Abstract
Objective: This research was designed to probe into the clinical significance of anti-mullerian hormone (AMH) levels in early missed abortion. Methods: Forty-six women with early missed abortions treated in our hospital from October 2018 to June 2019 were collected as the research subjects and included in the observation group (OG), while 51 normal pregnant women were included in the control group (CG) during the same period. The levels of AMH, human follicle stimulating hormone (FSH) and human luteinizing hormone (LH) in the serum of women of both groups were tested by enzyme-linked immunosorbent assay (ELISA). The diagnostic value of AMH in early missed abortion was analyzed by receiver operating characteristic (ROC). The correlation between AMH and FSH, LH was assessed via Pearson correlation. According to the median expression of AMH before treatment, patients were divided into high and low expression groups (HEG, LEG, respectively), and time of vaginal bleeding and menstrual resurgence, and the incidence of coagulation dysfunction were compared after operation. Results: The AMH and FSH levels in serum of patients in the OG were obviously lower than those in the CG, and the LH level was markedly higher. The area under the curve of serum AMH was 0.867. AMH was positively correlated with FSH and negatively correlated with LH. The time of vaginal bleeding and menstruation resurgence of the HEG patients were remarkably lower than those of the LEG (All P < 0.05). Conclusion: Serum AMH level is expected to be a good prognostic indicator in diagnosing early missed abortion.
Keywords: Anti-mullerian hormone, early missed abortion, diagnosis, prognosis
Introduction
Missed abortion is a maternal issue where an embryo remains in the uterine cavity because of the failure of discharge after death of the embryo [1,2]. There are many factors in the pregnancy process, which will lead to natural abortion; so, there are many factors causing missed abortions [3,4]. Because the dead embryo will also generate thrombin in the uterus, which enter the maternal blood, the harm of a missed abortion mainly includes damage to the maternal coagulation function and disseminated intravascular coagulation, while placenta organization may cause uterine perforation and cavity adhesion; thus, if the embryo tissue is not removed thoroughly and in time, it can cause harm to the woman [5-7].
At the moment, missed abortion can be treated clinically by surgery and drugs, with good efficacy. Therefore, if patients can be diagnosed in the early stage of the issue, effective treatment schemes can be taken in time, thus improving the physical and mental health [8,9]. Anti-mullerian hormone (AMH) is produced by granulosa cells of preantral and ovarian follicles. Because AMH can take part in the follicular formation process, it is often used as an index to predict the follicular density, ovarian reserve and some fertility-related indicators in women, and has been widely used in artificial pregnancy [10-12]. Previous studies have also found that AMH is related to the identification of some abortion events. For example, Hong et al. [13] have mentioned that AMH level and age are a powerful predictors of early abortion in infertile patients. However, it is still unclear whether AMH has a predictive value in early missed abortion, and there is little research on the relationship between AMH and prognosis of missed abortion. Thus, the AMH level of early missed abortion in patients was detected to explore its clinical value in diagnosis and prognosis.
Methods
Patient data
Forty-six patients with early missed abortion were treated by curettage in Hubei Maternal and Child Health Hospital from October 2018 to June 2019. They were included in the observation group (OG), with an average age of (28.9±5.7) years, while 51 normally pregnant women were included in the control group (CG) with an average age of (28.2±4.8) years. This research was approved by the Medical Ethics Committee of our hospital. All patients were informed about the study details in advance, and they signed an informed consent form.
Inclusion and exclusion criteria
Inclusion criteria were as follows: the participants had a normal menstrual cycle, and the pregnancies were naturally conceived. Missed abortion was indicated by ultrasound without embryo bud or fetal heart beat, and confirmed by pathological examination after curettage. The clinical data of the women was complete and they were willing to cooperate with treatment and follow-up.
Exclusion criteria were as follows: women who had ovarian insufficiency; patients who were complicated with other gynecological inflammation, severe endometriosis or adenomyosis; chromosome abnormality; uterine malformation; habitual abortion; those who withdrew from the study halfway.
