Abstract
The extracellular matrix (ECM) is a water-swollen, tissue-specific material environment in which biophysiochemical signals are organized and influence cell behaviors. Electrospun nanofibrous substrates have been pursued as platforms for tissue engineering and cell studies that recapitulate features of the native ECM, in particular its fibrous nature. In recent years, progress in the design of electrospun hydrogel systems has demonstrated that molecular design also enables unique studies of cellular behaviors. In comparison to the use of hydrophobic polymeric materials, electrospinning hydrophilic materials that crosslink to form hydrogels offer the potential to achieve the water-swollen, nanofibrous characteristics of endogenous ECM. Although electrospun hydrogels require an additional crosslinking step to stabilize the fibers (allowing fibers to swell with water instead of dissolving) in comparison to their hydrophobic counterparts, researchers have made significant advances in leveraging hydrogel chemistries to incorporate biochemical and dynamic functionalities within the fibers. Consequently, dynamic biophysical and biochemical properties can be engineered into hydrophilic nanofibers that would be difficult to engineer in hydrophobic systems without strategic and sometimes intensive post-processing techniques. This Review describes common methodologies to control biophysical and biochemical properties of both electrospun hydrophobic and hydrogel nanofibers, with an emphasis on highlighting recent progress using hydrogel nanofibers with engineered dynamic complexities to develop culture systems for the study of biological function, dysfunction, development, and regeneration.
Graphical Abstract
Hydrogel nanofibers build on established soft biomaterials to enable design and control of unique, dynamic cell culture systems.
1. Introduction
The extracellular matrix (ECM) is a complex, dynamic, and tissue-specific scaffolding system that presents a myriad of biophysical and biochemical cues that influence cellular behaviors1–4. The ECM is typically comprised of varying compositions of fibrous proteins and proteoglycans, coupled with soluble components such as growth factors5–7; however, the state of this structure is constantly in flux as it is simultaneously degraded and synthesized by the resident cellular population4–8. As the biophysical and biochemical attributes of the ECM at two distinct junctures are never identical, recapitulating tissue-specific milieus in vitro is challenging5–7. To better understand cellular behaviors and processes occurring in physiologically-relevant systems, in vitro culture systems must continue to advance to accurately model the ECM4,6,9–11.
Progress in developing more sophisticated in vitro culture platforms has advanced with new insights into the composition and properties of the ECM coupled with new technical capabilities to recreate its features. The heterogeneous material environment of the ECM is water-rich and nanofibrous in nature1,4,12, typically comprised of single-fiber diameters on the order of tens to hundreds of nanometers (10–500 nm)12–16. Electrospinning is an accessible technique for depositing fibrous substrates with diameters analogous to those comprising native ECM5–7, and has been established as an effective way to produce nanofibrous materials across many fields of research17–21, including tissue engineering22,23. Within tissue engineering and regenerative medicine, electrospun nanofibers have been applied to wound healing24 and the engineering of diverse tissue types including models of cardiac25, vascular26, neural27,28, and musculoskeletal29 environments. In research applications addressing fundamental biological and physiological questions, electrospun substrates have also been tactically engineered to tease out cellular responses to differing environmental cues and perturbations for in vitro studies2,3,30–32. For more information, Xue et al.33 and Rahmati et al.34 have recently published expansive reviews of the electrospinning process and extensive applications of electrospun materials.
Turning the focus from the process and applications onto the materials themselves, electrospun fibers utilized in tissue engineering applications throughout the years have been primarily comprised of hydrophobic polymers that were solubilized in organic solvents prior to electrospinning (Figure 1). These materials were prevalent in the early waves of electrospinning due to their favorable performance in the electrospinning process and their ability to form fibrous substrates for cell culture without further stabilization steps, such as interpolymer crosslinking12,35. A disadvantage of utilizing many of these hydrophobic polymers is they may lack desired cell-instructive biofunctionality in their fibrous form, and consequently require strategic chemistries to increase the bioactivity prior to seeding cells for culture36,37. Furthermore, since these materials are foreign to physiological systems, it may be necessary to engineer them further to mediate biological responses in vivo during transplantation and degradation. There are many established methods to modify the surfaces of these hydrophobic nanofibers36,37; however, a current shift towards using crosslinked polymers to develop hydrogel networks offers potential to reduce the complexity of post-processing (refer to Figure 1) by drawing on the diversity of hydrogel functionalities available for modifying and controlling microenvironmental features and establishing dynamic materials38.
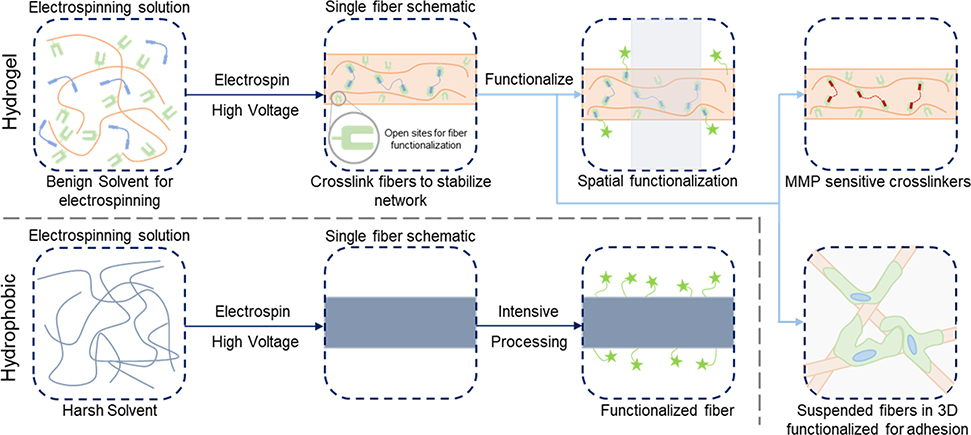
Figure 1. Functionalization of hydrogel versus hydrophobic nanofibers.
(Top, left to right): electrospinning precursor solution containing a hydrophilic polymer with a crosslinker to stabilize hydrogel nanofibers; solution is electrospun and crosslinked (e.g. with UV irradiation) with leftover sites for further functionalization; three example pathways to functionalize the fibers – spatial control over bioactivity (green stars, shaded area indicates unfunctionalized region)86, fibers crosslinked with matrix metalloproteinase (MMP) sensitive crosslinkers for tunable degradation8, suspended hydrogel fibers in a bulk gel for 3D models of the ECM123. (Bottom, left to right): electrospinning precursor solution containing hydrophobic polymer (typically in a harsh solvent); solution is electrospun and fibers are ready for processing; intensive chemical processing is typically needed for fiber functionalization.
Another advantage offered by electrospun hydrogel fibers compared to their hydrophobic material analogs is the water-swollen nature of native ECM and of natural fibers within ECM microenvironments1,4,12. Furthermore, the plethora of established chemistries used to modify polymeric backbones and engineer crosslinking in hydrogel fiber systems enables the facile development of functionality for controlling the biophysiochemical properties to recapitulate features of the endogenous ECM1,39–41. Hydrogel systems for cell culture were originally introduced as advancements from tissue culture polystyrene1, and as soon as they were developed for cell culture, researchers aimed to advance the technology towards dynamic culture systems4,38. Electrospun fibers are mirroring this progression first through the development of hydrogel fibers, and now in trends towards dynamic fibrous environments that allow for modeling and probing of biological processes, while also affording control over the complexity of culture systems to reconstitute natural tissue as closely as possible. Significant progress in the engineering of fibrous culture substrates has been made, with the potential for further developments in materials design to continue to advance towards recapitulating endogenous tissue42.
This Review focuses on the methods developed to modify the biophysical and biochemical properties of electrospun polymers – both hydrophobic and hydrophilic – with an emphasis on the strengths provided by crosslinkable, hydrophilic polymers that form hydrogels. We further focus on the chemistries developed to modify hydrogel nanofibers to manipulate the complexity of biological systems in space and time, while additionally highlighting the advancements being made by researchers towards the development of dynamic scaffolding that effectively reconstitutes physiologically-relevant ECM. Furthermore, we also provide light commentary highlighting the advantages and associated challenges within these systems to ideally inform the next phase of advancements in nanofibrillar hydrogel design.
2. Hydrophobic polymer fibers for cell culture
The use of hydrophobic polymers has been central to the development of fibrous culture systems43, and materials commonly used include polylactic acid (PLA)44–47, poly(lactic-co-glycolic acid) (PLGA)48, polycaprolactone (PCL)49, polyethylene terephthalate (PET)50, among many others51,52. Since these materials are characteristically hydrophobic, they require nonpolar organic solvents to facilitate the electrospinning process25,51,53,54. Therefore – in biomedical applications – water infiltration is limited to spaces between fibers, without substantially absorbing into the polymeric matrices of the fibers themselves51. Despite this challenge, these materials are well-suited to the electrospinning process and have seen extensive use in the tissue engineering space. Part of the strength of these materials in electrospinning is that the morphological features of the resulting nanofibers can be readily tailored by simply controlling process parameters12,54,55, yielding substrates with designed topographical characteristics that contribute to the biophysical properties that cells transduce. Similarly, post-electrospinning techniques have been employed to increase the bioactivity of the fibrous substrates. Since cells are heavily influenced by a combination of both biophysical and biochemical signals in their microenvironment6,7, techniques have continuously progressed to introduce relevant signals to nanofibers based on these hydrophobic materials in order to influence the cells interacting with them.
2.1. Hydrophobic nanofibers enabling control over physical properties
Work aiming to engineer and alter nanofibrous topographies is driven by cellular transduction of biophysical stimuli from their microenvironments to influence signaling pathways that direct downstream phenotypic fate decisions56. Therefore, control over physical properties of culture systems is a critical consideration in biomedical applications including tissue engineering, regenerative medicine, and fundamental investigations into cellular processes and development. The diameters of electrospun fibers can be readily controlled through solution properties and variable parameters of the electrospinning process – in particular solution viscosity, polymer molecular weight, applied voltage, and solution flow rate55,57,58. Even with this level of control, careful consideration is needed when developing fibers to match the tissue system of interest. For instance, Young’s modulus of electrospun fibers exhibits an inverse relationship with fiber diameter59; therefore, a balance is typically needed when engineering models that replicate tissue-specific systems in the body60.
Treatments for modulating fiber topography.
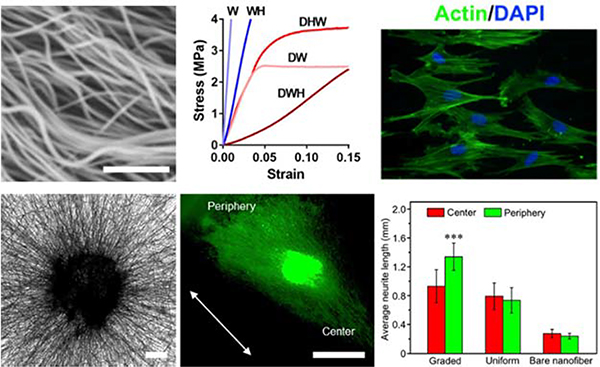
Hydrophobic polymeric fibers are relatively robust, which allows for diverse processing techniques to further control physical and topographical properties. For example, towards engineering topography to influence cell shape and localization through contact guidance, Park and coworkers demonstrated the ability to spatially control the deposition and alignment of PLA nanofibers on polymer surfaces61. The hydrophobicity of PLA was leveraged during the electrospinning process and an electrolyte solution of potassium chloride on the collection surface was utilized to focus the electric field during fiber collection – a process that wouldn’t be possible with hydrophilic polymers61. Moreover, from a post-processing perspective, Szczesny et al. heated poly(L-lactic acid) (PLLA) fibers to 85° C to induce contraction, yielding crimped fibrous substrates that recapitulated the crimped nature of tendinous tissue62. Further mechanical testing showed that the crimped fibers provided a nonlinear stress-strain regime, which mirrors that seen with natural tendon tissue upon initial mechanical loading62 (refer to Figure 2 Top). Towards a similar end, Chen et al. leveraged thermally-responsive materials that shrink upon the addition of heat to crimp fibers63. The waviness in the resultant fibers improved cellular infiltration into the scaffolds, and also promoted transcriptional growth factor-β (TGF-β) expression from human mesenchymal stromal cells (hMSCs) – an important regulator in the development of connective tissue63. While brief, these examples highlight the great potential hydrophobic fibers have to be tailored through modifications to the process, through post-processing, or through leveraging material properties such as thermal-responsiveness, to replicate natural tissue in vitro.
