Abstract
Objective: To study the clinical characteristics, changes in relevant test parameters, time of nucleic acid negative conversion, and effect of glucocorticoid treatment in Wuhan area patients with the novel coronavirus pneumonia (COVID-19). Methods: Data of 173 inpatients at Huoshenshan Hospital from February 10 to March 17, 2020, were analyzed retrospectively. Clinical characteristics, partial test results, and the influence of glucocorticoid therapy on the clinical outcomes of nucleic acid negative conversion and changes in lung CT images were compared. The patients were divided at admission into 4 groups according to the course of disease and glucocorticoid treatment. Differences among the groups were analyzed statistically. Results: The median age of 173 patients was 62 years, and 91.3% were over 40 years old. Underlying diseases occurred in 50.3% of patients, 32.6% had family gatherings, and 24.3% had exposure while shopping or at a hospital. Median times of nucleic acid negative conversion in group A+B (course of disease < 3 weeks) and group C+D (course of disease ≥ 3 weeks) were 23 days and 37 days, respectively (P < 0.05). Other group comparisons, i.e., of A+C with B+D, A with B, or C with D, were not statistically different. One week after reexamination, chest CT lesion area had changed by 52% in group C and 50% in group D (P > 0.05). In some patients, administration of glucocorticoid for more than 4 weeks significantly promoted the reduction of inflammatory shadow in the lung. Conclusion: Most patients hospitalized with COVID-19 in Wuhan were middle-aged and elderly people with underlying diseases and a history of family gatherings. Glucocorticoid therapy did not affect nor prolong the duration of nucleic acid negative conversion. Glucocorticoid therapy could promote improvement of lung lesions within 3 weeks after disease onset. Beyond 3 weeks, the treatment did not promote reduction in lung shadow area, however the density of shadow did decrease.
Keywords: COVID-19, clinical characteristics, glucocorticoid effect, nucleic acid negative conversion, Wuhan area
Introduction
The novel coronavirus pneumonia, which has spread rapidly around the world since December 2019 [1-4], was labeled by the World Health Organization (WHO) as coronavirus disease 2019 (COVID-19), and as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by the International Committee on Taxonomy of Viruses [5]. As of the end of October 2020, approximately 48 million people were infected and 1.2 million had died. Specific antiviral drugs were lacking, although chloroquine proved partially effective for COVID-19 virus [6], and Remdesivir and other antiviral drugs were also partially effective [7]. Approaches for COVID-19 treatment include mainly comprehensive and symptomatic treatments. The effectiveness of glucocorticoid treatment has been questioned, partly because of past experience in treating SARS. We found that glucocorticoid treatment was somewhat more effective in patients with COVID-19, which differs from SARS. More clinicians tend to use glucocorticoid therapy for COVID-19, and the recent literature has also evaluated glucocorticoid therapy in a positive light [8,9]. However, whether glucocorticoid therapy can improve clinical outcome and alter the time for nucleic acid negative conversion needs further verification. Corticosteroid therapy in patients with MERS was not associated with a difference in mortality after adjustment for time-varying confounders but was associated with delayed MERS coronavirus RNA clearance [10], During the 2003 SARS outbreak, high-dose glucocorticoid was widely used, and in cases where its use had limited effectiveness, it also brought many side effects, such as necrosis of the femoral head, aggravation of diabeteslung and skin infections, and increased fungal infections, which increased concerns about the use of glucocorticoid. When MERS broke out in the Middle East, it was reported that glucocorticoid could prolong the time for coronavirus clearance and affect patient prognosis [11]. As a result, many doctors expect treatment of COVID-19 virus with glucocorticoid to delay negative nucleic acid conversion. In order to provide further supporting evidence, this study reviewed and summarized the epidemiology, clinical manifestations, prognosis of lung lesions, and nucleic acid negative conversion times in 173 patients hospitalized with COVID-19 in the Wuhan area. An anti-inflammatory effect of glucocorticoid against COVID-19 pneumonia was evident, while the use glucocorticoid at low dosage had no complications.
Methods
Research subjects
The trial “The novel coronavirus pneumonia diagnosis and treatment scheme (Chinese)” (fourth, fifth, sixth, and seventh editions) was conducted among Wuhan municipal residents. Data of 200 inpatients from February 10 to March 17, 2020, at Huoshenshan Hospital were located by manual extraction and by verification of the electronic medical record system. All the cases were normal or heavy body weight, and none used invasive mechanical ventilation. Nasal catheters, masks, or high-flow oxygen therapy through the nose were given. Supportive symptomatic treatments were given according to the diagnosis and treatment plan. Routine examination was performed at admission, and chest CT was reexamined each week. A total of 173 COVID-19 patients who met the discharge criteria of diagnosis and treatment plan were included in the study of glucocorticoid treatment and time of nucleic acid negative conversion.
Research methods
The general situation, epidemiological history, chief complaint time, initial symptoms, first laboratory examination, nucleic acid negative conversion time, and chest CT data of the patients were analyzed retrospectively. Glucocorticoid treatment after admission was analyzed and recorded. The analysis of hospitalization data showed that, for some patients admitted within 3 weeks of the onset of disease, methylprednisolone intravenous drip therapy (40-80 mg/day) was started after admission (day 1-3), and stopped or gradually reduced to discontinuation within 3-7 days. In some patients who were hospitalized 3 weeks after onset of disease, oral prednisone treatment (15-20 mg/day) was started after admission (day 1-3), and stopped or gradually reduced to discontinuation within 3-7 days. Some patients continued to take corticosteroids for 4 weeks or longer according to the need of curative effect. Patients were divided into 4 groups, according to the disease duration at admission (groups A and B within 3 weeks, groups C and D more than 3 weeks) and whether they received glucocorticoid therapy (groups A and C with glucocorticoid, groups B and D without). There were 47 patients in group A, including 27 males and 20 females, with a median age of 63.5 years, and with 13 severe cases and 34 common cases. There were 45 patients in group B, including 26 males and 19 females, with a median age of 62.5 years, and with 2 severe cases and 43 common cases. There were 25 cases in group C, including 15 males and 10 females, with a median age of 66 years, and with 5 severe cases and 20 common cases. There were 56 cases in group D, including 35 males and 21 females, with a median age of 60.5 years, and with 7 severe cases and 49 common cases.
The results of nucleic acid detection were analyzed and compared among the patient groups, and changes in chest CT after 1 week of treatment with or without glucocorticoid were compared. Due to human factors, such as the unified arrangement of discharge time during the epidemic period, this study did not analyze the length of hospital stay. Novel coronavirus pneumonia, leukocyte count, interleukin-6 (IL-6) concentration, serum IgG, IgM level, and CRP level were analyzed statistically, and also in a simple comparison between early and late stages. Being of potential clinical significance, relationships between glucocorticoid treatment and coronavirus pneumonia, or changes in imaging data in certain cases, were analyzed.