Detection methods
At 7:00 a.m., the next day after the subjects were admitted to hospital, 5 mL sterile venous blood was taken and put into a coagulation promoting tube. The blood serum was collected by centrifuge (3000×g at 4°C for 10 min) and stored in a freezer at -80°C. The levels of AMH, human follicle stimulating hormone (FSH) and human luteinizing hormone (LH) were tested via ELISA. Based on the kit instructions, the concentration of cytokines was determined by absorbance value and standard curve. All samples were tested three times. AMH, FSH and LH ELISA kits were all provided by Abcam (ab267629, ab108678, ab178658), and the instrument was an E170 all-active immunity analyzer bought from Roche, Switzerland.
Outcome measures
Main outcome measures: the levels of AMH, FSH and LH in the OG and the CG were observed, and the diagnostic value of AMH in missed abortion was detected by receiver operating characteristic (ROC) curve.
Secondary outcome measures: the correlation between serum AMH, FSH and LH in the OG was analyzed by Pearson test. Patients in the OG were divided into high and low expression groups (HEG, LEG) based on the median AMH level, and the time of vaginal bleeding and menstrual resurgence of both groups were compared.
Statistical methods
SPS with Windows version 18.0 (SPSS, Chicago, Illinois, USA) was used for statistical analysis. All data were expressed as Mean ± SD. The comparison between groups was analyzed by Student’s t test. The diagnostic value of AMH in missed abortion was evaluated by ROC. Pearson correlation was employed for correlation analysis. P < 0.05 was considered to be statistically remarkable.
Results
Baseline data of patients
By comparing the clinical baseline data of both groups, we found that there was no marked difference in age, BMI, pregnancy complications (gestational hypertension, gestational diabetes mellitus), history of abortion, previous pregnancy, or number of birth, cervical diseases and medication history during pregnancy (P > 0.05) (Table 1).
Table 1.
Baseline data table
| Control group (n=51) | Observation group (n=46) | X2 | P | ||
|---|---|---|---|---|---|
| Age (years) | < 35 | 45 (88.24) | 37 (80.43) | 1.126 | 0.289 |
| ≥ 35 | 6 (11.76) | 9 (19.57) | |||
| BMI (kg/m2) | < 18.5 | 5 (9.80) | 9 (19.57) | 1.866 | 0.172 |
| ≥ 18.5 | 46 (90.20) | 37 (80.43) | |||
| Complications during pregnancy | Gestational hypertension | 4 (9.80) | 3 (6.52) | 0.344 | 0.557 |
| Gestational diabetes mellitus | 3 (5.88) | 1 (2.17) | 0.841 | 0.359 | |
| Abortion history | Yes | 6 (11.76) | 8 (17.39) | 0.620 | 0.431 |
| No | 45 (88.24) | 38 (82.61) | |||
| Previous pregnancy history (times) | < 2 | 45 (88.24) | 37 (80.43) | 1.126 | 0.289 |
| ≥ 2 | 6 (11.76) | 9 (19.57) | |||
| Birth history | Yes | 5 (9.80) | 7 (15.22) | 0.634 | 0.419 |
| No | 46 (90.20) | 39 (84.78) | |||
| Cervical diseases | Yes | 5 (9.80) | 9 (19.57) | 1.866 | 0.172 |
| No | 46 (90.20) | 37 (80.43) | |||
| Medication history during pregnancy | Yes | 18 (35.29) | 22 (47.83) | 1.567 | 0.211 |
| No | 33 (64.71) | 24 (52.17) |
Detection of patient indicators
We detected the AMH, FSH and LH levels in both groups, and found that the AMH and FSH levels in the OG were markedly lower than those in the CG (P < 0.05), while the LH level was obviously higher than that in the CG (P < 0.05). By drawing the ROC curve for diagnosing missed abortion with AMH, we found that the area under the curve of AMH was 0.867 (Figure 1).