Figure 2. Cell culture on modified hydrophobic fibrous scaffolds.
(Top, left to right): Crimped PLLA fibers synthesized via heat treatment with sacrificial fibers by Szczesny et al.62 to develop a tendinous/ligament-like tissue structure; the crimped system (DWH) exhibited a traditional non-linear stress-strain curve similar to that of native tendon/ligament tissue, whereas controls (W, WH, DHW, DW) all were unable to replicate this behavior; actin/DAPI staining of cells seeded on these crimped systems demonstrated less alignment with the fibers and reoriented significantly upon mechanical strain. Scalebar = 1 μm. (Top) Reprinted and adapted with permission from Szczesny et al., copyright 2017 American Chemical Society62. (Bottom, left to right): PCL fibers aligned radially due to a novel electrospinning collection setup, scalebar = 200 μm; Tuj-1 staining (green) of dorsal root ganglion cells shows significant neurite extension in the direction of fiber alignment (white arrow) and laminin gradient; quantification displaying average neurite length for the gradient experiments compared to controls of uniform laminin presentation and no laminin presentation. Scalebar = 1mm, ***p < 0.001. (Bottom) Reprinted and adapted with permission from Wu et al., copyright 2018 American Chemical Society76.
2.2. Hydrophobic fibers enabling modulation of biochemical properties
Pre-incubation (non-covalent) modifications.
In addition to responding to biophysical cues in cell fate decisions, cells also integrate biochemical cues from their local microenvironment7,39,64–66. Therefore, chemically modifying hydrophobic fibers that are otherwise inherently bioinert with relevant biomolecules is critical to influencing phenotypic outcomes 36,37. There is a plethora of studies expanding upon methods for introducing these biochemical cues into fibrous culture systems – many of which include some variation of a chemical coating as a preliminary step. For example, nonspecific adsorption of biomolecules on fibers, such as ECM-derived laminin27,28 and compounds contained within endothelial cell basal medium-249, supported neural and endothelial cell adhesion, respectively. Extending this pre-incubation one step further, Kador et al. adsorbed laminin and fibronectin onto PLA scaffolds and covalently bound Netrin-1 protein using carbodiimide (EDC/NHS) crosslinking between the carboxylic acids on laminin/fibronectin and the amines on Netrin-167. Kador and coworkers also demonstrated efficacy in conjugating Netrin-1 to the laminin/fibronectin on fibers utilizing a photo-based succinimidyl-diazirine (SDA) crosslinker67. The immobilization of Netrin-1 on these fibrous scaffolds resulted in increased polarity of retinal ganglion cells when compared to the non-functionalized controls67.
Polydopamine-based modifications.
Other methods aiming to improve the biofunctionality of fibrous substrates include a preliminary step of introducing reactive chemical functionalities to fiber surfaces. Similar to the aforementioned adsorption pathways, polydopamine surface coatings, naturally inspired by the adhesiveness of mussels, allow for the presentation of catechol/quinone groups on fibers68. These groups can then freely react with thiols and amines of biomolecules – such as bone morphogenetic protein-269, laminin70, or Arg-Gly-Asp (RGD) peptide motifs71 – undergoing either Schiff-base reactions or Michael additions72,73.
High-energy surface treatments.
High-energy surface treatments can also be used to introduce bioactivity. For example, Savoji and coworkers utilized plasma-polymerization to introduce a thin coating on PET nanofibers that presented reactive amine groups, which in turn supported the adhesion and subsequent proliferation of human umbilical vein endothelial cells50. In addition, Piai et al. treated PLA fibers with UV/ozone to introduce reactive oxygen groups prior to aminolysis via incubation in 1,6-hexamethylenediamine45. Chondroitin sulfate was then conjugated to the reactive amines on the PLA fibers by the aforementioned carbodiimide (EDC/NHS) crosslinking45. Plasma treatment has also been used in conjunction with the previously discussed polydopamine chemistry to graft another glycosaminoglycan, in this case heparin, onto polycarbonate-urethane grafts to improve bioactivity in vivo74. Moreover, Tanes et al.75 and Wu et al.76 both demonstrated the ability to introduce gradients of nerve growth factor (NGF)75 and epidermal growth factor (EGF)76 on PCL nanofibers using bovine serum albumin (BSA) as a bioinert blocking agent. Both methods utilized oxygen plasma to functionalize the surface, prior to the sequential introduction of BSA to block open sites, then either NGF/EGF was conjugated to fibers to confer bioactivity. In the presence of both an NGF gradient and aligned fibers, dorsal root ganglion cells exhibited a preferential alignment as well as increased average length of extended neurites 75 (refer to Figure 2 Bottom).
Click chemistries for biochemical modifications.
Click chemistries have been explored to functionalize hydrophobic fibers with biochemical cues. Reactions that have been successfully used for controlled presentation of biomolecules include copper-catalyzed azide-alkyne cycloaddition (CuAAC) and sans metal strain-promoted azide-alkyne cycloaddition (SPAAC)37. As their names reflect, CuAAC reactions require the presentation of alkynes and azides for conjugation77, whereas SPAAC reactions require the presentation of strained alkynes and azides for conjugation but proceed in the absence of a copper catalyst78. Examples include the functionalization of PLA with an alkyne by Shi et al. to facilitate conjugation of an azide-presenting enzyme onto fibers through CuAAC chemistry79. Examples of SPAAC reactions with nanofibers include works by Smith Callahan et al.80 and Zheng et al.81 where PLLA and PCL were functionalized with 4-dibenzo-cyclooctynol (DIBO) to provide reactive sites for conjugation of azide-containing molecules. In these works, both cell-adhesive peptides and fluorophores were conjugated to the DIBO-containing nanofibers. We refer to an excellent review by Kalaoglu-Altan et al. regarding ‘clickable’ electrospun fibers for further information on the use of bioorthogonal chemistries to modify nanofibers37.
Summary – controlling hydrophobic nanofiber biochemical properties.
Nanofibers based on hydrophobic materials have thus far been central to the development of biomedical electrospun materials and have demonstrated the progress of research in this area – becoming increasingly sophisticated, bioactive platforms with great potential in regenerative medicine. Nonetheless, these systems face certain challenges in biomedical applications that are inherent to the materials used, and can be addressed through the use of hydrogel material systems. A minor concern exists in the use of cytotoxic solvents during electrospinning to dissolve hydrophobic polymers82. Although the potential to leave behind residual solvent is addressed in work with these materials, water-soluble hydrogel materials that are electrospun from aqueous solutions do not face this challenge. More significant are challenges related to advancing the biomimetic and dynamic features of electrospun fibrous systems. For example, with respect to controlling the biophysical properties of nanofibrous environments, hydrophobic systems largely afford minimal direct control over the stiffness and viscoelasticity of the resultant fibers beyond modifying solution properties prior to electrospinning. Additionally, spatial control over the localization of biomolecules in these hydrophobic nanofibrous systems has been demonstrated through the aforementioned techniques to introduce gradients of growth factors75,76, but achieving complex spatiotemporal control over biochemical and biophysical features of a fibrous system remains challenging. Progressing towards polymers used in hydrogels offers a library of existent chemistries along with continual research to advance technology and address many of these concerns38,40,41 (Figure 1). This offers great potential to expand the possibilities within nanofibrous systems and to combine the strengths of hydrogel materials and nanofibers in engineering biomimetic environments38,40,41.
3. Hydrogel nanofibers
The opportunities for increased control over the biophysical properties and spatiotemporal presentation of biochemical functionality has been a driving factor in the progression towards electrospun hydrogel fibers. Hydrogel fibers build on the strengths of hydrogel materials that can be chemically modified with functional moieties – for both crosslinking and introducing biomolecules1,65. These strengths allow for the precise tailoring of mechanical and chemical properties to replicate the tissue system of choice1,38. Thus, hydrogel nanofibers offer not only the potential for superior control over fiber properties compared to their hydrophobic analogs83, but the fibers also have the potential to provide a microenvironment that more closely mirrors the water-swollen, fibrous characteristics of natural tissue13–15.
Fabrication of hydrogel nanofibers.
Hydrogel nanofibers are produced via electrospinning similarly to other variants of polymeric nanofibers. Commonly, the solution consists of the hydrophilic polymer of choice (e.g. hyaluronic acid (HA), poly(ethylene glycol) (PEG), or dextran), a crosslinker (for systems that require a linker molecule), a photoinitiator (for photomediated reactions), and water as a solvent2,84,85. For lower molecular weight polymers, like HA and PEG, a high molecular weight polymer, typically poly(ethylene oxide), is added to increase solution viscosity and induce chain entanglements32,84–86. For higher molecular weight polymers, like dextran, this is not typically needed2,31,87. This solution is then typically extruded though a needle at low flow rates, at the point of which an electric field is applied to the solution. This induces a competing interaction between polymer chain entanglements within the solution and electrostatic repulsion from the voltage – which due to solution extrusion, elongates into a Taylor cone. At the point of the Taylor cone, the solution vaporizes, which causes a polymeric fiber jet to form that whips and accelerates towards the grounded collection surface12,58. Following the deposition of the fibers, they must then be stabilized through some variation of crosslinking (to be described in depth-below) in order to facilitate water absorption into the polymeric networks as opposed to fibers solubilizing upon hydration2,84–86. Crosslinking also enables control over biophysical properties of hydrogel fibers, with degree of crosslinking directly affecting fiber parameters such as stiffness and diameter – which correlate with capacity for water swelling into the fibers86,88. Once crosslinking is complete, facile functionalization of fibers is possible to introduce bioactivity into the fibrous hydrogel system.
Introduction to hydrogel nanofiber crosslinking and stabilization.
One specific suite of hydrogel-forming materials represents natural polymers due in part to their innate biocompatibility and presentation of relevant ligands89,90. For example, collagen inherently presents bioactive sites for integrin-mediated cell adhesion12. However, other polymers can also intrinsically interact with cells – such as hyaluronic acid (HA) (typically produced through fermentation processes1) with CD4491–93. That being said, cells tend to exhibit low adhesion to HA without chemical modifications to improve bioactivity86. Therefore, HA, as well as other polysaccharide materials such as dextran2, need to be functionalized with bioactive molecules prior to being utilized for cell culture systems. It is also worth including other hydrophilic polymers in this category such as the synthetic polymer poly(ethylene glycol) (PEG)94. There are a whole host of established chemistries to modify the backbones of these exemplified hydrophilic polymers with pendant functional moieties, with these moieties doubling as both crosslinking sites and biomolecule conjugation sites. Therefore, strategic modification of these polymers thereby provides significant user control over the resultant biophysical and biochemical characteristics of the nanofibers.
Unlike hydrophobic materials, as discussed previously, polymeric materials used in hydrogels are soluble in water and fibers generated by electrospinning will dissolve upon hydration without stabilization. Thus, hydrogel-based systems must generally be stabilized through some form of intermolecular crosslinking between the polymers that comprise the nanofibers. In many cases, regulation of crosslinking enables control over physical properties, as will be discussed at greater length in the next section. Naturally-derived polymers such as collagen95 and gelatin96, for example, can be electrospun; however, though the native materials undergo physical crosslinking, the resultant nanofibers themselves typically are not robust enough for handling without further post-processing95,96. To circumvent this, crosslinking agents, like glutaraldehyde, have been utilized with collagen and gelatin to improve resultant mechanical properties95–99. Furthermore, Kishan et al. developed a platform for electrospinning gelatin that crosslinks on-the-fly using a diisocyanate crosslinker to retain fiber mechanical properties100. Another effective method to stabilize collagen/gelatin-based fibers leverages carbodiimide chemistry, such as EDC/NHS crosslinking, to introduce ‘zero-length’ crosslinks101–103. Chemical crosslinking has also been used to stabilize nanofibers formed from synthetic hydrophilic materials104, for example using glutaraldehyde to crosslink polyacrylamide (PA)105 and poly(vinyl alcohol) (PVA)106–108. Glutaraldehyde as a crosslinker readily reacts with pendant groups on PA and PVA to form linkages, and offers the potential to provide user-defined control over the stiffness and swelling of resultant electrospun fibers105,106.
Chemical modifications for covalent crosslinking of hydrophilic polymers.
