Abstract
Background
Patients with advanced gastroesophageal junction cancer (GEJC) have poor survival outcomes, and GEJC-specific data from trials evaluating agents in gastric cancers (GCs) as a whole are lacking. Trifluridine/tipiracil (FTD/TPI) was approved for previously treated metastatic GC or GEJC (mGC/mGEJC) based on results of the phase 3 TAGS trial. Subgroup analyses by primary tumor type (GC or GEJC) in TAGS are reported here.
Methods
Pa tients with mGC/mGEJC treated with ≥ 2 prior chemotherapy regimens were randomized (2:1) to receive FTD/TPI or placebo, plus best supportive care. A pre-planned sub-analysis was performed to evaluate efficacy and safety outcomes by primary tumor type (GEJC or GC).
Results
Of 507 randomized patients, 145 (29%) had GEJC and 360 (71%) had GC as the primary disease site. Baseline characteristics were generally similar between the GEJC and GC subgroups, except that more patients in the GEJC subgroup had received ≥ 3 prior regimens (72 vs. 59% in the GC subgroup). Survival benefit with FTD/TPI was observed in both subgroups. The overall survival hazard ratio for FTD/TPI vs placebo was 0.75 (95% CI 0.50–1.11) and 0.67 (95% CI 0.52–0.87) in the GEJC and GC subgroups, respectively. Grade ≥ 3 adverse events of any cause were reported in 75 (77%) and 192 (81%) FTD/TPI-treated patients in the GEJC and GC subgroups, respectively. No new safety concerns were noted with FTD/TPI.
Conclusion
As in patients with GC, FTD/TPI showed an efficacy benefit in patients with GEJC in the TAGS trial, along with demonstrating a manageable safety profile.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10120-021-01156-x.
Keywords: Trifluridine/tipiracil, Gastroesophageal junction cancer, TAGS, Phase 3, Subgroup analysis
Introduction
Gastroesophageal junction cancer (GEJC), though often grouped under gastric cancer (GC) in clinical trial and registries, has distinct clinical features, risk factors, and diagnosis and treatment challenges [1]. The incidence of GEJC has been increasing over several decades, doubling in the United States from 16% in 1973 to 32% in 2013 [2, 3]. GEJC is often diagnosed at a relatively late stage when the disease has become unresectable, and patients with advanced/metastatic GEJC generally require multiple lines of therapy, as recurrence is common [4].
In a real-world analysis of over 3000 patients with advanced GC/GEJC (43% with GEJC); median OS with first-line therapy, composed primarily of chemotherapy combinations, was 10.7 months and declined with each subsequent line of therapy (7.6–2.8 months) [5]. Additional real-world data suggest that patients with GEJC may have reduced landmark survival rates compared with GC at 6 (20 vs. 30%) and 12 months (11 vs. 16%) [6].
Trifluridine/tipiracil (FTD/TPI) is an oral therapy comprising the thymidine analog trifluridine and tipiracil, which prevents trifluridine degradation [7]. FTD/TPI received approval in the United States, Europe, and Japan for previously treated metastatic GC/GEJC based on OS benefit observed in the phase 3 TAGS (TAS-102 Gastric Study; NCT02500043) [8, 9]. Here, we present data from a pre-planned subgroup analysis that was conducted to evaluate the efficacy and safety of FTD/TPI in patients with GEJC.
Materials and methods
TAGS, a global phase 3 randomized placebo-controlled clinical trial, enrolled patients with non-resectable metastatic GC/GEJC who had received at least two previous chemotherapy regimens and had an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1. GEJ involvement was documented by endoscopic, radiologic, surgical, or pathology report.
Patients were randomized (2:1) to receive FTD/TPI 35 mg/m2 or placebo, both twice daily with best supportive care, on days 1–5 and 8–12 of each 28-day treatment cycle. The primary endpoint was OS; secondary endpoints included progression-free survival (PFS), time to deterioration (TTD) of ECOG PS to ≥ 2, safety, and tolerability. The protocol was approved by the institutional review board/independent ethics committee at each participating site. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. All patients provided written informed consent. Additional details about the conduct of this study have been reported previously [6].
Although pre-planned, the subgroup analyses described in this report were not powered for statistical significance and are not intended to be used to compare results between primary tumor locations with GEJC vs GC involvement. Hazard ratios (HRs) and associated 95% confidence intervals (CIs) for time-to-event endpoints were based on a stratified Cox proportional hazards model; median values were Kaplan–Meier estimates.
