Abstract
Background: Cyanotic congenital heart disease (CCHD) is one of the most common birth anomalies, in which chronic hypoxia is the basic pathophysiological process. Methods: To investigate the heart’s metabolic remodeling to hypoxia, we performed an untargeted metabolomic analysis of cardiac tissue from 20 CCHD patients and 15 patients with acyanotic congenital heart disease (ACHD). Results: A total of 71 (63%) metabolites from 113 detected substances in cardiac tissue differed between the CCHD and ACHD groups. A partial least squares discriminant analysis showed separation between the CCHD and ACHD groups. A pathway enrichment analysis revealed that the most enriched metabolic pathways were amino acid metabolism and energy metabolism. Eleven amino acids were increased in CCHD patients, indicating that protein synthesis was down-regulated. Most of the metabolites in Krebs circle were increased in CCHD patients, suggesting down regulation of aerobic energy metabolism. Hierarchical cluster analysis showed that nicotinamide adenine dinucleotide (NAD) was clustered with Krebs cycle related substrates and its level was significantly higher in CCHD than that in ACHD patients. These analyses suggest that NAD might play an important role in response to hypoxia in CCHD patients. Conclusion: Our data showed a significantly different metabolic profile in CCHD patients compared to ACHD patients, including reduced protein synthesis and aerobic energy production, and the increased level of NAD in the myocardium may be a response mechanism to hypoxia.
Keywords: Congenital heart disease, chronic hypoxia, metabolomics, Krebs cycle, nicotinamide adenine dinucleotide
Introduction
Congenital heart disease (CHD) is the most common birth anomaly. The prevalence is about 1%, which is similar around the world [1-3]. CHD occurs in 10% of aborted fetuses, and is the leading cause of mortality from congenital disabilities [4,5]. Cyanotic CHD (CCHD) account for approximately 25% of all CHDs [2], and chronic hypoxia is the basic pathophysiological process. Under such circumstances, the heart itself adapts to hypoxia and survives in a certain period. Thus, research into the protective mechanisms of cardiomyocytes against chronic hypoxia can lead to novel treatment strategies for many patients.
Metabolomics is the study of metabolism globally which captures global biochemical events by assaying thousands of small molecules in tissues, followed by applying of informatics techniques to define metabolomic signatures of the targets [6]. Gas and liquid chromatography coupled with mass spectrometry is well suited for metabolomic research to identify and accurately quantify the small-molecular-weight metabolites [7]. Metabolomics is widely used in studies on cancers, where significant discoveries have potential therapeutic value [8]. However, there are few metabolomic studies of CCHD patients using human cardiac tissues.
Our study evaluates the effects of hypoxia on the metabolism of the human heart by comparing cardiac tissue from CCHD patients with cardiac tissue from acyanotic CHD (ACHD) patients to describe the metabolic remodeling of cardiac tissue in CCHD. Significantly differential metabolic profiling in the cardiac tissue shown between CCHD and ACHD patients. Interestingly, the content of nicotinamide adenine dinucleotide (NAD) was significantly higher in myocardium from CCHD than that from ACHD patients. The present study provides a valuable result for a better understanding of the pathogenesis mechanism of CCHD.
Methods
Patients
Thirty-five children who had cardiac surgery for CHD at Fuwai Hospital were enrolled in this study. Our sample included 15 patients with ACHD and 20 patients with CCHD were grouped into CCHD group. A small piece of tissue from the patients’ left atrial was excised when a cardiopulmonary bypass was performed. The study was approved by the Institutional Ethics Committee of Fuwai Hospital and was conducted following the Declaration of Helsinki principles. Written informed consent was obtained from the guardians of each patient.
Sample preparation for metabolomics analysis
Cardiac tissue was excised during the cardiopulmonary bypass, washed in ice-cold PBS to remove excess blood, and snap-frozen in liquid nitrogen. Metabolites were extracted with methanol and then prepared for LC-MS.
