Abstract
Objective: To investigate the safety, efficacy, and prognosis of advanced gastric cancer patients treated with molecular targeted drug therapy. Methods: A total of 200 patients with metastatic gastric cancer admitted to our hospital from March 2018 to December 2018 were randomly selected and divided into the control group, group A, group B and group C, with 50 patients in each group. Patients in the control group received surgical treatment combined with conventional chemotherapy. Patients in group A were provided with surgical treatment combined with bevacizumab, patients in group B received surgical treatment combined with apatinib, and patients in group C received surgical treatment combined with recombinant human endostatin (RHE). Clinical efficacy, vascular endothelial growth factor (VEGF) and vascular endothelial growth factor receptor 2 (VEGFR-2) levels, Response Evaluation Criteria in Solid Tumors (RECIST), sentinel lymph node (SLD) metastasis, and adverse reactions were compared among different groups of patients with metastatic gastric cancer. Results: There were no significant differences in treatment efficiency, VEGF and VEGFR-2 levels, RECIST, SLD metastasis value and adverse reactions among group A, group B and group C, and the results were not statistically significant (P>0.05). The levels of VEGF, VEGFR-2, SLD metastasis, and adverse reactions in group A, B, and C were significantly lower than those in the control group (P<0.05). The effective rate of treatment and RECIST in group A, B and C were significantly higher than those in the control group, and the comparison results were statistically significant (P<0.05). Conclusion: Molecular targeted drug therapy is effective and safe in patients with advanced gastric cancer, and the prognosis of patients is satisfactory, without the proliferation and metastasis of cancer cells.
Keywords: Molecular-targeted drugs, advanced gastric cancer, security, effectiveness, the prognosis of outcome
Introduction
Gastric cancer currently ranks top in cancer-related mortalities worldwide, and patients with it always have a somber prognosis [1-3]. Currently, tumor resection, radiotherapy, and chemotherapy are the mainstay of treatment. However, it is most often diagnosed at an advanced stage, after becoming metastatic at distant sites [4-6]. Therefore, early screening of biological indicators for tumor metastasis is necessary. Studies have shown that VEGF and VEGFR-2 levels can serve as indicators to predict whether the tumor will have lymphatic metastasis [5,6]. Targeted therapy is a type of therapy that customizes the usage of medication based on patients’ individual conditions. Molecular targeted drug therapy is relatively common in treating malignant tumors in current medical research; it mainly focuses on molecular targeted drug’s mechanism and clinical effects on treating malignant tumors. Relevant study states that molecular targeted drug therapy is superior to routine radiotherapy and chemotherapy in treating malignant tumors, because it remarkably extends patients’ survival time and improves the cure rate from gastric cancer [7-9]. Response Evaluation Criteria in Solid Tumors (RECIST) emphasizes that tumor markers are expected to reach the normal level in addition to the complete disappearance of the tumor stipulated by the criteria for clinical recovery. Additionally, the RECIST 1.1 version issued new criteria on the progress and the clinical stability. The new regulations have further expanded the clinical stability criteria for some tumors, suggesting the importance of survival with tumors, and making the efficacy evaluation more accurate by calculating the number of people with SLD metastasis. The primary aim of the present study was to investigate the safety, efficacy, and prognosis of molecular targeted drug therapy in advanced gastric cancer.
Materials and methods
General materials
A total of 200 patients with metastatic gastric cancer admitted to our hospital from March 2018 to December 2018 were randomly selected and divided into the control group, group A, group B and group C, with 50 patients in each group. The control group included 23 males and 27 females with an average age of (56.75±2.54) years old and an average disease course of (1.05±0.34) years. There were 6 cases of hypertension, 7 cases of diabetes, and 3 cases of hyperlipidemia. Group A included 25 males and 25 females with an average age of (57.51±2.01) years old and an average disease course of (1.13±0.19) years. There were 5 cases of hypertension, 7 cases of diabetes, and 5 cases of hyperlipidemia. Group B included 24 males and 26 females with an average age of (55.29±3.99) years old and an average disease course of (1.25±0.28) years. There were 6 cases of hypertension, 8 cases of diabetes, and 2 cases of hyperlipidemia. Group C included 26 males and 24 females with an average age of (56.88±2.54) years old and an average disease course of (1.35±0.31) years. There were 8 cases of hypertension, 8 cases of diabetes, and 1 cases of hyperlipidemia. There were no significant differences among the four groups’ general information (P>0.05).
Inclusion/exclusion criteria
Inclusion criteria
① Patients who met the clinical manifestation of advanced gastric cancer. ② Patients aged 40 to 60 years old. ③ No other malignant tumors and with normal cardio, pulmonary, and renal function. ④ No major surgeries recently. ⑤ Patients participated in this research voluntarily and signed the informed consent form. This study obtained approval from our hospital’s research ethics committee.
