Abstract
Objective: To explore the application value of goal-directed fluid therapy (GDFT) in patients undergoing laparoscopy-assisted radical gastrectomy with fast-track anesthesia. Methods: From December 2016 to December 2019, 74 patients who underwent laparoscopy-assisted radical gastrectomy under the concept of enhanced recovery after surgery (ERAS) in gastrointestinal Surgery department of Tongling People’s Hospital were selected as research participants. They were divided into two groups: the routine group (patients were treated with conventional fluids) (n=37) and the GDFT group (patients were treated with GDFT) (n=37). In the two groups, patients were compared in terms of intraoperative fluid inflow and outflow, hemodynamic indexes before operation for 30 min (T0), after anesthesia induction for 30 min (T1), during operation for 0.5 h (T2) and 1.5 h (T3) and after operation (T4), postoperative complications, postoperative recovery, mini-mental state examination (MMSE) scores on the first day (d0) before operation and the first day (d1), the third day (d2) and the seventh day (d3) after operation, and inflammatory factor levels. Results: The amount of crystal input, colloid, blood loss, fluid replacement and urine volume in the GDFT group were significantly less than those in the routine group (P < 0.05). From T1 to T4, the values of mean arterial pressure (MAP) and central venous pressure (CVP) in the GDFT group were higher than those in the routine group (p < 0.05). The total incidence of postoperative complications in the GDFT group was lower than that in the routine group (P < 0.05). Compared with those in the routine group, the postoperative anus exhaust time, the first time of starting to eat, the time of leaving bed, the duration of stay in the postanesthesia care unit and the hospital stay were significantly shorter in the GDFT group (P < 0.05). From D1 to D3, the MMSE score in the GDFT group was higher than that in the routine group, while the levels of C-reactive protein (CPR), interleukin 6 (IL-6) and procalcitonin (PCT) were lower than those in the routine group (P < 0.05). Conclusion: GDFT has a better effect on the rapid rehabilitation of patients undergoing laparoscopy-assisted radical gastrectomy during fast-track anesthesia, and it also has a positive effect on maintaining the stability of hemodynamics, reducing systemic inflammation and decreasing postoperative complications.
Keywords: Goal-directed fluid, radical gastrectomy, fast-track anesthesia, inflammation
Introduction
Gastric cancer is a common malignant tumor and the third leading cause of cancer-related death in the world [1]. According to statistics, there were 679,100 new cases and 498,000 deaths in China in 2015 [2]. Radical surgery is the first treatment for gastric cancer, which can be divided into two types: open surgery and laparoscopic surgery [3]. Compared with open surgery, laparoscopic surgery has the advantages of minimal trauma, quick recovery and mild pain, so it is widely recognized by doctors and patients [4]. However, the operation space of laparoscopic surgery is limited, which requires high level of surgery technique of the operator. Moreover, laparoscopic surgery needs to establish carbon dioxide pneumoperitoneum, which can cause hypercapnia, intraperitoneal hypertension syndrome and other stress reactions [5,6]. Therefore, it is critical to keep good perioperative management of patients undergoing radical gastrectomy .
The concept of enhanced recovery after surgery (ERAS) was first proposed by Danish surgeon Kehlet, and it has been widely used in various surgical operations [7]. This concept is a standard, evidence-based medicine based perioperative management plan, which is coordinated by multidisciplinary medical staff and aims at reducing surgical stress, accelerating recovery of physiological functions, reducing complications and shortening hospitalization time [8]. Because both insufficient and excess blood volume may increase postoperative complications, the intraoperative fluid-supplement therapy in ERAS is controversial at present [9]. Goal-directed fluid therapy (GDFT) refers to improving cardiac output or tissue oxygen supply to an abnormal state, with this as the primary objective for fluid therapy [10]. Up to now, more and more studies have shown that GDFT can bring better clinical benefits during perioperative period. For example, GDFT can not only reduce the incidence of postoperative complications and postoperative bleeding in hip revision operation, but also reduce the hospitalization and ICU stay time [11]. Among the high-risk patients undergoing brain surgery, intraoperative GDFR is related to the decrease of hospitalization days and expenses in ICU and the decreased rate of postoperative morbidity [12]. Fast-track anesthesia is an internationally respected anesthesia program in recent years, which advocates applying multi-mode perioperative recovery program to speed up the recovery of patients, so it coincides with ERAS concept.
