Abstract
Introduction:
Although calcium pyrophosphate deposition (CPPD) disease is common, there are no validated outcome measures for clinical research in this condition. The aim of this study was to generate a list of outcome domains as reported by patients, their caregivers, healthcare professionals (HCPs) and stakeholders to inform the development of an Outcome Measures in Rheumatology (OMERACT) Core Domain Set for CPPD.
Methods:
Patients with CPPD and their caregivers, HCPs and stakeholders took part in semi-structured qualitative interviews to explore potential outcome domains for CPPD clinical research relevant to their lived experience and knowledge of CPPD. Interviews were conducted in six countries across three continents. Data was analysed using manifest content analysis to identify outcome domains, which were tabulated and mapped to the core areas as defined by the OMERACT Filter 2.1.
Results:
Thirty-six interviews were conducted in total. Participants comprised of 28 patients (six of which included a caregiver), seven HCPs and one stakeholder. The commonly identified (sub-) domains (d) across the [1] abnormalities/manifestations core area were joint pain (d=35), joint swelling (d=27), joint stiffness (d=25), CPPD flares (d=25); [2] life-impact core area were overall function (d=35), and specifically the ability to complete daily tasks (d=25); and [3] societal/resource use core area were use of analgesic medicines (d=26). Patients more commonly reported joint swelling, stiffness and range of movement, and use of analgesics while HCPs more commonly reported domains relating to presence of CPP crystals, radiologic calcification, joint damage, time to diagnosis and suitability of treatment.
Conclusion:
Among a number of potential outcome domains identified, articular manifestations, function and analgesic use were most frequently mentioned by participants. These findings will be used to develop an OMERACT Core Domain Set for CPPD.
Keywords: CPPD, outcome domains, content analysis
INTRODUCTION
Calcium pyrophosphate (CPP) deposition (CPPD) manifests as acute CPP crystal arthritis, chronic CPP crystal inflammatory arthritis, and osteoarthritis (OA) with CPPD, and is commonly detected as articular chondrocalcinosis on radiographs [1]. Although prevalent [2], CPPD is under-researched, with only a handful of clinical trials and no validated core outcome domains or measures [3]. For instance, a double-blind, placebo-controlled, randomised-controlled trial of methotrexate for CPPD used the 44-joint disease activity score (DAS-44) as the primary outcome measure [4]. This outcome measure was developed for rheumatoid arthritis (RA) and does not map to the clinical manifestations of CPPD that are typically characterised by a mix of chronic mechanical and, acute and chronic inflammatory symptoms. The lack of a core domain set is an important barrier to clinical research in CPPD.
A core set of outcome measures is needed to assess the benefits and harms of interventions, and to better understand the natural history of a disease in terms of disease progression and its impact on the individual. The first phase in developing core outcome measures is to identify and prioritise the core outcome domains taking account of the published research, and input from patients, healthcare professionals (HCPs) and other stakeholders [5, 6]. Working under the auspices of the Outcome Measures in Rheumatology (OMERACT) initiative framework [7] the OMERACT CPPD Working Group has completed a scoping review of published studies, which identified outcome domains used in prior research studies [8].
The aim of this study was to generate a list of outcome domains reported by patients and their caregivers, HCPs, and other stakeholders that would complement the outcome domains identified in the literature search and inform the development of a core set of outcome domains for future CPPD trials and observational research studies.
METHODS
Participants:
This study involved patients with a diagnosis of CPPD, their caregivers, HCPs and other stakeholders. Participants were identified and invited to participate in a semi-structured interview at one of the six sites across the world, including three continents (University of Auckland/Auckland District Health Board [New Zealand], Nottingham University Hospital NHS Trust [UK], Brigham and Women’s Hospital [USA], Hospital de la Santa Creu I Sant Pau [Spain], University of Ferrara [Italy], and Lille Catholic University [France]). Other stakeholders from the pharmaceutical industry and research funders were invited to participate based on the co-chairs’ knowledge of the field.