Exclusion criteria
① Patients with incomplete liver function. ② Patients with cognitive disorder, unconsciousness or mental disorder. ③ Patients with allergies or were allergic to related medicines. ④ Patients with autoimmune diseases.
Methods
The margin was required to be negative for surgical treatment of advanced gastric cancer in 4 groups (control group, group A, B, C). Surgical resection methods include subtotal gastrectomy and total gastrectomy; for distal gastric cancer, subtotal gastric resection is preferred. The surgical method of proximal gastric cancer is based on the actual situation of the patient, and proximal gastrectomy or subtotal gastrectomy is selected. Conventional chemotherapy treatments include mitomycin or cisplatin as basic chemotherapy drugs. The control group had surgery combined with conventional chemotherapy. Group A received surgery combined with bevacizumab (manufacturer: Roche Pharma (Switzerland) Ltd, batch number: S20120069, specification: 400 ml/bottle) for treatment, intravenous injection, 5 mg/kg, once two weeks. Group B was provided surgery combined with apatinib (manufacturer: Jiangsu Hengrui Medical Corp., Ltd, SFDA approval number: H20140103, specification: 850 mg) for treatment, 2 pills per time, once a day, per os. Group C received surgery combined with RHE (manufacturer: Shandong Xiansheng Maidejin Bio Pharmaceutical Ltd, SFDA approval number: S20050088, specification: 15 mg/2.4×105 U/3 ml/bottle) for treatment, intravenous injection, dissolve 1.2×105 U/m2 in 250 ml 0.9% sodium chloride injection, intravenous drip at constant speed for 3 h, keep using RHE for 14 days, stop for a week and continue delivering for 14 days. After two weeks of treatment, 3 ml fasting venous blood was collected, centrifuged at 3000 r/min for 10 min, and the supernatant was collected to test serum’s VEGF and VEGFR-2 levels.
Observation indexes
The treatment efficacy, VEGF and VEGFR-2 levels, RECIST, SLD metastasis value and adverse reactions among different groups were compared. Excellent means the target lesions basically disappeared. Effective means no increase in target lesions. Ineffective means there is an increase in target lesions. The lower the VEGF and VEGFR-2 levels are, the higher patients’ survival rate is.
Complete remission (CR): all the target lesions disappear; partial remission (PR): the sum of maximum diameters reduce by at least 30%; progressive disease (PD): the sum of maximum diameters reduce by at least 20% or new lesions appear; stable disease (SD): there are certain reduction in the sum of maximum diameters, but the reduction fails to reach PR; or there are certain increase in the sum of maximum diameters, but the increase fails to reach PD [10-12]. Objective remission rate (ORR)=CR+PR, disease control rat (DCR)=CR+PR+SD.
The modified Clavien-Dindo classification system was applied to evaluate the adverse reactions after treatment. Level 0: no complications occur after surgery. Level 1: complications don’t need drug intervention. Level 2: complications need drug intervention. Level 3 complications need other interventions besides drug intervention. Level 4: complications are life-threatening. Level 5: complications result in death.
Statistical analysis
Statistical analysis was performed using the SPSS statistical software 20.0. The measurement data were represented by (x̅ ± sd). For inter-group comparisons, data were analyzed with LSD-t test. For multiple-group comparisons, data were analyzed with one-way analysis of variance or repeated measures analysis of variance (RMANOVA). The enumeration data were examined by χ2 and represented by [% (n)]. And P<0.05 was considered as statistically significant.
Results
Comparison of the treatment efficiency
For the control group, there were 15 excellent cases, 15 effective cases, and 20 ineffective cases. For group A, there were 26 excellent cases, 19 effective cases, and 5 ineffective cases. For group B, there were 25 excellent cases, 17 effective cases, and 8 ineffective cases. For group C, there were 22 excellent cases, 22 effective cases, and 6 ineffective cases. There were no significant differences among the treatment efficiency of group A, B and C (P>0.05). The treatment efficiency of group A, B and C were significantly greater than that of the control group (P<0.05). See Figure 1.
Figure 1.
