with a microscope at 0 h, 6 h, 12 h, 24 h, and 48 h after administration.
Transwell migration assay
The transwell chambers were used for migration analysis. Methods: the cells (2×105 cells) were added into serum-free medium upper chamber, and the lower chamber had complete FBS medium. Then chambers were incubated at 37°C in 5% CO2 atmosphere for 24 hours, then the inside cells in the upper chamber were wiped off with cotton swabs. 0.5 mL paraformaldehyde was added into the empty well of the 24 well culture plate, the chamber was put in it for 30 min, and then taken out and put on a super clean table to dry for about 10 min. The membrane was dyed with 0.1% crystal violet for 10 min, washed with PBS twice, and then dried for 10 min. Visual fields were randomly selected under a high power microscope to count the number of membrane-penetrating cells.
Transwell invasion assay
Cells were digested and adjusted to 2×105 cells/mL. 100 µL cell suspension was added into Matrigel pre-treated upper chamber, and 500 µL culture medium containing 30% FBS into the bottom chamber of the 24-well plate. Cell plates were cultured for 24 h at 37°C, then the inside cells in the upper chamber were wiped off with cotton swabs. 0.5 mL paraformaldehyde was added into the empty hole of the 24 well culture plate, chamber was placed in it for 30 min, and then taken out and put on a super clean table to dry for about 10 min. The membrane was dyed with 0.1% crystal violet for 10 min, washed with PBS twice, and then dried for 10 min. Visual fields were randomly selected under a highpower microscope to count the number of membrane penetrating cells.
Adhesion experiment
Rat tail collagen (50 μL/well) was added into 96 well culture plate and set overnight at 4°C. After washing PBS for three times, it was sealed with 2% BSA at room temperature for 2 hours, and then washed three times with RPMI 1640. 100 μL cell suspension (3×105 cells/mL) was added to each well. This was washed out after 30 minutes. 10 μL MTT (5 mg/mL) was added to each well, and the cells were further cultured in the incubator for 4 h. After 4 h, 100 μL/well solution was added and it was put into an incubator overnight. The OD value was detected at 570 nm. The percentage of adherent cells (%) = (ODcontrol well - ODmeasurement well)/ODcontrol well ×100%.
Western blot
The protein was extracted using cell lysis buffer, then protein concentration was determined with BCA protein detection kit. An equal amount of protein solubilization was separated by 10% sodium dodecyl sulfate (SDS) polyacrylamide gel. After SDS-PAGE, protein was transferred to a polyvinylidene fluoride (PVDF) membrane (Millipore Corporation). It was cultured in skim milk (5%) containing TBST (0.1%) for 1 h. The membrane was incubated with primary antibodies against human p-PI3K (1:1000, Affinity Biosciences, USA), PI3K, AKT, p-AKT, NF-κB, MMP-9 (1:1000, Santa Cruz Biotechnology, USA), or GAPDH (1:1000, Proteintech Group, USA) overnight at 4°C. Then it was incubated with secondary antibody at room temperature for 1 h. The expression level of target protein was normalized to GAPDH.
Statistical analysis
Results are expressed as mean ± standard deviation. One-way ANOVA was used to determine the significance of differences between groups. We used GraphPad Prism 7 for all statistical analyses, with P<0.05 as the threshold for significance.
Results
Effect of QLD on proliferation of gastric cancer cells
MTT assay showed that 1000 μg/mL QLD promoted the proliferation of BGC-823 at 48 h and 72 h (Figure 1A), while QLD with different concentrations had no detectable effect on the proliferation of SGC-7901 (Figure 1B). To examine whether QLD has influences on the apoptosis of BGC-823, flow cytometry and immunofluorescence were performed, and the results showed that QLD had no obvious effect on cell apoptosis (Figure 1C and 1D).
Figure 1.
Effect of QLD on the proliferation of cells. (A) BGC-823 at 24 h, 48 h, 72 h and (B) SGC-7901 at 24 h, 48 h, 72 h. (mean ± sd, n=6; *P<0.05, **P<0.01). (C) Flow cytometric analysis of apoptotic cells using the annexin V-FITC reagent (above) and quantification of images (below). (D) Changes in nuclear morphology after QLD treatment for 24 h as determined by Hoechst 33324 staining.
