Abstract
Classical aluminum adjuvant is a deficient antigen carrier for cross-presentation and cross-priming of CD8+ cytotoxic T cells. Our previous research has demonstrated that cross-presentation efficiency significantly increased when antigens are conjugated covalently to α-Al2O3 nanoparticles. Here we found that coating conventional aluminum adjuvants with polyethyleneimine (PEI) could enhance antigen cross-presentation of DCs (dendritic cells) in vitro and in vivo. PEIs exerted differential effects on antigen cross-presentation. These findings provided an alternative approach to promote the rapid translation of alumina nanoparticles adjuvants into clinical application.
Keywords: Aluminum adjuvant, PEI, dendritic cells, antigen cross-presentation
Introduction
Dendritic cell (DC)-based vaccines have recently attracted extensive attention due to their promising application in tumor immunotherapy. DCs are the most powerful professional antigen-presenting cells (APCs) for initiating specific antigen immune responses. However, clinical efficacy of DCs-based vaccines is often limited by the lack of effective antigens that induce sufficient immune response [1,2]. Therefore, maximizing the efficiency of cross-presentation is the key strategy for the successful clinical applicability of DCs-based vaccines.
Adjuvants are often combined with antigens to boost immune responses in veterinary and human vaccines [3,4]. Aluminum hydroxide is the most widely used adjuvant for decades. Moreover, aluminum-containing adjuvants have been approved by the US Food and Drug Administration for human use due to their excellent safety profile [3-7]. However, classical alum adjuvants only trigger moderate antigen-specific antibody responses, serving as deficient antigen carriers for cross-presentation and cross-priming of CD8+ cytotoxic T cells [8,9].
Rehydragel, an aluminum hydroxide wet gel suspension, is composed of micro-crystalline clusters of nanofibers and exhibits excellent protein adsorption properties [3,10]. Many studies have shown that Rehydragel has significant advantages, such as large effective surface area, excellent biocompatibility and great antigen-loading capacity [3,7,10]. Our previous work demonstrated that cross-presentation efficiency was significantly increased when antigens were covalently conjugated to α-Al2O3 nanoparticles [11]. Recent studies show that cationic polyethyleneimine (PEI) of proper molecular weight (MW) can lead to high surface charge and membrane interference effect that facilitates effective binding when applied to delivery system [12,13]. In this study, we investigated whether coating Rehydragel with PEI could improve the antigen cross-presentation and cross-priming of antigen specific T cells.
Materials and methods
PEI coating
A total of 0.5-mL of Rehydragels (LV, HS, HPA, 5 mg/mL) was mixed with 1.5-mL of PEIs (25 mg/mL). The mixture was then diluted into protein solution and incubated at RT 60 minutes. LV, HS and HPA were brought from Chemtrade Chemicals. Linear PEI with a mean MW of 2.5, 25, 40 and 160 kDa (lPEI2.5k, lPEI25k, lPEI40k and lPEI160k) was obtained from PolyScience. Branched PEI with a mean MW of 0.8, 1.8, 25 and 50-100 kDa (bPEI0.8k, bPEI25k and bPEI50-100k) was purchased from Sigma-Aldrich.
Mice
Eight-week-old C57BL/6 and OT-I mice (whose T cells recognize the H-2Kb-restricted OVA257-264 peptide) were purchased from the Jackson Laboratory. All mice were bred and maintained in a specific pathogen-free environment. All animal experiments were reviewed and approved by the Earle A. Chiles Research Institute Animal Care and Use Committee.
Cell lines and bone marrow-derived DC
Prof Hong-ming Hu offered the mutu DC cell line which was derived from the spleens of CD11c:SV40LgT-transgenic C57BL/6 mice [14] and B3Z cell line, the H-2Kb-OVA257-264 specific CD8+ T cell hybridoma with LacZ expression [15]. Culture of mutu DCs was performed in DMEM containing 10% fetal bovine serum, 100 units/mL penicillin, 2 mML-glutamine, and 100 mg/mL streptomycin (Thermo Fisher Scientific).
Bone marrow-derived dendritic cells (BMDCs) were generated from bone marrow precursor cells collected from C57BL/6 mice, transporter associated with antigen processing1 (TAP1) knockout mice and CD40 knockout mice. Briefly, femurs were dissected from mice, followed by flushing bone marrow with RPMI 1640 medium. After twice of washing with PBS, the cells (1×106 cell/well) were treated with a 5-day culture with RPMI 1640 medium containing IL-4 (1 ng/mL, PeproTech) and GM-CSF (10 ng/mL, granulocyte-macrophage colony stimulating factor, Gibco). The medium was relinquished by half on days 2 and 4.
Cross-presentation assay by chlorophenol Red-β-D-galactopyranoside (CPRG) assay in vitro
Mutu DCs and TAP1 knockout DCs (2×104), CD40 knockout DCs (2×104) were loaded with Rehydragel already coated with PEI of different MWs and OVA protein (10 μg/mL, Sigma-Aldrich) in the presence or absence of proteasomal inhibitors, velcade (200 nM, Millennium) and lactacysin (20 μM, Sigma-Aldrich), and lysosomal inhibitors, NH4Cl (20 μM, Sigma-Aldrich), tunicamycin (20 μM, Sigma-Aldrich) and concanamycin A (20 μM, Sigma-Aldrich). From six hours after loading, DCs were cocultured with B3Z cells (2×105) overnight. The activation of B3Z cells was determined by β-galactosidase activity at 595 nm absorbance of the products after CPRG (Sigma-Aldrich) cleavage. In this study we called the assay of antigen cross-presentation CPRG assay.
