Abstract
Objective: The purpose of this research is to probe the mechanism of Jatrorrhizine (JAT) improving Aβ 25-35-induced nerve cell injury through the miR-223-3p/HDAC4 axis. Methods: SH-SY5Y cells were treated with Aβ 25-35 to simulate nerve injury in the pathogenesis of Alzheimer’s disease (AD), and JAT-treated SH-SY5Y cells were assessed for HDAC4 and miR-223-3p. The HDAC4 and miR-223-3p levels were tested by qRT-PCR. Proliferation was determined through MTT. Apoptosis was assessed by flow cytometry, and the related indexes of oxidative stress (OS) were examined by an OS kit. Results: Compared with AD group, OD value increased, apoptosis rate decreased, and OS was inhibited in the AD+JAT group (all P<0.05). In SH-SY5Y cells, miR-223-3p can specifically inhibit the HDAC4 expression. The miR-223-3p expression increased and HDAC4 decreased after JAT acted on SH-SY5Y cells stimulated by Aβ 25-35 (all P<0.05). The addition of over-expression HDAC4 vector or miR-223-3p inhibitor could inhibit proliferation, and promote apoptosis and OS on the basis of JAT (all P<0.05). In addition, over-expressing miR-223-3p can suppress over-expressed HDAC4’s effects on proliferation, apoptosis, and OS of SH-SY5Y cells (all P<0.05). Conclusion: JAT can improve the nerve injury induced by Aβ 25-35 by up-regulating miR-223-3p and inhibiting the HDAC4 expression, suppress apoptosis and OS, and induce proliferation. This research further clarified the mechanism of JAT in AD.
Keywords: Jatrorrhizine, Aβ 25-35, SH-SY5Y, miR-223-3p, nerve cell injury
Introduction
Alzheimer’s disease (AD) is a familiar chronic neurodegenerative disease marked by memory loss and progressive cognitive dysfunction [1]. With the continuous growth of the elderly population, the morbidity of AD is also rising rapidly [2]. At the moment, there is no effective treatment. The current treatment can only appropriately slow down the disease progression, and the individual difference of patients receiving treatment is great. It is urgent to analyze the pathogenesis of AD and explore new molecular therapeutic targets for treatment.
The deposition of β-amyloid peptide (Aβ) and hyperphosphorylation of senile plaques (SP) and Tau proteins leading to neurofibrillary tangles are two main pathologic manifestations of AD [3]. The accumulation of Aβ in brain and cerebral vessels is effective in the occurrence and progression of AD. There is evidence that many factors including apoptosis, inflammation, and oxidative stress (OS) are relevant to AD’s pathogenesis [4].
Jatrorrhizine (JAT) is a tetrahydroisoquinoline alkaloid with bactericidal effect extracted from Coptis chinensis, which is often used as a detoxifying and hypoglycemic agent [5]. Research has shown that JAT has many biologic functions such as anti-inflammatory and antioxidant [6]. Previous research has shown that JAT can be used to protect neurons from cell damage induced by hydrogen peroxide or Aβ oligomer [7]. JAT’s mechanism in AD needs to be explored in depth. Therefore, this study aims to explore the regulatory mechanism of JAT in AD by combining traditional Chinese medicine treatment with molecular biology.
It has been reported that HDAC4, a member of HDAC family IIa, can regulate the survival of neurons. Under the action of some proteins in neurodegenerative diseases, HDAC4 can translocate from the cytoplasm to the nucleus and inhibit the activity of MEF2 after binding to myocyte specific enhancer 2 (MEF2), a transcription factor related to neuronal survival in the nucleus, resulting in neuronal death [8]. The expression of HDAD4 in the frontal cortex of patients with cognitive impairment of AD is enhanced, which is related to early neuron dysfunction and cognitive impairment [9]. It is speculated that JAT may control AD progression by regulating HDAC4, and HDAD4 is chosen as an important research point to find the possible upstream regulation mechanism.
microRNAs (miRNAs), non-coding RNAs with 18-22 nucleotides, exert a variety of biological functions by targeting the 3’ untranslated region (3’UTR) of their target mRNA [10,11]. Recently, they have become new therapeutic targets for neurodegenerative diseases [12]. This research predicted that HDAC4 could specifically bind miR-223-3p by using TargetScan and StarBase online prediction websites. It has been reported that miR-223-3p can protect isolated cortical neurons from conditioned medium-mediated degeneration in multiple sclerosis [13]. Furthermore, miR-223 in serum exosomes can be used as a crucial diagnostic and prognostic marker of dementia [14]. Nevertheless, a functional study of miR-223-3p in AD has not been confirmed. It is speculated that miR-223-3p may regulate AD development and progression.
