Abstract
Background:
Timing of eating relative to the dim light melatonin onset (DLMO) may serve as a modifiable risk factor for adverse cardiometabolic outcomes. The primary aim of this study was to examine whether the timing of eating relative to DLMO is associated with BMI, body fat, and diet in healthy adults without the confound of sleep deprivation.
Methods:
Healthy men and women (N = 97), ages 18–50, with a habitual sleep duration of ≥ 6.5 hours and ≤ 8.5 hours completed 7 days of actigraphy and daily sleep and food diaries. Participants underwent a DXA scan and blood draws to assess DLMO in the clinical research unit.
Results:
A shorter duration between DLMO and the average clock time of the last meal (last meal-DLMO) was related to a higher number of meals consumed, b = .25, SEb = .06, p < .001, longer feeding duration, b = .84, SEb = .06, p < .001, greater carbohydrate intake, b = 9.08, SEb = 3.55, p = .01, and greater sugar intake, b = 4.73, SEb = 1.83, p = .01. Last meal-DLMO was not associated with BMI in the full sample; however, among those with later DLMO (after 10:30pm) last meal-DLMO was related to higher BMI, b = .92, SEb =.36, p = .02.
Conclusion:
These results suggest that timing of last meal relative to DLMO may serve as a marker of circadian misalignment and that eating the last meal closer to DLMO may negatively impact dietary habits.
Keywords: melatonin, body composition, diet
Introduction
Obesity represents a growing public health concern, as the prevalence in the United States is estimated to have reached 36.5% [1]. An emerging area of interest is how meal timing relates to cardiometabolic outcomes, although existing studies have focused on the clock timing of feeding and results have been mixed [2–5]. If meal timing is related to outcomes such as obesity, it is a modifiable risk factor, and thus, crucial to study further.
The mismatch between meal timing and intrinsic circadian rhythms (a form of circadian misalignment) may link late eating to diet and weight outcomes. Circadian misalignment has previously been associated with adverse metabolic outcomes [6]; however, much of the research examining the impact of circadian misalignment on health has focused on mis-timed sleep in relation to the intrinsic circadian rhythm and only one prior study has examined meal timing in relation to objective markers of circadian rhythms. In this study, McHill and colleagues found that participants with a diurnal rhythm of calorie consumption closer to the onset of endogenous melatonin marked by the Dim Light Melatonin Onset (DLMO) had higher BMIs and increased body fat percentage [7]. Additionally, those who consumed a greater percentage of calories within 4 hours of the DLMO and sleep onset had significantly higher percentages of body fat. These findings are limited as the study utilized a convenience sample of college students, and therefore, cannot be generalized.
We sought to extend these previous findings to assess whether eating at a later biological time impacts health in a sample of healthy adults. More specifically, the goal of this study is to evaluate whether the timing of eating relative to biological time is associated with BMI, body fat, and diet quality in a sample of healthy adults with average and late sleep timing practices who were free of depression and short sleep duration. We hypothesized that meal timing closer to DLMO (e.g. eating at a later biological time) would be associated with higher BMI, greater body fat, higher total caloric intake, greater meal frequency and worse dietary quality. Exploratory post-hoc analyses were conducted, stratifying the sample into two groups by time of DLMO to determine whether these relationships differed based on DLMO time. Given that DLMO is a variable that can be costly to measure, we also examined the associations between last meal relative to sleep midpoint and BMI, body fat, and diet quality. We hypothesized that results of the analyses examining the relationship between timing of eating relative to biological time and BMI, body fat, and diet quality would be comparable to the results of analyses examining the relationship between timing of eating relative to the sleep midpoint and BMI, body fat, and diet quality.
Methods
Participants
This is a secondary analysis of a study designed to evaluate the role of circadian timing with BMI, body fat, and obesity related behaviors [8]. The protocol for the study was approved by the Northwestern University Institutional Review Board (IRB# 55383) and all participants completed written informed consent prior to participation in this study. Participants included healthy men and women, ages 18–50 years with habitual sleep duration ≥ 6.5 hours and ≤ 8.5 hours. Exclusionary criteria included: high risk or presence of obstructive sleep apnea, insomnia, restless legs syndrome as assessed by the screening questionnaires and/or home sleep monitoring, history of cognitive or other neurological disorders, presence of any major psychiatric disorder, current alcohol or substance abuse as assessed by the Structured Clinical Interview for the DSM-IV Axis I Disorders [9], history of or concurrent unstable or serious medical illness (cancer, diabetes or cardiovascular disease), current use of psychoactive medications including antidepressants, anxiolytics, neuroleptics, anticonvulsants, hypnotics, stimulants, or beta blockers, shift work or travel over 2 time zones in the past 6 months, caffeine >300 mg per day, smoking, pregnancy or the desire to become pregnant during the study period or inability to read and write in English.
