Abstract
Objective: To explore the effect of exosomes containing miR-122-5p secreted by lipopolysaccharide (LPS)-induced neutrophils on the apoptosis and permeability of brain microvascular endothelial cells (BMECs). Methods: Neutrophils in blood were isolated, purified and identified. LPS-induced neutrophils were co-cultured with BMECs. Untreated or LPS-induced neutrophil exosomes were isolated and identified with a transmission electron microscope. miR-122-5p expressions in the exosomes were detected by real-time quantitative polymerase chain reaction, and then the exosomes were co-cultured with BMECs. Bioinformatics analysis was performed to predict the downstream target gene of miR-122-5p, and OCLN was selected as the subject. Dual luciferase reporter assay was carried out to verify the interactive relationship between OCLN and miR-122-5p. LPS and miR-122-5p were used to treat neutrophils, and then exosomes were collected. Exosome or OCLN was embedded in BMECs. The proliferation, colony forming ability and apoptosis of BMECs were detected by cholecystokinin octopeptide, clone formation assay and flow cytometry, respectively. Corresponding kits were used to detect the activities of reactive oxygen species, superoxide dismutase, malondialdehyde and catalase. Vascular endothelial growth factor and tight junction proteins (ZO-1 and Claudin-5) expressions were measured by Western blot for cell permeability evaluation. Results: miR-122-5p had an increased expression in LPS-induced neutrophil exosomes and could promote oxidative stress, apoptosis and permeability increase of BMECs and the inhibition of BMECs proliferation and colony formation (P<0.05). miR-122-5p targeted the binding with OCLN and down-regulated OCLN expression. OCLN overexpression partly decreased the malignant effect of miR-122-5p on BMECs (P<0.05). Conclusion: LPS can induce neutrophils to secrete exosomes containing miR-122-5p. The down-regulation of OLCN expression can aggravate BMECs injury.
Keywords: Neutrophils, exosomes, brain microvascular endothelial cells, permeability
Introduction
Cerebrovascular disease is a global health concern. Cerebrovascular disease is the second common cause of death and disability according to the World Health Organization [1]. Brain microvascular endothelial cells (BMECs) impairment is considered to be the initial event of vascular disease and runs throughout the process of disease occurrence and development [2,3]. BMECs injury can lead to the change in angiotasis and hemodynamics, the activation of blood platelets and coagulation system, and the increase of vascular permeability, thus inducing a series of pathophysiological changes. The change in BMECs permeability is also an important manifestation of blood brain barrier [4-6]. Therefore, BMECs are widely used in the study of cerebrovascular diseases.
Neutrophils play a key role in the occurrence of all kinds of inflammatory responses. Neutrophils maintain a balance level under normal status. When the body is infected with pathogens, lipopolysaccharide (LPS) in the body will be activated and directly act on neutrophils. Neutrophils resist extraneous germs, but also generate active components that damage themselves [7]. LPS can act on neutrophils to increase the adhesive ability of neutrophils, stimulate the secretion of inflammatory factors, and generate anti-apoptosis effect [8]. Neutrophils also play an important effect in LPS-induced neuroinflammation [9]. In addition, the arrest of neutrophils has been shown to be associated with LPS-induced blood brain barrier [10]. In this study, the specific potential mechanism of LPS-induced neutrophils participating in blood brain barrier was further explored on the basis of previous studies.
Exosomes are cell-derived extracellular spherical vesicles of 30-150 nm and complexes containing various DNAs, RNAs and proteins [11]. Exosomes exist in serum, plasma and other biological fluids and is secreted by immune cells, fibroblasts and epithelial cells [12]. The exosome in different cells has differences in components, and the biological activity of exosome-secreted functional molecule is greater than that of the soluble form of exosomes. Exosomes are involved in signal transmission among cells and mainly act on inflammation, information transfer, tumor growth, metastasis, antigen presenting and angiogenesis [13]. In addition, exosomes have been widely investigated in many diseases. For example, the miRNA of exosome serve as a biomarker for predicting chemotherapy resistance in diffuse large B cell lymphoma [14]. Activated exosome lncRNAs serve as competitive endogenous RNAs in renal cancer cells to promote the resistance to sunitinib in renal cancer [15].
In this study, RNA sequencing expression profile was used to perform differential genes expression analysis, and OCLN was selected as the subject. OCLN-coded transmembrane protein is the first confirmed tight junction protein, which is the main structural protein of tight junction, and continuously distributes in tight junction, with a molecular weight of 65 kD [16,17]. OCLN-coded transmembrane proteins play a key regulation role in protein tight junction formed cell permeability barrier and are important components of blood brain barrier [18]. On the online target relation prediction website, miR-122-5p, the upstream target of OCLN was found. Significant miR-122-5p overexpression exists in exosomes from 14 cell lines [19], and significant up-regulation of miR-122-5p is observed after cerebral ischemia [20]. However, it needs to further explore miR-122-5p’s mechanism in cerebrovascular diseases.
In this study, the effect of exosomes containing miR-122-5p secreted by neutrophils on the proliferation, cloning, apoptosis, oxidative stress and permeability of BMECs by regulating OCLN was explored to provide a certain basis for the study of cerebrovascular diseases.
Materials and methods
Bioinformatics analysis
Transcriptome expression profile microarray data of BMECs (GSE5883) from Gene Expression Omnibus were obtained and differential genes expression analysis was performed with the limma package. The microarray data contained neutrophil-treated BMECs samples and negative control (NC). |log2FC| >1.5 and false discovery rate <0.05 were set as the threshold values to screen differential genes. Then differential genes received GO and KEGG analyses on the DAVID online website (https://david.ncifcrf.gov/). RNA22 (https://cm.jefferson.edu/rna22/), miRWalk (http://mirwalk.umm.uni-heidelberg.de) and miRDB (http://mirdb.org) were used to predict miRNAs that might bind to differential genes, and the intersection was obtained.
Purification and cultivation of neutrophils
Neutrophils were purified first. Peripheral venous blood of 10 mL from healthy people was obtained and treated within 2 h. Neutrophils were extracted with the Histopaque-1077 (Sigma, USA) and Histopaque-1119 (Sigma, USA). Cells (cell activity >90%) were re-suspended in the 1640 medium (2-5×106 cells/mL) containing 10% fetal bovine serum (Thermo Fisher Scientific Inc., USA). Neutrophils were incubated under 5% CO2 at 37°C or co-incubated with LPS for 2 h for further analysis.
Exosome isolation and identification
Exosomes were separated from neutrophil culture supernatant with exoEasy Maxi Kit (Shanghai Duma Biological Technology Co. Ltd., China) in accordance with the instruction. Cell culture supernatant was pre-filtered, and 600 µL EXOs buffer solutions were obtained finally and preserved at 80°C for analysis. Exosome morphology had been identified with a transmission electron microscope (Thermo Fisher, USA).