Thus, this research speculated that JAT might influence the neural cell behavior of AD by adjusting the miR-223-3p/HDAD4 axis. This provides a new research basis for further understanding the role of JAT in the occurrence and development of AD, and also creates a new target for treatment.
Materials and methods
Cell culture
Human neuroblastoma cell line SH-SH-SY5Y was provided by American Type Culture Collection (ATCC) and stored in DMEM basic medium. It was added with 10% FBS and 1% penicillin/streptomycin, and placed in a 5% CO2 incubator at 37°C. SH-SY5Y cells were pretreated by 20 μM/L Aβ 25-35 (80-90% fusion) for 24 h to construct AD cell models [15].
For in vitro cell research, JAT (CAS: 3621-38-3, National Institute of Food and Drug Administration, China) was dissolved in dimethyl sulfoxide (DMSO) to form 10 mM concentration, which was stored in the dark at 4°C and further diluted to 10 μM in fresh cell culture medium for later use [16]. Cells were cultured under 5% CO2 and 95% humidity, and they were in logarithmic growth phase.
Cell transfection and grouping
miR-223-3p mimics, inhibitors, and their negative controls (miR-NC), HDAC4 overexpression vectors (pcDNA3.1-HDAC4), and their negative controls (pcDNA3.1-NC) were constructed at Yuanjing Biotechnology Co., Ltd., China. According to the instructions, miR-223-3p mimic/inhibitor and its negative control (miR-NC) and pcDNA3.1-HDAC4 (pcDNA3.1-NC) were transfected into SH-SY5Y cells through Lipofectamine 3000.
The cell groups were as follows: control group (blank control SH-SY5Y cells), AD group (SH-SY5Y cells induced by Aβ 25-35), AD+JAT group (those induced cells+JAT), AD+JAT+pcDNA3.1-HDAC4 group (those induced cells+JAT+HDAC4 over-expression vector), AD+JAT+pcDNA3.1-NC group (those induced cells+JAT+HDAC4 negative control), AD+JAT+miR-223-3p inhibitor group (those induced cells+miR-223-3p inhibitor), AD+JAT+miR-NC group (those induced cells+miR-223-3p negative control), AD+JAT+pcDNA3.1-HDAC4+miR-223-3p mimic group (those induced cells+JAT+HDAC4 over-expression vector+miR-223-3p mimics) and AD+JAT+pcDNA3.1-HDAC4+miR-NC group (those induced cells+JAT+HDAC4 over-expression vector+miR-223-3p negative control).
MTT
Cell proliferation was measured by the MTT cell proliferation test kit (C0009M, Shanghai Beyotime Biotechnology Co., Ltd., China). SH-SH-SY5Y cells grown in logarithmic phase were collected and inoculated on 96-well plates with 1×104 cells per well. A total of 20 μL MTT (5.0 mg/mL) was added to each well at 24, 48 and 72 h and cultured at 37°C for 4 h. The MTT solution was removed and 100 μL dimethyl sulfoxide (DMSO) was added. The absorbance at 490 nm was tested by a strip reader after shaking for about 10 min.
Flow cytometry
Apoptosis was assessed by Annexin V-FITC/PI apoptosis detection kit (C1062S, Shanghai Beyotime Biotechnology Co., Ltd., China) based on the instructions. Transfected cells were dissolved with trypsin, cleaned with cold PBS, centrifuged, and resuspended in buffer. They were double-stained with Annexin-V-FITC (10 μL) and propidium iodide (PI; 5 μL) under dark conditions for 15 min. Then, apoptosis was tested by flow cytometry (RS001, Shanghai Ruishi Technology Co., Ltd., China).
Determination of OS levels
The OS levels were tested by micro method, using malondialdehyde (MDA) content detection (BC0025), lactate dehydrogenase (LDH) (BC0685) and superoxide dismutase (SOD) activity detection kits (BC0175) (Beijing Solarbio Technology Co., Ltd., China). The supernatant was collected by centrifugation at 3000 rpm at 4°C for 15 min. The MDA levels and the LDH and SOD activities were determined by using the corresponding test kits in light of the manufacturer’s instructions.
qRT-PCR
Total RNA was obtained from SH-SY5Y cells by TRIzol® reagent, and the same amount was transcribed into cDNA by cDNA synthesis kit (BTN131005, Beijing Biolab Technology Co., Ltd., China). The SYBRPremixEx-Taq kit was applied for real-time PCR in Step One Plus™ (RR420A, Beijing Zhijie Fangyuan Technology Co., Ltd., China). The reaction conditions were as follows: pre-denaturation at 95°C for 30 s, pre-denaturation at 95°C for 35 s, pre-denaturation at 60°C for 60 s. The gene relative expression was calculated by 2-ΔΔCt method, and GAPDH and U6 were regarded as internal references. Primer sequences are shown in Table 1.