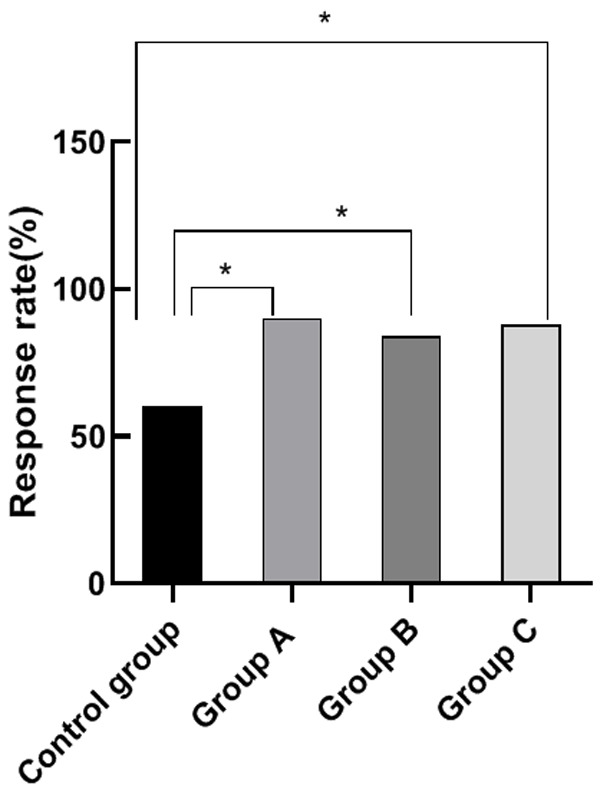
Comparison of treatment efficiency. Note: The horizontal axis stands for the groups, and the vertical axis stands for treatment efficiency. From left to right, the treatment efficiency of the four groups respectively is 60%, 90%, 84%, and 88%. a, comparison between the control group and group A (x2=12.00, P=0.001) (P<0.05). b, comparison between the control group and the group B, (x2=7.14, P=0.008) (P<0.05). c, comparison between the control group and the group C, (x2=10.19, P=0.001) (P<0.05).
Comparison of VEGF, VEGFR-2 levels
There were no significant differences among the VEGF and VEGFR-2 levels of group A, B and C (P>0.05). The VEGF and VEGFR-2 levels of group A, B and C were significantly lower than those of the control group (P<0.05), indicating a higher survival rate for patients. This result demonstrates that molecular targeted drug therapy can effectively increase the survival rate for patients with advanced gastric cancer. See Figures 2 and 3.
Figure 2.
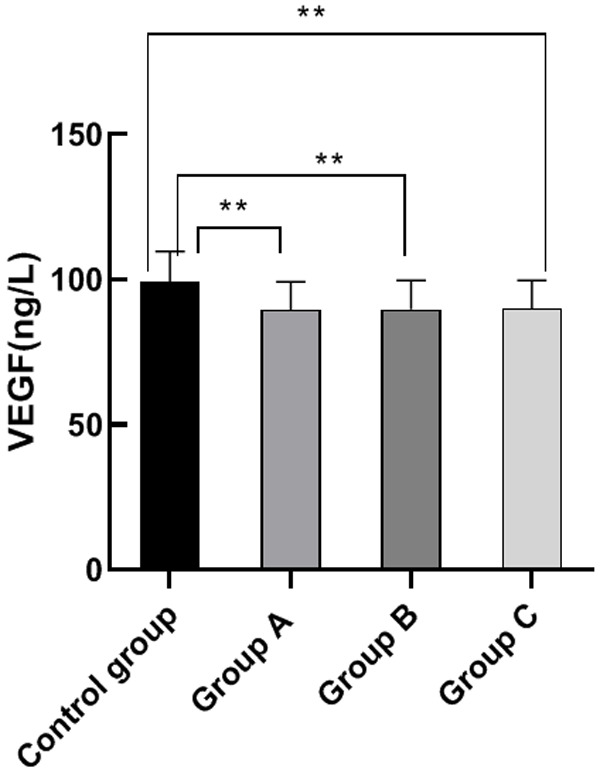
Comparison of VEG level. Note: The horizontal axis stands for the groups, and the vertical axis stands for VEGF level. From left to right, the VEGF level of the four groups respectively is (99.28±10.35) ng/L, (89.52±9.66) ng/L, (89.69±10.02) ng/L, and (90.00±9.68) ng/L. a, comparison between the control group and the group A, (t=4.87, P<0.001) (P<0.05). b, comparison between the control group and the group B, (t=4.71, P<0.001) (P<0.05). c, comparison between the control group and the group C, (t=4.63, P<0.001) (P<0.05).
Figure 3.
