Abstract
Objective: To investigate the regulatory mechanism of sevoflurane-induced neuronal apoptosis through analyzing the expression of glial cell-derived neurotrophic factor (GDNF) mediated by miR-133, sponged by long non-coding RNA (lncRNA) CDKN2B-AS1. Methods: An in vitro cell injury model was established by using different concentrations of sevoflurane and primary hippocampal neurons. Cell proliferation was detected by Cell Counting Kit-8 (CCK-8); caspase-3 and caspase-9 activities were detected by colorimetry, and apoptotic cells were determined by Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay. Fluorescence in situ hybridization (FISH) analysis was used to detect localized expression of CDKN2B antisense RNA 1 (CDKN2B-AS1), and dual-luciferase reporter assay was employed to verify the correlation of CDKN2B-AS1 and miR-133, and of miR-133 and GDNF. The expression of CDKN2B-AS1, miR-133, and GDNF mRNA in the cell injury model were measured by quantitative reverse transcription-polymerase chain reaction (qRT-PCR). Western blot was utilized to detect the expression of GDNF protein in the cell injury model. Results: In the cell injury model, CDKN2B-AS1 was highly expressed in the cytoplasm, and CDKN2B-AS1 and GDNF were downregulated and miR-133 was upregulated as detected by qRT-PCR (all P<0.05). The connections between CDKN2B-AS1 and miR-133, and between miR-133 and GDNF were confirmed. That is, CDKN2B-AS1 regulated the expression of GDNF by adsorbing miR-133 (all P<0.05). In cells treated with sevoflurane, overexpression of CDKN2B-AS1 could inhibit caspase-3 and caspase-9 activities and the degree of apoptosis. miR-133 could partially alleviate the effect of overexpressing CDKN2B-AS1 on cells, and si-GDNF the effect of miR-133 inhibitor (all P<0.05). Conclusion: lncRNA CDKN2B-AS1 can up-regulate the expression of GDNF, inhibit neuronal apoptosis, and ease the toxic effect of sevoflurane on neural cells by acting as a sponge to adsorb miR-133.
Keywords: LncRNA CDKN2B-AS1, miR-133, GDNF, sevoflurane, apoptosis, neurotoxicity
Introduction
Postoperative cognitive dysfunction (POCD) is a common complication after anesthesia. It is a central nervous system injury disease that usually occurs in the elderly, leading to a decline in memory, mobility, cognition and other basic capabilities, prolonged disease course, inconvenience to the patient’s life, and increased medical costs [1,2]. Sevoflurane is a commonly used inhaled anesthetic, and inappropriate inhalation of sevoflurane has been shown to cause cognitive impairment [3]. Studies have found that sevoflurane can lead to neuronal apoptosis in aged rats, to trigger neuronal inflammation, brain damage, and further evoke POCD [4]. Liu et al. demonstrated that sevoflurane can inhibit the activity of the AMPK-SIRT1 pathway, thereby inducing neuronal apoptosis and POCD [5].
Long non-coding RNAs (lncRNA) have been the target over the years. Aberrant lncRNAs have also been found in POCD rats; for example, dexmedetomidine can target Npas4 to regulate POCD in rats by regulating LOC102546895 [6]. Chen et al. found that the development of POCD is very likely to be associated with downregulation of lncRNA PCAI expression [7]. LncRNA CDKN2B Antisense RNA 1 (CDKN2B-AS1), also known as ANRIL, is located at chr9:21, 994, 139-22, 239, 122 (GRCh38/hg38), and downregulation of this gene has been found to be associated with stroke [8]. Sevoflurane can downregulate ANRIL in a concentration-dependent manner [9]. However, the specific mechanism by which lncRNA CDKN2B-AS1 regulates sevoflurane-induced POCD remains unclear, and will be further explored in this study.
By searching RNA22 website (https://cm.jefferson.edu/rna22/), we found that miR-133 may act as a downstream target of CDKN2B-AS1. Once expressed in the cytoplasm, lncRNAs have the potential to competitively adsorb microRNAs and regulate the expression of microRNAs, thus affecting biologic functions [10]. It has been demonstrated that inhibition of miR-133a expression can attenuate sevoflurane-induced neuronal apoptosis [11]. The downstream target gene of miR-133, glial cell line derived neurotrophic factor (GDNF), was found and included by searching the Targetscan website and using a dual-luciferase reporter assay. Studies have reported that enhanced GDNF expression can improve cognitive function in mice [12].
In this study, we will investigate the protective role of lncRNA CDKN2B-AS1 sponging miR-133 to regulate GDNF expression in sevoflurane-induced neuronal cell injury.
Materials and methods
Culturing hippocampal neurons
Thirty Wistar rats of either sex weighing 16.25±2.14 g within 48 hours of birth were obtained from the Laboratory Animal Center of Southern Medical University. The rats were sacrificed under aseptic conditions. Then the scalp and skull were cut open, and the brain tissue was exposed. After that, the hippocampus was dissected with the help of a microscope and ophthalmic toothless forceps, placed in anatomic solution for cleaning and mincing, and digested with 0.125% trypsin (Procell, China) at 37°C for 20 min. Subsequently, the supernatant was removed, centrifuged, and resuspended, and the cells were collected and inoculated into a Poly-D-Lysine 96-well plate with a density of 1*106 cells/mL. A total of 0.15 mL suspension was added to each well, allowed to stand in an incubator containing 5% CO2 at 37°C for 24 h, and removed into the culture medium without fetal bovine serum (FBS). Then 2% cytarabine (Shanghai Kanglang Biotechnology Co., Ltd., China) was added after 72 h of culture, and the medium was changed every 3 days. After 7 days, the purity of hippocampal neurons was measured. Neuronal cell markers CD24 and tyrosine hydroxylase (TH) were detected by flow cytometry, and cells with a positive rate of more than 95% were included in subsequent experiments [13]. This study was approved by the local hospital Ethics Committee.