Results
Of 507 patients enrolled in the TAGS trial, 145 (29%) and 360 (71%) had a sole primary tumor location of GEJ or GC, respectively (2 patients had both gastric and GEJC tumors and were excluded from this analysis). Although baseline patient characteristics in these two subgroups were generally similar, there were some notable differences (Table 1). A higher proportion of patients in the GEJC than the GC subgroup were male (85 vs. 68%), were White (83 vs. 65%), had an ECOG PS of 1 (70 vs. 59%), or were more heavily pretreated (72 vs. 59% completing ≥ 3 prior regimens). Within both subgroups, baseline characteristics were generally similar between the treatment groups, with some exceptions in the GEJC subgroup. In this subgroup, patients randomized to FTD/TPI versus placebo were more heavily pretreated (74 vs. 66% had received ≥ 3 prior regimens), and a smaller proportion had undergone prior gastrectomy (40 vs. 55%).
Table 1.
Baseline clinical and disease characteristics
| GEJCa | GCa | |||
|---|---|---|---|---|
| FTD/TPI | Placebo | FTD/TPI | Placebo | |
| (n = 98) | (n = 47) | (n = 239) | (n = 121) | |
| Age, years | ||||
| Mean | 61 | 62 | 63.4 | 61.9 |
| Median (range) | 62.0 (24–89) | 62.0 (42–80) | 64 (27–86) | 63 (32–82) |
| Sex, n (%) | ||||
| Male | 83 (85) | 40 (85) | 169 (71) | 76 (63) |
| Race, n (%) | ||||
| White | 83 (85) | 37 (79) | 161 (67) | 74 (61) |
| Asian | 6 (6) | 4 (9) | 45 (19) | 25 (21) |
| Black | 0 | 0 | 1 (< 1) | 2 (2) |
| Not collected | 8 (8) | 4 (9) | 30 (13) | 20 (17) |
| Other | 1 (1) | 2 (4) | 2 (1) | 0 |
| ECOG PS, n (%) | ||||
| 0 | 28 (29) | 15 (32) | 95 (40) | 53 (44) |
| 1 | 70 (71) | 32 (68) | 144 (60) | 68 (56) |
| Geographic region, n (%) | ||||
| Japan | 6 (6) | 4 (9) | 40 (17) | 23 (19) |
| USA | 13 (13) | 3 (6) | 8 (3) | 2 (2) |
| EU | 79 (81) | 40 (85) | 191 (80) | 96 (79) |
| Previous gastrectomy, n (%) | ||||
| Yes | 39 (40) | 26 (55) | 108 (45) | 156 (40) |
| No | 59 (60) | 21 (45) | 131 (55 | 73 (60) |
| Prior radiotherapy, n (%) | ||||
| Yes | 36 (37) | 17 (36) | 35 (15) | 9 (7) |
| No | 62 (63) | 30 (64) | 204 (85) | 112 (93) |
| Number of metastatic sites, n (%) | ||||
| 1–2 | 50 (52) | 25 (53) | 105 (44) | 47 (39) |
| ≥ 3 | 48 (49) | 22 (47) | 134 (56) | 74 (61) |
| Number of prior regimens, n (%) | ||||
| 2 | 25 (26) | 16 (34) | 101 (42) | 47 (39) |
| 3 | 41 (42) | 15 (32) | 93 (39) | 45 (37) |
| ≥ 4 | 32 (33) | 16 (34) | 45 (19) | 29 (24) |
EU Europe, FTD/TPI trifluridine/tipiracil, GEJC gastroesophageal junction cancer, GC gastric cancer, USA United States of America
aTwo patients had both gastric and GEJC tumors and were excluded from this analysis
At data cutoff (31 March 2018), ≥ 94% of patients in both treatment arms in each tumor-type subgroup had discontinued treatment (Supplementary Table). The most common reason for discontinuation in both the GEJC and GC subgroups was disease progression (78% of GEJC and 72% of GC in FTD/TPI-treated arm; GEJC of 87% and 86% of GC in placebo-treated arm).