Procedure
A full description of the procedures are reported elsewhere [8]. Briefly, participants completed screening for study criteria via questionnaires, 7 days of actigraphy, and 1 night home apnea monitoring. After screening, participants were scheduled for a 1 night overnight in the clinical research unit (CRU). Participants completed 7 days of actigraphy, as well as daily sleep and food diaries prior to the CRU session. At the overnight laboratory session, participants completed a dual energy X-ray absorptiometry (DXA) scan to measure body fat. Then blood samples were taken every 30 min from Circadian Time (CT; where 0 is normal wake time) 11 to CT 18 to assess DLMO. Participants were discharged in the morning after breakfast.
Measures
Demographics:
Participants reported their age, sex, race, ethnicity, income, employment and marital status on a study-specific demographic questionnaire.
Obstructive sleep apnea risk:
Participants completed an overnight screening at home using a portable sleep apnea screening device (ApneaLink, Resmed Inc. Poway, CA). Participants who had an Apnea Hypopnea Index >5 on the Apnea Link recording were excluded from the study.
Sleep:
Sleep/wake patterns were estimated using 7 days of wrist actigraphy using the Actiwatch Spectrum (Philips/Respironics, Inc, Bend, OR). Actiwatches were worn on the non-dominant wrist and set with 30 second epoch length and medium sensitivity. Actigraphic sleep parameters (sleep onset, sleep offset, and sleep duration) were calculated using Actiware-Sleep 6.0 software with default settings. In order to be included in analyses, participants needed at least 5 days of valid actigraphy data. Average sleep midpoint (middle time point between sleep onset and sleep offset) was calculated from actigraphy data.
Circadian timing (DLMO):
Plasma melatonin levels were assayed using a commercially available radioimmunoassay for the in-vitro diagnostic quantitative determination of melatonin from IBL (IBL International GmbH, Hamburg, Germany). Dim light melatonin onset was determined using 2 SD above the baseline + 15% of the 3 highest values [10].
Body Mass index:
Body Mass Index (kg/m2) was calculated using measurements from the laboratory session: height measured at admission by nursing and weight taken in light clothing, without eating or drinking after the morning void.
Body fat and body fat distribution:
Body fat was measured using DXA on a whole body Hologic scanner (Version 13.1). Body fat values were calculated for total body fat mass, trunk fat mass, android and gynoid regions using automated calculations provided by Hologic. Values were calculated for total body fat % and the gynoid/android ratio (a measure of central adiposity).
Dietary Intake:
Participants completed 7 days of written food diaries during the week before the laboratory assessment. All participants were required to have tracked intake for at least two week days and one weekend day for their data to be included. On the food diaries, participants recorded time, location, type of food, amount consumed and a description of each component including brands and restaurant names. During the laboratory session, study staff reviewed food diaries with participants and queried for missing data. We calculated the average meal timing (including last meal) using all 7 days of data. The total caloric intake was calculated using a random sample of 3 days (2 work/school days, 1 free day). Caloric intake and macronutrients were analyzed using the Food Processor software (ESHA, Inc, Salem, OR). Total daily caloric intake, grams of protein, fat, sugar, and carbohydrates each day was computed and averaged. Midpoint of caloric intake was calculated as the average time when 50% of calories had been consumed. Meal frequency was calculated as the number of eating occasions each day, with an eating occasion defined as >50 calories consumed >30 minutes after the previous meal or snack. Feeding duration was calculated as the average duration of time between the first meal and the last meal.
Meal timing relative to biological time:
We calculated two measures of meal timing relative to DLMO: (1) the duration between DLMO and the average clock time of the last meal (last meal-DLMO); and (2) the duration between the average clock time of each participant’s midpoint of caloric intake and DLMO (caloric midpoint-DLMO). We selected the first measure of last meal-DLMO in an attempt to capture a meal that may have been consumed when energy expenditure was at its lowest [11], and when diet-induced thermogenesis is lower [12, 13, 14]. Additionally, prior research has examined the last meal relative to measures of sleep timing [15], but it has yet to be assessed in comparison to a measure of circadian timing. Caloric midpoint-DLMO was selected as an additional measure of meal timing relative to DLMO, to replicate the findings of McHill and colleagues [7].
Meal timing relative to the sleep midpoint.
The midpoint of sleep has been correlated with DLMO [16], and may be a more clinically relevant variable, given the high cost of assessing DLMO. Thus, we calculated a variable of the average duration between the last meal and the midpoint of sleep (last meal-sleep midpoint).