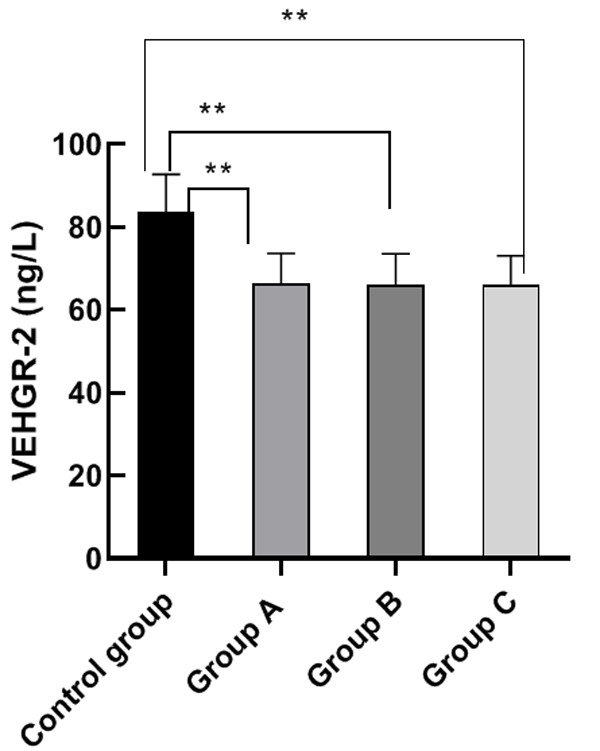
VEGFR-2 levels. Note: The horizontal axis stands for the groups, and the vertical axis stands for VEGFR-2 level. From left to right, the VEGFR-2 level of the four groups respectively is (83.50±9.21) ng/L, (66.35±7.24) ng/L, (65.98±7.54) ng/L, (66.08±7.00) ng/L. a, comparison between the control group and the group A (t=10.35, P<0.001) (P<0.05). b, comparison between the control group and the group B (t=10.41, P<0.001) (P<0.05). c, comparison between the control group and the group C (t=10.65, P<0.001) (P<0.05).
Comparison of RECIST standards
There were no significant differences among the RECIST standards’ ORR and DCR of group A, B and C (P>0.05). The RECIST standards’ ORR and DCR of group A, B and C were significantly higher than those of the control group (P<0.05). This comparison shows that molecular targeted drug therapy can effectively improve the clinical effects and prolong the survival time for patients with advanced gastric cancer. See Figure 4.
Figure 4.
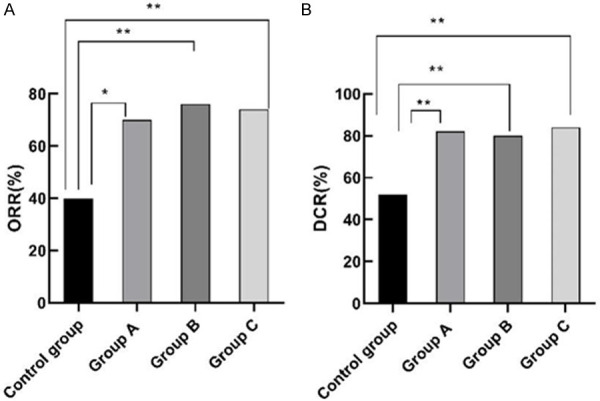
Comparison of RECIST standards. Note: For (A), the horizontal axis stands for the groups, and the vertical axis stands for ORR. From left to right, the ORR of the four groups respectively is 40%, 70%, 76%, 74%. a, comparison between the control group and the group A (x2=9.09, P=0.003) (P<0.05). b, comparison between the control group and the group B x2=26.60, P<0.001 (P<0.05). c, comparison between the control group and the group C (x2=23.58, P<0.001) (P<0.05). For (B), the horizontal axis stands for the groups, and the vertical axis stands for DCR. From left to right, the DCR in the four groups respectively is 52%, 82%, 80%, 84%. a , comparison between the control group and the group A (x2=20.35, P<0.001) (P<0.05). b, comparison between the control group and the group B (x2=17.47, P<0.001) (P<0.05). c, comparison of control group and group C (x2=23.53, P<0.001) (P<0.05).
Comparison of SLD metastasis value
Control group’s SLD metastasis value was significantly high than that of group A, B and C (P<0.05). There were no significant differences among the SLD metastasis value of group A, B and C (P>0.05). This result proves that molecular targeted drug therapy can effectively inhibit the metastasis of cancer cells for patients with advanced gastric cancer. See Figure 5.
Figure 5.