Table 1.
qRT-PCR sequences
| Name | Sequence (5’-3’) | |
|---|---|---|
| miR-223-3p | Forward | CGCUAUCUUUCUAUUAACUGACCAUAA |
| Reverse | CGCUAUCUUUCUAUUAUGACUCCAUAA | |
| HDAC4 | Forward | CGGTCCAGGCTAAAGCAGAA |
| Reverse | TCTGTGACATCCAACGGACG | |
| GAPDH | Forward | ACCACAGTC CATGCCATCAC |
| Reverse | TCCACCACCCT GTT GCTGTA | |
| U6 | Forward | GCUUCGGCAGCACAUAUACUAAA |
| Reverse | CGCUUCACGAAUUUGCGUGUCAU |
Dual luciferase report experiment
The targeted binding relationship between miR-223-3p and HDAC4 was examined by a dual luciferase gene detection kit (D0010, Beijing Solarbio Technology Co., Ltd., China). The 3’UTR fragments of wild-type and mutant HDAC4 containing miR-223-3p binding site were inserted into pGL3 vector and named HDAC4-WT and HDAC4-MUT, respectively. For luciferase report analysis, SH-SY5Y cells were co-transfected with HDAC4-WT or HDAC4-MUT and miR-223-3p mimics or simulated NC under Lipofectamine 3,000. The relative luciferase activity was normalized by Renilla luciferase activity.
Statistical analysis
SPSS 22.0 was applied in statistical analysis. The measurement data were under normality test through Jarque-Bera, and those in accordance with normal distribution were represented in the form of mean ± standard deviation (x̅ ± sd). The inter-group comparison was made by t-test, and that among multiple groups was assessed through one-way analysis of variance and Tukey back testing. If they did not conform to the normal distribution, the inter-group comparison was made by Mann-Whitney U test, and that among multiple groups was evaluated by Bonferroni method. P<0.05 denotes a significant difference.
Results
JAT can improve SH-SY5Y cell injury stimulated by Aβ 25-35
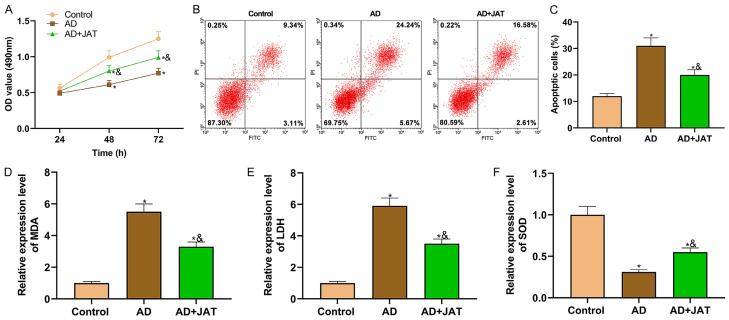
In order to probe into JAT’s role in AD progression, after SH-SY5Y cells were stimulated by Aβ 25-35 to construct AD cell models in vitro, 10 μM JAT was added to Aβ 25-35 induced SH-SY5Y cell culture medium to observe JAT’s effect. Cell proliferation experiments manifested that the proliferation of SH-SY5Y cells was enhanced after JAT was added (all P<0.05; Figure 1A). Flow cytometry revealed that JAT reduced the apoptosis rate of SH-SY5Y cells stimulated by Aβ 25-35 (P<0.05; Figure 1B, 1C). The OS parameters showed that the levels of oxidative products (MDA and LDH) decreased and those of antioxidant substances (SOD) increased after JAT treatment, suggesting that JAT enhanced the antioxidant capacity of SH-SY5Y cells and reduced cell damage (all P<0.05; Figure 1D-F).
Figure 1.
Effect of JAT on SH-SY5Y cells in AD. A: Cell proliferation is tested by MTT assay; B and C: Apoptosis is tested by flow cytometry; D-F: Detection results of OS parameters (MDA, LDH and SOD). Compared with control group, *P<0.05, while compared with AD group, &P<0.05. JAT: Jatrorrhizine; MDA: malondialdehyde; LDH: lactate dehydrogenase; SOD: superoxide dismutase.