In many cases, the polymers forming the molecular backbones of these hydrogel materials are chemically modified using various strategies that enable their stabilization after electrospinning for use as fibrous hydrogel systems. Photoinitiated reactions represent a major platform for the stabilization of these hydrogel fibrous networks, and the common methodologies for photoinduced reactions leverage differing versions of the ene-ene scheme – for example through acrylate-based functional groups – and thiol-ene reactions. In the presence of light and a photoinitiator, ene-ene reactions undergo a chain-growth mechanism and form kinetic chains that crosslink the backbone polymers109. In the case of the thiol-ene reaction, photoinitiation produces a thiyl radical, which opens and subsequently binds with an adjacent alkene enabling stoichiometric crosslinking11,110–112. In addition to the crosslinking type, the degree of substitution on the polymeric backbone itself plays an important role in the regulation of downstream fiber mechanics113,114 – therefore, careful consideration is needed when designing the specific material system.
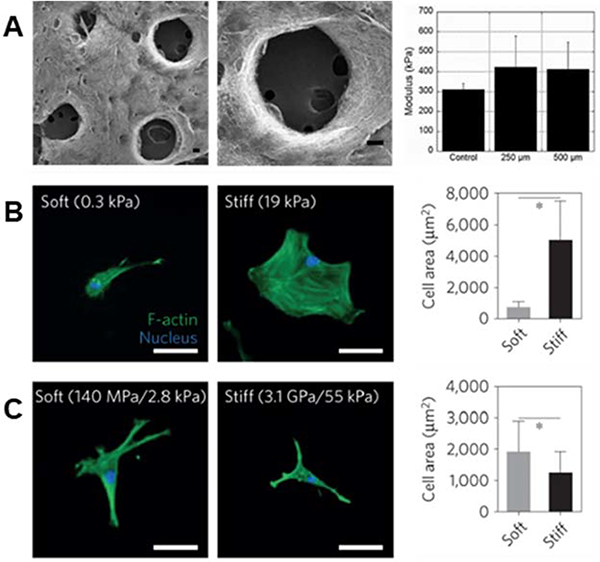
Many of these hydrophilic polymers have been modified to present pendant alkenes (using methacrylates and vinyl sulfones, for example) for crosslinking post-electrospinning. Gelatin is commonly modified with methacrylate moieties to create a material (GelMA) that can be stabilized by photoinitiated crosslinking of electrospun fibers115–117. Similar chemistry has been used to modify HA30,118, silk fibroin119,120, and PEG32,94. Dextran, another polysaccharide, can also be modified with methacrylate2,3,31 or vinyl sulfone87 functional groups for crosslinking and subsequent reactions that aim at improving bioactivity. In most cases, alkene groups within nanofibers allow for anhydrous radical-induced polymerization within fibers to stabilize the polymeric networks prior to hydration121. One of the strengths of photochemistries is the great potential for spatial control of reactions. Crosslinking, and therefore fiber stability (and ultimately mechanics), can be specified via selective irradiation of electrospun nanofibers through photomasks. Sundararaghavan et al. used this to introduce porosity within thick fibrous substrates that would aid in cell infiltration. By masking regions of fibers during anhydrous crosslinking of methacrylated HA nanofibers, leaving them unexposed to light, regions of fibers could be selectively dissolved during hydration122 (see Figure 3A).
Figure 3. Importance of fiber physical properties for cell culture.
(A, left to right): SEM micrographs of MeHA fibers with user-specified photopatterned pores, zoomed in micrograph of a photopatterned pore, and a column chart displaying modulus of scaffolds – with no significant difference between scaffolds with pores and scaffolds without pores. (A) Reprinted and adapted with permission from Sundararaghavan et al., copyright 2010 John Wiley and Sons122; scalebars = 100 μm. (B, left to right): hMSCs show increased cell spreading on stiff hydrogels as opposed to soft hydrogels – quantified by the column chart illustrating cell area (*p < 0.05). (C, left to right): hMSCs demonstrate increased spreading on soft rather than stiff hydrogel fibers – quantified by the column chart showing cell area (*p < 0.05). These differing results emphasize the need for careful consideration when designing the biophysical properties of fibrous hydrogels for cell culture. (B) and (C) Reprinted and adapted with permission from Baker et al., copyright 2015 Springer Nature2; scalebars = 50 μm.
Disadvantages and considerations when electrospinning hydrogels.
Although hydrogel materials have stark advantages over their non-hydrogel counterparts, there are some associated disadvantages that need to be considered when designing these material systems for electrospinning. For example, an important consideration when using some lower molecular weight polymers, like HA and PEG, is that a carrier polymer may be required during the electrospinning process to induce chain entanglements in the solution85,118. High molecular weight polymers – like poly(ethylene oxide) – may be added to the electrospinning solution to facilitate fiber formation and subsequently be washed away when the scaffolds are hydrated123. Furthermore, many biomaterials that form hydrogels are not ready for electrospinning ‘out-of-the-box’1. Specifically, many of the materials require chemical functionalization to introduce reactive moieties such as methacrylates2, vinyl sulfones124, or norbornenes86 to the polymeric backbones. An additional disadvantage of using these functionalized materials is the batch-to-batch variation in their synthesis, which can potentially alter fiber properties1. We refer to an excellent review by Caliari and Burdick1 for further information regarding synthesis and considerations of common hydrogel biomaterials. Finally, an inherent issue with these hydrophilic materials is the need to crosslink the fibers immediately post-electrospinning, prior to any further functionalization2,85,86. Once the material and crosslinking strategy are chosen, however, the resultant biophysical and biochemical properties can be easily modulated – as described in the following sections. Please refer to Table 1 for a representative list of hydrogel biomaterials that have been electrospun, along with a few established methods for crosslinking and modulating the resultant biophysiochemical properties.
Table 1.
Representative list of hydrophilic materials used to form hydrogel nanofibers with post-processing techniques.
| Material | Example crosslinking method(s) | Modulation of biophysiochemical properties |
|---|---|---|
| Fully-Synthetic Materials | ||
| Polyacrylamide (PA) |
Chemical: • Glutaraldehyde crosslinker105 |
Biochemical: • Likely adsorption-based modifications Biophysical: • Degree (extent) of crosslinking105 |
| Poly(vinyl alcohol) (PVA) |
Chemical: • Glutaraldehyde crosslinker106 • PVA composites for crosslinking107 Physical: • Controlling hydrophobicity through PVA modifications108 |
Biochemical: • Likely adsorption-based modifications Biophysical: • Degree (extent) of crosslinking106 or PVA modification108 • Degree of hydrolysis (i.e. quantity of pendant reactive groups)107 |
| Poly(ethylene glycol) (PEG) |
Chemical: • Pendant norbornenes (step-growth polymerization)85,136 • Pendant methacrylates (chain-growth polymerization)32 |
Biochemical • Adsorption-based modifications32 • Pendant norbornenes provide sites for addition of biomolecules ○ Light-mediated thiol-ene conjugation85 Biophysical: • Stiffness controlled via irradiation and crosslinker– for example: norbornenes136 and methacrylates32 |
| Naturally-Derived Materials | ||
| Collagen |
Chemical: • Glutaraldehyde crosslinker95,97,99 • Carbodiimide crosslinking (EDC/NHS)102 |
Biochemical: • Collagen provides natural bioactive sites for cell adhesion and interaction95 Biophysical: • Degree (extent) of chemical crosslinking97 |
| Gelatin |
Chemical: • Glutaraldehyde98 and diisocyanate crosslinkers100 • Carbodiimide crosslinking (EDC/NHS)101,103 • Pendant methacrylates (chain-growth polymerization)115–117 Physical: • Dehydrothermal crosslinking (generally weaker fibers)96 |
Biochemical: • Gelatin provides natural bioactive sites for cell adhesion and interaction96 Biophysical: • Degree (extent) of chemical crosslinking96 • Degree of chain-growth polymerization (e.g. with methacrylates)115,116 |
| Hyaluronic Acid (HA) |
Chemical: • Pendant norbornenes (step-growth polymerization)86 • Pendant methacrylates (chain-growth polymerization)30,88,118,126,144 • Pendant maleimides (chain-growth polymerization)8 • Hydrazide/aldehyde proximity reactions to crosslink adjacent fibers145 |
Biochemical: • Pendant molecules provide sites for addition of biomolecules ○ Michael addition: thiolated biomolecules react with pendant alkenes in basic conditions8,118 ○ Light-mediated thiol-ene conjugation86 Biophysical: • Stiffness also controlled via irradiation time – for example: methacrylates88,122 • Stiffness within norbornene modified systems can conceivably be controlled via crosslinker added, following from Gramlich et al.112 |
| Dextran |
Chemical: • Pendant methacrylates (chain-growth polymerization)2,3,31 • Pendant vinyl sulfones (chain-growth polymerization)87,124,146 |
Biochemical: • Pendant molecules provide sites for addition of biomolecules ○ Methacrylated heparin conjugated to free methacrylates within methacrylated-dextran fibers87 ○ Michael addition: thiolated biomolecules react with pendant alkenes in basic conditions2,3,31,87,124,146 Biophysical: • Stiffness also controlled via irradiation time – for example: chain-growth polymerization2 |
3.1. Hydrogel nanofibers enabling control over physical properties
As noted, the physical properties of cellular microenvironments exert strong influences over cell behaviors and phenotypes125,126. In nanofibrous systems, hydrogel-based materials offer possibilities for engineering these properties, such as the mechanical and viscoelastic environments with which cells interact, within a fiber-based environment to achieve certain outcomes or interrogate biological questions.
Ene-ene mechanism for controlling physical properties.
Within systems crosslinked via chain-growth polymerizations, the possibility to propagate kinetic chains after an initial fiber-stabilizing crosslinking allows further light exposures to generate increasingly stiff fibrous networks113 as well as spatially control mechanical features. This property allows for direct-user control over resultant fiber crosslinking density, and consequently fiber stiffness, via irradiation duration87.
Following the deposition and stabilization of hydrogel fibers, cell behaviors can be analyzed in in vitro tissue models that more closely mirror physiological features and enable experiments that assess cellular responses to perturbations of these environments. In ene-ene systems, control over mechanical properties, such as Young’s modulus, has allowed cellular responses to environments of differing fiber stiffnesses to be assessed2,3,30,31. For example, Baker et al. leveraged a methacrylated-dextran system and demonstrated that cell spreading behaviors on 2D stiff fibers (55 kPa, network stiffness) were inhibited in comparison to 2D soft fibers (2.8 kPa, network stiffness) – a phenomenon that is the inverse of what is seen on 2D hydrogels (Figure 3B and C)2. Baker et al. propose that this is due to the cells’ superior ability to recruit fibers on soft substrates as opposed to stiff2, a notion that is corroborated by a computational model presented by Cao et al. that suggests increased focal adhesion size when matrix fibers are recruited by cells42. Highlighting the complexity of mechanoresponsive cellular behaviors that can be influenced and interrogated in these systems, modulating fiber stiffness allows for design of 3D environments with high cell infiltration, combating the poor infiltration typically seen through the small pores of electrospun scaffolds127–129. Interestingly, Song et al. demonstrated that cellular infiltration can be improved by utilizing stiffer methacrylated-hyaluronic acid (MeHA) fibers88, a concept that is seemingly contradictory to more cell spreading exhibited on soft fibers. This phenomenon can likely be attributed to the tendency of cells to recruit matrix fibers88,130, which in turn decreases downstream pore size88. In fact, Song et al. demonstrate that on short time scales, cells invade soft fibers quickly, but then are stagnant at longer time scales – whereas cells continually invade stiff fibers across these longer time scales88. Furthering this, Heo et al. investigated the effect of nuclear stiffening as a response to matrix mechanics on cellular infiltration into these dense fibrous scaffolds131. The result of this work demonstrated that momentary softening of the nucleus improves infiltration – suggesting that a combination of nuclear softening in conjunction with stiffer fibers can aid in cell migration into thick fibrous matrices131.
The ene-ene chain-growth polymerization is a common method for developing hydrogel fibers; however, in utilizing a chain-growth polymerization technique for crosslinking fibers and controlling mechanics, one must account for the continued growth and formation of kinetic chains in subsequent exposures to light. This additional exposure can result in increasingly stiff material environments and can cause heterogeneities leading to an inconsistent global network – an issue seen in aqueous chain-growth polymerization132,133.
Thiol-ene mechanism for controlling physical properties.