Statistical methods
The data were analyzed using GraphPad Prism software. For data with non-normal distributions, medians are presented. Counted data are expressed as numbers of cases and percentages, and the comparison between groups was performed by continuous-correction chi-squared test and by independent sample median test. P < 0.05 was considered statistically significant.
Results
General patient information and characteristics of the disease
Of the 173 patients, 103 (59.5%) were male and 70 (40.5%) were female. The median age was 62 years (25-82 years), and 158 cases (91.3%) were ≥ 40 years old (Table 1). All patients were positive for 2019-nCoV nucleic acid on nasopharyngeal or oropharyngeal swabs. Twenty-seven cases (15.6%) were severe, and 146 cases (84.4%) were of the common type. All of the patients lived in Wuhan and came from areas or communities affected by the new epidemic situation. History of family gathering occurred in 58 patients (32.6%), and 42 (24.3%) had visited supermarkets, shopping malls, or hospitals before onset of the disease. Chronic underlying diseases such as hypertension, diabetes, cardiovascular disease, or chronic lung disease occurred in 89 patients (51.4%). From the onset of symptoms, fever accounted for 83.2% (144/173), cough accounted for 67.7% (117/173), fatigue accounted for 54.9% (95/173), loss of appetite accounted for 49.1% (85/173), others such as sore throat, runny nose, muscle soreness, headache accounted for 29.4% (51/173), accompanied by diarrhea, abdominal discomfort accounted for 26.6% (46/173) (See Table 1).
Table 1.
Demographic and baseline characteristics of 173 patients with new crown
| Features | All patients (n = 173) | A (n = 47) | B (n = 45) | P-volueΔ | C (n = 25) | D (n = 56) | P volueΔΔ |
|---|---|---|---|---|---|---|---|
| Age (years) | 62 (25~82) | 63.5 (25~82) | 62.5 (29~81) | 0.6366 | 66 (33~79) | 60.5 (26~79) | 0.1882 |
| ≥ 40 years old | 158 (91.3%) | 44 (93.6%) | 42 (93.3%) | 0.5554 | 24 (96%) | 50 (89.2%) | 0.3485 |
| Gender | |||||||
| male | 103 (59.5%) | 27 (57.4%) | 26 (57.8%) | 0.9747 | 15 (60%) | 35 (62.5%) | 0.8332 |
| female | 70 (40.5%) | 20 (42.6%) | 19 (42.2%) | 0.9747 | 10 (40%) | 21 (37.5%) | 0.8332 |
| Family gathering history | 58 (32.6%) | 14 (29.8%) | 15 (33.3%) | 0.7180 | 9 (36%) | 20 (35.7%) | 0.9805 |
| History of shopping or hospital exposure | 42 (24.3%) | 10 (21.3%) | 10 (22.2%) | 0.9137 | 7 (28%) | 15 (26.8%) | 0.911 |
| Diabetes history | 31 (17.9%) | 5 (10.6%) | 5 (11.1%) | 0.9427 | 7 (28%) | 14 (25%) | 0.7793 |
| Cardiovascular disease | 10 (5.8%) | 3 (6.4%) | 2 (4.4%) | 0.6858 | 1 (4%) | 4 (7%) | 0.5927 |
| Chronic lung disease | 5 (2.9%) | 1 (2.1%) | 1 (2.2%) | 0.9755 | 1 (4%) | 2 (3.6%) | 0.9260 |
| Clinical classification | |||||||
| Severe | 27 (15.6%) | 13 (27.7%) | 2 (4.4%) | 0.0023 | 7 (28%) | 5 (8.9%) | 0.0256 |
| Common | 146 (84.4%) | 34 (72.3%) | 43 (95.6%) | 0.0023 | 20 (72%) | 49 (91.1%) | 0.3864 |
| Symptom | |||||||
| Time of chief complaint (d) | 17.5 (3-64) | 16 (7-20) | 14 (3-20) | 0.8439 | 34 (21-61) | 32.5 (22-64) | 0.2824 |
| No fever | 28 (16.2%) | 7 (14.9%) | 6 (13.3%) | 0.8322 | 4 (16%) | 11 (19.6%) | 0.7010 |
| fever process (d) | 12 (3-64) | 14 (5-21) | 13 (1-21) | 0.4114 | 10 (1-43) | 12 (3-64) | 0.0845 |
| Cough | 117 (67.7%) | 34 (72.3%) | 25 (55.6%) | 0.0953 | 20 (80%) | 38 (67.9%) | 0.2685 |
| Fatigue | 95 (54.9%) | 26 (55.3%) | 25 (55.6%) | 0.9821 | 14 (56%) | 30 (53.4%) | 0.8418 |
| Loss of appetite | 85 (49.1%) | 24 (51.1%) | 22 (48.9%) | 0.8370 | 12 (48%) | 27 (48.2%) | 0.9860 |
| Sore throat, headache, etc | 51 (29.4%) | 14 (29.8%) | 13 (28.9%) | 0.9257 | 7 (28%) | 17 (30.4%) | 0.3339 |
| diarrhea | 46 (26.6%) | 13 (27.7%) | 12 (26.7%) | 0.9159 | 7 (28%) | 14 (25%) | 0.6029 |
(GraphPad Prism software, t-test).
Statistical comparison between group A and group B.
Statistical comparison between group C and group D.
Among the 173 patients, the onset of disease was from January 10 to February 25, 2020, and admission occurred from February 10 to March 17, 2020. On admission, the median chief complaint time was 17.5 days (3-64 days) for all patients, 16 days (7-20 days) in group A, 14 days (3-20 days) in group B, 34 days (21-61 days) in group C and 32.5 days (22-64 days) in Group D. Severe cases accounted for 27.2% (13/47) of patients in group A, but only 4.4% (2/45) in group B, P < 0.05. Cough cases accounted for 27.2% (13/47) in group A and 4.4% (2/45) in group B, P < 0.05. There was no significant difference between groups A and B in the remaining items. Comparing group C with group D, the median age in group C was 66 years (33-79) and in group D was 60.5 years (26-79), P < 0.05. Patients over 40 years old accounted for 96% in group C and 89.2% in group D, P < 0.05. Severe cases in groups C and D accounted for 28% and 8.9%, respectively, P < 0.05. Cough accounted for 80% of cases (20/25) in group C and 67.9% (38/56) in group D. There were no significant differences between the two groups in the remaining items (see Table 1).
Levels of IgM, IgG, IL-6, and routine blood chemistry values before and after treatment, and at different stages of disease
The median IgM and IgG levels, blood cell counts, and CRP levels (with ranges) for the four patient groups are shown in Table 2. Compared with group B, the levels of IgM and IgG in group A were higher (P < 0.05), while the lymphocyte count was lower (1.09 × 109/L versus 1.52 × 109/L, P < 0.05), the lymphocyte percentage was lower (21.9% versus 28.65%, P < 0.05), and the CRP level was higher (14.39 mg/L versus 3.28 mg/L, P < 0.01; Table 2). There were no significant differences in these levels between group C and group D. The level of IL-6 is group A 3.91 pg/mL, group B 2.54 pg/mL, group C 4.41 pg/mL, goup D 2.23 pg/mL, There were no significant differences between goup A with goup B, group C with goup D.