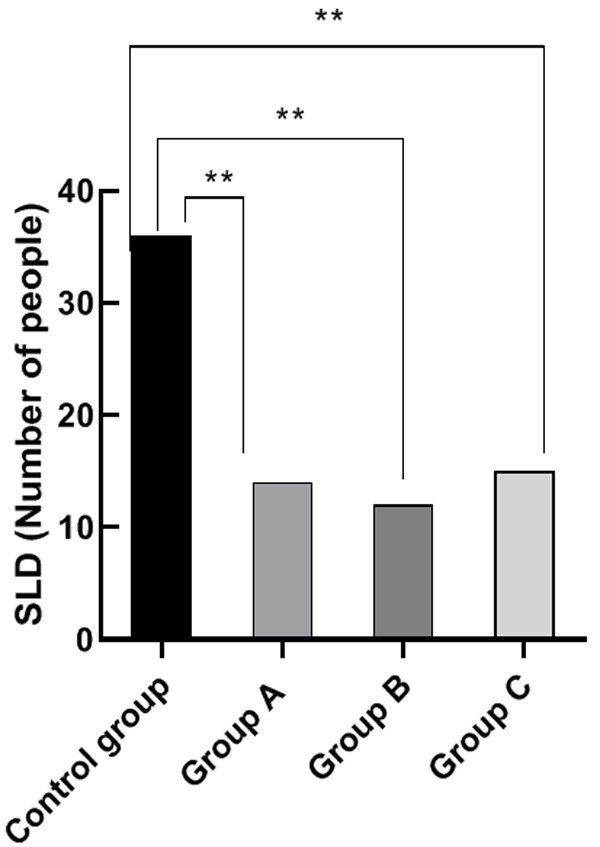
Comparison of SLD metastasis value. Note: The horizontal axis stands for the groups, and the vertical axis stands for SLD metastasis value. From left to right, the SLD metastasis in the four groups respectively is 36 cases, 14 cases, 12 cases and 15 cases. a, comparison between the control group and the group A (x2=19.36, P<0.001) (P<0.05). b, comparison of control group and group B (x2=23.08, P<0.001) (P<0.05). c, comparison between the control group and group C (x2=17.65, P<0.001) (P<0.05).
Comparison of adverse reactions rate
During treatment, all the groups exhibited neutropenia, nausea, vomiting, and rash. The adverse reactions rate of the control group was significantly higher than that of group A, B and C (P<0.05). There were no significant differences among the adverse reactions rate of group A, B and C (P>0.05). This comparison reveals that molecular targeted drug therapy results in high safety and has a better prognosis for patients with advanced gastric cancer. See Figure 6.
Figure 6.
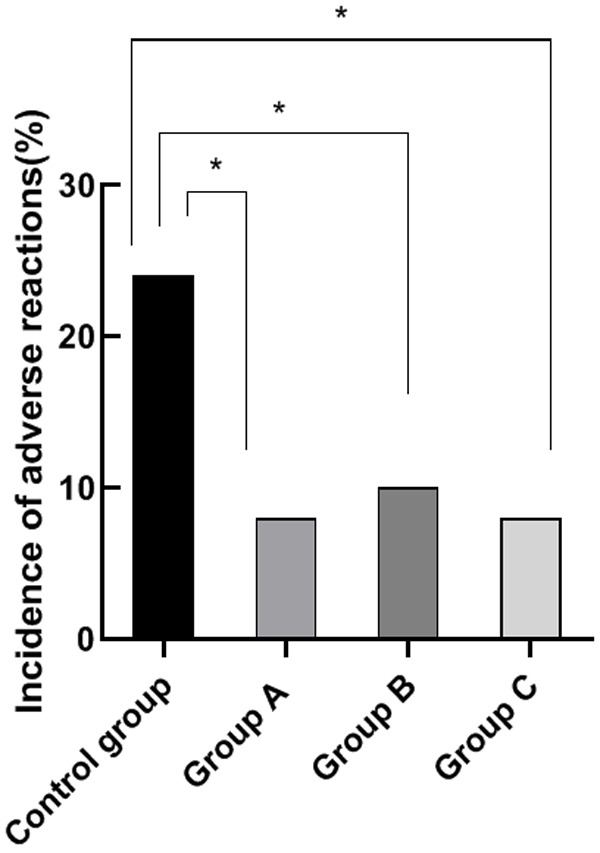
Comparison of adverse reactions rate. Note: The horizontal axis stands for the groups, and the vertical axis stands for adverse reactions rate. From left to right, the adverse reactions rate of the four groups respectively are 24%, 8%, 10%, 8%. a, comparison between the control group and the group A (x2=9.52, P=0.002) (P<0.05). b, comparison between the control group and the group B (x2=6.95, P=0.008) (P<0.05). c, comparison between the control group and the group C (x2=9.52, P=0.002) (P<0.05).
Discussion
Molecular targeted drug therapy has been attracting a great deal of attention in the context of malignant tumors [13-15]. According to related research, the application of apatinib in advanced gastric cancer has garnered a remarkable outcome. Through oral administration of apatinib, patients’ symptoms can be improved, and the deterioration can be postponed [16-18]. Additionally, prior research has stated that the application of bevacizumab and RHE has better clinical effects and better safety regarding treating malignant tumors. Gastric cancer is a malignant tumor with high mortality rate and morbidity rate. Patients with advanced gastric cancer suffer from great pain, and the adverse reactions owing to tumor resection easily lead to recurrence, proliferation, and metastasis. Moreover, radiotherapy and chemotherapy, if adopted, not only have low efficiency, but also have strong negative influence on patients’ health, triggering oral disease, retinal diseases, skin diseases, etc. [19-22]. Therefore, molecular targeted drug therapy is a great topic in the treatment of patients with advanced gastric cancer.
