Abstract
Background:
The purpose of this study was to evaluate brain structure and function in participants with iCL/P and unaffected controls. Effects of cleft presence and reading status (average vs impaired) were evaluated.
Methods:
Males, ages 8 – 11 years old, including 26 with iCL/P and 57 unaffected peers were recruited and coded for reading status (average vs impaired). All participants underwent a volumetric and task-based functional MRI. Volumes and significant regions of activation during the decoding task were obtained. Main effects of cleft and reading status, and their interaction were evaluated.
Results:
Participants with iCL/P had significantly increased frontal gray matter volume (associated with average reading) and occipital gray and white matter volume (associated with impaired reading). Impaired readers with iCL/P had a distinctive activation pattern in visual association and motor regions relative to other groups.
Conclusions:
Findings suggest that increases in frontal gray matter volume may be associated with effective compensation during reading, while posterior increases in occipital volume may be associated with ineffective compensation for participants with iCL/P. These patterns were different from idiopathic dyslexia. Further work in a larger sample is needed to determine if these differences are associated with cleft type and with sex.
While thirty percent of oral clefts can be tied to a known genetic syndrome, roughly 70% occur in “isolation”, i.e., without a known genetic cause. However, the diagnosis of an isolated cleft of the lip and/or palate (iCL/P) is not necessarily “isolated” as there are a variety of associated problems. Research has documented varying rates of academic,1–7 behavioral,8 cognitive,9,10 emotional,11–14 and social15,16 long-term issues associated with iCL/P. It has been argued that these late outcomes are due to a mixture of medical (e.g., frequent surgeries17,18), sensory (e.g., hearing loss and speech disruption19,20), and psychosocial (e.g., stigma and teasing21) influences. While this complex and multifaceted combination of factors certainly plays a role, results are mixed in the level of impact each variable has. Researchers have suggested the additional (and possibly more primary) impact of abnormal neural migration.22,23
The hypothesis of abnormal brain development is grounded in the observation that cells of the face and brain originate from the same prenatal location and migrate at roughly the same developmental time point. This migration of cells is disrupted in oral clefting, resulting in a cleft of the lip (CL), the palate (CP), or both (CLP). Because there is abnormal migration of facial cells, it is hypothesized that the same disruption occurs in the migration of cells during development of the central nervous system. The resulting abnormalities in brain development are subtle, not resulting in major neurological disabilities, but instead leading to high incidence/low severity disruptions in behavioral and cognitive functioning.
Neuroimaging work among children and adolescents with iCL/P has demonstrated a global decrease in both cerebral and cerebellar volumes compared to unaffected controls, with increased gray matter and decreased white matter for boys24 and decreased cortical thickness in early adolescence.25 Within the past decade, researchers have extended neural imaging work to toddlers and infants. Yang and colleagues26 found decreased gray matter in the left auditory cortex and thalamus in participants 6 – 24 months old. A pilot study on 7 – 11 weeks old infants27 reported a trend of less myelinated cerebral white matter among boys with iCL/P before exposure to anesthesia relative to unaffected infants. Regional structural differences have been found to be associated with impairments in attention,28 cognition,29 speech,30 and social skills.15,31
Preliminary work has also evaluated differential neural activation. Adult males with iCLP had increased blood flow in the inferior frontal lobe, occipital lobe, and cerebellum during reading tasks of increasing complexity. This over-activation was interpreted as neural inefficiency during the task.32 A functional MRI task was conducted in a Chinese sample of men post-palatoplasty where they were asked to subvocalize Chinese characters.33 Despite equal accuracy and activation in traditional language systems, there was a slower response among men with iCL/P and increased activation in the left hippocampus relative to unaffected peers. Finally, as a feasibility project for the current study, Conrad and colleagues34 had 8 – 16 year old boys with iCP complete a traditional nonword rhyming task during a functional MRI scan. Areas of over and under-activation were found across fronto-temporal, parieto-temporal, and temporal-occipital systems compared to unaffected controls.
One essential element that has been missing from this line of research is the contrast of neurological measures in iCL/P to a disease-control group that has the disability of interest, but no cleft. Such a design determines if phenotypic patterns (e.g., impaired reading) are similar to known neural disruption or a different pattern of disruption. Given the high rate of reading impairment among children with iCL/P4,7,35 and the extensive research on patterns of disruption in specific left hemisphere reading networks (parieto-temporal and occipito-temporal regions36,37,38) among children with dyslexia, this is an ideal comparison group. The purpose of the current study was to evaluate neural patterns of reading achievement in children with iCL/P using structural and functional MRI to two contrast groups: unaffected children with either average (uAR) or impaired reading (uIR). The primary question was: Do neural patterns related to impaired reading in iCL/P match those of unaffected children with impaired reading? Specific research questions evaluated the impact of both cleft status (affected vs unaffected) and reading status (average vs impaired) on brain structure and activation. It was hypothesized that structural differences would be stronger by cleft status, with regional differences in cerebral white and gray matter and cerebellum volume. Given the exploratory nature of the activation task in this study design, no specific hypotheses were made regarding findings.
Methods
Participants
Participants were recruited through the Cleft Clinic patient registry (iCL/P) and advertisements through local staff/student email list serves (uAR) and websites/Facebook pages of state-wide dyslexia associations (uIR). Participants were male, ages 8 – 11 years old, predominately Caucasian (84%), and right-handed. Given previous literature finding different patterns of gray vs white matter among boys and girls with iCL/P, the current study was limited to boys to avoid potential differences to sex and increase the power to detect differences due to reading ability. There was no history of neurological insult or injury, and there were no major medical or mental diagnoses (aside from cleft in the iCL/P groups). For the iCL/P sample, children with a diagnosed genetic syndrome or multiple co-morbidities were excluded. In contrast to recruitment of the two unaffected groups, reading skill was not screened during recruitment of participants with iCL/P. This was done to ensure a wide range of reading ability in this group, which permitted the evaluation of factors related to poor vs average reading among children with iCL/P. Further details of recruitment has been previously described.39
The study visit included assessment of neuropsychological skills and reading achievement, in addition to structural and functional MRI protocols. In the majority of participants, testing and imaging was conducted on the same day. Results of neuropsychological and achievement testing has been presented previously.39 The current sample is a subset that successfully completed structural and functional imaging protocols during their visit. Of the original sample, 89% (28 uAR, 29 uIR, and 26 iCL/P [6 iCL, 9 iCP, and 11 iCLP]) had successful structural scans and were included in the current analyses. The most frequent reason for not completing a scan was participant discomfort, in addition to scheduling conflicts or significant motion artifacts. Comparisons of the participants who had a successful MRI scan (N = 83) and those who did not (N = 10), did not yield any significant differences in age (F (1, 89) = 0.352, p = .555) or socioeconomic status (SES; F (1, 87) = 0.168, p = .683) using a modified Hollingshead scale.40
All procedures were approved by the Institutional Review Board. Legal guardians provided written consent and participants provided verbal and written assent. The neuropsychological and achievement battery (lasting 4 hours) was conducted in the same day as the MRI scan (lasting 35 minutes). Guardians were reimbursed for travel expenses and participants were compensated monetarily.