Western blot analysis
In order to detect OVA expression in DCs, mutu DCs (5×106) were loaded with Rehydragel/PEI-OVA for 6 hours, and treated with Perfringolysin O (PFO, 100 ng/mL) at 37°C for 30 min. Afterward, the cytosol was collected. Bicinchoninic acid (BCA) protein assay kit (Thermo Fisher Scientific) was used to measure protein concentration. Ub-proteins isolated from cytosol extracts of mutu DCs cocultured with OVA, LV-OVA or LV/bPEI25k-OVA were enriched as previously published [16]. The Vx3GFP fusion protein (30 μg/mL) with a His6 tag was added to the equal quantified PFO extract, followed by an overnight incubation at 4°C. The mixture was then subjected to Ni-Sepharose excel resin (GE Healthcare), followed by an overnight incubation at 4°C. After centrifugation, the resin was washed with Tris-NaCl buffer (20 mM Tris-Cl, 300 mM NaCl, pH 8.0) containing 5 mM imidazole, then the his6-Vx-bound ubiquitinated proteins were eluted with Tris-NaCl buffer containing 250 mM imidazole. The eluate was dialyzed against PBS overnight at 4°C and stored at -80°C as enriched Ub-proteins (ubiquitinated proteins). The eluted fractions were subjected to Western blot analysis with anti-OVA antibody. Protein was separated by SDS-PAGE, transferred to a nitrocellulose membrane, and incubated with primary anti-OVA (Sigma-Aldrich) and HRP-conjugated secondary antibody (Thermo Scientific) for one hour at room temperature. Membranes were subsequently visualized using chemiluminescent reagents and X-ray films (Bio-Rad).
Animal experiments
B16-OVA tumor cells (2×105) were injected subcutaneously (s.c.) into naïve C57BL/6 mice. On the 7th day, mice with palpable tumors were immunized with intranodal injection with LV-OVA, LV/bPEI25k-OVA, or OVA alone (30 μg protein each). On the 10th day, 5×106 DCs loaded with LV-OVA, LV/bPEI25k-OVA, or OVA alone were injected intravenously. PBS was used as control. Intracellular cytokine staining (ICS) was employed to testify the frequency of OVA peptide-specific CD8+ T cells. On the 16th day, lymphocytes in lymph nodes and spleens were fetched and seeded into 96-well round bottom plates (1×106 cells/well). SIINFEKL (1 μg/mL, OVA257-264 peptide) was added to each well. PBS was used as a negative control, and anti-CD3 (BD Biosciences) was used as a positive control to induce the lymphocytes activation. After a 12-hour culture, brefeldin A (10 mg/mL, Sigma-Aldrich) was added, followed by another 6-hour culture. Then, the cells were collected for flow cytometry analysis.
In some experiments, some B16-OVA tumor-bearing mice received adoptive transfer of OT-I spleen cells (1×107) when LV-OVA, LV/bPEI25k-OVA, or OVA alone were injected into inguinal lymph nodes on the 7th day after tumor cell inoculation. On day 10, DCs loaded with LV-OVA, LV/bPEI25k-OVA, or OVA alone were injected intravenously similarly. On the 16th day, the lymphocytes were stained with APC-labeled anti-mouse Thy1.1 antibody (eBioscience), PerCP-labeled anti-mouse CD8 antibody (eBioscience) and MHC Dextramer H-2Kb/SIINFEKL conjugated with PE (ImmuDEX) for flow cytometry analysis.
Statistical analysis
A 2-tailed Student t test (GraphPad Prism 7.0) was carried out. Error bars denoted mean ± SD. P<0.05 was considered statistically significant (ns, P>0.05, *P<0.05; **, P<0.01; ***, P<0.001).
Results
Effectiveness of Rehydragels on the cross-presentation of DCs
We first tested whether different Rehydragels (HPA, HS and LV) coated with the same PEI could facilitate the cross-presentation of OVA protein. Mutu DCs were loaded with OVA protein/nanoparticles with different concentration ratios for 6 hours and co-cultured with B3Z hybridoma cells overnight. The concentration of OVA protein was fixed at 10 μg/mL and we titrated the concentration of Rehydragel, branched PEI25k and branched PEI25k-coated Rehydragel, respectively. As shown in Figure 1A, PEI-coated Rehydragel could trigger stronger B3Z response than Rehydragel alone or PEI alone. And we found that when the concentration of OVA protein was 10 μg/mL, the optimal concentration of PEI-coated Rehydragel was 1 μg/mL (Figure 1A), which was sufficient for the effective cross-presentation and used for the following experiments. Furthermore, PEI-coated LV or HS particles triggered stronger B3Z response than PEI-coated HPA (Figure 1B). These results indicated that the cross-presentation of OVA protein was obviously enhanced when OVA protein was combined to bPEI25k-coated Rehydragel before being loaded onto DCs, but the effectiveness of Rehydragel varied with its type.