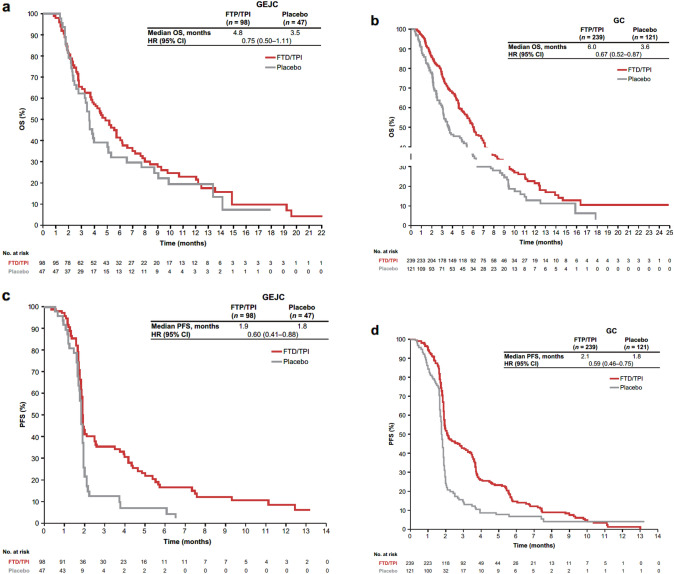
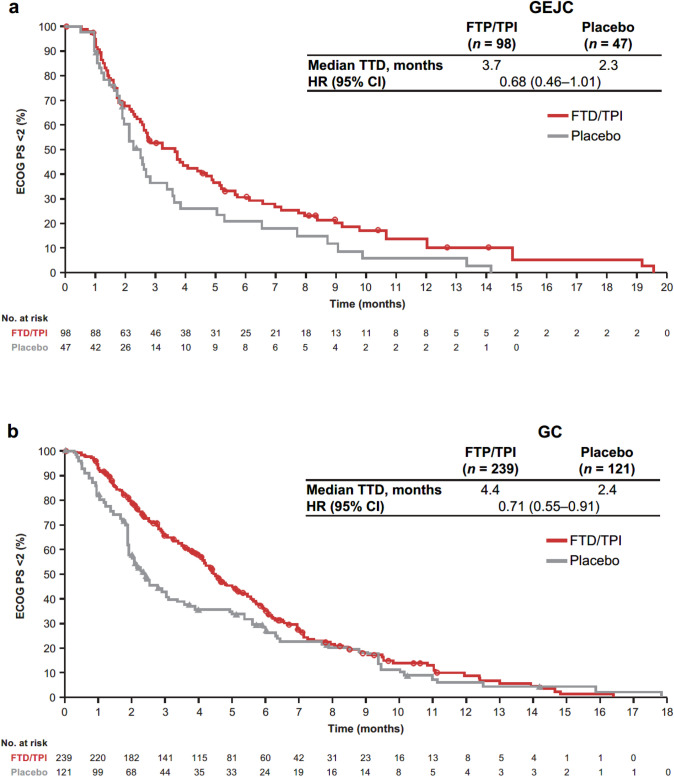
In both the GEJC and GC subgroups, efficacy outcomes were improved with FTD/TPI compared with placebo (Fig. 1). In the GEJC subgroup, OS and PFS HRs were 0.75 (95% CI 0.50–1.11) and 0.60 (95% CI 0.41–0.88), respectively. In the GC subgroup, OS and PFS HRs were 0.67 (95% CI 0.52–0.87) and 0.59 (95% CI 0.46–0.75). Median OS in the FTD/TPI group was numerically lower in the GEJC than the GC subgroup (4.8 vs. 6.0 months). The HR for TTD of ECOG PS to ≥ 2 for FTD/TPI vs placebo was 0.68 (95% CI 0.46–1.01) in the GEJC subgroup and 0.71 (95% CI 0.55–0.91) in the GC subgroup (Fig. 2).
Fig. 1.
Efficacy outcomes in the GEJC and GC subgroups. a OS in the GEJC subgroup. b OS in the GC subgroup. c PFS in the GEJC subgroup. d PFS in the GC subgroup. CI: confidence interval, ECOG PS: Eastern Cooperative Oncology Group performance status, GEJC: gastroesophageal junction cancer, GC: gastric cancer, HR: hazard ratio, PFS: progression-free survival, OS: overall survival
Fig. 2.