In comparison, the light-mediated thiol-ene step-growth polymerization offers many of the same strengths of photochemical reactions, but with increased spatiotemporal control over the formation of hydrogel networks109,112. Similar to the ene-ene chemistry, hydrophilic polymers have been modified with functional groups for thiol-ene photopolymerization. This reaction relies on a functional alkene that readily reacts with nearby thiyl radicals that are typically induced by a photoinitiator110. Commonly, these polymeric backbones for electrospinning include, or are modified with, alkenes such as norbornenes85,86 and acrylates134 – among others135. To crosslink the fibers, the electrospinning precursor solution must include a crosslinking molecule with multiple thiols, and after electrospinning but before hydration, fibers should be exposed to light to stabilize the fibers, similar to ene-ene chain-growth polymerization. As before, light-initiated chemistry allows spatial control over the reaction, with unexposed regions able to be dissolved away upon hydration. As mentioned, the thiol-ene reaction is advantageous because it can be designed stoichiometrically to directly control crosslinking density via molar ratios of reactive groups within the crosslinker relative to the polymeric backbone, with near ideal networks forming through a step-growth mechanism132. The ability to control the level of crosslinking also enables residual alkenes to be preserved after crosslinking for subsequent reaction with molecules containing thiols – for example, in the addition of biomolecules86,112, which will be discussed in further depth in the next section, or in introducing additional crosslinking molecules to modify mechanics with the spatiotemporal control afforded by photochemistry.
Aiming to utilize thiol-ene chemistries to engineer the mechanical environment cells interacted with, Iglesias-Echevarria et al. designed a coaxial electrospinning method with PCL as the core polymer for structural stability, and PEG-norbornene (PEGNB) as the sheath for tunability136. The PEGNB outer layer afforded control over resultant stiffness of the fibers, while also leaving behind residual norbornene groups for subsequent conjugation of thiolated RGD motifs for increased cell adhesion. The stiffness of the PEGNB sheath was modulated to investigate cellular response to differing environments. When bovine pulmonary artery endothelial cells were seeded on fibers of varying stiffnesses, higher cell infiltration and deposition of matrix materials (e.g. collagen, elastin) were seen on fibers with greater Young’s moduli136 – a result in line with those mentioned above by Song et al. utilizing a MeHA fibrous system88. Another interesting approach employed by Yang et al. involved electrospun poly((3-mercaptopropyl)methylsiloxane) (PMMS) with triallyl cyanurate (TAC) as the crosslinker137. PMMS has pendant thiol groups that can react with any of the alkenes on TAC to form a crosslink that stabilizes the fibers, with residual thiols available for further modification. In addition to the flexibility in the crosslinking afforded by this system, Yang et al. leveraged the residual thiols on TAC to conjugate a maleimide-modified poly(N-isopropylacrylamide) (PNIPAAm) to the fibers – exploiting the thermal-responsiveness of PNIPAAm for user-control over resultant fiber hydrophobicity137. In regard to physical properties, the thiol-ene reaction is a facile, powerful platform for the formation of hydrogel fibers for cell culture, providing high levels of control over the resultant fibrous scaffolds.
Summary – controlling hydrogel nanofiber physical properties.
The physical properties of hydrogel nanofibers can be particularly well-regulated through photochemistries developed for bulk hydrogels; however, these platforms typically yield static fibers without the inclusion of further processing for dynamic complexity. There exists potential for other chemistries, including in situ reactions to be expanded upon below in the section outlining dynamic fiber systems – which can perhaps be used in conjunction with the aforementioned photoinduced chemistries in dual-crosslinking systems. It is worth reiterating that while these hydrogel fiber systems allow strategic control over physical properties that cells experience, regardless of how these fibers are crosslinked, the nanofiber diameters will increase upon fiber hydration – a phenomenon that is directly correlated with polymer hydrophilicity and crosslinking density88. Thus, careful balance and consideration are required when designing a hydrogel fiber system that recapitulates the physical properties of the tissue system of choice. However, the physical properties only tell half the story of physiologically-relevant ECM. To design an in vitro system that is truly indicative of natural tissue, a synergistic approach that incorporates both the relevant biophysical and biochemical signals is required. Fortunately, the crosslinking methods described above not only provide direct control over the physical properties, they can also be used to spatiotemporally incorporate desired biomolecules into the nanofibrillar environment.
3.2. Hydrogel fibers enabling modulation of biochemical properties
Within hydrogel materials, modifications such as those described above allow for spatiotemporal modulation not just of the biophysical properties, as there has been considerable progress in utilizing the same chemistries in controlling biochemical properties too. Hydrogels can be designed such that the functional groups used to bind crosslinking molecules might also bind biofunctional molecules, and careful control of the crosslinking process can leave unreacted sites within the hydrogel after crosslinking to couple molecules that increase bioactivity for cellular studies86,87. The ene-ene and thiol-ene reaction pathways that have been described above are also commonly utilized to introduce these biochemical signals; however, there are alternative chemistries under development that achieve similar results. We aim to provide an overview of chemistries for incorporating biomolecules into nanofibrous scaffolds based on hydrogel materials, where, in comparison to hydrophobic polymers, aqueous media can be used for all reactions36,37,86.
Ene-ene mechanism for controlling biochemical properties.
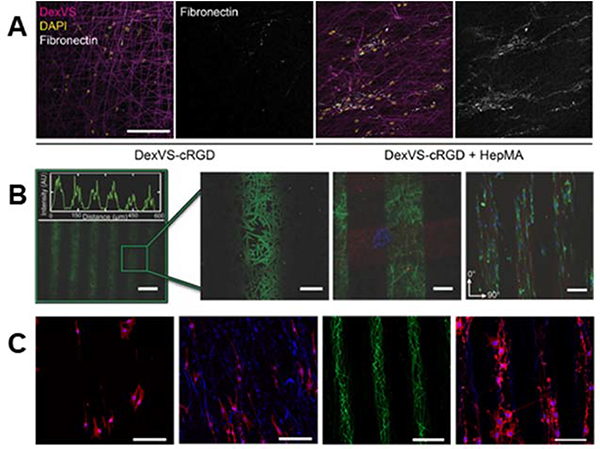
Ene-ene chain-growth, though more commonly employed in crosslinking fibers without further functionalization via the mechanism, can be used to introduce biochemical cues. For example, Davidson et al. conjugated methacrylated heparin to free vinyl sulfone groups on dextran fibers through ene-ene photopolymerization to investigate the influence of heparin presentation on resultant cell adhesion and matrix protein sequestration87. The addition of heparin was demonstrated to correlate with improved cell adhesion, as well as improved binding of cell-secreted fibronectin to the dextran fibers87 (Figure 4A). Extending the use of heparin to trap biomolecules such as the aforementioned cell-secreted fibronectin, Mays et al. conjugated methacrylated heparin to hyaluronic acid fibers to facilitate growth factor sequestration in order to promote chick dorsal root ganglia neurite length138.
Figure 4. Introducing biochemical cues into fibrous hydrogels.
(A, left to right): Dextran-vinyl sulfone (DexVS) fibers (magenta) were seeded with human lung fibroblasts (nuclei shown in yellow) in the presence of RGD or RGD + heparin. Conjugation of RGD + heparin to DexVS fibers increased the secretion and subsequent binding of fibronectin (white) onto the fibrous matrix. (A) Reprinted and adapted with permission from Davidson et al., copyright 2020 Elsevier87; scalebar = 200 μm. (B, left to right): spatial patterning of thiolated fluorophores onto NorHA fibers via thiol-ene click chemistry. Zoomed in images show high pattern fidelity, and the ability to pattern multiple biomolecules on the same scaffold – indicated by the red, green, and blue fluorophores on the fibers. The ability to pattern adhesive regions, using an RGD motif, allows for preferential cellular localization in RGD+ regions that elongate in the direction of fiber alignment. (B) Reprinted and adapted with permission from Wade et al., copyright 2015 John Wiley and Sons86; scalebars (left to right) = 100 μm, 25 μm, 100 μm, and 100 μm. (C, left to right): Patterning of bioactivity on synthetic fibers using UV irradiation. Rat Schwann cells exhibited a less elongated morphology on non-bioactive substrates (far left) when compared to substrates that were activated with UV light (middle left). The use of photomasks allowed for introduction of linear bioactive regions (middle right) which promoted cell attachment over non-bioactive regions (far right). (C) Reprinted and adapted with permission from Girão et al. 2019147; scalebars (left to right) = 200 μm, 200 μm, 100 μm, and 100 μm.
An important consideration in methods that functionalize fibers that were crosslinked via photoinitiated chain-growth polymerization through another photoinitiated reaction, is the effect of the subsequent reaction on kinetic chains formed during crosslinking. These kinetic chains can continue to propagate with the continued addition of radicals87, and this may increase the Young’s modulus of the fibers through additional crosslinking. To surmount this challenge, researchers may leverage the Michael-type addition reaction, where thiolated molecules bind to double bonds at slightly elevated pH, in order to incorporate functional molecules onto the pendant alkenes within these systems, avoiding further polymerization.
Thiol-ene (Michael Addition) for controlling biochemical properties.
The Michael addition is often used to conjugate thiols to pendant alkenes in hydrogel systems139–143. This chemistry allows for facile, homogenous conjugation of thiolated biomolecules to fibrous networks containing alkenes3,87,118. This conjugation can be calculated stoichiometrically, allowing for precise control over the level of functionalization. Therefore, this reaction can occur either pre-electrospinning, to modify polymeric materials that will be used in the electrospinning processs118, or after the crosslinking step that typically follows electrospinning2. For example, although HA is a naturally-occurring polymer that interacts with cells via the CD44 surface receptor, HA hydrogel substrates still require modification with ligands that can bind adhesive proteins on cell surfaces to improve cell adhesion86. Kim et al. used the based-catalyzed Michael addition to controllably introduce RGD motifs onto electrospun MeHA fibers, and demonstrated that higher presentations of RGD resulted in increased hMSC spreading, proliferation, and formation of focal adhesions118. Furthermore, Sundararaghavan and Burdick were able to introduce gradients of RGD in the Z direction into dense fibrous substrates using a novel electrospinning setup that deposited unmodified MeHA and high-RGD-modified MeHA at varying flow rates144. The thiol-Michael addition is a powerful and versatile method to introduce controlled densities of biomolecules into fibrous hydrogel systems; however, due to the requirement of a basic pH for the reaction to proceed, there is minimal spatial control over the presentation of these molecules2,3,30,87,118, as materials that are undergoing modification are often uniformly immersed into a basic buffer containing the thiolated molecule of interest. For spatially controlled addition of bioactivity into fibrous systems, the radical-induced thiol-ene conjugation is preferable.
Thiol-ene (radical induced) for controlling biochemical properties.
Due to the inherent complexity of natural ECM5–7, as well as the desire – in many experiments – to study cellular responses to differential signals in their microenvironments, the ability to tightly control the heterogeneity of biochemical functionalization of in vitro tissue culture scaffolds is desired. The radial-induced coupling of thiolated molecules onto pendant alkenes of hydrogel fibers allows for the precise localization of bioactive molecules that control cellular behaviors, such as adhesion, at high fidelity86,135. As discussed previously, this photochemistry allows light exposure to control the positioning of these molecules, so strategically designed photomasks, or carefully focused light, can be employed to control where coupling occurs in XY space. Wade and coworkers demonstrated the former using aligned electrospun nanofibers created from norbornene-functionalized hyaluronic acid (NorHA)86. In this seminal work, Wade et al. showed that through stoichiometric calculations, multiple thiolated peptides (in this case, red/green/blue fluorophores) can be conjugated to fibrous NorHA surfaces – indicating that multiple bioactive molecules can be controllably introduced86. Furthermore, using a thiolated RGD motif, Wade et al. demonstrated how 3T3 fibroblasts responded to a combination of microenvironmental cues: a controlled spatial presentation of RGD on an aligned nanofibrous topography86 (Figure 4B). Moreover, Sharma and coworkers demonstrated the relative ease in employing this chemistry with PEG-norbornene fibers in a microarray system. This high-throughput platform allowed for investigation of multiple thiolated peptides with a multitude of cell types to probe cellular responses to differing microenvironments85. These results, taken together, clearly support the power of this chemistry scheme to control the biochemical cues that are necessary to incorporate into cell culture systems.
UV-irradiation for controlling biochemical properties.
In addition to radical-induced coupling, selective UV irradiation has been used to control localization of relevant biomolecules on hydrogel fibers. Similar to the UV functionalization of PLA nanofibers, Girao et al. used the block copolymer poly(ethylene oxide terephthalate)/poly(butylene terephthalate) (PEOT/PBT) to synthesize nanofibers147. This block copolymer provides a hydrophilic region (PEOT) and a brittle, hydrophobic region (PBT) – meaning the resultant fibers can absorb high percentages of water. The surfaces of these water-swollen fibers were then subsequently functionalized via selective UV irradiation to spatially control the introduction of reactive groups for biomolecule and cell adhesion. Biomolecules – such as fluorescein isothiocyanate (FITC)-tagged BSA – were conjugated vertically through the material in the XY plane and rat Schwann cells adhered selectively to functionalized regions147 (Figure 4C). The ability to tailor mechanical properties of the resultant fibers by modulating block lengths in the copolymer, in addition to spatial control over presentation of biochemical cues, makes this platform particularly attractive in the use for tissue engineering scaffolds.