Cell culture, transfection and grouping
Human BMECs (ATCC, USA) were cultured at a density of 1×105 cells/well inEBM-2 medium containing fetal bovine serum (10%) and endothelial cell growth supplement (40 µg/mL). Cells were stored under 5% CO2 at 37°C.
pcDNA3.1-OCLN and its corresponding empty vector were transfected into BMECs which were co-cultured with LPS-induced neutrophils. Neutrophils were transfected with miR-122-5p mimic, inhibitor and NC. Lipofectamine 3,000 (Thermo Fisher Scientific Inc., USA) was employed to perform transfection in accordance with the instruction. Cells were collected 48h after transfection for subsequent study.
BMECs were grouped as follows. Neutrophils-treated BMECs were divided into the neutrophil group (untreated neutrophils + BMECs), LPS + neutrophil group (LPS-induced neutrophils + BMECs), LPS + neutrophil EXOs group (LPS-induced neutrophil-derived exosomes + BMECs), and neutrophil EXOs group (untreated neutrophil-derived exosomes + BMECs).
miR-122-5p-treated BMECs were grouped into the blank group (miR-122-5p blank control + BMECs), miR-NC group (miR-122-5p NC + BMECs), miR-mimic group (miR-122-5p mimic + BMECs), and miR-inhibitor group (miR-122-5p inhibitor + BMECs).
BMECs treated with exosomes containing miR-122-5p were divided into the control group (untreated BMECs), miR-NC-exo group (BMECs + LPS and NC vector-treated neutrophil-derived exosomes), miR-inhibitor-exo group (BMECs + LPS and miR-122-5p inhibitor-treated neutrophil-derived exosomes), and miR-mimic-exo group (BMECs + LPS and miR-122-5p mimic-treated neutrophil-derived exosomes).
OCLN-treated BMECs were divided into the vector group (empty vector-treated BMECs) and OCLN group (pcDNA3.1-OCLN-treated BMECs).
BMECs with combined treatment were divided into the miR-mimic-exo + vector group (BMECs + LPS, miR-122-5p mimic and empty vector-treated neutrophil-derived exosomes) and miR-mimic-exo + OCLN group (pcDNA3.1-OCLN-treated BMECs + LPS and miR-122-5p mimic-treated neutrophil-derived exosomes).
All mimics, inhibitors and transfection vectors in this study were provided by the Shanghai GenePharma Co. Ltd., China.
Real-time quantitative polymerase chain reaction
Cells were isolated with the TRIzol kit (Thermo Fisher Scientific Inc., USA) to obtain total RNAs. After the purity and concentration of RNA were measured, a reverse transcription kit (Thermo Fisher, USA) was utilized to reversely transcribe RNA. The instruction of fluorescent quantitative polymerase chain reaction kit (Thermo Fisher, USA) was followed to perform polymerase chain reaction. The experiment was repeated thrice for each sample. The results were analyzed by using 2-ΔΔCT. GAPDH and U6were served as internal references of OCLN and miR-122-5p, respectively. Specific amplification primers of quantitative polymerase chain reaction are shown in Table 1.
Table 1.
Primer sequence
| Gene | Sequence (5’-3’) |
|---|---|
| miR-122-5p | F: ACACTCCAGCTGGGAA |
| R: GTGCAGGGTCCGAGGT | |
| OCLN | F: AAGACGATGAGGTGCAGAAG |
| R: GTGAAGAGAGCCTGACCAAA | |
| GAPDH | F: GGAGCGAGATCCCTCCAAAAT |
| R: GGCTGTTGTCATACTTCTCATGG | |
| U6 | F: AGCATGATATTTGCTGATGCTGT |
| R: TGGCTCAGGTGTGCCTTAATGGA |
Cholecystokinin octopeptide
Cell proliferation ability was measured with a cholecystokinin octopeptide (CCK-8) cell counting kit (Beyotime Biotechnology, China). BMECs of 100 mL were added in each well of the 96-well plate (2×104 cells/mL) and cultured in a humidifying 5% CO2 incubator at 37°C. Supernatant was transferred to a medium containing 2.5% neutrophil exosomes. After 24, 48 and 72 h, 10 mL CCK-8 solutions were added in each well. Optical density at 450 nm was read with a microplate reader 2 h later. The optical density value was corrected according to cell count.
Oxidative stress level determination
Indicators of oxidative stress employed malondialdehyde (MDA), catalase (CAT), reactive oxygen species (ROS), and superoxide dismutase (SOD) and were detected by fluorescent probe method, thiobarbituric acid method, WST-1 assay and colorimetric method, respectively, with the ROS detection kit (HR8786), MDA detection kit (KFS380), SOD detection kit (SNM203) and CAT detection kit (YT292, all kits from Beijing Biolab Technology Co. Ltd., China) according to the corresponding instruction.
Western blot
Tissues or cells were obtained to extract total proteins with RIPA lysis buffer. BCA kit was utilized to measure protein concentration. SDS-PAGE was used to isolate 20 μg protein samples, which were then transferred to the PVDF membranes for 90 min. The membrane was sealed for 2 h with 10% skimmed milk powder solution and primary antibodies Claudin-5 (1:1000, ABCAM, UK), ZO-1 (1:1000, ABCAM, UK) and VEGF (1:1000, ABCAM, UK). The membrane was washed thrice (for 15 min per time) using tris buffered saline tween (TBST) in the next morning. Then goat anti-rabbit IgG second antibody (1:1500, ab197780, ABCAM, UK) was used to incubate the membranes at room temperature for 2 h and washed thrice with TBST. ECL kit (Beyotime Biotechnology, China) was employed to observe protein bands, which weree then photographed. Gray values were analyzed using I-mage J software. GAPDH was taken as the internal reference.
Flow cytometry
Annexin V-FITC/PI staining kit (Beyotime Biotechnology, China) was utilized to detect cell apoptosis. Stably transfected cells were dissolved by trypsin and washed with cold phosphate buffered saline. Buffer solutions were used to re-suspend the dissolved cells after centrifugation. Annexin V-FITC (5 μL) and PI (5 μL) were added successively into the buffer solutions in a dark place and incubated. Then cell apoptosis was measuring using flow cytometry (Thermo Fisher Scientific Inc., USA).