Table 2.
Comparison of initial laboratory examination, chest CT changes and nucleic acid negative conversion in 173 patients with new crown (GraphPad Prism software, t test)
| Features | A (n = 47) | B (n = 45) | P valueΔ | C (n = 25) | D (n = 56) | P ValueΔΔ |
|---|---|---|---|---|---|---|
| Time of complaint (d) | 16 (7-20) | 14 (3-20) | 0.8439 | 34 (21-61) | 32.5 (22-64) | 0.2824 |
| Nucleic acid negative conversion time (d) | 23 (13-48) | 22 (5-42) | 0.1979 | 38 (13-50) | 37 (25-67) | 0.2589 |
| IgM* (IU/L) | 59.015 (2.98-210.34) | 51.6 (1.53-123.85) | 0.0484 | 56.29 (6.13-212.57) | 40.095 (0.69-1193.9) | 0.7541 |
| IgG** (IU/L) | 150.43 (1.4-224.52) | 134.84 (0.84-211.17) | 0.0470 | 156.07 (79.07-220.5) | 176.465 (54.05-219.94) | 0.5811 |
| WBC *109/L | 5.3 (1.4-19) | 5.4 (3.2-10.1) | 0.6428 | 5.3 (3.48-10.3) | 5.6 (3.4-11.3) | 0.5749 |
| Lymphocyte count *109/L | 1.09 (0.39-2.67) | 1.52 (0.48-2.6) | < 0.0001 | 1.5 (0.36-2.53) | 1.57 (0.77-3.66) | 0.8585 |
| Lymphocyte ratio (%) | 21.9 (3.1-50.9) | 28.65 (9.3-46.4) | 0.0407 | 26.2 (9.6-35.9) | 29.2 (4.6-56.1) | 0.1800 |
| CRP mg/L | 14.39 (0.19-254.93) | 3.28 (0.02-82.64) | 0.0094 | 2.52 (0.21-98.15) | 1.57 (0.09-28.03) | 0.0886 |
| IL-6 pg/mL*** | 3.91 (0.03-104.5) | 2.54 (< 1.5-139.6) | 0.6786 | 4.41 (< 1.5-6.53) | 2.23 (< 1.5-14.99) | 0.7991 |
| PCT ng/ml | 0.05 (0.01-0.33) | 0.03 (0.01-0.08) | 0.1926 | 0.04 (0.01-0.1) | 0.04 (0.01-0.1) | 0.4771 |
| Proportion of pulmonary infection (%) | 12.6 (1.2-38) | 11.3 (0.1-35) | 0.6987 | 15.6 (4-38.5) | 15.3 (0.1-43.2) | 0.6953 |
| Percentage of lung changes (%)*** | 33.05 (5.5-100) | 22.5 (1-100) | 0.0386 | 52 (1.4-75) | 50 (1-85) | 0.5118 |
P value of group A compared with group B;
P value of group C compared with group D;
IgM cases were n = 30 in group A, n = 45 in group B, n = 25 in group C, n = 56 in group D;
IgG cases were n = 30 in group A, n = 45 in group B, n = 25 in group C, n = 56 in group D;
IL-6 cases were n = 30 in group A, n = 45 in group B, n = 25 in group C, n = 56 in group D;
percentage of pulmonary infection decreased or increased after 1 week interval.
The median IgM level was similar for early stage Covid-19 (< 3 weeks, combined group A+B), compared to late stage disease (≥ 3 weeks, group C+D; see Table 4). The median levels of IgG were 147.675 IU/L for early stage (group A+B) versus 174.17 IU/L for late stage disease (group C+D), P < 0.05 (Table 4). Levels of CRP were also higher for early than for late stage, 9.42 mg/L (group A+B) versus 1.68 mg/L (group C+D), P < 0.05 (see Table 3). There were no other significant differences between the goup A with group B, group C with group D (See Table 2).
Table 4.
Comparison of nucleic acid negative conversion in 173 patients with new crown (GraphPad Prism software, t test)
| Group | n | Nucleic acid negative coversion time (d) | P value |
|---|---|---|---|
| A | 47 | 23 (13-48) | 0.1979Δ |
| B | 45 | 22 (5-42) | |
| C | 25 | 38 (13-50) | 0.2589ΔΔ |
| D | 56 | 37 (25-67) | |
| A+B | 92 | 23 (5-48) | < 0.0001ΔΔΔ |
| C+D | 81 | 37 (13-67) | |
| A+C | 72 | 26 (13-50) | 0.0704ΔΔΔΔ |
| B+D | 101 | 31 (5-67) |
P value of group A compared with group B;
P value of group C compared with group D;
P value of group A+B compared with group C+D;
P value of group A+C compared with group B+D.
Table 3.
Comparison of initial laboratory examination and nucleic acid negative conversion of hospitalized patients in early stage (< 3 weeks) and late stage (≥ 3 weeks) (GraphPad Prism software, t test)
| Features | A+B (n = 92) | C+D (n = 81) | P valueΔ |
|---|---|---|---|
| Complaint time (d) | 14 (3~20) | 34 (21-64) | < 0.0001 |
| Nucleic acid negative conversion time (d) | 23 (5-48) | 37 (13-67) | < 0.0001 |
| IgM* (IU/L) | 51.6 (1.53-210.34) | 45.27 (0.69-1193.9) | 0.3159 |
| IgG** (IU/L) | 147.675 (0.84-224.52) | 174.17 (54.05-220.5) | 0.0036 |
| WBC *109/L | 5.25 (1.4-19) | 5.4 (3.4-11.3) | 0.5338 |
| Lymphocyte count *109/L | 1.2 (0.39-2.67) | 1.535 (0.36-3.66) | 0.0085 |
| Lymphocyte ratio (%) | 26.1 (3.1-50.9) | 28.25 (4.6-56.1) | 0.0488 |
| CRP mg/L | 9.42 (0.02-254.93) | 1.68 (0.09-98.15) | < 0.0001 |
| IL-6 pg/mL*** | 2.87 (0.03-139.6) | 2.34 (< 1.5-14.99) | 0.0122 |
| PCT ng/ml | 0.04 (0.01-0.33) | 0.04 (0.01-0.1) | 0.6762 |
| Proportion of pulmonary infection (%) | 12.6 (0.1-38) | 15.3 (0.1-43.2) | 0.7676 |
P value of A+B group compared with C+D group;
IgM cases were A+B group n = 75, C+D group n = 81;
IgG cases were A+B group n = 75, C+D group n = 81;
IL-6 cases were A+B group n = 75, C+D group n = 81.