Summary – controlling hydrogel nanofiber biochemical properties.
Methods like those described above allow for easy and controllable incorporation of relevant biochemical signals into fibrous hydrogel tissue culture systems, and demonstrate strengths and potential of hydrogel-based nanofibrous platforms. It is of note that the thiol-ene reaction allows for calculated, stoichiometric crosslinking, leaving residual alkenes available for biomolecule conjugation86, although similar control might be exerted through careful regulation of other reactions. Light-based mechanisms offer strengths in enabling selective spatial specification of reactions. UV functionalization of fibers has demonstrated the potential to achieve the same end goal, albeit in hydrophobic materials147, whose properties such as biocompatibility, degradation, and amenability to modification must be carefully considered in material design. Other hydrophilic materials, such as hyaluronic acid and dextran, have strong track records in these areas, but the chemical structure and properties of the backbone polymer are predetermined1,148. Regardless of the material selection and chemistry design, hydrogel fibers offer possibilities for high resolution spatial control over the heterogeneity of tissue culture platforms, and materials might easily be combined for next-generation fibrous systems.
4 Towards dynamic complexity and mimicking natural tissue
With technologies established to engineer nanofibrous substrates with specific biophysiochemical properties, it is now possible to precisely control the spatial heterogeneity of biophysical and biochemical cues within the scaffolds. Because of this, there is exciting progress in the development of fibrous hydrogel systems that mimic natural tissue, with an emphasis on dynamic complexity – where properties of these systems might be designed to change or be controlled over time.
Engineering degradability into hydrogel nanofibers.
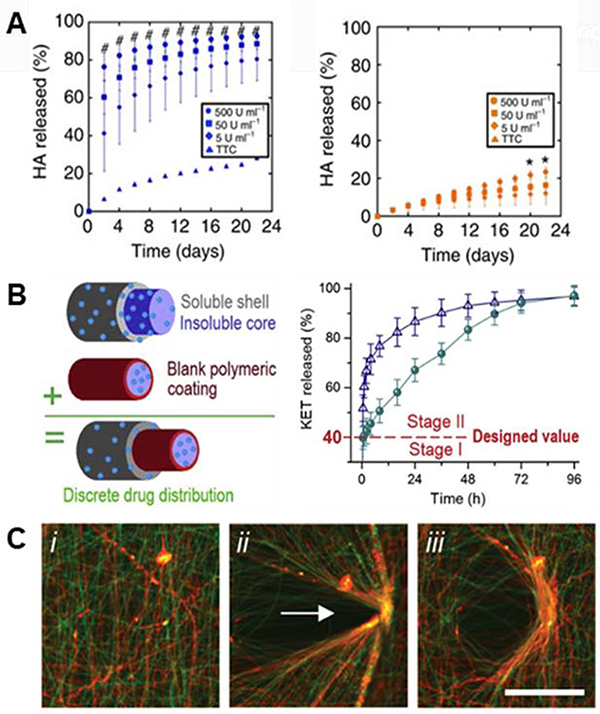
Advances in the engineering of bulk hydrogels, both in 2D and 3D, have demonstrated unique strengths in this area – for example in material designs using enzymatically degradable crosslinkers to allow for physiologically-mediated decomposition of the scaffolds149–151 – and it follows that nanofibers based on hydrogel systems would have similar potential. The potential to engineer materials technologies established in bulk hydrogels into hydrogel-based nanofibers is illustrated by the development of electrospun HA fibers crosslinked with a protease-sensitive crosslinker8, establishing enzymatic degradability based on materials first used as bulk hydrogels152. Wade and coworkers leveraged a maleimide-functionalized HA that was electrospun with a crosslinker peptide that was degradable enzymatically by rhMMP-2 and Type II collagenase8 (Figure 5A). The addition of this degradability into fibrous hydrogels allows for dynamic restructuring of the fibrous ECM by resident cells via the secretion of enzymes and subsequent deposition of new matrix proteins. Wade et al. furthered this work by demonstrating efficacy of degradation in vivo – highlighting aspects important to translation in a subcutaneous implantation model8.
Figure 5. Dynamic complexity in electrospun fibers.
(A): HA hydrogel fibers were crosslinked with a peptide crosslinker that was susceptible to degradation via matrix metalloproteinases (MMPs). (Left): degradation of MMP-sensitive HA fibers in the presence of differing concentrations of Type II collagenase (# p < 0.05, for all test groups versus control), and (right): degradation of HA fibers crosslinked with a peptide that is not sensitive to Type II collagenase (* p < 0.05, for 500 U/mL group versus control). There is a clear positive degradation effect when using an MMP-sensitive crosslinker. (A) Reprinted and adapted with permission from Wade et al., copyright 2015 Springer Nature8. (B): Triaxial electrospun fibers for sustained drug release. (Left): schematic of the triaxial fibers that include a polymeric coating around the innermost fiber to slow drug release. (Right): Model drug release (KET) from core-shell fibers (blue triangles) and triaxial fibers (green circles). Core-shell and tri-layered fibers both exhibited quick release past stage I (40% of release), but tri-layered fibers slowed the release throughout stage II compared to core-shell fibers – due to the polymeric coating introduced around the core. (B) Reprinted and adapted with permission from Yang et al., copyright 2020 Elsevier158. (C, left to right): Hydrazide and aldehyde-functionalized NorHA fibers (i) that react to form hydrazone bonds when in contact (ii) – allowing for permanent, covalent rearrangement of fibrous scaffolds (iii). (C) Reprinted and adapted with permission from Davidson et al., copyright 2019 John Wiley and Sons145; scalebars = 100 μm.
Dynamic fibers for selective molecule delivery.
Dynamic properties in fibrous hydrogels are also embodied in applications that load the fibers with bioactive molecules to create temporal signaling. Temporal control over the release of chemokines or cytokines represent technologies with great potential for nanofibrous systems to influence cellular behavior and regeneration. Applications of controlled release from nanofibrous systems predominantly center on drug delivery applications, and there are several comprehensive reviews on this topic55,82,153; we highlight a few systems here to illustrate technologies that might be applied in nanofibrous systems designed for tissue engineering and regenerative medicine.
Non-hydrogel fibers have demonstrated effectiveness in the delivery of molecules by both coating fibers154,155 and incorporating bioactive molecules in the precursor solution155. Ahire and coworkers adsorbed HA to the surface of poly(D,L, lactide) fibers and demonstrated a sustained, linear release of HA over time154. Xia et al. also showed efficacy in the sustained delivery of adsorbed vascular endothelial growth factor (VEGF) to the surface of poly (L-lactic acid) fibers that included nerve growth factor (NGF) in the core155. This two-step release allowed for sequential addition of biomolecules to the local environment and can, in theory, be applied to a multitude of growth/soluble factors.
Hydrogel fibers have also demonstrated promising results in the field of drug delivery. For example, Kishan and coworkers developed a platform that provides a sustained release of proteins to the local environment using different types of crosslinked gelatin fibers156. Their methacrylated gelatin system relied on traditional mass transfer for the release of a model protein incorporated within the fibers. On the other hand, gelatin crosslinked using a diisocyanate molecule was loaded with a model protein that reacted with the gelatin backbone, and protein release in this scenario relied on gelatin degradation to free the protein from the fibers156. These two gelatin systems can be employed together to provide a tunable, sustained release of desired proteins from hydrogel fibers to support tissue growth and regeneration.
Core-shell fibers have also proven to be advantageous in the release of bioactive molecules to the adjacent environment. In the spirit of hydrogel fibers, a core-shell fibrous system was developed for the thermally-responsive release of rhodamine B157. The shell was comprised of poly-L-lactide-co-caprolactone (PLCL) and the core of poly(N-isopropylacrylamide-co-N-isopropylmethacrylamide) (P(NIPAAm-co-NIPMAAm)) – a thermally responsive polymer. The addition of the thermally-responsive P(NIPAAm-co-NIPMAAm) core allowed for a slower, more sustained release when compared to just a PLCL control157. Extending this, Yang and coworkers developed triaxial nanofibers comprised of polyvinylpyrrolidone (PVP) and cellulose acetate (CA), using ketoprofen (KET) as a model drug158. Yang et al. assert that the use of a tri-layered electrospun fiber yielded a more beneficial release profile initially, and the use of a CA blocking layer around the core provided a longer, more sustained release than a two layered system158 (Figure 5B). While these are select examples of the extensive work in this area55,82,153, they illustrate the potential to engineer nanofibers to control release profiles and deliver important bioactive molecules relevant in cellular systems. Continuing work in designing dynamic delivery systems has direct implications for engineering temporal complexity into electrospun fibers.
Improving cell infiltration.
Incorporating dynamicity into electrospun fibers is an important consideration in developing nanofibrous scaffolds that interface with cells and natural tissue, especially in translation of regenerative materials, as touched on above with respect to controlled release. Efforts to develop dynamic fibrous structures have sought to overcome a challenge faced by electrospun fibers in implantation: small pore sizes between fibers in larger, dense mats that are of clinically relevant dimensions prevent efficient cell infiltration into the scaffolds127–129. One way to surmount this challenge, in addition to the aforementioned intrafiber modifications such as enzymatically degradable crosslinks, is to spin multiple fiber types into a single substrate, where a fiber type might confer dynamic features into the substrate, such as increasing its porosity upon implantation. Specifically, water-soluble poly(ethylene oxide) (PEO) sacrificial fibers that dissolve in water, but take up space during fiber deposition and contribute to the initial structure of a larger electrospun substrate, can be co-spun with a material that is stable and persists over longer timescales159–161. This method has shown to improve infiltration, without hindering cellular transduction of microenvironmental cues160. This technique has been extended to the development of an engineered intervertebral disc, where an annulus geometry was designed with PCL fibers as the outer shell and hydrogel as the inner core162. The addition of PEO sacrificial fibers helped increase cell infiltration into this disc model which yielded superior matrix deposition when compared to the control that did not include sacrificial fibers162.
Molecular-level dynamic complexities.
Dynamic chemistries at the molecular level also offer the potential for engineering dynamic behaviors that emerge at the scales of individual fibers and fibrous systems. Chemical crosslinking approaches that allow for fibers to rearrange in response to outside perturbations—either during assembly of structures or through interactions with cells—have been demonstrated to enable the creation of complex fibrous constructs and to allow cells to modify the physical environment they experience over time. For example, dynamic supramolecular crosslinking, where non-covalent, reversible interactions occur between complementary molecules on different polymers, can be used to assemble nanofibrous substrates and create structures with biomimetic complexity. Hyaluronic acid functionalized with methacrylates for covalent stabilization of fibers and also β-cyclodextrin (CD) (CD-MeHA) can be used to create nanofibers that form reversible bonds at interfaces with materials similarly functionalized with adamantane through supramolecular host-guest interactions84. CD is a cyclic host molecule with a hydrophobic core that hydrophobically interacts with guest molecules, such as adamantane (Ad) in noncovalent bonds that can be dynamically disrupted and restored163–166. By designing nanofibers that present complementary functionalities on their surfaces, a nanofibrous substrate presenting CD could be adhered to another presenting Ad, offering capabilities to generate layers of aligned fibers that might be useful in cartilage or cardiac tissue engineering applications, where they might reproduce fibrous tissue structures84.