This study aims at investigating the safety, efficacy, and prognosis of molecular targeted drugs, including apatinib, bevacizumab and RHE, in advanced gastric cancer. Apatinib is a small-molecule targeted anti-VEGFR-2 drug for advanced gastric cancer patients who have failed second-line chemotherapy. It blocks downstream signal transduction and inhibits the formation of tyrosine kinase through highly selective competition for the ATP binding site of intracellular receptor-2, thereby inhibiting the formation of new blood vessels in tumor tissues, and ultimately achieves the purpose of treating tumors [23]. Additionally, Bevacizumab is a recombinant human monoclonal IgG1 antibody that acts by inhibiting the biological activity of human vascular endothelial growth factor. In other words, Bevacizumab can bind to VEGF and prevent it from binding to receptors on the surface of endothelial cells. In an in vitro angiogenesis model, the binding of VEGF to its corresponding receptor can lead to endothelial cell proliferation and angiogenesis [24]. Furthermore, RHE can inhibit the expression of vascular endothelial growth factor and can inhibit tumor growth. Vascular endothelial growth factor is a cytokine that promotes the growth of blood vessels. It plays a role in the occurrence and development of tumors and other tissues. Therefore, targeted inhibition of the expression of vascular endothelial growth factor can inhibit the occurrence and development of tumors and other diseases [25].
The results of the present study show that the treatment efficiency of groups treated with molecular targeted drugs was significantly higher than that of the control group (P<0.05). The VEGF and VEGFR-2 levels of groups treated with molecular targeted drugs was significantly lower than that of the control group (P<0.05). The lower the VEGF and VEGFR-2 levels, the higher the patients’ survival odds. Thus, molecular targeted drug therapy can obviously increase the survival rate for patients with advanced gastric cancer. We also found that the ORR and DCR of the control group were obviously lower than those of groups treated with molecular targeted drugs (P<0.05). Furthermore, the smaller the SLD metastasis value is, the less patients’ metastatic cancer cells are, and the better patients’ conditions are. We observed that the SLD metastasis value of the control group was significantly higher than that of groups treated with molecular targeted drugs (P<0.05), which shows that molecular targeted drug therapy can effectively control the conditions and reduce the odds of cancer cells metastasis.
A prior trial proposed that [26] apatinib can obviously inhibit VEGFR-2 level, wherein the mouse xenografts transplantation model, apatinib strongly inhibit the growth of cell CT26 by inhibiting catenin signaling pathways and angiogenesis, hence achieving the result of treating rectal cancer with targeted therapy. This result resonates with our result that patients treated with molecular targeted drug therapy has lower VEGFR-2 level. The limitation of this study is that the long-term follow-up is not conducted, and the long-term disease-free survival time cannot be thus calculated. In the future, long-term follow-up trials are needed to provide guidance for advanced gastric cancer treatment.
In conclusion, molecular targeted drug, including apatinib, bevacizumab and RHE, is a preferred method for patients with advanced gastric cancer in terms of high efficacy and safety. Importantly, the prognosis outcome is satisfactory without proliferation and metastasis.
Disclosure of conflict of interest
None.