Establishing sevoflurane model
Hippocampal cells were placed in a self-made anesthesia glass box, and then the box was placed at a constant temperature of about 37°C in a water bath box with a hole on both sides, of which one hole was connected to the anesthesia machine (Nanjing Beden Medical Co., Ltd., China) to regulate the concentration of sevoflurane (Shanghai Aladdin Biochemical Technology Co., Ltd., China) (1%, 2%, 4%), and the other one was connected to anesthetic gas monitor (Shanghai Draeger Medical Instrument Co., Ltd., China) to monitor the concentration of sevoflurane. Both treatments were continued for 6 hours, while the control group was given inhaled air.
Grouping and transfection
The lncRNA CDKN2B-AS1 overexpression vector, miR-133 mimics or inhibitor, and siRNA vectors for silencing GDNF were all provided by Shanghai GenePharma Co., Ltd. (China). Groups include NC (negative control), CDKN2B-AS1 (transfection of overexpression plasmid), CDKN2B-AS1 + miR-NC (transfection of overexpression plasmid + miRNA negative control), CDKN2B-AS1 + miR-133 (transfection of overexpression plasmid + miR-133 mimics), and miR-133 inhibitor (transfection of miR-133 inhibitor). When the cells were cultured to 80% confluence, the designed plasmids were transfected using Lipofectamine 3,000 transfection reagent (Thermo Fisher Scientific, China) according to the manufacturer’s instructions, followed by corresponding experiments 48 hours later.
qRT-PCR
Total RNA was extracted using TRIzol reagent kit (Thermo Fisher Scientific, USA), and the purity of total RNA was evaluated using a spectrophotometer; cDNA was synthesized using a reverse transcription kit (Shanghai Yisheng Biotechnology Co., Ltd., China); quantitative reverse transcription-polymerase chain reaction (qRT-PCR) was detected using Brilliant II SYBR ROX qRT-PCR premixed solution (Agilent Technologies, USA); PCR-grade water free of nuclease, premixed solution, primers and probes were added into sample solution and gently mixed, followed by the transfer into a polymerase chain reaction (PCR) tube. Altogether, 400 ng RNA was added, briefly centrifuged, and placed into the qRT-PCR instrument, with reactions set at 50°C for 30 min, 95°C for 10 min, 95°C for 15 s, and 60°C for 1 min. All steps were performed according to kit instructions. The results were calculated using a relative quantification (2-ΔΔCt) method and with GAPDH as an internal reference for CDKN2B-AS1, and GDNF and U6 for miR-133. Primers were designed and synthesized with the assistance of Shanghai GenePharma Co., Ltd. (China), and the sequences are shown in Table 1.
Table 1.
List of sequencing primers
| Gene | Primer Sequences (5’-3’) upstream | Primer Sequences (5’-3’) downstream |
|---|---|---|
| CDKN2B-AS1 | AGGAGGCTGAATGTCAGTTTT | AGCGGTTTAGTTTAATTTCGCTT |
| miR-133 | TTTGGTCCCCTTCAACC | GAGCAGGGTCCGAGGT |
| GDNF | CGAACTCGTGCCCCTAACC | AGACCCCCAGCTGGACATTA |
| GAPDH | GCGAGATCGCACTCATCATCT | TCAGTGGTGGACCTGACC |
| U6 | CTCGCTTCGGCAGCACA | AACGCTTCACGAATTTGCGT |
Note: CDKN2B-AS1: CDKN2B Antisense RNA 1; GDNF: glial cell-derived neurotrophic factor.
FISH
Fluorescence in situ hybridization (FISH) kit (Guangzhou Boxin Biotechnology Co., Ltd., China) was used. Cells were fixed in paraformaldehyde (Shanghai Beyotime Biotechnology Co., Ltd., China) for 30 min at room temperature and washed twice with 0.1% diethyl pyrocarbonate (DEPC)/PBS, 5 min/time. Proteinase K mixture (Beijing Solarbio Science & Technology Co., Ltd., China) was dripped into the sample, and incubated by covering the membrane for an even distribution for 10 min, followed by continued rinsing with PBS twice at room temperature, 5 min/time. Another fixation with paraformaldehyde, and washing with PBS were performed, and then sequential washing with precooled gradient alcohol was conducted, 5 min/time. Afterwards, 15 μL of the prehybridization solution was added dropwise to the sample and evenly incubated at 37°C, then the hybridization reaction solution was added dropwise, and a membrane was covered to allow an uniform contact with incubation overnight at 42°C. After that, the samples were washed twice at 53°C using 50% formamide (Sigma, USA), 5 min each time (2*SSC at 42°C for 5 min, and 0.5*SSC at 42°C for 5 min). Then 15 μL of 4’, 6-diamidino-2-phenylindole (DAPI) staining solution (Shanghai Beyotime Biotechnology Co., Ltd., China) was dripped into the samples, kept in a dark room for reaction for 10 min, washed with PBS (three times, 5 min each), dried, mounted, and observed under a confocal microscope (Olympus Sales & Service Co., Ltd., Beijing, China) for staining results.