Changes in chest CT shadow before and after treatment
The proportion of pulmonary infection was determined as lung shadow on the day of admission. Median percentages for the four patient groups are shown in Table 2. There was no significant difference between group A and group B or between group C and group D (P > 0.05). The percentage change in lung infection (shadow area) after one week differed significantly between group A and group B (P < 0.05), but there was no significant difference between group C and group D (P > 0.05, see Table 2). However, shadow area cannot fully represent treatment effectiveness. In many patients, especially in early-admission patients, the shadow may become less dense even though its area changes little, accompanied by symptom relief, and reflecting an obvious curative effect (see Tables 2 and 3). Some patients continued glucocorticoid treatment for 4 weeks beyond the first week, with obvious improvements of inflammatory shadow in the lung (Figures 4, 5 and 6).
Figure 4.
Case 3. Imaging changes of chest CT, Female, 71 years old, taking prednisone 10 mg/D orally for 2 weeks, although there were some residual shadows.
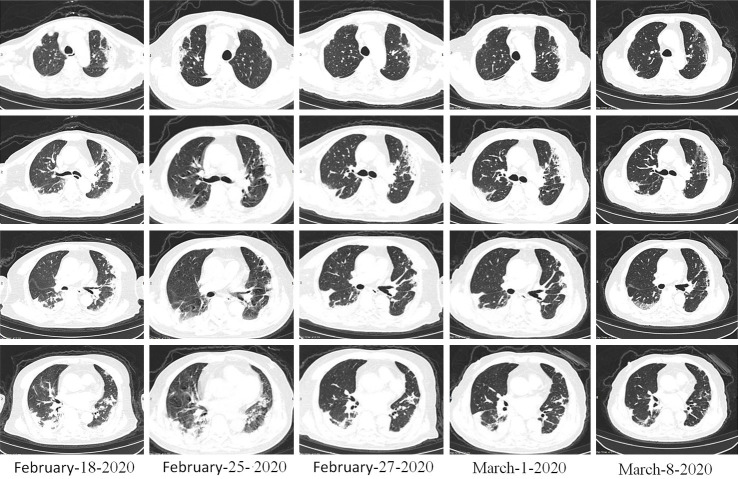
Figure 5.
Case 4. Imaging changes of chest CT, Female, 67 years old, oral prednisone 20 mg/day, 4 weeks, Pulmonary shadow is mostly absorbed.
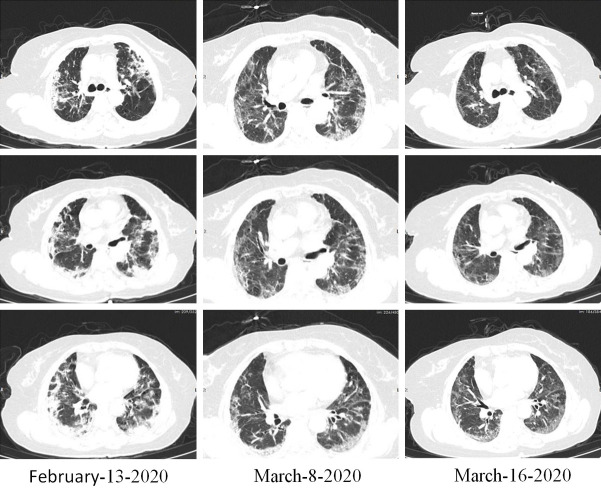
Figure 6.
Case 5. Imaging changes of chest CT, Male, 81 years old, chest CT, February-18 methylprednisolone 40 mg/d, February-25 shadow absorption, stop hormone, February-27 and March-1 shadow no change, oral prednisone 20 mg/D, shadow absorption.
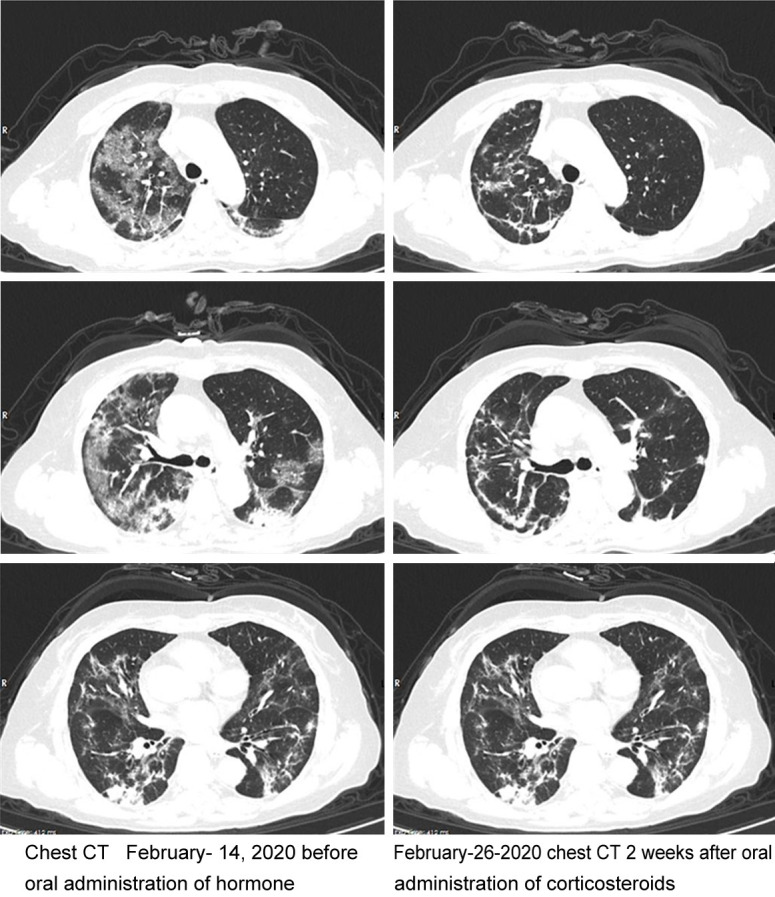
Case 1
Novel coronavirus pneumonia (common type) in a female patient, age 41 years, date of onset January 25th, was treated early with glucocorticoid. Chest CT images in the same plane were compared before and after treatment (Figure 2). The healthy adult female experienced onset of fever for half a month, which did not subside during 10 days in the hospital, as in other confirmed cases. Several measures were taken without obvious effect (admission 6 L/min oxygen, oxygen saturation 95%). There was a risk of severe pneumonia. Glucocorticoid (40 mg/day) was given for 4 days. By the third day, the symptoms improved, and continued to improve after stopping the drug. An image after 2 weeks showed the infection absorbed partly. Conclusion: glucocorticoid therapy plays a key role in mid-term patients, and the mid-term effect is good.
Figure 2.
Case 1. Chest CT image changes of case 1 before and after early application of Glucocorticoid (in the same plane).