Neuropsychological and Achievement Battery
Intelligence.
The Wechsler Intelligence Scale for Children, 5th Edition (WISC-V41) is a widely used tool for assessing general cognitive abilities. Select subtests were administered to obtain a Global Abilities Index (GAI) for each participant.
Reading Achievement.
The Woodcock-Johnson Reading Mastery Test, 3rd Edition (WRMT-III42) is designed to assess various aspects of reading achievement. Subtests of Word Identification, Word Attack (pseudoword decoding), and Oral Reading Fluency were administered.
MRI Scanner
The first 47 participants (20 uAR, 11uIR, 16 iCL/P) were scanned using a 3.0 Tesla Siemens Trio MR scanner. Halfway through recruitment, the scanner was replaced with a 3.0 Tesla GE Premier MRI Scanner. The final 32 participants completed scanning on this scanner. Both scanners used a 12-channel head coil. The COMBAT harmonization method was applied to adjust for scanner-induced variation.43,44
Structural Protocol.
Anatomical T1-weighted images were acquired as follows for Siemens (GE parameters in parentheses): coronal MPRAGE (BRAVO), TR = 2300 (8.392) ms, TE = 2.82 (3.184) ms, TI = 900 (450) ms, flip angle = 10 (12)°, FOV = 282 x 282 x 264 mm, matrix = 256 x 256 x 240. Parameters for T2-weighted were: coronal, TR = 4800 (3000) ms, TE = 430 (85.925) ms, FOV = 256 x 256 x 224 mm, matrix = 256 x 256 x 160.
Functional Protocol.
After structural sequences, subjects were shown stimuli using the program E-Prime to assess regions of brain activity during word reading tasks. Stimuli included a control task (modified line orientation; e.g., Do [//\] and [//\] match?) and the target task (rhyme judgment with non-words; e.g., Do [VUS] and [PUX] rhyme?). These are existing tasks for evaluation of neural activity during reading among children and adolescents.45 The stimuli for each task was presented simultaneously, one below the other, and subjects determined if the stimuli were the same/rhymed. Participant answers and response time were registered with a button press of the right index finger. Training on this task was conducted in a quiet office prior to entering the scanner.
Each task set had 4 line/word pairs, “YES” and “NO” correct responses were presented in a random order within each task set. The presentation of each pair lasted 2500 msec followed by 2000 msec of blank screen (for response time), for a total time of 18 seconds per task set. The control and target tasks were presented in a block design (ABAB), with 4 epochs of each task and an 18 second fixation (+) between each epoch for each run (visually presented in Figure 1). There was a total of 2 runs with a 90 second fixation (+) between the two runs. Total time for the task was 10.5 minutes.
Figure 1.
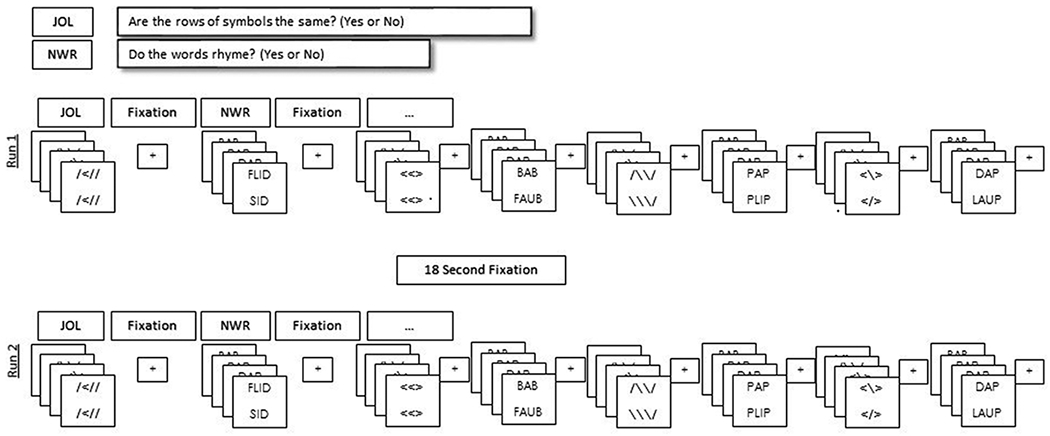
fMRI Protocol. Visual representation of the sequence of JOL and NWR task presentation across both runs of the fMRI protocol.
Data Processing.
DICOM files were reoriented to RPI, skull-striped, and registered to the NIHPD asymetric 4.5-18.5 2mm atlas.46 After conversion to NIFTI, images were despiked, smoothed, and motion corrected using Analysis of Functional Neuroimages (AFNI47). Bias field inhomogeneity was corrected using the N4 algorithm implemented in Advanced Normalization Tools software.48 Images were processed using the BRAINSAutoWorkup pipeline which optimizes tissue classification through an iterative framework and produces robust parcellation of brain regions in a multi-site setting.49 BRAINSAutoWorkup labels brain regions using a multi-atlas, similarity-weighted, majority-vote procedure (joint label fusion50) using a set of expert-segmented templates adapted from the Desikan-Killiany atlas.51 Brain regions include cortical and subcortical regions, separated by hemispheres and tissue type (gray or white matter) where appropriate. Residual inter-scanner variation was harmonized using an empirical Bayesian approach43,52 as implemented by the ez.combat toolbox in R.53 We confirmed that scanner did not predict regional volume by conducting Kolmogorov-Smirnov tests and visualizing the empirical cumulative distributions for each scanner across ROIs and groups. Statistical analyses were performed on the harmonized neuroimaging data.
Analyses
Between Subject Factors.
Two separate coding variables were created to assess main effects of cleft status and reading status, as well as their potential interactions. Cleft Status was coded as unaffected (0) or iCL/P (1). As reported previously, Reading Status was coded as average (0) or impaired (1) based on performance on reading tasks presented in the original paper.39 Participants were coded with “impaired reading” if they had at least one score at or below the 25th Percentile on any reading accuracy of fluency subtest. This resulted in four separate groups: unaffected participants with either impaired (uIR) or average (uAR) reading and participants with iCL/P who had either impaired (cIR) or average (cAR) reading.