Dual-luciferase reporter assay
Wild-type or mutant 3’UTR containing CDKN2B-AS1/GDNF with binding sites for miR-133 were amplified and cloned into pGL3 vector (Wuhan Miaoling Bioscience & Technology Co., Ltd, China) to construct CDKN2B-AS1/GDNF-WT and CDKN2B-AS1/GDNF-MUT plasmids, which were co-transfected into 293T cells with miR-133 NC and miR-133 mimics using Lipofectamine 3,000 transfection reagent (Thermo Fisher Scientific, China), and luciferase activity in the cells was detected using a dual-luciferase reporter assay. Transfection and detection procedures were performed according to the manufacturer’s instructions of the kit (Shanghai Yisheng Biotechnology Co., Ltd., China).
CCK8
Hippocampal neuronal cells were seeded in a 96-well plate at 5×103 cells/well, and the wells containing no cells were set as blank control. The plate was placed in an incubator, and CCK8 solution (Shanghai Omer Biotechnology Co., Ltd., China) was added at 0 h, 24 h, 48 h, and 72 h of culture. The absorbance at 450 nm was measured by an enzyme-labeled analyzer (NanJing DeTie Laboratory Equipment Co., Ltd., China) after 2 hours of culture.
Detection of caspase-3 and caspase-9 activities
The experiments were completed using caspase-3 and caspase-9 activity assay kits (Beijing Solarbio Science & Technology Co., Ltd., China). Hippocampal neuronal cells were first centrifuged for 5 min. The supernatant was discarded and the samples were washed with PBS, and then lysed by adding lysis solution on ice with sufficient shaking. The activities of caspase-3 and caspase-9 were measured using a colorimetric assay as indicated in the kit instructions, and the absorbance was evaluated at 405 nm using a spectrophotometer.
TUNEL staining
The experiment was performed using Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) apoptosis detection kit (Shanghai Sangon Biological Engineering Technology & Services Co, Ltd., China). Cells were implanted on a 96-well plate, 5×104 cells per well, and 100 μL 4% paraformaldehyde was added to each well for fixation. Then the plate was let to stand for reaction at room temperature for 20 min. The paraformaldehyde buffer was sucked off, discarded, and washed with PBS. Subsequently, PBS containing 0.01% Triton was added for 2 min in an ice bath. After washing with PBS, the reaction solution (0.5 μL 100X TF3-dUTP + 50 μL reaction buffer) was added to the wells, and the reaction was performed at 37°C for 1 hour in the dark. The reaction solution was discarded, washed with PBS and mounted using antifade mounting medium, and observed for fluorescence intensity at 590 nm on a flow cytometer (Agilent Biotechnology Co., Ltd., USA).
Western blot
RIPA lysate (Beijing Solarbio Science & Technology Co., Ltd., China) was used to fully lyse hippocampal neuronal cells for isolated proteins, and BCA protein assay kit (Nanjing BioChannel Biotechnology Co., Ltd, China) was used to quantitatively detect proteins, which were added to 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis for separation at a voltage of 120 V for 2 h and then transferred onto polyvinylidene difluoride membranes (Thermo Fisher Scientific, USA). Membranes were blocked after 1 h, then GDNF and GAPDH primary antibodies (1:2000 and 1:10000, respectively; ABCAM, UK) were added for overnight incubation at a reaction temperature of 4°C. Catalase labeled goat anti-rabbit IgG secondary antibody (1:1000; ABCAM, UK) was added for another 2-hour incubation. Finally, enhanced chemiluminescence (ECL) substrate (Shanghai Yisheng Bio-Technology Co., Ltd., China) was added and analyzed on the Fluor-Chem M system, and the relative protein expression was described as the ratio of the gray value of the target protein to that of the internal reference protein.
Statistical analysis
SPSS 25.0 was applied to analyze the data, and the results were expressed in the form of mean ± standard deviation. An independent samples t-test was utilized for comparison between two groups. Comparison of more than two groups was conducted by one-way analysis of variance. Bonferonni method was employed for multiple pairwise comparison among groups. Differences were considered significant if P<0.05.
Results
Detection of lncRNA CDKN2B-AS1 expression
An analysis was first performed in GeneCards (https://www.genecards.org/) to find the specific location of lncRNA CDKN2B-AS1 on the chromosome (Figure 1A), and CDKN2B-AS1 was examined for localized expression by FISH experiments, which revealed that the cytoplasm contains more CDKN2B-AS1 (Figure 1B). Hippocampal neuronal cells were treated with different concentrations of sevoflurane, and then the expression of CDKN2B-AS1 in the cells was detected by qRT-PCR. The results showed that the higher the concentration of sevoflurane, the lower the expression of CDKN2B-AS1, with the lowest in the 4% sevoflurane group (Figure 1C, all P<0.05).
Figure 1.
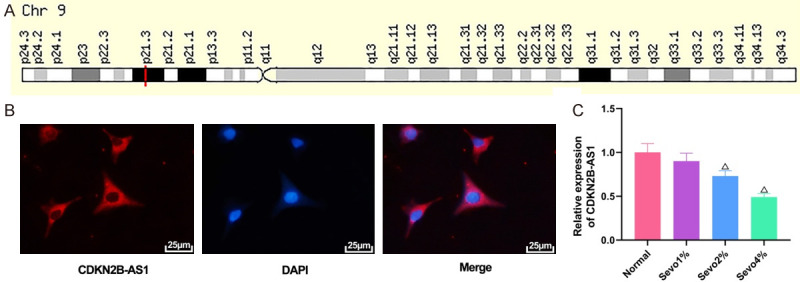
Expression of CDKN2B-AS1 by qRT-PCR. A: Location of CDKN2B-AS1 on the chromosome; B: FISH experimental plot (×400); C: Expression of CDKN2B-AS1 in cells treated with different concentrations of sevoflurane. Compared with normal group, ΔP<0.05. CDKN2B-AS1: CDKN2B antisense RNA 1; qRT-PCR: quantitative reverse transcription-polymerase chain reaction; FISH: fluorescence in situ hybridization.