This study was designed to evaluate the application value of GDFT in patients undergoing laparoscopy-assisted radical gastrectomy with fast-track anesthesia, so as to provide reference data for clinical practice.
Materials and methods
Research objects
From December 2016 to December 2019, 74 patients who underwent laparoscopy-assisted radical gastrectomy under the concept of enhanced recovery after surgery (ERAS) in gastrointestinal Surgery department of Tongling People’s Hospital were selected as research participants. According to the order of visits, they were assigned numbers and randomly divided into routine fluid treatment group (routine group) and GDFT group by computer software for a prospective case-control study.
Inclusion criteria: All selected patients underwent routine preoperative examination, immune function and abdominal CT examination, and there was no distant organ metastasis such as liver, spleen, lung and brain, and the cardiac function was evaluated by cardiac ultrasound. The complications that might develop in two different preoperative fluid therapy methods were fully explained to patients and their families, and the patients and their families agreed with them, and signed an informed consent form.
Exclusion criteria were as follows: Patients younger than 30 years old or older than 70 years old; serious dysfunction of heart, lung, brain, kidney and other organs; severe arrhythmia; serious allergy; digestive tract obstruction or immune diseases; patients who had received preoperative radiotherapy and chemotherapy; severe malnutrition; patients who had received plasma or human serum albumin for many times during perioperative period; those who needed organs joint excision; those who were transferred from laparoscope to open surgery.
This research has been approved by the Ethics Committee of our hospital, and the Helsinki Declaration was strictly followed in the process of research.
Methods
Preoperative preparation
a. Before operation for 1 day, patients were visited for preoperative counseling and psychological counseling to reduce anxiety and improve compliance. b. Before surgery, the organ functions were evaluated and optimized to reduce surgical risks. c. Preoperative intestinal preparation: Before surgery, the mechanical bowel preparation was not performed. Only at 17:00 before surgery, the patient was given 2 packets of compound polyethylene glycol electrolyte powder plus 1500 ml of warm water orally. d. No drinking time before operation: 10% of warm sugar water (250 ml) was given to the patient orally before operation for 2 h. e. Before operation, the serum albumin, C-reactive protein and procalcitonin levels were examined in the laboratory.
Methods and procedures of anesthesia
The anesthesia was thoracic segment (T8-9) continuous epidural anesthesia combined with intravenous compound general anesthesia.
Preoperative treatment: After entering the operating room, all the surgical patients were given indwelling needles to establish intravenous channels, and they were instilled compound electrolyte solution to supplement physiological requirements and lost body fluid caused by fasting and intestinal preparation. The vital signs were monitored, including: (1) Noninvasive blood pressure; (2) Electrocardiogram; (3) Finger pulse oxygen saturation; (4) BIS monitoring anesthesia depth; (5) No preoperative medication. First, the thoracic spinal canal block puncture was performed, and then radial artery puncture catheterization was condcuted and connected with pressure transducer to monitor arterial blood pressure (In GDFT group, FloTrac sensor was connected with Vigileo monitor to monitor the cardiac index and variability of stroke volume). In order to avoid preoperative stress, the patients in the two groups were given midazolam (1~1.5 mg) for intravenous drip and oxygen inhalation by mask after entering the room, and the baseline values of each monitoring index were recorded after stabilization.
The anesthesia was induced by total intravenous anesthesia. The patients were slowly injected with sufentanil (0.5 ug/kg), and the patients were injected with propofol (1.5-2 min/kg) 2 minutes later, and the rocuronium (1 mg/kg) was given to patients when BIS was less than 60.
Tracheal intubation: The tracheal intubation was completed and mechanical ventilation was conducted after the patient’s eyelash reflection disappeared, muscle relaxation improved, and BIS was maintained at about 45. The tidal volume was 8 ml/kg, PETC0235-45 mmhg was maintained, and the nasopharyngeal temperature was monitored. After endotracheal intubation, the catheterization of the right internal jugular vein was performed to monitor CVP.
Anesthesia maintenance: The dexmedetomidine (0.8 ug/kg), propofol (50-100 ug/kg/min), remifentanil (0.02-0.12 ug/kg/min) and rocuronium was injected intermittently, and 0.375% of ropivacaine was injected every 1 h to maintain analgesia. The dosage of opioid analgesics was reduced, and BIS values were maintained between 45 and 60. The injection of rocuronium was stopped 30 min before the end of operation, and the propofol and remifentanil were stopped 10 min before the end of operation.
