Abstract
Objective: This study aims to investigate the regulatory role of exosome lncRNA OIP5-AS1 in tumor progression and autophagy. Methods: Seventy-three cases of osteosarcoma (OS) tissues and 56 cases of adjacent normal tissues were collected to culture human OS cell line HOS. The exosomes secreted by OS cell line were isolated and collected. Apoptosis and exosome markers were detected by flow cytometry. A nude mouse model of OS was established. The gene expression levels of lncRNA OIP5-AS1, miR-153 and autophagy-related protein 5 (ATG5) were quantified by real-time quantitative PCR (RT-PCR). The binding sites of lncRNA OIP5-AS1 and miR-153 were predicted by Starbase3.0, and the binding sites of miR-153 and ATG5 were predicted by Targetscan7.2. The gene binding sites were verified by luciferase reporter gene detection or RNA immunoprecipitation (RIP). The relative level of protein was tested by Western blot. Transwell was applied to test migration and invasion of OS cells. The angiogenesis of OS cells was tested by tubule formation test. Results: The results of RT-PCR showed that lncRNA OIP5-AS1 levels were elevated in OS cells and exosomes secreted by cells. Cell function experiments revealed that the proliferation, migration, and invasion of OS cells were promoted by exosomal lncRNA OIP5-AS1. In exosomes, lncRNA OIP5-AS1 inhibited the expression of LC3-II and Beclin 1 proteins, indicating that exosomal lncRNA OIP5-AS1 inhibited autophagy. According to the results of bioinformatics tools and dual-luciferase reporter (DLR) assay or RNA immunoprecipitation (RIP), miR-153 targeted the 3’-UTR of lncRNA OIP5-AS1 and autophagy-related protein 5 (ATG5). The results of western blot (WB) assay showed that exosomal lncRNA OIP5-AS1 and down-regulated miR-153 led to the enhancement of ATG5 protein expression, while up-regulated miR-153 resulted in the decrease of ATG5 protein expression. ATG5 was negatively correlated with miR-153 and positively correlated with lncRNA OIP5-AS1. The results of tubule formation assay disclosed an increase in the angiogenesis level caused by the exosomal lncRNA OIP5-AS1, which was then reversed by the increase of miR-153 and decrease of ATG5. Conclusion: Highly enriched exosomal lncRNA OIP5-AS1 can regulate OS tumor angiogenesis and autophagy through miR-153 and ATG5.
Keywords: Osteosarcoma, exosomal lncRNA OIP5, miR-153, ATG5, angiogenesis, autophagy
Introduction
Osteosarcoma (OS) is a primary bone cancer prevalent among children and adolescents [1], with an incidence of 5.2 cases per million people aged 0-19 years [2]. The formation and development of tumors are accompanied by angiogenesis. Abundant nutrients and oxygen are indispensable to the growth and metastasis of cancer cells, which could be supplied by blood vessels [3]. Angiogenesis plays a key role in the progression of OS [4]. The study by Tsai et al. [5] has suggested that the inhibition on angiogenesis can suppress OS. Autophagy is a process for cells to remove and degrade their damaged proteins or organelles [6]. Its function is both positive and negative in the formation and progression of malignant tumors [7]. In the early stage of cancer formation, autophagy can inhibit the canceration of normal cells [8]. However, autophagy can facilitate immune escape of cancer cells and promote cancer cell metabolism during cancer progression [9]. Autophagy-related protein 5 (ATG5) is a key player in autophagy. The study by Kim et al. [10] has suggested that ATG5-mediated autophagy is involved in the regulation of mitochondrial function, proliferation, apoptosis, and adhesion. The reduction of ATG5 protein expression can inhibit autophagy and cell growth of OS [11]. Therefore, the understanding of the mechanism underlying OS angiogenesis and autophagy can improve the treatment of OS.
There is abundant evidence that non-coding RNAs (ncRNAs) are involved in the expression and functions of proteins. Long ncRNAs (lncRNAs) and miRNAs are two major members of ncRNAs, which play a key role in the development and progression of OS [12]. LncRNAs are leading regulators of cell biological processes such as cell aging, oxidative stress and differentiation [13]. LncRNA OIP5-AS1 is expressed at a markedly higher level in OS [14-16], which suggests that it is deeply involved in the proliferation and apoptosis of OS cells and can regulate the formation and development of malignant tumors. MiRNAs regulate 90% of protein-coding genes by inducing mRNA degradation or inhibiting miRNA translation. In the human genome, miR-153 is found in chromosome 6, with 87 bp in length. Studies [18-20] have revealed that the differential expression of miR-153 is correlated with cisplatin resistance, oxidative stress and proliferation of OS cells.
In this study, we detected highly enriched lncRNA OIP5-AS1 in exosomes secreted by OS cells based on the quantitative reverse transcription PCR (RT-qPCR) and discovered enhanced lncRNA OIP5-AS1 expression in OS tissue samples. Besides, Starbase and Targetscan database analysis predicted binding sites between lncRNA OIP-AS1 and miR-153. Here, we cultured exosomes together with OS cells, seeking to study the role of lncRNA OIP5-AS1 in OS angiogenesis and autophagy.
Methods
OS patients
Totally 73 samples of OS tissue and 56 samples of adjacent normal tissue were collected from patients with OS. Inclusion criteria: The patients were diagnosed with OS based on clinical symptoms or results of pathological biopsy; The patient had a complete case. Exclusion criteria were as below: Patients with tumors other than OS; Patients who had received radiotherapy and chemotherapy or surgical resection of OS in the past; Patients with chronic orthopedic diseases; Patients with other systemic immune diseases; Patients with severe cardiovascular and cerebrovascular diseases and hepatic and renal insufficiency. All participants in the study signed the written informed consent. This study was carried out under the approval of the ethics committee of our hospital. Sliced tissue samples were stored at -80°C.