Patients were sampled using a maximum variation technique to ensure the various clinical presentations of CPPD were represented. Participants with different clinical presentations were recruited until sufficient numbers were reached that reflected approximately their proportions within the CPPD population. The clinical phenotypes of CPPD were defined according to the expert opinion of their rheumatologist, and comprised a) acute CPP crystal arthritis, b) chronic CPP crystal inflammatory arthritis, and c) CPPD + OA [1]. In addition, the rheumatologist also specified whether axial and/or appendicular joints were involved. As the manifestations of CPPD vary from one phenotype to another over time, and both appendicular and axial joints may be affected sequentially or simultaneously, it was possible to classify a patient to more than one group.
Ethical approval:
The study received ethical approval from West Midlands-Coventry and Warwick Research Ethics Committee, UK (19/WM/0264) and was registered on clinicaltrials.gov (NCT04176003). Ethical approval was also received to conduct interviews at the other five sites (Auckland Health Research Ethics Committee (000131) and Auckland District Health Board (A+8575), New Zealand; Partners HealthCare Institutional Review Board (2019P002136), USA; Universitat Autonòma de Barcelona (EC/19/266/5667), Spain; Comitato Etico Indipendente di Area Vasta Emilia Centro della Regione Emilia-Romanga (644/2019/Oss/AOUFe), Italy; and, Lille Catholic Hospitals Institutional Review Board (CIER2019-34), France).
Interview guides:
Three separate semi-structured interview guides were developed for patients and their caregivers, HCPs and stakeholders with involvement of the patient research partners in the OMERACT CPPD working group. All interview guides listed the outcome domains identified in scoping literature review and participants were asked to agree or disagree on which domains they perceived to be relevant. Additionally, patients and caregivers were asked about their lived experience of CPPD, and HCPs and stakeholders were asked about their experience of managing CPPD patients and views of patients lived experience, from which further outcome domains could be identified. The interview guides can be found in the supplementary material.
Interview procedure:
Participants were invited to take part in a one-to-one interview after giving written informed consent. Patients had the option to do this as a paired interview with their caregiver. Interviews were conducted face-to-face or by telephone in a private room by a researcher local to each site (AF, KC, CDT, GF, TP, and ST). The median (IQR) length of the interviews was 20 (16 – 28) minutes. All discussions were digitally audio-recorded.
Data analysis
Interviews were transcribed clean verbatim, translated into English where necessary and identifiers removed. Data were analysed using manifest content analysis to identify potential outcome domains and count how often they were present across the interviews [9]. Outcomes were coded deductively using the list of outcome domains identified in the scoping review [8], and inductively from the text. AF and KC independently coded 10% of transcripts and compared findings. Any differences were resolved by discussion between the two coders. A third coder (AA) was available to clarify any disagreements between the two coders, but this was not needed. After consensus was reached, AF coded the remaining transcripts. The third coder was consulted to discuss any additional potential domains identified in the remaining transcripts. All domains identified from the transcripts were then categorised according to one of four core areas: manifestations or abnormalities, life impact, longevity and societal or resource use, as defined by the OMERACT 2.1 Filter [10].
The frequency of each reported domain was tabulated by interviewee groups, to explore differences in domains reported by patients and their caregivers, and by HCPs and other stakeholders. Patient interviews were further subdivided according to their clinical presentation. As patients and their caregivers were interviewed at the same time, data for these were analysed together. Mean (standard deviation [SD]) were calculated for descriptive statistics.
RESULTS
In total, 36 interviews were conducted across the six sites, in three continents. The participants comprised of 28 patients (16 female) of which six included a caregiver, seven HCPs and one stakeholder from a non-profit arthritis-focused organisation. Seven additional stakeholders from pharmaceutical companies, research funders, hospital management and regulatory authorities were invited to participate. Of these, six declined without a reason or stated that their organisation was not interested in CPPD research currently and, one stakeholder reported no previous experience of CPPD.