Fluid management
Fluid supplementation scheme in group A: FloTrac/Vigileo monitoring system (Edwards Company, USA) was used to monitor CO, CI, SV, SVI and SVV. The fluid supplementation target was CI 2.5~4.0 L•min-1•m-2, SVV 2%~13%, MAP 65~110 mmHg and SVI 35~47 ml/m2. Specific liquid infusion scheme: When CI was greater than 2.5 L•min-1•m-2, SVV was less than 13%, and MAP was greater than 65 mmHg, the infusion was slowed down. When CI was less than 2.5 L•min-1•m-2, SVV was more than 13%, MAP was less than 65 mmHg, and SVI was less than 35 ml/m2, patients were rapidly injected with compound electrolyte or 130/0.4 of hydroxyethyl starch solution (250 ml) (rate: 250 ml/30 min). If SVV and SVI changed obviously (the decrease of SVV was more than 2%), 250 ml of liquid could be injected again. If SVV and SVI did not change significantly (the decrease in SVV was less than 2%), patients could be intravenously injected with dobutamine at 10 ml/h (the drug concentration was 50 mg/50 ml). When CI was less than 2.5 L•min-1•m-2, SVV was less than 13%, MAP was less than 65 mmhg, and SVI was less than 35 ml/m2, patients were intravenously injected with 3-10 ml/h of dobutamine (the drug concentration was 50 mg/50 ml). When CI was greater than 2.5 L•min-1•m-2, SVV was greater than 13%, and SVI was less than 35 ml/m2, patients were rapidly injected with compound electrolyte or 130/0.4 of hydroxyethyl starch solution (250 ml) (rate: 250 ml/30 min). If SVV and SVI changed obviously (the decrease of SVV was more than 2%), the liquid (250 ml) could be injected again. If SVV and SVI did not change significantly, the liquid infusion could be slowed down. When CI was greater than 2.5 L•min-1•m-2, SVV was less than 13%, SVI was greater than 35 ml/m2, and MAP was less than 65 mmHg, the patients were intravenously injected with norepinerepine (3-10 ml/h) (the drug concentration was 2 mg/50 ml), and the fluid infusion was slowed or suspended.
Fluid supplementation scheme in group B: The infusion volume was composed of compensatory vascular dilatation, fluid loss during fasting, physiological maintenance, third gap loss, blood and body fluid loss during operation. The MAP was maintained at 60~110 mmHg and CVP was maintained at 6~12 cmH2O. The crystal solution was compound electrolyte solution and the colloid solution was 130/0. The ratio of 4-hydroxyethyl starch solution and input crystal solution to colloid solution was 2:1. The infusion rate was 1000 ml/h at the first hour after the patient entered the operating room, and then slowed down (the rate was maintained at 250~500 ml/h).
Post-operative treatment
After the operation, the patient was extubated (breathing > 8 times/min and PetCO2 < 45 mmHg) after the recovery of spontaneous breathing and response to the instruction to open eyes, and then sent to PACU.
Outcome measures
The mean arterial pressure (MAP) and central venous pressure (CVP) were recorded by Drage multifunctional monitor at 30 min before operation (T0), 30 min after anesthesia induction (T1), 0.5 h during operation (T2), 1.5 h during operation (T3) and after operation (T4).
The incidence of postoperative complications and recovery indicators were recorded in the two groups. The postoperative complications mainly included incision infection, inflammatory intestinal obstruction, anastomotic leakage and cholecystitis. The postoperative recovery indicators mainly included anal exhaust time, the first time of starting to eat, the time of leaving bed, the time of PACU stay and hospitalization time.
The fasting venous blood (3 ml) were collected from patients in the two groups on the first day (d0) before operation and the first day (d1), the third day (d2) and the seventh day (d3) after operation, and the serum was obtained by centrifugation. The levels of C-reactive protein (CRP), interleukin-6 (IL-6) and procalcitonin (PCT) in serum were measured by enzyme-linked immunosorbent assay.
The mini-mental state scale (MMSE) [13] was used to score the cognitive function of patients in the two groups before anesthesia for 24 hours and after anesthesia for 6 hours, 12 hours and 24 hours, with a full score of 30. Patients with less than 27 points meant cognitive impairment. The lower the score, the higher the degree of impairment.