The LC-MS analysis used a Waters ACQUITY UPLC (Waters Corp., Massachusetts, USA) and a LTQ-Orbitrap mass spectrometer (ThermoFisher Scientific, Massachusetts, USA). The sample extract was split into two aliquots, dried, and then reconstituted in either acidic or basic LC-compatible solvents. One aliquot was analyzed using acidic positive ion optimized conditions and the other using basic negative ion optimized conditions in two independent injections using separate dedicated columns. Extracts reconstituted in acidic conditions were gradient eluted using water and methanol both containing 0.1% formic acid, while the basic extracts, which also used water and methanol, contained 6.5 mM ammonium bicarbonate.
The LC-MS data were analyzed with the Micromass MarkerLynx Applications Manager, Version 4.1 (Waters, Milford, MA, USA), which allowed deconvolution, alignment, and data reduction. The data were collated into a table of mass and retention time pairs with associated intensities for all the detected peaks.
Statistical analysis
The Shapiro-Wilk tests was applied to determine whether data were normally distributed. Continuous variables were expressed as either mean ± SD or median (interquartile range), while discrete variables were expressed with counts and percentages. We used t-tests combined with Levene’s tests to compare each group’s mean as well as Mann-Whitney U tests to compare each group’s median. Discrete variables were compared with the χ2 or Fisher’s exact tests. SPSS 23.0 software (IBM Corp., Armonk, NY) was used for these analyses. Partial least squares discriminant analysis (PLS-DA) and hierarchical cluster analysis were performed using the online software Metaboanalyst 4.0 (Wisehart Research Group, Ottowa; http://www. metaboanalyst.ca/). Reported probability values were for two-sided tests, and p values < 0.05 were considered statistically significant.
Results
Study population
Thirty-five patients with congenital heart disease were included in our study, 20 with CCHD and 15 with ACHD. The baseline characteristics of the patients are shown in Table 1. The median age of all the patients, CCHD patients and ACHD patients was 10, 14 and 9 months. The overall average weight of all the patients, CCHD patients and ACHD patients was 9.1 kg, 10 kg and 7.8 kg. As expected, the average arterial oxygen saturation was lower, and the hemoglobin was higher in in CCHD group than in the ACHD group. There were no differences in creatinine, blood urea nitrogen and left ventricular ejection fraction between the groups. In the CCHD group, 11 (55%) patients had tetralogy of Fallot and nine (45%) had complete transposition of the great arteries. In the ACHD group, six (40%) had an atrial septal defect, six (40%) had a ventricular septal defect and three (20%) had an atrial and ventricular septal defect (Table 2).
Table 1.
Baseline characteristics
| All (n=35) | CCHDa (n=20) | ACHDb (n=15) | P value | |
|---|---|---|---|---|
| Age (Months) | 10 (8, 16) | 14 (9.5, 23.5) | 9 (7, 10) | 0.003 |
| Males | 22 (63) | 15 (75) | 7 (47) | 0.086 |
| Body weight (Kg) | 9.1±3.0 | 10.0±3.5 | 7.8±1.6 | 0.028 |
| Body length (cm) | 74.3±12.1 | 77.8±14.6 | 69.6±5.2 | 0.028 |
| BSAc (kg/m2) | 0.42±0.11 | 0.45±0.13 | 0.37±0.05 | 0.039 |
| SaO2 d (%) | 86.3±13.2 | 77.7±11.0 | 97.9±2.9 | < 0.001 |
| Hbe (g/dL) | 130.5±34.7 | 145.2±39.6 | 110.9±9.0 | 0.001 |
| Creatinine (mg/dL) | 26.2±6.7 | 27.7±7.8 | 24.4±4.6 | 0.125 |
| BUNf (mg/dL) | 3.5±1.4 | 3.9±1.5 | 3.0±1.3 | 0.061 |
| LVEFg (%) | 64.9±5.2 | 65.5±4.5 | 64.0±6.1 | 0.406 |
CCHD, cyanotic congenital heart disease;
ACHD, acyanotic congenital heart disease;
BSA, body surface area;
SaO2, arterial oxygen saturation;
Hb, hemoglobin;
BUN, blood urea nitrogen;
LVEF, left ventricle ejection fraction.
Table 2.
Congenital heart defects of the patients
CCHD, cyanotic congenital heart disease;
TOF, tetralogy of Fallot;
d-TGA, transposition of great arteries;
ACHD, acyanotic congenital heart disease;
ASD, atrial septal defect;
VSD, ventricular septal defect.
