Abstract
Objective: To investigate the efficacy of transcatheter arterial chemoembolization (TACE) combined with thalidomide-mediated adjuvant therapy on the expression levels of vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) in hepatocellular carcinoma (HCC) patients. Methods: A prospective study was designed, by which 134 HCC patients from our hospital who underwent treatment were selected and randomly divided into an observation group and a control group, 67 participants per group. The control group was administered hepatic TACE, while the observation group was given TACE in combination with thalidomide. The total disease control rate (DCR) and the rate of adverse effects were analyzed and compared between the two groups of patients. The expression levels of CD3+, CD4+, CD8+, CD4+/CD8+, VEGF, VEGFA, and bFGF were measured between the two groups before and after treatment. The overall survival rate of the two groups were also compared after a follow-up for 3 years. Results: The rate of adverse effects and DCR in the control group were 44.78% and 61.19%, respectively, whereas these rates were 22.39% and 89.55% in the observation group, respectively. Of note, the differences in terms of the rate of adverse effects and DCR were statistically significant between the two groups (P<0.05). Before treatment, no significant difference was shown regarding the expression levels of CD3+, CD4+, CD8+, CD4+/CD8+, VEGF, VEGFA, and bFGF between the two groups (P>0.05). After treatment, the expression levels of CD3+, CD4+, and CD4+/CD8+ were significantly upregulated in the two groups, while the levels of CD8+, VEGF, VEGFA, and bFGF were considerably downregulated (P<0.05). In addition, compared with the control group, the expression levels of CD3+, CD4+, and CD4+/CD8+ were significantly higher, whereas the levels of CD8+, VEGF, VEGFA, and bFGF were notably lower in the observation group (P<0.05). After the follow-up for 3 years, the overall survival rate of the observation group was significantly higher in comparison to the control group (P<0.05). Conclusion: TACE in combination with thalidomide-mediated adjuvant treatment has revealed a promising clinical outcome on HCC patients by downregulating the levels of VEGF and bFGF.
Keywords: Transcatheter arterial chemoembolization, thalidomide, hepatocellular carcinoma, vascular endothelial growth factor, basic fibroblast growth factor
Introduction
Liver cancer is a common malignancy in our digestive system. It has been shown that the incidence and the mortality of this disease rank the 5th and 3rd, respectively among the malignant tumors worldwide [1,2]. With the development of our society, the morbidity of hepatocellular carcinoma (HCC) is becoming higher. The routine therapeutic strategy for liver cancer is the hepatectomy, which has made some progress for liver cancer treatment. However, post-surgery disadvantages such as high chance of complications and pains are of concerns among patients. In clinical practice, the gold standard to treat advanced HCC is transcatheter arterial chemoembolizalion (TACE), which can slowly release the drug to the target organs and maintain a high blood concentration of the medication to control the rapid growth of tumors. However, studies have shown that TACE may have some negative impacts on the immune function of patients [3-6]. Thalidomide belongs to a group of immunosuppressants, which functions by regulating T lymphocytes, preventing tumor angiogenesis, and inducing tumor apoptosis and has been widely used as an auxiliary anti-tumor medication in the treatment of intermediate and advanced malignant tumors [7]. Previous studies have elucidated the clinical effects of the TACE combined with thalidomide in the treatment of HCC, but the underlying mechanism remains elusive [8]. Angiogenesis is one of the fundamental steps for tumor initiation and progression, in which vascular endothelial growth factor (VEGF) is a key angiogenic mediator to drive the neovascularization [9-11]. The fibroblast growth factor (FGF) family and its related receptors are involved in a variety of biological functions, such as regulating cell proliferation, differentiation and migration, participating in the initiation and development of tumors, and playing roles in the tissue repair [12]. Based on previous studies, this study aimed to elucidate the efficacy of TACE in combination with thalidomide-mediated adjuvant therapy on the levels of VEGF and bFGF in patients with HCC and to provide therapeutic insights into HCC treatment.