Effect of QLD on the migration, invasion, and adhesion of gastric cancer cells
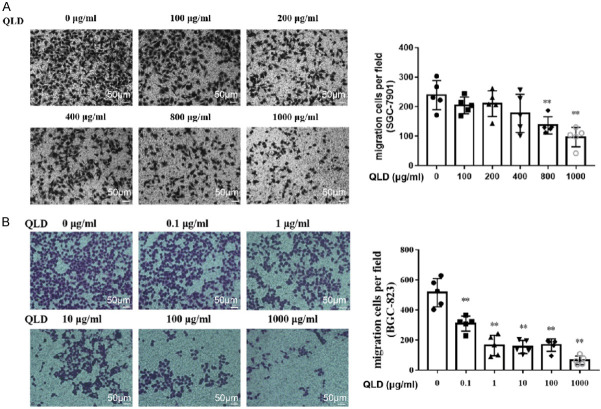
The scratch test showed no obvious inhibitory effect on the healing ability of SGC-7901 (Figure 2A). The transwell migration assays indicated that QLD could inhibit SGC-7901 cells passing through the basement membrane, suggesting that it could strongly repress the migration of SGC-7901 (Figure 3A). For BGC-823 cells, QLD could significantly reduce their number in a concentration-dependent manner (Figure 2B). In other words, QLD can arrest the migration of BGC-823, and starts to work at a low dose (Figure 3B). The transwell invasion test showed that QLD could significantly restrain the invasion of SGC-7901 (Figure 4A) and BGC-823 (Figure 4B). QLD could significantly prevent the adhesion of gastric cancer SGC-7901 (Figure 5A) and BGC-823 cells (Figure 5B).
Figure 2.
Effect of QLD on healing of SGC-7901 and BGC-823. Scratch test to detect the effect of QLD on the healing ability of SGC-7901 (A) and BGC-823 (B) (magnification: ×200; scale bar: 100 μm; mean ± sd, n=4, **P<0.01, ***P<0.001 vs. 0 μg/mL).
Figure 3.
Effect of QLD on migration of SGC-7901 and BGC-823. Transwell migration assay to detect the effect of QLD on the migration of SGC-7901 (A) and BGC-823 (B) (magnification: ×200; scale bar: 50 μm; mean ± sd, n=5, **P<0.01 vs. 0 μg/mL).
Figure 4.
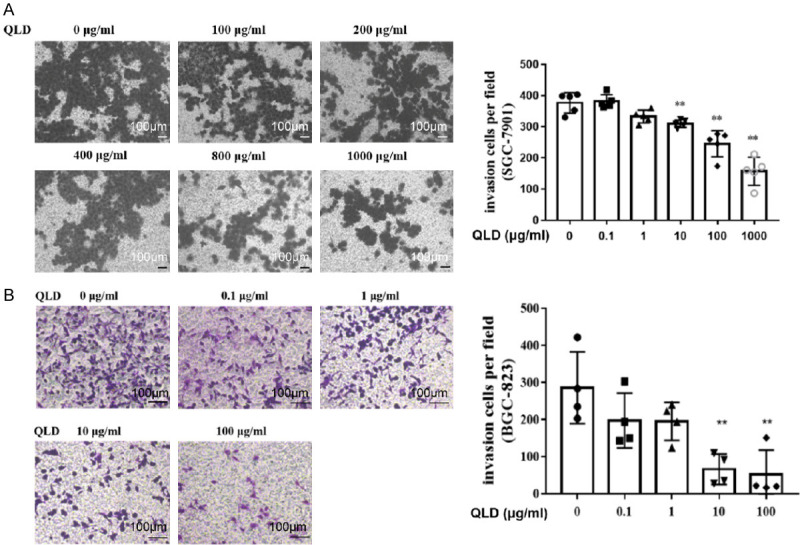
Effect of QLD on invasion of SGC-7901 and BGC-823. Transwell invasion assay to detect the effect of QLD on the invasion of SGC-7901 (A) (magnification: ×200; scale bar: 100 μm; mean ± sd, **P<0.01 vs. 0 μg/mL) and BGC-823 (B) (magnification: ×200; scale bar: 100 μm; mean ± sd, **P<0.01 vs. 0 μg/mL).
Figure 5.
Effect of QLD on adhesion of SGC-7901 and BGC-823. Cell adhesion assay to detect the effect of QLD on the invasion of SGC-7901 (A) and BGC-823 (B) (mean ± sd, n=5, *P<0.05, **P<0.01, ***P<0.001, vs. 0 μg/mL).