Isolation, extraction and characterization of exosomes
The OS cell suspension was centrifuged at 1 × 105 g for 8 hours, and then the cells were cultured in the DMEM supplemented by 10% FBS (37°C, with 5% CO2). After culture, the supernatant was collected when cell adherence reached 80-90% to extract exosomes. Following the 1.5 hour-centrifugation of exosomes (1 × 105 g), the sediment was collected. The exosomes pellet was dissolved in phosphate-buffered saline (PBS) by repeatedly pipetting. Exosomal markers (TSG101, CD63, and CD81) were tested on a flow cytometry (FCM) system. The morphology and particle size of exosomes were characterized under the scanning electron microscope (SEM).
Models of xenograft tumor
BALB/C male nude mice (16-18 g, aged 5-6 days) were purchased for the modeling of OS in nude mice. The HOS cell line (200 μL) (5 × 106) was subcutaneously injected into the back of mice daily for 8 days, and then the mice were randomly divided into the control group (the NC group) and the observation groups (the exo group, exo+NC siRNA group, and the exo+OIP5-AS1 group), with 10 mice in each group. Rats from the NC group represented the control group and were injected with PBS via tail vein. Rats from the exo group received the injection of exosomes secreted by OS (10 mg) into the tail; Rats from the exo+NC siRNA group received the tail injection of the negative control and exosomes; Rats from the exo+OIP5-AS1 group received the tail injection of OIP5-AS1 and exosomes. The tumor size was measured every 3 days until after 24 days, and then the tumor was weighed and took out. Gene expression in the tumor tissue was quantified by RT-qPCR. In this study, all the animal experiments were approved by the Ethics Committee, and the relevant experimental operations were strictly in accordance with the Implementation Rules for the Management of Medical Laboratory Animals (Order No. 55 of the Ministry of Health of the People’s Republic of China).
Cell culture
Human osteosarcoma (HOS) cell strains were purchased from ATCC. The exosomes secreted by OS cells were co-cultured with HOS. Cells were seeded in a 6-well plate with 1 × 105 cells per well. Then HOS cells were transfected using the Lipofectamine 3000 transfection kit (Invitrogen, Carlsbad, CA, USA) following the kit instructions. NC siRNA, NC mimics, OIP5-AS1 siRNA, and miR-153 mimics were provided by Sangon Biotech (Shanghai) Co., Ltd.
Tests of cell migration and invasion
The tests of cell migration and invasion were measured by Transwell assay. Migration: Firstly, we seed cell into transwell upper room, and added DMEM with 15% FBS in transwell lower room. Then, the cells on the upper layer of the filter membrane were wiped off with cotton swabs after culture for 24 hours at 37°C and 5% CO2; The filter membrane was fixed with methanol for 5 min and stained with crystal violet for 15 mins. Finally, 5 visual fields were randomly selected for calculating the cell numbers of migration. Invasion: Matrigel was added into Transwell upper chamber, and cells were added after matrigel was solidified. The following steps are the same as the migration experiment.
Test of cell apoptosis
Firstly, cells were exposed to 2 mM hydrogen peroxide for 4 hours. Then, these cells were treated with Apoptosis Kit with Annexin V FITC and PI (ThermoFisher, Waltham, Massachusetts, USA). Finally, Beamcyte flow cytometer (China) and CytoSYS 1.1 were used to test the apoptosis.
Test of angiogenesis
Human umbilical vein endothelial cell (HUVEC) strains purchased from ATCC were seeded into the cell plate pre-coated with matrigel at a density of 1 × 104 cells/well. The extracted exosomes were co-cultured with HUVECs and incubated at 37°C for 4 hours. The angiogenesis level was evaluated under an optical microscope. Relative angiogenesis level = number of new blood vessels in an observation group/number of new blood vessels in the NC group.
WB assay
Protein antibodies: Beclin 1 (1:1000), LC3B (1:2000), ATG5 (1:1000) and GAPDH (1:1000) were purchased from Abcam (UK). Firstly, cells were lysed by RIPA buffer and then centrifuged for 20 minutes. The precipitate was discarded and 50 μL of the supernatant was collected to test the protein concentration via BCA Protein Assay Kit (Abcam, UK). Then, the total protein was separated by SDS-PAGE electrophoresis, and the separated protein was transferred to a polyvinylidene fluoride membrane (EMD millipore, Burlington, MA, USA). Finally, it was incubated with protein antibodies for overnight at 4°C and goat anti-rabbit antibody at room temperature for 1 hour, and the membrane was treated with the ECL luminescent solution for visualization. GAPDH worked as the internal reference protein. In this study, Bio-rad gel imaging system was used to develop and quantitatively analyze protein bands.
RT-qPCR
The separation of the total RNA from tissues or cells followed the instructions of the Trizon reagent (Invitrogen, Carlsbad, CA, USA). LncRNA OIP5-AS1, miR-153, and ATG5 mRNA were subjected to the reverse transcription and quantified using the TaqMan One Step RT-qPCR kit (Solarbio, Beijing, China). The reaction system and procedures referred to the kit instructions. U6 and GAPDH genes worked as control genes. RNA expression was quantified by the QuantStudio™ 7 Flex Real-Time PCR system (Applied Biosystems, Foster City, CA, USA). The relative expression of RNA was standardized by the 2-ΔΔt method. The primers were designed and synthesized by Tiangen Biotech (Beijing) Co, Ltd.
Target prediction and verification
The sequences of lncRNA OIP5-AS1, miR-153, and ATG5 mRNA were analyzed and compared on the Targetscan7.2 (http://www.targetscan.org/vert_72/) and Starbase3.0 (http://starbase.sysu.edu.cn/). OIP5-AS1 wild and mutant types and ATG5 wild and mutant types were constructed. After the transfection of those vectors, the luciferase intensity was tested using the dual-luciferase reporter assay system (Promega Corporation, Madison, WI, USA). The enrichment of MS2-RNA was evaluated by the RNA immunoprecipitation (RIP) assay. As shown in Figure 4, the wild types of OIP5-AS1 and ATG5 could be bound to miR-153, and the mutant type underwent base mutation processing at the predicted site.