Materials and methods
General information
A total of 134 HCC patients who underwent treatment in our hospital from January 2015 to January 2017 were selected in this prospective research and divided into an observation group and a control group based on a random number table method, with 67 participants in each group. Our study was approved by the ethics committee of our hospital, and patients as well as their family members were informed and signed the consent form.
Inclusion and exclusion criteria
Inclusion criteria: (1) Met the relevant diagnostic and staging criteria of HCC [13]; (2) Without targeted drug therapy, radiotherapy, chemotherapy or other drug treatments in the past; (3) No indication for surgery; (4) No history of liver surgery; (5) With complete clinical data; (6) No other malignant tumors; (7) Age >18 years.
Exclusion criteria: (1) Missing data or specimens; (2) With other severe diseases; (3) Pregnancy or lactation; (4) Poor compliance; (5) Intolerant with this treatment plan.
Methods
Both groups’ patients were treated with TACE, which was conducted by a modified Seldinger method [14]. The chemotherapy regimen included 5-Fu (0.75-1.25 g) + cisplatin (80-120 mg) + oxaliplatin (200 mg) + episoft Bistar (80-120 mg). The embolization agent contained gelatin and super-liquefied lipiodol. The dosage of chemotherapy and embolization agents depended on the size of the tumor and blood supply.
The observation group was administered with thalidomide based on the therapy of the control group. Thalidomide (Changzhou Pharmaceutical Factory Co., Ltd., 25 mg × 20 tablets) was taken orally from 7 days after TACE, 100-200 mg each time, once per day, continuously for 3 months. The control group was given the placebo from 7 days after TACE, 100-200 mg each time, once per day for 3 months.
Outcome measurements
The levels of VEGF, VEGF-A, and bFGF
Two tubes of 5 mL fasting venous blood were collected from patients at one day before treatment and the morning after treatment. The sample was centrifuged for 5 min at 3,000 r/min to isolate the serum. One of the venous blood samples was subjected to the flow cytometry (BD FACSCalibur, US) to detect CD3+, CD4+, CD8+, and CD4+/CD8+ levels, while the other one was used for enzyme-linked immunosorbent assay (ELISA; Hamilton microlab star multi-functional microplate reader, Switzerland) to measure the levels of VEGF, VEGF-A and bFGF [15].
Tumor response rate
The clinical efficacy of the treatment was investigated based on the Response Evaluation Criteria in Solid Tumors (RECIST) [16]. Complete response (CR): the lesions disappeared completely and no new lesions generated; partial response (PR): no new lesions generated for 4 weeks and the sum of the longest diameter of the lesions reduced by more than 30%; stable disease (SD): the sum of the longest diameters of the lesions decreased less than 30%; progressive disease (PD): new lesions appeared or the sum of longest diameters of the lesions increased by more than 20%. Objective response rate (RR) = (CR + PR)/total number of patients of this group ×100%; disease control rate (DCR) = (CR + PR + SD)/total number of patients of this group ×100%.
Adverse effects and survival
The occurrence of adverse effects such as gastrointestinal reactions, anemia, fever, fatigue, bone marrow suppression, and leukopenia were recorded in the two groups. Adverse effects rate = number of patients with adverse effects/total number of patients of each group ×100%.
After a follow-up for 3 years, the survival time and survival rate were calculated, which were compared between the two groups of patients.
Statistical analysis
Statistical analysis was processed using the SPSS 22.0 software. The counting data were presented as the number of cases and percentage (n, %), while the quantitative data was presented as mean ± standard deviation (x̅ ± sd). The quantitative data that followed normal distribution were analyzed by t test, whereas the counting data were compared by χ2 test. The Kaplan-Meier (K-M) analysis was applied to compare the overall survival rates of the two groups. P<0.05 was considered as significantly different.
Results
Comparison of general information between the two groups of patients
The data demonstrated that no significant difference was found regarding the general information such as age and gender between the two groups of patients (P>0.05, Table 1).
Table 1.