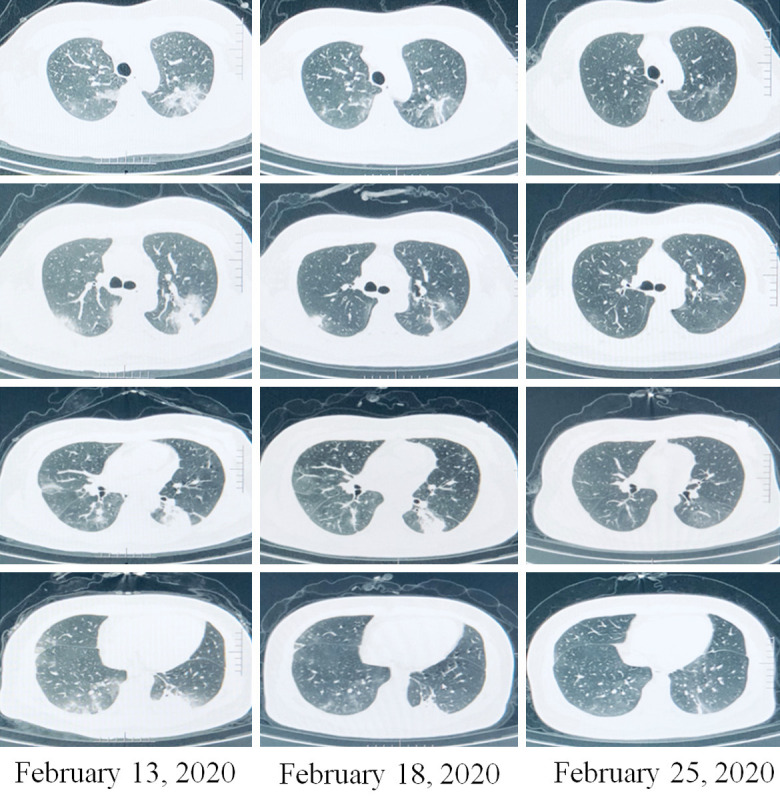
Case 2
A 58-year-old female was hospitalized on February 10, 2020, for seven days of fever and shortness of breath. Seven days earlier, her fever was 39°C, and was accompanied by shortness of breath and chills. CT indicated novel coronavirus pneumonia of the common type, with a few shadows in both lungs. The diagnosis was clear: coronavirus pneumonia (common type), consistent with reports in many external hospitals that recorded temperatures of 38-39°C, with poor mental function, fatigue, loss of appetite, and loss of sleep. She was admitted to the hospital at 6 L/min, with oxygen saturation 95%, and an oxygenation index estimated to be approximately 200 mm Hg. After admission, prednisone (10 mg/day) was given orally, and was reduced to 5 mg/day after 1 week. Body temperature was normal within 2 days, and symptoms were completely improved in 5 days. Chest CT showed the disappearance of most of the lung shadows (Figure 3). She was discharged from the hospital on March 2. She had been suffering from “left breast cancer” for 5 years and had diabetes mellitus for more than 10 years. The severe symptoms toward severe pneumonia were relieved, without aggravation of her diabetes. This patient’s outcome suggested that the early application of low-dose glucocorticoid had good effect.
Figure 3.
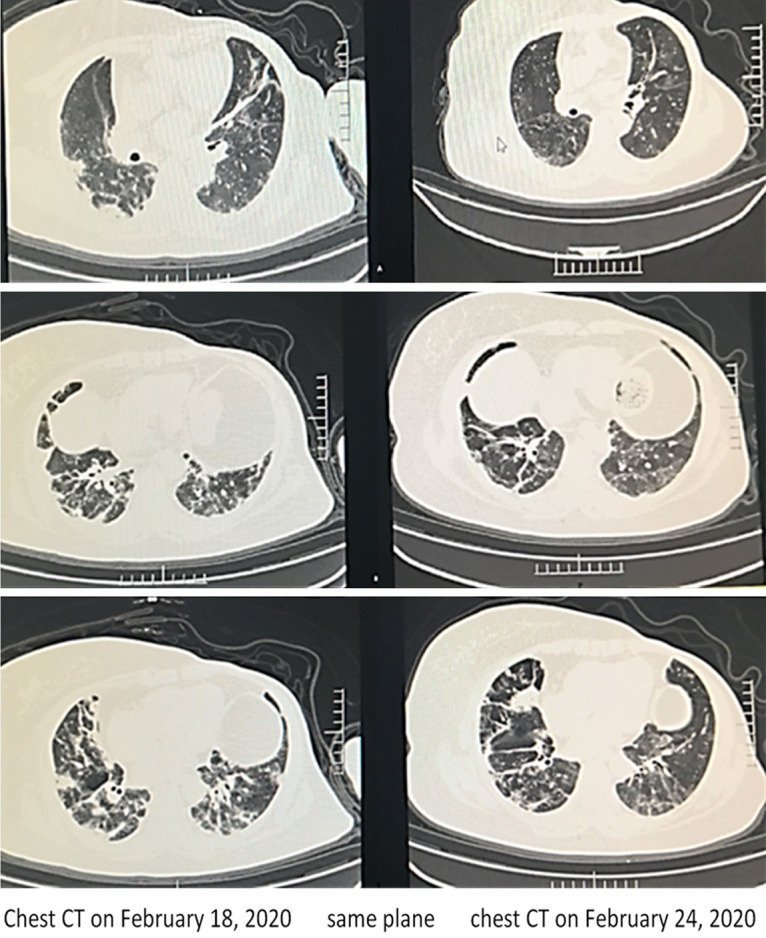
Case 2. Imaging changes of case 2 before and after glucocorticoid treatment (in the same plane), Most of the shadow absorption, the area does not shrink, but the density becomes low.
Case 3
A novel coronavirus pneumonia (common type) was diagnosed in a 71-year-old woman who had experienced intermittent fever, cough, and shortness of breath for 19 days. The highest temperature was 38.2°C. She was given anti-infection treatment. The lung shadow did not appear to recede. Prednisone (10 mg/day) was given orally. Two weeks later, the lung shadow had conspicuously improved (Figure 4), the dyspnea condition was improved, and oxygen saturation had increased from 89% to 95%. The use of glucocorticoids in the medium term can diminish inflammatory shadows in the lung.
Case 4
Diagnosis of novel coronavirus pneumonia (severe) was given to a patient, age 67 years, who had been admitted for symptoms of fever, cough, sputum, and shortness of breath lasting 21 days. Oral prednisone (20 mg/day) was given, with a weekly reduction of 5 mg/day. After 4 weeks, inflammatory shadow in the lungs had gradually diminished (Figure 5), oxygen saturation had increased to more than 95%, and symptoms were completely relieved.