Ove-expressing HDAC4 can partially reverse JAT’s protective effect on SH-SY5Y cells
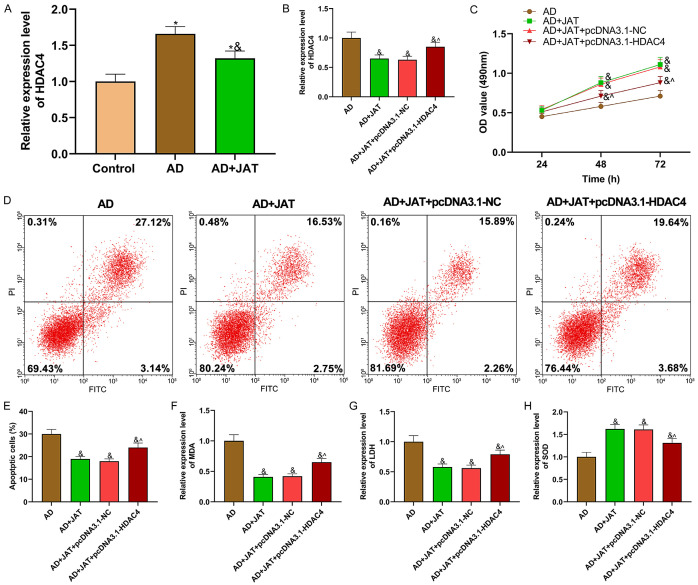
qRT-PCR manifested that the HDAC4 expression decreased after JAT was supplemented to SH-SY5Y cell culture medium stimulated by Aβ 25-35 (P<0.05; Figure 2A). To study JAT and HDAC4’s mechanism in AD, HDAC4 was overexpressed and transfected into SH-SY5Y cells treated with JAT and Aβ 25-35 for functional experiments. The HDAC4 expression in each group was shown in Figure 2B. Compared with AD group, the HDAD4 expression in AD+ JAT group was inhibited, while compared with AD+JAT+pcDNA3.1-NC group, the expression in AD+JAT+pcDNA3.1-HDAC4 group was enhanced (all P<0.05). MTT assay manifested that JAT improved the proliferation of SH-SY5Y cells decreased by Aβ 25-35, while HDAC4 over-expression vector partially reversed it (P<0.05; Figure 2C). The experimental data of apoptosis revealed that the apoptosis rate decreased by JAT increased by pcDNA3.1-HDAC4 (Figure 2D, 2E). Compared with AD+JAT+pcDNA3.1-NC group, MDA, LDH increased and SOD decreased in AD+JAT+pcDNA3.1-HDAC4 group, suggesting that HDAC4 overexpression vector aggravated the OS of SH-SY5Y cells stimulated by Aβ 25-35 (P<0.05; Figure 2F-H).
Figure 2.
Overexpressing HDAC4 can partially reverse JAT’s protective effect on SH-SY5Y cells. A, B: Expression of HDAC4 in each group of cells; C: Cell proliferation is tested by MTT assay; D, E: Apoptosis is tested by flow cytometry; F-H: Detection results of OS parameters (MDA, LDH and SOD). Compared with control group, *P<0.05, compared with AD group, &P<0.05, while compared with AD+JAT+pcDNA3.1-NC group, ^P<0.05. JAT: Jatrorrhizine; MDA: malondialdehyde; LDH: lactate dehydrogenase; SOD: superoxide dismutase.
HDAC4 targets miR-223-3p
HDAC4 was chosen as the end point and the possible upstream regulation mechanism was sought. With HDAC4 as the research target, Targetscan and Starbase were used to predict the upstream miRNA that might regulate HDAC4, and the top 50 with higher binding strength in Starbase and those with binding scores below -0.05 in Targetscan were selected and intersected. Three miRNAs (miR-29a-3p, miR-29b-3p and miR-223-3p) were obtained (Figure 3A). We focused on miR-223-3p (Figure 3B). Research has shown that miR-223 in serum exosomes can be used as an important diagnostic and prognostic marker of dementia [14]. The expression of serum miR-223-3p can be used as a key diagnostic index of AD [17]. Hence, miR-223-3p was selected for follow-up study.
Figure 3.
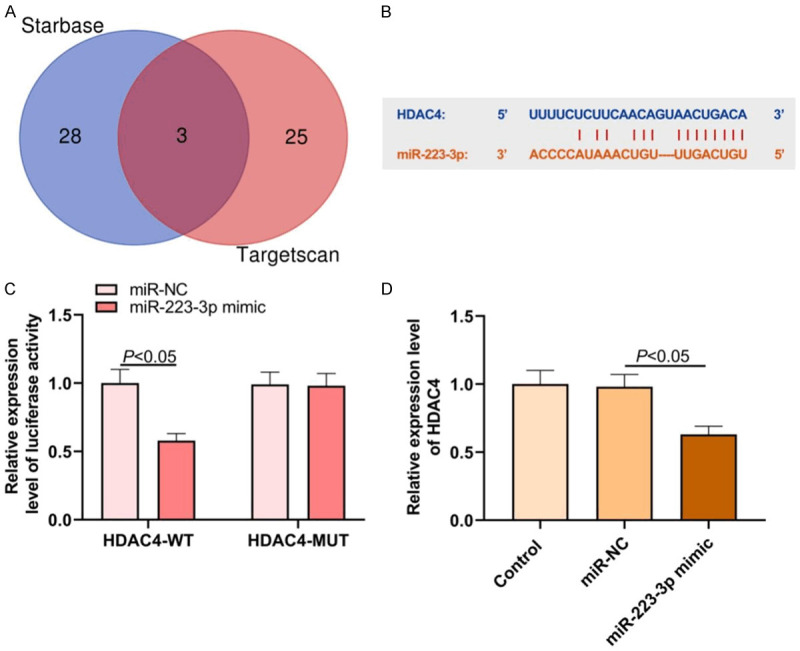
Bioinformatic tools predict possible molecular mechanisms in AD. A: Common targeted miRNAs predicted by HDAC4 in Targetscan and StarBase databases; B: Binding site of HDAC4 and miR-223-3p; C: Dual luciferase report experimental results; D: Effect of miR-223-3p on HDAC4 expression in SH-SY5Y cells.