Head Circumference and Global Structural Differences.
Measures of head circumference, inter-cranial volume (icv), whole brain tissue, as well as cerebrum and cerebellum volume were compared using univariate analysis of covariance (ANCOVA; controlling for SES) with cleft and reading status as between subject factors. For this and subsequent analyses, main and interaction effects were evaluated at p < .05 and p < .01, respectively. Partial eta2 (η2) is provided for an estimate of effect size; where η2 * 100 is interpreted as the percentage of variance associated with the effect and associated error.54
Cerebral Lobe Differences.
Volume within the cerebrum was divided into frontal, parietal, temporal and occipital lobes. Univariate ANCOVAs evaluated potential main and interaction effects for Total volume in each lobe. To limit the risk of type I error due to multiple comparisons, four separate MANCOVAs evaluated gray and white matter in the left and right hemispheres within each lobe.
Functional Differences during Decoding Task.
Descriptive values for Accuracy and Response Time for JOL and NWR tasks were calculated. Repeated Measures ANOVA was used to explore any potential between group (i.e., cleft and reading status) and within group (i.e., stimuli [JOL vs NWR] and temporal [Run 1 vs Run 2] differences. Finally, possible relationship to age was evaluated using Pearson correlation.
Analysis of functional data was conducted using Analysis of Functional Neuroimages (AFNI47). The BOLD signal is convolved with an ideal hemodynamic response function (HRF) and intra-subject motion parameters. Individuals’ data were slice-time corrected, despiked, then motion-corrected, spatially Gaussian smoothed (10.5mm FWHM), normalized, and analyzed using general linear modeling. The resultant beta coefficients were subjected to ANOVA testing (controlling for scanner) to extract significant voxels of neural activity during the visual control (JOL) and target decoding (NWR) tasks across the whole brain. Next, contrasts of NWR>JOL were evaluated; effectively removing regional activity associated with simple visualization of words and isolating findings to regions associated specifically with decoding the words. Significant regions of activation for each sub-group (i.e., uAR, uIR, cAR, and cIR) as well as between group differences to evaluate main effects of cleft (i.e., unaffected vs iCL/P) and reading (i.e., average vs impaired reading) within the NWR>JOL contrast were identified. This sub sample was not powerful enough to evaluate interaction effects.
Results
Sample Characteristics
The sample included 79 participants (Table 1). Among unaffected participants, 4 had reading scores incongruent with their target recruitment group (i.e., reading scores for 2 uAR participants fell in the impaired range and reading scores for 2 uIR participants fell in the average range), and were excluded from analyses. Eleven participants in the iCL/P sample (44%) had reading achievement that fell within the impaired range (0 iCL, 6 iCP, and 5 iCLP) while 15 had average reading scores (6 iCL, 4 iCP, and 5 iCLP).
Table 1.
Demographics.
| Unaffected | iCL/P | Cleft Status | Reading Status | Interaction | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AR | IR | AR | IR | |||||||||||
| N = 26 | N = 27 | N = 15 | N = 11 | |||||||||||
| M | SE | M | SE | M | SE | M | SE | F | η2 | F | η2 | F | η2 | |
| Age | 10.00 | 0.22 | 9.99 | 0.21 | 10.19 | 0.28 | 9.65 | 0.33 | 0.08 | <.01 | 1.06 | .01 | 1.00 | .01 |
| SES | 2.04 | 0.10 | 2.19 | 0.10 | 2.40 | 0.13 | 2.55 | 0.15 | 8.66 | .10 | 1.42 | .02 | <0.01 | <.01 |
| 1 (n) | (1) | (1) | (0) | (0) | ||||||||||
| 2 (n) | (23) | (20) | (10) | (6) | ||||||||||
| 3 (n) | (2) | (6) | (4) | (4) | ||||||||||
| 4 (n) | (0) | (0) | (1) | (1) | ||||||||||
| 5 (n) | (0) | (0) | (0) | (0) | ||||||||||
| GA^ | 110.29 | 1.86 | 104.53 | 1.71 | 109.41 | 2.32 | 99.14 | 2.91 | 1.80 | .03 | 12.92 | .15 | 1.05 | .02 |
| W^ | 104.32 | 2.07 | 80.61 | 1.99 | 105.58 | 2.70 | 87.88 | 3.20 | 2.63 | .03 | 67.76 | .48 | 1.45 | .02 |
| W^ | 105.41 | 2.28 | 84.81 | 2.19 | 102.82 | 2.97 | 87.94 | 3.53 | 0.01 | <.01 | 40.93 | .36 | 1.08 | .01 |
| ORF^ | 101.61 | 1.63 | 78.91 | 1.59 | 102.79 | 2.11 | 86.98 | 2.51 | 5.02 | .06 | 94.78 | .57 | 3.10 | .04 |
Note. SES (socioeconomic status) is based on a reversed-scored, 5 point Hollingshead scale (higher values reflect lower SES). Due to small cell sizes, effects of cleft and reading status were evaluated using ANOVA and SES as a continuous variable. GAI (Global Abilities Index), WI (Word Identification), WA (Word Attack), and ORF (Oral Reading Fluency); with mean of 100 and standard deviation of 15.
Mean and standard error values are estimated, controlling for socioeconomic status.
Main effects were significant at p < .05 (bold) and interaction effects were significant at p < .10 (bold).
ANOVA indicated no cleft or reading status main effects or interaction for age. The proportion of Caucasian participants did not significantly differ between unaffected participants (88.7%) and those with iCL/P (73.1%; χ2 (1, 79) = 3.089, p = .079). However, the groups differed significantly on SES (F (1, 75) = 8.658, p = .004), where the iCL/P group had lower SES. There was a main effect of reading status for GAI (F (1, 71) = 12.922, p = .001; those with impaired reading had lower GAI). As expected, Impaired Readers had significantly lower performance on reading measures. Using a p < .01 cut-off for interaction effects, the cleft type by reading interaction effect was significant for Oral Reading Fluency (F (1, 73) = 3.103, p = .082); the uIR group had lower fluency scores than the cIR group (mean = 78.906 vs 86.983, respectively).
Head Circumference and Global Structural Differences
The ANCOVAs evaluating global structural differences resulted in no significant main effects or cleft by reading status interaction (See Table 2).
Table 2.