Figure 4.
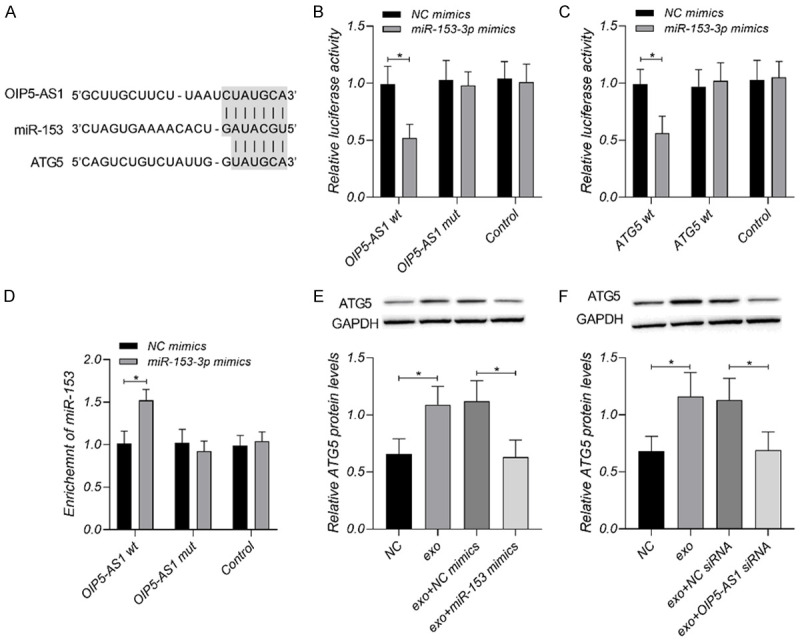
Exosomal lncRNA OIP5-AS1 regulates OS cells through miR-153 and ATG5. A. Prediction of base pairing between lncRNA OIP5-AS1, miR-153, and ATG5 mRNA. B. Co-cultured OIP5-AS1 mutant or wild type with NC mimics or miR-153 mimics, and highly enriched miR-153 on lncRNA OIP5-AS1 according to RNA quantification results. C, D. MiR-153 as a target gene of lncRNA OIP5-AS1 and ATG5 mRNA as a downstream target gene of miR-153 verified by the DLR assay results and RIP assay. E, F. Decreased ATG5 mRNA and protein levels caused by the up-regulation of miR-153 and down-regulation of lncRNA OIP5-AS1. *P < 0.05. Each experiment was repeated three times.
Statistical analysis
The 5-year survival of patients with OS was analyzed by the K-M analysis and the log-rank test. If data conformed to the normal distribution via the K-S test, the comparison among three and more groups was analyzed by the one-way analysis of variance, and the post-hoc comparison was analyzed by the Dunnett-t test; The comparison between the two groups was analyzed by the independent samples t-test. The pairwise correlation between lncRNA OIP5-AS1, miR-153, and ATG5 was identified by the Pearson. A difference in statistics was recognized when the P value was below 0.05.
Results
LncRNA OIP5-AS1 was enriched in exosomes secreted by OS cells
In this study, the exosomes were isolated and extracted from OS cells. The results of characterization parameters and RT-qPCR are shown in Figure 1. Exosomes were about 40-200 nm in size according to the SEM and DLS results. The results of FCM revealed positive results for exosomal surface markers (CD63, CD81, and TSG101). CD63, CD81 and TSG101 were all positive, with the percentages of 66.73%, 64.02% and 65.15% (mean value of three tests) respectively. According to the RT-qPCR results, compared with the control group, lncRNA OIP5-AS1 was enriched in exosomes secreted by OS cells.
Figure 1.
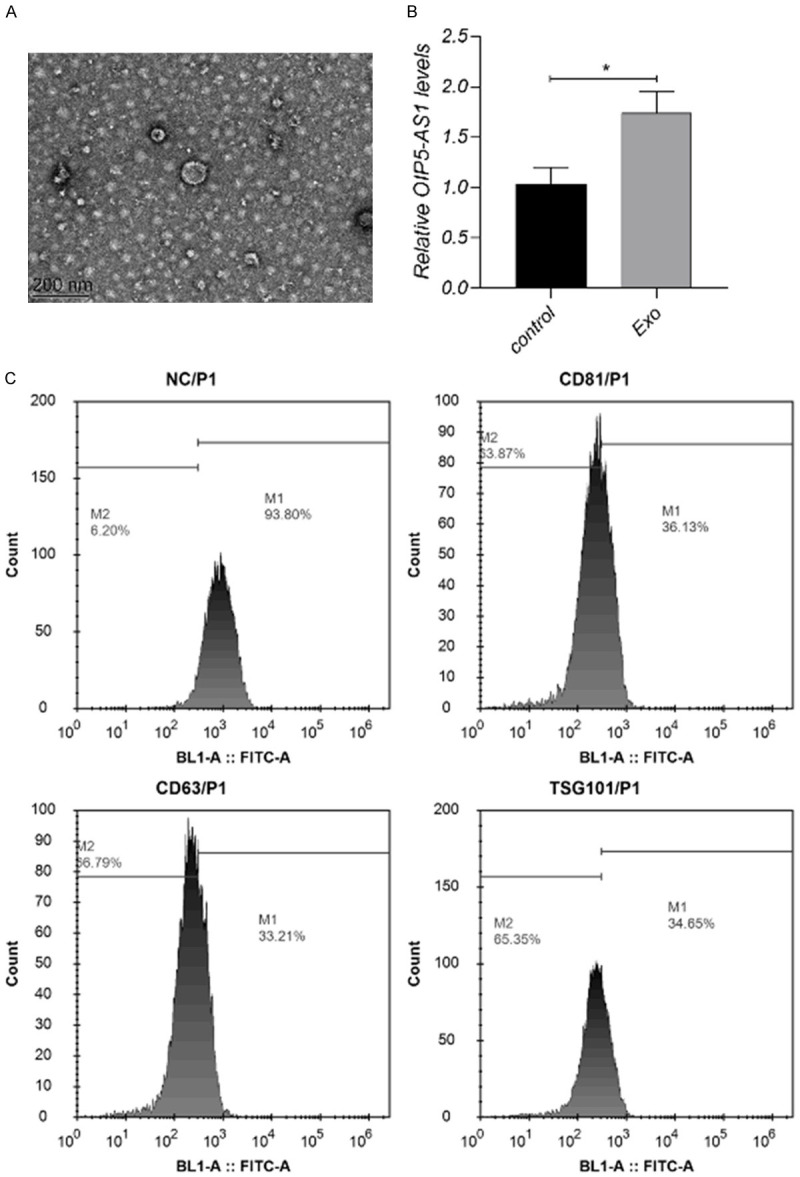
LncRNA OIP5-AS1 is enriched in exosomes secreted by OS cells. A. SEM image of exosomes, scale bar = 200 nm. B. Highly enriched lncRNA OIP5-AS1 in exosomes. C. FCM test of exosomal markers including CD63, CD81, and TSG101. CD63, CD81 and TSG101 were all positive, with the percentages of 66.79%, 63.87% and 65.35% respectively. Each experiment was repeated three times.