Figure 1.
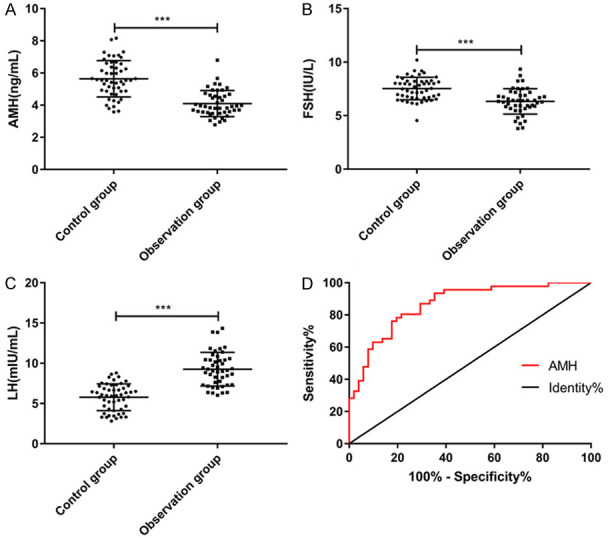
Expression levels of AMH, FSH and LH. A. The level of AMH in the observation group is markedly lower than that in the control group (t=7.658, P < 0.001). B. The FSH level in the observation group is dramatically lower than that in the control group (t=5.310, P < 0.001). C. The LH level in the observation group is remarkably higher than that in the control group (t=9.132, P < 0.001). *** means P < 0.001. D. The diagnostic ROC curve of AMH in early missed abortion shows that the area under the curve is 0.867, and the sensitivity and specificity are 80.43% and 78.43%.
Correlation between AMH and FSH, LH
Through Pearson correlation analysis of the relationship between AMH, FSH and LH in the OG, we found that the AMH and FSH expression was positively correlated and negatively correlated with LH (Figure 2).
Figure 2.
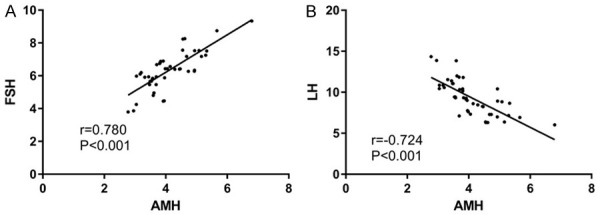
Correlation between AMH and FSH, LH. A. There is a positive correlation between AMH and FSH (r=0.780, P < 0.001). B. AMH is positively correlated with LH (r=-0.724, P < 0.001).
Postoperative adverse reactions
In view of the median AMH level of patients in the OG, they were divided into HEG and LEG. The postoperative adverse reactions of the two groups were compared. It was found that the time of vaginal bleeding and menstrual resurgence of patients in the HEG were obviously lower than those in the LEG (Table 2).
Table 2.
Adverse reaction time
| High expression group (n=23) | Low expression group (n=23) | t | P | |
|---|---|---|---|---|
| Vaginal bleeding time (d) | 6.32±1.10 | 8.71±1.43 | 6.353 | < 0.001 |
| Menstruation resurgence time (d) | 32.46±3.47 | 37.24±4.76 | 3.862 | < 0.001 |
Discussion
Missed abortion is a special kind of spontaneous abortion, which may be caused by reproductive tract infection, abnormal sex and endocrine hormones, immune factors and abnormal thyroid function during pregnancy [14,15]. The greater the gestational weeks of missed abortion women are, the closer the adhesion between placenta and uterine wall is and the greater the harm and pain of abortion is for patients. It will also increase the difficulty of treatment for patients, so early diagnosis is needed and it’s better to remove the non-viable as soon as possible [16].