Head Circumference and Global Brain Volumes after COMBAT Harmonization and Power Proportion Correction.
| Unaffected | iCL/P | Cleft Status | Reading Status | Interaction | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
AR N = 26 |
IR N = 27 |
AR N = 15 |
IR N = 11 |
|||||||||||
| M | SE | M | SE | M | SE | M | SE | F | η2 | F | η2 | F | η2 | |
| HC | 21.351 | 0.161 | 21.014 | 0.152 | 21.741 | 0.206 | 21.417 | 0.244 | 3.874 | .050 | 2.953 | .039 | 0.001 | < .001 |
| ICV | 1,514,927 | 23,435 | 1,512,957 | 22,491 | 1,520,194 | 30,480 | 1,535,257 | 36186 | 0.215 | .003 | 0.053 | .001 | 0.091 | .001 |
| Whole Brain* | 1,345,921 | 19,419 | 1,310,591 | 18,637 | 1,358,700 | 25,257 | 1,361,093 | 29985 | 1.647 | .022 | 0.488 | .007 | 0.653 | .009 |
| Cerebrum* | 1,175,749 | 18,547 | 1,154,699 | 17,799 | 1,205,293 | 24,122 | 1,199,900 | 28638 | 2.519 | .033 | 0.345 | .005 | 0.123 | .002 |
| Cerebellum* | 144,493 | 2,249 | 141,936 | 2,158 | 138,325 | 2,925 | 140,991 | 3472 | 1.552 | .021 | <0.001 | < .001 | 0.933 | .012 |
Note. HC (Head Circumference) is in inches. ICV (Inner-Cranial Volume) is COMBAT harmonized in cubic mm.
Volumes are COMBAT harmonized and power proportions to ICV in cubic mm.
All mean and standard error values are estimated, controlling for socioeconomic status.
Cerebral Lobe Differences
There were no significant main or interaction effects when the total volume within each of the 4 cerebral lobes were assessed (See Table 3). However, significant differences were observed for the frontal and occipital lobes when gray and white matter were examined separately for the left and right hemisphere. Within the frontal lobe, there was a main effect for cleft type (F (1, 74) = 4.927, p = .030) where participants with a cleft were more likely to have more left gray matter than those who were unaffected. There was also a main effect for reading status (F (1, 74) = 5.342, p = .024) where participants with average reading had more gray matter in the right hemisphere than those with impaired reading. In the occipital lobe, the cleft by reading interaction effect significantly predicted gray F (1, 74) = 6.067, p = .016) and white matter (F (1, 74) = 5.296, p = .024) volume in the right hemisphere. The cIR group had increased right gray and white matter where these volumes for cAR participants were either equivalent to or lower than the volumes for the uIR and uAR groups (See Figure 2).
Table 3.
Gray and White Matter Volumes by Cerebral Lobe and Hemisphere after COMBAT Harmonization and Power Proportion Correction.
| Unaffected | iCL/P | Cleft Status | Reading Status | Interaction | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
AR N = 26 |
IR N = 27 |
AR N = 15 |
IR N = 11 |
|||||||||||
| M | SE | M | SE | M | SE | M | SE | F | η2 | F | η2 | F | η2 | |
| Frontal | 494,686 | 7,896 | 482,898 | 7,578 | 514,427 | 10,270 | 499,125 | 12,193 | 3.217 | .042 | 1.998 | .026 | 0.034 | < .001 |
| Left Gray | 142,409 | 1,972 | 139,657 | 1,892 | 148,984 | 2,564 | 144,196 | 3,044 | 4.927 | .062 | 2.482 | .032 | 0.184 | .002 |
| Right Gray | 142,770 | 1,983 | 140,437 | 1,903 | 149,745 | 2,579 | 140,954 | 3,062 | 2.214 | .029 | 5.342 | .067 | 1.834 | .024 |
| Left White | 103,975 | 2,111 | 102,960 | 2,026 | 104,451 | 2,745 | 107,392 | 3,259 | 0.839 | .011 | 0.141 | .002 | 0.608 | .008 |
| Right White | 104,704 | 2,046 | 102,488 | 1,964 | 104,352 | 2,662 | 105,628 | 3,160 | 0.288 | .004 | 0.036 | .000 | 0.504 | .007 |
| Parietal | 269,546 | 4,082 | 267,613 | 3,918 | 268,126 | 5,310 | 266,225 | 6,304 | 0.073 | .001 | 0.150 | .002 | <.001 | < .001 |
| Left Gray | 86,330 | 1,236 | 85,691 | 1,186 | 85,652 | 1,608 | 87,154 | 1,909 | 0.063 | .001 | 0.083 | .001 | 0.519 | .007 |
| Right Gray | 83,296 | 1,150 | 83,009 | 1,103 | 84,906 | 1,495 | 81,223 | 1,775 | 0.004 | < .001 | 2.025 | < .001 | 1.509 | .020 |
| Left White | 47,856 | 977 | 47,736 | 938 | 46,234 | 1,271 | 47,616 | 1,509 | 0.493 | .007 | 0.283 | .007 | 0.409 | .005 |
| Right White | 51,184 | 1,056 | 51,110 | 1,014 | 50,137 | 1,374 | 50,228 | 1,631 | 0.518 | .007 | <0.001 | .007 | 0.004 | < .001 |
| Temporal | 223.011 | 3.406 | 226.158 | 3.269 | 222.604 | 4.430 | 221.304 | 5.259 | 0.370 | .005 | 0.050 | .001 | 0.295 | .004 |
| Left Gray | 82,496 | 1,314 | 84,314 | 1,261 | 83,858 | 1,710 | 82,523 | 2,030 | 0.016 | < .001 | 0.023 | < .001 | 0.996 | .013 |
| Right Gray | 84,337 | 1,161 | 85,500 | 1,114 | 84,312 | 1,510 | 83,233 | 1,793 | 0.605 | .008 | 0.001 | < .001 | 0.645 | .009 |
| Left White | 29,184 | 613 | 28,962 | 588 | 28,617 | 797 | 28,910 | 946 | 0.158 | .002 | 0.002 | < .001 | 0.122 | .002 |
| Right White | 27,058 | 492 | 27,061 | 472 | 26,177 | 639 | 27,064 | 759 | 0.496 | .007 | 0.557 | .007 | 0.559 | .008 |
| Occipital | 131,643 | 2,878 | 129,962 | 2,762 | 132,700 | 3,743 | 141,975 | 4,443 | 3.199 | .041 | 1.182 | .016 | 2.507 | .033 |
| Left Gray | 47,812 | 1,141 | 48,236 | 1,095 | 49,675 | 1,484 | 50,210 | 1,761 | 1.755 | .023 | 0.120 | .002 | 0.002 | .000 |
| Right Gray | 48,739 | 952 | 47,275 | 914 | 49,266 | 1,238 | 53,441 | 1,470 | 7.663 | .094 | 1.376 | .018 | 6.067 | .076 |
| Left White | 17,405 | 505 | 17,288 | 485 | 16,785 | 657 | 17,578 | 780 | 0.066 | .001 | 0.304 | .004 | 0.561 | .008 |
| Right White | 17,709 | 493 | 17,475 | 473 | 16,804 | 641 | 19,295 | 761 | 0.534 | .007 | 3.565 | .046 | 5.296 | .067 |
Note. All measures are COMBAT harmonized and power proportions to ICV in cubic mm, controlling for socioeconomic status. Main effects were significant at p < .05 (bold) and interaction effects were significant at p < .10 (bold).