The interaction between HDAC4 and miR-223-3p was verified by dual luciferase report experiment. Compared with miR-NC and HDAC4-WT co-transfection group, the luciferase activity of miR-223-3p mimic and HDAC4-WT co-transfection group decreased (P<0.05; Figure 3C). This manifested that after miR-223-3p was over-expressed in SH-SY5Y cells, the HDAC4 expression decreased, and miR-223-3p could negatively regulate the expression (P<0.05; Figure 3D).
miR-223-3p inhibitor can partially reverse JAT’s protective effect on SH-SY5Y cells
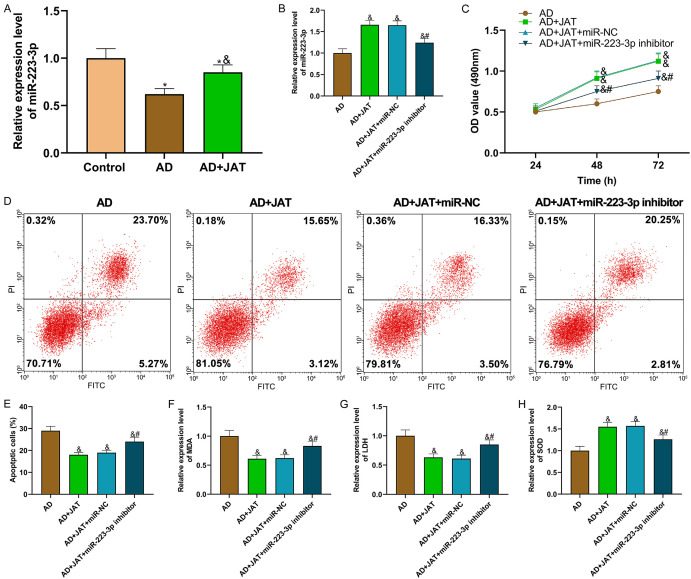
qRT-PCR detection revealed that the miR-223-3p expression in SH-SY5Y cells treated with Aβ 25-35 increased after JAT was added (P<0.05; Figure 4A). To study JAT and miR-223-3p’s mechanism in AD, the miR-223-3p inhibitor was transfected into SH-SY5Y cells for functional analysis. The data showed the miR-223-3p expression in each group of cells (Figure 4B). Compared with AD group, the cell proliferation of AD+JAT group increased (P<0.05), and that of SH-SY5Y cells decreased after miR-223-3p was inhibited (P<0.05; Figure 4C). Flow cytometry analysis manifested that the apoptosis rate in the AD+JAT group was lower than that in the AD group (P<0.05), while transfecting miR-223-3p inhibitor increased the apoptosis rate that was decreased by JAT (P<0.05; Figure 4D, 4E). The OS parameters manifested that miR-223-3p inhibitor partially reversed the decreased levels of oxidation products (MDA and LDH) and increased those of antioxidant substances (SOD) in JAT (P<0.05; Figure 4F-H).
Figure 4.
miR-223-3p inhibitor can partially reverse JAT’s protective effect on SH-SY5Y cells. A, B: Relative expression of miR-223-3p in each group of cells; C: Cell proliferation is tested by MTT assay; D, E: Apoptosis is tested by flow cytometry; F-H: Detection results of OS parameters (MDA, LDH and SOD). Compared with control group, *P<0.05; compared with AD group, &P<0.05; compared with AD+JAT+miR-NC group, #P<0.05. JAT: Jatrorrhizine; MDA: malondialdehyde; LDH: lactate dehydrogenase; SOD: superoxide dismutase.