Figure 1.

Rehydragels (HPA, HS, LV) enhanced cross-presentation of murine DCs. A. Rehydragel alone, PEI alone and PEI-coated Rehydragel with different concentrations (0.03, 0.1, 0.3, 1, 3 μg/mL) were mixed with OVA protein (10 μg/mL) for 1 hour at room temperature. Mutu DCs were loaded with OVA/nanoparticles for 6 hours at 37°C and then cultured with B3Z cells overnight. The B3Z responses were analyzed by CPRG assay. Groups of PBS, OVA peptide (1 μg/mL) and B3Z only were used as control in these experiments. B. The changes of DC cross-presentation by different Rehydragels were determined by CPRG assay when the concentration of PEI-coated Rehydragel was 1 μg/mL. *P<0.05, **P<0.01, ***P<0.001.
Effectiveness of different PEIs on the cross-presentation of DCs
Next, we examined how different PEIs coated onto the same Rehydragel affected the cross-presentation of OVA protein. To determine the optimal HS/PEI concentration ratio, we coated HS with branched PEI 0.8k and PEI 25k at different ratios. We found that the cross-presentation was the most effective when the concentration ratio of HS/bPEI (branched PEI) was 10:1 (Figure 2A). Moreover, CPRG results showed that combined with OVA protein, bPEI0.8k-coated or bPEI25k-coated HS could elicit stronger B3Z response than any other branched PEIs and linear PEIs (Figure 2B). These results showed that HS coated with branched PEI0.8k or PEI25k could yield stronger responses of OVA antigen cross-presentation by DCs.
Figure 2.

Different PEIs enhanced cross-presentation of murine DCs. A. HS particles were coated with bPEI0.8k or bPEI25k at different ratios and then mixed with OVA protein (10 μg/mL); B. HS particles were coated with different branched PEIs or linear PEIs and then mixed with OVA protein (10 μg/mL). Mutu DCs were loaded with HS/PEI-OVA for 6 hours at 37°C and then cultured with B3Z cells overnight. The B3Z responses were analyzed by CPRG assay. Groups of PBS, OVA protein (10 μg/mL), OVA peptide (1 μg/mL) and B3Z only, B3Z mixed with OVA were used as control in these experiments. ns, P>0.05, *P<0.05, **P<0.01.
Effectiveness of Rehydragels and bPEIs mixture on the cross-presentation of DCs
We have found that HS and LV particles performed better among the tested Rehydragels, and the branched PEI 0.8k and 25k were also more effective than other PEIs. Next, we examined the effectiveness of different mixture of Rehydragels and bPEIs on the cross-presentation of OVA protein. HS/bPEI0.8k (HS particles coated with bPEI0.8k), HS/bPEI25k (HS particles coated with bPEI25k), HS/bPEI0.8k+25k (HS particles coated with bPEI0.8k and bPEI25k simultaneously) and HS/bPEI0.8k+HS/bPEI25k (pure mixture of HS/bPEI0.8k and HS/bPEI25k together) were prepared. According to CPRG results, HS/bPEI25k was stronger than HS/bPEI0.8k in inducing OVA cross-presentation, but HS/bPEI25k, HS/bPEI0.8k+25k and HS/bPEI0.8k+HS/25k showed no difference (Figure 3A). Moreover, we found that LV/bPEI25k was the most powerful combination (Figure 3B). Thus, LV/bPEI25k was used for the following experiments.
Figure 3.

Different Rehydragels and bPEIs mixtures enhanced cross-presentation of murine DCs. A. HS/bPEI0.8k (HS particles coated with bPEI0.8k), HS/bPEI25k (HS particles coated with bPEI25k), HS/bPEI0.8k+25k (HS particles coated with bPEI0.8k and bPEI25k simultaneously) and HS/bPEI0.8k+HS/bPEI25k (pure mixture of HS/bPEI0.8k and HS/bPEI25k together) were mixed with OVA protein (10 μg/mL), respectively. B. HS or LV particles were coated with bPEI0.8k or bPEI25k respectively, and then mixed with OVA protein (10 μg/mL). Mutu DCs were loaded with the Rehydragel/bPEI-OVA for 6 hours at 37°C and then cultured with B3Z cells overnight. The B3Z responses were analyzed by CPRG assay. Groups of PBS and OVA peptide (1 μg/mL) were used as control in these experiments. ns, P>0.05, *P<0.05, **P<0.01.
To understand why Rehydragel/bPEI could facilitate DCs cross-presentation, we performed Western blot analysis using antibodies against OVA to probe the cytosolic delivery of OVA in the Ub-protein fractions isolated from cytosol extracts of mutu DCs cocultured with OVA, LV-OVA or LV/bPEI25k-OVA, respectively. Our previous work has shown that Ub-proteins can enhance cross-presentation compared with cell lysates [16]. So we purified Ub-proteins from the cytosol extracts collected from the supernatant of PFO-treated DCs. For affinity purification of Ub-proteins, we used the Vx3GFP fusion protein containing triple ubiquitin-interacting motifs (UIMs) that could bind ubiquitinated protein. As expected, when the same total protein concentration of PFO extract was used, Ub-OVA displayed an obviously stronger signal in the LV/bPEI25k-OVA-cocultured DCs compared with LV-OVA- or OVA-cocultured DCs (Figure 4A).