In this research, we explored the clinical significance of AMH in early missed abortion, and proved that it had better diagnostic value, and the AMH level of missed abortion patients was positively correlated with the FSH and LH levels. Meanwhile, the time of vaginal bleeding and menstrual resurgence of patients with higher AMH level were less than those with a lower level.
In the first place, we compared the AMH, FSH and LH levels of normal pregnant women and missed abortion patients, and discovered that the levels of all three were remarkably lower in the latter group than those of the former. A recent study has shown that inflammation is one of the leading conditions for missed abortion, and patients often have inflammatory reactions [17]. Zhang et al. [18] mentioned that the ovarian reserve and fertility of rats transplanted with human amniotic epithelial cells were dramatically improved, and the AMH level increased and inflammation was reduced. Therefore, compared with normal pregnant women, the inflammatory reactions in patients with missed abortion may reduce their AMH level, achieving AMH decrease. Then, by drawing a ROC curve, we found that the area under the curve of AMH in diagnosing missed abortion was 0.867, and the sensitivity and specificity were 80.43% and 78.43%, which indicated that AMH had better diagnostic value and might be used as a potential diagnostic index.
Curettage is a common surgical method to treat missed abortion clinically, which helps remove stillbirth from patients by suction curettage [19,20]. However, uterine curettage damages patients’ endometrium, which leads to vaginal bleeding, abdominal pain and poor menstruation, and it also affects their sex hormone secretion [21,22]. Some studies have shown that the secretion of some hormones will affect the regeneration of endometrium after operation. FSH and LH are both involved in the regulation of uterine function and can promote the growth and repair of the endometrium [23-25]. AMH is considered as a good index of ovarian reserve function. In the meantime, recent research has found that AMH is also expressed in the endometrium, and AMH regulates the cell vitality and proliferation of endometriosis cells, which may be used as a therapeutic agent for endometriosis [26]. We suspect that AMH may improve postoperative outcomes by repairing the endometrium, and reducing time of vaginal bleeding time and menstrual resurgence. Because it has been verified to be positively correlated with the FSH and LH levels before, higher AMH indicates that patients tend to have higher FSH and LH, and that endometrial repair is better and more conducive to recovery. In the light of the median level of AMH, we divided the patients into HEG and LEG, and compared the postoperative adverse reactions between both groups. We found that the time of vaginal bleeding and menstrual resurgence of patients in the HEG were obviously lower than those in the LEG, which also verified our guess that patients with higher AMH level had better repair ability.
Nevertheless, this research also has some shortcomings. First of all, we did not detect the changes of AMH before and after treatment, so it is vague whether different treatment methods will affect AMH level. Secondly, in some studies, some indicators, such as average neutrophil count, can be used as predictive indicators of missed abortion; hence, we hope to discuss and compare the advantages and disadvantages of these other indicators in future studies.
To sum up, serum AMH level is expected to be a good prognostic index for diagnosing early missed abortion.
Disclosure of conflict of interest
None.