Clone formation assay
Clone formation assay was performed to evaluate cell colony formation ability. After cell transfection in each group, trypsin (0.25%) was used to digest BMECs in the log phase. Collected cells (1×103 cells) were seeded in a 6-well plate in normal BMECs medium and incubated under the above oxygen deficient condition. Cell culture medium was refreshed per two days. Cells were incubated for 2 weeks and rinsed using phosphate buffered saline. Then methanol (2 mL) was added to fix BMECs, and crystal violet solution (0.1%) was added to dye cells for 30 min at room temperature. Then cells in each group were counted and photographed.
Dual luciferase reporter assay
Luciferase reporter vectors (OCLN-WT/OCLN-MT) were constructed. BMECs were seeded in a 48-well plate and cultured to the confluence of 70% cells. OCLN-WT or OCLN-MT vector was co-transfected with miR-NC or miR-122-5p mimic using Lipofectamine 3000. Luciferase activity was measured 48 h after transfection by reference to the instruction.
Statistical analysis
Data excluding bioinformatics data were analyzed by GraphPad Prism 8. All experiments were repeated thrice. Data were shown by mean ± standard deviation. One-way analysis of variance was employed to perform comparison among groups, followed by Tukey post hoc test. Independent-samples t test was employed to perform comparison between groups. P<0.05 indicated a significant difference.
Results
LPS-induced neutrophils promoted the increase of BMECs permeability
Vascular endothelial growth factor (VEGF) and tight junction protein expressions were important parameters for the evaluation of cell permeability. In this study, VEGF, ZO-1 and Claudin-5 were used as indicators of cell permeability. Un-induced and LPS-induced neutrophils were co-cultured with BMECs. Real-time quantitative polymerase chain reaction (RT-qPCR) and Western blot revealed that there were significantly decreased ZO-1 and Claudin-5 expressions at the transcription and translation level, increased mRNA and protein expressions of VEGF (all P<0.05) and increased cell permeability in LPS + neutrophil group versus in neutrophil group (Figure 1). Results in the above indicated that LPS-induced neutrophils promoted the increase of BMECs permeability.
Figure 1.
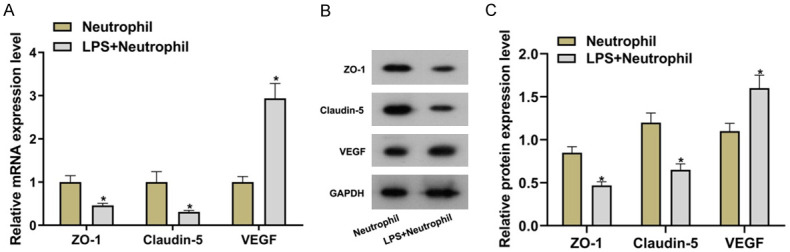
LPS-induced neutrophils promoted the increase of BMECs permeability. A: mRNA expression levels of VEGF, ZO-1 and Claudin-5; B: Protein bands; C: Protein expression levels of VEGF, ZO-1 and Claudin-5 and semiquantitative results. Compared with the neutrophil group, *P<0.05. LPS: lipopolysaccharide; VEGF: vascular endothelial growth factor.
LPS-induced neutrophil exosomes promoted the increase of BMECs apoptosis and permeability
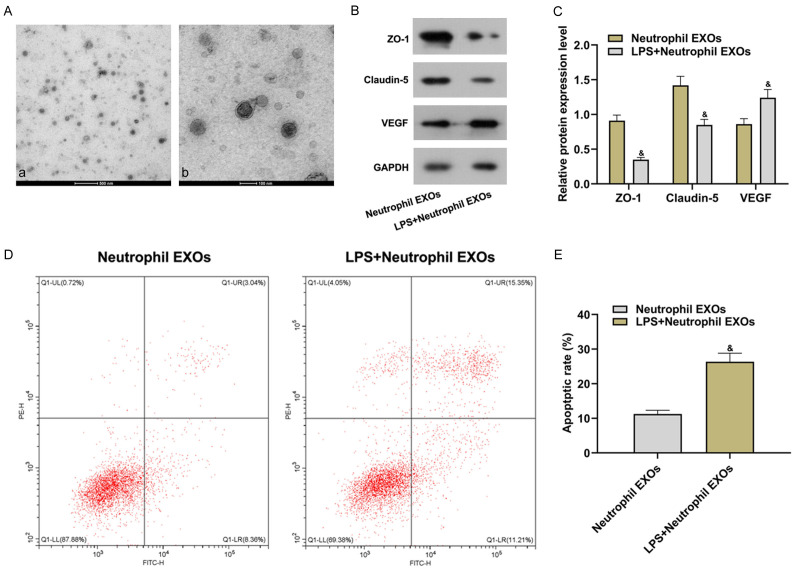
All neutrophil exosomes were identified with a transmission electron microscope to verify the successful isolation of exosomes. These neutrophil exosomes showed a typical cotyliform, with a size of about 130 nm (Figure 2A). Then un-induced and LPS-induced neutrophil exosomes were co-cultured with BMECs. Claudin-5 and ZO-1 expressions in LPS + neutrophil EXOs group versus in Neutrophil EXOs group lowered significantly, and VEGF expression increased (all P<0.05). It indicated that BMECs permeability increased significantly (Figure 2B and 2C). LPS + neutrophil EXOs group compared with Neutrophil EXOs group showed higher cell apoptotic rate (P<0.05, Figure 2D and 2E), suggesting that LPS-induced neutrophil exosomes promoted the increase of BMECs apoptosis and permeability.
Figure 2.
LPS-induced neutrophil exosomes promoted the increase of BMECs apoptosis and permeability. (A) Isolation results of neutrophil-secreted exosomes (a: 10,000×; b: 40,000×); (B) Relative protein expression detected by Western blot; (C) Relative protein expression; (D) Flow cytometry scatter plot; (E) Cell apoptotic rate detected by flow cytometry. Compared with the neutrophil EXOs group, &P<0.05. LPS: lipopolysaccharide; VEGF: vascular endothelial growth factor.