Effects of three components of QLD on proliferation, migration, invasion, and adhesion of BGC-823
The previous network pharmacology study showed that thecalycosin in Astragalus membranaceus, diosgenin in Smilaxglabra, and catechins in Agrimoniapilosa may be the material basis of QLD. To verify whether these compounds are bioactive, their effect on proliferation, migration, invasion, and adhesion of BGC-823 were determined. It showed that 100 μg/mL diosgenin could significantly reduce the proliferation of BGC-823, while calycosin and catechin had no notable effect on the proliferation of BGC-823 (Figure 6A). To exclude the influence of cell number, all the following experiments were carried out in 0.01-10 μg/mL. Diosgenin, calycosin, and catechin could all inhibit the migration (Figure 6B) and invasion (Figure 6C) of gastric cancer cells. Both diosgenin and catechin could significantly prevent the adhesion of gastric cancer cells (Figure 6D). Notably, the inhibition of diosgenin was stronger than that of calycosin.
Figure 6.
Effects of three components in QLD on proliferation, migration, invasion, and adhesion of BGC-823. A: Effect of Calycosin, Catechin, and Diosgenin of BGC-823 by MTT assay (mean ± sd, n=4, *P<0.05, **P<0.01 vs. DMSO). B: Effect of Calycosin, Catechin, and Diosgenin on BGC-823 migration. Images of cell migration after treatment with compounds and 0.1% EtOH which was assessed by wound-healing assay in BGC-823 cells (left). Data represent mean ± sd of four duplication rate in 48 hours (middle). The scratch test results of cell migration (right) (magnification: ×200; scale bar: 100 μm; mean ± sd, n=4, *P<0.05, **P<0.01 vs. DMSO or ethanol). C: Representative photos of transwell migration assay and quantitation analysis (magnification: ×200; scale bar: 100 μm; mean ± sd, n=5, *P<0.05 vs. DMSO or ethanol). D: Effect of Calycosin, Catechin and Diosgenin on adhesion of BGC-823 cells (mean ± sd, n=4, *P<0.05, **P<0.01 vs. DMSO or ethanol).
QLD inhibits MMP-9 and affects the PI3K/Akt signaling pathway
Previous studies have shown that MMP-9 can induce the activation of upstream PI3K/Akt signaling, thus mediating the progression of a variety of tumors. Therefore, we detected the proteins of PI3K, AKT, and MMP-9. With the increase of the dosage of QLD, the proteins of PI3K, Akt, and MMP-9 decreased gradually (Figure 7). These results suggested that QLD could reduce the expression of MMP-9 and the mechanism might be related to inhibiting the activation of the PI3K/Akt pathway.
Figure 7.

QLD inhibits MMP-9 and affects the PI3K/AKT pathway. Effect of QLD on protein expression in SGC7901.
Discussion
In recent years, gastric cancer has attracted wide attention due to its high morbidity and mortality [1]. Tumor distant metastasis is the main cause of cancer-related death and suppression of metastasis would ameliorate patient prognosis [23]. Tumor metastasis is regarded with the characteristics of migration, invasion, and adhesion [24]. The metastasis of tumor is an important cause of low survival rate in cancer patients and is studied by trying to inhibit migration.
At present, there are no clear treatment measures to stop the metastasis of tumor, but TCM has provided many ideas. Cancer toxicity theory is one of the most important theories in TCM oncology. Yu Rencun, a famous national TCM doctor, believes that “deficiency syndrome” is the root cause of the occurrence of tumors, and cancer toxicity is developed based on deficiency of qi and of organs [25]. Cancer toxicity consumes qi and blood, and changes from pathologic products into pathogenic factors, forming a vicious circle. Therefore, anti-toxicity is an important part of cancer theory and also a key link in the treatment of malignant tumor. QLD is a traditional Chinese medicine, which takes “detoxification” as its principle. It can effectively reduce the recurrence rate of gastric cancer and improve the quality of life of patients with gastric cancer after operation clinically. Hence, it is significant to discuss the function and mechanism of QLD.