JAT regulates miR-223-3p/HDAC4 to reduce SH-SY5Y cell injury stimulated by Aβ 25-35
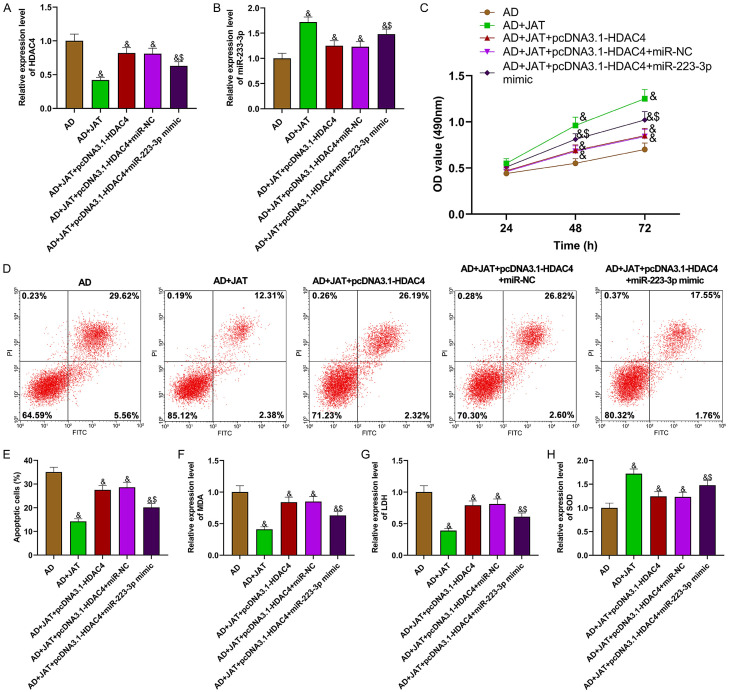
To study the mechanism of JAT combined with miR-223-3p/HDAC4 in AD, HDAC4 and miR-223-3p overexpression vectors were co-transfected into JAT-treated AD cell models. Figure 5A, 5B shows the HDAC4 and miR-223-3p expression in each group of cells. The functional experimental data signify that overexpressing HDAC4 can inhibit the proliferation of JAT, increase the apoptosis rate, and aggravate OS, while over-expressing miR-223-3p can partially reverse the effect mediated by pcDNA3.1-HDAC4, improve the proliferation and antioxidant capacity of SH-SY5Y cells in AD, and reduce the apoptosis rate (P<0.05; Figure 5C-H). The above results suggest that JAT can protect SH-SY5Y cells induced by Aβ 25-35 by up-regulating miR-223-3p and inhibiting HDAC4.
Figure 5.
JAT regulates miR-223-3p/HDAC4 to reduce SH-SY5Y cell injury stimulated by Aβ 25-35. A: Relative expression of HDAC4 in each group of cells; B: Relative expression of miR-223-3p in each group of cells; C: Cell proliferation is tested by MTT assay; D, E: Apoptosis is tested by flow cytometry; F-H: Detection results of OS parameters (MDA, LDH and SOD). Compared with AD group, &P<0.05; compared with AD+JAT+pcDNA3.1-HDAC4+miR-NC group, $P<0.05. JAT: Jatrorrhizine; MDA: malondialdehyde; LDH: lactate dehydrogenase; SOD: superoxide dismutase.
The mechanism diagram of this research is shown (Figure 6).
Figure 6.
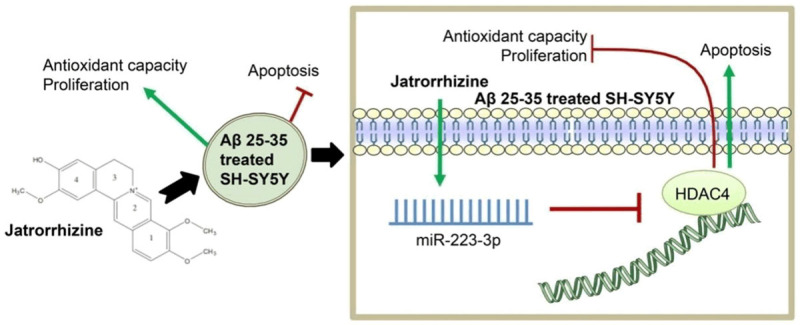
Jatrorrhizine ameliorates A miR223-induced neuronal injury through the miR-223-3p/HDAC4 axis.
Discussion
A toxic effect of Aβ on AD neurons has been confirmed [4]. Aβ 25-35 is a short toxic fragment with high cytotoxicity and neurotoxicity, that has been widely used to establish AD models in vitro [18]. JAT has been proven to have the effect of scavenging oxygen free radicals and reducing neuronal stress response, and its therapeutic effect on AD has been preliminarily reported [16]. Compared with the control group, JAT treatment of SH-SY5Y cells stimulated by Aβ 25-35 can improve cell proliferation and inhibit apoptosis, inflammation and OS, suggesting the value of JAT in AD treatment. Thus, we studied the mechanism of JAT in AD with molecular biology.