Alterations in myocardial metabolism in CCHD
The relative concentrations of myocardial metabolites were measured by mass spectrometry using an unbiased, non-targeted metabolomic approach. A total of 113 metabolites in cardiac tissue were quantified. Multivariate and univariate analyses were used to investigate changes in myocardial metabolism in the ACHD and CCHD groups. Seventy-one metabolites (63%) that differed between the groups were involved in different pathways, as shown in Figure 1A. The metabolites that changed significantly were in Krebs cycle, glycometabolism and amino acid metabolism (Figure 1B). PLS-DA score plot showed a separation between ACHD and CCHD groups (Figure 2). These analyses indicated a significant difference in metabolic profiling between CCHD and ACHD patients.
Figure 1.
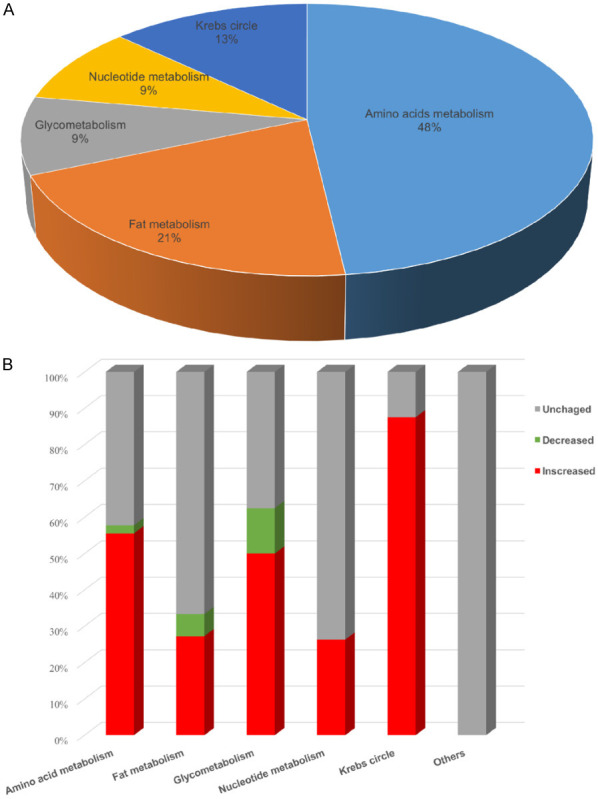
Metabolites distribution and directional changes in heart tissue. A. Class distribution of differential identified metabolites. B. Percentage of metabolites that increased, decreased and unchanged in each class.
Figure 2.
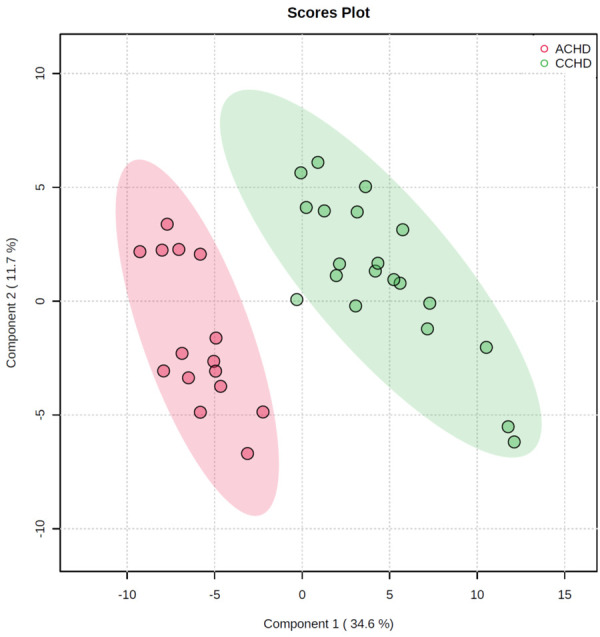
Partial Least Squares Discriminant Analysis (PLS-DA) of cardiac tissue metabolites.