Statistical processing
SPSS 18.0 (IBM Corp, Armonk, NY, USA) was used for statistical analysis, and GraphPad Prism 7 was used to draw this data picture. The comparison between these counting data was conducted by Chi-square test or Fisher exact test. The measurement data between two groups were compared by independent t test. Single factor analysis of variance was used to compare the mean among the multiple groups, and the subsequent pairwise comparison was conducted by Dunnett-t test. The difference was statistically significant with P < 0.05.
Results
Comparison of clinical data
By comparing the clinical data in the two groups, it was found that there was no significant difference in terms of gender, age, ASA grade, tumor location, BM and preoperative body temperature between the two groups (P > 0.05) (Table 1).
Table 1.
Comparison of clinical data between the two groups
| Grouping | Routine group (n=37) | GDFT group (n=37) | t/χ2 | P |
|---|---|---|---|---|
| Gender | - | - | ||
| Male | 24 (64.86) | 24 (64.86) | ||
| Female | 13 (35.14) | 13 (35.14) | ||
| Age | 0.510 | 0.475 | ||
| < 60 years old | 13 (35.14) | 16 (43.24) | ||
| ≥ 60 years old | 24 (64.86) | 21 (56.76) | ||
| ASA grading | 1.369 | 0.504 | ||
| I | 5 (13.51) | 7 (18.92) | ||
| II | 21 (56.76) | 16 (43.24) | ||
| III | 11 (29.73) | 14 (37.84) | ||
| TNM staging | 0.898 | 0.638 | ||
| I | 4 (10.81) | 3 (8.11) | ||
| II | 20 (54.05) | 24 (64.86) | ||
| III | 13 (35.14) | 10 (27.03) | ||
| Tumor location | 0.244 | 0.885 | ||
| Upper stomach | 6 (16.22) | 7 (18.92) | ||
| Middle stomach | 12 (32.43) | 10 (27.03) | ||
| Lower stomach | 19 (51.35) | 17 (45.95) | ||
| BMI (kg/m2) | 22.81±3.21 | 23.12±2.96 | 0.432 | 0.667 |
| Preoperative body temperature (°C) | 36.52±0.37 | 36.38±0.39 | 1.584 | 0.118 |
Comparison of intraoperative intake and output and use of vasoactive drug
In both groups, the operation was successfully completed. By recording the intraoperative intake and output of patients in the two groups, it was found that the amount of crystal input, colloid, blood loss, fluid replacement and urine volume in the GDFT group were significantly less than those in the routine group (P < 0.05). By recording the number of patients receiving vasoactive drugs during operation between the two groups, it was found that there was no significant difference in the proportion of patients receiving vasoactive drugs between the two groups (P > 0.05) (Table 2).
Table 2.
Comparison of intraoperative intake and output and use of vasoactive drug
| Grouping | Routine group (n=37) | GDFT group (n=37) | t/χ2 | P |
|---|---|---|---|---|
| Crystal input (ml) | 1182.56±341.85 | 786.71±305.98 | 5.248 | < 0.001 |
| Colloid (ml) | 712.85±296.21 | 556.82±246.59 | 2.463 | 0.016 |
| Blood loss (ml) | 201.69±63.85 | 156.05±47.36 | 3.492 | < 0.001 |
| Fluid replacement volume (ml) | 1451.48±308.63 | 1145.36±263.36 | 4.589 | < 0.001 |
| Urine volume (ml) | 367.64±209.12 | 245.42±180.59 | 2.691 | 0.009 |
| Vasoactive drugs | 21 (56.76) | 16 (43.24) | 1.351 | 0.245 |
Comparison of hemodynamic indexes
The hemodynamic indexes of patients were measured in the two groups at different time points. It was found that there was no significant difference in HR, MAP and CVP values between the two groups at T0 (P > 0.05). From T1 to T4, MAP and CVP values in the GDFT group were higher than those in the routine group (P < 0.05) (Figure 1).
Figure 1.
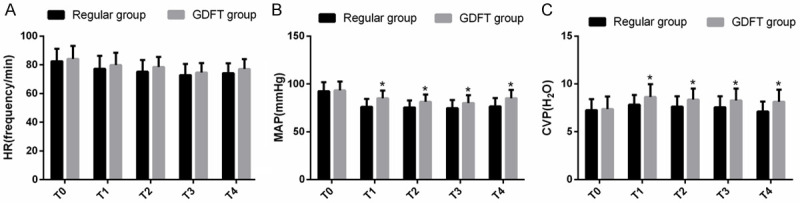
Comparison of hemodynamic indexes. A. Comparison of HR between the two groups at different time points. B. Comparison of MAP between the two groups at different time points. C. Comparison of CVP between the two groups at different time points. Note: * means that compared with routine group, P < 0.05.