Next, we explored HDAC4’s effects on SH-SY5Y cells stimulated by Aβ 25-35. Our results manifested that the HDAC4 expression increased in SH-SY5Y cells treated with Aβ 25-35, while JAT could decrease the expression. Overexpressing HDAC4 can aggravate apoptosis and OS stimulated by Aβ 25-35, inhibit cell proliferation, and partially reverse the therapeutic effect of JAT on nerve cells in AD. HDAC4 mainly exists in the cytoplasm of neurons and shuttles between cytoplasm and nucleus induced by incoming and outgoing signals [19]. HDAC has been reported to regulate the survival of neurons. Some studies have found that ApoE4 exerts its harmful effects by affecting the deposition of Aβ and neurofibrillary tangles in the brain, and it reduces the expression of brain-derived neurotrophic factors by up-regulating the expression of HDAC4 [20]. These results indicate that JAT regulates HDAC4 to participate in the occurrence and development of AD, which further proves the previous studies on the nerve damage function of HDAC4.
Many miRNAs are effective in AD progression [21]. In this research, miR-223-3p was selected as HDAC4’s target miRNA and the targeted relationship between them was verified. Previous research manifested that the serum miR-223-3p expression could be used as an important diagnostic index for AD [22]. miR-223-3p is a latent therapeutic target in AD progression, but its mechanism is vague. This research found that the interaction between miR-223-3p and HDAC4 was verified by dual luciferase reporter experiment. In addition, JAT can up-regulate the down-regulated miR-223-3p level in AD cell models. miR-223-3p inhibitor can partially reverse JAT’s efficacy on AD, and overexpressing miR-223-3p can partially reverse the nerve cell injury aggravated by overexpression of HDAC4. These results reveal that JAT can protect nerve cells in AD progression by up-regulating miR-223-3p and inhibiting HDAC4. However, in vivo experiments have not been discussed in this experiment, and animal experiments are needed for further verification.
In general, this research confirmed that JAT regulated the miR-223-3p/HDAC4 axis to protect neurons induced by Aβ 25-35. JAT/miR-223-3p/HDAC4 may be an effective target for AD.
Disclosure of conflict of interest
None.
References
- 1.Wang LL, Song YP, Mi JH, Ding ML. Peptidyl arginine deiminase 4 and its potential role in Alzheimer’s disease. Med Hypotheses. 2020;146:110466. doi: 10.1016/j.mehy.2020.110466. [DOI] [PubMed] [Google Scholar]
- 2.Mazzi C, Massironi G, Sanchez-Lopez J, De Togni L, Savazzi S. Face recognition deficits in a patient with Alzheimer’s disease: amnesia or agnosia? The importance of electrophysiological markers for differential diagnosis. Front Aging Neurosci. 2020;12:580609. doi: 10.3389/fnagi.2020.580609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhuang JC, Chen ZJ, Cai PP, Wang R, Yang QW, Li LL, Yang HL, Zhu RJ. Targeting microRNA-125b promotes neurite outgrowth but represses cell apoptosis and inflammation via blocking PTGS2 and CDK5 in a FOXQ1-dependent way in Alzheimer disease. Front Cell Neurosci. 2020;14:587747. doi: 10.3389/fncel.2020.587747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu CC, Du YJ, Wang SQ, Liu LB, Shen F, Wang L, Lin YF, Kong LH. Experimental evidence of the benefits of acupuncture for Alzheimer’s disease: an updated review. Front Neurosci. 2020;14:549772. doi: 10.3389/fnins.2020.549772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu KB, Wei WJ, Hu JQ, Wen D, Ma B, Liu WL, Wang Y, Lu ZW. Apoptosis activation in thyroid cancer cells by jatrorrhizine-platinum (II) complex via downregulation of PI3K/AKT/mammalian target of rapamycin (mtor) pathway. Med Sci Monit. 2020;26:e922518. doi: 10.12659/MSM.922518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu GJ, Mu T, Zhang LH, Chen XL. Jatrorrhizine hydrochloride alleviates tert-butyl hydroperoxide-induced endothelial cell injury through its anti-inflammatory activity and ppar-γ activation. Cell Mol Biol (Noisy-le-grand) 2020;66:125–129. [PubMed] [Google Scholar]