Our results showed that QLD has a significant influence on migration, invasion, and adhesion of gastric cancer cells BGC-823 and SGC7901. It suggests that QLD can inhibit the migration, invasion, and adhesion of gastric cancer cells. Adhesion function reflects the interaction between tumor cells and extracellular matrix, vascular endothelial cells, or parenchymal organ cells. Tumor crossing the basement membrane and invading surrounding tissues needs the ability for invasion. In addition, active mobility is also an important factor for tumor metastasis. Therefore, the effects of QLD on migration, invasion, and adhesion indicate its role in inhibiting tumor metastasis.
To further explore the components that play important roles in inhibiting metastasis in QLD, we performed network pharmacology on QLD. These results showed that the calycosin in Astragalus membranaceus, diosgenin in Smilax glabra, and catechins in Agrimoniapilosa may be the key component of QLD. Diosgenin has shown a vast range of pharmacologic activities in preclinical studies of tumor treatment and has an efffect on tumor invasion, metastasis, and angiogenesis [26,27]. Sahar et al. showed that catechins have an effect on 7,12-dimethylbenzanthracene-induced tumor metastasis, and angiogenesis and cancer stem cells [28]. Zhou observed the effect of “Yiqi Huayu Jiedu” recipe on colorectal cancer metastasis. In this recipe, calycosin is one of the main components, which plays an important role in inhibiting the metastasis of cancer [29]. These three active components have some research basis in the control of tumor metastasis. However, there has been limited information on the mechanisms of the three components for preventing gastric tumor distant metastasis in recent years. In our research, we investigated that diosgenin, catechins, and calycosin may influence migration, invasion, and adhesion of gastric tumor cells. As our results shown, diosgenin could significantly reduce the proliferation of BGC-823. Diosgenin, calycosin, and catechin could all reduce the speed of closure and the number of migrated cells to inhibit the invasion and migration of BGC-823. Both diosgenin and catechin could significantly prevent the adhesion of BGC-823. We demonstrated that the three components are the main active components in QLD that could inhibit the lateral and longitudinal migration of gastric cancer cells.
Recent studies have shown that matrix metalloproteinases (MMPs) play a crucial role in the subsequent invasion and metastasis of tumor cells, and MMP-9 is regarded as one of the progression markers of gastric cancer [30-32]. The most common signaling upstream of MMP-9 is the PI3K/Akt signal pathway, which manages metastasis [33,34]. PI3K/Akt signaling pathway inhibitors have been clinically used in cancer treatment [35]. Thus, we investigated the expression and activation of MMP-9, PI3K, and Akt. The results suggest that QLD could regulate the activation of PI3K and Akt to reduce the expression of MMP-9, and thus inhibit the process of gastric cancer cell migration.
In this study, we demonstrated that QLD can stop tumor metastasis. The mechanism of QLD inhibition on BGC-823 and SGC-7901 cell migration, invasion, and adhesion may be by the inhibition of MMP-9 expression through the PI3K/Akt signaling pathway. As the prominent effect of QLD is proven in treating gastric cancer clinically and theoretically, QLD might serve as a novel treatment in tumor therapy. Additionally, the mechanism of QLD still needs to be further studied, thereby to explore more targets of tumor therapy.
Therefore, traditional Chinese medicine compound QLD inhibits the metastasis of gastric cancer cells through a complex procedure. The mechanism is suggested to be that the main active components (diosgenin, catechins, and calycosin) regulate the expression of target (MMP-9) through the PI3K Akt signaling pathway, and inhibit the invasion, migration, and adhesion of gastric cancer cells.
Acknowledgements
This work was supported by the Science and Technology Development Project of Nanjing (YKK18171).