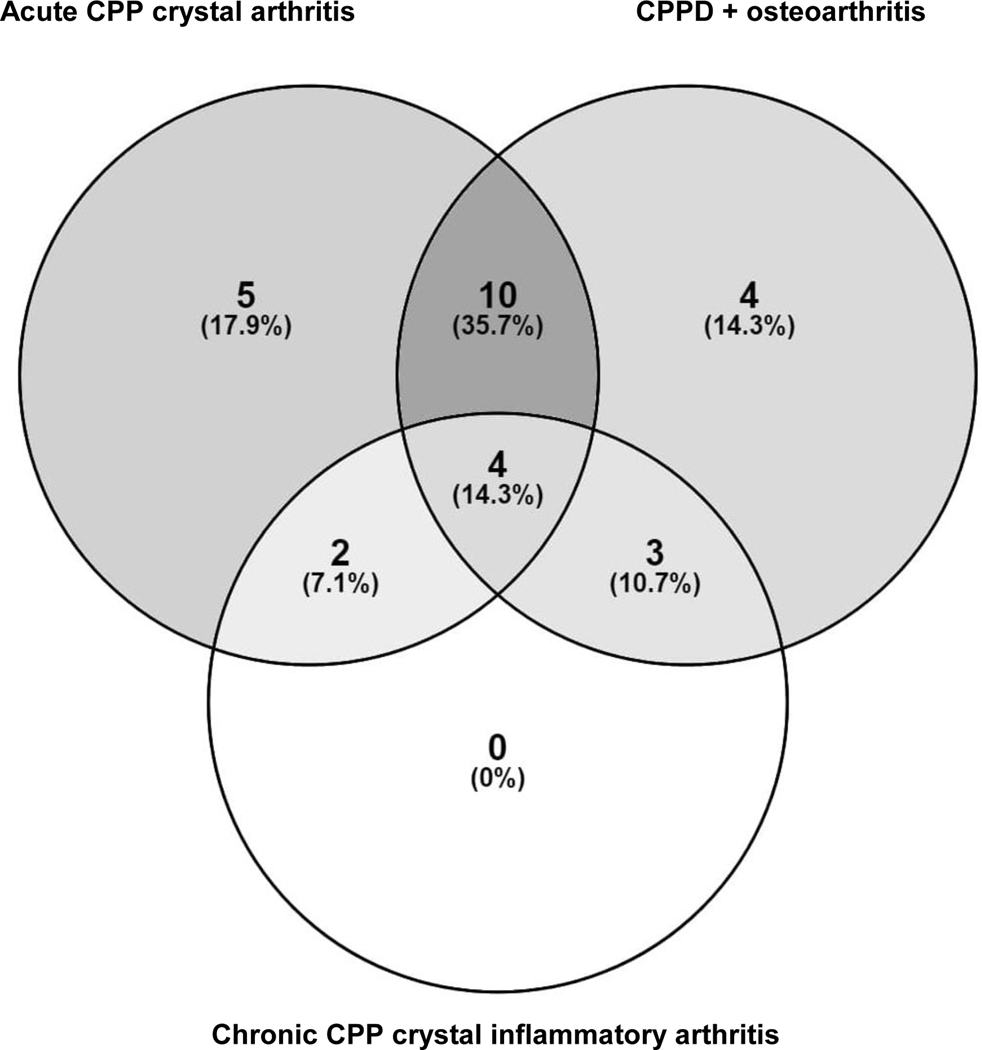
Patients’ mean (SD) age, and age at disease onset was 73.3 (9.8) and 67.5 (11.5) years respectively. They represented a range of CPPD manifestations, and several participants had more than one manifestation; 21 had episodes of acute CPP crystal arthritis, 9 had chronic CPP crystal inflammatory arthritis, and 21 had CPPD + OA (Figure 1). Twenty-six had appendicular and six had axial joint involvement. The HCPs included rheumatologists (four), general practitioners (two), and a rheumatology nurse. The other stakeholder was from an advocacy group for people with arthritis.
Figure 1.
Clinical presentations of patients with calcium pyrophosphate deposition (CPPD) included in the study, n (%)
Outcome domains identified across all interviews
Outcome items and statements that mapped to 44 domains (d) were identified. Table 1 shows the domains identified across the patient and caregiver, and HCPs and stakeholder interviews mapped to the core areas and domains of OMERACT Filter 2.1.
Table 1.