Data Analysis Plan
Preliminary analyses (means, SD, and frequencies) were computed to characterize the study sample. Descriptive analyses (correlations, t-tests, ANOVA) were conducted to examine relationships between demographics with meal timing. A series of multiple linear regression analyses with the meal timing variables (last meal-DLMO and caloric midpoint-DLMO) as predictor variables and BMI, body fat, and diet variables as outcomes were used to assess our primary aims. Regression models controlled for age, sex, and sleep duration. Models examining dietary variables also controlled for BMI. Post-hoc exploratory stratified analyses were conducted among groups with earlier or later DLMO (DLMO earlier or later than 10:30 pm) to examine group differences in the relationships between meal timing and diet, and BMI/body fat. To create the two groups DLMO was divided into earlier and later using a median split. These groups were created, as earlier or later DLMO may be reflective of two distinct groups. Additionally, regression analyses with last meal-sleep midpoint as predictor variables and BMI, body fat, and diet variables as outcomes were run.
For all data, outliers that were greater than 3 SDs from the mean were removed from the analyses. One outlier was removed for all analyses and three additional outliers were removed for analyses examining carbohydrate, protein, and sugar intake. We conducted sensitivity analyses with and without the outliers, and results were similar. Therefore we are presenting the analyses without outliers.
Results
Participants
Table 1 reports participant characteristics for the entire sample, as well as participants stratified into earlier and later DLMO groups. The final sample was comprised of 97 participants. On average, participants were 26.78 (SD = 7.25) years of age and there were more female participants (62.9%) than male (37.1%). Participants were primarily White (62.9%). Average BMI was in the normal range (M = 24.05; SD = 4.58). Participants had an average sleep onset time of 12:47 am (SD = 1:22), wake time of 8:05 am (SD = 1:16), and sleep duration of 443.71 minutes (SD = 50.45). DLMO ranged from 7:30 pm – 4:00 am, with an average of 10:37 pm (SD = 1:28). Participants reported consuming breakfast at 9:22 am (SD = 1:22) and reported consuming their last meal at 8:26 pm (SD = 1:53). There was an average of −2:07 hrs (SD = 2:09) between the last meal and DLMO. Very few participants (9.2%) ate their last meal after DLMO.
Table 1.
Participant Characteristics
| Overall Sample M (SD) | Earlier DLMO (<10:30 pm) M (SD) | Later DLMO (>10:30 pm) M (SD) | |
|---|---|---|---|
| Age | 26.8 (7.3) | 28.5 (7.2)* | 25.3 (7.1)* |
| Sex | 61F/36M | 31F/14M | 30F/22M |
| BMI | 24.0 (4.6) | 24.3 (5.2) | 23.8 (4.1) |
| DLMO (HH:MM) | 10:37 pm (1:28) | 9:24 pm (0:42) | 11:40 pm (1:05) |
| Last meal (HH:MM) | 8:26 pm (1:53) | 8:21 pm (2:27) | 8:31 pm (1:15) |
| Feeding Duration (hrs) | 11.1 (2.2) | 11.6 hrs (2.5)* | 10.6 hrs (1.8)* |
| Caloric Midpoint (HH:MM) | 3:24 pm (2:02) | 3:11 pm (2:04) | 3:35 pm (1:57) |
| Last Meal-DLMO (HH:MM) | −2:07 (2:09) | −0:52 (2:06)* | −3:11 (1:32)* |
| Sleep duration (minutes) | 443.7 (50.5) | 451.5 (46.2) | 437.0 (53.4) |
| Daily Caloric Intake | 2079.2 (656.0) | 2079.5 (587.0) | 2079.0 (715.9) |
| Protein (g) | 92.1 (61.6) | 79.7 (25.5) | 102.6 (79.1) |
| Carbohydrates (g) | 236.4 (71.5) | 238.5 (66.3) | 234.5 (76.3) |
| Fat (g) | 79.5 (32.8) | 82.4 (32.4) | 77.1 (33.3) |
| Sugar (g) | 80.6 (38.1) | 89.0 (38.8) | 73.6 (36.5) |
| Fiber (g) | 25.4 (41.3) | 27.2 (49.0) | 23.8 (34.2) |
Note: Overall sample N = 97; however, last meal and feeding duration were missing data from seven participants, and caloric midpoint was missing data from four participants.
p < .05 indicates difference between earlier and later DLMO
Relationships between demographics, DLMO, and meal timing
Greater age was associated with shorter duration between last meal and DLMO (r = .23, p = .05). Last meal-DLMO was not associated with sex, race, ethnicity, or education level. Caloric midpoint-DLMO was not associated with age, race, ethnicity, or education level.