Regulation of exosomal lncRNA OIP5-AS1 in OS cells
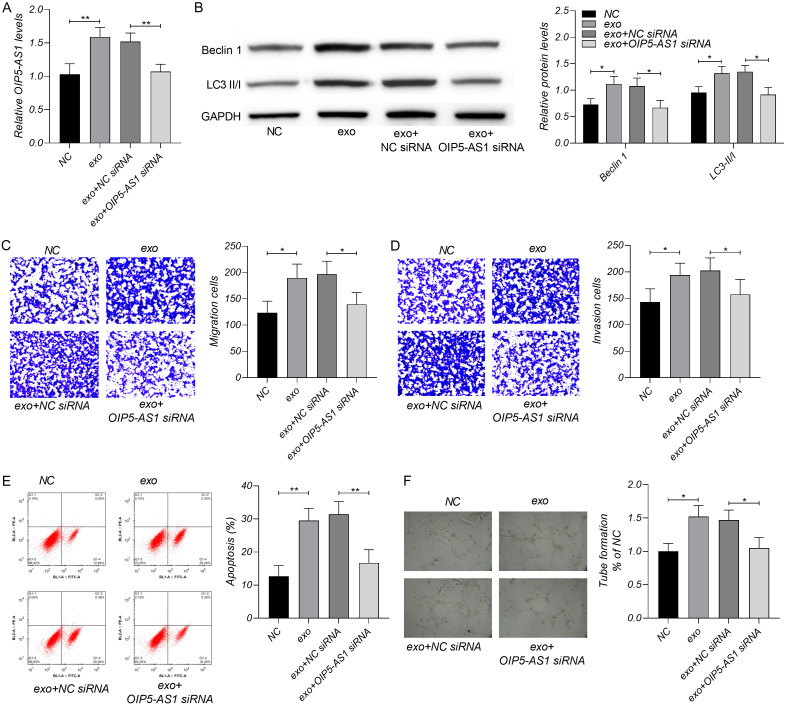
In order to study the regulatory influence of exosomal lncRNA OIP5-AS1 on OS, we measured the biological processes, including migration, invasion, apoptosis, autophagy and angiogenesis of OS cells (Figure 2). The exosmoes enhanced cell migration, invasion, angiogenesis and autophagy, and decreased cell apoptosis. However, the decrease of OIP5-AS1 could offset the role of exosomes on OS biological processes. The level of lncRNA OIP5-AS1 was rise in the exo group than in the NC group, and reduced in the exo+OIP5-AS1 group than in the exo+NC siRNA group.
Figure 2.
Influence of exosomal lncRNA OIP5-AS1 in OS. A. The exosomes increased OIP5-AS1, and OIP5-AS1 siRNA could offset that decrease resulted from exosomes. B. The exosomes increased the levels of Beclin 1 and LC3 II/I, and OIP5-AS1 siRNA offset the increase by exosomes. C, D. The exosomes increased the cell numbers of migration and invasion, and OIP5-AS1 siRNA offset the influence of exosomes on migration and invasion. E. The exosomes inhibited OS apoptosis, and miR-153 mimics offset the regulation of exosomes on apoptosis. F. The exosomes enhanced the angiogenesis of OS which could be offset by OIP5-AS1 siRNA. *P < 0.05, **P < 0.01. Each experiment was repeated three times.
Exosomal lncRNA OIP5-AS1 regulated OS progression through miR-153 and ATG5
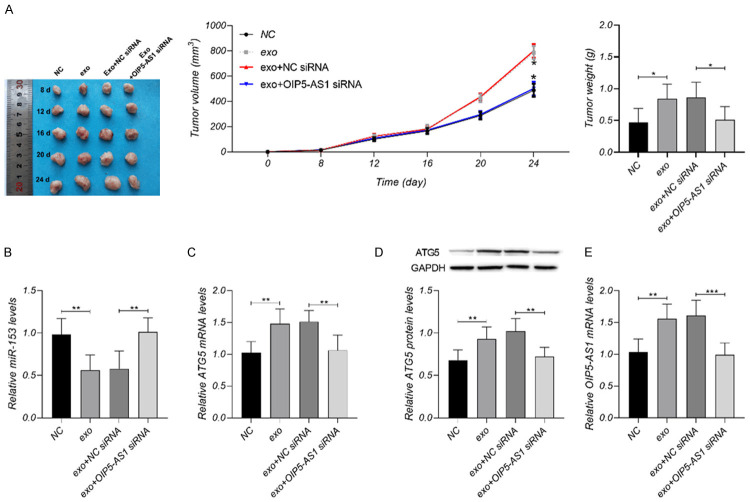
Nude mice models for OS were constructed to determine the effect of lncRNA OIP5-AS1 on OS in vivo. Details about the tumor size, weight, and differentially expressed genes within 24 days are shown in Figure 3. The exosomes increased OIP5-AS1, ATG5, tumors size and weight, and decreased miR-153. The decrease of OIP5-AS1 could offset the influence of exosomes on OS tumors.