Figure 2.
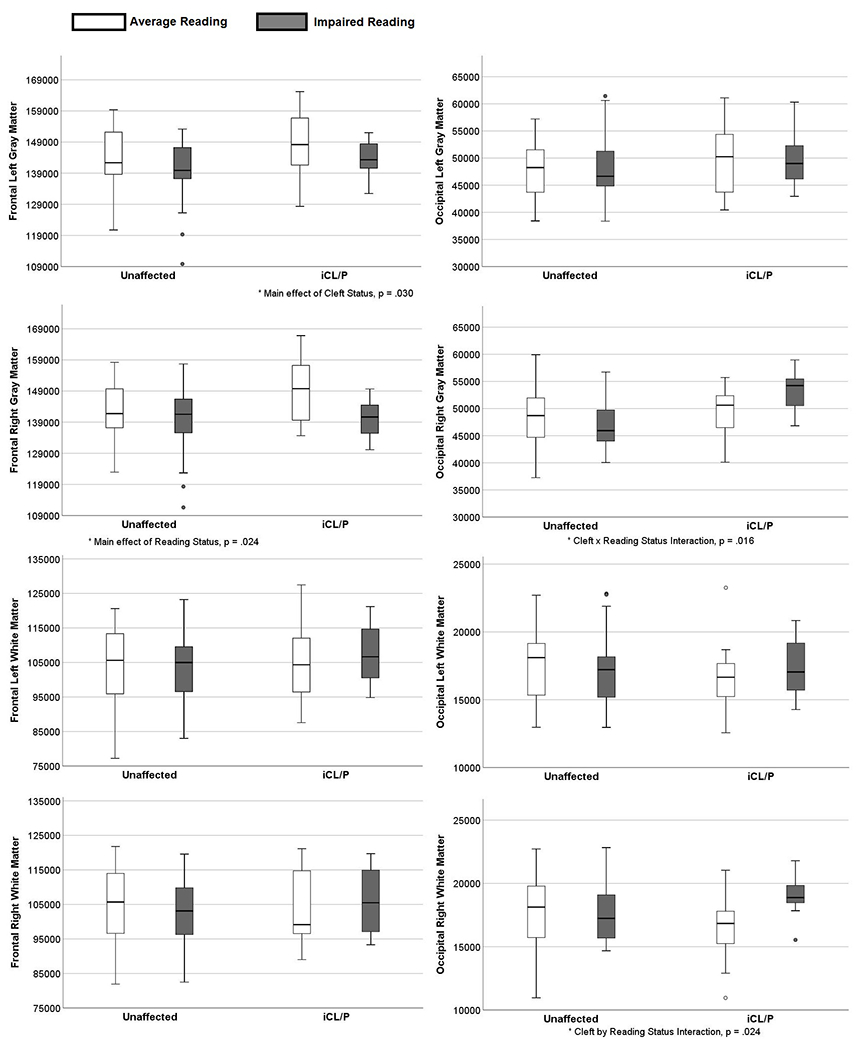
COMBAT Harmonized and Power Proportions for Gray and White Matter in Frontal and Occipital Lobes by Group.
Functional Differences during Decoding Task
fMRI data was successfully obtained and processed for 67 participants (22 uAR, 27 uIR, 9 cAR [3 iCL, 1 iCP, 5 iCLP], 9 cIR [4 iCP, 5 iCLP]). Cases of uncessessful processing was due to excessive motion. Accuracy and Response Time across Runs 1 and 2 of JOL and NWR tasks are presented in Table 4. Results of Repeated Measures ANOVA indicated that all participants demonstrated improved accuracy on both JOL and NWR tasks between Run 1 and 2 (F (1, 61) = 21.547, p < .001). Additionally, while all participants took longer to respond (F (1, 59) = 87.648, p < .001) and performed lower (F (1, 61) = 41.694, p < .001) on NWR items compared to JOL items, the difference in accuracy was significantly greater in those with impaired reading (F (1, 61) = 8.129, p = .006). Finally, results of Pearson correlation indicated that age was significantly correlated to accuracy on JOL (i.e., accuracy increased with age; r (67) = .436, p < .001), but not for NWR (r (67) = .212, p = .085).
Table 4.
In-Scanner Task Average Accuracy (Percent Correct) and Response Time (ms).