Protection by CDKN2B-AS1 overexpression for neuronal cell injury induced by sevoflurane
The CDKN2B-AS1 overexpression plasmid was constructed and transfected into cells to observe the biologic characteristics of CDKN2B-AS1. Transfection efficiency was first examined, and qRT-PCR experiments showed that transfection of CDKN2B-AS1 overexpression plasmid in 4% sevofluorane (Sevo 4%) treated cells could elevate CDKN2B-AS1 expression (Figure 2A, P=0.0008). Compared with the NC group of cells transfected with an empty plasmid, the proliferation of cells treated with Sevo 4% was inhibited, while such proliferation could be enhanced after the addition of CDKN2B-AS1 overexpression compared with the Sevo 4% + NC group (Figure 2B, P<0.05). In colorimetric and TUNEL staining experiments, it was found that sevofluorane with a concentration of 4% could promote caspase-3 and caspase-9 activities and increase the apoptosis rate in hippocampal neuronal cells compared with the NC group, while Sevo 4% plus CDKN2B-AS1 could reduce caspase-3 and caspase-9 activities and the apoptosis rate compared with the Sevo 4% + NC group (Figure 2C and 2D, all P<0.05). These results indicate that CDKN2B-AS1 can protect hippocampal neuronal cells.
Figure 2.
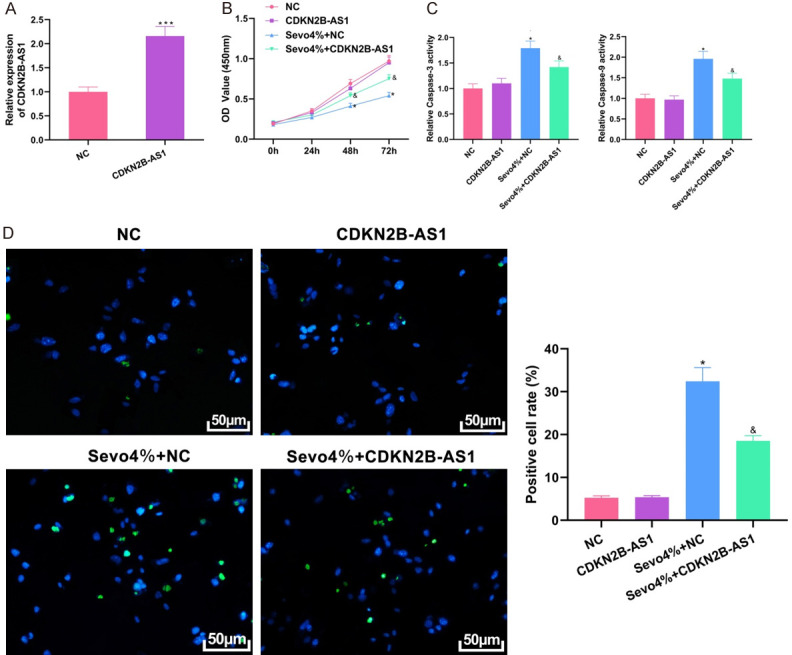
Protective effect of CDKN2B-AS1 on hippocampal neuronal cells. A: Transfection efficiency of overexpression plasmid by qRT-PCR; B: Evaluation of cell proliferation by CCK8; C: Colorimetric assay results; D: TUNEL staining results (×200). Compared with NC group, *P<0.05, ***P<0.001; compared with Sevo 4% + NC group, &P<0.05. CDKN2B-AS1: CDKN2B Antisense RNA 1; qRT-PCR: quantitative reverse transcription-polymerase chain reaction; TUNEL: transferase dUTP nick end labeling.
MiR-133 as a downstream target of CDKN2B-AS1
At the RNA22 prediction website (https://cm.jefferson.edu/rna22/), miR-133, a downstream target of CDKN2B-AS1, which has complementary base sequences (Figure 3A), was searched. Then a dual-luciferase reporter assay was designed in accordance with the sequence. The results showed that co-transfection of miR-133 mimic plus CDKN2B-AS1-WT could reduce luciferase activity compared with that of miR-133 mimic plus CDKN2B-AS1-MUT (Figure 3B, P<0.05). Subsequently, the expression level of miR-133 in hippocampal neuronal cells treated with different concentrations of sevoflurane was determined and the results demonstrated that the expression of miR-133 was upregulated with increasing sevoflurane concentration, with the highest in Sevo 4% (Figure 3C, all P<0.05). Then upregulation of the expression of CDKN2B-AS1 in cells exhibited an inhibitory effect on the expression level of miR-133 (Figure 3D, P<0.05). The above experiments indicate that miR-133 is a downstream target of CDKN2B-AS1, that can negatively regulate miR-133.
Figure 3.