Figure 3.
Regulatory effect of exosomal lncRNA OIP5-AS1 on OS in vivo. A. The exosomes increased tumors size and weight, and OIP5-AS1 siRNA could offset the influence of exosomes on OS tumors. B-E. The exosomes increased OIP5-AS1 and ATG5, and decreased miR-153; OIP5-AS1 siRNA offset the influence of exosomes on OIP5-AS1, ATG5 and miR-153. *P < 0.05, **P < 0.01, ***P < 0.001. Each group: n = 10; Each experiment was repeated 3 times.
LncRNA OIP5-AS1 may regulate OS tumors through miR-153 and ATG5 according the result above. To determine the hypothesis, we analyzed the sequences of the three genes on Targetscan7.2 and Starbase3.0 and predicted their binding sites. As shown in Figure 4, there were binding sites between miR-153 and the 3’untranslated regions of lncRNA OIP5-AS1 and ATG5 mRNA, respectively. LncRNA OIP5-AS1 could target miR-153 and negatively regulate its expression, and miR-153 could inhibit the post-transcriptional expression of ATG5 through the target.
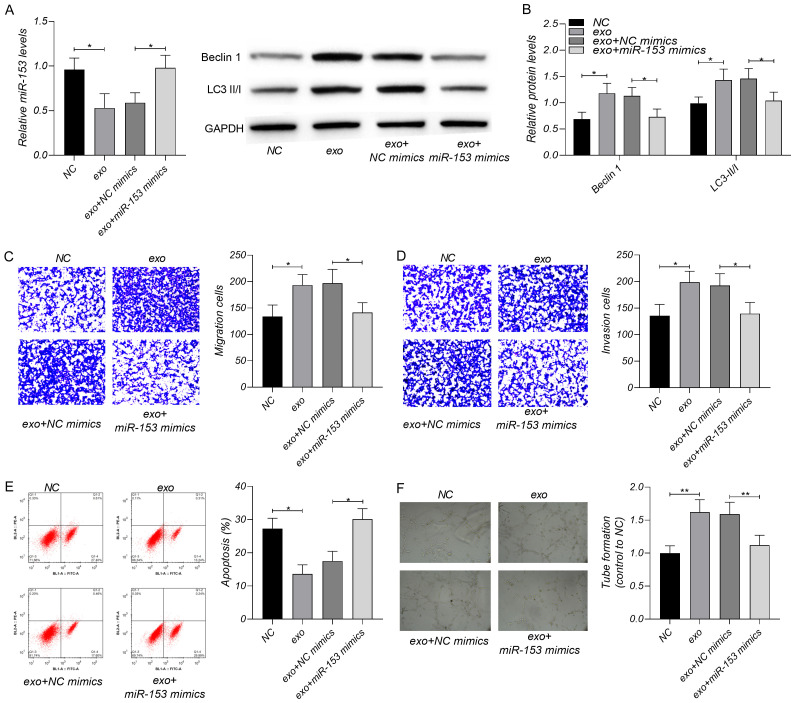
To verify whether lncRNA OIP5-AS1 regulates OS cells through miR-153 and ATG5, we interfered with miR-153 expression in OS using miR-153 mimics and then tested cell migration, invasion, autophagy, apoptosis and angiogenesis. As shown in Figure 5, the exosomes enhanced cell migration, invasion, angiogenesis and autophagy, and decreased cell apoptosis; The increase of miR-153 could neutralize the influence of exosomes on OS.
Figure 5.
Regulatory effect of miR-153 on OS. A. The exosomes decreased miR-153, and miR-153 mimics could offset the reduction induced by exosomes. B. The exosomes increased the levels of Beclin 1 and LC3 II/I, and miR-153 mimics offset the increase by exosomes. C, D. The exosomes increased the cell numbers of migration and invasion, and miR-153 mimics offset the influence of exosomes on migration and invasion. E. The exosomes inhibited OS apoptosis, and miR-153 mimics offset the regulation of exosomes on apoptosis. F. The exosomes enhanced the angiogenesis of OS which could be offset by miR-153 mimics. *P < 0.05, **P < 0.01. Each experiment was repeated three times.
Influence of lncRNA OIP5-AS1 in the prognosis of OS
The levels of lncRNA OIP5-AS1, miR-153, and ATG5 mRNA in OS tumor tissues are shown in Figure 6. Higher levels of lncRNA OIP5-AS1 and ATG5 mRNA and lower miR-153 levels were detected in OS tissues than in non-OS tissues. LncRNA OIP5-AS1 levels were higher in OS tissues with a diameter > 8 cm than in OS tissues with a diameter < 8 cm. The 5-year survival in the high OIP5-AS1 group was better than that in the low OIP5-AS1 group. The Pearson method revealed that OIP5-AS1 was negatively related with miR-153, and miR-153 was negatively related with ATG5 mRNA in 73 samples of OS tissues.
Figure 6.