Differential gene screening and target relation prediction
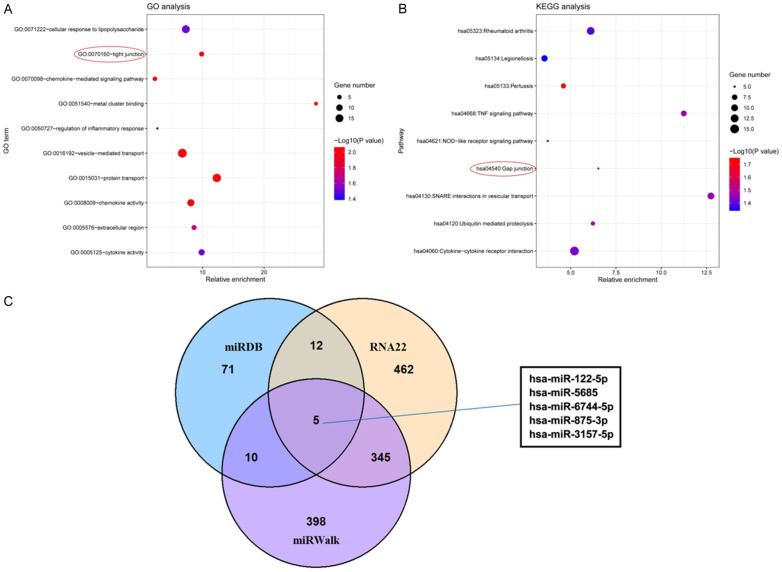
A total of 256 differential expression genes were found in the microarray data, including 102 up-regulated genes and 154 down-regulated genes. Significant enrichment of cell tight junction related GO term (Fold enrichment: 9.863142438, P=0.008649819) and pathway (Fold enrichment: 6.525946305, P=0.031474141) was found by GO and KEGG analyses (Figure 3A and 3B), indicating that neutrophils might regulate BMECs permeability by secreting micro-vesicles. It was interesting that occludin was found in the above GO term and was selected as the focus of this study. OCLN was taken as the target gene to explore the possible upstream molecular mechanism of miRNA-mRNA interaction. RNA22, miRDB and miRWalk were used to predict miRNAs that might bind to OCLN, and the intersection was obtained. hsa-miR-122-5p, hsa-miR-5685, hsa-miR-6744-5p, hsa-miR-875-3p and hsa-miR-3157-5p were found in the results obtained by the three prediction software (Figure 3C). In this study we focused on miR-122-5p. In BMECs, mechanism of miR-122-5p and OCLN was further explored.
Figure 3.
RNA sequencing and target relation prediction. A: GO analysis results; B: KEGG analysis results; C: Target sites of OCLN predicted by RNA22, miRDB and miRWalk.
miR-122-5p targeted the binding with OCLN and down-regulated OCLN expression
Through the RNA22, miRDB and miRWalk target relation prediction websites specific sites of miR-122-5p binding to OCLN were found (Figure 4A). The results of dual luciferase reporter assay indicated that miR-mimic versus miR-NC suppressed the luciferase activity of OCLN-WT (P<0.05) while had little effect on the luciferase activity of OCLN-MUT (P>0.05, Figure 4B). OCLN expression decreased significantly in the miR-mimic group and increased in the miR-inhibitor group as compared to miR-NC group, and no significant difference was found between the miR-NC group and blank group (Figure 4C). The results suggested that OCLN was the target gene of miR-122-5p and regulated by miR-122-5p.
Figure 4.
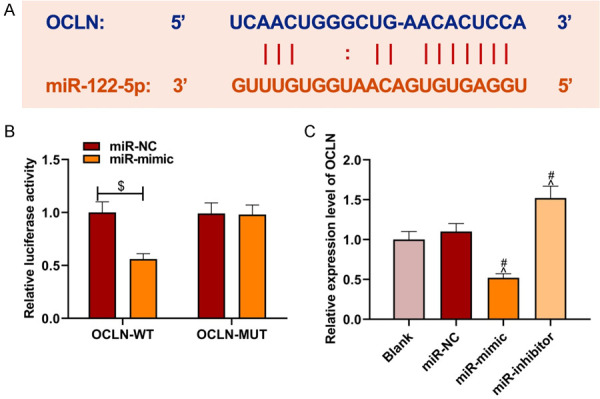
miR-122-5p targeted the binding with OCLN and down-regulated OCLN expression. A: Binding sites of miR-122-5p and OCLN; B: Dual luciferase reporter assay results; C: OCLN expression regulated by miR-122-5p. Compared with the miR-NC and OCLN-WT co-transfection group, $P<0.05; compared with the blank group, ^P<0.05; compared with the miR-NC group, #P<0.05. NC: negative control.
miR-122-5p in over-expressed neutrophil exosomes aggravated BMECs injury
Exosomes in the LPS + neutrophil EXOs and neutrophil EXOs groups were detected by RT-qPCR to verify miR-122-5p expression in neutrophil-secreted exosomes. miR-122-5p expression in LPS + neutrophil EXOs group versus neutrophil EXOs group was higher (P<0.05, Figure 5A), indicating that LPS might regulate downstream biological process by stimulating neutrophils secreting miR-122-5p. miR-122-5p expression in LPS-induced neutrophil-secreted exosomes was intervened and shown in Figure 5B. As compared to miR-NC-exo group, BMECs proliferation and colony formation ability decreased significantly in the miR-mimic-exo group and increased in the miR-inhibitor-exo group (P<0.05, Figure 5C-E). The expressions of antioxidant substances (SOD and CAT) in the miR-mimic-exo group versus the miR-NC-exo group were lower, and the expressions of oxidation products (ROS and MDA) increased significantly (P<0.05, Figure 5F-H). miR-122-5p overexpression had been shown to aggravate the oxidative injury of BMECs. Overexpressed miR-122-5p had been further confirmed to inhibit the growth of BMECs and aggravated the oxidative injury of BMECs.
Figure 5.
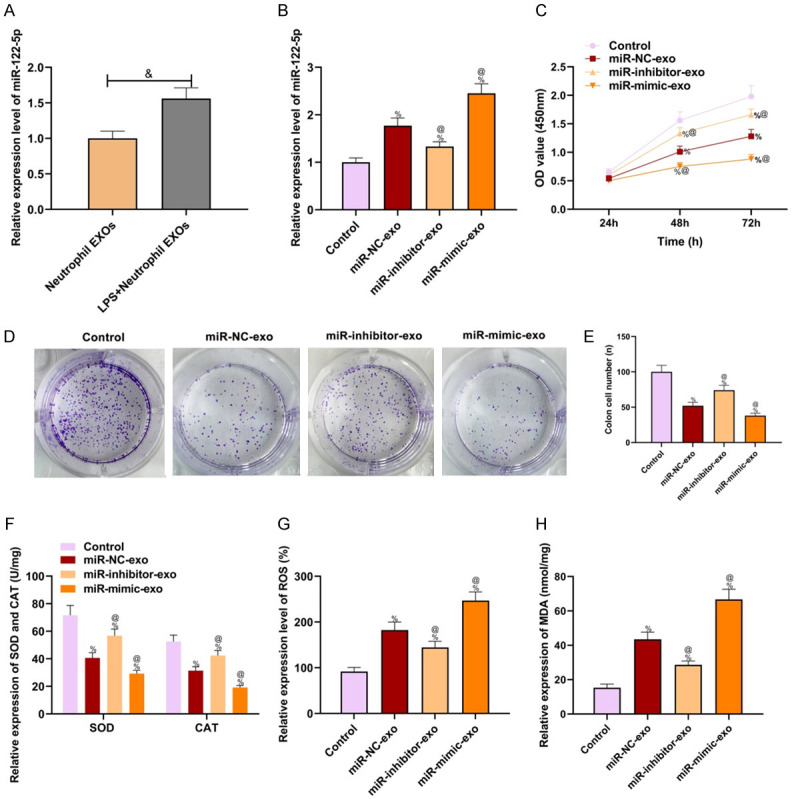
Over-expressed miR-122-5p aggravated BMECs injury. A: miR-122-5p expression in un-induced and LPS-induced neutrophil exosomes; B: miR-122-5p expression in BMECs detected by RT-qPCR; C: Cell proliferation detected by CCK-8; D: Clone formation assay results; E: Clone cell number; F: Expressions of antioxidant substances (SOD and CAT); G: ROS expression; H: MDA expression. Compared with the neutrophil EXOs group, &P<0.05; compared with the control group, %P<0.05; compared with the miR-NC-exo group, @P<0.05. RT-qPCR: real-time quantitative polymerase chain reaction; CCK-8: cholecystokinin octopeptide; LPS: lipopolysaccharide; ROS: reactive oxygen species; MDA: malondialdehyde; SOD: superoxide dismutase; CAT: catalase; NC: negative control.