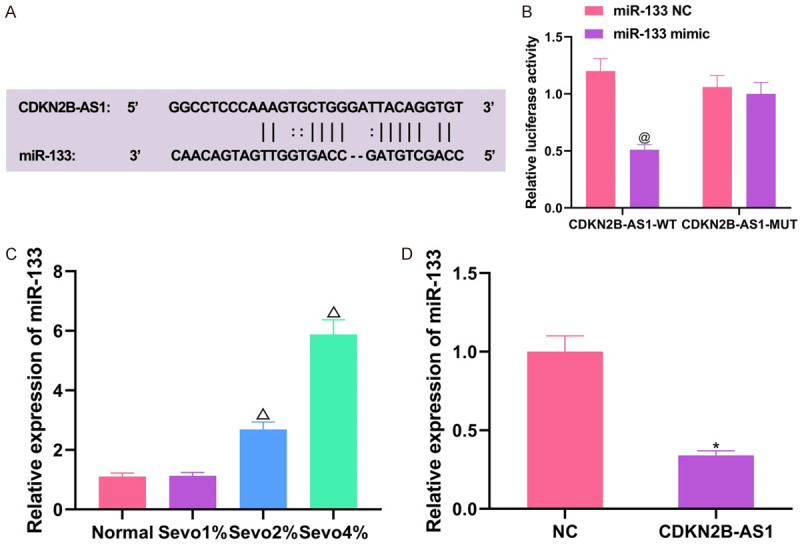
Validation of association between CDKN2B-AS1 and miR-133. A: Specific binding site of CDKN2B-AS1 to miR-133; B: Dual-luciferase reporter assay results; C: Expression of miR-133 in cells treated with different concentrations of sevoflurane; D: Influences of the expression of miR-133 by CDKN2B-AS1. Compared with miR-133-NC + CDKN2B-AS1-WT co-transfection groups, @P<0.05; compared with normal group, ΔP<0.05; compared with NC group, *P<0.05. CDKN2B-AS1: CDKN2B Antisense RNA 1.
Partial reversing of the protective effect by miR-133 for CDKN2B-AS1 on cells
Through CCK8, colorimetric assay, and TUNEL staining experiments, it was found that overexpression of CDKN2B-AS1 could protect hippocampal neuronal cells by promoting cell proliferation, inhibiting caspase-3 and caspase-9 expression, and reducing apoptosis of cells treated with 4% sevoflurane (all P<0.05). After the addition of miR-133, the proliferation of cells was inhibited and the apoptotic rate was promoted (all P<0.05), and the protective effect of CDKN2B-AS1 on cells was partially inhibited by miR-133 (Figure 4).
Figure 4.
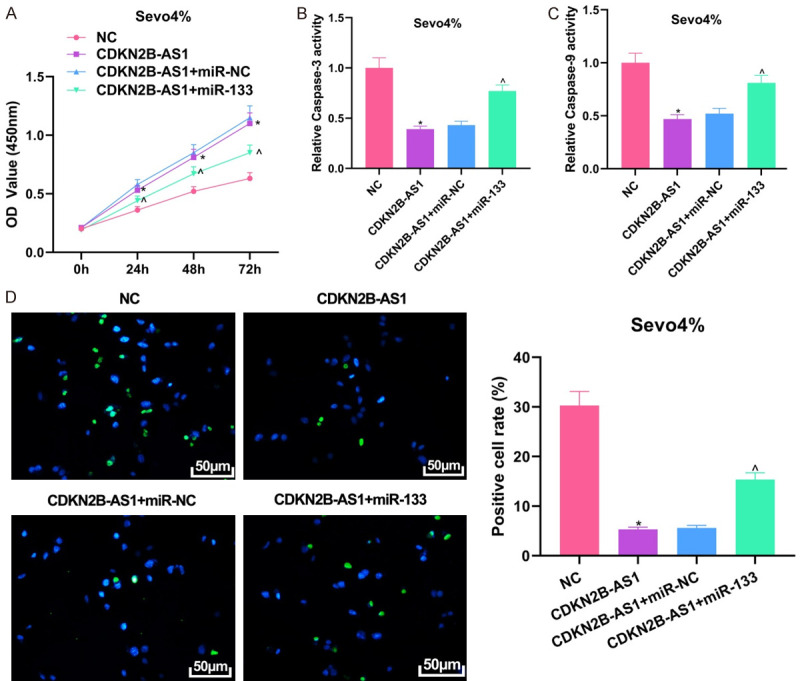
Partial reversal of the protective effect of miR-133 for CDKN2B-AS1 on hippocampal neuronal cells. A: Plasmid transfection efficiency by qRT-PCR; B: CCK8 assessment of cell proliferation ability; C: Colorimetric assay result; D: TUNEL staining result (×200). Compared with NC group, *P<0.05; compared with CDKN2B-AS1 + miR-NC group, ^P<0.05. CDKN2B-AS1: CDKN2B Antisense RNA 1; qRT-PCR: quantitative reverse transcription-polymerase chain reaction; TUNEL: transferase dUTP nick end labeling.
Negative regulation of miR-133 for GDNF
TargetscanHuman7.2 (http://www.targetscan.org/vert_72/), the online prediction tool showed that there is a specific complementary base sequence between miR-133 and GDNF (Figure 5A), and dual-luciferase reporter assay based on the sequence further confirmed that GDNF is a downstream target gene of miR-133 (Figure 5B). Overexpression of miR-133 in cells showed that both mRNA and GDNF proteins could be downregulated, while downregulation of miR-133 yielded opposite results (Figure 5C-E, all P<0.05). Detection of GDNF expression in sevoflurane-treated cells revealed that GDNF mRNA and protein expression levels decreased dependently with increasing sevoflurane concentration (Figure 5F-H, P<0.05). These experimental results illustrate that GDNF is a downstream target gene of miR-133 and is negatively regulated by miR-133, suggesting that CDKN2B-AS1/miR-133 effect on sevoflurane-induced neuronal apoptosis may be achieved by the regulation of GDNF.