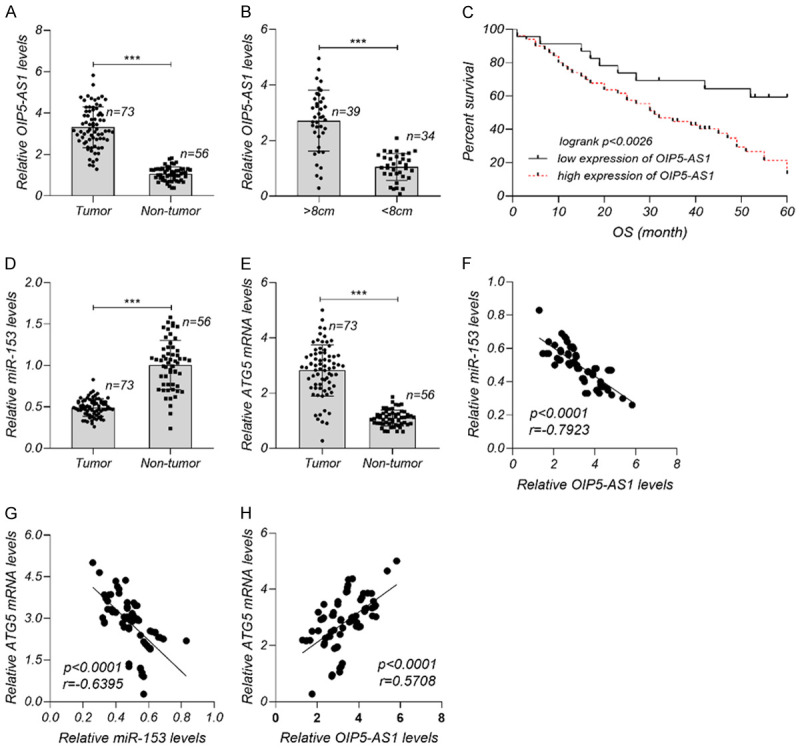
Role of lncRNA-TMPO-AS1 in OS prognosis. A. Markedly higher lncRNA OIP5-AS1 in OS tissues than in non-OS tissues. B. Markedly higher lncRNA OIP5-AS1 in OS tissues with a diameter > 8 cm than in OS tissues with a diameter < 8 cm. C. Better 5-year survival in the high-OIP5-AS1 group than in the low-OIP5-AS1 group. D, E. Higher ATG5 mRNA levels and lower miR-153 levels in OS tissues than in non-OS tissues. F-H. Relationship of OIP5-AS1/miR-153, OIP5-AS1/ATG5 mRNA and miR-153/ATG5 mRNA in 73 samples of OS tissues. ***P < 0.001. There were 73 samples of OS tissues and 56 samples of non-OS tissues. Each experiment was repeated three times.
Discussion
Tumors are closely affected by their microenvironment because cancer cells can secrete important functional factors for the tumor microenvironment during cell growth and progression, including cytokines and chemokines [21]. The microenvironment of OS cells transmits information through exosomes secreted by the cells [22], so exosomes play an important role in the development and progression of OS. Tao et al. [23] hold that EWSAT1 in exosomes can stimulate the sensitivity and reactivity of vascular endothelial cells, thereby inducing OS angiogenesis. The study by Li et al. [24] has suggested that linc00852 delivered by exosomes can regulate the proliferation, migration and invasion of OS cells. In this study, lncRNA OIP5-AS1 was expressed at markedly higher levels in OS tissues and was highly enriched in exosomes secreted by OS cells. Such results indicate that lncRNA OIP5-AS1 may participate in the development of OS malignant tumors through exosomal delivery.
LncRNA/miRNA/mRNA axis is an important regulation path in the cell life cycle. LncRNA OIP5-AS1 is an important regulator in OS, which can monitor and affect the biological functions of cancer cells by sponging miRNAs such as miR-340-5p, miR-137-3p, and miR-337-3p [14-16]. Here, we noted that lncRNA OIP5-AS1 bound to miR-153 through sequence pairing and hence regulated the downstream target gene of miR-153, ATG5. During autophagy, the ULK1 complex activates the PI3K pathway, thereby inducing the ATG5-mediated ATG12 and LC3 coupling systems and triggering a series of cascade reactions to form autophagosomes and to begin the subsequent autophagy process [25]. Therefore, ATG5 is an important member of autophagy and any changed in its expression level can affect autophagy activity. This study has revealed that the exosomal lncRNA OIP5-AS1 enhances ATG5 expression by inhibiting the expression of miR-153 in OS cells and hence promotes cell autophagy.
Angiogenesis also plays an important role in OS. The results of this study showed that exosomes promoted angiogenesis, down-regulated exosomal lncRNA OIP5-AS1 and decreased OS angiogenesis, suggesting that lncRNA OIP5-AS1 plays a significant regulatory role in the angiogenesis of OS. Cancer cells rely on active energy metabolism. Good blood circulation can provide sufficient oxygen and nutrients for the tumor microenvironment to promote cancer cell growth [26]. Endothelial cells are the main component of blood vessels and participate in the transportation of nutrients and oxygen [27]. Sprott et al. [28] has suggested that ATG5 not only mediates autophagy, but also regulates angiogenesis in vascular endothelial cells through reactive oxygen species-dependent signaling pathways. Therefore, we speculate that the exosomal lncRNA OIP5-AS1 may affect the endothelial cell’s angiogenesis by regulating the expression of ATG5 in endothelial cells.
Clinically, the 5-year survival of different lncRNA OIP5-AS1 expression groups was recorded and compared in this study, and a poor survival outcome was noted in OS patients with higher OIP5-AS1 expression, which implied that lncRNA OIP5-AS1 has a possible connection with OS prognosis. We will conduct research and analysis on this possible connection in the future. ATG5-mediated autophagy also involves NF-κB and p53/Rb signaling pathways [10,29]. Whether lncRNA OIP5-AS1 regulates these pathways in OS requires further research and discussion. Autophagy can facilitate immune escape during the development of cancer cells. The regulation of OS cell autophagy by lncRNA OIP5-AS1 may change the immune system’s monitoring mechanism for OS cells, so the relationship between lncRNA OIP5-AS1 and OS tumor immunity also deserves further analysis. The above problems may be beneficial to promote the development of OS treatment, and the deficiency of this study is that it fails to discuss these issues in depth.