Comparison of postoperative complications
By recording the postoperative complications in the two groups, it was found that there were 5 cases with incision infection, 3 cases with inflammatory intestinal obstruction, 4 cases with anastomotic leakage and 3 cases with cholecystitis in the routine group, and the total incidence of postoperative complications was 40.54%. In the GDFT group, there were 2 cases with incision infection, 2 cases with inflammatory intestinal obstruction, 2 cases with anastomotic leakage and 1 cases with cholecystitis, and the total incidence of postoperative complications was 18.92%. The total incidence of postoperative complications in the GDFT group was lower than that in the routine group (P < 0.05) (Table 3).
Table 3.
Comparison of postoperative complications
| Grouping | Routine group (n=37) | GDFT group (n=37) | χ2 | P |
|---|---|---|---|---|
| Incision infection | 5 (13.51) | 2 (5.41) | 1.420 | 0.233 |
| Inflammatory intestinal obstruction | 3 (8.11) | 2 (5.41) | 2.145 | 0.643 |
| Anastomotic leakage | 4 (10.81) | 2 (5.41) | 0.726 | 0.394 |
| Cholecystitis | 3 (8.11) | 1 (2.70) | 1.057 | 0.304 |
| Total number of people affected | 15 (40.54) | 7 (18.92) | 4.140 | 0.042 |
Comparison of cognitive function scores
By evaluating the cognitive function in the two groups at different time points, it was found that there was no significant difference in MMSE scores between the two groups at d0 (P > 0.05). Compared with that at d0, the MMSE scores at d1-d3 were obviously decreased in both groups, but the MMSE scores in the GDFT group at d1-d3 were higher than those in the routine group (P < 0.05) (Table 4).
Table 4.
Comparison of cognitive function scores
| Grouping | d0 | d1 | d2 | d3 |
|---|---|---|---|---|
| Routine group (n=37) | 29.35±0.46 | 21.34±1.33* | 24.18±2.12* | 27.26±1.25* |
| GDFT group (n=37) | 29.24±0.41 | 24.12±1.84* | 27.21±1.53* | 28.32±1.03* |
| t | 1.306 | 9.065 | 8.429 | 4.776 |
| P | 0.195 | < 0.001 | < 0.001 | < 0.001 |
represents the comparison with d0 within the group, P < 0.05.
Comparison of inflammatory factor levels
The levels of inflammatory factors in serum of the two groups were detected at different time points, and there was no significant difference in the levels of CPR, IL-6 and PCT between the two groups at d0 (P > 0.05). From D1 to D3, the levels of CPR, IL-6 and PCT in the GDFT group were lower than those in the routine group (P < 0.05) (Figure 2).
Figure 2.
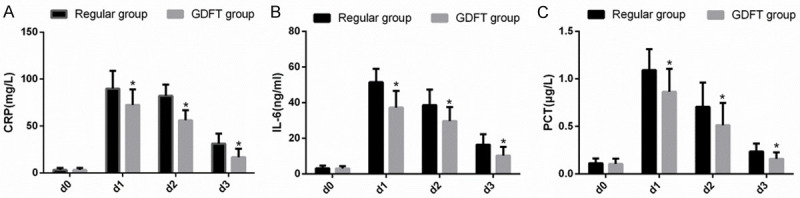
Comparison of inflammatory factor levels. A. Comparison of serum CPR level between the two groups at different time points. B. Comparison of serum IL-6 level between the two groups at different time points. C. Comparison of serum PCT level between the two groups at different time points. Note: * means that compared with routine group, P < 0.05.
Comparison of postoperative recovery
By observing the postoperative recovery indexes in the two groups, it was found that the postoperative anal exhaust time, the first time of starting to eat, the time of leaving bed, the time of PACU stay and the hospitalization time in the GDFT group were significantly shorter than those in the routine group (P < 0.05) (Table 5).
Table 5.