Pathway impact analyses
A pathway enrichment analysis revealed that the significantly enriched metabolic pathways included aminoacyl-tRNA biosynthesis, histidine metabolism, D-glutamine and D-glutamate metabolism, glutathione metabolism, phenylalanine, tyrosine and tryptophan biosynthesis, alanine, aspartate and glutamate metabolism, pyruvate metabolism, arginine biosynthesis, and nicotinate and nicotinamide metabolism (Figure 3).
Figure 3.
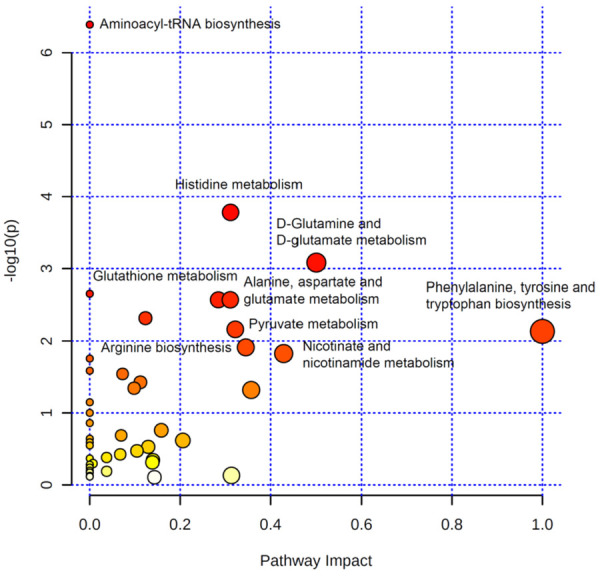
Pathway impact analysis of metabolic changes.
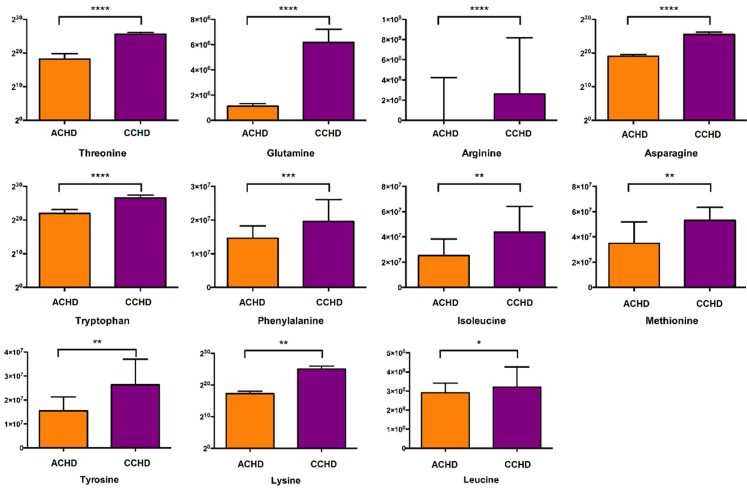
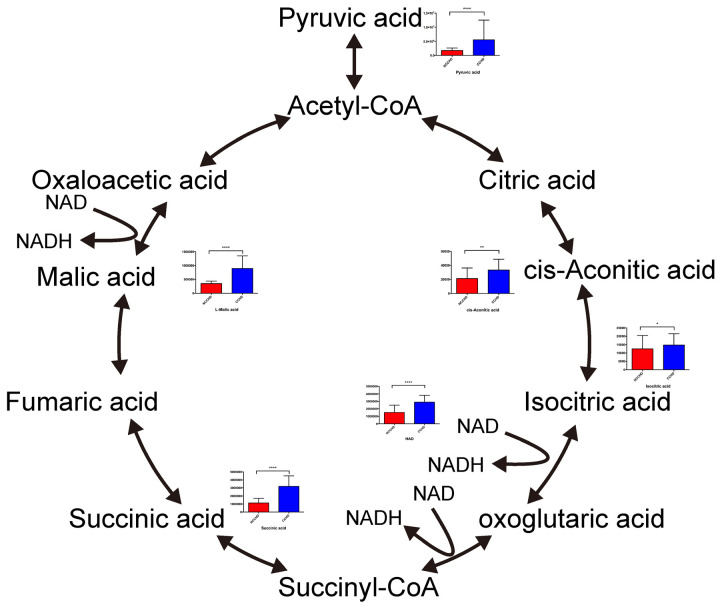
The results showed that 11 amino acids, including glutamine, arginine, asparagine, tryptophan, phenylalanine, isoleucine, leucine, methionine, tyrosine, threonine and lysine, were increased in CCHD patients, indicating that protein synthesis was down-regulated (Figure 4). Most of the metabolites involved in the Krebs cycle were increased in CCHD patients (Figure 5). These findings implied that aerobic energy metabolism was down regulated in these patients. On the other hand, lactate was higher in the CCHD group, which indicated that anaerobic energy metabolism was up-regulated (Figure 6). The metabolites involved in glycolysis, including glucose 6-phosphate and pyruvate, were higher in the CCHD group, suggesting decreased glucose metabolism (Figure 6). In fatty acid metabolism, acyl-carnitines such as palmitoyl-carnitine, butyryl-carnitine, hexanoyl-carnitine, 2-methylbutyroyl-carnitine, dodecanoyl-carnitine and propionyl-carnitine, were increased in the CCHD group, indicating the down regulation of beta-oxygenation of fatty acid (Figure 6).