Figure 4.

The mechanisms involved in enhanced DCs cross-presentation by Rehydragel/bPEI. A. Ub-OVA expression in the cytosol extract of mutu DCs. Mutu DCs cocultured with OVA protein (10 μg/mL), LV-OVA, LV/bPEI25k-OVA for 6 hours, and then the Ub-proteins from the cytosol extract were collected for Western blot analysis. The pure OVA protein (100 ng/mL) was used as control in this experiment. B. Rehydragel/bPEI increased IL-12 secretion by murine DCs. The secretion of IL-12 cytokine was detected by ELISA after mutu DCs were loaded with OVA, bPEI25k-OVA, LV-OVA or LV/bPEI25k-OVA. PBS was used as control in this experiment. *P<0.05, **P<0.01.
In mice, CD8a+ DCs are superior to CD8+ T cells in cross-presenting antigens, and can produce interleukin-12 (IL-12) that promotes cytotoxicity [17,18]. In this study, we found the release of IL-12 by DCs was increased also by either LV-OVA or LV/bPEI25k-OVA, more significantly by the latter (Figure 4B).
Taken together, these results indicated that coating conventional aluminum adjuvants with PEI could enhance antigen cross-presentation of DCs in vitro.
DCs cross-presentation was inhibited by 1IFNR
Toll-like receptor 3 (TLR3) and I-IFNs can regulate DCs maturation, cross-presentation, and antiviral and antitumor responses [19-28]. Next, we investigated if the TLR3 and 1IFNR pathways contributed to Rehydragel/bPEI-enhanced DCs cross-presentation. WT mutu DCs, TLR3-/- mutu DCs and 1IFNR-/- mutu DCs were loaded with LV/bPEI25k-OVA for CPRG assay. As shown in Figure 5A, the cross-presentation of OVA protein was inhibited in the absence of IFNR, but unchanged in other groups. The results suggested a critical role of IFNR in Rehydragel/bPEI-enhanced DCs cross-presentation.
Figure 5.

Cross-presentation of OVA protein by DCs was inhibited in the absence of 1IFNR. A. Mutu DCs, TLR3-/- mutu DCs and IFNR-/- mutu DCs were loaded with LV/bPEI25k-OVA for 6 hours for CPRG assay. B. Mutu DCs, murine BMDCs and CD40-/- DCs were loaded with LV/bPEI25k-OVA for 6 hours for CPRG assay. Groups of PBS and OVA peptide (1 μg/mL) were used as control in these experiments. ns, P>0.05, *P<0.05.
CD40 signaling is implicated in cytotoxic T lymphocyte (CTL) induction by cross-priming through activated antigen presenting cells (APCs) [29,30]. To determine whether the Rehydragel/bPEI-enhanced DCs cross-presentation was specific to CD40 signaling, mouse BMDCs were produced from CD40 knockout mice, co-cultured with LV/bPEI25k-OVA, and then used to stimulate B3Z cells. Interestingly, the CPRG results revealed that CD40 deficiency exerted no significant effect on OVA cross-presentation activated by LV/bPEI25k (Figure 5B).
Rehydragel/bPEI enhanced OVA cross-presentation in DCs through proteasome, lysosome and TAP1 pathways
The cross-presentation of phagocytosed antigens by MHC class I (MHC-I) molecules is known to be mediated through the recruitment of TAP transporters from the endoplasmic reticulum [31,32]. We next evaluated cross-presentation of LV/bPEI25k-OVA by TAP1 KO BMDCs (TAP1 knockout BMDCs). The CPRG results showed that TAP1-/- DCs loaded with LV/bPEI25k-OVA failed to activate the B3Z cells (Figure 6A), suggesting that TAP1 was absolutely indispensable for Rehydragel/bPEI-enhanced OVA cross-presentation in DCs.
Figure 6.

Rehydragel/bPEI enhanced OVA cross-presentation in mutu DCs through proteasome, lysosome and TAP1 pathways. A. Mutu DCs, murine BMDCs and Tap1-/- DCs were loaded with LV/bPEI25k-OVA for 6 hours for CPRG assay. B. Mutu DCs were treated with proteasome inhibitors and lysosome inhibitors, and then loaded with LV/bPEI25k-OVA for 6 hours for CPRG assay. Groups of PBS and OVA peptide (1 μg/mL) were used as control in these experiments. ns, P>0.05, *P<0.05, ***P<0.001.
To further assess whether Rehydragel/bPEI-enhanced OVA cross-presentation required the degradation of proteasomes or lysosomes, we used proteasome inhibitors (velcade and lactacysin) and lysosome inhibitors (NH4Cl, tunicamycin and concanamycin A). After incubated with LV/bPEI25k-treated OVA and different inhibitors for six hours, the cross-presentation in DCs was strongly inhibited both by proteasome inhibitors and lysosome inhibitors (Figure 6B). These results showed that both proteasomes and lysosomes participated in TAP1-mediated Rehydragel/bPEI-enhanced OVA cross-presentation.