References
- 1.Kale K, Vishwekar P, Balsarkar G, Jassawalla MJ, Alkahtani S, Kishore U, Sawant G, Madan T. Serum levels of collectins are sustained during pregnancy: surfactant protein D levels are dysregulated prior to missed abortion. Reprod Sci. 2020;27:1894–1908. doi: 10.1007/s43032-020-00209-3. [DOI] [PubMed] [Google Scholar]
- 2.Luan X, Yan Y, Zheng Q, Wang M, Chen W, Yu J, Fang J. Excessive reactive oxygen species induce apoptosis via the APPL1-Nrf2/HO-1 antioxidant signalling pathway in trophoblasts with missed abortion. Life Sci. 2020;254:117781. doi: 10.1016/j.lfs.2020.117781. [DOI] [PubMed] [Google Scholar]
- 3.Li T, Li X, Guo Y, Zheng G, Yu T, Zeng W, Qiu L, He X, Yang Y, Zheng X, Li Y, Huang H, Liu X. Distinct mRNA and long non-coding RNA expression profiles of decidual natural killer cells in patients with early missed abortion. FASEB J. 2020;34:14264–14286. doi: 10.1096/fj.202000621R. [DOI] [PubMed] [Google Scholar]
- 4.Biyik I, Albayrak M, Keskin F. Platelet to lymphocyte ratio and neutrophil to lymphocyte ratio in missed abortion. Rev Bras Ginecol Obstet. 2020;42:235–239. doi: 10.1055/s-0040-1709693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cramer S, Hatem F, Heller DS. Placenta increta presenting as retained placenta: a report of 3 cases. Fetal Pediatr Pathol. 2019;38:215–225. doi: 10.1080/15513815.2019.1582121. [DOI] [PubMed] [Google Scholar]
- 6.Bespalova O, Bakleicheva M, Kovaleva I, Tolibova G, Tral T, Kogan I. Expression of vitamin D and vitamin D receptor in chorionic villous in missed abortion. Gynecol Endocrinol. 2019;35:49–55. doi: 10.1080/09513590.2019.1653563. [DOI] [PubMed] [Google Scholar]
- 7.Alnafisah F, Alalfy SA. Unilateral uterine and ovarian arterial ligations in a case of missed abortion at 12 weeks of gestation with undiagnosed placenta accreta. Cureus. 2018;10:e3562. doi: 10.7759/cureus.3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yousuf S, Akhter N, Arzoo S, Ferdous B. Efficacy and safety of intravaginal misoprostol for mid-trimester medical termination of pregnancy. Mymensingh Med J. 2018;27:544–549. [PubMed] [Google Scholar]
- 9.Serdinsek T, Reljic M, Kovac V. Medical management of first trimester missed miscarriage: the efficacy and complication rate. J Obstet Gynaecol. 2019;39:647–651. doi: 10.1080/01443615.2018.1535577. [DOI] [PubMed] [Google Scholar]
- 10.Eubanks AA, Nobles CJ, Hill MJ, DeCherney AH, Kim K, Sjaarda LA, Perkins NJ, Ye A, Zolton JR, Silver RM, Schisterman EF, Mumford SL. Recalled maternal lifestyle behaviors associated with anti-mullerian hormone of adult female offspring. Reprod Toxicol. 2020;98:75–81. doi: 10.1016/j.reprotox.2020.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moolhuijsen LME, Visser JA. Anti-mullerian hormone and ovarian reserve: update on assessing ovarian function. J Clin Endocrinol Metab. 2020;105:3361–73. doi: 10.1210/clinem/dgaa513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song J, Gu L, Ren X, Liu Y, Qian K, Lan R, Wang T, Jin L, Yang J, Liu J. Prediction model for clinical pregnancy for ICSI after surgical sperm retrieval in different types of azoospermia. Hum Reprod. 2020;35:1972–1982. doi: 10.1093/humrep/deaa163. [DOI] [PubMed] [Google Scholar]
- 13.Hong S, Chang E, Han EJ, Min SG, Kim S, Kang MK, Cha DH, Shim SH, Park HJ. The anti-Mullerian hormone as a predictor of early pregnancy loss in subfertile women. Syst Biol Reprod Med. 2020;66:370–377. doi: 10.1080/19396368.2020.1806944. [DOI] [PubMed] [Google Scholar]