Summary of outcome domains (d) identified from the patient and caregiver (n=28), and healthcare professional and other stakeholder (n=8) interviews, mapped to core areas and domains of OMERACT Filter 2.1 [9]
| Concepts | Pathophysiology | Impact of health conditions | ||
|---|---|---|---|---|
| Core Areas | Abnormalities/Manifestations | Life Impact | Longevity | Societal/Resource Use |
| Outcomes domains |
Symptoms/Signs: Joint pain (28, 7) Joint swelling (23, 4) CPPD flares (19, 6) Joint stiffness (21, 4) Joint movement (19, 3) Fever(3, 1) Joint heat (7, 2) Joint redness (0, 1), Biomarkers - Imaging or Soluble: Inflammation in the blood or joint fluid (2, 4) Crystals in the joint fluid (8, 5) Joint damage on imaging tests (7, 2) Joint calcification on imaging tests (8, 5) Other manifestations: Related medical conditions such as osteoarthritis (14, 6) Side effects of treatment (9, 2) |
Impact of manifestations on: Overall function (27, 8): Ability to complete daily tasks (20, 5) Ability to do usual hobbies, leisure, exercise, and social activities (18, 3) Ability to work (9, 5) Adapting to physical ability (7,0) Balance (2, 0) Reliance on family members (11, 1) Quality of life (2, 5) Sleep quality (6,0) Emotional or psychological wellbeing (9, 2) Patients’ financial wellbeing (3, 2) |
Survival (0) Mortality (0) |
Healthcare utilisation: Use of analgesics (23, 3) Use of anti-inflammatory medicines: Colchicine (12, 4) NSAIDs (9, 4) Corticosteroids (12, 6) Use of immune-suppressive medicines: Anakinra (1, 1) Methotrexate (3,1) Leflunomide (0,1) Hydroxychloroquine (1, 0) Nutritional/visco-supplementation: Hyaluronic acid (1, 0) Folic acid (1, 0) Number of medications (7, 2) Number of treatments (3, 2) Need for joint surgery (6, 4) Duration of hospital stay (2, 2) |
| Response to treatment (7, 4) Satisfaction with treatment (6, 3) |
||||
(d, d): number of patient and caregiver interviews, number of healthcare professional and other stakeholder interviews; NSAIDs: non-steroidal anti-inflammatory drugs
Manifestations
Outcome domains within this core area were categorised into symptoms/signs, biomarkers, and other manifestations. Common domains (or subdomains) identified that mapped to symptoms/signs were joint pain (d=35), joint swelling (d=27), CPPD flares (d=25), joint stiffness (d=25) and joint movement (d=22). Outcome domains that mapped to biomarkers were joint calcification (d=13), joint damage (d=9), crystals in joint fluid (d=13) and inflammation in blood or joint fluid (d=6). Outcome domains that mapped to other manifestations were related medical conditions such as osteoarthritis (d=20) and side effects of treatments (d=11). Joint swelling, stiffness and movement were more often reported by patients, and domains mapping to biomarkers and related medical conditions were more often reported by HCPs (Table 1). The stakeholder also reported joint pain and stiffness, inflammation in blood or joint fluid and related medical conditions.
Life impact
Identified outcome domains that mapped to the life impact category were overall function (d=35), emotional and psychological wellbeing (d=11), quality of sleep (d=6) and financial wellbeing (d=5). More specific outcome domains that related to the overall function domain were identified. Common domains were ability to complete daily tasks (d=25); ability to do usual hobbies, leisure, exercise, and social activities (d=21); and ability to work (d=14). All participants, except one patient, reported one or more of these life impacts. Other outcome domains identified within life impact were response to treatment (d=11) and satisfaction with treatment (d=9). These were more commonly reported by HCPs (Table 1).
Longevity
No outcome domains were identified from the interviews relating to survival or mortality.