Rehydragel/bPEI increased the efficiency of cross-presentation in vivo
Next, we examined whether Rehydragel/bPEI-antigen could elicit an endogenous T cell response. Tumor-bearing C57BL/6 mice were modeled via subcutaneous injection of B16-OVA tumor cells. Mice with palpable tumors were immunized with intranodal injection of LV-OVA, LV/bPEI25k-OVA, or OVA alone. The intravenous injection of DCs loaded with LV-OVA, LV/bPEI25k-OVA, or OVA alone was given at day 3 after the first intranodal injection. Intracellular IFN-γ staining was used to detect the frequency of the OVA-specific CD8+ T cells in the vaccinated mice. After ex vivo stimulation with SIINFEKL, we found that CD8+ T cells displayed a significantly higher level of IFN-γ in the mice vaccinated with LV/bPEI25k-OVA (Figure 7A and 7B). In contrast, these vaccinations did not change the level of IFN-γ when re-stimulated with anti-CD3 (Figure 7B).
Figure 7.
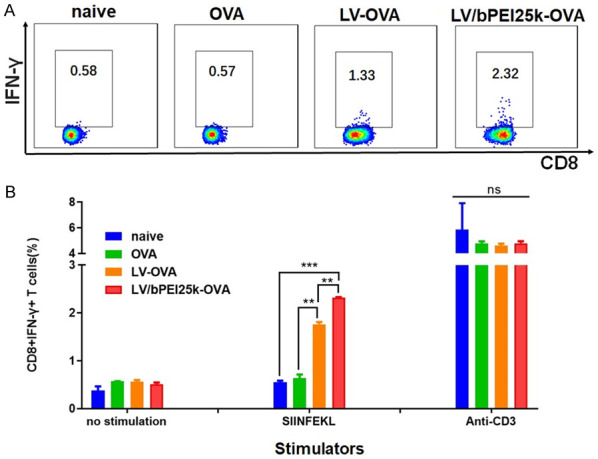
Vaccination with Rehydragel/bPEI-OVA induced high frequency of OVA-specific IFN-γ producing CD8+ T cells in the B16-OVA tumor-bearing mice. Lymphocytes collected from the lymph nodes and spleens of B16-OVA tumor-bearing mice vaccinated with PBS, LV-OVA, LV/bPEI25k-OVA or OVA alone were co-incubated with SIINFEKL and anti-CD3. Intracellular IFN-γ staining of T cells was detected by flow cytometry. PBS and anti-CD3 were used as control in this experiment. A. Representative cytoflourograph. B. Cumulative data from three experiments. ns, P>0.05, **P<0.01, ***P<0.001.
In addition, when the B16-OVA tumor-bearing mice received simultaneously adoptive transfer of OT-I spleen cells and intranodal immunization with LV-OVA, LV/bPEI25k-OVA, or OVA alone, Kb-OVA257-264 Dextramer was used to stain CD8+ T cells (Thy1.1+) that specifically recognize OVA257-264 in the context of H-2Kb. Flow cytometric analysis further revealed an obvious increase in the percentage of CD8+ Dextramer+ cells in mice vaccinated with LV/bPEI25k-OVA (Figure 8A and 8B).
Figure 8.

Detection of the percentages of CD8+ T cells (Thy1.1+) that specifically recognize OVA257-264 in the context of H-2Kb by Flow cytometry analysis. The B16-OVA tumor-bearing mice received adoptive transfer of OT-I spleen cells when LV-OVA, LV/bPEI25k-OVA, or OVA alone were injected into inguinal lymph nodes. The lymphocytes were stained with APC-labeled anti-mouse Thy1.1 antibody, PerCP-labeled anti-mouse CD8 antibody and MHC Dextramer H-2Kb/SIINFEKL conjugated with PE. A. Representative cytoflourograph. B. Cumulative data from three experiments. *P<0.05, **P<0.01.
Together, these results demonstrated that PEI-modified Rehydragel could increase the efficiency of cross-presentation in vivo.
Discussion
Aluminum adjuvants serve as immunopotentiators in vaccines clinically [3-7]. However classical aluminum-containing adjuvants only enhance antibody responses, but not cell-mediated immunity [9,33]. We previously showed that a-Al2O3 nanoparticles were more powerful than alum in inducing antigen cross-presentation and the antitumor ability [11]. Aluminum hydroxide nanoparticles have an adsorbability strong enough to carry soluble proteins [3,10]. PEI is a nanomaterial carrying strong positive charge that can enhance the surface charge when modifying nanoparticles [12]. In this study, we determined whether alum-based adjuvant Rehydragel coated with PEI could promote antigen cross-presentation mediated by DCs. Mutu DCs, an immortal mouse DC cell line, was chosen as APC. We selected OVA protein as the model antigen because OVA-specific B3Z hybridoma T cells were available. Meanwhile the activation of B3Z cells was detected by CPRG assay. We found that the mixture of low-concentration OVA with Rehydragel LV or HS coated with bPEI25k could elicit strong B3Z responses, suggesting that coating conventional aluminum adjuvant particles with PEI could increase the efficiency of antigen cross-presentation. Furthermore, we found that this efficiency varied with aluminum particles and PEI mixtures. Our results also showed that branched PEIs were more effective than linear PEIs in enhancing the antigen cross-presentation of DCs, which might be explained by the differences in membrane interference effects between the two polymers.