- 14.Roberts SCM, Upadhyay UD, Liu G, Kerns JL, Ba D, Beam N, Leslie DL. Association of facility type with procedural-related morbidities and adverse events among patients undergoing induced abortions. JAMA. 2018;319:2497–2506. doi: 10.1001/jama.2018.7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Radovic Janosevic D, Popovic J, Krstic M, Tubic-Pavlovic A, Stefanovic M, Pop-Trajkovic S. The structure of immunocompetent decidual cells in recurrent missed abortions. Vojnosanit Pregl. 2016;73:306–311. doi: 10.2298/VSP141226018R. [DOI] [PubMed] [Google Scholar]
- 16.Chen C, Liu X, Chen D, Huang S, Yan X, Liu H, Chang Q, Liang Z. A risk model to predict severe postpartum hemorrhage in patients with placenta previa: a single-center retrospective study. Ann Palliat Med. 2019;8:611–621. doi: 10.21037/apm.2019.09.04. [DOI] [PubMed] [Google Scholar]
- 17.Zhang X, Zhang K, Zhang Y. Pigment epithelium-derived factor facilitates NLRP3 inflammasome activation through downregulating cytidine monophosphate kinase 2: a potential treatment strategy for missed abortion. Int J Mol Med. 2020;45:1436–1446. doi: 10.3892/ijmm.2020.4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Ouyang X, You S, Zou H, Shao X, Zhang G, Zhang C, Hu L. Effect of human amniotic epithelial cells on ovarian function, fertility and ovarian reserve in primary ovarian insufficiency rats and analysis of underlying mechanisms by mRNA sequencing. Am J Transl Res. 2020;12:3234–3254. [PMC free article] [PubMed] [Google Scholar]
- 19.Chaikof M, Lazer T, Gat I, Quach K, Alkudmani B, Zohni K, Baratz A, Glass K, Sharma P, Librach C. Lower complication rates with office-based D&C under ultrasound guidance for missed abortion. Minerva Ginecol. 2017;69:23–28. doi: 10.23736/S0026-4784.16.03935-6. [DOI] [PubMed] [Google Scholar]
- 20.Wu XQ, Zhang HW, Fang XL, Ding H, Piao L, Joseph Huang S. Factors associated with successful transabdominal sonography-guided dilation and curettage for early cesarean scar pregnancy. Int J Gynaecol Obstet. 2015;131:281–284. doi: 10.1016/j.ijgo.2015.06.029. [DOI] [PubMed] [Google Scholar]
- 21.Jiang J, Wang C, Xue M. High-intensity focused ultrasound versus uterine artery embolization for patients with retained placenta accreta. Eur J Obstet Gynecol Reprod Biol. 2020;252:82–86. doi: 10.1016/j.ejogrb.2020.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Wei SS, Li DH, Zhang ZF, Sun WC, Jia CL. Type II caesarean scar pregnancy management by ultrasound-guided local lauromacrogol injection in combination with suction curettage: a case report. Medicine (Baltimore) 2020;99:e19743. doi: 10.1097/MD.0000000000019743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piotrowska-Tomala KK, Jonczyk AW, Skarzynski DJ, Szostek-Mioduchowska AZ. Luteinizing hormone and ovarian steroids affect in vitro prostaglandin production in the equine myometrium and endometrium. Theriogenology. 2020;153:1–8. doi: 10.1016/j.theriogenology.2020.04.039. [DOI] [PubMed] [Google Scholar]
- 24.Kaczmarek MM, Blitek A, Schams D, Ziecik AJ. The effect of insulin-like growth factor-I, relaxin and luteinizing hormone on vascular endothelial growth factor secretion by cultured endometrial stromal cells on different days of early pregnancy in pigs. Reprod Biol. 2008;8:163–170. doi: 10.1016/s1642-431x(12)60011-4. [DOI] [PubMed] [Google Scholar]
- 25.James K, Bhartiya D, Ganguly R, Kaushik A, Gala K, Singh P, Metkari SM. Gonadotropin and steroid hormones regulate pluripotent very small embryonic-like stem cells in adult mouse uterine endometrium. J Ovarian Res. 2018;11:83. doi: 10.1186/s13048-018-0454-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Signorile PG, Petraglia F, Baldi A. Anti-mullerian hormone is expressed by endometriosis tissues and induces cell cycle arrest and apoptosis in endometriosis cells. J Exp Clin Cancer Res. 2014;33:46. doi: 10.1186/1756-9966-33-46. [DOI] [PMC free article] [PubMed] [Google Scholar]