Primary Analyses: Last meal-DLMO
BMI and Body Fat.
Multiple regression analysis revealed that neither measure of meal timing relative to DLMO (last meal-DLMO or caloric midpoint-DLMO) was associated with BMI, body fat percentage, or Android/Gynoid fat ratio when controlling for age, sex, and sleep duration (see Table 2).
Table 2.
Relationships between Last Meal-DLMO, and BMI, body fat, and dietary habits and Caloric Midpoint-DLMO, and BMI, body fat, and dietary habits
| Last Meal-DLMO |
|||
|---|---|---|---|
| Unstandardized B | SEB | p | |
| BMI | .31 | .23 | .18 |
| Body Fat Percentage | −.16 | .33 | .63 |
| Android/Gynoid Fat Ratio | .01 | .01 | .65 |
| Total Caloric Intake | 56.84 | 32.08 | .08 |
| Number of Meals | .25 | .06 | < .001 |
| Feeding Duration | .84 | .06 | < .001 |
| Protein | .41 | 5.60 | .94 |
| Sugar | 4.73 | 1.83 | .01 |
| Carbohydrates | 9.08 | 3.55 | .01* |
| Fat | 2.57 | 1.66 | .12 |
| Fiber | 2.64 | 2.19 | .23 |
| Caloric Midpoint-DLMO |
|||
| Unstandardized B | SEB | p | |
| BMI | .06 | .21 | .79 |
| Body Fat Percentage | .17 | .30 | .57 |
| Android/Gynoid Fat Ratio | .01 | .01 | .96 |
| Total Caloric Intake | −26.82 | 29.14 | .36 |
| Number of Meals | .07 | .06 | .25 |
| Feeding Duration | .10 | .10 | .33 |
| Protein | −5.63 | 4.98 | .26 |
| Sugar | 1.37 | 1.71 | .43 |
| Carbohydrates | −2.93 | 3.29 | .38 |
| Fat | −.83 | 1.50 | .58 |
| Fiber | −2.19 | 1.96 | .27 |
Note: All models controlled for age, sex, race, and sleep duration. Models examining caloric intake, feeding duration, number of meals, and nutrition data also controlled for BMI.
Denotes a result that was not significant when outliers were included in the data.
Eating Patterns.
Last meal-DLMO was not related to total caloric intake, when controlling for age, sex, sleep duration, and BMI. However, consuming the last meal closer to DLMO (i.e., eating at a later biological time) was related to consuming a greater number of meals, b = .25, SEb = .06, p < .001 and having a longer feeding duration, b = .84, SEb = .06, p < .001, when controlling for age, sex, sleep duration, and BMI. Caloric midpoint-DLMO was not related to total caloric intake, feeding duration, or mean number of meals when controlling for age, sex, sleep duration, and BMI.
Diet.
Eating the last meal closer to DLMO was associated with greater carbohydrate intake, b = 9.08, SEb = 3.55, p = .01, and greater sugar intake, b = 4.73, SEb = 1.83, p = .01, when controlling for age, sex, sleep duration, and BMI. There were no relationships between last meal-DLMO and protein, fat, or fiber intake. Caloric midpoint-DLMO was not associated with intake of carbohydrates, sugar, protein, fat, or fiber.
Exploratory Analyses
Earlier vs Later DLMO.
BMI and Body Fat.
In those with a later DLMO, eating the last meal closer to DLMO was associated with a higher BMI, b = .92, SEb =.36, p = .02, but this was not the case in those with an earlier DLMO, when controlling for sex, age, and sleep duration (see Figure 1). Last meal-DLMO was not related to body fat percentage or Android/Gynoid fat ratio in either group. Caloric midpoint-DLMO was not associated with BMI, body fat percentage, or Android/Gynoid fat ratio in those with earlier or later DLMO.
Figure 1.