Comparison of general information between the two groups of patients (x̅ ± sd, n)
| Indicator | Observation group (n=67) | Control group (n=67) | t/χ2 | P |
|---|---|---|---|---|
| Age (years) | 53.4±5.7 | 53.9±5.2 | 0.530 | 0.597 |
| Gender | 0.308 | 0.579 | ||
| Male | 44 | 47 | ||
| Female | 23 | 20 | ||
| Pathological type | 0.729 | 0.695 | ||
| Nodular | 33 | 31 | ||
| Bulky | 28 | 32 | ||
| Diffuse | 6 | 4 | ||
| Child-pugh staging [13] | 0.121 | 0.728 | ||
| A | 31 | 29 | ||
| B | 36 | 38 | ||
| Clinical stage | 0.191 | 0.662 | ||
| II b-III | 53 | 55 | ||
| IV | 14 | 12 |
Comparison of clinical outcome between the two groups of patients
The results revealed that the RR and DCR of the observation group were 41.79% and 89.55%, respectively, while they were 29.85% and 61.19%, respectively in the control group. Compared with the control group, the DCR was significantly higher in the observation group (P<0.05, Table 2).
Table 2.
Comparison of clinical efficacy between the two groups of patients (n, %)
| Group | CR (%) | PR (%) | SD (%) | PD (%) | RR (%) | DCR (%) |
|---|---|---|---|---|---|---|
| Observation group (n=67) | 1 (1.49) | 27 (40.30) | 32 (47.76) | 7 (10.45) | 28 (41.79) | 60 (89.55) |
| Control group (n=67) | 0 (0.00) | 20 (29.85) | 21 (31.34) | 26 (33.81) | 20 (29.85) | 41 (61.19) |
| χ2 | 2.078 | 4.787 | ||||
| P | 0.150 | 0.029 |
Comparison of CD3+, CD4+, CD8+, and CD4+/CD8+ levels between the two groups before and after treatment
Before treatment, the expression levels of CD3+, CD4+, CD8+, and CD4+/CD8+ between the two groups did not reveal any significant difference (P>0.05). After treatment, the expression levels of CD3+, CD4+, and CD4+/CD8+ were significantly increased in both groups, while the level of CD8+ was notably decreased (P<0.05). In comparison to the control group, the observation group showed significantly higher levels of CD3+, CD4+, and CD4+/CD8+, while significantly lower level of the CD8+ (P<0.001, Table 3).
Table 3.
Comparison of CD3+, CD4+, CD8+, and CD4+/CD8+ levels between the two groups before and after treatment (x̅ ± sd)
| Group | Observation group (n=67) | Control group (n=67) | t | P |
|---|---|---|---|---|
| CD3+ | ||||
| Before treatment | 42.34±5.57 | 42.78±5.24 | 0.471 | 0.638 |
| After treatment | 71.39±6.94* | 53.28±6.71* | 15.356 | <0.001 |
| CD4+ | ||||
| Before treatment | 31.25±3.51 | 31.74±3.22 | 0.842 | 0.401 |
| After treatment | 44.98±4.06* | 38.21±4.03* | 9.687 | <0.001 |
| CD8+ | ||||
| Before treatment | 37.83±4.42 | 37.91±4.34 | 0.106 | 0.916 |
| After treatment | 30.01±2.59* | 33.56±2.71* | 7.752 | <0.001 |
| CD4+/CD8+ | ||||
| Before treatment | 0.87±0.20 | 0.83±0.24 | 1.048 | 0.297 |
| After treatment | 1.44±0.31* | 1.19±0.22* | 5.383 | <0.001 |
Note: compared with before treatment within the same group;
P<0.05.
Comparison of VEGF, VEGFA and bFGF levels between the two groups before and after treatment
Before treatment, no significant difference was found in terms of the levels of VEGF, VEGFA, and bFGF between the two groups (P>0.05). After treatment, the levels of VEGF, VEGFA, and bFGF were significantly decreased in both groups (P<0.05). Additionally, VEGF, VEGFA, and bFGF levels of the observation group were remarkably downregulated in comparison to those of the control group (P<0.001, Table 4).