In summary, highly enriched exosomal lncRNA OIP5-AS1 regulates angiogenesis and ATG5-mediated autophagy in OS through miR-153 (Figure 7), thereby participating in the formation and development of malignant tumors. LncRNA OIP5-AS1 may affect the clinical prognosis of OS. Therefore, lncRNA OIP5-AS1 has high research and application value in OS.
Figure 7.
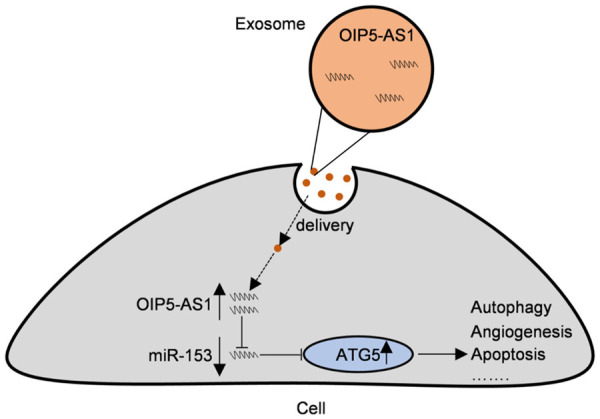
Regulatory mechanism of lncRNA OIP5-AS1/miR-153/ATG5 axis in OS. LncRNA OIP5-AS1, which was highly enriched in exosomes, regulated angiogenesis, autophagy, apoptosis and other biological functions through miR-153, thus participating in the formation and development of OS malignancies.
Acknowledgements
A project of the National Natural Science Foundation of China to investigate the mechanism of propolis inhibition of lung metastasis of osteosarcoma based on Wnt/β-catenin signal transduction pathway (No. 81573995), The National Natural Science Foundation of China Youth Project: Research on the Mechanism of Propolis Inhibiting Bone Metastasis and Osteolytic Bone Destruction in Breast Cancer (No. 81403412).
Disclosure of conflict of interest
None.
References
- 1.Czarnecka AM, Synoradzki K, Firlej W, Bartnik E, Sobczuk P, Fiedorowicz M, Grieb P, Rutkowski P. Molecular biology of osteosarcoma. Cancers (Basel) 2020;12:2130. doi: 10.3390/cancers12082130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sadykova LR, Ntekim AI, Muyangwa-Semenova M, Rutland CS, Jeyapalan JN, Blatt N, Rizvanov AA. Epidemiology and risk factors of osteosarcoma. Cancer Invest. 2020;38:259–269. doi: 10.1080/07357907.2020.1768401. [DOI] [PubMed] [Google Scholar]
- 3.Lugano R, Ramachandran M, Dimberg A. Tumor angiogenesis: causes, consequences, challenges and opportunities. Cell Mol Life Sci. 2020;77:1745–1770. doi: 10.1007/s00018-019-03351-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perut F, Roncuzzi L, Zini N, Massa A, Baldini N. Extracellular nanovesicles secreted by human osteosarcoma cells promote angiogenesis. Cancers (Basel) 2019;11:779. doi: 10.3390/cancers11060779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsai HC, Cheng SP, Han CK, Huang YL, Wang SW, Lee JJ, Lai CT, Fong YC, Tang CH. Resistin enhances angiogenesis in osteosarcoma via the MAPK signaling pathway. Aging (Albany NY) 2019;11:9767–9777. doi: 10.18632/aging.102423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Camuzard O, Santucci-Darmanin S, Carle GF, Pierrefite-Carle V. Role of autophagy in osteosarcoma. J Bone Oncol. 2019;16:100235. doi: 10.1016/j.jbo.2019.100235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jamali Z, Taheri-Anganeh M, Shabaninejad Z, Keshavarzi A, Taghizadeh H, Razavi ZS, Mottaghi R, Abolhassan M, Movahedpour A, Mirzaei H. Autophagy regulation by microRNAs: novel insights into osteosarcoma therapy. IUBMB Life. 2020;72:1306–1321. doi: 10.1002/iub.2277. [DOI] [PubMed] [Google Scholar]
- 8.Colhado Rodrigues BL, Lallo MA, Perez EC. The controversial role of autophagy in tumor development: a systematic review. Immunol Invest. 2020;49:386–396. doi: 10.1080/08820139.2019.1682600. [DOI] [PubMed] [Google Scholar]
- 9.Poillet-Perez L, White E. Role of tumor and host autophagy in cancer metabolism. Genes Dev. 2019;33:610–619. doi: 10.1101/gad.325514.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim J, Chee WY, Yabuta N, Kajiwara K, Nada S, Okada M. Atg5-mediated autophagy controls apoptosis/anoikis via p53/Rb pathway in naked mole-rat fibroblasts. Biochem Biophys Res Commun. 2020;528:146–153. doi: 10.1016/j.bbrc.2020.05.083. [DOI] [PubMed] [Google Scholar]
- 11.Gu Z, Hou Z, Zheng L, Wang X, Wu L, Zhang C. LncRNA DICER1-AS1 promotes the proliferation, invasion and autophagy of osteosarcoma cells via miR-30b/ATG5. Biomed Pharmacother. 2018;104:110–118. doi: 10.1016/j.biopha.2018.04.193. [DOI] [PubMed] [Google Scholar]
- 12.Wang JY, Yang Y, Ma Y, Wang F, Xue A, Zhu J, Yang H, Chen Q, Chen M, Ye L, Wu H, Zhang Q. Potential regulatory role of lncRNA-miRNA-mRNA axis in osteosarcoma. Biomed Pharmacother. 2020;121:109627. doi: 10.1016/j.biopha.2019.109627. [DOI] [PubMed] [Google Scholar]
- 13.Kim J, Abdelmohsen K, Yang X, De S, Grammatikakis I, Noh JH, Gorospe M. LncRNA OIP5-AS1/cyrano sponges RNA-binding protein HuR. Nucleic Acids Res. 2016;44:2378–2392. doi: 10.1093/nar/gkw017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun X, Tian C, Zhang H, Han K, Zhou M, Gan Z, Zhu H, Min D. Long noncoding RNA OIP5-AS1 mediates resistance to doxorubicin by regulating miR-137-3p/PTN axis in osteosarcoma. Biomed Pharmacother. 2020;128:110201. doi: 10.1016/j.biopha.2020.110201. [DOI] [PubMed] [Google Scholar]