Reversible bonds, like the Ad-CD guest-host system, have been demonstrated to introduce viscoelasticity into hydrogel tissue culture systems – allowing for cells to easily deform and remodel the local microenvironment157,167,168. Nanofibrous systems with dynamic properties that enable cells to remodel their physical surroundings offer unique capabilities beyond bulk hydrogels, to observe, study, and perturb cellular behaviors through their interaction with fibrous materials. As discussed extensively here, these materials can be designed to offer ECM-like topographies as well as ECM-mimetic biophysical and biochemical features which offer cells more freedom of motion than might be achieved by encapsulating cells within a 3D hydrogel network. Towards establishing nanofibrous systems that allow dynamic, cell-responsive rearrangements of microenvironmental physical features, Davidson et al. used NorHA that was additionally modified with either hydrazide or aldehyde groups (NorHA-Hyd and NorHA-Ald, respectively) to dual-electrospin a fibrous blend of NorHA-Hyd and NorHA-Ald145. At the fiber surfaces, hydrazide and aldehyde functional groups reacted to form hydrazone bonds when the two fiber types were in contact, i.e. an adhesive interaction145,152,169 (Figure 5C). The interaction is proposed to allow cells to dynamically remodel the surrounding matrix by recruiting fibers with traction forces – with the recruited fibers subsequently reacting to preserve the structure145. Xu et al. also employed this chemical functionality within poly(oligoethylene glycol methacrylate) (POEGMA) fibers. POEGMA was functionalized with hydrazide/aldehyde moieties, which allowed for immediate in situ crosslinking following double-barrel electrospinning169. Xu et al. found that the hydrazide/aldehyde reaction allowed for the quick formation of crosslinks that were degradable both hydrolytically and enzymatically169.
Hydrogel fibers in the third dimension.
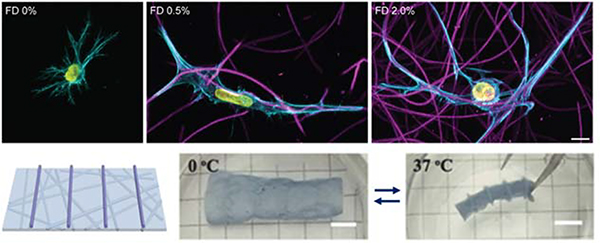
Towards increasing the dimensionality of fibrous constructs or adding fibrous features to 3D tissue models, electrospun fibers have also been employed in 3D contexts – such as dispersion into bulk hydrogels124 and shape-shifting 3D scaffolds170, as highlighted here. The addition of fibrous networks dispersed within amorphous bulk hydrogels allows for recapitulation of the fibrillar nature of endogenous ECM, in a physiologically relevant 3D environment4. For example, Matera et al. demonstrated increased human dermal fibroblast spreading in hydrogels with dispersed dextran fibers, as well as cellular morphological changes in a fiber density-dependent manner124 (Figure 6 Top). This example reinforces the stark influence of the biophysical signals that fibers provide within 3D cell culture systems as researchers progress towards perfecting models of ECM in vitro.
Figure 6. Fiber suspensions in 3D hydrogels.
(Top): Dispersion of DexVS fibers in 3D GelMA hydrogels. Increasing concentrations of suspended fibers (from left to right) demonstrates stark influence of fiber density on cell morphology – 0% and 2% show high levels of spread, whereas 0.5% shows a uniaxial morphology. (Top) Reprinted and adapted with permission from Matera et al., copyright 2019 American Chemical Society124; scalebar = 10 μm. (Bottom): P(NIPAAm-ABP) electrospun fibers with 3D printed supports. (from left to right): schematic of 3D printed supports atop of the nanofibrous P(NIPAAm-ABP) substrate; scaffold is suspended in water and adopts a relaxed conformation since the temperature is below the LCST (0° C); scaffold rolls and deforms when suspended in water with a temperature above the LCST (37° C) – thus acting as a shape-shifting hydrogel nanofiber system. (Bottom) Reprinted and adapted with permission from Chen et al., copyright 2018 John Wiley and Sons170; scalebars = 5 mm.
From a biofabrication-specific standpoint, Chen and coworkers demonstrated the ability to electrospin poly(N-isopropylacrylamide) (P(NIPAAm)) hydrogel nanofibrous scaffolds that were secondarily crosslinked via UV light with acryloylbenzophenone (P(NIPAAm-ABP)) to form thermo-responsive mats170. Photocrosslinkable P(NIPAAm) solutions were also 3D printed onto these electrospun mats to provide rigid structure (i.e. trusses) to the mats. Due to P(NIPAAm)’s conformational changes above and below its lower critical solution temperature (LCST), the electrospun mats with supports exhibit shape changes upon temperature transition around the LCST due to the amount of water that is contained within the fibrous network. Below the LCST (0° C), P(NIPAAm-ABP) scaffolds demonstrated a relaxed structure; however, once the temperature was increased to above the LCST (37° C), the scaffolds rolled into shapes that were dictated by the structures 3D printed atop of the mats – hence shape-shifting nanofibrous hydrogel scaffolds (Figure 6 Bottom)170. This system demonstrates efficacy in controlling the topography of nanofibrous hydrogel culture systems and can be extended to virtually any tissue system where 3D geometric structure is of interest.
Summary – dynamic complexity and mimicking natural tissue.
Work in the field continues to advance dynamic features in fibrous cell culture systems that will be central to mimicking natural tissue systems, probing fundamental biological questions, and successfully designing systems for regenerative medicine. The inclusion of protease degradable crosslinkers, dynamic remodeling, sacrificial fibers for increased cellular infiltration, and the extension towards 3D scaffolds are key progressions in the development of fiber systems. However, the field of electrospun fibers – namely hydrogel fibers – is still trending behind the progress seen with 2D/3D bulk hydrogel systems, and there exists clear potential for hydrogel-based nanofibers to continue to be engineered to better recapitulate native physiology and control cell behaviors.
5. Next generation hydrogel fibers
As the field continues to progress towards fibrous hydrogel systems that recapture the salient features of a tissue system of interest, technology developed for engineering 2D/3D bulk hydrogels offers considerable opportunities for application in electrospun hydrogel systems. For example, expanding upon chemistries enabling dynamic degradation via the usage of a protease-sensitive crosslinker, chemical functionalities exist that allow directed degradation, such as photocleavable crosslinking through nitrobenzyl ether groups developed and demonstrated by the Anseth group171,172. These have allowed for user-defined degradation at extremely short timescales relative to protease degradation.
Technologies that allow reversible biochemical cues to be incorporated into bulk hydrogels offer the potential for dynamic spatiotemporal control over microenvironmental features. The presentation of relevant biomolecules within the ECM is constantly in flux4–7, and the ability to replicate this signaling complexity within an engineered microenvironment is critical to studying and replicating biological processes. Work that has reversibly, and repeatedly, introduced bioactive molecules into culture systems has utilized both covalent and supramolecular chemistries. Light-based approaches include nitrobenzyl ether techniques to photocleave the molecules from the scaffolds10,173, while the Anseth group designed an allyl-sulfide that mediates multiple thiol-ene click reactions for incorporation and subsequent removal of desired molecules11,111. These studies were conducted with bulk PEG hydrogels, but can conceivably be applied to PEG electrospun fibers or other hydrogel fibers that are modified to support these chemistries.
Groups have also employed supramolecular chemistries to reversibly incorporate bioactive molecules in hydrogel materials. Guest-host interactions allow for self-assembly of molecules, but can be easily disrupted via the addition of a competing molecule174. For example, Boekhoven et al. utilized β-cyclodextrin as a host molecule and took advantage of differing affinities of naphthyl and adamantane to reversibly incorporate biomolecules174. To develop technology enabling greater control over these reversible interactions, oligonucleotides with toeholds have been employed for their ability to provide bioactive domains on hydrogel surfaces175. Bioactivity was removed via the addition of complementary oligonucleotides that took advantage of the toehold region – providing a system with defined bioactivity by cyclical addition of these oligonucleotides175. Both of these examples demonstrated the ability to control cell morphology and spreading based on the presentation of these bioactive ligands on alginate surfaces174,175. Extending technologies such as these onto established hydrogel fibers would broaden opportunities to dynamically modulate complexity in water-swollen fibrous networks.
With continued progress and innovation in the materials design of fibrous hydrogel systems – and building upon exciting observations enabled by these platforms – we believe that it is inevitable that the technologies mentioned above will pave the way for platforms that truly recapitulate the endogenous ECM. With the growing understanding of the hydrated, fibrillar structure and function of the extracellular matrix, this progress is needed before we can truly probe fundamental physiological processes in vitro. As we progress forward, the growing ability to precisely define the biophysiochemical properties of an in vitro system offers the unique capability of engineering biomimetic environments for controlled perturbations to homeostasis in order to understand fundamental physiological function, dysfunction, development, and regeneration. Moreover, in addition to this fundamental experimentation, the ability to replicate natural tissue would be a significant stride towards the seamless integration of engineered therapeutics for successful tissue regeneration. With applications ranging the full scale of tissue engineering – from fundamental studies, to clinical translation – the development of dynamic, fibrillar hydrogels offers seemingly limitless potential as the field continues to develop.
Acknowledgements
This work was supported by the University of Virginia and the National Institutes of Health through UVA Biotechnology Training Program NIGMS 5T32 GM008715. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of interest
There are no conflicts to declare.
References
- 1.Caliari SR and Burdick JA, Nat. Methods, 2016, 13, 405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker BM, Trappmann B, Wang WY, Sakar MS, Kim IL, Shenoy VB, Burdick JA and Chen CS, Nat. Mater, 2015, 14, 1262–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davidson CD, Wang WY, Zaimi I, Jayco DKP and Baker BM, Sci. Rep, 2019, 9, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker BM and Chen CS, J. Cell Sci, 2012, 125, 3015–3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frantz C, Stewart KM and Weaver VM, J. Cell Sci, 2010, 123, 4195–4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wade RJ and Burdick JA, Mater. Today, 2012, 15, 454–459. [Google Scholar]