Table 4.
Comparison of VEGF, VEGFA and bFGF levels between the two groups before and after treatment (x̅ ± sd)
| Group | Observation group (n=67) | Control group (n=67) | t | P |
|---|---|---|---|---|
| VEGF (pg/mL) | ||||
| Before treatment | 447.24±76.55 | 452.83±81.32 | 0.410 | 0.683 |
| After treatment | 208.79±43.11* | 294.45±40.17* | 11.899 | <0.001 |
| VEGFA (pg/mL) | ||||
| Before treatment | 232.56±33.42 | 237.81±34.27 | 0.898 | 0.371 |
| After treatment | 154.89±21.03* | 187.33±23.72* | 8.376 | <0.001 |
| bFGF (pg/mL) | ||||
| Before treatment | 121.46±13.35 | 124.51±14.76 | 1.254 | 0.212 |
| After treatment | 71.34±8.89* | 92.75±8.02* | 14.637 | <0.001 |
Note: compared with before treatment within the same group;
P<0.05.
Comparison of the rate of adverse effects between the two groups of patients
The results revealed that the incidence of adverse effects of the observation group was significantly lower in comparison to that of the control group (22.39% vs. 44.78%, P<0.05, Table 5).
Table 5.
Comparison of the incidence of adverse effects between the two groups of patients (n, %)
| Symptom | Observation group (n=67) | Control group (n=67) | χ2 | P |
|---|---|---|---|---|
| Gastrointestinal reactions | 4 (5.97) | 7 (10.45) | 0.891 | 0.345 |
| Anemia | 1 (1.49) | 7 (10.45) | 4.786 | 0.029 |
| Fever | 1 (1.49) | 2 (2.98) | 0.341 | 0.559 |
| Fatigue | 2 (2.98) | 1 (1.49) | 0.341 | 0.559 |
| Bone marrow suppression | 6 (8.96) | 8 (11.94) | 0.319 | 0.572 |
| Leukopenia | 1 (1.49) | 5 (7.46) | 2.792 | 0.095 |
| Total | 15 (22.39) | 30 (44.78) | 4.540 | 0.033 |
Comparison of overall survival rate between the two groups of patients
After the follow-up for 3 years, the median survival time of the observation group was 32.86 months, with an overall survival rate of 61.19%, while the median survival time of the control group was 23.83 months, with an overall survival rate of 41.79%. Statistical analysis showed that the overall survival rate was significantly higher in the observation group than that of the control group (χ2=21.150, P=0.001, Figure 1).
Figure 1.
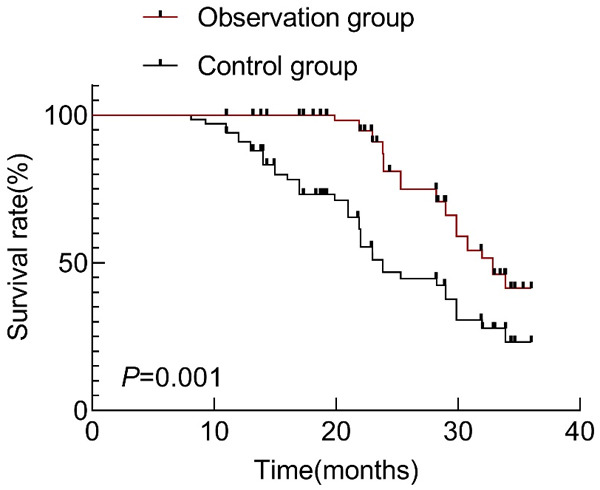
Comparison of overall survival rate between the two groups of patients.