- 15.Liu L, Wang S. Long non-coding RNA OIP5-AS1 knockdown enhances CDDP sensitivity in osteosarcoma via miR-377-3p/FOSL2 axis. Onco Targets Ther. 2020;13:3853–3866. doi: 10.2147/OTT.S232918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song L, Zhou Z, Gan Y, Li P, Xu Y, Zhang Z, Luo F, Xu J, Zhou Q, Dai F. Long noncoding RNA OIP5-AS1 causes cisplatin resistance in osteosarcoma through inducing the LPAATβ/PI3K/AKT/mTOR signaling pathway by sponging the miR-340-5p. J Cell Biochem. 2019;120:9656–9666. doi: 10.1002/jcb.28244. [DOI] [PubMed] [Google Scholar]
- 17.Otoukesh B, Abbasi M, Gorgani HO, Farahini H, Moghtadaei M, Boddouhi B, Kaghazian P, Hosseinzadeh S, Alaee A. MicroRNAs signatures, bioinformatics analysis of miRNAs, miRNA mimics and antagonists, and miRNA therapeutics in osteosarcoma. Cancer Cell Int. 2020;20:254. doi: 10.1186/s12935-020-01342-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng FH, Zhao ZS, Liu WD. Long non-coding RNA ROR regulated ABCB1 to induce cisplatin resistance in osteosarcoma by sponging miR-153-3p. Eur Rev Med Pharmacol Sci. 2019;23:7256–7265. doi: 10.26355/eurrev_201909_18828. [DOI] [PubMed] [Google Scholar]
- 19.Wen JF, Jiang YQ, Li C, Dai XK, Wu T, Yin WZ. LncRNA-XIST promotes the oxidative stress-induced migration, invasion, and epithelial-to-mesenchymal transition of osteosarcoma cancer cells through miR-153-SNAI1 axis. Cell Biol Int. 2020;44:1991–2001. doi: 10.1002/cbin.11405. [DOI] [PubMed] [Google Scholar]
- 20.Wang H, Yu Y, Fan S, Luo L. Knockdown of long noncoding RNA TUG1 inhibits the proliferation and cellular invasion of osteosarcoma cells by sponging miR-153. Oncol Res. 2018;26:665–673. doi: 10.3727/096504017X14908298412505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hinshaw DC, Shevde LA. The tumor microenvironment innately modulates cancer progression. Cancer Res. 2019;79:4557–4566. doi: 10.1158/0008-5472.CAN-18-3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raimondi L, De Luca A, Gallo A, Costa V, Russelli G, Cuscino N, Manno M, Raccosta S, Carina V, Bellavia D, Conigliaro A, Alessandro R, Fini M, Conaldi PG, Giavaresi G. Osteosarcoma cell-derived exosomes affect tumor microenvironment by specific packaging of microRNAs. Carcinogenesis. 2020;41:666–677. doi: 10.1093/carcin/bgz130. [DOI] [PubMed] [Google Scholar]
- 23.Tao SC, Huang JY, Wei ZY, Li ZX, Guo SC. EWSAT1 acts in concert with exosomes in osteosarcoma progression and tumor-induced angiogenesis: the “double stacking effect”. Adv Biosyst. 2020;4:e2000152. doi: 10.1002/adbi.202000152. [DOI] [PubMed] [Google Scholar]
- 24.Li Q, Wang X, Jiang N, Xie X, Liu N, Liu J, Shen J, Peng T. Exosome-transmitted linc00852 associated with receptor tyrosine kinase AXL dysregulates the proliferation and invasion of osteosarcoma. Cancer Med. 2020;9:6354–6366. doi: 10.1002/cam4.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Camuzard O, Santucci-Darmanin S, Carle GF, Pierrefite-Carle V. Autophagy in the crosstalk between tumor and microenvironment. Cancer Lett. 2020;490:143–153. doi: 10.1016/j.canlet.2020.06.015. [DOI] [PubMed] [Google Scholar]
- 26.Jiang X, Wang J, Deng X, Xiong F, Zhang S, Gong Z, Li X, Cao K, Deng H, He Y, Liao Q, Xiang B, Zhou M, Guo C, Zeng Z, Li G, Li X, Xiong W. The role of microenvironment in tumor angiogenesis. J Exp Clin Cancer Res. 2020;39:204. doi: 10.1186/s13046-020-01709-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schaaf MB, Houbaert D, Mece O, Agostinis P. Autophagy in endothelial cells and tumor angiogenesis. Cell Death Differ. 2019;26:665–679. doi: 10.1038/s41418-019-0287-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sprott D, Poitz DM, Korovina I, Ziogas A, Phieler J, Chatzigeorgiou A, Mitroulis I, Deussen A, Chavakis T, Klotzsche-von Ameln A. Endothelial-specific deficiency of ATG5 (autophagy protein 5) attenuates ischemia-related angiogenesis. Arterioscler Thromb Vasc Biol. 2019;39:1137–1148. doi: 10.1161/ATVBAHA.119.309973. [DOI] [PubMed] [Google Scholar]
- 29.Peng X, Wang Y, Li H, Fan J, Shen J, Yu X, Zhou Y, Mao H. ATG5-mediated autophagy suppresses NF-kappaB signaling to limit epithelial inflammatory response to kidney injury. Cell Death Dis. 2019;10:253. doi: 10.1038/s41419-019-1483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]