Comparison of postoperative recovery
| Grouping | Routine group (n=37) | GDFT group (n=37) | t | P |
|---|---|---|---|---|
| Anus exhaust time (d) | 4.09±0.76 | 3.52±0.79 | 3.163 | 0.002 |
| The first time of starting to eat (d) | 4.53±0.85 | 3.85±0.96 | 3.226 | 0.002 |
| Time of leaving bed (d) | 2.01±0.88 | 1.56±0.69 | 2.448 | 0.017 |
| Time of PACU stay (min) | 61.64±17.69 | 42.56±13.84 | 5.167 | < 0.001 |
| Hospitalization time (d) | 11.29±4.43 | 8.86±3.67 | 2.569 | 0.012 |
Discussion
In this study, we analyzed the application value of GDFT in patients undergoing laparoscopy-assisted radical gastrectomy with fast-track anesthesia. The results revealed that compared with conventional liquid therapy, GDFT could achieve better clinical effects for patients undergoing laparoscopy-assisted radical gastrectomy with fast-track anesthesia, including maintaining hemodynamic stability, reducing postoperative complications, promoting postoperative recovery and accelerating postoperative inflammation resolution.
In clinical work, fluid therapy during the operation has always been one of the important issues for surgeons and anesthesiologists. For complicated surgery, major surgery and critically illed patients, the postoperative outcome is often related to too much or too little perioperative fluid input [14]. Less blood volume may lead to insufficient tissue perfusion, and more blood volume may lead to tissue edema, both of which will lead to postoperative complications and affect the recovery of patients [15]. GDFT can provide guidance for fluid therapy by monitoring hemodynamic changes, thus effectively preventing excessive or insufficient infusion [16,17]. The results of this study revealed that the MAP and CVP values in the GDFT group were higher than those in the routine group at each time point from T1 to T4. This indicated that GDFT could make the hemodynamics of patients more stable in laparoscopy-assisted radical gastrectomy. GDFT can achieve optimal oxygen delivery by maintaining or increasing cardiac output, maintain the internal environment of immune cells, protect tissues from the risk of preoperative hypoperfusion, and avoid intestinal barrier disorder and intestinal related lymphoid tissue damage, thus promoting tissue repair and reducing infection rate [18,19]. Previous studies have revealed that GDFT reduced the incidence of surgical site infection after abdominal surgery [20]. Other studies have shown that the GDFT can reduce the postoperative morbidity in patients undergoing gastrointestinal surgery [21]. The results of this study showed that the total incidence of postoperative complications in the GDFT group was lower than that in the routine group, and the MMSE score in the GDFT group was higher than that in the routine group at each time point from D1 to D3.
There are many factors that affect the prognosis of patients undergoing surgery, among which the type and total amount of infusion will also affect the prognosis of patients to a certain extent [22]. In traditional liquid therapy, large amounts of crystoloid solution are often injected, which easily leads to tissue edema and low blood pressure after operation, and may affect tissue repair, increase the incidence of complications such as lung infection, so it is not conducive to postoperative recovery of patients. This study showed that the amount of crystal input, colloid, blood loss, fluid replacement and urine volume in the GDFT group were significantly less than those in the routine group, and the postoperative anal exhaust time, the first time of starting to eat, the time of leaving bed, the time of PACU stay and hospitalization time in the GDFT group were significantly shorter than those in the routine group. In addition, the score of postoperative cognitive function in the GDFT group was higher than that in the routine group. This indicated that GDFT could promote postoperative recovery in patients undergoing laparoscopy-assisted radical gastrectomy. Surgical trauma will cause the patient’s body to release a large amount of inflammatory mediators, thus mediating systemic inflammatory response, affecting the body’s immune function and increasing the incidence of postoperative complications such as infection and organ dysfunction [23]. At present, there are few reports about inflammatory reaction that were caused by GDFT and surgery. However, studies have revealed that intravenous infusion of a large amount of crystoloid solution can promote inflammation and accelerate the dissolution of collagenase [24]. CPR, IL-6 and PCT are commonly used indicators to reflect the degree of inflammatory reaction, which usually increase with the aggravation of inflammatory reaction [25,26]. The results of this study showed that the levels of CPR, IL-6 and PCT in the GDFT group were lower than those in the routine group from D1 to D3. This indicated that GDFT could reduce the degree of inflammatory reaction in patients undergoing laparoscopy-assisted radical gastrectomy.