Discussion
Due to the lack of specific biomarkers for early diagnosis of HCC, patients have already progressed to the intermediate and late-stage HCC when diagnosed, resulting in missing the best time for treatment. Currently, TACE is often applied to treat HCC in clinical practice. Although this approach has been approved to have promising outcome, it has been reported that tumors are prone to relapse after TACE treatment [17]. The possible reason is that TACE can cause hypoxia in tumor microenvironment, which favors the growth of tumors and induces the angiogenesis. Neovascularization is the fundamental step for the initiation and development of tumors, which is medicated by hypoxia in tumor microenvironment [18]. Studies have reported that when the body is under hypoxic conditions, hypoxia-inducible factor (HIF) upregulates VEGF expression, which in turn promotes the proliferation of vascular endothelial cells and the neovascularization, reduces extracellular matrix (EXM), and increases the permeability of tumor blood vessels [19]. VEGFA is a member of the VEGF family, which can interact with VEGFR2 to induce the tumor angiogenesis [20]. bFGF refer to a type of polypeptide with a wide range of biological functions. Studies have shown that bFGF can promote the cell division and proliferation of mesoderm and ectoderm, thereby inducing the formation of new blood vessels [21]. In addition, studies have also revealed that bFGF can upregulate the expression of VEGF, increase the level of VEGF in vascular smooth muscle, and indirectly induce the angiogenesis [22]. Therefore, this study uses VEGF, VEGFA, and bFGF as biological indicators to evaluate clinical outcome of TACE in combination with thalidomide-mediated adjuvant therapy in the treatment of HCC.
Thalidomide is a class of glutamic acid derivatives with sedative and antiemetic effects, which has been initially used to control early pregnancy reactions. However, with the occurrence of serious adverse effects, its use during pregnancy has been banned. Recently, researchers have demonstrated that thalidomide can effectively inhibit the expression of VEGF and bFGF, thereby suggesting an anti-angiogenic effect [23]. In addition, thalidomide can also regulate the expression of some cytokines, improve the body’s immune function, and play an anti-tumor effect. Therefore, studies have been conducted in exploring its clinical effects in the treatment of cancer [24,25]. This study has applied thalidomide combined with TACE to treat HCC, which demonstrates that compared with the control group, the DCR is significantly higher in the observation group. By either TACE alone or combined therapy, the levels of VEGF, VEGFA, and bFGF in patients are considerably downregulated, and the combined therapy has revealed more reduction regarding the levels of these biological indicators. Our data indicate that thalidomide combined with TACE can suppress the tumor angiogenesis by significantly inhibiting the expression levels of VEGF and bFGF. In addition, the occurrence of adverse effects has also been compared between the two groups during the treatment, which shows that adverse effects such as gastrointestinal reactions, anemia, fever, fatigue, bone marrow suppression, and leukopenia are found among some patients in both groups. However, the total adverse effects are significantly higher in the control group than those of the observation group, which suggests that in order to have the timely treatment abnormal responses of patients should be taken into account.
T lymphocytes play important roles in our body’s immune function. CD3+ represents the total T lymphocytes, which can be divided into CD4+ and CD8+ subgroups. CD4+CD25+ Tr of CD4+ cells are regulatory T cells, which mainly function in the modulation of the immune system. On the contrary, CD8+ cells together with CD4+ cells maintain the balance of the immune response. Our data have shown that the level of T lymphocyte subgroups in patients has been improved after treatment, and the improvement is more significant for the treatment via thalidomide combined with TACE, suggesting that the combined treatment can significantly improve patients’ immune function. In addition, this study has compared the overall survival rate through a 3-year follow-up between the two groups, which reveals that the median survival time of the observation group is 32.86 months (overall survival rate of 61.19%), while the median survival time of patients treated with TACE alone is 23.83 months (overall survival rate of 41.79%), indicating a better long-term outcome with combined treatment.
In summary, TACE in combination with thalidomide-mediated adjuvant treatment of patients with HCC has shown a promising clinical outcome via downregulating the levels of VEGF and bFGF. However, this study has not observed the dynamic changes of various biological indicators, the sample size is small, and other indicators are not included to comprehensively evaluate the effect of this combined approach, leading to limitations of this study. Therefore, follow-up studies are warranted.
Disclosure of conflict of interest
None.