Time to deterioration in the GEJC and GC subgroups. a TTD of ECOG PS to ≥ 2 in the GEJC subgroup. b TTD of ECOG PS to ≥ 2 in the GC subgroup. CIL: confidence interval, ECOG PS: Eastern Cooperative Oncology Group performance status, GEJC: gastroesophageal junction cancer, GC: gastric cancer, HR: hazard ratio, TTD: time to deterioration
Grade ≥ 3 adverse events (AEs) of any cause with FTD/TPI were reported in 75 (77%) and 192 (81%) patients in the GEJC and GC subgroups, respectively (Table 2). The most frequently reported grade ≥ 3 AEs with FTD/TPI in the GEJC group were neutropenia (25%) and anemia (13%); incidences of these AEs in the GC subgroup were 38 and 21%, respectively. In the GEJC subgroup, dosing modifications and discontinuations due to AEs of any cause with FTD/TPI were 41 (42%) and 7 (7%), respectively, and in the GC subgroup, were 107 (45%) and 29 (12%). Treatment-related deaths were reported in 1 (< 1%) FTD/TPI-treated patient (attributed to cardiopulmonary arrest) and 1 (1%) placebo-treated patient (attributed to toxic hepatitis), both in the GC subgroup.
Table 2.
Adverse events
| GEJC | GC | |||||||
|---|---|---|---|---|---|---|---|---|
| FTD/TPI (n = 97)a | Placebo (n = 46)a | FTD/TPI (n = 238)a | Placebo (n = 120)a | |||||
| Any grade | Grade ≥ 3 | Any grade | Grade ≥ 3 | Any grade | Grade ≥ 3 | Any grade | Grade ≥ 3 | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Any AE of any cause | 96 (99) | 75 (77) | 44 (96) | 27 (59) | 230 (97) | 192 (81) | 111 (93) | 69 (58) |
| Any treatment-related AE | 73 (75) | 40 (41) | 23 (50) | 2 (4) | 198 (83) | 136 (57) | 71 (59) | 19 (16) |
| Action taken due to AEs of any cause | ||||||||
| Dosing modification (dosing delay or dose reduction) | 52 (54) | 41 (42) | 11 (24) | 9 (20) | 143 (60) | 107 (45) | 25 (21) | 19 (16) |
| Treatment discontinuation | 9 (9) | 7 (7) | 5 (11) | 3 (7) | 34 (14) | 29 (12) | 23 (20) | 18 (15) |
| AEs of any cause in ≥ 10% of patients | ||||||||
| Hematologic AEs | ||||||||
| Neutropeniab | 41 (42) | 24 (25) | 0 | 0 | 135 (57) | 90 (38) | 7 (6) | 0 |
| Anemiac | 36 (37) | 13 (13) | 6 (13) | 2 (4) | 114 (48) | 51 (21) | 25 (21) | 10 (8) |
| Leukopeniad | 16 (17) | 1 (1) | 0 | 0 | 62 (26) | 30 (13) | 3 (3) | 0 |
| Thrombocytopeniae | 12 (12) | 1 (1) | 0 | 0 | 48 (20) | 10 (4) | 8 (7) | 0 |
| Gastrointestinal AEs | ||||||||
| Nausea | 43 (44) | 5 (5) | 13 (28) | 1 (2) | 81 (34) | 5 (2) | 40 (33) | 4 (3) |
| Vomiting | 26 (27) | 4 (4) | 11 (24) | 0 | 57 (24) | 8 (3) | 22 (18) | 3 (3) |
| Diarrhea | 22 (23) | 2 (2) | 6 (13) | 1 (2) | 54 (23) | 7 (3) | 17 (14) | 2 (2) |
| Abdominal pain | 19 (20) | 4 (4) | 10 (22) | 7 (15) | 36 (15) | 10 (4) | 21 (18) | 8 (7) |
| Ascites | 4 (4) | 1 (1) | 0 | 0 | 15 (6) | 11 (5) | 16 (13) | 11 (9) |
| Constipation | 27 (28) | 3 (3) | 12 (26) | 2 (4) | 18 (8) | 1 (< 1) | 13 (11) | 2 (2) |
| Dysphagia | 15 (15) | 5 (5) | 4 (9) | 3 (7) | 5 (2) | 2 (1) | 3 (3) | 1 (1) |
| Other AEs | ||||||||
| Decreased appetite | 28 (29) | 5 (5) | 15 (33) | 2 (4) | 87 (37) | 24 (10) | 36 (30) | 9 (8) |
| Fatigue | 35 (36) | 10 (10) | 11 (24) | 0 | 54 (23) | 13 (6) | 23 (19) | 10 (8) |
| Asthenia | 18 (19) | 4 (4) | 8 (17) | 1 (2) | 47 (20) | 12 (5) | 32 (27) | 10 (8) |
| Back pain | 8 (8) | 1 (1) | 5 (11) | 3 (7) | 17 (7) | 1 (< 1) | 6 (5) | 1 (1) |
| Dyspnea | 12 (12) | 5 (5) | 5 (11) | 1 (2) | 12 (5) | 1 (< 1) | 12 (10) | 5 (4) |
| General physical health deterioration | 9 (9) | 8 (8) | 4 (9) | 4 (9) | 14 (6) | 14 (6) | 12 (10) | 10 (8) |
AE adverse event, FTD/TPI trifluridine/tipiracil, GEJC gastroesophageal junction cancer, GC gastric cancer
aAs treated population
bNeutropenia and/or decreased neutrophil count
cAnemia and/or decreased hemoglobin level
dLeukopenia and/or decreased white blood cell count
eThrombocytopenia and/or decreased platelet count
Discussion
This subgroup analysis of the TAGS trial provides detailed efficacy and safety data in patients with metastatic GEJC treated with FTD/TPI. The analysis demonstrated efficacy benefits with FTD/TPI in both the GEJC and GC subgroups.