OCLN overexpression in BMECs partly reversed the regulation of neutrophil-derived miR-122-5p on BMECs growth and oxidative stress
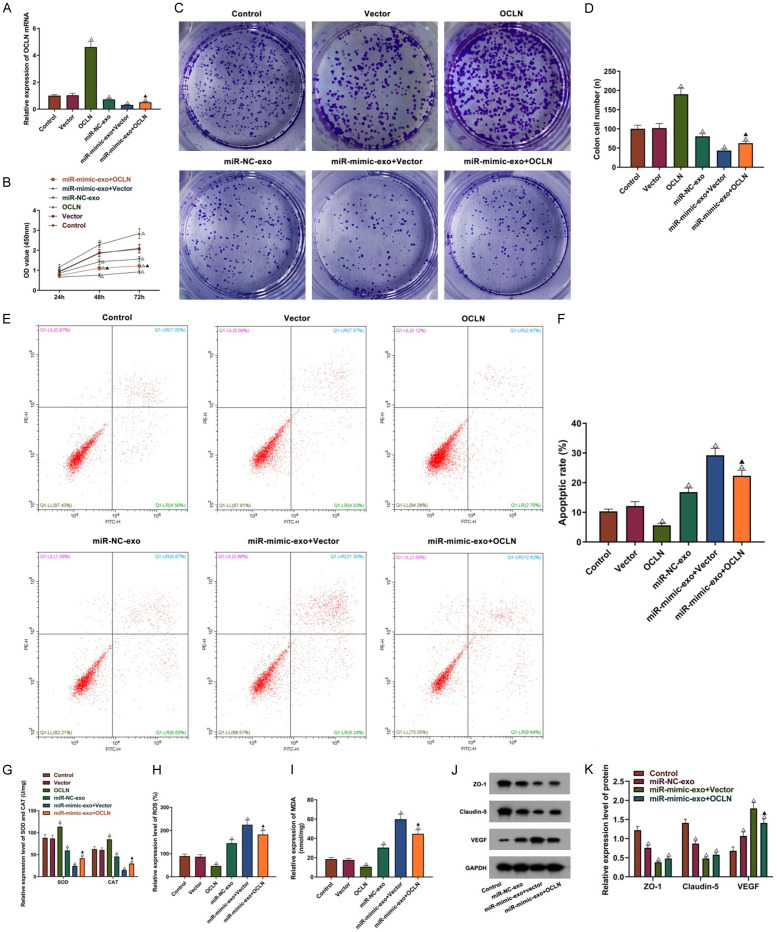
Combined with the results in the section “miR-122-5p targeted the binding with OCLN and down-regulated OCLN expression”, BMECs were treated with exosomes containing miR-122-5p or OCLN, and the internal mechanism of miR-122-5p in the regulation of BMECs growth and oxidative stress was further explored. OCLN expression in BMECs was detected. Exosomes containing miR-122-5p had been shown to be successfully absorbed by BMECs and reduced OCLN expression. Moreover, OCLN was over-expressed successfully in BMECs (P<0.05, Figure 6A). Cell phenotype test showed that compared with the vector group, OCLN overexpression significantly increased BMECs activity, colony formation ability SOD and CAT expressions and inhibited BMECs apoptosis, ROS generation and MDA level (all P<0.05, Figure 6B-I). As compared to miR-mimic-exo + vector group, OCLN overexpression in BMECs partly decreased the blocking of BMECs activity, colony formation ability, SOD and CAT expressions and the induction of BMECs apoptosis, ROS and MDA generation by miR-122-5p in neutrophil-derived exosomes (all P<0.05). After BMECs absorbed miR-mimic-exo, their regulation on Claudin-5 and ZO-1 of blood brain barrier was inhibited significantly, and VEGF related to endothelial cell proliferation showed an increasing trend (all P<0.05, Figure 6J, 6K). Compared with the miR-mimic-exo + vector group, OCLN overexpression in BMECs partly decreased miR-mimic-exo-induced VEGF up-regulation (P<0.05), but ZO-1 and Claudin-5 expressions showed no significant differences (both P>0.05).
Figure 6.
OCLN overexpression partly reversed the effect of miR-122-5p on BMECs. A: OCLN mRNA expression in BMECs treated with miR-122-5p exosomes or OCLN; B: Cell activity; C, D: Colony formation ability; E, F: Cell apoptosis; G-I: SOD, CAT, ROS and MDA levels; J, K: ZO-1, Claudin-5 and VEGF protein expressions. Compared with the control or vector group, ΔP<0.05; compared with the miR-mimic-exo + vector group, ▲P<0.05. OD: optical density; ROS: reactive oxygen species; MDA: malondialdehyde; SOD: superoxide dismutase; CAT: catalase; NC: negative control; VEGF: vascular endothelial growth factor.
Discussion
The relation between exosomes and cerebrovascular diseases has gradually become a research hotspot in recent years [21]. The effect of miR-122-5p exosomes secreted by LPS-induced neutrophils on BEMCs permeability by targeting downstream gene OCLN was mainly analyzed in this study.
miRNA is a kind of small RNA with a length of about 20-24 nt [22,23]. An increasing number of miRNAs participate in regulating cerebrovascular diseases [24-26]. Although miRNAs exert a key role in cerebrovascular diseases, the effect of miRNA exosomes on BEMCs is still unclear.