References
- 1.Wu FX, Chen J, Bal T, Zhu SL, Yang TB, Qi LN, Zou L, Li ZH, Ye JZ, Li LQ. The safety and efficacy of transarterial chemoembolization combined with sorafenib and sorafenib mono- therapyin patients with BCLC Stage B/C hepatocellular carcinoma. BMC Cancer. 2017;17:645. doi: 10.1186/s12885-017-3545-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao J, Gray SG, Wabitsch M, Greene CM, Lawless MW. The therapeutic properties of resminostat for hepatocellular carcinoma. Oncoscience. 2018;5:196. doi: 10.18632/oncoscience.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kwan SW, Harris WP, Gold LS, Hebert PL. Comparative effectiveness of transarterial embolization and sorafenib for hepatocellular carcinoma: a population-based study. AJR Am J Roentgenol. 2018;210:1359. doi: 10.2214/AJR.17.19094. [DOI] [PubMed] [Google Scholar]
- 4.Wu X, Chen R, Zheng W, Hu J. Comprehensive analysis of factors affecting clinical response and short-term survival to drugeluting bead transarterialchernoembolization for treatment in patients with livercancer. Technol Cancer Res Treat. 2018;17:1533033818759878. doi: 10.1177/1533033818759878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang H, Du PC, Wu MC, Cong WM. Postoperative adjuvant transarterial chemoembolization for multinodular hepatocellular carcinoma within the barcelona clinic liver cancer early stage and microvascular invasion. Hepatobiliary Surg Nutr. 2018;7:418–428. doi: 10.21037/hbsn.2018.09.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad H, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 7.Liang PC, Ch’ang HJ, Hsu C, Chen LT, Shih TTF, Liu TW. Perfusion parameters of dynamic contrast-enhanced magnetic resonance imaging predict outcomes of hepatocellular carcinoma receiving radiotherapy with or without thalidomide. Hepatol Int. 2015;9:258–268. doi: 10.1007/s12072-014-9557-1. [DOI] [PubMed] [Google Scholar]
- 8.Wu J, Ng J, Christos PJ, Goldenberg AS, Sparano J, Sung MW, Hochster HS, Muggia FM. Chronic thalidomide and chemoembolization for hepatocellular carcinoma. Oncologist. 2014;19:1229–1230. doi: 10.1634/theoncologist.2014-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramjiawan RR, Griffioen AW, Duda DG. Anti-angiogenesis for cancer revisited: is there a role for combinations with immunotherapy? Angiogenesis. 2017;20:185–204. doi: 10.1007/s10456-017-9552-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu S, Sun J, Zhang J, Xu XF, Li H, Shan BZ, Tian T, Wang HC, Ma DX, Ji CY. Aberrant expression and association of VEGF and Dll4/notch pathway molecules under hypoxia in patients with lung cancer. Histol Histopathol. 2013;28:277–284. doi: 10.14670/HH-28.277. [DOI] [PubMed] [Google Scholar]
- 11.Zhang S, Gao X, Fu W, Li SW, Yue LM. Immunoglobulin-like domain 4-mediated ligand-independent dimerization triggers VEGFR-2 activation in HUVECs and VEGFR2- positive breast cancer cells. Breast Cancer Res Treat. 2017;163:423–434. doi: 10.1007/s10549-017-4189-5. [DOI] [PubMed] [Google Scholar]
- 12.Chen YC, Zhang L, Li EN, Ding LX, Zhang GA, Hou Y, Yuan W. Association of the insulin-like growth factor-1 single nucleotide polymorphisms rs35767, rs2288377, and rs5742612 with osteoporosis risk: a meta-analysis. Medicine (Baltimore) 2017;96:e9229–e9231. doi: 10.1097/MD.0000000000009231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ministry of Health of the People’s Republic of China. Standards for diagnosis and treatment of hepatocellular carcinoma (2011) J. Clin. Oncol. 2011;16:929–946. [Google Scholar]