References
- 1.Wrobel P, Ahmed S. Current status of immunotherapy in metastatic colorectal cancer. Int J Colorectal Dis. 2019;34:13–25. doi: 10.1007/s00384-018-3202-8. [DOI] [PubMed] [Google Scholar]
- 2.Cottu P, Coudert B, Perol D, Doly A, Manson J, Aujoulat O, Barletta H, Chalabi N, Samelson L, Pivot X. Evolution in the real-world therapeutic strategies in more than 20,000 women with breast cancer having received human epidermal growth factor receptor 2-targeted treatments: results from the french personalized reimbursement model database (2011-2018) Eur J Cancer. 2020;141:209–217. doi: 10.1016/j.ejca.2020.10.012. [DOI] [PubMed] [Google Scholar]
- 3.Ding D, Javed AA, Cunningham D, Teinor J, Wright M, Javed ZN, Wilt C, Parish L, Hodgin M, Ryan A, Judkins C, McIntyre K, Klein R, Azad N, Lee V, Donehower R, De Jesus-Acosta A, Murphy A, Le DT, Shin EJ, Lennon AM, Khashab M, Singh V, Klein AP, Roberts NJ, Hacker-Prietz A, Manos L, Walsh C, Groshek L, Brown C, Yuan C, Blair AB, Groot V, Gemenetzis G, Yu J, Weiss MJ, Burkhart RA, Burns WR, He J, Cameron JL, Narang A, Zaheer A, Fishman EK, Thompson ED, Anders R, Hruban RH, Jaffee E, Wolfgang CL, Zheng L, Laheru DA. Challenges of the current precision medicine approach for pancreatic cancer: a single institution experience between 2013 and 2017. Cancer Lett. 2021;497:221–228. doi: 10.1016/j.canlet.2020.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D, Li H, Chen W, Yang H, Liu Y, You B, Zhang X. Efficient tumor-targeting delivery of siRNA via folate-receptor mediated biomimetic albumin nanoparticles enhanced by all-trans retinoic acid. Mater Sci Eng C Mater Biol Appl. 2021;119:111583. doi: 10.1016/j.msec.2020.111583. [DOI] [PubMed] [Google Scholar]
- 5.Koemans WJ, Luijten JCHBM, van der Kaaij RT, Grootscholten C, Snaebjornsson P, Verhoeven RHA, van Sandick JW. The metastatic pattern of intestinal and diffuse type gastric carcinoma - A Dutch national cohort study. Cancer Epidemiol. 2020;69:101846. doi: 10.1016/j.canep.2020.101846. [DOI] [PubMed] [Google Scholar]
- 6.Ding S, Wang R, Peng S, Luo X, Zhong L, Yang H, Ma Y, Chen S, Wang W. Targeted therapies for RET-fusion cancer: dilemmas and breakthrough. Biomed Pharmacother. 2020;132:110901. doi: 10.1016/j.biopha.2020.110901. [DOI] [PubMed] [Google Scholar]
- 7.Zheng R, Li M, Wang S, Liu Y. Advances of target therapy on NOTCH1 signaling pathway in T-cell acute lymphoblastic leukemia. Exp Hematol Oncol. 2020;9:31. doi: 10.1186/s40164-020-00187-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yin Z, Wu D, Shi J, Wei X, Jin N, Lu X, Ren X. Identification of ALDH3A2 as a novel prognostic biomarker in gastric adenocarcinoma using integrated bioinformatics analysis. BMC Cancer. 2020;20:1062. doi: 10.1186/s12885-020-07493-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Angeli M, Carosi M, Vizza E, Corrado G. Sister Mary Joseph’s nodule in endometrial cancer: a case report and review of the literature. J Cancer Res Ther. 2019;15:1408–1410. doi: 10.4103/jcrt.JCRT_523_18. [DOI] [PubMed] [Google Scholar]
- 10.Guo T, Liu P, Yang J, Wu P, Chen B, Liu Z, Li Z. Evaluation of targeted agents for advanced and unresectable hepatocellular carcinoma: a network meta-analysis. J Cancer. 2019;10:4671–4678. doi: 10.7150/jca.32828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma J, Pi G, Bi J, Li Y, He H, Li Y, Hu D, Verma V, Han G. Concurrent apatinib and brain radiotherapy in patients with brain metastases from driver mutation-negative non-small-cell lung cancer: study protocol for an open-label randomized controlled trial. Clin Lung Cancer. 2020;22:e211–e214. doi: 10.1016/j.cllc.2020.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Katai H, Ishikawa T, Akazawa K, Fukagawa T, Isobe Y, Miyashiro I, Oda I, Tsujitani S, Ono H, Tanabe S, Nunobe S, Suzuki S, Kakeji Y Registration Committee of the Japanese Gastric Cancer Association. Optimal extent of lymph node dissection for remnant advanced gastric carcinoma after distal gastrectomy: a retrospective analysis of more than 3000 patients from the nationwide registry of the Japanese Gastric Cancer Association. Gastric Cancer. 2020;23:1091–1101. doi: 10.1007/s10120-020-01081-5. [DOI] [PubMed] [Google Scholar]