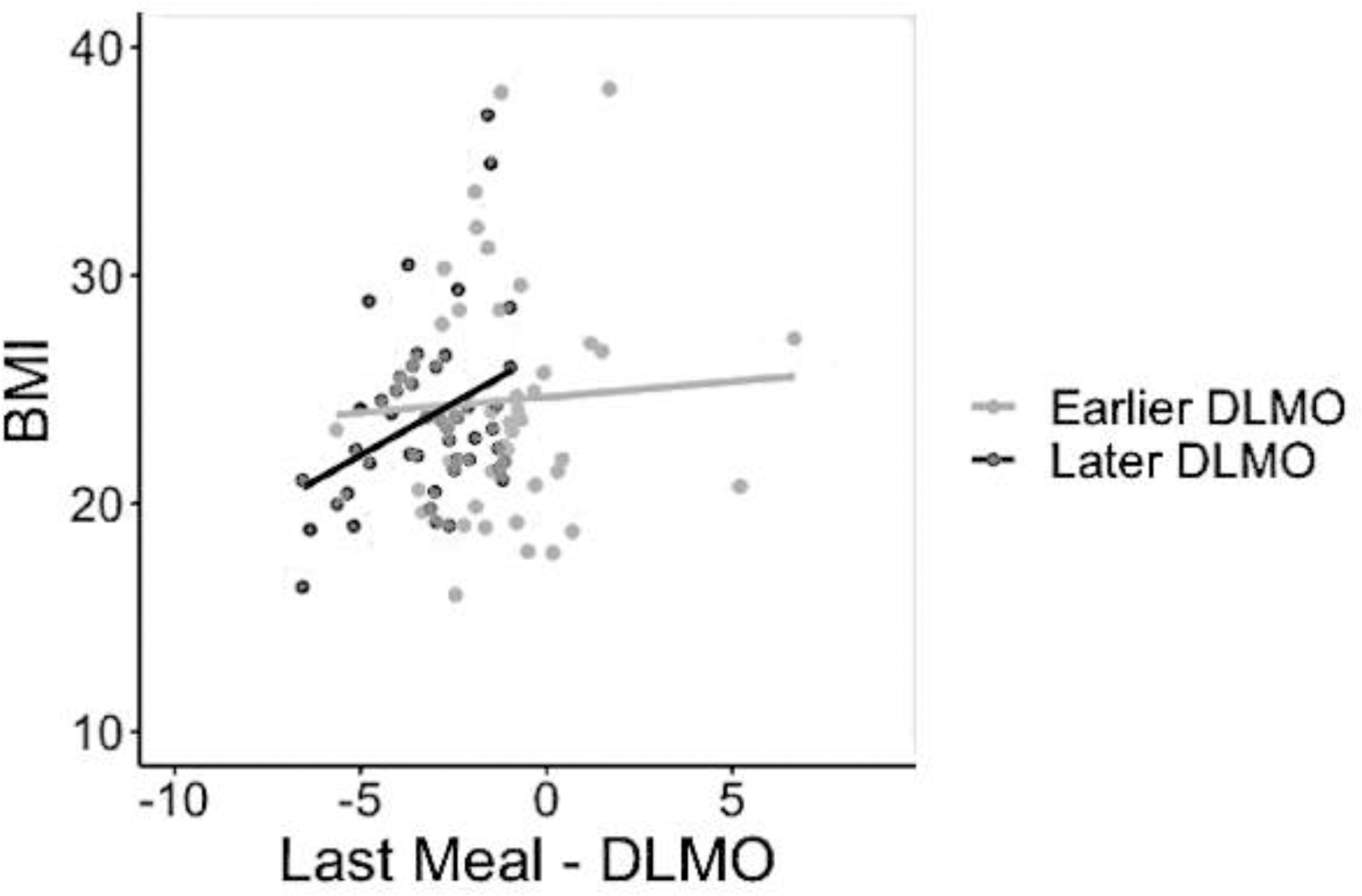
Relationships between Last meal-DLMO and BMI among those with earlier and later DLMO
Note: last meal-DLMO is displayed as the hours of difference between the last meal and DLMO. BMI units are plotted on the Y axis.
Eating patterns.
Last meal-DLMO was not related to total caloric intake, when controlling for age, sex, sleep duration, and BMI in either those with earlier or later DLMO. Eating the last meal closer to DLMO was significantly associated with greater mean number of meals among participants with later DLMOs, b = .36, SEb = .11, p = .01, but not those with earlier DLMOs. A longer feeding duration was related to eating the last meal closer to DLMO among both those with earlier DLMOs, b = .97, SEb = .09, p < .001, and those with later DLMOs, b = .83, SEb = .13, p < .001. Caloric midpoint-DLMO was not related to total caloric intake, feeding duration, or mean number of meals when controlling for age, sex, sleep duration, and BMI in either those with earlier or later DLMO.
Last meal-Sleep Midpoint.
BMI and Body Fat.
Multiple regression analysis revealed that meal timing relative to the midpoint of sleep (last meal-Sleep Midpoint) was not associated with BMI, body fat percentage, or Android/Gynoid fat ratio when controlling for age, sex, and sleep duration.
Eating Patterns.