Case 5
Novel coronavirus pneumonia (severe) was diagnosed in a male, age 81 years, who was admitted to the hospital for 20 days due to fever, cough, shortness of breath, and dyspnea lasting 20 days. The hospital determined severe lung shadow and oxygen saturation in the range of 85%-90%. The patient was given anti-infection treatment combined with methylprednisolone (40 mg/day) by intravenous drip for one week, when the symptoms had improved, his condition was stable, and the lung shadow was improved (Figure 6). On February 27 and March 1, after stopping the drug, the chest CT shadow had not changed. Oral prednisone (20 mg/day) was initiated for one week. The shadow was clearly reduced, the symptoms gradually improved, and the drug was gradually discontinued. It is suggested that the prompt treatment with advanced glucocorticoid can promote the clearance of pulmonary inflammatory shadow.
Comparison of negative conversion intervals of each group before and after treatment
Times of nucleic acid negative conversion were determined for all 173 patients (Table 2). There was no significant difference in median conversion time between group A and group B, or between group C and group D (P > 0.05, Table 2). The median time of nucleic acid negative conversion was significantly shorter for patients treated at early stage (< 3 weeks, combined group A+B) than for those treated at late stage (≥ 3 weeks, combined group C+D); P < 0.0001 (Tables 3 and 4). These results suggest that early treatment is directly related to the course of disease, prognosis and nucleic acid clearance rate. There was no significant difference between group A+C and group B+D (P = 0.0704) (See Tables 3 and 4, and Figure 1).
Figure 1.
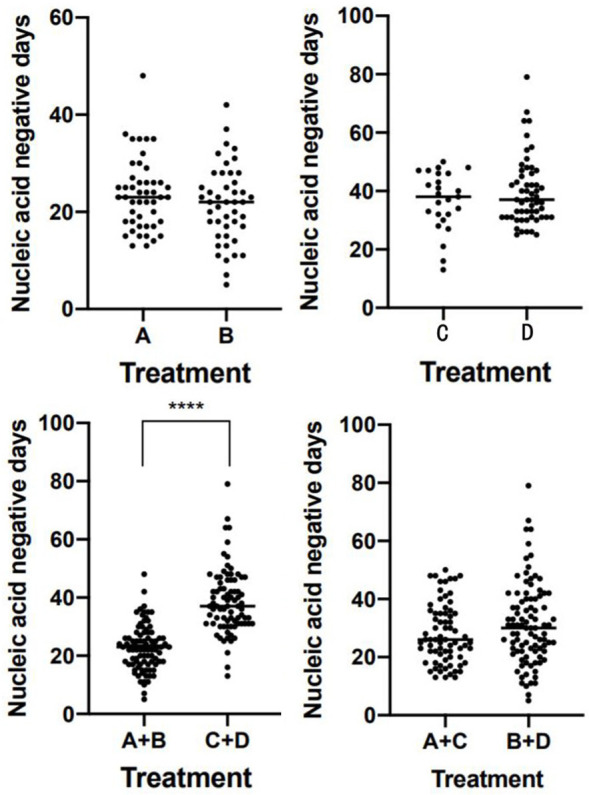
Statistical chart of nucleic acid negative conversion in 173 patients with new crown, ★★★★There was significant difference between group A+B and group C+D (P = 0.0001). The time of nucleic acid turning negative was directly related to the duration of the disease, but not related to the application of glucocorticoid. There was no significant difference between groups A and B, C and D, A+C and B+D.
Discussion
Coronavirus infection disease-19 (COVID-19) is a worldwide pandemic, which seriously endangers human health and disturbs global economic development and order. It is urgent to deeply understand the COVID-19 disease process, and to seek effective treatments and prevention and control measures. This study retrospectively analyzed the data of 173 patients in the Wuhan area who were hospitalized with COVID-19 from January 10 to February 25. The epidemiological and clinical characteristics were basically consistent with numerous literature reports [12-18]. At the same time, some new characteristics emerged. Among patients in the study, most were middle-aged and elderly people, 91.3% being over 40 years old, and most had chronic underlying diseases, 51.4% of them having hypertension, diabetes, cardiovascular disease, or chronic lung disease. In terms of disease transmission, history of family gathering was evident, accounting for 32.6% of cases. In terms of clinical symptoms, the predominant respiratory symptoms were fever and cough [15,17], a different presentation than in European and American COVID-19 patients [19,20]. No loss of taste and smell was reported. It is worth noting that 16.2% of patients had no fever, while 26.6% of patients had diarrhea, abdominal discomfort, and other gastrointestinal symptoms.
Patients in this study were divided into groups according to the duration of symptoms before hospital admission (groups A and B within 3 weeks, groups C and D more than 3 weeks) and whether or not their treatment included glucocorticoid (groups A and C with glucocorticoid, groups B and D without). Median levels of IgM (IU/L) and IgG (IU/L) were higher in group A compared with group B (P < 0.05), the lymphocyte count was lower [1.09 × 109/L (0.39-2.67 × 109/L) versus 1.52 × 109/L (0.48-2.6 × 109/L), P < 0.05], the lymphocyte percentage was lower [21.9% (3.1%-50.9%) versus 28.65% (9.3%-46.4%), P < 0.05], and the CRP level was higher [14.39 mg/L (0.19-254.93 mg/L) versus 3.28 mg/L (0.02-82.64 mg/L), P < 0.01; see Table 2]. These findings indicated the decline in inflammatory factors related to glucocorticoid treatment, especially among patients treated with glucocorticoid earlier in the progress of the disease. There were no significant differences between group C and group D, suggesting that glucocorticoid treatment may have less impact on inflammatory factors when given later in the course of COVID-19 disease. The median IgM level for group A+B (patients treated within the first 3 weeks of disease) was higher than that of group C+D (patients treated at a later stage, ≥ 3 weeks). The median levels of IgG were 147.675 (0.84-224.52) for group A+B, and 174.17 (54.05-220.5) for group C+D, P < 0.05 (Table 3), suggesting that IgG levels may be related to duration of the disease. Median levels of CRP were 9.42 mg/L (0.02-254.93 mg/L) in group A+B, and 1.68 mg/L (0.09-98.15 mg/L) in group C+D (P < 0.01). This suggests that CRP is closely related to the degree of inflammation and anti-inflammatory treatment, and can be used as an indicator of Inflammation and disease.