In multivariate Cox regression analyses of OS in TAGS, which included stratification factors and primary tumor site, primary tumor site (gastric or GEJ) was not identified as being prognostic or predictive of OS with FTD/TPI treatment (Pinteraction = 0.29). In the current analysis, median OS with FTD/TPI was marginally lower in the GEJC (4.8 months) than the GC subgroup (6.0 months), although OS was similar with placebo in both subgroups (3.5 and 3.6 months). This could be attributed to patients in the GEJC subgroup overall being more heavily pretreated overall (72 vs. 58% of patients in the GC subgroup having received ≥ 3 previous lines of therapy), as well as differences in the proportion of patients receiving ≥ 3 prior lines treatment between FTD/TPI-treated (74%) and placebo-treated patients (66%) within the GEJC subgroup.
To date, data in GEJC subgroups in trials of other anticancer agents have been limited to mostly HRs of survival, with few studies reporting survival data. The KEYNOTE-059 study, one of the few with survival data, reported similar median OS in the GEJC and GC subgroups (5.7 months [95% CI 4.2–8.4) and 5.6 months [3.8–7.2]) in the GC subgroups with pembrolizumab [10]. OS HRs for the GEJC and GC subgroups reported in other phase 3 studies, such as KEYNOTE-061 (pembrolizumab vs paclitaxel; 0.61 [0.41–0.90] and 0.94 [0.71–1.23] for GEJC and GC), ATTRACTION-2 (nivolumab vs placebo; 0.44 [0.20–0.97] and 0.69 [0.55–0.87], respectively) and RAINBOW (ramucirumab plus paclitaxel vs placebo plus paclitaxel; 0.52 [0.35–0.78] and 0.90 [0.70–1.10], respectively) each indicated a marginally greater death risk reduction with the investigational regimen in the GEJC subgroup than in the GC subgroup [11–13]. In contrast, earlier trials testing non-immune-related agents showed trends towards better survival outcomes in the GC subgroup. For example, the ToGA trial in which the location of the primary cancer was stratified for reported OS HRs for GEJC and GC for chemotherapy/ trastuzumab versus chemotherapy as 0.67 (95% CI 0.42–1.08) vs. 0.76 (95% CI 0.60–0.96), respectively [14]. Possible mechanism for why GEJC does better or worse than GC is difficult based on the current evidence base. Many earlier trials were not stratified for the two anatomical sites, thus, making safe comparative conclusions difficult. There are differences in molecular characteristics between GEJC and GC as identified in the Cancer Genome Atlas (TGCA) which may explain differences in responsiveness to cancer [14]. As discussed, studies testing the emerging immune checkpoint inhibitors may demonstrate a clearer difference in survival outcomes predicated on the molecular differences of the two anatomical sites [15].
In the current sub-analysis, no new safety concerns were noted with FTD/TPI in the GEJC subgroup, and the incidence of grade ≥ 3 hematologic AEs appeared to be lower than in the FTD/TPI-treated GC subgroup. Comparable safety data have not been reported by these subgroups in trials of other agents, including those mentioned above.