Figure 5.
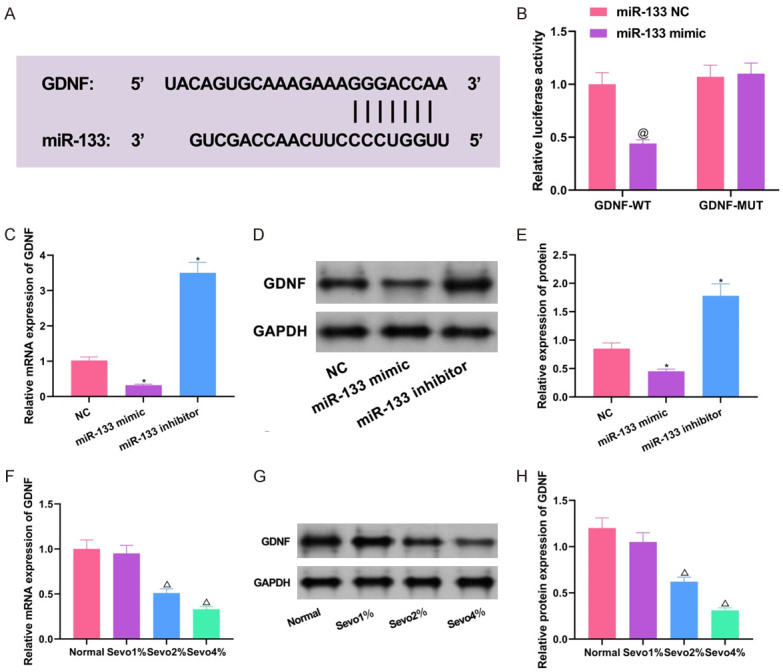
Validation of the association between miR-133 and GDNF. A: Specific binding sites of miR-133 and GDNF; B: Dual-luciferase reporter assay results; C: qRT-PCR detection of the regulation of GDNF mRNA expression by miR-133; D, E: Western blot detection of the regulation of GDNF protein expression by miR-133; F: GDNF mRNA expression in cells treated with different concentrations of sevoflurane; G, H: GDNF protein expression in cells treated with different concentrations of sevoflurane. Compared with co-transfection of miR-133-NC + GDNF-WT group, @P<0.05; compared with normal group, ΔP<0.05; compared with NC group, *P<0.05. GDNF: glial cell-derived neurotrophic factor; qRT-PCR: quantitative reverse transcription-polymerase chain reaction.
Discussion
The onset of POCD is highly related to age. With the aging of population, the incidence of POCD is also increasing, especially the risk of death in the first year after surgery, thus POCD has begun to attract much attention. However, the pathogenesis of POCD has not yet been clarified. Studies have found that POCD may be associated with oxidative stress damage, nerve regeneration, and so on [14]. LncRNAs play a key role in regulating cellular inflammation, proliferation and apoptosis. In the study by Cheng et al., for example, it was pointed out that lncRNA TUG1 can promote an inflammatory response in parkinsonian microglia [15]. It was also highlighted by Zhang et al. that lncRNA MEG3 can reduce apoptosis of hippocampal neurons by regulating the PI3K/AKT/mTOR pathway [16]. LncRNA CDKN2B-AS1 is a newly discovered lncRNA that not only plays a role in cancer, but also in brain diseases [17,18]. It has been demonstrated that sevoflurane can directly cause cognitive dysfunction in aged rats [19]. Therefore, in this study, sevoflurane was used to induce neurotoxicity in hippocampal neuronal cells to investigate the effect of lncRNA CDKN2B-AS1 on neuronal cells.
Previous studies have shown that sevoflurane is able to promote neuronal apoptosis, and result in cognitive impairment [20]. Through our current study, it was found that neurotoxicity was greatly increased, the degree of apoptosis was elevated, and the expression of CDKN2B-AS1 was decreased in sevoflurane-treated hippocampal neuronal cells. However, these changes were improved after overexpression of CDKN2B-AS1 in the cells; that is, the anesthetic neurotoxicity of hippocampal neuronal cells was alleviated, cell proliferation was increased, and the cells were effectively treated. This suggests that CDKN2B-AS1 plays a potential neuroprotective role in POCD. While the mechanism of action of CDKN2B-AS1 in cells is varied, and mainly depends on its location. For example, it demonstrates its biologic characteristics through the regulation of microRNAs once present in the cytoplasm [21]. Through experiments, we found that CDKN2B-AS1 is mainly expressed in the cytoplasm and can adsorb microRNAs to regulate mRNAs, which is also the most discussed mechanism. In this study, we searched for downstream factors that can make CDKN2B-AS1 serve as a sponge at a biologic prediction website and found that miR-133 is a likely downstream target of CDKN2B-AS1, with specific base complementary sequences. We then designed dual-luciferase experiments to verify this prediction.