| Unaffected | iCL/P | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AR | IR | AR | IR | |||||||
| N = 22 | N = 27 | N = 9 | N = 9 | N = 67 | ||||||
| Mean [SD] | Range | Mean [SD] | Range | Mean [SD] | Range | Mean [SD] | Range | Mean [SD] | Range | |
| Judgement of Line | ||||||||||
| Total Accuracy | 77 [14] | 44 - 97 | 74 [15] | 31 - 97 | 83 [26] | 19 - 100 | 72 [13] | 56 - 97 | 76 [16] | 19 - 100 |
| Run 1 | 74 [16] | 44 - 100 | 70 [18] | 13 - 94 | 81 [24] | 25 - 100 | 67 [17] | 44 - 94 | 73 [18] | 13 - 100 |
| Run 2 | 81 [16] | 38 - 100 | 78 [14] | 50 - 100 | 85 [29] | 13 - 100 | 76 [12] | 56 - 100 | 80 [17] | 13 - 100 |
| Total Response Time | 1754 [223] | 1434 - 2334 | 1904 [244] | 1459 - 2463 | 1818 [183] | 1502 - 2025 | 2081 [318] | 1592 - 2571 | 1867 [259] | 1434 - 2571 |
| Run 1 | 1760 [296] | 1381 - 2436 | 1913 [286] | 1215 - 2585 | 1927 [182] | 1679 - 2178 | 2095 [389] | 1434 - 2598 | 1889 [307] | 1215 - 2598 |
| Run 2 | 1749 [211] | 1396 - 2336 | 1896 [271] | 1342 - 2413 | 1709 [204] | 1326 - 1905 | 2068 [344] | 1749 - 2544 | 1846 [276] | 1326 - 2544 |
| Non-Word Rhyming | ||||||||||
| Total Accuracy | 69 [12] | 34 - 84 | 49 [17] | 0 - 72 | 74 [16] | 41 - 94 | 52 [13] | 34 - 72 | 59 [18] | 0 - 94 |
| Run 1 | 62 [18] | 0 - 88 | 47 [17] | 0 - 69 | 71 [18] | 31 - 94 | 47 [19] | 19 - 81 | 55 [20] | 0 - 94 |
| Run 2 | 75 [11] | 44 - 94 | 50 [20] | 0 - 88 | 77 [14] | 50 - 94 | 57 [16] | 31 - 81 | 63 [20] | 0 - 94 |
| Total Response Time | 2189 [266] | 1587 - 2616 | 2352 [429] | 1325 - 3220 | 2316 [398] | 1745 - 3128 | 2276 [476] | 1574 - 2905 | 2282 [382] | 1325 - 3220 |
| Run 1 | 2191 [363] | 1582 - 2986 | 2308 [422] | 1367 - 3126 | 2307 [341] | 1769 - 2916 | 2089 [496] | 1110 - 2788 | 2240 [403] | 1110 - 3126 |
| Run 2 | 2180 [263] | 1593 - 2714 | 2395 [488] | 1282 - 3390 | 2325 [478] | 1721 - 3340 | 2464 [545] | 1917 - 3148 | 2323 [436] | 1282 - 3390 |
Z-Max values for significant clusters of activation during NWR (removing the effects of JOL) are presented for each group in Figure 3 and Table 5. Between group differences in clusters of activation were not significant for the main effect of cleft. Post-hoc analysis evaluating participants with and without iCL/P separately for average vs impaired readers found that among average readers, those who were unaffected had significantly higher activation bilaterally in Wernicke’s area. No significant differences were found between impaired readers with or without iCL/P.
Figure 3.
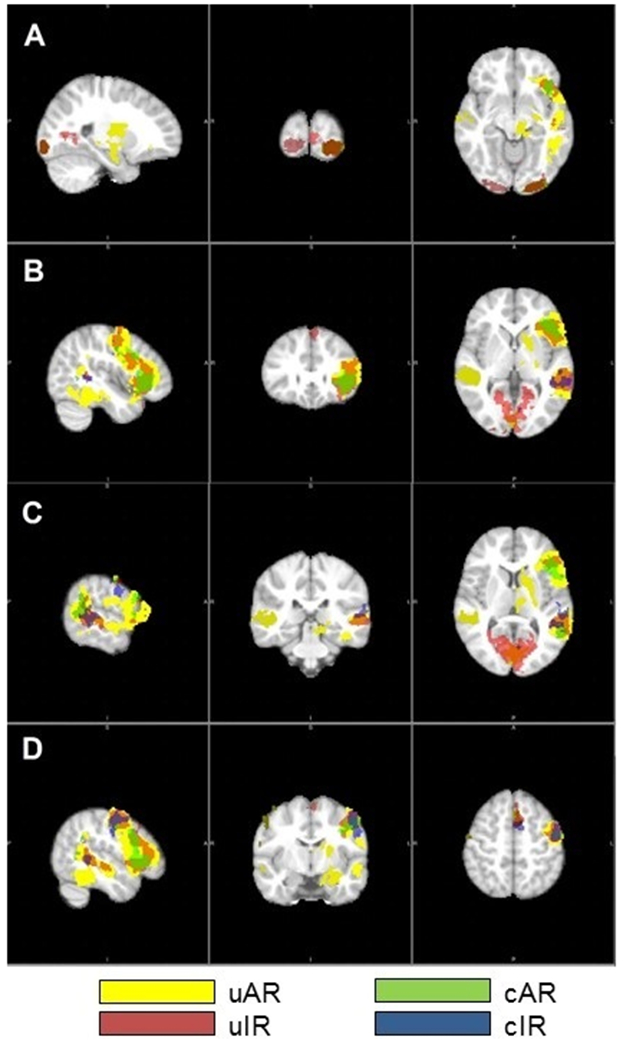
Clusters of Significant Activation during NWR Task. Images are presented in radiological orientation (LAS) with coordinates listed as x/y/z. A) Left Visual Association Area (BA 18: −24/−96/−10), B) Left Broca’s Area (BA 45: −44/28/2), C) Left Wernicke’s Area (BA 22: −60/−32/8), and D) Left Primary Motor Cortex (BA 4: −50/−8/−54).
Table 5.
Significant Clusters of Activation (NWR>JOL) for each Group.
| Unaffected | iCL/P | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
AR N = 22 |
IR N = 27 |
AR N = 9 |
IR N = 9 |
||||||||||||||
| Name | BA | Z-Max | x | y | z | Z-Max | x | y | z | Z-Max | x | y | z | Z-Max | x | y | z |
| Left Hemisphere | |||||||||||||||||
| Triangularis (Broca’s Area) | 45 | 3.72 | −44 | 28 | 2 | ||||||||||||
| Orbitalis | 47 | 6.32 | −42 | 30 | −2 | 6.26 | −40 | 32 | −4 | ||||||||
| Superior Temporal Gyrus (Wernicke’s Area)^ | 22 | 5.04 | −62 | −40 | 10 | 3.88 | −60 | −32 | 8 | ||||||||
| Angular Gyrus | 39 | 4.75 | −56 | −56 | 8 | ||||||||||||
| Supramarginal Gyrus# | 40 | ||||||||||||||||
| Premotor Cortex# | 6 | 5.95 | −2 | 6 | 66 | 5.64 | −2 | −2 | 64 | 3.71 | 0 | 2 | 66 | ||||
| Primary Motor Cortex | 4 | 4.05 | −50 | −8 | 54 | ||||||||||||
| Visual Association Area# | 19 | ||||||||||||||||
| Visual Association Area | 18 | 6.40 | −24 | −96 | −10 | 6.73 | −24 | −96 | −6 | ||||||||
| Hippocampus | 54 | 4.41 | −20 | 2 | 8 | ||||||||||||
| Right Hemisphere | |||||||||||||||||
| Frontal Cortex | 9 | 4.12 | 34 | 44 | 36 | ||||||||||||
| Superior Temporal Gyrus (Wernicke’s Area)^ | 22 | 4.66 | 62 | −38 | 6 | ||||||||||||
| Primary Motor Cortex | 4 | 3.33 | 66 | 0 | 16 | ||||||||||||
| Visual Association Area | 18 | 3.61 | 2 | −88 | 14 | 6.06 | 24 | −96 | −8 | 3.52 | 6 | −82 | 18 | ||||
| Visual Association Area | 17 | 4.86 | 6 | −74 | −18 | ||||||||||||
| Cerebellum | − | 4.01 | 10 | −80 | −38 | ||||||||||||
Note. BA (Brodman’s Area).