Societal or resource use
Outcome domains were categorised into healthcare utilisation and costs. Common domains identified in the healthcare utilisation category were the use of analgesics (d=26), corticosteroids (d=18), colchicine (d=16), non-steroidal anti-inflammatory drugs (NSAID) (d=13), and the need for joint surgery (d=10). Analgesic use was more often reported as a potential outcome domain by patients while HCPs more commonly reported time to diagnosis and the need for joint surgery (Table 1). The other stakeholder reported NSAID and corticosteroid use, and the need for joint surgery as relevant outcome domains. Under costs, direct and indirect costs were identified as relevant by one patient.
There were broad similarities in the pattern of outcome domains identified across the three main phenotypes of CPPD, with joint pain, overall function and analgesic use as the most commonly identified domains in manifestations, life impact and societal or resource use areas (Tables S1-S3). However, as expected patients with acute CPP crystal arthritis more often reported CPPD flares than those with chronic CPP crystal arthritis or CPPD + OA.
DISCUSSION
This is the first study to report on the potential outcome domains for CPPD encompassing perspectives of patients, caregivers, HCPs and other stakeholders. The most commonly reported outcome domains by patients and their caregivers, HCPs and the other stakeholder were joint pain, joint swelling, movement and stiffness, CPPD flares, joint calcification, inflammation in blood or synovial fluid, function and medication use. Pain and overall function were the most often reported outcome domains, each reported by all bar one of the participants. Similar outcome domains were identified by people with different CPPD phenotypes.
While the outcome domains were broadly comparable across the patient and caregiver, and HCP and other stakeholder interviews, there were some differences in opinion between them. For instance, people with CPPD more commonly identified joint swelling, stiffness, range of movement, and the use of analgesic medicines or polypharmacy as important outcome domains while HCPs more commonly identified calcification, joint damage and levels of inflammation as well as treatments such as corticosteroids. This difference relates to the fact that people with CPPD reflect on their day-to-day experience of living with CPPD while HCPs build on their experience of managing CPPD. Similar differences in outcome domains identified by patients and HCPs have been observed for other diseases [11].
CPPD flares were more often identified as an outcome domain by patients with acute CPP crystal arthritis than people with other clinical presentation of CPPD. However, other outcome domains were reported by comparable proportions of people with different clinical manifestations of CPPD. The lack of any other discernible difference in the outcome domains reported by patients with different CPPD phenotypes can be explained by the fact the majority of patients were classified into more than one group based on their disease manifestations. We could recruit only five patients with isolated acute CPP crystal arthritis as the sole manifestation of CPPD. This is not surprising as most people with flares of acute CPP crystal arthritis also have OA. However, in addition to differences in CPPD flares as an outcome domain across different clinical presentations of CPPD, manifestations and biomarkers of both acute and chronic arthritis were identified as potential outcome domains in this study suggesting that different core domain sets may need to be drawn up for acute (e.g. acute CPP crystal arthritis) and chronic manifestations (e.g. chronic CPP crystal inflammatory arthritis or CPPD + OA) of CPPD. Such a strategy has been adopted in RA, with different outcome domains for RA and RA flare [12, 13].
The potential outcome domains identified in our study are broadly similar to the outcome domains endorsed by OMERACT for other conditions such as gout, RA and OA [12–16]. Of interest, neither the patients nor the HCPs identified patient or physician global assessment as outcome domains in our study. Flares of disease were identified as an outcome domain. However, this is likely to be a composite domain and further research is needed to identify the outcome domains for CPPD flares. Such a study may examine in more detail the experience of participants when they are experiencing a flare of acute CPP crystal inflammatory arthritis. Given the older age of our sample, and the association between OA and CPPD, OA was commonly reported as a comorbidity of CPPD.