Glucocorticoid has been previously studied for use in many viral infectious diseases, but its clinical benefits have been widely debated [10,11,21,22], especially because in the treatment of SARS, MERS, and influenza, administration of glucocorticoid did not reduce 90 day mortality, and also increased the risk of secondary infection, osteoporosis, hyperglycemia, and other complications, as well as possibly prolonging virus clearance time [10,11], and increasing the risk of death after 2 weeks of glucocorticoid treatment [22]. At present, drugs for the effective treatment of COVID-19 are still lacking. Many clinicians continue to use glucocorticoid for treatment of COVID-19, and most of them choose low dosage and a short course of treatment. Recent studies have given glucocorticoid therapy a positive evaluation. Studies show that among patients who receive mechanical ventilation for COVID-19, glucocorticoid treatment reduces the time of ventilator use, and reduces 28-day mortality [8,9,23]. In our retrospective analysis of 173 patients without mechanical ventilation, glucocorticoid treatment occurred in some patients in acute stage and within the first 3 weeks of disease, who received methylprednisolone (40-80 mg/day) by intravenous drip, which was stopped after 1 week or gradually reduced over 3-7 days, and also in some patients treated more than 3 weeks after disease onset, who received oral prednisone (15-20 mg/day), which was stopped after 1 week or gradually reduced within 3-7 days. Patients in acute stage (within the first 3 weeks, groups A and B) were more likely to be treated with glucocorticoid if they were severe, having low lymphocyte ratio or elevated CRP or other inflammatory factors (see Tables 1 and 2). Patients in late stage (more than 3 weeks, groups C and D) were also more likely to be treated with glucocorticoid if severe (see Tables 1 and 2). In contrast to previous reports that found glucocorticoid to be ineffective in the treatment of SARS and MERS, the present study suggested that low-dose and short course glucocorticoid treatments showed good effect for COVID-19. In patients whose course of disease was less than 3 weeks, the rate of improvement of lung lesions after 1 week of glucocorticoid treatment was significantly higher than that in patients whose treatment did not include glucocorticoid (33.05% and 22.5%, respectively). For patients whose course of disease was ≥ 3 weeks, there was no significant difference in changes in lung lesions with glucocorticoid therapy. Our findings suggest that the initiation of glucocorticoid therapy during the acute stage of COVID-19 can improve clinical symptoms, but whether glucocorticoid therapy can improve the final clinical outcome still needs further study. The length of glucocorticoid treatment may be shorter in patients who are in the advanced stage, perhaps accounting for its weaker effect on lung shadow. Some patients who continued the treatment for an additional 4 weeks showed good improvements in lung shadow (Figures 4, 5 and 6). These cases suggest, preliminarily, that glucocorticoid use can also improve lung shadow in advanced patients, with corresponding improvement of dyspnea, but this needs to be evaluated in a greater number of cases.
On the other hand, it remains to be clarified whether glucocorticoid treatment will affect nucleic acid negative conversion time in patients with COVID-19. Previous studies on SARS and MERS showed that high viral load was associated with early administration of glucocorticoid, which prolonged the clearance of coronavirus [10,11]. The present study found that, regardless of the disease stage being less than or greater than 3 weeks, glucocorticoid treatment did not significantly prolong or otherwise alter nucleic acid negative conversion times (see Tables 3 and 4, and Figures 3 and 4), whether methylprednisolone or prednisone was administered. This could be related to the dosage and timing of glucocorticoid application, because in this study, low-dose and short-course glucocorticoid treatments were adopted. Either for patients admitted in the first 3 weeks of disease or those admitted at a later stage, the median times of chief complaint were 16 days and 14 days, respectively (see Table 2). Thus, the median time until initiating glucocorticoid treatment was about 2 weeks, suggesting that the disease had already progressed to a very high level in the acute stage of COVID-19. It may prove better to administer glucocorticoid in the early stage, but this needs to be confirmed in a greater number of cases.
Our study also found that nucleic acid negative conversion time was significantly longer for patients with a disease course of more than 3 weeks than for patients with disease course less than 3 weeks (37 d versus 23 d, P < 0.01). Higher median IgG level and lower CRP level in the former group (Table 3) were consistent with the disease having developed to the late stage. However, the prolonged duration of nucleic acid positivity among these patients cannot yet be explained. Secretion patterns have been reported [24,25]. These studies suggest that COVID-specific antibodies level may decrease or disappear within a short time, and whether this is related to the longer duration of nucleic acid positivity still needs further study. Our analysis of chest CT scans showed that, although the proportion of pulmonary infection did not differ significantly between patients at different stages of disease (15.3% versus 12.6%), the pulmonary fibrosis lesions were more serious in patients with course of disease beyond 3 weeks. On the one hand, this was consistent with pulmonary fibrosis having progressed beyond the acute stage, and on the other hand, it also suggested that the disease was more severe in these patients. In addition, the lymphocyte count and proportion were at a normal, low level, suggesting that poor recovery of immune function may also be a reason.
In summary, most COVID-19 patients in the Wuhan area are middle-aged and elderly people with chronic underlying diseases and a history of family gathering. Respiratory symptoms are the main symptoms. In this study, we retrospectively analyzed patients with concurrent onset who had been admitted at different stages of the disease. We found that treatment with glucocorticoid did not prolong the time of nucleic acid negative conversion, and that glucocorticoid administration during acute phase can promote improvement of lung lesions. However, due to the small number of cases, further research is needed to verify these findings.
Acknowledgements
We thank all patients involved in this research for allowing us to utilize their data.
Disclosure of conflict of interest
None.