Disclosure of conflict of interest
None.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Song M, Rabkin CS, Camargo MC. Gastric cancer: an evolving disease. Curr Treat Options Gastroenterol. 2018;16:561–569. doi: 10.1007/s11938-018-0203-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang QS. The role of DNA repair protein Ku80 in the occurrence and development of esophageal cancer. Peking Union Med College. 2008 [Google Scholar]
- 4.Zhong YT, Zhan YH. The mechanism of antitumor effectiveness of dark plum and its compound preparation. Shaanxi J Tradit Chin Med. 2018;39:406–409. [Google Scholar]
- 5.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li L, Li W. Epithelial-mesenchymal transition in human cancer: comprehensive reprogramming of metabolism, epigenetics, and differentiation. Pharmacol Ther. 2015;150:33–46. doi: 10.1016/j.pharmthera.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Bosch-Fortea M, Martín-Belmonte F. Mechanosensitive adhesion complexes in epithelial architecture and cancer onset. Curr Opin Cell Biol. 2018;50:42–49. doi: 10.1016/j.ceb.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 8.Gkountela S, Castro-Giner F, Szczerba BM, Vetter M, Landin J, Scherrer R, Krol I, Scheidmann MC, Beisel C, Stirnimann CU, Kurzeder C, Heinzelmann-Schwarz V, Rochlitz C, Weber WP, Aceto N. Circulating tumor cell clustering shapes DNA methylation to enable metastasis seeding. Cell. 2019;176:98–112. e114. doi: 10.1016/j.cell.2018.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedl P, Locker J, Sahai E, Segall JE. Classifying collective cancer cell invasion. Nat Cell Biol. 2012;14:777–783. doi: 10.1038/ncb2548. [DOI] [PubMed] [Google Scholar]
- 10.Sun CX, Li DG, Liu SJ, Liu JX. Pathogenesis and treatment of Turbid Toxin in gastric cancer. Hebei J Tradit Chin Med. 2011;33:673–674. [Google Scholar]
- 11.Wan CX. Dynamic evolution of TCM syndrome distribution before and after operation based on pathological types and stages of gastric cancer. Beijing Univ Chin Med. 2019 [Google Scholar]
- 12.Sun XL, Wang KY. A review on evolution law of TCM syndromes in patients with postoperative gastrointestinal cancer. Henan Tradit Chin Med. 2019;39:1475–1478. [Google Scholar]
- 13.Wang Z, Dong L, Zhen Y, Wang Y, Qi D, Xu A, Meng X, Li W. Astragalus extract inhibits proliferation but enhances apoptosis in gastric cancer. Pak J Pharm Sci. 2016;29:1473–1482. [PubMed] [Google Scholar]
- 14.Yu J, Ji H, Dong X, Feng Y, Liu A. Apoptosis of human gastric carcinoma MGC-803 cells induced by a novel Astragalus membranaceus polysaccharide via intrinsic mitochondrial pathways. Int J Biol Macromol. 2019;126:811–819. doi: 10.1016/j.ijbiomac.2018.12.268. [DOI] [PubMed] [Google Scholar]
- 15.Auyeung KK, Woo PK, Law PC, Ko JK. Astragalus saponins modulate cell invasiveness and angiogenesis in human gastric adenocarcinoma cells. J Ethnopharmacol. 2012;141:635–641. doi: 10.1016/j.jep.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 16.Guo CX, Liu JB, Zhao Y, Chen SH, Liu Y, Qian J. Atractylode inhibits proliferation and promotes apoptosis in gastric cancer cell line SGC-7901. Chin J Histochem Cytochem. 2017;26:468–474. [Google Scholar]
- 17.Kan X, Zhang W, You R, Niu Y, Guo J, Xue J. Scutellaria barbata D. Don extract inhibits the tumor growth through down-regulating of Treg cells and manipulating Th1/Th17 immune response in hepatoma H22-bearing mice. BMC Complement Altern Med. 2017;17:41. doi: 10.1186/s12906-016-1551-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu HY. SBP(Salvia chinensisBenth. Polysaccharides) inhibits proliferation, migration of human gastric cancer cell line MGC-803 cells and it’s effects on the expression level of interleukin-8 of the cells. Cheng Med Univ. 2013 [Google Scholar]
- 19.Yu CH, Huan FW, Bai XJ. Advances in molecular pharmacognosy of eupolyphagasteleophaga. J Economic Animal. 2018;22:118–121. 124. [Google Scholar]
- 20.Jiang HQ, Zhong WC, Zhu GF. Research progress on antineoplastic effects of woodlouse worm. Hebei J Tradit Chin Med. 2012;34:455–458. [Google Scholar]