Eating the last meal closer to the sleep midpoint was associated with greater total caloric intake when controlling for age, sex, sleep duration and BMI, b = 91.3, SEb = 31.6, p = .005. Similarly, eating the last meal closer to the sleep midpoint was related to consuming a greater number of meals, b = .3, SEb = .1, p < .001, and having a longer feeding duration, b = .9, SEb = .1, p <.001 when controlling for age, sex, sleep duration, and BMI.
Diet.
Eating the last meal closer to the sleep midpoint was associated with greater carbohydrate intake, b = 12.3, SEb = 3.5, p = .001, greater fat intake, b = 3.7, SEb = 1.7, p = .03, greater fiber intake, b = 4.9, SEb = 2.2, p = .03, and greater sugar intake, b = 6.1, SEb = 1.8, p = .001 when controlling for age, sex, sleep duration, and BMI. It was not associated with protein consumption.
Earlier vs Later DLMO.
BMI and Body Fat.
Last meal-midpoint of sleep was not related to BMI, body fat percentage, or Android/Gynoid fat ratio in those with later or earlier DLMO when controlling for age, sex, and sleep duration.
Eating Patterns.
In those with a later DLMO, eating the last meal closer to the midpoint of sleep was associated with greater caloric intake, b = 173.2, SEb = 72.8, p = .02, but this was not the case in those with an earlier DLMO, when controlling for sex, age, sleep duration, and BMI. Eating the last meal closer to the midpoint of sleep was associated with greater number of meals in both earlier, b = .26, SEb = .08, p = .002, and later, b = .45, SEb = .11, p < .001, DLMO groups when controlling for sex, age, sleep duration, and BMI.
Discussion
The goal of this study was to examine the relationships between the timing of food intake in relation to internal biological time (e.g., meal timing relative to a circadian marker, DLMO) and BMI, body fat percentage, and eating patterns (e.g., caloric intake; feeding duration) in a sample of adults with average and late sleep timing, who were free of depression and short sleep duration. Results demonstrated that each biological hour later the last meal was consumed was related to consuming .25 more meals and was related to an increase in feeding duration of .85 hours across the day. Additionally, each biological hour later the last meal was consumed was associated with a 9 gram increase in carbohydrate consumption and 5 gram increase in sugar consumption, despite not being associated with a greater total caloric intake. Biological timing of the caloric midpoint was not associated with any of our outcome measures.
Later biological timing of the last meal was also related to increased feeding duration (resulting in a shorter overnight fasting duration) and consuming a greater number of meals. This is similar to the results of previous research, demonstrating that consuming the last meal closer to sleep onset was related to increased caloric intake through increased meal frequency [15]. Eating the last meal at a later biological time may also negatively impact BMI or body composition over time, given the circadian variation of resting energy expenditure [11] and the fact that diet-induced thermogenesis is lower at later circadian times [12, 13, 14]. Over time, this also may impact the ability to lose weight. For instance, Garaulet and colleagues engaged participants in a weight loss intervention and found that those individuals who ate lunch after 3:00 PM lost less weight when compared to those who ate earlier, although energy intake, dietary composition, energy expenditure, and physical activity were similar between the two groups [17]. Similarly, Gill and Panda explored feeding duration (time between first and last eating episode) as a potential mechanism for weight status [18]. Although no relationships between feeding duration and BMI were found at baseline, a subset of participants with a BMI of >25 and a feeding duration of >14 hours per day were asked to restrict feeding duration to 10–12 hours per day. Participants were not instructed to alter caloric or macronutrient intake, yet this change in feeding duration resulted in an average decrease in caloric intake of 20.26%.
Additionally, these results are similar to those found in this sample in a prior analysis; phase angle (sleep onset time relative to DLMO, a marker of circadian alignment) was related to consuming a greater number of meals [8]. Given this, it may be that timing of last meal relative to DLMO serves as a similar marker of circadian misalignment. Future studies that seek to examine the relationships between biological timing of intake, body composition, and eating patterns over longer periods of time may be warranted.
Later biological timing of the last meal was also associated with greater carbohydrate intake and greater sugar intake, even when controlling for sleep duration. Foods high in sugar and carbohydrates are often calorically dense, and eating more of these foods over time may result in weight gain [19]. Greater sugar intake and higher glycemic load from refined carbohydrates have also been associated with increased cardiovascular disease mortality [20] and coronary heart disease [21], thus attending to biological timing of intake may improve cardiovascular health through these avenues as well. The mechanism between increased intake of carbohydrates and sugar when eating the last meal at a later biological time is unclear. It may be that it is more difficult to make healthy choices during a later circadian time of day. To our knowledge, no studies have examined the relationship between circadian timing and decision-making processes surrounding food choice. A third variable, such as chronotype, may also be contributing to this relationship. A recent study that found that evening chronotype was related to having higher odds of not meeting the American Heart Association’s dietary recommendations when compared to those who are neutral or morning type [22], likely due to the commonly found association between eveningness and worse health behaviors.