Efficient cross-presentation of soluble antigens often requires high-concentration protein in the DC’s cytosol. We have shown that Ub-proteins can increase cross-presentation efficiency, compared with cell lysates [16]. So, OVA protein expression was examined in the Ub-protein fraction isolated from the cytosol of DCs by Western blot analysis in our study. We used the membrane pore forming protein (PFO), which can perforate the cytomembrane and release cytosolic proteins into the medium [34]. Western blot analysis revealed that Ub-OVA released into the cytosol increased obviously when the antigen was combined to bPEI-coated Rehydragel in DCs.
We next investigated whether OVA protein combined with Rehydragel/bPEI could induce DCs to secrete Th1-cytokines IL-12 that promotes cytotoxicity [17,18]. ELISA analysis showed that LV/bPEI25k was more powerful than LV alone or bPEI25k alone in promoting the secretion of IL-12 by DCs.
Type I IFN could directly enhance the function of DCs, a process implicated in the initiation of adaptive immune responses [22]. IFN-α/β induces the maturation of DCs, up-regulates their co-stimulatory activity, and enhances the cross-presentation of antigens to CD8+ T cells [23-26]. Our findings also verified the effect of IFN-α/β on the Rehydragel/bPEI-enhanced cross-presentation of murine DCs.
TLR stimulation promotes cross-presentation in type I IFN-dependent fashion. Among TLRs, TLR3 can recognize viral double-stranded RNA and is involved in the high-level induction of IFN-α/β [35]. Meanwhile, cross-presentation by DCs requires the participation of surface molecules, such as CD40, lectin, langerin, mannose receptor and heat shock protein [29,30]. Interestingly, in our experiments, TLR3 or CD40 deficiency posed no significant effects on DCs cross-presentation activated by Rehydragel/bPEI. Our results revealed that the antigen cross-presentation activated by Rehydragel/bPEI was not only achieved by TLR3 and CD40 stimulation. Further studies should be conducted to reveal the roles of other TLRs (such as TLR4, TLR7, TLR9) and surface molecules of DCs (such as langerin, lectin, heat shock protein).
Cytosolic antigens can be degraded by the proteasomes, transported to the ER (endoplasmic reticulum) by TAP transporters, and then loaded onto MHC class I molecules. In contrast, some peptides derived from exogenous proteins can also be degraded by proteases in endocytic compartments, and then loaded onto MHC class II molecules in endosomes and lysosomes [36,37]. A recent study shows that lysosomal cysteine proteases, with the help of MHC class II molecules and the MHC class I-like molecule CD1D, can regulate antigen presentation and elicit the activation of a subgroup of T cells [38]. Here, we also confirmed that TAP1 was absolutely indispensable for Rehydragel/bPEI-enhanced OVA cross-presentation by DCs. To further elucidate the antigen delivery pathways of dendritic cells, proteasomal and lysosomal inhibitors were used in the study. Our results showed that OVA protein combined with Rehydragel/bPEI could be presented by DCs via both the proteasome and the lysosome pathways. However, it remains to determine the precise role of the proteasome and the lysosome pathway, and the proteolytic mechanism that regulates antigen presentation.
Furthermore, we also explored whether Rehydragel/bPEI-OVA could trigger the response of endogenous T cell. It was found that the level of OVA-specific T cells and the number of Kb-OVA257-264 Dextramer positive CD8+ T cells increased significantly in the mice vaccinated with LV/bPEI25k-OVA. Thus, these results proved that PEI-coated Rehydragel greatly enhanced the efficiency of cross-presentation in vivo.
In summary, our studies demonstrated that coating conventional aluminum adjuvant particles with PEI could enhance antigen cross-presentation of DCs in vitro and in vivo. These findings provided an alternative approach to promote rapid translation of alumina nanoparticles adjuvants into clinical application for cancer treatment and prevention of infection by the intracellular pathogens.
Acknowledgements
This work was funded by grants from the National Natural Sciences Foundation of China (81502681), Providence Portland Medical Foundation, National Institutes of Health (U43CA165048).
Disclosure of conflict of interest
None.