- 21.Ye CL, Munyon TP, Huang PP, Li JJ, Jing J, Fang H. Optimization of extraction process of total flavonoids from Agrimonia pilosa by response surface methodology. Food Res Develop. 2017;38:52–57. [Google Scholar]
- 22.Zhao L, Yan X, Shi J, Ren FZ, Liu LH, Sun SP, Shan BE. Ethanol extract of Forsythia suspensa root induces apoptosis of esophageal carcinoma cells via the mitochondrial apoptotic pathway. Mol Med Rep. 2015;11:871–80. doi: 10.3892/mmr.2014.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Félix U, Ramiro AM. Targeting metastasis with snake toxins: molecular mechanisms. Toxins. 2019;9:390. doi: 10.3390/toxins9120390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anborgh PH, Mutrie JC, Tuck AB, Chambers AF. Role of the metastasis-promoting protein osteopontin in the tumour microenvironment. J Cell Mol Med. 2010;14:2037–2044. doi: 10.1111/j.1582-4934.2010.01115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang WJ, Wang XM. A preliminary study on the theory of “internal deficiency” in the treatment of tumor by Yu Renchun. Beijing J Tradit Chin Med. 2011;30:186–188. [Google Scholar]
- 26.Mao ZJ, Tang QJ, Zhang CA, Qin ZF, Pang B, Wei PK, Liu B, Chou YN. Anti-proliferation and anti-invasion effects of diosgenin on gastric cancer BGC-823 cells with HIF-1α shRNAs. Int J Mol Sci. 2012;13:6521–6533. doi: 10.3390/ijms13056521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Y, Tang YM, Yu SL, Han YW, Kou JP, Liu BL, Yu BY. Advances in the pharmacological activities and mechanisms of diosgenin. Chin J Nat Med. 2015;13:578–87. doi: 10.1016/S1875-5364(15)30053-4. [DOI] [PubMed] [Google Scholar]
- 28.Abd El-Rahman SS, Shehab G, Nashaat H. Epigallocatechin-3-gallate: the prospective targeting of cancer stem cells and preventing metastasis of chemically-induced mammary cancer in rats. Am J Med Sci. 2017;354:54–63. doi: 10.1016/j.amjms.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Zhou JY. Study on inhibition of colon cancer metastasis and its mechanism by YiqiHuayuJiedu decoction. Nanjing Univ Chin Med. 2017 [Google Scholar]
- 30.Mao W, Sun Y, Zhang H, Cao L, Wang J, He P. A combined modality of carboplatin and photodynamic therapy suppresses epithelial-mesenchymal transition and matrix metalloproteinase-2 (mmp-2)/mmp-9 expression in hep-2 human laryngeal cancer cells via ros-mediated inhibition of mek/erk signalling pathway. Lasers Med Sci. 2016;31:1697–1705. doi: 10.1007/s10103-016-2040-6. [DOI] [PubMed] [Google Scholar]
- 31.Duffy MJ, Maguire TM, Hill A, McDermott E, O’Higgins N. Metalloproteinases: role in breast carcinogenesis, invasion and metastasis. Breast Cancer Res. 2000;2:252–257. doi: 10.1186/bcr65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu L, Ye Y, Zhu X. MMP-9 secreted by tumor associated macrophages promoted gastric cancer metastasis through a PI3K/AKT/Snail pathway. Biomed Pharmacother. 2019;117:109096. doi: 10.1016/j.biopha.2019.109096. [DOI] [PubMed] [Google Scholar]
- 33.Lee YC, Cheng TH, Lee JS, Chen JH, Liao YC, Fong Y, Wu CH, Shih YW. Nobiletin, a citrus flavonoid, suppresses invasion and migration involving FAK/PI3K/Akt and small GTPase signals in human gastric adenocarcinoma AGS cells. Mol Cell Biochem. 2011;347:103–115. doi: 10.1007/s11010-010-0618-z. [DOI] [PubMed] [Google Scholar]
- 34.Chang F, Lee JT, Navolanic PM, Steelman LS, Shelton JG, Blalock WL, Franklin RA, McCubrey JA. Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: a target for cancer chemotherapy. Leukemia. 2003;17:590–603. doi: 10.1038/sj.leu.2402824. [DOI] [PubMed] [Google Scholar]
- 35.Will M, Qin AC, Toy W, Yao Z, Rodrik-Outmezguine V, Schneider C, Huang X, Monian P, Jiang X, de Stanchina E, Baselga J, Liu N, Chandarlapaty S, Rosen N. Rapid induction of apoptosis by PI3K inhibitors is dependent upon their transient inhibition of RAS-ERK signaling. Cancer Discov. 2014;4:334–347. doi: 10.1158/2159-8290.CD-13-0611. [DOI] [PMC free article] [PubMed] [Google Scholar]