Unaffected Average Readers > Unaffected Impaired Readers.
Unaffected Average Readers > Average Readers with iCL/P.
Differences significant at p < .05.
For the main effect of reading, those with average reading scores had more activation in 3 clusters: the left premotor cortex, visual association area, and supramarginal gyrus. Post-hoc analysis evaluating average vs impaired readers separately for participants with and without iCL/P indicated that these findings were driven by the unaffected group; no significant differences between average and impaired readers with iCL/P were found.
Discussion
The current study sought to increase understanding of brain structure and function related to reading in boys with iCL/P contrasted to unaffected participants with average or impaired reading. Our approach offered the opportunity to examine differences related to the presence of a cleft, the presence of impaired reading, and potential interactions.
Expected Activation in Unaffected Readers
Unaffected average readers demonstrated significant activation in expected fronto-temporal (Broca’s area) and occipito (visual association area) systems. Activation for this group was higher in the left supramarginal gyrus, visual association area, and premotor cortex compared to unaffected impaired readers. In contrast, unaffected impaired readers also had activation in the occipito (visual association area) system, and fronto-temporal and parieto-temporal activity was stronger in the orbitalis and Wernicke’s area (respectively). These activation patterns support the validity of the nonword rhyming task in eliciting word decoding networks.
Increased Frontal Gray Matter
Results demonstrated increased gray matter volume in the left frontal lobe among participants with iCL/P and in the right frontal lobe for those with average reading. Both of these increases are moderate in effect size and appear to be driven by the cAR group. Previous research on frontal volumes among children with cleft has been mixed; Adamson55 found increased volumes while Nopoulos24 found decreased volumes. However, both of these studies included males and females and sex differences may have impacted overall findings.
In contrast to findings of increased frontal gray volume among participants with cleft and average reading scores, their frontal lobe activation during the decoding task was limited. Those with average reading had activation in the left orbitalis (similar to those with idiopathic dyslexia) and the left angular gyrus. Those with impaired reading had activation in the left superior temporal gyrus (Wernicke’s Area; similar to those with idiopathic dyslexia). The lack of activation in the left inferior frontal gyrus (Broca’s Area) is consistent with findings of hypo-activation within Broca’s Area among boys with iCP compared to unaffected controls with average reading,56 though the differences in activation did not reach significance for the current sample.
It is unclear if the increased frontal volume offers a compensatory element for those with cleft. A previous publication on this sample39 found that for participants with iCL/P, better reading was associated with increased attention (a skill associated with frontal lobe function). This hypothesis could be evaluated with a larger sample, region of interest segmentation within the frontal lobe, and measures of resting-state fMRI and white matter tractogrophy that would provide information on frontal lobe connectivity and relation to reading.
Increased Right Occipital Matter and Activation Patterns for Impaired Readers with iCL/P
A key finding in brain structure and function was that cIR boys had significantly increased gray and white matter in the right occipital lobe. (This increase was not found in the uIR group.) Increased occipital matter among children with cleft was also found by Adamson55 and Nopoulos,24 while Bodini25 found decreased volume in the right occipital pole. This is the first time those findings have been viewed by reading abilitiy.
Further, the cIR group demonstrated significant activation in the right visual association area during the reading task. In the previous pilot study,56 it was found that in addition to decreased activation within the left inferior frontal gyrus (fronto-temporal system), impaired reading in iCP was also associated with increased activation in the left lingual gyrus (the occipito-temporal system). The current findings are consistent with lack of fronto-temporal system activation, but demonstrate occipito-temporal activation more posteriorally and limited to the right hemisphere. These differences may be due to the fact that groups were not divided by reading ability in the pilot study as they were in the current analyses.
One possible interpretation of this connection is that for children with iCL/P with poor reading, increased connections within the occipital lobe (visual processing) are being used to compensate for poor phonological processing (a fronto-temporal process). Neuropsychological testing in this sample identified a significant deficit in auditory rote memory for participants with iCL/P, as well as deficits in reading being associated with issues in phonological awareness, rapid automatized naming, rote memory, and auditory working memory.39 A connection between poor auditory memory and other early speech/language skills have also been found in other samples of boys and girls with iCL/P.3,57
A significant limitation of this study is the lower power for analyzing functional data; 12 participants did not have usable fMRI scans which reduced numbers within the cAR and cIR subgroups (n = 9 each). It is possible that true differences in activation for participants with iCL/P were not identified. Additionally, the sample size of participants with iCL/P was not large enough to evaluate if findings were due to possible cleft type differences. Previous work has suggested that males with iCL are less likely to demonstrate reading deficits compared to those with iCLP and iCP.35,39 Moreover, socioeconomic status was significantly lower for participants with iCL/P vs unaffected controls. Lower SES was associated with lower intelligence (F (1, 71) = 9.190, p = .003) and Oral Reading Fluency scores (F (1, 73) = 8.731, p = .004) as well as lower inner-cranial volume (F (1, 74) = 5.403, p = .023). While socioeconomic was controlled for statistically and the relationship to brain structure/activation was only significant for ICV, the possibility that lower SES among participants with iCL/P may have had some contribution to outcomes cannot be fully ruled out. Contrasts between groups from equivalent socioeconomic backgrounds would better control for any impact this may have on outcomes and should be included in future studies. Finally, the sample is limited to males, restricting generalization of these findings. Future work will need to obtain a larger and more diverse sample, so that potential cleft type and sex effects can adequately be explored.
Conclusion
This study adds to the growing work evaluating neuronal associations to language and reading deficits commonly found among patients with iCL/P. Overall, structural differences were driven by participants with iCL/P, adding to literature supporting disrupted neural development. Among participants with iCL/P, increased frontal gray matter was associated with average reading skills – possibly reflective of efficient compensation of attention/executive functioning networks. However, increased volume in the occipital lobe among participants with iCL/P was associated with impaired reading and disrupted activation during the decoding task. This suggest altered pathways being used for reading – possibly inefficient compensation of the visual system. Further exploration of these findings can help improve clinical screening of reading issues among children with iCL/P (e.g., including measures of phonological awareness, auditory memory, and attention/executive function) and guide appropriate intervention.