There are some deficiencies in this study. Firstly, the number of subjects in this study is small and all of them are from the same hospital, which may lead to certain bias in the results. Secondly, GDFT will bring economic burden to patients from the perspective of economics. In addition, patients with gastric cancer younger than 30 years old or older than 70 years old were not included in this study. Whether GDFT can exert the same effect on patients at other age stages is unknown.
To sum up, GDFT has a better effect on the rapid rehabilitation of patients undergoing laparoscopy-assisted radical gastrectomy during fast-track anesthesia, and it also has a positive effect on maintaining the stability of hemodynamics, reducing systemic inflammatory and decreasing postoperative complications during anesthesia.
Acknowledgements
Hygiene scientific research project of Tongling City, Anhui Province [Health Research (2016) No. 3].
Disclosure of conflict of interest
None.
References
- 1.Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388:2654–2664. doi: 10.1016/S0140-6736(16)30354-3. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Wang WK, Tu CY, Shao CX, Chen W, Zhou QY, Zhu JD, Xu HT. Impact of enhanced recovery after surgery on postoperative rehabilitation, inflammation, and immunity in gastric carcinoma patients: a randomized clinical trial. Braz J Med Biol Res. 2019;52:e8265. doi: 10.1590/1414-431X20198265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keus F, de Jong JA, Gooszen HG, van Laarhoven CJ. Laparoscopic versus open cholecystectomy for patients with symptomatic cholecystolithiasis. Cochrane Database Syst Rev. 2006;18:CD006231. doi: 10.1002/14651858.CD006231. [DOI] [PubMed] [Google Scholar]
- 5.Buskens CJ, Sahami S, Tanis PJ, Bemelman WA. The potential benefits and disadvantages of laparoscopic surgery for ulcerative colitis: a review of current evidence. Best Pract Res Clin Gastroenterol. 2014;28:19–27. doi: 10.1016/j.bpg.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Noshiro H, Shimizu S, Nagai E, Ohuchida K, Tanaka M. Laparoscopy-assisted distal gastrectomy for early gastric cancer: is it beneficial for patients of heavier weight? Ann Surg. 2003;238:680–685. doi: 10.1097/01.sla.0000094302.51616.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth. 1997;78:606–617. doi: 10.1093/bja/78.5.606. [DOI] [PubMed] [Google Scholar]
- 8.Ljungqvist O, Scott M, Fearon KC. Enhanced recovery after surgery: a review. JAMA Surg. 2017;152:292–298. doi: 10.1001/jamasurg.2016.4952. [DOI] [PubMed] [Google Scholar]
- 9.Lassen K, Soop M, Nygren J, Cox PB, Hendry PO, Spies C, von Meyenfeldt MF, Fearon KC, Revhaug A, Norderval S, Ljungqvist O, Lobo DN, Dejong CH Enhanced Recovery After Surgery (ERAS) Group. Consensus review of optimal perioperative care in colorectal surgery: Enhanced Recovery After Surgery (ERAS) Group recommendations. Arch Surg. 2009;144:961–969. doi: 10.1001/archsurg.2009.170. [DOI] [PubMed] [Google Scholar]
- 10.Shoemaker WC, Appel PL, Kram HB, Waxman K, Lee TS. Prospective trial of supranormal values of survivors as therapeutic goals in high-risk surgical patients. Chest. 1988;94:1176–1186. doi: 10.1378/chest.94.6.1176. [DOI] [PubMed] [Google Scholar]
- 11.Habicher M, Balzer F, Mezger V, Niclas J, Muller M, Perka C, Kramer M, Sander M. Implementation of goal-directed fluid therapy during hip revision arthroplasty: a matched cohort study. Perioper Med (Lond) 2016;5:31. doi: 10.1186/s13741-016-0056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo J, Xue J, Liu J, Liu B, Liu L, Chen G. Goal-directed fluid restriction during brain surgery: a prospective randomized controlled trial. Ann Intensive Care. 2017;7:16. doi: 10.1186/s13613-017-0239-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arevalo-Rodriguez I, Smailagic N, Roque IFM, Ciapponi A, Sanchez-Perez E, Giannakou A, Pedraza OL, Bonfill Cosp X, Cullum S. Mini-Mental State Examination (MMSE) for the detection of Alzheimer’s disease and other dementias in people with mild cognitive impairment (MCI) Cochrane Database Syst Rev. 2015;2015:CD010783. doi: 10.1002/14651858.CD010783.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bellamy MC. Wet, dry or something else? Br J Anaesth. 2006;97:755–757. doi: 10.1093/bja/ael290. [DOI] [PubMed] [Google Scholar]