Our recently published scoping review to identify outcome domains used in previous studies on CPPD included 112 published articles [8]. The most commonly reported outcome domains were imaging manifestations of joint damage (59 studies) and joint calcification (28 studies), followed by joint pain (26 studies) and response to treatment (23 studies). The findings of our current study suggest that imaging manifestations and response to treatment are less important for people with CPPD, who more often identify joint pain, swelling, stiffness and movement, and CPPD flares. Joint pain was identified as an outcome domain in just under a quarter of previous CPPD articles, while joint swelling, stiffness and movement, and CPPD flares were outcomes in 5 – 10% of these articles. That many participants report joint symptoms demonstrates the need to include such outcome domains in a core domain set for CPPD. In addition, domains relating to life impact and healthcare utilisation have also been reported less often in previous studies, whereas many participants reported outcomes within these core areas, highlighting too their importance in a future CPPD Core Domain Set. We also identified domains relating more specifically to the nature of CPPD’s impact, such as on patients’ ability to carry out usual daily activities, exercise, socialise or work, as well as other life impacts such as emotional wellbeing. Given the limited data available so far on the impact of this disease on function, quality of life, participation and productivity [8], the findings of this study further highlight the need for future studies exploring the impact of CPPD on patients’ lives. Similar to other studies seeking to define outcome domains for rheumatic disorders [12], soliciting patient perspectives identified domains that are important to measure in CPPD trials but have not previously been considered.
Although no outcome domains were identified from the interviews relating to the core area of longevity, and the scoping review only identified two studies with outcomes mapped to survival and none for mortality [8], as the latter is a mandatory domain for OMERACT [10] it will be added to the core set automatically.
Strengths of this study include the inclusion of patients that represent the entire spectrum of CPPD clinical presentations and from multiple countries across Europe, North America and Australasia. This increases the generalisability of our findings. Patients with the disease, caregivers, HCPs, and other stakeholders who advocate on their behalf provide unique insights about the symptoms and impact of a disease, and the priorities and concerns that need to be addressed. Including them in the process of identifying outcome domains ensures that the final core outcome domain set is relevant and meaningful. Limitations of the study include absence of people with isolated chronic CPP crystal inflammatory arthritis, and that only one stakeholder took part. Despite its high prevalence, CPPD is not currently on the research and development agenda for most pharmaceutical companies, nor the advocacy agenda for the national arthritis organisations. Consequently, we were unable to solicit interest in this study from a number of pharmaceutical stakeholders and arthritis-focused organisations including non-profit groups and governmental agencies. Finally, as the aim of this study was to simply identify potential outcome domains, it is not possible from the findings to derive any statistical inferences to compare differences in reported outcomes between participant groups, or rank their relative importance, but they provide an indication of outcome domains to be considered as the OMERACT CPPD Core Domain Set is developed.
In conclusion, a number of potential outcome domains related to CPPD were identified, the most often reported were domains relating to articular manifestations, function and analgesic use. These findings will be used to inform the development of the OMERACT Core Domain Set for CPPD.
Supplementary Material
Highlights.
A number of outcome domains that are important to measure in CPPD trials, relevant to the patient experience but not previously considered, were identified
Most commonly reported domains related to articular manifestations, function and analgesic use
No discernible differences in domains reported by patients with different CPPD phenotypes
Acknowledgments
Funding details: This research was funded by the NIHR Nottingham Biomedical Research Centre. Dr Tedeschi is supported by NIH-NIAMS (K23 AR075070, L30 AR070514). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care. Prof. R. Christensen reports that the Musculoskeletal Statistics Unit, the Parker Institute, Bispebjerg and Frederiksberg Hospital is supported by a core grant from the Oak Foundation (OCAY-18-774-OFIL).
KC reports a research fellowship grant from Arthritis Australia. RC is a member of the Technical Advisory Group of OMERACT, an organization that develops outcome measures in rheumatology and receives arms-length funding from 12 companies. JAS has received consultant fees from Crealta/Horizon, Medisys, Fidia, UBM LLC, Trio health, Medscape, WebMD, Clinical Care options, Clearview healthcare partners, Putnam associates, Focus forward, Navigant consulting, Spherix, Practice Point communications, the National Institutes of Health and the American College of Rheumatology. JAS owns stock options in Amarin pharmaceuticals and Viking therapeutics. JAS is on the speaker’s bureau of Simply Speaking. JAS is a member of the executive of OMERACT, an organization that develops outcome measures in rheumatology and receives arms-length funding from 12 companies. JAS serves on the FDA Arthritis Advisory Committee. JAS is the chair of the Veterans Affairs Rheumatology Field Advisory Committee. JAS is the editor and the Director of the UAB Cochrane Musculoskeletal Group Satellite Center on Network Meta-analysis. JAS previously served as a member of the following committees: member, the American College of Rheumatology’s (ACR) Annual Meeting Planning Committee (AMPC) and Quality of Care Committees, the Chair of the ACR Meet-the-Professor, Workshop and Study Group Subcommittee and the co-chair of the ACR Criteria and Response Criteria subcommittee.