References
- 1.Cintolo JA, Datta J, Mathew SJ, Czerniecki BJ. Dendritic cell-based vaccines: barriers and opportunities. Future Ooncol. 2012;8:1273–1299. doi: 10.2217/fon.12.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santos PM, Butterfield LH. Dendritic cell-based cancer vaccines. J Immunol. 2018;200:443–449. doi: 10.4049/jimmunol.1701024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.He P, Zou Y, Hu Z. Advances in aluminum hydroxide-based adjuvant research and its mechanism. Hum Vaccin Immunother. 2015;11:477–488. doi: 10.1080/21645515.2014.1004026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuroda E, Coban C, Ishii KJ. Particulate adjuvant and innate immunity: past achievements, present findings, and future prospects. Int Rev Immunol. 2013;32:209–220. doi: 10.3109/08830185.2013.773326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kool M, Soullie T, van Nimwegen M, Willart MA, Muskens F, Jung S, Hoogsteden HC, Hammad H, Lambrecht BN. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J Exp Med. 2008;205:869–882. doi: 10.1084/jem.20071087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Exley C, Siesjo P, Eriksson H. The immunobiology of aluminium adjuvants: how do they really work? Trends Immunol. 2010;31:103–109. doi: 10.1016/j.it.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 7.Maughan CN, Preston SG, Williams GR. Particulate inorganic adjuvants: recent developments and future outlook. J Pharm Pharmacol. 2015;67:426–449. doi: 10.1111/jphp.12352. [DOI] [PubMed] [Google Scholar]
- 8.Kool M, Fierens K, Lambrecht BN. Alum adjuvant: some of the tricks of the oldest adjuvant. J Med Micorobiol. 2012;61:927–934. doi: 10.1099/jmm.0.038943-0. [DOI] [PubMed] [Google Scholar]
- 9.Marrack P, McKee AS, Munks MW. Towards an understanding of the adjuvant action of aluminium. Nat Rev Immunol. 2009;9:287–293. doi: 10.1038/nri2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colaprico A, Senesi S, Ferlicca F, Brunelli B, Ugozzoli M, Pallaoro M, O’Hagan DT. Adsorption onto aluminum hydroxide adjuvant protects antigens from degradation. Vaccine. 2020;38:3600–3609. doi: 10.1016/j.vaccine.2020.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Li H, Li Y, Jiao J, Hu HM. Alpha-alumina nanoparticles induce efficient autophagy-dependent cross-presentation and potent antitumour response. Nat Nanotechnol. 2011;6:645–650. doi: 10.1038/nnano.2011.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, Chen Y, Wang M, Ma Y, Xia W, Gu H. A mesoporous silica nanoparticle--PEI--fusogenic peptide system for siRNA delivery in cancer therapy. Biomaterials. 2013;34:1391–1401. doi: 10.1016/j.biomaterials.2012.10.072. [DOI] [PubMed] [Google Scholar]
- 13.Zhupanyn P, Ewe A, Buch T, Malek A, Rademacher P, Muller C, Reinert A, Jaimes Y, Aigner A. Extracellular vesicle (ECV)-modified polyethylenimine (PEI) complexes for enhanced siRNA delivery in vitro and in vivo. J Control Release. 2020;319:63–76. doi: 10.1016/j.jconrel.2019.12.032. [DOI] [PubMed] [Google Scholar]
- 14.Steiner QG, Otten LA, Hicks MJ, Kaya G, Grosjean F, Saeuberli E, Lavanchy C, Beermann F, McClain KL, Acha-Orbea H. In vivo transformation of mouse conventional CD8alpha+ dendritic cells leads to progressive multisystem histiocytosis. Blood. 2008;111:2073–2082. doi: 10.1182/blood-2007-06-097576. [DOI] [PubMed] [Google Scholar]
- 15.Molino NM, Anderson AK, Nelson EL, Wang SW. Biomimetic protein nanoparticles facilitate enhanced dendritic cell activation and cross-presentation. Acs Nano. 2013;7:9743–9752. doi: 10.1021/nn403085w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu G, Moudgil T, Cui Z, Mou Y, Wang L, Fox BA, Hu HM. Ubiquitinated proteins isolated from tumor cells are efficient substrates for antigen cross-presentation. J Immunother. 2017;40:155–163. doi: 10.1097/CJI.0000000000000165. [DOI] [PubMed] [Google Scholar]
- 17.Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz VR, Trumpfheller C, Yamazaki S, Cheong C, Liu K, Lee HW, Park CG, Steinman RM, Nussenzweig MC. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 18.Hochrein H, Shortman K, Vremec D, Scott B, Hertzog P, O’Keeffe M. Differential production of IL-12, IFN-alpha, and IFN-gamma by mouse dendritic cell subsets. J Immunol. 2001;166:5448–5455. doi: 10.4049/jimmunol.166.9.5448. [DOI] [PubMed] [Google Scholar]
- 19.Han HD, Byeon Y, Kang TH, Jung ID, Lee JW, Shin BC, Lee YJ, Sood AK, Park YM. Toll-like receptor 3-induced immune response by poly(d,l-lactide-co-glycolide) nanoparticles for dendritic cell-based cancer immunotherapy. Int J Nanomedicine. 2016;11:5729–5742. doi: 10.2147/IJN.S109001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watts C, West MA, Zaru R. TLR signalling regulated antigen presentation in dendritic cells. Curr Opin Immunol. 2010;22:124–130. doi: 10.1016/j.coi.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Szeles L, Meissner F, Dunand-Sauthier I, Thelemann C, Hersch M, Singovski S, Haller S, Gobet F, Fuertes MS, Mann M, Garcin D, Acha-Orbea H, Reith W. TLR3-mediated CD8+ dendritic cell activation is coupled with establishment of a cell-intrinsic antiviral state. J Immunol. 2015;195:1025–1033. doi: 10.4049/jimmunol.1402033. [DOI] [PubMed] [Google Scholar]