- 7.Lutolf MP and Hubbell JA, Nat. Biotechnol, 2005, 23, 47–55. [DOI] [PubMed] [Google Scholar]
- 8.Wade RJ, Bassin EJ, Rodell CB and Burdick JA, Nat. Commun, 2015, 6, 6639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Velasco-Hogan A, Xu J and Meyers MA, Adv. Mater, , DOI: 10.1002/adma.201800940. [DOI] [PubMed] [Google Scholar]
- 10.Deforest CA and Anseth KS, Angew. Chemie Int. Ed, 2012, 51, 1816–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grim JC, Brown TE, Aguado BA, Chapnick DA, Viert AL, Liu X and Anseth KS, ACS Cent. Sci, 2018, 4, 909–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wade RJ and Burdick JA, Nano Today, 2014, 9, 722–742. [Google Scholar]
- 13.Shoulders MD and Raines RT, Annu. Rev. Biochem, 2009, 78, 929–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hynes RO, Science (80-. )., 2009, 326, 1216–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffith LG and Swartz MA, Mol. Cell Biol, 2006, 7, 211–224. [DOI] [PubMed] [Google Scholar]
- 16.Stevens MM and George JH, Science (80-. )., 2005, 310, 1135–1138. [DOI] [PubMed] [Google Scholar]
- 17.Liu C, Hsu P-C, Lee H-W, Ye M, Zheng G, Liu N, Li W and Cui Y, Nat. Commun, 2015, 6, 6205. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Li W, Xia Y, Jiao X and Chen D, J. Mater. Chem. A, 2014, 2, 15124–15131. [Google Scholar]
- 19.Herricks TE, Kim S-H, Kim J, Li D, Kwak JH, Grate JW, Kim SH and Xia Y, J. Mater. Chem, 2005, 15, 3241–3245. [Google Scholar]
- 20.Ji X, Wang P, Su Z, Ma G and Zhang S, J. Mater. Chem. B, 2014, 2, 181–190. [DOI] [PubMed] [Google Scholar]
- 21.Islam MS, Ang BC, Andriyana A and Afifi AM, SN Appl. Sci, 2019, 1, 1248. [Google Scholar]
- 22.Liu H, Ding X, Zhou G, Li P, Wei X and Fan Y, J. Nanomater, , DOI:. [DOI] [Google Scholar]
- 23.Kishan AP and Cosgriff-Hernandez EM, J. Biomed. Mater. Res. A, 2017, 105, 2892–2905. [DOI] [PubMed] [Google Scholar]
- 24.Sun L, Gao W, Fu X, Shi M, Xie W, Zhang W, Zhao F and Chen X, Biomater. Sci, 2018, 6, 340–349. [DOI] [PubMed] [Google Scholar]
- 25.Schoen B, Avrahami R, Baruch L, Efraim Y, Goldfracht I, Elul O, Davidov T, Gepstein L, Zussman E and Machluf M, Adv. Funct. Mater, , DOI: 10.1002/adfm.201700427. [DOI] [Google Scholar]
- 26.De Valence S, Tille JC, Giliberto JP, Mrowczynski W, Gurny R, Walpoth BH and Möller M, Acta Biomater, 2012, 8, 3914–3920. [DOI] [PubMed] [Google Scholar]
- 27.Christopherson GT, Song H and Mao H, Biomaterials, 2009, 30, 556–564. [DOI] [PubMed] [Google Scholar]
- 28.Lim SH, Liu XY, Song H, Yarema KJ and Mao H, Biomaterials, 2010, 31, 9031–9039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chamundeswari VN, Yuan Siang L, Jin Chuah Y, Shi Tan J, Wang DA and Loo SCJ, Biomed. Mater, , DOI: 10.1088/1748-605X/aa8bcd. [DOI] [PubMed] [Google Scholar]
- 30.Davidson MD, Song KH, Lee MH, Llewellyn J, Du Y, Baker BM, Wells RG and Burdick JA, ACS Biomater. Sci. Eng, 2019, 5, 3899–3908. [DOI] [PubMed] [Google Scholar]
- 31.Wang WY, Davidson CD, Lin D and Baker BM, Nat. Commun, 2019, 10, 1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wingate K, Bonani W, Tan Y, Bryant SJ and Tan W, Acta Biomater, 2012, 8, 1440–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xue J, Wu T, Dai Y and Xia Y, Chem. Rev, 2019, 119, 5298–5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rahmati M, Mills DK, Urbanska AM, Saeb MR, Venugopal JR, Ramakrishna S and Mozafari M, Prog. Mater. Sci, , DOI: 10.1016/j.pmatsci.2020.100721. [DOI] [Google Scholar]
- 35.Schiffman JD and Schauer CL, Polym. Rev, 2008, 48, 317–352. [Google Scholar]
- 36.Jordan AM, Viswanath V, Kim S, Pokorski JK and Korley LTJ, J. Mater. Chem. B, 2016, 4, 5958–5974. [DOI] [PubMed] [Google Scholar]
- 37.Kalaoglu-Altan OI, Sanyal R and Sanyal A, Polym. Chem, 2015, 6, 3372–3381. [Google Scholar]
- 38.Burdick JA and Murphy WL, Nat. Commun, 2012, 3, 1269. [DOI] [PubMed] [Google Scholar]
- 39.Tibbitt MW and Anseth KS, Biotechnol. Bioeng, 2009, 103, 655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Highley CB, Prestwich GD and Burdick JA, Curr. Opin. Biotechnol, 2016, 40, 35–40. [DOI] [PubMed] [Google Scholar]
- 41.Burdick JA and Prestwich GD, Adv. Healthc. Mater, 2011, 23, H41–H56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cao X, Ban E, Baker BM, Lin Y, Burdick JA, Chen CS and Shenoy VB, Proc. Natl. Acad. Sci. U. S. A, 2017, 114, E4549–E4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li W-J, Laurencin CT, Caterson EJ, Tuan RS and Ko FK, J. Biomed. Mater. Res, 2002, 60, 613–621. [DOI] [PubMed] [Google Scholar]
- 44.Lee BL-P, Jeon H, Wang A, Yan Z, Yu J, Grigoropoulos C and Li S, Acta Biomater, 2012, 8, 2648–2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Piai JF, da Silva MA, Martins A, Torres AB, Faria S, Reis RL, Muniz EC and Neves NM, Appl. Surf. Sci, 2017, 403, 112–125. [Google Scholar]
- 46.Shabani I, Haddadi-Asl V, Seyedjafari E and Soleimani M, Biochem. Biophys. Res. Commun, 2012, 423, 50–54. [DOI] [PubMed] [Google Scholar]
- 47.He L, Tang S, Prabhakaran MP, Liao S, Tian L, Zhang Y, Xue W and Ramakrishna S, Macromol. Biosci, 2013, 13, 1601–1609. [DOI] [PubMed] [Google Scholar]
- 48.Ko Y-G and Kwon OH, J. Ind. Eng. Chem, 2020, 89, 147–155. [Google Scholar]
- 49.Soliman S, Sant S, Nichol JW, Khabiry M, Traversa E and Khademhosseini A, J. Biomed. Mater. Res. A, 2011, 96, 566–574. [DOI] [PubMed] [Google Scholar]
- 50.Savoji H, Hadjizadeh A, Maire M, Ajji A, Wertheimer MR and Lerouge S, Macromol. Biosci, 2014, 14, 1084–1095. [DOI] [PubMed] [Google Scholar]
- 51.Hu X, Liu S, Zhou G, Huang Y, Xie Z and Jing X, J. Control. Release, 2014, 185, 12–21. [DOI] [PubMed] [Google Scholar]
- 52.Bashur CA, Shaffer RD, Dahlgren LA, Guelcher SA and Goldstein AS, Tissue Eng. Part A, 2009, 15, 2435–2445. [DOI] [PubMed] [Google Scholar]
- 53.Haider A, Haider S and Kang IK, Arab. J. Chem, 2018, 11, 1165–1188. [Google Scholar]
- 54.Agarwal S, Wendorff JH and Greiner A, Polymer (Guildf), 2008, 49, 5603–5621. [Google Scholar]
- 55.Chakraborty S, Liao I-C, Adler A and Leong KW, Adv. Drug Deliv. Rev, 2009, 61, 1043–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miller AE, Hu P and Barker TH, Adv. Healthc. Mater, 2020, 9, 1901445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peijs T, in Comprehensive Composite Materials II, 2018, pp. 162–200. [Google Scholar]
- 58.Pham QP, Sharma U and Mikos AG, Tissue Eng, 2006, 12, 1197–1211. [DOI] [PubMed] [Google Scholar]
- 59.Ojha SS, in Electospun Nanofibers, 2017, pp. 239–253. [Google Scholar]
- 60.Nezarati RM, Eifert MB, Dempsey DK and Cosgriff-Hernandez E, J. Biomed. Mater. Res. - Part B Appl. Biomater, 2015, 103, 313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Park SM, Eom S, Choi D, Han SJ, Park SJ and Kim DS, Chem. Eng. J, 2018, 335, 712–719. [Google Scholar]
- 62.Szczesny SE, Driscoll TP, Tseng H-Y, Liu P-C, Heo S-J, Mauck RL and Chao P-HG, ACS Biomater. Sci. Eng, 2017, 3, 2869–2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen H, Baptista DF, Criscenti G, Crispim J, Fernandes H, van Blitterswijk C, Truckenmüller R and Moroni L, Nanoscale, 2019, 11, 14312–14321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Seliktar D, Science (80-. )., 2012, 336, 1124–1129. [DOI] [PubMed] [Google Scholar]
- 65.Murphy WL, McDevitt TC and Engler AJ, Nat. Mater, 2014, 13, 547–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Griffith LG and Naughton G, Science (80-. )., 2002, 295, 1009–1014. [DOI] [PubMed] [Google Scholar]
- 67.Kador KE, Alsehli HS, Zindell AN, Lau LW, Andreopoulos FM, Watson BD and Goldberg JL, Acta Biomater, 2014, 10, 4939–4946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee H, Rho J and Messersmith PB, Adv. Mater, 2009, 21, 431–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cho H, Madhurakkat Perikamana SK, Lee J, Lee J, Lee K-M, Shin CS and Shin H, ACS Appl. Mater. Interfaces, 2014, 6, 11225–11235. [DOI] [PubMed] [Google Scholar]
- 70.Nazeri N, Karimi R and Ghanbari H, J. Biomed. Mater. Res. Part A, , DOI: 10.1002/jbm.a.37013. [DOI] [PubMed] [Google Scholar]
- 71.Shin YM, Jun I, Lim Y-M, Rhim T and Shin H, Macromol. Mater. Eng, 2013, 298, 555–564. [Google Scholar]
- 72.Lynge ME, van der Westen R, Postma A and Städler B, Nanoscale, 2011, 3, 4916–4928. [DOI] [PubMed] [Google Scholar]
- 73.Ryu JH, Messersmith PB and Lee H, ACS Appl. Mater. Interfaces, 2018, 10, 7523–7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Qiu X, Lee BL-P, Ning X, Murthy N, Dong N and Li S, Acta Biomater, 2017, 51, 138–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tanes ML, Xue J and Xia Y, J. Mater. Chem. B, 2017, 5, 5580–5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu T, Xue J, Li H, Zhu C, Mo X and Xia Y, ACS Appl. Mater. Interfaces, 2018, 10, 8536–8545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Haldón E, Nicasio MC and Pérez PJ, Org. Biomol. Chem, 2015, 13, 9528–9550. [DOI] [PubMed] [Google Scholar]
- 78.Mbua NE, Guo J, Wolfert MA, Steet R and Boons G-J, ChemBioChem, 2011, 12, 1912–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shi Q, Chen X, Lu T and Jing X, Biomaterials, 2008, 29, 1118–1126. [DOI] [PubMed] [Google Scholar]
- 80.Smith Callahan LA, Xie S, Barker IA, Zheng J, Reneker DH, Dove AP and Becker ML, Biomaterials, 2013, 34, 9089–9095. [DOI] [PubMed] [Google Scholar]
- 81.Zheng J, Xie S, Lin F, Hua G, Yu T, Reneker DH and Becker ML, Polym. Chem, 2013, 4, 2215–2218. [Google Scholar]
- 82.Pillay V, Dott C, Choonara YE, Tyagi C, Tomar L, Kumar P, du Toit LC and Ndesendo VMK, J. Nanomater, 2013, 789289. [Google Scholar]
- 83.de Lima GG, Lyons S, Devine DM and Nugent MJD, in Hydrogels, Springer Singapore, 2018, pp. 219–258. [Google Scholar]
- 84.Highley CB, Rodell CB, Kim IL, Wade RJ and Burdick JA, J. Mater. Chem. B, 2015, 2, 8110–8115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sharma S, Floren M, Ding Y, Stenmark KR, Tan W and Bryant SJ, Biomaterials, 2017, 143, 17–28. [DOI] [PubMed] [Google Scholar]
- 86.Wade RJ, Bassin EJ, Gramlich WM and Burdick JA, Adv. Mater, 2015, 27, 1356–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Davidson CD, Jayco DKP, Matera DL, DePalma SJ, Hiraki HL, Wang WY and Baker BM, Acta Biomater, 2020, 105, 78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Song KH, Heo S-J, Peredo AP, Davidson MD, Mauck RL and Burdick JA, Adv. Healthc. Mater, 2019, 9, 1901228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Moroni L, Burdick JA, Highley C, Lee SJ, Morimoto Y, Takeuchi S and Yoo JJ, Nat. Rev. Mater, 2018, 3, 21–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li M, Mondrinos MJ, Gandhi MR, Ko FK, Weiss AS and Lelkes PI, Biomaterials, 2005, 26, 5999–6008. [DOI] [PubMed] [Google Scholar]
- 91.Kwon MY, Wang C, Galarraga JH, Puré E, Han L and Burdick JA, Biomaterials, 2019, 222, 119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Misra S, Hascall VC, Markwald RR and Ghatak S, Front. Immunol, 2015, 6, 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chung C, Beecham M, Mauck RL and Burdick JA, Biomaterials, 2009, 30, 4287–4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Roberts JJ and Bryant SJ, Biomaterials, 2013, 34, 9969–9979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Castilla-Casadiego DA, Ramos-Avilez HV, Herrera-Posada S, Calcagno B, Loyo L, Shipmon J, Acevedo A, Quintana A and Almodovar J, Macromol. Mater. Eng, 2016, 301, 1064–1075. [Google Scholar]