Later biological timing of the last meal was not related to measures of BMI or body composition in our full sample, which is in contrast to what was found by McHill and colleagues [7]. This may be due to differences within the study samples. For instance, participants in McHill’s study were younger, had a later sleep onset, reported a shorter average total sleep time, and later average DLMO. Given these group differences and our null findings, we split our sample into those with earlier and later DLMO. Those in the group with later DLMO ate their last meal an average of 3:11 prior to DLMO, whereas those with an earlier DLMO ate their last meal an average of 52 minutes prior to DLMO. When examining those with later DLMO separately, results demonstrated that eating the last meal at a later biological time was associated with greater BMI. No such relationships emerged among those with an earlier DLMO. This may indicate that eating the last meal at a later biological time may be more likely to impact health in groups with later DLMO, such as the college students studied by McHill and colleagues. It may be that those with later DLMO experience multiple forms of misalignment, and that eating closer to DLMO is one form of misalignment that is impacting health in a cumulative way with other forms of misalignment. The relationship seen in this group may also be mediated or moderated by another variable, such as impulsivity, altered light exposure, or consistency of meal times. It may also be that this study was underpowered to detect effects if they existed within the sample as a whole. Regardless, these findings provide further support that the biological timing of the last meal may be a crucial variable to intervene on in future intervention studies.
When examining Last meal-sleep midpoint, some findings were similar to Last meal-DLMO. Thus, studies that are not able to assess DLMO may use Last meal-sleep midpoint as a similar measure. Relationships between Last meal-sleep midpoint and eating patterns may even be stronger than those between Last meal-DLMO and these variables. Of note, Last meal-sleep midpoint was associated with total caloric intake, where Last meal-DLMO was not, which may also explain why a greater number of dietary variables were related to Last meal-sleep midpoint. In other words, those participants who consumed their last meal closer to the sleep midpoint consumed more calories, which not surprisingly resulted in consumption of a great number of grams of different nutrients. Last meal-DLMO appears to be the more relevant variable if examining relationships with BMI.
This study is not without limitations, first, the sample was comprised of healthy individuals who slept ≥ 6.5 hours and ≤ 8.5 hours, which limits generalizability of the study findings. Average sleep duration in our sample was 7.4 hours (SD = 50.5 minutes), which indicates that some within our sample were receiving less sleep than what is recommended, particularly for younger adults [23]. However, we statistically controlled for sleep duration to ensure our results were not attributable to sleep deprivation. Future studies should seek to include those with a wide range of sleep duration, and also those with very short sleep and those with medical disorders who may be more at risk for poor cardiometabolic outcomes. Second, the cross sectional nature of the study does not allow for causative statements to be made. Future studies should seek to examine the relationships between DLMO, meal timing, BMI and body fat across several months. Third, the study sample was not powered to detect mediating or moderating effects. Thus, future research should include studies with larger sample sizes.
In conclusion, these data suggest that a later biological timing of the last meal is associated with increased feeding duration, consumption of more meals throughout the day, and greater carbohydrate and sugar intake, which may impact cardiometabolic health over time. Additionally, those with later DLMO appear to have a relationship between biological timing of feeding and body composition. Additional research is needed to explore the mechanisms between these relationships.
Acknowledgements:
We are grateful for Lisa Wolfe, MD and Hryar Attarian, MD for assistance in data collection in the clinical research unit. We also acknowledge the assistance of David Clough, Lori Koch, Tiffany Kim and Leland Bardsley for their assistance in data collection. Research reported in this publication was supported in part by the National Institutes of Health’s National Heart Lung and Blood Institute 1K23HL109110–01 (KGB), 1R01HL141706–01A1 (KGB) and the National Center for Advancing Translational Sciences, Grant Number UL1TR001422. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors declare no conflicts of interest.