Figure 4.
The difference of amino acids involved in protein synthesis.
Figure 5.
Changed metabolites involved in Krebs circle. *The metabolites without bar graph were not detected.
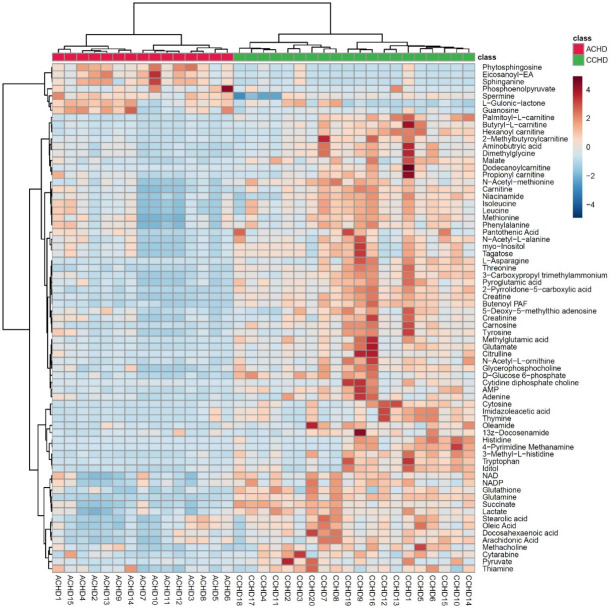
Figure 6.
Hierarchal cluster analysis of the patients with CHD based on their metabolic profile.
Hierarchical cluster analysis of the metabolites separated the groups (Figure 6). We found that NAD was in the same cluster with Krebs cycle related substrates, indicating that the level of NAD was significantly correlated with the condition of the Krebs cycle. These results suggest that NAD may be an endogenous myocardial protective substance that plays an essential role in CCHD patients adapting to the chronic hypoxic condition. We further quantitatively verified the level of NAD in myocardium using a method based on liquid chromatography/triple-stage quadrupole mass spectrometry (LC/TSQ-MS). The CCHD group levels were approximately two times higher than those of the ACHD group (Figure 7), supporting the hypothesis that NAD may play an important role in myocardial energy metabolism during the chronic hypoxia process in CCHD patients.
Figure 7.
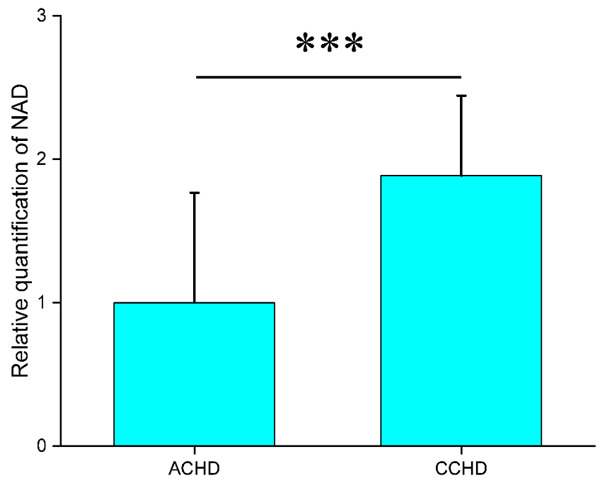
The relative quantification of NAD in myocardium from CCHD and ACHD. ***P < 0.001.