MicroRNA is also a class of non-coding RNAs. It has about 20 nucleotide units, which can further regulate mRNAs or signaling pathways to produce biologic effects [22]. miR-133 is also involved in the development of a variety of diseases, and its increased expression in the serum can be used as a marker for the diagnosis of acute cerebral infarction [23]. It has also been reported that downregulation of miR-133a expression can inhibit sevoflurane-induced neuronal apoptosis and inflammatory response by regulating the expression of Sox4 protein [11]. In this study, miR-133 expression was found to be elevated in sevoflurane-induced hippocampal neuronal cells, and it partially reversed the protective effect of CDKN2B-AS1 on neuronal cells. Bioinformatic analysis of miR-133-regulated downstream target genes, and the presence of binding sites between GDNF and miR-133 suggested that GDNF mRNA is likely to be a downstream target gene of miR-133, which was confirmed using dual-luciferase reporter assays. In addition, qRT-PCR and western blot experiments also verified that miR-133 regulated the expression of GDNF. It has been shown that the mechanism of stem cell therapy for Parkinson’s disease lies in the fact that stem cells can increase neurotrophic factors in the striatum, such as GDNF and BDNF, inhibit apoptosis of neurons, and restore the number of neurons [24]. There is a correlation between high expression of GDNF and enhanced neurocognitive function [25]. Yun et al. also revealed the mechanism of GDNF in neuroprotection in the hippocampus [26]. It has also been shown that Cnidium monnieri can inhibit neuronal apoptosis and improve cognitive impairment in mice by increasing the expression of GDNF [12]. Therefore, it is likely that GDNF is also a factor that plays a protective role for neuronal cells. In our experiments, GDNF was underexpressed in sevoflurane-treated neuronal cells, and was regulated by miR-133, so we hypothesize that the regulation of sevoflurane-induced neuronal apoptosis by CDKN2B-AS1 and miR-133 may be achieved by the regulation of GDNF.
However, we merely discussed CDKN2B-AS1 in detail. The protective effect of GDNF has not been thoroughly investigated, and the absence of in vivo animal experiments is also a limitation of this study. In summary, the expression of CDKN2B-AS1 was downregulated in sevoflurane-treated neuronal cells, and CDKN2B-AS1 could reduce sevoflurane-treated apoptosis and promote cell proliferation. Through experimental analysis, it can be seen that CDKN2B-AS1 may act as a sponge for miR-133 and up-regulate the expression of GDNF. This provides a new molecular pathway for exploring the mechanism of CDKN2B-AS1.
Acknowledgements
This work was supported by Regional Science Fund Project for The role and mechanism of Klotho mediated NF-kappa B and FGF23 signaling pathway to regulate the invasion of POCD macrophages (81860211).
Disclosure of conflict of interest
None.
References
- 1.Zhong J, Li J, Miao C, Zuo Z. A novel individual-based determination of postoperative cognitive dysfunction in mice. Aging Dis. 2020;11:1133–1145. doi: 10.14336/AD.2019.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ding L, Chen DX, Li Q. Effects of electroencephalography and regional cerebral oxygen saturation monitoring on perioperative neurocognitive disorders: a systematic review and meta-analysis. BMC Anesthesiol. 2020;20:254. doi: 10.1186/s12871-020-01163-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chauhan R, Panda N, Bhagat H, Bharti N, Luthra A, Soni SL, Kaloria N, Salunke P, Bhaire V, Bloria SD. Comparison of propofol and sevoflurane on cerebral oxygenation using juglar venous oximetery (SjVo(2)) in patients undergoing surgery for traumatic brain injury. Asian J Neurosurg. 2020;15:614–619. doi: 10.4103/ajns.AJNS_348_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu L, Liu C, Fang L. AMPK-SIRT1 pathway dysfunction contributes to neuron apoptosis and cognitive impairment induced by sevoflurane. Mol Med Rep. 2021;23:56. doi: 10.3892/mmr.2020.11694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang LH, Xu YC, Zhang W. Neuroprotective effect of CTRP3 overexpression against sevoflurane anesthesia-induced cognitive dysfunction in aged rats through activating AMPK/SIRT1 and PI3K/AKT signaling pathways. Eur Rev Med Pharmacol Sci. 2020;24:5091–5100. doi: 10.26355/eurrev_202005_21202. [DOI] [PubMed] [Google Scholar]
- 6.Deng F, Cai L, Zhou B, Zhou Z, Xu G. Whole transcriptome sequencing reveals dexmedetomidine-improves postoperative cognitive dysfunction in rats via modulating lncRNA. 3 Biotech. 2020;10:202. doi: 10.1007/s13205-020-02190-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y, Zhang Y, Ye G, Sheng C, Kong L, Yuan L. Knockdown of lncRNA PCAI protects against cognitive decline induced by hippocampal neuroinflammation via regulating SUZ12. Life Sci. 2020;253:117626. doi: 10.1016/j.lfs.2020.117626. [DOI] [PubMed] [Google Scholar]