References
- 1.Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of obesity among adults and youth: United States, 2011–2014. NCHS data briefs. 2015: 219, 1–8. [PubMed] [Google Scholar]
- 2.Baron KG, Reid KJ, Kern AS, Zee PC. Role of sleep timing in caloric intake and BMI. Obesity. 2011: 19, 1374–1381. [DOI] [PubMed] [Google Scholar]
- 3.Kant AK, Schatzkin A, Ballard-Barbash R Evening eating and subsequent long-term weight change in a national cohort. Int J Obesity. 1997: 21, 407. [DOI] [PubMed] [Google Scholar]
- 4.Kant AK, Ballard-Barbash R, Schatzkin A. Evening eating and its relation to self-reported body weight and nutrient intake in women, CSFII 1985–86. J Am Coll Nutr. 1995: 14, 358–363. [DOI] [PubMed] [Google Scholar]
- 5.Wang JB, Patterson RE, Ang A, Emond JA, Shetty N, Arab L. Timing of energy intake during the day is associated with the risk of obesity in adults. J Hum Nutr Diet. 2014: 27, 255–262. [DOI] [PubMed] [Google Scholar]
- 6.Baron KG, Reid KJ. Circadian misalignment and health. Int Rev Psychiatr. 2014: 26, 139–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McHill AW, Phillips AJ, Czeisler CA, Keating L, Yee K, Barger LK, Garaulet M, Scheer FA, Klerman EB. Later circadian timing of food intake is associated with increased body fat. Am J Clin Nutr. 2017:106, 1213–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baron KG, Reid KJ, Kim T, Van Horn L, Attarian H, Wolfe L, Siddique J, Santostasi G, Zee PC. Circadian timing and alignment in healthy adults: associations with BMI, body fat, caloric intake and physical activity. Int J Obesity. 2017: 41, 203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV axis I disorders research version (SCID-I). New York, NY: Biometrics Research, New York State Psychiatric Institute. 1996. [Google Scholar]
- 10.Voultsios A, Kennaway DJ, Dawson D. Salivary melatonin as a circadian phase marker: validation and comparison to plasma melatonin. J Biol Rhythms. 1997: 12, 457–466. [DOI] [PubMed] [Google Scholar]
- 11.Zitting KM, Vujovic N, Yuan RK, Isherwood CM, Medina JE, Wang W, Buxton OM, Williams JS, Czeisler CA, Duffy JF. Human resting energy expenditure varies with circadian phase. Curr Biol,. 2018: 28, 3685–3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morris CJ, Garcia JI, Myers S, Yang JN, Trienekens N, & Scheer FA (2015). The human circadian system has a dominating role in causing the morning/evening difference in diet-induced thermogenesis. Obesity, 23(10), 2053–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romon M, Edme JL, Boulenguez C, Lescroart JL, & Frimat P (1993). Circadian variation of diet-induced thermogenesis. The American journal of clinical nutrition, 57(4), 476–480. [DOI] [PubMed] [Google Scholar]
- 14.McHill AW, Melanson EL, Higgins J, Connick E, Moehlman TM, Stothard ER, & Wright KP (2014). Impact of circadian misalignment on energy metabolism during simulated nightshift work. Proceedings of the National Academy of Sciences, 111(48), 17302–17307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reid KJ, Baron KG, Zee PC. Meal timing influences daily caloric intake in healthy adults. Nutr Res. 2014: 34, 930–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin SK, & Eastman CI (2002). Sleep logs of young adults with self-selected sleep times predict the dim light melatonin onset. Chronobiology International, 19(4), 695–707. [DOI] [PubMed] [Google Scholar]
- 17.Garaulet M, Gómez-Abellán P, Alburquerque-Béjar JJ, Lee YC, Ordovás JM, Scheer FA. Timing of food intake predicts weight loss effectiveness. Int J Obesity. 2013: 37, 604–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gill S, Panda S. A smartphone app reveals erratic diurnal eating patterns in humans that can be modulated for health benefits. Cell Metab. 2015: 22, 789–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. New Engl J Med. 2011: 364, 2392–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang Q, Zhang Z, Gregg EW, Flanders WD, Merritt R, Hu FB. Added sugar intake and cardiovascular diseases mortality among US adults. JAMA Int Med, 2014, 174, 516–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu S, Willett WC, Stampfer M, Hu FB, Franz M, Sampson L … & Manson JE. A prospective study of dietary glycemic load, carbohydrate intake, and risk of coronary heart disease in US women–. The American journal of clinical nutrition. 2000: 71(6), 1455–1461. [DOI] [PubMed] [Google Scholar]
- 22.Makarem N, Paul J, Giardina EGV, Liao M, Aggarwal B. Evening chronotype is associated with poor cardiovascular health and adverse health behaviors in a diverse population of women. Chronobiology International. 2020: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Consensus Conference Panel, Watson NF, Badr MS, Belenky G, Bliwise DL, Buxton OM, … & Kushida C (2015). Joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society on the recommended amount of sleep for a healthy adult: methodology and discussion. Sleep, 38(8), 1161–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]