- 13.Zhang X, Peng Y, Yuan Y, Gao Y, Hu F, Wang J, Zhu X, Feng X, Cheng Y, Wei Y, Fan X, Xie Y, Lv Y, Ashktorab H, Smoot D, Li S, Meltzer SJ, Hou G, Jin Z. Histone methyltransferase SET8 is regulated by miR-192/215 and induces oncogene-induced senescence via p53-dependent DNA damage in human gastric carcinoma cells. Cell Death Dis. 2020;11:937. doi: 10.1038/s41419-020-03130-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yanwei L, Feng H, Ren P, Yue J, Zhang W, Tang P, Shang X, Pang Q, Liu D, Chen C, Pan Z, Tao YZ. Safety and efficacy of apatinib monotherapy for unresectable, metastatic esophageal cancer: a single-arm, open-label, phase II study. Oncologist. 2020;25:e1464–e1472. doi: 10.1634/theoncologist.2020-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tian Z, Liu H, Zhao Y, Wang X, Ren H, Zhang F, Li P, Zhang P, Wang J, Yao W. Secondary pneumothorax as a potential marker of apatinib efficacy in osteosarcoma: a multicenter analysis. Anticancer Drugs. 2021;32:82–87. doi: 10.1097/CAD.0000000000001016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan H, Li X, Peng Y, Zhang P, Zou N, Liu X. Apatinib and fractionated stereotactic radiotherapy for the treatment of limited brain metastases from primary lung mucoepidermoid carcinoma: a case report. Medicine (Baltimore) 2020;99:e22925. doi: 10.1097/MD.0000000000022925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Díaz Del Arco C, Estrada Muñoz L, Sánchez Pernaute A, Ortega Medina L, García Gómez de Las Heras S, García Martínez R, Fernández Aceñero MJ. Which lymph node staging system better predicts prognosis in patients with gastric carcinoma? A comparative study between 3 different lymph node classifications for resected gastric cancer in a western tertiary center. Am J Clin Oncol. 2021;44:1–9. doi: 10.1097/COC.0000000000000770. [DOI] [PubMed] [Google Scholar]
- 18.Yang J, Liu Z, Zeng B, Hu G, Gan R. Epstein-Barr virus-associated gastric cancer: a distinct subtype. Cancer Lett. 2020;495:191–199. doi: 10.1016/j.canlet.2020.09.019. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi Y, Tanabe H, Ando K, Fujiya M, Okumura T. Endoscopic finding of a lace pattern in a case of Epstein-Barr virus-associated early gastric carcinoma. Gastrointest Endosc. 2021;93:768–769. doi: 10.1016/j.gie.2020.10.008. [DOI] [PubMed] [Google Scholar]
- 20.Huang X, Xie C, Tang J, He W, Yang F, Tian W, Li J, Yang Q, Shen J, Xia L, Lan C. Adipose tissue area as a predictor for the efficacy of apatinib in platinum-resistant ovarian cancer: an exploratory imaging biomarker analysis of the AEROC trial. BMC Med. 2020;18:267. doi: 10.1186/s12916-020-01733-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lan C, Shen J, Wang Y, Li J, Liu Z, He M, Cao X, Ling J, Huang J, Zheng M, Zou G, Yan H, Liu Q, Yang F, Wei W, Deng Y, Xiong Y, Huang X. Camrelizumab plus apatinib in patients with advanced cervical cancer (CLAP): a multicenter, open-label, single-arm, phase II trial. J. Clin. Oncol. 2020;38:4095–4106. doi: 10.1200/JCO.20.01920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo Q, Sun Y, Kong E, Rao L, Chen J, Wu Q, Zhang T, Liu N, Li M, Sun L. Apatinib combined with chemotherapy or concurrent chemo-brachytherapy in patients with recurrent or advanced cervical cancer: a phase 2, randomized controlled, prospective study. Medicine (Baltimore) 2020;99:e19372. doi: 10.1097/MD.0000000000019372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu Q, Fu Y, Wen W, Xi T, Zhao G. Efficacy and prognosis analyses of apatinib combined with S-1 in third-line chemotherapy for advanced gastric cancer. J BUON. 2020;25:987–994. [PubMed] [Google Scholar]
- 24.Rebelo de Almeida C, Mendes RV, Pezzarossa A, Gago J, Carvalho C, Alves A, Nunes V, Brito MJ, Cardoso MJ, Ribeiro J, Cardoso F, Ferreira MG, Fior R. Zebrafish xenografts as a fast screening platform for bevacizumab cancer therapy. Commun Biol. 2020;3:299. doi: 10.1038/s42003-020-1015-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang SL, Han CB, Sun L, Huang LT, Ma JT. Efficacy and safety of recombinant human endostatin combined with radiotherapy or chemoradiotherapy in patients with locally advanced non-small cell lung cancer: a pooled analysis. Radiat Oncol. 2020;15:205. doi: 10.1186/s13014-020-01646-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cai X, Wei B, Li L, Chen X, Yang J, Li X, Jiang X, Lv M, Li M, Lin Y, Xu Q, Guo W, Gu Y. Therapeutic potential of apatinib against colorectal cancer by inhibiting VEGFR2-mediated angiogenesis and β-catenin signaling. Onco Targets Ther. 2020;13:11031–11044. doi: 10.2147/OTT.S266549. [DOI] [PMC free article] [PubMed] [Google Scholar]