- 96.Campiglio CE, Negrini NC, Farè S and Draghi L, Materials (Basel), 2019, 12, 2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jha BS, Ayres CE, Bowman JR, Telemeco TA, Sell SA, Bowlin GL and Simpson DG, J. Nanomater, 2011, 348268. [Google Scholar]
- 98.Aoki H, Miyoshi H and Yamagata Y, Polym. J, 2015, 47, 267–277. [Google Scholar]
- 99.Zhang X, Tang K and Zheng X, J. Bionic Eng, 2016, 13, 143–149. [Google Scholar]
- 100.Kishan AP, Nezarati RM, Radzicki CM, Renfro AL, Robinson JL, Whitely ME and Cosgriff-Hernandez EM, J. Mater. Chem. B, 2015, 3, 7930–7938. [DOI] [PubMed] [Google Scholar]
- 101.Liu L, Kamei K, Yoshioka M, Nakajima M, Li J, Fujimoto N, Terada S, Tokunaga Y, Koyama Y, Sato H, Hasegawa K, Nakatsuji N and Chen Y, Biomaterials, 2017, 124, 47–54. [DOI] [PubMed] [Google Scholar]
- 102.Fischer RL, McCoy MG and Grant SA, J. Mater. Sci. Mater. Med, 2012, 23, 1645–1654. [DOI] [PubMed] [Google Scholar]
- 103.Ghassemi Z and Slaughter G, Conf. Proc. IEEE Eng. Med. Biol. Soc., 2018, 6088–6091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Young RE, Graf J, Miserocchi I, Van Horn RM, Gordon MB, Anderson CR and Sefcik LS, PLoS One, 2019, 14, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lu P and Hsieh Y-L, Polymer (Guildf), 2009, 50, 3670–3679. [Google Scholar]
- 106.Destaye AG, Lin CK and Lee CK, ACS Appl. Mater. Interfaces, 2013, 5, 4745–4752. [DOI] [PubMed] [Google Scholar]
- 107.Park JC, Ito T, Kim KO, Kim KW, Kim BS, Khil MS, Kim HY and Kim IS, Polym. J, 2010, 42, 273–276. [Google Scholar]
- 108.Kim CK, Kim BS, Sheikh FA, Lee US, Khil MS and Kim HY, Macromolecules, 2007, 40, 4823–4828. [Google Scholar]
- 109.Hui E, Gimeno KI, Guan G and Caliari SR, Biomacromolecules, 2019, 20, 4126–4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hoyle CE and Bowman CN, Angew. Chemie, 2010, 49, 1540–1573. [DOI] [PubMed] [Google Scholar]
- 111.Grim JC, Marozas IA and Anseth KS, J. Control. Release, 2015, 219, 95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gramlich WM, Kim IL and Burdick JA, Biomaterials, 2013, 34, 9803–9811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ifkovits JL and Burdick JA, Tissue Eng, 2007, 13, 2369–2385. [DOI] [PubMed] [Google Scholar]
- 114.Van Den Bulcke AI, Bogdanov B, De Rooze N, Schacht EH, Cornelissen M and Berghmans H, Biomacromolecules, 2000, 1, 31–38. [DOI] [PubMed] [Google Scholar]
- 115.Aldana AA, Malatto L, Rehman MAU, Boccaccini AR and Abraham GA, Nanomaterials, 2019, 9, 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhao X, Sun X, Yildirimer L, Lang Q, (William) Lin ZY, Zheng R, Zhang Y, Cui W, Annabi N and Khademhosseini A, Acta Biomater, 2017, 49, 66–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lobo AO, Afewerki S, de Paula MMM, Ghannadian P, Marciano FR, Zhang YS, Webster TJ and Khademhosseini A, Int. J. Nanomedicine, 2018, 13, 7891–7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kim IL, Khetan S, Baker BM, Chen CS and Burdick JA, Biomaterials, 2013, 34, 5571–5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bessonov IV, Rochev YA, Arkhipova AY, Kopitsyna MN, Bagrov DV, Karpushkin EA, Bibikova TN, Moysenovich AM, Soldatenko AS, Nikishin II, Kotliarova MS, Bogush VG, Shaitan KV and Moisenovich MM, Biomed. Mater, 2019, 14, 034102. [DOI] [PubMed] [Google Scholar]
- 120.Bin Bae S, Kim MH and Park WH, Polym. Degrad. Stab, , DOI: 10.1016/j.polymdegradstab.2020.109304. [DOI] [Google Scholar]
- 121.Tan AR, Ifkovits JL, Baker BM, Brey DM, Mauck RL and Burdick JA, J. Biomed. Mater. Res. - Part A, 2008, 87, 1034–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sundararaghavan HG, Metter RB and Burdick JA, Macromol. Biosci, 2010, 10, 265–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dong B, Arnoult O, Smith ME and Wnek GE, Macromol. Rapid Commun, 2009, 30, 539–542. [DOI] [PubMed] [Google Scholar]
- 124.Matera DL, Wang WY, Smith MR, Shikanov A and Baker BM, ACS Biomater. Sci. Eng, 2019, 5, 2965–2975. [DOI] [PubMed] [Google Scholar]
- 125.Hall MS, Alisafaei F, Ban E, Feng X, Hui CY, Shenoy VB and Wu M, Proc. Natl. Acad. Sci. U. S. A, 2016, 113, 14043–14048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sundararaghavan HG, Saunders RL, Hammer DA and Burdick JA, Biotechnol. Bioeng, 2013, 110, 1249–1254. [DOI] [PubMed] [Google Scholar]
- 127.Baker BM, Nathan AS, Huffman GR and Mauck RL, Osteoarthr. Cartil, 2009, 17, 336–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Qu F, Guilak F and Mauck RL, Nat. Rev. Rheumatol, 2019, 15, 167–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Baker BM and Mauck RL, Biomaterials, 2007, 28, 1967–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Abhilash AS, Baker BM, Trappmann B, Chen CS and Shenoy VB, Biophys. J, 2014, 107, 1829–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Heo SJ, Song KH, Thakur S, Miller LM, Cao X, Peredo AP, Seiber BN, Qu F, Driscoll TP, Shenoy VB, Lakadamyali M, Burdick JA and Mauck RL, Sci. Adv, 2020, 6, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kloxin AM, Kloxin CJ, Bowman CN and Anseth KS, Adv. Mater, 2010, 22, 3484–3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lin-Gibson S, Jones RL, Washburn NR and Horkay F, Macromolecules, 2005, 38, 2897–2902. [Google Scholar]
- 134.Shanmuganathan K, Sankhagowit RK, Iyer P and Ellison CJ, Chem. Mater, 2011, 23, 4726–4732. [Google Scholar]
- 135.Kalaoglu-Altan OI, Verbraeken B, Lava K, Gevrek TN, Sanyal R, Dargaville T, De Clerck K, Hoogenboom R and Sanyal A, ACS Macro Lett, 2016, 5, 676–681. [DOI] [PubMed] [Google Scholar]
- 136.Iglesias-Echevarria M, Durante L, Johnson R, Rafuse M, Ding Y, Bonani W, Maniglio D and Tan W, Biomater. Sci, 2019, 7, 3640–3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yang H, Zhang Q, Lin B, Fu G, Zhang X and Guo L, J. Polym. Sci. Part A Polym. Chem, 2012, 50, 4182–4190. [Google Scholar]
- 138.Mays EA, Kallakuri SS and Sundararaghavan HG, J. Biomed. Mater. Res. - Part A, 2020, 1–9. [DOI] [PubMed] [Google Scholar]
- 139.Nair DP, Podgórski M, Chatani S, Gong T, Xi W, Fenoli CR and Bowman CN, Chem. Mater, 2014, 26, 724–744. [Google Scholar]
- 140.Petrou CL, D’Ovidio TJ, Bolukbas DA, Tas S, Brown RD, Allawzi A, Lindstedt S, Nozik-Grayck E, Stenmark KR, Wagner DE and Magin CM, J. Mater. Chem. B, 2020, 8, 6814–6826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Trappmann B, Baker BM, Polacheck WJ, Choi CK, Burdick JA and Chen CS, Nat. Commun, 2017, 8, 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zustiak SP, Boukari H and Leach JB, Soft Matter, 2010, 6, 3609–3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Han K, Yin W-N, Fan J-X, Cao F-Y and Zhang X-Z, ACS Appl. Mater. Interfaces, 2015, 7, 23679–23684. [DOI] [PubMed] [Google Scholar]
- 144.Sundararaghavan HG and Burdick JA, Biomacromolecules, 2011, 12, 2344–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Davidson MD, Ban E, Schoonen ACM, Lee M-H, D’Este M, Shenoy VB and Burdick JA, Adv. Mater, , DOI: 10.1002/adma.201905719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Davidson CD, Jayco DKP, Wang WY, Shikanov A and Baker BM, J. Biomech. Eng, 2020, 142, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Girão AF, Wieringa P, Pinto SC, Marques PAAP, Micera S, van Wezel R, Ahmed M, Truckenmueller R and Moroni L, Front. Bioeng. Biotechnol, , DOI: 10.3389/fbioe.2019.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Saha K, Pollock JF, Schaffer DV and Healy KE, Curr. Opin. Chem. Biol, 2007, 11, 381–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Nih LR, Moshayedi P, Llorente IL, Berg AR, Cinkornpumin J, Lowry WE, Segura T and Carmichael ST, Data Br, 2017, 10, 202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nih LR, Sideris E, Carmichael ST and Segura T, Adv. Mater, , DOI: 10.1002/adma.201606471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Holloway JL, Ma H, Rai R and Burdick JA, J. Control. Release, 2014, 191, 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Purcell BP, Lobb D, Charati MB, Dorsey SM, Wade RJ, Zellars KN, Doviak H, Pettaway S, Logdon CB, Shuman JA, Freels PD, Gorman III JH, Gorman RC, Spinale FG and Burdick JA, Nat. Mater, 2014, 13, 653–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bhattarai RS, Bachu RD, Boddu SHS and Bhaduri S, Pharmaceutics, , DOI: 10.3390/pharmaceutics11010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ahire JJ, Robertson D, Neveling DP, Van Reenen AJ and Dicks LMT, RSC Adv, 2016, 6, 34791–34796. [Google Scholar]
- 155.Xia B and Lv Y, Mater. Sci. Eng. C, 2018, 82, 253–264. [DOI] [PubMed] [Google Scholar]
- 156.Kishan A, Walker T, Sears N, Wilems T and Cosgriff-Hernandez E, J. Biomed. Mater. Res. - Part A, 2018, 106, 1155–1164. [DOI] [PubMed] [Google Scholar]
- 157.Pawłowska S, Rinoldi C, Nakielski P, Ziai Y, Urbanek O, Li X, Kowalewski TA, Ding B and Pierini F, Adv. Mater. Interfaces, , DOI: 10.1002/admi.202000247. [DOI] [Google Scholar]
- 158.Yang Y, Chang S, Bai Y, Du Y and Yu D-G, Carbohydr. Polym, 2020, 243, 116477. [DOI] [PubMed] [Google Scholar]
- 159.Phipps MC, Clem WC, Grunda JM, Clines GA and Bellis SL, Biomaterials, 2012, 33, 524–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Baker BM, Shah RP, Silverstein AM, Esterhai JL, Burdick JA and Mauck RL, Proc. Natl. Acad. Sci. U. S. A, 2012, 109, 14176–14181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Baker BM, Gee AO, Metter RB, Nathan AS, Marklein RA, Burdick JA and Mauck RL, Biomaterials, 2008, 29, 2348–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ashinsky BG, Gullbrand SE, Bonnevie ED, Wang C, Kim DH, Han L, Mauck RL and Smith HE, Acta Biomater, 2020, 111, 232–241. [DOI] [PubMed] [Google Scholar]
- 163.Gomes ME, Domingues RMA and Reis RL, Tissue Eng. Part B, 2017, 23, 211–224. [DOI] [PubMed] [Google Scholar]
- 164.Highley CB, Rodell CB and Burdick JA, Adv. Mater, 2015, 27, 5075–5079. [DOI] [PubMed] [Google Scholar]
- 165.Sinawang G, Osaki M, Takashima Y, Yamaguchi H and Harada A, Polym. J, 2020, 52, 839–859. [Google Scholar]
- 166.Tanaka M, Nakahata M, Linke P and Kaufmann S, Polym. J, 2020, 52, 861–870. [Google Scholar]
- 167.Loebel C, Mauck RL and Burdick JA, Nat. Mater, 2019, 18, 883–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Vining KH, Stafford A and Mooney DJ, Biomaterials, 2019, 188, 187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Xu F, Sheardown H and Hoare T, Chem. Commun, 2016, 52, 1451–1454. [DOI] [PubMed] [Google Scholar]
- 170.Chen T, Bakhshi H, Liu L, Ji J and Agarwal S, Adv. Funct. Mater, 2018, 28, 3–9. [Google Scholar]
- 171.Kloxin AM, Kasko AM, Salinas CN and Anseth KS, Science (80-. )., 2009, 324, 59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.DeForest CA and Anseth KS, Nat. Chem, 2011, 3, 925–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Deforest CA and Tirrell DA, Nat. Mater, 2015, 14, 523–531. [DOI] [PubMed] [Google Scholar]
- 174.Boekhoven J, Rubert Pérez CM, Sur S, Worthy A and Stupp SI, Angew. Chemie - Int. Ed, 2013, 52, 12077–12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Freeman R, Stephanopoulos N, Álvarez Z, Lewis JA, Sur S, Serrano CM, Boekhoven J, Lee SS and Stupp SI, Nat. Commun, 2017, 8, 15982. [DOI] [PMC free article] [PubMed] [Google Scholar]