References
- 1.Van Cuong L, Giang HTN, Linh LK, Shah J, Van Sy L, Hung TH, Reda A, Truong LN, Tien DX, Huy NT. The first Vietnamese case of COVID-19 acquired from China. Lancet Infect Dis. 2020;20:408–409. doi: 10.1016/S1473-3099(20)30111-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen X, Tian J, Li G. Initiation of a new infection control system for the COVID-19 outbreak. Lancet Infect Dis. 2020;20:397–398. doi: 10.1016/S1473-3099(20)30110-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Machhi J, Herskovitz J, Senan AM, Dutta D, Nath B, Oleynikov MD, Blomberg WR, Meigs DD, Hasan M, Patel M, Kline P, Chang RC, Chang L, Gendelman HE, Kevadiya BD. The natural history, pathobiology, and clinical manifestations of SARS-CoV-2 infections. J Neuroimmune Pharmacol. 2020;15:359–386. doi: 10.1007/s11481-020-09944-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tizaoui K, Zidi I, Lee KH, Ghayda RA, Hong SH, Li H, Smith L, Koyanagi A, Jacob L, Kronbichler A, Shin JI. Update of the current knowledge on genetics, evolution, immunopathogenesis, and transmission for coronavirus disease 19 (COVID-19) Int J Biol Sci. 2020;16:2906–2923. doi: 10.7150/ijbs.48812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meo SA, Klonoff DC, Akram J. Efficacy of chloroquine and hydroxychloroquine in the treatment of COVID-19. Eur Rev Med Pharmacol Sci. 2020;24:4539–4547. doi: 10.26355/eurrev_202004_21038. [DOI] [PubMed] [Google Scholar]
- 7.Ortiz-Prado E, Simbana-Rivera K, Gomez-Barreno L, Rubio-Neira M, Guaman LP, Kyriakidis NC, Muslin C, Jaramillo AMG, Barba-Ostria C, Cevallos-Robalino D, Sanches-SanMiguel H, Unigarro L, Zalakeviciute R, Gadian N, Lopez-Cortes A. Clinical, molecular, and epidemiological characterization of the SARS-CoV-2 virus and the Coronavirus Disease 2019 (COVID-19), a comprehensive literature review. Diagn Microbiol Infect Dis. 2020;98:115094. doi: 10.1016/j.diagmicrobio.2020.115094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tomazini BM, Maia IS, Cavalcanti AB, Berwanger O, Rosa RG, Veiga VC, Avezum A, Lopes RD, Bueno FR, Silva MVAO, Baldassare FP, Costa ELV, Moura RAB, Honorato MO, Costa AN, Damiani LP, Lisboa T, Kawano-Dourado L, Zampieri FG, Olivato GB, Righy C, Amendola CP, Roepke RML, Freitas DHM, Forte DN, Freitas FGR, Fernandes CCF, Melro LMG, Junior GFS, Morais DC, Zung S, Machado FR, Azevedo LCP COALITION COVID-19 Brazil III Investigators. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA. 2020;324:1307–1316. doi: 10.1001/jama.2020.17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee N, Allen Chan KC, Hui DS, Ng EK, Wu A, Chiu RW, Wong VW, Chan PK, Wong KT, Wong E, Cockram CS, Tam JS, Sung JJ, Lo YM. Effects of early corticosteroid treatment on plasma SARS-associated coronavirus RNA concentrations in adult patients. J Clin Virol. 2004;31:304–309. doi: 10.1016/j.jcv.2004.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arabi YM, Mandourah Y, Al-Hameed F, Sindi AA, Almekhlafi GA, Hussein MA, Jose J, Pinto R, Al-Omari A, Kharaba A, Almotairi A, Al Khatib K, Alraddadi B, Shalhoub S, Abdulmomen A, Qushmaq I, Mady A, Solaiman O, Al-Aithan AM, Al-Raddadi R, Ragab A, Balkhy HH, Al Harthy A, Deeb AM, Al Mutairi H, Al-Dawood A, Merson L, Hayden FG, Fowler RA. Corticosteroid therapy for critically Ill patients with middle east respiratory syndrome. Am J Respir Crit Care Med. 2018;197:757–767. doi: 10.1164/rccm.201706-1172OC. [DOI] [PubMed] [Google Scholar]
- 12.Brosnahan SB, Jonkman AH, Kugler MC, Munger JS, Kaufman DA. COVID-19 and respiratory system disorders: current knowledge, future clinical and translational research questions. Arterioscler Thromb Vasc Biol. 2020;40:2586–2597. doi: 10.1161/ATVBAHA.120.314515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abohamr SI, Abazid RM, Aldossari MA, Amer HA, Badhawi OS, Aljunaidi OM, Alzarzour SH, Saadeddin HM, Bhat FA, Elsheikh E. Clinical characteristics and in-hospital mortality of COVID-19 adult patients in Saudi Arabia. Saudi Med J. 2020;41:1217–1226. doi: 10.15537/smj.2020.11.25495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yao T, Gao Y, Cui Q, Peng B, Chen Y, Li J, Huang C, He C, Pu J, Wei J, Zhan Y, Yan J, Tian J, Zhang Z, Liu Z. Clinical characteristics of a group of deaths with COVID-19 pneumonia in Wuhan, China: a retrospective case series. BMC Infect Dis. 2020;20:695. doi: 10.1186/s12879-020-05423-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Lu X, Li Y, Chen H, Chen T, Su N, Huang F, Zhou J, Zhang B, Yan F, Wang J. Clinical course and outcomes of 344 intensive care patients with COVID-19. Am J Respir Crit Care Med. 2020;201:1430–1434. doi: 10.1164/rccm.202003-0736LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J, Gong X, Wang Z, Chen R, Li T, Zeng D, Li M. Clinical features of familial clustering in patients infected with 2019 novel coronavirus in Wuhan, China. Virus Res. 2020;286:198043. doi: 10.1016/j.virusres.2020.198043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zubair AS, McAlpine LS, Gardin T, Farhadian S, Kuruvilla DE, Spudich S. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA Neurol. 2020;77:1018–1027. doi: 10.1001/jamaneurol.2020.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Konstantinidis I, Delides A, Tsakiropoulou E, Maragoudakis P, Sapounas S, Tsiodras S. Short-term follow-up of self-isolated covid-19 patients with smell and taste dysfunction in greece: two phenotypes of recovery. ORL J Otorhinolaryngol Relat Spec. 2020;82:295–303. doi: 10.1159/000511436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ni YN, Chen G, Sun J, Liang BM, Liang ZA. The effect of corticosteroids on mortality of patients with influenza pneumonia: a systematic review and meta-analysis. Crit Care. 2019;23:99. doi: 10.1186/s13054-019-2395-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steinberg KP, Hudson LD, Goodman RB, Hough CL, Lanken PN, Hyzy R, Thompson BT, Ancukiewicz M. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med. 2006;354:1671–1684. doi: 10.1056/NEJMoa051693. [DOI] [PubMed] [Google Scholar]
- 23.Villar J, Ferrando C, Martinez D, Ambros A, Munoz T, Soler JA, Aguilar G, Alba F, Gonzalez-Higueras E, Conesa LA, Martin-Rodriguez C, Diaz-Dominguez FJ, Serna-Grande P, Rivas R, Ferreres J, Belda J, Capilla L, Tallet A, Anon JM, Fernandez RL, Gonzalez-Martin JM. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020;8:267–276. doi: 10.1016/S2213-2600(19)30417-5. [DOI] [PubMed] [Google Scholar]
- 24.Chirathaworn C, Sripramote M, Chalongviriyalert P, Jirajariyavej S, Kiatpanabhikul P, Saiyarin J, Soudon C, Thienfaidee O, Palakawong Na Ayuthaya T, Brukesawan C, Chaiwanichsiri D, Intharasongkroh D, Wanlapakorn N, Chansaenroj J, Puenpa J, Yorsaeng R, Thitithanyanont A, Kitphati R, Mungaomklang A, Nagavajara P, Poovorawan Y. SARS-CoV-2 RNA shedding in recovered COVID-19 cases and the presence of antibodies against SARS-CoV-2 in recovered COVID-19 cases and close contacts, Thailand, April-June 2020. PLoS One. 2020;15:e0236905. doi: 10.1371/journal.pone.0236905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imai K, Tabata S, Ikeda M, Noguchi S, Kitagawa Y, Matuoka M, Miyoshi K, Tarumoto N, Sakai J, Ito T, Maesaki S, Tamura K, Maeda T. Clinical evaluation of an immunochromatographic IgM/IgG antibody assay and chest computed tomography for the diagnosis of COVID-19. J Clin Virol. 2020;128:104393. doi: 10.1016/j.jcv.2020.104393. [DOI] [PMC free article] [PubMed] [Google Scholar]