- 8.da Silva CF, Schwartz J, Belli VDS, Ferreira LE, Cabral NL, França PHC. Ischemic stroke and genetic variants: in search of association with severity and recurrence in a brazilian population. J Stroke Cerebrovasc Dis. 2020;29:104487. doi: 10.1016/j.jstrokecerebrovasdis.2019.104487. [DOI] [PubMed] [Google Scholar]
- 9.Gao Y, Ma H, Hou D. Sevoflurane represses proliferation and migration of glioma cells by regulating the ANRIL/let-7b-5p axis. Cancer Biother Radiopharm. 2020:19. doi: 10.1089/cbr.2020.3596. [DOI] [PubMed] [Google Scholar]
- 10.Zhang S, Wan H, Zhang X. LncRNA LHFPL3-AS1 contributes to tumorigenesis of melanoma stem cells via the miR-181a-5p/BCL2 pathway. Cell Death Dis. 2020;11:950. doi: 10.1038/s41419-020-03141-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao Y, Ai Y. Overexpression of lncRNA Gm15621 alleviates apoptosis and inflammation response resulting from sevoflurane treatment through inhibiting miR-133a/Sox4. J Cell Physiol. 2020;235:957–965. doi: 10.1002/jcp.29011. [DOI] [PubMed] [Google Scholar]
- 12.Xiao H, Wang Y, Wu Y, Li H, Liang X, Lin Y, Kong L, Ni Y, Deng Y, Li Y, Li W, Yang J. Osthole ameliorates cognitive impairments via augmenting neuronal population in APP/PS1 transgenic mice. Neurosci Res. 2021;164:33–45. doi: 10.1016/j.neures.2020.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Monaco S, Baur K, Hellwig A, Hölzl-Wenig G, Mandl C, Ciccolini F. A flow cytometry-based approach for the isolation and characterization of neural stem cell primary cilia. Front Cell Neurosci. 2018;12:519. doi: 10.3389/fncel.2018.00519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang CX, Bao F, Zhong J, Zhang L, Deng LB, Sha Q, Jiang H. The inhibitory effects of class I histone deacetylases on hippocampal neuroinflammatory regulation in aging mice with postoperative cognitive dysfunction. Eur Rev Med Pharmacol Sci. 2020;24:10194–10202. doi: 10.26355/eurrev_202010_23240. [DOI] [PubMed] [Google Scholar]
- 15.Cheng J, Duan Y, Zhang F, Shi J, Li H, Wang F, Li H. The role of lncRNA TUG1 in the parkinson disease and its effect on microglial inflammatory response. Neuromolecular Med. 2021;23:327–334. doi: 10.1007/s12017-020-08626-y. [DOI] [PubMed] [Google Scholar]
- 16.Zhang H, Tao J, Zhang S, Lv X. LncRNA MEG3 reduces hippocampal neuron apoptosis via the PI3K/AKT/mTOR pathway in a rat model of temporal lobe epilepsy. Neuropsychiatr Dis Treat. 2020;16:2519–2528. doi: 10.2147/NDT.S270614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dasgupta P, Kulkarni P, Majid S, Hashimoto Y, Shiina M, Shahryari V, Bhat NS, Tabatabai L, Yamamura S, Saini S, Tanaka Y, Dahiya R. LncRNA CDKN2B-AS1/miR-141/cyclin D network regulates tumor progression and metastasis of renal cell carcinoma. Cell Death Dis. 2020;11:660. doi: 10.1038/s41419-020-02877-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song C, Qi Y, Zhang J, Guo C, Yuan C. CDKN2B-AS1: an indispensable long non-coding RNA in multiple diseases. Curr Pharm Des. 2020;26:5335–5346. doi: 10.2174/1381612826666200806102424. [DOI] [PubMed] [Google Scholar]
- 19.Chen Y, Zhang P, Lin X, Zhang H, Miao J, Zhou Y, Chen G. Mitophagy impairment is involved in sevoflurane-induced cognitive dysfunction in aged rats. Aging (Albany NY) 2020;12:17235–17256. doi: 10.18632/aging.103673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lv G, Li C, Wang W, Li N, Wang K. Silencing SP1 alleviated sevoflurane-induced POCD development via cholinergic anti-inflammatory pathway. Neurochem Res. 2020;45:2082–2090. doi: 10.1007/s11064-020-03070-7. [DOI] [PubMed] [Google Scholar]
- 21.Xu C, Zhai J, Fu Y. LncRNA CDKN2B-AS1 promotes the progression of ovarian cancer by miR-143-3p/SMAD3 axis and predicts a poor prognosis. Neoplasma. 2020;67:782–793. doi: 10.4149/neo_2020_190617N515. [DOI] [PubMed] [Google Scholar]
- 22.Zhang T, Ma S, Liu C, Hu K, Xu M, Wang R. Rosmarinic acid prevents radiation-induced pulmonary fibrosis through attenuation of ROS/MYPT1/TGFβ1 signaling via miR-19b-3p. Dose Response. 2020;18:1559325820968413. doi: 10.1177/1559325820968413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu P, Xin J, Song L, Chen Y, Ma J, Liu L, Qi Z, Pan X, Zhou S. Serum miR-133 as a potential biomarker in acute cerebral infarction patients. Clin Lab. 2020;66 doi: 10.7754/Clin.Lab.2019.190933. [DOI] [PubMed] [Google Scholar]
- 24.Park H, Chang KA. Therapeutic potential of repeated intravenous transplantation of human adipose-derived stem cells in subchronic MPTP-induced Parkinson’s disease mouse model. Int J Mol Sci. 2020;21:8129. doi: 10.3390/ijms21218129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang X, Zhou C, Gao J, Duan W, Yu M, Xiao W, Zhang X, Dong H, Wang X, Zhang X. Serum BDNF and GDNF in Chinese male patients with deficit schizophrenia and their relationships with neurocognitive dysfunction. BMC Psychiatry. 2019;19:254. doi: 10.1186/s12888-019-2231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yun D, Jeon MT, Kim HJ, Moon GJ, Lee S, Ha CM, Shin M, Kim SR. Induction of GDNF and GFRα-1 following AAV1-Rheb(S16H) administration in the hippocampus in vivo. Exp Neurobiol. 2020;29:164–175. doi: 10.5607/en19075. [DOI] [PMC free article] [PubMed] [Google Scholar]