The main limitation of the current analyses was that although they were pre-planned, they were not powered for statistical significance. This precluded a robust evaluation of the efficacy and safety of FTD/TPI in the GEJC or GC subgroups.
Conclusion
In summary, the results of this analysis indicate that FTD/TPI is an effective treatment option with a manageable safety profile in patients with metastatic GEJC, similar to what was observed in GC. FTD/TPI resulted in an efficacy benefit in the GEJC subgroup despite patients in the FTD/TPI group being more heavily pretreated than in the placebo group.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the patients and families who made this trial possible and the clinical study teams who were involved in this trial, as well as the data and safety monitoring board members.
Funding
This study was sponsored by Taiho Oncology, Inc., and Taiho Pharmaceutical Co., Ltd. Professional medical writing and editorial assistance were provided by Sara Thier, PhD, Wendy Sacks, PhD, and Jennifer L. Robertson, PhD, at Ashfield Healthcare Communications (Lyndhurst, NJ, USA), funded by Taiho Oncology, Inc.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Li-Chang HH, Kasaian K, Ng Y, Lum A, Kong E, Lim H, et al. Retrospective review using targeted deep sequencing reveals mutational differences between gastroesophageal junction and gastric carcinomas. BMC Cancer. 2015;15:32. doi: 10.1186/s12885-015-1021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartel M, Brahmbhatt B, Bhurwal A. Incidence of gastroesophageal junction cancer continues to rise: analysis of surveillance, epidemiology, and end results (SEER) database. J Clin Oncol. 2019;37(4 suppl):40. doi: 10.1200/JCO.2019.37.4_suppl.40. [DOI] [Google Scholar]
- 3.Casamayor M, Morlock R, Maeda H, Ajani J. Targeted literature review of the global burden of gastric cancer. Ecancer. 2018;12:883. doi: 10.3332/ecancer.2018.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toihata T, Imamura Y, Watanabe M, Baba H. Management of metastatic esophagogastric junction adenocarcinoma. J Cancer Metastasis Treat. 2018;4:24. doi: 10.20517/2394-4722.2017.82. [DOI] [Google Scholar]
- 5.Le DT, Ott PA, Korytowsky B, Le H, Le TK, Zhang Y, et al. Real-world treatment patterns and clinical outcomes across lines of therapy in patients with advanced/metastatic gastric or gastroesophageal junction cancer. Clin Colorectal Cancer. 2020;19:32–38. doi: 10.1016/j.clcc.2019.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Chau I, Le DT, Ott PA, Korytowsky B, Le H, Le TK, et al. Developing real-world comparators for clinical trials in chemotherapy-refractory patients with gastric cancer or gastroesophageal junction cancer. Gastric Cancer. 2020;23:133–141. doi: 10.1007/s10120-019-01008-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Emura T, Murakami Y, Nakagawa F, Fukushima M, Kitazato K. A novel antimetabolite, TAS-102 retains its effect on FU-related resistant cancer cells. Int J Mol Med. 2004;13:545–549. [PubMed] [Google Scholar]
- 8.LONSURF (trifluridine and tipiracil) tablets, for oral use [prescribing information]. Princeton: Taiho Oncology; 2019.
- 9.Shitara K, Doi T, Dvorokin M, Mansoor W, Arkenau H-T, Prokharau A, et al. Trifluridine/tipiracil versus placebo in patients with heavily pretreated metastatic gastric cancer (TAGS): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018;19:1437–1438. doi: 10.1016/S1470-2045(18)30739-3. [DOI] [PubMed] [Google Scholar]
- 10.Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018;4:e180013. doi: 10.1001/jamaoncol.2018.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shitara K, Özgüroğlu M, Bang YJ, Di Bartolomeo M, Mandalà M, Ryu M-H, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet. 2018;392:123–133. doi: 10.1016/S0140-6736(18)31257-1. [DOI] [PubMed] [Google Scholar]
- 12.Kang Y-K, Boku N, Satoh T, Ryu M-H, Chao Y, Kato K, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:2461–2471. doi: 10.1016/S0140-6736(17)31827-5. [DOI] [PubMed] [Google Scholar]
- 13.Wilke H, Muro K, Van Cutsem E, Oh S-C, Bodsky G, Shimada Y, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224–1235. doi: 10.1016/S1470-2045(14)70420-6. [DOI] [PubMed] [Google Scholar]
- 14.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 15.Cancer Genome Atlas Research Network Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513(7517):202–209. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.