- 7.Wang S, Jiang W, Ouyang T, Shen XY, Wang F, Qu YH, Zhang M, Luo T, Wang HQ. Jatrorrhizine balances the gut microbiota and reverses learning and memory deficits in APP/PS1 transgenic mice. Sci Rep. 2019;9:19575. doi: 10.1038/s41598-019-56149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang W, Xiao FL, Huang WJ, Wei QS, Li XS. MicroRNA-29a-3p strengthens the effect of dexmedetomidine on improving neurologic damage in newborn rats with hypoxic-ischemic brain damage by inhibiting HDAC4. Brain Res Bull. 2020;167:71–79. doi: 10.1016/j.brainresbull.2020.11.011. [DOI] [PubMed] [Google Scholar]
- 9.Mahady L, Nadeem M, Malek-Ahmadi M, Chen KW, Perez SE, Mufson EJ. Frontal cortex epigenetic dysregulation during the progression of Alzheimer’s disease. J Alzheimers Dis. 2018;62:115–131. doi: 10.3233/JAD-171032. [DOI] [PubMed] [Google Scholar]
- 10.Ricciardi A, Bennuru S, Tariq S, Kaur S, Wu WW, Elkahloun AG, Arakelyan A, Shaik J, Dorward DW, Nutman TB, Semnani RT. Extracellular vesicles released from the filarial parasite brugiamalayi downregulate the host mTOR pathway. PLoS Negl Trop Dis. 2021;15:e0008884. doi: 10.1371/journal.pntd.0008884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu YZ, Liu X, Lin CW, Jia XH, Zhu HM, Song J, Zhang Y. Noncoding RNAs regulate alternative splicing in cancer. J Exp Clin Cancer Res. 2021;40:11. doi: 10.1186/s13046-020-01798-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nunomura A, Perry G. RNA and oxidative stress in Alzheimer’s disease: focus on microRNAs. Oxid Med Cell Longev. 2020;2020:2638130. doi: 10.1155/2020/2638130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morquette B, Juźwik CA, Drake SS, Charabati M, Zhang Y, Lécuyer MA, Galloway DA, Dumas A, de Faria Junior O, Paradis-Isler N, Bueno M, Rambaldi I, Zandee S, Moore C, Bar-Or A, Vallières L, Prat A, Fournier AE. MicroRNA-223 protects neurons from degeneration in experimental autoimmune encephalomyelitis. Brain. 2019;142:2979–2995. doi: 10.1093/brain/awz245. [DOI] [PubMed] [Google Scholar]
- 14.Wei H, Xu YH, Xu WL, Zhou QW, Chen Q, Yang ML, Feng F, Liu YQ, Zhu XL, Yu M, Li YF. Serum exosomal miR-223 serves as a potential diagnostic and prognostic biomarker for dementia. Neuroscience. 2018;379:167–176. doi: 10.1016/j.neuroscience.2018.03.016. [DOI] [PubMed] [Google Scholar]
- 15.Wang XP, Li XJ, Huang B, Yang LL, Chen K, Zhao DD, Luo XD, Wang YJ. Downregulation of miR-33 has protective effect against Aβ25-35-induced injury in SH-SH-SY5Y cells. Med Sci Monit. 2020;26:e921026. doi: 10.12659/MSM.921026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang P, Gao XY, Yang SQ, Sun ZX, Dian LL, Qasim M, Phyo AT, Liang ZS, Sun YF. Jatrorrhizine inhibits colorectal carcinoma proliferation and metastasis through Wnt/β-catenin signaling pathway and epithelial-mesenchymal transition. Drug Des Devel Ther. 2019;13:2235–2247. doi: 10.2147/DDDT.S207315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mancuso R, Agostini S, Hernis A, Zanzottera M, Bianchi A, Clerici M. Circulatory miR-223-3p discriminates between Parkinson’s and Alzheimer’s patients. Sci Rep. 2019;9:9393. doi: 10.1038/s41598-019-45687-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu WW, Jin HQ, Huang YN. Mitochondria-associated Membranes (MAMS): a potential therapeutic target for treating ALZheimer’s disease. Clin Sci (Lond) 2021;135:109–126. doi: 10.1042/CS20200844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neuner SM, Wilmott LA, Hoffmann BR, Mozhui K, Kaczorowski CC. Hippocampal proteomics defines pathways associated with memory decline and resilience in normal aging and Alzheimer’s disease mouse models. Behav Brain Res. 2017;322:288–298. doi: 10.1016/j.bbr.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sen A, Nelson TJ, Alkon DL. ApoE4 and Aβ oligomers reduce BDNF expression via HDAC nuclear translocation. J Neurosci. 2015;35:7538–51. doi: 10.1523/JNEUROSCI.0260-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu QL, Lei CB. Neuroprotective effects of miR-331-3p through improved cell viability and inflammatory marker expression: correlation of serum miR-331-3p levels with diagnosis and severity of Alzheimer’s disease. Exp Gerontol. 2020;144:111187. doi: 10.1016/j.exger.2020.111187. [DOI] [PubMed] [Google Scholar]
- 22.Jia LH, Liu YN. Downregulated serum miR-223 servers as biomarker in Alzheimer’s disease. Cell Biochem Funct. 2016;34:233–237. doi: 10.1002/cbf.3184. [DOI] [PubMed] [Google Scholar]