Exosomes are vesicles secreted by different types of cells. Exosomes exert a key part in mediating intercellular communication and can cross the blood brain barrier [27]. Exosomes have been proved to participate in multiple physiological and pathological processes [28]. In addition, exosome is one of the therapeutic strategies for cerebrovascular diseases. For example, rats with cerebral ischemia had abundant mature miRNAs in brain endothelial cells and more selective miRNAs up-regulation than non-ischemic rats [29]. miR-34c exosomes showed a neuroprotective effect on cerebral ischemia by the TLR7/MAPK pathway [30]. However, there were few reports on the change in exosome miRNAs and their regulation on BEMCs.
This was the first study to explore the regulation of neutrophil exosome miR-122-5p on BEMCs. miR-122-5p has played a role as a tumor suppressor in liver and colorectal cancer progression [31,32]. In addition, drug combination for mice with autoimmune encephalomyelitis could reduce miR-122-5p expression level [33]. Up-regulation of miR-122-5p occurred after cerebral ischemia. miR-122-5p inhibited activation-related receptors expressions in natural killer cells and reduce natural killer cells to produce cytotoxic effect [20]. In this study, miR-122-5p showed a high expression in LPS-induced neutrophil-secreted exosomes, which could inhibit Claudin-5 and ZO-1 expressions in BEMCs, promote VEGF expression, and increase cell permeability.
Moreover, LPS-induced neutrophil-secreted miR-122-5p exosomes could facilitate BEMCs apoptosis. CCK-8 and clone formation assay indicated that overexpressed miR-122-5p significantly suppressed BEMCs proliferation and colony formation. The endothelium of blood brain barrier has been confirmed to be the main site for ROS generation, and ROS can act on the mediation of oxidative damage caused by cerebral ischemia, the impairing of cerebrovascular endothelial barrier integrity and the increase of endothelial cell permeability [34]. Therefore, we detected oxidative stress factors and found that miR-122-5p overexpression could significantly accelerate the oxidative stress process.
A study about renal cell carcinoma showed that the regulation of OCLN by miR-122 resulted in the malignant phenotype of renal cell carcinoma cells but reduced the invasion of hepatitis C virus into liver cells [35]. In addition, miR-122-5p expression had a negative correlation with OCLN expression in patients with oligospermia [36]. It indicated that such target relation might also exist in other organs, tissues or cells. miR-122-5p and the downstream target gene OCLN were used for further analysis to further explore mechanism of miR-122-5p exosomes in BEMCs injury. OCLN as a main structural protein of tight junction has a protective effect on cell barrier [18]. A study showed that in mice in vivo and in vitro, OCLN is important in restricting macromolecules (10-70 kDa) passing through the epithelium without affecting transepithelial resistance [37]. Truncation of OCLN expression in xenopus laevis increased paracellular leakage, and OCLN in cultured epithelial cells reduced transepithelial resistance [38]. In this study, dual luciferase reporter assay was performed to confirm the interactive relationship between miR-122-5p and OCLN. miR-122-5p targeted the binding with OCLN and down-regulated OCLN expression. The results of functional analysis experiment showed that miR-122-5p exosomes overexpression inhibited BEMCs proliferation and colony formation and promote the increase of cell apoptosis, oxidative stress and cell permeability. OCLN overexpression vector transfected in overexpressed miR-122-5p exosomes partly reversed the effect of miR-122-5p exosomes on BEMCs oxidative stress, apoptosis and proliferation. Combined with previous reports to analyze the mechanism, miR-200b could reduce endothelial cell permeability by targeting VEGF, and VEGF could regulate retinal endothelial cell permeability by inducing Claudin protein phosphorylation [39,40]. Therefore, we speculated that VEGF and tight junction protein OLCN had a coordination effect in regulating endothelial cell permeability. As common detection indicators in permeability analysis, OCLN, ZO-1 and Claudin-5 were not reported to have a regulatory relationship. In this study, we also did not confirm that intervention in OCLN alone would have an effect on ZO-1 and Claudin-5. A previous review showed that OLCN family phosphorylation was closely related to oxidative stress, which had been extensively researched, and played an important role in oxidative stress related pathology and corresponding drug intervention [41]. The possible regulation of miR-122-5p/OCLN in the oxidative stress of BEMCs had been further explained.
In conclusion, miR-122-5p in LPS-induced neutrophil exosomes can down-regulate OCLN expression and promote BEMCs injury. However, animal experiments are needed to further verify the experimental reliability, hoping to further clarify the effect of neutrophil-secreted miR-122-5p exosomes in BEMCs. The mechanism flow chart of this study is shown in Figure 7.
Figure 7.
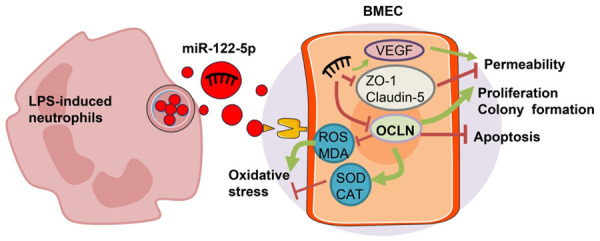
Mechanism flow chart of LPS-induced neutrophil-secreted miR-122-5p exosomes regulating BEMCs proliferation, apoptosis and permeability by targeting OCLN. LPS: lipopolysaccharide; VEGF: vascular endothelial growth factor; BEMC: brain microvascular endothelial cell.
Disclosure of conflict of interest
None.