- 22.Swiecki M, Colonna M. Type I interferons: diversity of sources, production pathways and effects on immune responses. Curr Opin Virol. 2011;1:463–475. doi: 10.1016/j.coviro.2011.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Honda K, Sakaguchi S, Nakajima C, Watanabe A, Yanai H, Matsumoto M, Ohteki T, Kaisho T, Takaoka A, Akira S, Seya T, Taniguchi T. Selective contribution of IFN-alpha/beta signaling to the maturation of dendritic cells induced by double-stranded RNA or viral infection. Proc Natl Acad Sci U S A. 2003;100:10872–10877. doi: 10.1073/pnas.1934678100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 25.Sprooten J, Agostinis P, Garg AD. Type I interferons and dendritic cells in cancer immunotherapy. Int Rev Cell Mol Biol. 2019;348:217–262. doi: 10.1016/bs.ircmb.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Shirley JL, Keeler GD, Sherman A, Zolotukhin I, Markusic DM, Hoffman BE, Morel LM, Wallet MA, Terhorst C, Herzog RW. Type I IFN sensing by cDCs and CD4(+) T cell help are both requisite for cross-priming of AAV capsid-specific CD8(+) T cells. Mol Ther. 2020;28:758–770. doi: 10.1016/j.ymthe.2019.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skold AE, Mathan T, van Beek J, Florez-Grau G, van den Beukel MD, Sittig SP, Wimmers F, Bakdash G, Schreibelt G, de Vries I. Naturally produced type I IFNs enhance human myeloid dendritic cell maturation and IL-12p70 production and mediate elevated effector functions in innate and adaptive immune cells. Cancer Immunol Immunother. 2018;67:1425–1436. doi: 10.1007/s00262-018-2204-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montoya M, Schiavoni G, Mattei F, Gresser I, Belardelli F, Borrow P, Tough DF. Type I interferons produced by dendritic cells promote their phenotypic and functional activation. Blood. 2002;99:3263–3271. doi: 10.1182/blood.v99.9.3263. [DOI] [PubMed] [Google Scholar]
- 29.Katakam AK, Brightbill H, Franci C, Kung C, Nunez V, Jones CR, Peng I, Jeet S, Wu LC, Mellman I, Delamarre L, Austin CD. Dendritic cells require NIK for CD40-dependent cross-priming of CD8+ T cells. Proc Natl Acad Sci U S A. 2015;112:14664–14669. doi: 10.1073/pnas.1520627112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fernandez-Ruiz D, Lau LS, Ghazanfari N, Jones CM, Ng WY, Davey GM, Berthold D, Holz L, Kato Y, Enders MH, Bayarsaikhan G, Hendriks SH, Lansink L, Engel JA, Soon M, James KR, Cozijnsen A, Mollard V, Uboldi AD, Tonkin CJ, de Koning-Ward TF, Gilson PR, Kaisho T, Haque A, Crabb BS, Carbone FR, McFadden GI, Heath WR. Development of a novel CD4(+) TCR transgenic line that reveals a dominant role for CD8(+) dendritic cells and CD40 signaling in the generation of helper and CTL responses to blood-stage malaria. J Immunol. 2017;199:4165–4179. doi: 10.4049/jimmunol.1700186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lawand M, Abramova A, Manceau V, Springer S, van Endert P. TAP-dependent and -independent peptide import into dendritic cell phagosomes. J Immunol. 2016;197:3454–3463. doi: 10.4049/jimmunol.1501925. [DOI] [PubMed] [Google Scholar]
- 32.Guermonprez P, Saveanu L, Kleijmeer M, Davoust J, Van Endert P, Amigorena S. ER-phagosome fusion defines an MHC class I cross-presentation compartment in dendritic cells. Nature. 2003;425:397–402. doi: 10.1038/nature01911. [DOI] [PubMed] [Google Scholar]
- 33.Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33:492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanyal S, Claessen JH, Ploegh HL. A viral deubiquitylating enzyme restores dislocation of substrates from the endoplasmic reticulum (ER) in semi-intact cells. J Biol Chem. 2012;287:23594–23603. doi: 10.1074/jbc.M112.365312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu J, Lee MH, Chakhtoura M, Green BL, Kotredes KP, Chain RW, Sriram U, Gamero AM, Gallucci S. STAT2 is required for tlr-induced murine dendritic cell activation and cross-presentation. J Immunol. 2016;197:326–336. doi: 10.4049/jimmunol.1500152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joffre OP, Segura E, Savina A, Amigorena S. Cross-presentation by dendritic cells. Nat Rev Immunol. 2012;12:557–569. doi: 10.1038/nri3254. [DOI] [PubMed] [Google Scholar]
- 37.Segura E, Amigorena S. Cross-presentation in mouse and human dendritic cells. Adv Immunol. 2015;127:1–31. doi: 10.1016/bs.ai.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 38.Honey K, Rudensky AY. Lysosomal cysteine proteases regulate antigen presentation. Nat Rev Immunol. 2003;3:472–482. doi: 10.1038/nri1110. [DOI] [PubMed] [Google Scholar]