Discussion
This study’s results reveal profound and progressive changes in the hearts’ metabolic profiles when comparing patients with CCHD to those with ACHD. Hearts affected by ACHD displayed significant changes in Krebs cycle, amino acid and carbohydrate metabolism, with relatively little fatty acid and nucleotide metabolism changes. These findings reveal the different metabolic features of ACHD patients and CCHD patients.
Compared with ACHD patients, CCHD patients were ina state of relative hypoxic condition. Since the oxygen supply was insufficient in the heart, mitochondrial oxidative phosphorylation declined. An increased level of many metabolites in the Krebs cycle was detected in CCHD patients. This may indicate a down regulation of energy metabolism that lead to the accumulation of intermediates of Krebs cycle. However, we also found that lactic acid and pyruvic acid were increase in CCHD patients. We was demonstrated that anaerobic respiration and glycolysis were up-regulated in CCHD patients. Limited oxygen supply might lead to a decline in fatty acid oxygenation, which stimulates the cells in heart to uptake more fatty acid for compensation. This could explain, the increased level of acyl carnitine in the CCHD group.
Protein synthesis is a process that need energy support. Under such conditions as CCHD, with a limited energy supply, protein synthesis should be down-regulated. As our data shows, the amount of protein synthetic amino acids was increased in CCHD patients, indicating that the process was down-regulated. In addition, some branched-chain amino acids (BCAAs) such as isoleucine and leucine were increased in CCHD patients. Previous studies showed that BCAAs could improve metabolism and recover protein content in injured tissue [9,10], and supplement of BCAAs could promote the contraction of cardiac and skeletal muscle [11-13]. D’Antona and her colleagues found that BCAA regulate metabolism by increasing insulin release and promoting mitochondrial biogenesis to keep the morphology of myocytes and improve their function. The increased level of BACCs may be a protective response to the hypoxia stress, and the molecular mechanism should be further investigated.
Several studies have demonstrated that NAD could provide myocardial preservation during the ischemia/reperfusion process [14]. NAD is essential for the mitochondrial electron transport reaction. Excessive NAD depletion will lead to the opening of the mitochondrial permeability transition pore and, eventually, to cell death [15,16]. Our study found that the significant metabolic characteristics of the myocardium from CCHD patients were high-level NAD. Compared to other metabolites, the hierarchical cluster analysis showed that NAD was tightly clustered with several Krebs cycle intermediates. This result indicated that the level of NAD in the myocardium might positively correlate with energy metabolism in CCHD patients. However, the collaborative accumulation of NAD and the Krebs cycle intermediates did not show cause and effect in CCHD patients. Whether the endogenous elevation of NAD was proactive myocardial protection, passive accumulation, or both still needs further investigation. A previous study showed that NAD’s exogenous supplement could attenuate myocardial cell apoptosis and increase viability [14]. The hypoxia condition during cardiopulmonary bypass may lead to a depletion of NAD. This may cause a higher postcardiac surgery injury susceptibility of CCHD patients than ACHD patients because of the low tolerance of depletion of NAD.
The present study still has several limitations. First, even though many metabolites were detected, some important metabolites could not be detected due to technical limitations. Second, cross-section metabolites were measured so that the available database deduced the metabolites’ fluxes. Third, it is possible that tissue sample collection and metabolites extraction could introduce artificial changes.
Acknowledgements
This study was sponsored by Shanghai Municipal Science and Technology Major Project (Grant No. 2017SHZDZX01), the Science and Technology Innovation Committee of Shenzhen (Grant No. JCYJ20140414124506130) and Longgang Distract Science and Technology Foundation (Grant No. LGKCYLWS2019000873). We would like to thank TopEdit (www.topeditsci.com) for English language editing of this manuscript.
Disclosure of conflict of interest
None.