References
- 1.Wang Z, Yang Y, Liang X, Gao B, Liu M, Li W, Chen Z, Wang Z. COVID-19 associated ischemic stroke and hemorrhagic stroke: incidence, potential pathological mechanism, and management. Front Neurol. 2020;11:571996. doi: 10.3389/fneur.2020.571996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harun MSR, Marsh V, Elsaied NA, Webb KF, Elsheikha HM. Effects of Toxoplasma gondii infection on the function and integrity of human cerebrovascular endothelial cells and the influence of verapamil treatment in vitro. Brain Res. 2020;1746:147002. doi: 10.1016/j.brainres.2020.147002. [DOI] [PubMed] [Google Scholar]
- 3.Zhang L, Luo X, Chen F, Yuan W, Xiao X, Zhang X, Dong Y, Zhang Y, Liu Y. LncRNA SNHG1 regulates cerebrovascular pathologies as a competing endogenous RNA through HIF-1α/VEGF signaling in ischemic stroke. J Cell Biochem. 2018;119:5460–5472. doi: 10.1002/jcb.26705. [DOI] [PubMed] [Google Scholar]
- 4.Kulczar C, Lubin KE, Lefebvre S, Miller DW, Knipp GT. Development of a direct contact astrocyte-human cerebral microvessel endothelial cells blood-brain barrier coculture model. J Pharm Pharmacol. 2017;69:1684–1696. doi: 10.1111/jphp.12803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang M, Wang XY, Zhou WY, Li J, Wang J, Guo LP. Cerebral protection of salvianolic acid A by the inhibition of granulocyte adherence. Am J Chin Med. 2011;39:111–120. doi: 10.1142/S0192415X11008683. [DOI] [PubMed] [Google Scholar]
- 6.Lyck R, Ruderisch N, Moll AG, Steiner O, Cohen CD, Engelhardt B, Makrides V, Verrey F. Culture-induced changes in blood-brain barrier transcriptome: implications for amino-acid transporters in vivo. J Cereb Blood Flow Metab. 2009;29:1491–1502. doi: 10.1038/jcbfm.2009.72. [DOI] [PubMed] [Google Scholar]
- 7.Sadiku P, Willson JA, Ryan EM, Sammut D, Coelho P, Watts ER, Grecian R, Young JM, Bewley M, Arienti S, Mirchandani AS, Sanchez Garcia MA, Morrison T, Zhang A, Reyes L, Griessler T, Jheeta P, Paterson GG, Graham CJ, Thomson JP, Baillie K, Thompson AAR, Morgan JM, Acosta-Sanchez A, Dardé VM, Duran J, Guinovart JJ, Rodriguez-Blanco G, Von Kriegsheim A, Meehan RR, Mazzone M, Dockrell DH, Ghesquiere B, Carmeliet P, Whyte MKB, Walmsley SR. Neutrophils fuel effective immune responses through gluconeogenesis and glycogenesis. Cell Metab. 2021;33:411–423. e4. doi: 10.1016/j.cmet.2020.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albrecht J, Sonnewald U, Waagepetersen HS, Schousboe A. Glutamine in the central nervous system: function and dysfunction. Front Biosci. 2007;12:332–343. doi: 10.2741/2067. [DOI] [PubMed] [Google Scholar]
- 9.Kim YR, Kim YM, Lee J, Park J, Lee JE, Hyun YM. Neutrophils return to bloodstream through the brain blood vessel after crosstalk with microglia during LPS-induced neuroinflammation. Front Cell Dev Biol. 2020;8:613733. doi: 10.3389/fcell.2020.613733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gorina R, Lyck R, Vestweber D, Engelhardt B. β2 integrin-mediated crawling on endothelial ICAM-1 and ICAM-2 is a prerequisite for transcellular neutrophil diapedesis across the inflamed blood-brain barrier. J Immunol. 2014;192:324–337. doi: 10.4049/jimmunol.1300858. [DOI] [PubMed] [Google Scholar]
- 11.Fang S, He T, Jiang J, Li Y, Chen P. Osteogenic effect of tsRNA-10277-loaded exosome derived from bone mesenchymal stem cells on steroid-induced osteonecrosis of the femoral head. Drug Des Devel Ther. 2020;14:4579–4591. doi: 10.2147/DDDT.S258024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J, Zhao J, Zhu J, Zhang S. Hypoxic non-small-cell lung cancer cell-secreted exosomal microRNA-582-3p drives cancer cell malignant phenotypes by targeting secreted frizzled-related protein 1. Cancer Manag Res. 2020;12:10151–10161. doi: 10.2147/CMAR.S263768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng C, Xiong Z, Wang C, Xiao W, Xiao H, Xie K, Chen K, Liang H, Zhang X, Yang H. Folic acid-modified Exosome-PH20 enhances the efficiency of therapy via modulation of the tumor microenvironment and directly inhibits tumor cell metastasis. Bioact Mater. 2021;6:963–974. doi: 10.1016/j.bioactmat.2020.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng Y, Zhong M, Zeng S, Wang L, Liu P, Xiao X, Liu Y. Exosome-derived miRNAs as predictive biomarkers for diffuse large B-cell lymphoma chemotherapy resistance. Epigenomics. 2019;11:35–51. doi: 10.2217/epi-2018-0123. [DOI] [PubMed] [Google Scholar]
- 15.Qu L, Ding J, Chen C, Wu ZJ, Liu B, Gao Y, Chen W, Liu F, Sun W, Li XF, Wang X, Wang Y, Xu ZY, Gao L, Yang Q, Xu B, Li YM, Fang ZY, Xu ZP, Bao Y, Wu DS, Miao X, Sun HY, Sun YH, Wang HY, Wang LH. Exosome-transmitted lncARSR promotes sunitinib resistance in renal cancer by acting as a competing endogenous RNA. Cancer Cell. 2016;29:653–668. doi: 10.1016/j.ccell.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Zhu M, Lu J, Shen J, Fei L, Chen D. A 22-amino-acid peptide regulates tight junctions through occludin and cell apoptosis. PeerJ. 2020;8:e10147. doi: 10.7717/peerj.10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rom S, Heldt NA, Gajghate S, Seliga A, Reichenbach NL, Persidsky Y. Author correction: hyperglycemia and advanced glycation end products disrupt BBB and promote occludin and claudin-5 protein secretion on extracellular microvesicles. Sci Rep. 2020;10:18828. doi: 10.1038/s41598-020-64349-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Correale J, Villa A. Cellular elements of the blood-brain barrier. Neurochem Res. 2009;34:2067–2077. doi: 10.1007/s11064-009-0081-y. [DOI] [PubMed] [Google Scholar]