- 15.Bighamian R, Parvinian B, Scully CG, Kramer G, Hahn JO. Control-oriented physiological modeling of hemodynamic responses to blood volume perturbation. Control Eng Pract. 2018;73:149–160. doi: 10.1016/j.conengprac.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ripolles-Melchor J, Casans-Frances R, Espinosa A, Abad-Gurumeta A, Feldheiser A, Lopez-Timoneda F, Calvo-Vecino JM EAR Group, Evidence Anesthesia Review Group. Goal directed hemodynamic therapy based in esophageal Doppler flow parameters: a systematic review, meta-analysis and trial sequential analysis. Rev Esp Anestesiol Reanim. 2016;63:384–405. doi: 10.1016/j.redar.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 17.Eriksen JK, Nielsen LH, Moeslund N, Keller AK, Krag S, Pedersen M, Pedersen JAK, Birn H, Jespersen B, Norregaard R. Goal-directed fluid therapy does not improve early glomerular filtration rate in a porcine renal transplantation model. Anesth Analg. 2020;130:599–609. doi: 10.1213/ANE.0000000000004453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rollins KE, Mathias NC, Lobo DN. Meta-analysis of goal-directed fluid therapy using transoesophageal Doppler monitoring in patients undergoing elective colorectal surgery. BJS Open. 2019;3:606–616. doi: 10.1002/bjs5.50188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chong MA, Wang Y, Berbenetz NM, McConachie I. Does goal-directed haemodynamic and fluid therapy improve peri-operative outcomes?: a systematic review and meta-analysis. Eur J Anaesthesiol. 2018;35:469–483. doi: 10.1097/EJA.0000000000000778. [DOI] [PubMed] [Google Scholar]
- 20.Yuan J, Sun Y, Pan C, Li T. Goal-directed fluid therapy for reducing risk of surgical site infections following abdominal surgery - A systematic review and meta-analysis of randomized controlled trials. Int J Surg. 2017;39:74–87. doi: 10.1016/j.ijsu.2017.01.081. [DOI] [PubMed] [Google Scholar]
- 21.Jin J, Min S, Liu D, Liu L, Lv B. Clinical and economic impact of goal-directed fluid therapy during elective gastrointestinal surgery. Perioper Med (Lond) 2018;7:22. doi: 10.1186/s13741-018-0102-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joosten A, Coeckelenbergh S, Alexander B, Delaporte A, Cannesson M, Duranteau J, Saugel B, Vincent JL, Van der Linden P. Hydroxyethyl starch for perioperative goal-directed fluid therapy in 2020: a narrative review. BMC Anesthesiol. 2020;20:209. doi: 10.1186/s12871-020-01128-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Obradovic M, Kurz A, Kabon B, Roth G, Kimberger O, Zotti O, Bayoumi A, Reiterer C, Stift A, Fleischmann E. The effect of intraoperative goal-directed crystalloid versus colloid administration on perioperative inflammatory markers - a substudy of a randomized controlled trial. BMC Anesthesiol. 2020;20:210. doi: 10.1186/s12871-020-01126-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kulemann B, Timme S, Seifert G, Holzner PA, Glatz T, Sick O, Chikhladze S, Bronsert P, Hoeppner J, Werner M, Hopt UT, Marjanovic G. Intraoperative crystalloid overload leads to substantial inflammatory infiltration of intestinal anastomoses-a histomorphological analysis. Surgery. 2013;154:596–603. doi: 10.1016/j.surg.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 25.Ma L, Zhang H, Yin YL, Guo WZ, Ma YQ, Wang YB, Shu C, Dong LQ. Role of interleukin-6 to differentiate sepsis from non-infectious systemic inflammatory response syndrome. Cytokine. 2016;88:126–135. doi: 10.1016/j.cyto.2016.08.033. [DOI] [PubMed] [Google Scholar]
- 26.Wu F, Hou XQ, Sun RR, Cui XJ. The predictive value of joint detection of serum amyloid protein A, PCT, and Hs-CRP in the diagnosis and efficacy of neonatal septicemia. Eur Rev Med Pharmacol Sci. 2019;23:5904–5911. doi: 10.26355/eurrev_201907_18335. [DOI] [PubMed] [Google Scholar]