References
- 1.van der Linde D, Konings EE, Slager MA, Witsenburg M, Helbing WA, Takkenberg JJ, Roos-Hesselink JW. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;58:2241–7. doi: 10.1016/j.jacc.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- 3.Bernier PL, Stefanescu A, Samoukovic G, Tchervenkov CI. The challenge of congenital heart disease worldwide: epidemiologic and demographic facts. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2010;13:26–34. doi: 10.1053/j.pcsu.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Jorgensen M, McPherson E, Zaleski C, Shivaram P, Cold C. Stillbirth: the heart of the matter. Am J Med Genet A. 2014;164A:691–9. doi: 10.1002/ajmg.a.36366. [DOI] [PubMed] [Google Scholar]
- 5.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaddurah-Daouk R, Kristal BS, Weinshilboum RM. Metabolomics: a global biochemical approach to drug response and disease. Annu Rev Pharmacol Toxicol. 2008;48:653–83. doi: 10.1146/annurev.pharmtox.48.113006.094715. [DOI] [PubMed] [Google Scholar]
- 7.Weckwerth W. Metabolomics in systems biology. Annu Rev Plant Biol. 2003;54:669–89. doi: 10.1146/annurev.arplant.54.031902.135014. [DOI] [PubMed] [Google Scholar]
- 8.Tomita M, Kami K. Cancer. Systems biology, metabolomics, and cancer metabolism. Science. 2012;336:990–1. doi: 10.1126/science.1223066. [DOI] [PubMed] [Google Scholar]
- 9.Dillon EL, Sheffield-Moore M, Paddon-Jones D, Gilkison C, Sanford AP, Casperson SL, Jiang J, Chinkes DL, Urban RJ. Amino acid supplementation increases lean body mass, basal muscle protein synthesis, and insulin-like growth factor-I expression in older women. J Clin Endocrinol Metab. 2009;94:1630–7. doi: 10.1210/jc.2008-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Volpi E, Ferrando AA, Yeckel CW, Tipton KD, Wolfe RR. Exogenous amino acids stimulate net muscle protein synthesis in the elderly. J Clin Invest. 1998;101:2000–7. doi: 10.1172/JCI939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solerte SB, Gazzaruso C, Bonacasa R, Rondanelli M, Zamboni M, Basso C, Locatelli E, Schifino N, Giustina A, Fioravanti M. Nutritional supplements with oral amino acid mixtures increases whole-body lean mass and insulin sensitivity in elderly subjects with sarcopenia. Am J Cardiol. 2008;101:69E–77E. doi: 10.1016/j.amjcard.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Solerte SB, Fioravanti M, Locatelli E, Bonacasa R, Zamboni M, Basso C, Mazzoleni A, Mansi V, Geroutis N, Gazzaruso C. Improvement of blood glucose control and insulin sensitivity during a long-term (60 weeks) randomized study with amino acid dietary supplements in elderly subjects with type 2 diabetes mellitus. Am J Cardiol. 2008;101:82E–88E. doi: 10.1016/j.amjcard.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 13.D’Antona G, Ragni M, Cardile A, Tedesco L, Dossena M, Bruttini F, Caliaro F, Corsetti G, Bottinelli R, Carruba MO, Valerio A, Nisoli E. Branched-chain amino acid supplementation promotes survival and supports cardiac and skeletal muscle mitochondrial biogenesis in middle-aged mice. Cell Metab. 2010;12:362–72. doi: 10.1016/j.cmet.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 14.Liu L, Wang P, Liu X, He D, Liang C, Yu Y. Exogenous NAD(+) supplementation protects H9c2 cardiac myoblasts against hypoxia/reoxygenation injury via Sirt1-p53 pathway. Fundam Clin Pharmacol. 2014;28:180–189. doi: 10.1111/fcp.12016. [DOI] [PubMed] [Google Scholar]
- 15.Xia W, Wang Z, Wang Q, Han J, Zhao C, Hong Y, Zeng L, Tang L, Ying W. Roles of NAD(+)/NADH and NADP(+)/NADPH in cell death. Curr Pharm Des. 2009;15:12–19. doi: 10.2174/138161209787185832. [DOI] [PubMed] [Google Scholar]
- 16.Lappalainen Z. Sirtuins: a family of proteins with implications for human performance and exercise physiology. Res Sports Med. 2011;19:53–65. doi: 10.1080/15438627.2011.536068. [DOI] [PubMed] [Google Scholar]