- 19.Tong F, Andress A, Tang G, Liu P, Wang X. Comprehensive profiling of extracellular RNA in HPV-induced cancers using an improved pipeline for small RNA-seq analysis. Sci Rep. 2020;10:19450. doi: 10.1038/s41598-020-76623-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kong Y, Li S, Cheng X, Ren H, Zhang B, Ma H, Li M, Zhang XA. Brain ischemia significantly alters microRNA expression in human peripheral blood natural killer cells. Front Immunol. 2020;11:759. doi: 10.3389/fimmu.2020.00759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thakur A, Sidu RK, Zou H, Alam MK, Yang M, Lee Y. Inhibition of glioma cells’ proliferation by doxorubicin-loaded exosomes via microfluidics. Int J Nanomedicine. 2020;15:8331–8343. doi: 10.2147/IJN.S263956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duică F, Condrat CE, Dănila CA, Boboc AE, Radu MR, Xiao J, Li X, Creţoiu SM, Suciu N, Creţoiu D, Predescu DV. MiRNAs: a powerful tool in deciphering gynecological malignancies. Front Oncol. 2020;10:591181. doi: 10.3389/fonc.2020.591181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, Zhou Y, Gao Q, Ping D, Wang Y, Wu W, Lin X, Fang Y, Zhang J, Shao A. The role of exosomal microRNAs and oxidative stress in neurodegenerative diseases. Oxid Med Cell Longev. 2020;2020:3232869. doi: 10.1155/2020/3232869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang H, Wang Y, Lian L, Zhang C, He Z. Glycine-Histidine-Lysine (GHK) alleviates astrocytes injury of intracerebral hemorrhage via the Akt/miR-146a-3p/AQP4 pathway. Front Neurosci. 2020;14:576389. doi: 10.3389/fnins.2020.576389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao W, Zhang T, Wang L, Fu J, Jin H. Diagnostic performance of circulating MicroRNAs in acute ischemic stroke: a protocol for systematic review and meta-analysis. Medicine (Baltimore) 2020;99:e22353. doi: 10.1097/MD.0000000000022353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu J, Zhou Z, Yang Q, Yang K. Differential expression of miR-30a-5p in post stroke depression and bioinformatics analysis of the possible mechanism. Nan Fang Yi Ke Da Xue Xue Bao. 2020;40:922–929. doi: 10.12122/j.issn.1673-4254.2020.07.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rao Y, Fang Y, Tan W, Liu D, Pang Y, Wu X, Zhang C, Li G. Delivery of long non-coding RNA NEAT1 by peripheral blood monouclear cells-derived exosomes promotes the occurrence of rheumatoid arthritis via the microRNA-23a/MDM2/SIRT6 axis. Front Cell Dev Biol. 2020;8:551681. doi: 10.3389/fcell.2020.551681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu L, Xia J, Li D, Kang Y, Fang W, Huang P. Mechanisms of M2 macrophage-derived exosomal long non-coding RNA PVT1 in regulating Th17 cell response in experimental autoimmune encephalomyelitisa. Front Immunol. 2020;11:1934. doi: 10.3389/fimmu.2020.01934. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Zhang Y, Qin Y, Chopp M, Li C, Kemper A, Liu X, Wang X, Zhang L, Zhang ZG. Ischemic cerebral endothelial cell-derived exosomes promote axonal growth. Stroke. 2020;51:3701–3712. doi: 10.1161/STROKEAHA.120.031728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu W, Liu J, Yang C, Xu Z, Huang J, Lin J. Astrocyte-derived exosome-transported microRNA-34c is neuroprotective against cerebral ischemia/reperfusion injury via TLR7 and the NF-κB/MAPK pathways. Brain Res Bull. 2020;163:84–94. doi: 10.1016/j.brainresbull.2020.07.013. [DOI] [PubMed] [Google Scholar]
- 31.Liang Y, Zhang D, Zheng T, Yang G, Wang J, Meng F, Liu Y, Zhang G, Zhang L, Han J, Hui P, Chen Z, Liu Y, Wang M, Jiang H, Liu L. lncRNA-SOX2OT promotes hepatocellular carcinoma invasion and metastasis through miR-122-5p-mediated activation of PKM2. Oncogenesis. 2020;9:54. doi: 10.1038/s41389-020-0242-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yin W, Xu J, Li C, Dai X, Wu T, Wen J. Circular RNA circ_0007142 facilitates colorectal cancer progression by modulating CDC25A expression via miR-122-5p. Onco Targets Ther. 2020;13:3689–3701. doi: 10.2147/OTT.S238338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al-Ghezi ZZ, Miranda K, Nagarkatti M, Nagarkatti PS. Combination of cannabinoids, Δ9-tetrahydrocannabinol and cannabidiol, ameliorates experimental multiple sclerosis by suppressing neuroinflammation through regulation of miRNA-mediated signaling pathways. Front Immunol. 2019;10:1921. doi: 10.3389/fimmu.2019.01921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen WX, Guo C, Feng H, Chen YJ. Mitochondria: novel mechanisms and therapeutic targets for secondary brain injury after intracerebral hemorrhage. Front Aging Neurosci. 2020;12:615451. doi: 10.3389/fnagi.2020.615451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sendi H, Mehrab-Mohseni M, Foureau DM, Ghosh S, Walling TL, Steuerwald N, Zamor PJ, Kaplan KJ, Jacobs C, Ahrens WA, Russo MW, Clemens MG, Schrum LW, Bonkovsky HL. MiR-122 decreases HCV entry into hepatocytes through binding to the 3’ UTR of OCLN mRNA. Liver Int. 2015;35:1315–1323. doi: 10.1111/liv.12698. [DOI] [PubMed] [Google Scholar]
- 36.Jingushi K, Kashiwagi Y, Ueda Y, Kitae K, Hase H, Nakata W, Fujita K, Uemura M, Nonomura N, Tsujikawa K. High miR-122 expression promotes malignant phenotypes in ccRCC by targeting occludin. Int J Oncol. 2017;51:289–297. doi: 10.3892/ijo.2017.4016. [DOI] [PubMed] [Google Scholar]
- 37.Zhang LN, Feng T, Spicer Leon J. The role of tight junction proteins in ovarian follicular development and ovarian cancer. Reproduction. 2018;155:R183–R198. doi: 10.1530/REP-17-0503. [DOI] [PubMed] [Google Scholar]
- 38.Cyr DG, Gregory M, Dubé E, Dufresne J, Chan PT, Hermo L. Orchestration of occludins, claudins, catenins and cadherins as players involved in maintenance of the blood-epididymal barrier in animals and humans. Asian J Androl. 2007;9:463–475. doi: 10.1111/j.1745-7262.2007.00308.x. [DOI] [PubMed] [Google Scholar]
- 39.Yang MC, You FL, Wang Z, Liu XN, Wang YF. Salvianolic acid B improves the disruption of high glucose-mediated brain microvascular endothelial cells via the ROS/HIF-1α/VEGF and miR-200b/VEGF signaling pathways. Neurosci Lett. 2016;630:233–240. doi: 10.1016/j.neulet.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 40.Harhaj NS, Felinski EA, Wolpert EB, Sundstrom JM, Gardner TW, Antonetti DA. VEGF activation of protein kinase C stimulates occludin phosphorylation and contributes to endothelial permeability. Invest Ophthalmol Vis Sci. 2006;47:5106–5115. doi: 10.1167/iovs.06-0322. [DOI] [PubMed] [Google Scholar]
- 41.Blasig IE, Bellmann C, Cording J, Del Vecchio G, Zwanziger D, Huber O, Haseloff RF. Occludin protein family: oxidative stress and reducing conditions. Antioxid Redox Signal. 2011;15:1195–1219. doi: 10.1089/ars.2010.3542. [DOI] [PubMed] [Google Scholar]