Footnotes
Conflict of interest statement: AF, CDT, GF, TP, OH, DG, BS, SKT, ND and AA have no disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zhang W, et al. , European League Against Rheumatism recommendations for calcium pyrophosphate deposition. Part I: terminology and diagnosis. Ann Rheum Dis, 2011. 70(4): p. 563–70. [DOI] [PubMed] [Google Scholar]
- 2.Abhishek A, Calcium pyrophosphate deposition disease: a review of epidemiologic findings. Curr Opin Rheumatol, 2016. 28(2): p. 133–9. [DOI] [PubMed] [Google Scholar]
- 3.Abhishek A, et al. , Review: Unmet Needs and the Path Forward in Joint Disease Associated With Calcium Pyrophosphate Crystal Deposition. Arthritis Rheumatol, 2018. 70(8): p. 1182–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finckh A, et al. , Methotrexate in chronic-recurrent calcium pyrophosphate deposition disease: no significant effect in a randomized crossover trial. Arthritis Res Ther, 2014. 16(5): p. 458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maxwell LJ, et al. , Core Domain Set Selection According to OMERACT Filter 2.1: The OMERACT Methodology. The Journal of Rheumatology, 2019. 46(8): p. 1014–1020. [DOI] [PubMed] [Google Scholar]
- 6.Boers M, et al. OMERACT Handbook Chapter 4: Developing Core Domain Sets. Available from: https://omeracthandbook.org/handbook (Accessed online 23 June 2020).
- 7.Boers M, et al. , Developing core outcome measurement sets for clinical trials: OMERACT filter 2.0. J Clin Epidemiol, 2014. 67(7): p. 745–53. [DOI] [PubMed] [Google Scholar]
- 8.Cai K, et al. , Outcome Domains reported in Calcium Pyrophosphate Deposition Studies: A Scoping Review by the OMERACT CPPD Working Group. Seminars in Arthritis and Rheumatism, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kondracki NL, Wellman NS, and Amundson DR, Content analysis: review of methods and their applications in nutrition education. J Nutr Educ Behav, 2002. 34(4): p. 224–30. [DOI] [PubMed] [Google Scholar]
- 10.Boers M, et al. , OMERACT Filter 2.1: Elaboration of the Conceptual Framework for Outcome Measurement in Health Intervention Studies. The Journal of Rheumatology, 2019. 46(8): p. 1021–1027. [DOI] [PubMed] [Google Scholar]
- 11.Mecoli CA, et al. , Perceptions of Patients, Caregivers, and Healthcare Providers of Idiopathic Inflammatory Myopathies: An International OMERACT Study. J Rheumatol, 2019. 46(1): p. 106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bykerk VP, et al. , Establishing a core domain set to measure rheumatoid arthritis flares: report of the OMERACT 11 RA flare Workshop. J Rheumatol, 2014. 41(4): p. 799–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boers M, et al. , World Health Organization and International League of Associations for Rheumatology core endpoints for symptom modifying antirheumatic drugs in rheumatoid arthritis clinical trials. J Rheumatol Suppl, 1994. 41: p. 86–9. [PubMed] [Google Scholar]
- 14.Schumacher HR, et al. , Outcome domains for studies of acute and chronic gout. J Rheumatol, 2009. 36(10): p. 2342–5. [DOI] [PubMed] [Google Scholar]
- 15.Singh JA, et al. , OMERACT endorsement of measures of outcome for studies of acute gout. J Rheumatol, 2014. 41(3): p. 569–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith TO, et al. , The OMERACT-OARSI Core Domain Set for Measurement in Clinical Trials of Hip and/or Knee Osteoarthritis. J Rheumatol, 2019. 46(8): p. 981–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.