- 14.Ao M, Xiao X, Yan Y, Shi Y, Lv XY. The effect of thalidomide on vascular endothelial growth factor and quality of life after interventional primary liver cancer. Adv Mod Biomed. 2017;17:4928–4931. [Google Scholar]
- 15.Spagnuolo RD, Brich S, Bozzi F, Conca E, Castelli C, Tazzari M, Maestro R, Brenca M, Gualeni AV, Gloghini A, Stacchiotti S, Pierotti MA, Pilotti S, Negri T. Sunitinib-induced morph of unctional changes and drug effectiveness in malignant solitary fibrous tumours. Oncotarget. 2016;7:45015–45026. doi: 10.18632/oncotarget.7523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang XN, Wu YL. Evaluation criteria for therapeutic efficacy of solid tumors-recist. Evidence-Based Med. 2004;4:85–90. 111. [Google Scholar]
- 17.Huang WK, Yang SF, You LN, Liu M, Liu DY, Gu P, Fan XW. Transcatheter arterial chemoembolisation (TACE) plus S-1 for the treatment of BCLC Stage B hepatocellular carcinoma refractory to TACE. Contemp Oncol (Pozn) 2016;20:468–474. doi: 10.5114/wo.2016.65607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tohme S, Chidi AP, Sud V, Tsung A. Prognostic nutritional index is associated with survival in patients with unresectable hepatocellular carcinoma treated with radioembolization. J Vasc Interv Radiol. 2017;28:470–472. doi: 10.1016/j.jvir.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 19.Poluzzi C, Iozzo RV, Schaefer L. Endostatin and endorepellin: a common route of action for similar angiostatic cancer avengers. Adv Drug Deliv Rev. 2016;97:156–173. doi: 10.1016/j.addr.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang S, Tang D, Zang W, Yin G, Dai JG, Sun YU, Yang ZJ, Hoffman RM, Guo XX. Synergistic inhibitory effect of traditional chinese medicine astragaloside iv and curcumin on tumor growth and angiogenesis in an orthotopic nude-mouse model of human hepatocellular carcinoma. Anticancer Res. 2017;37:465–473. doi: 10.21873/anticanres.11338. [DOI] [PubMed] [Google Scholar]
- 21.El-Raggal NM, El-Farrash RA, Saad AA, Attia EAS, Saafan HA, Shaaban IS. Circulating levels of vascular endothelial growth factor and basicfibroblastic growth factor in infantile hemangioma versus vascular malformations. Clin Appl Thromb Hemost. 2017;24:663–668. doi: 10.1177/1076029617710333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang JQ, Song HL, Lu YQ, Chen HY, Jiang S, Li LL. Effects of Estradiol on VEGF and bFGF by Akt in endometrial cancer cells are mediated through the NF-xB pathway. Oncol Rep. 2016;36:705–714. doi: 10.3892/or.2016.4888. [DOI] [PubMed] [Google Scholar]
- 23.Decourt B, Drumm-Gurnee D, Wilson J, Jacobson S, Belden C, Sirrel S, Ahmadi M, Shill H, Powell J, Walker A, Gonzales A, Macias M, Sabbagh MN. Poor safety and tolerability hamper reaching a potentially therapeutic dose in the use of thalidomide for Alzheimer’s disease: results from a double-blind, placebo-controlled trial. Curr Alzheimer Res. 2017;14:403–411. doi: 10.2174/1567205014666170117141330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raturi R, Patel AA, Carter JD. Two cases demonstrating thalidomide’s efficacy in refractory lupus nephritis. Clin Rheumatol. 2017;36:725–728. doi: 10.1007/s10067-016-3511-7. [DOI] [PubMed] [Google Scholar]
- 25.Tokida H, Kanaya Y, Shimoe Y, Yamori S, Tagawa K, Kuriyama M. Lateral geniculate body presenting only hemorrhage homonymous hemianopia-a case report. Rinsho Shinkeigaku. 2016;56:781–784. doi: 10.5692/clinicalneurol.cn-000935. [DOI] [PubMed] [Google Scholar]