Acknowledgements:
The authors would like to thank Decoding Dyslexia (Iowa and Illinois chapters) and the University of Iowa Craniofacial Clinic for their assistance in recruitment. We also would like to express our appreciation to the families who gave their time and effort to this study.
Funding Source: Research reported in this publication was supported by the National Institutes of Health [grant numbers DE024511 and UL1TR002537]. This work was conducted on an MRI instrument funded by 1S10OD025025-01.
Role of the Funder/Sponsor: The National Institutes of Health had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
Abbreviations:
- iCL/P
isolated cleft lip and/or palate
- iCL
isolated cleft lip only
- iCLP
isolated cleft lip and palate
- iCP
isolated cleft palate only
- uAR
(unaffected average reader
- uIR
unaffected impaired reader
- cAR
average reader with iCL/P
- cIR
impaired reader with iCL/P
Footnotes
Potential Conflicts of Interest: The authors have no conflicts of interest relevant to this article to disclose.
References
- 1.Clausen NG, Pedersen DA, Pedersen JK, et al. Oral Clefts and Academic Performance in Adolescence: The Impact of Anesthesia-Related Neurotoxicity, Timing of Surgery, and Type of Oral Clefts. The Cleft palate-craniofacial journal : official publication of the American Cleft Palate-Craniofacial Association. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collett BR, Stott-Miller M, Kapp-Simon KA, Cunningham ML, Speltz ML. Reading in children with orofacial clefts versus controls. J Pediatr Psychol. 2010;35(2):199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conrad AL, McCoy TE, DeVolder I, Richman LC, Nopoulos P. Reading in subjects with an oral cleft: speech, hearing and neuropsychological skills. Neuropsychology. 2014;28(3):415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gallagher ER, Collett BR. Neurodevelopmental and Academic Outcomes in Children With Orofacial Clefts: A Systematic Review. Pediatrics. 2019;144(1). [DOI] [PubMed] [Google Scholar]
- 5.Goodwin JW, Conrad AL, Ansley T, Nopoulos P. Arithmetical calculation and related neuropsychological skills in subjects with isolated oral clefts. Neuropsychology. 2017;31(7):834–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Persson M, Becker M, Conrad AL, Svensson H. Female and Male Differences in Academic Achievement in Individuals With Cleft: A Population-Based Register Study. The Cleft palate-craniofacial journal : official publication of the American Cleft Palate-Craniofacial Association. 2018;55(2):196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wehby GL, Collett BR, Barron S, Romitti P, Ansley T. Children with oral clefts are at greater risk for persistent low achievement in school than classmates. Archives of disease in childhood. 2015;100(12):1148–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hopkins EE, Wallace ML, Conley YP, Marazita ML. Symptoms of attention-deficit hyperactivity disorder, nonsyndromic orofacial cleft children, and dopamine polymorphisms: a pilot study. Biological research for nursing. 2015;17(3):257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conrad AL, Richman L, Nopoulos P, Dailey S. Neuropsychological functioning in children with non-syndromic cleft of the lip and/or palate. Child Neuropsychol. 2009;15(5):471–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Persson M, Becker M, Svensson H. General intellectual capacity of young men with cleft lip with or without cleft palate and cleft palate alone. Scand J Plast Reconstr Surg Hand Surg. 2008;42(1):14–16. [DOI] [PubMed] [Google Scholar]
- 11.Hunt O, Burden D, Hepper P, Stevenson M, Johnston C. Self-reports of psychosocial functioning among children and young adults with cleft lip and palate. The Cleft palate-craniofacial journal : official publication of the American Cleft Palate-Craniofacial Association. 2006;43(5):598–605. [DOI] [PubMed] [Google Scholar]
- 12.Hunt O, Burden D, Hepper P, Stevenson M, Johnston C. Parent reports of the psychosocial functioning of children with cleft lip and/or palate. The Cleft palate-craniofacial journal : official publication of the American Cleft Palate-Craniofacial Association. 2007;44(3):304–311. [DOI] [PubMed] [Google Scholar]
- 13.Kapp-Simon KA. A brief overview of psychological issues in cleft lip and palate. In: Berkowitz S, ed. Cleft Lip and Palate. 2nd ed. New York: Springer; 2006:257–261. [Google Scholar]
- 14.Nicholls W, Selvey LA, Harper C, Persson M, Robinson S. The Psychosocial Impact of Cleft in a Western Australian Cohort Across 3 Age Groups. The Cleft palate-craniofacial journal : official publication of the American Cleft Palate-Craniofacial Association. 2018:1055665618769660. [DOI] [PubMed] [Google Scholar]
- 15.Boes AD, Murko V, Wood JL, et al. Social function in boys with cleft lip and palate: relationship to ventral frontal cortex morphology. Behav Brain Res. 2007;181(2):224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van der Plas E, Koscik TR, Conrad AL, Moser DJ, Nopoulos P. Social motivation in individuals with isolated cleft lip and palate. J Clin Exp Neuropsychol. 2013;35(5):489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conrad AL, Goodwin JW, Choi J, Block RI, Nopoulos P. The Relationship of Exposure to Anesthesia on Outcomes in Children With Isolated Oral Clefts. Journal of child neurology. 2017;32(3):308–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laub DR Jr., Williams RK. Neonatal Anesthesia Neurotoxicity: A Review for Cleft and Craniofacial Surgeons. The Cleft palate-craniofacial journal : official publication of the American Cleft Palate-Craniofacial Association. 2015;52(4):494–498. [DOI] [PubMed] [Google Scholar]
- 19.Jocelyn LJ, Penko MA, Rode HL. Cognition, communication, and hearing in young children with cleft lip and palate and in control children: a longitudinal study. Pediatrics. 1996;97(4):529–534. [PubMed] [Google Scholar]
- 20.Lamb MM, Wilson FB, Leeper HA Jr. A comparison of selected cleft palate children and their siblings on the variables of intelligence, hearing loss, and visual-perceptual-motor abilities. Cleft Palate Journal. 1972;9:218–228. [PubMed] [Google Scholar]
- 21.Schonweiler R, Lisson JA, Schonweiler B, et al. A retrospective study of hearing, speech and language function in children with clefts following palatoplasty and veloplasty procedures at 18–24 months of age. Int J Pediatr Otorhinolaryngol. 1999;50(3):205–217. [DOI] [PubMed] [Google Scholar]
- 22.Conrad AL, Nopoulos P, Richman LC. Neuropsychological and Neuroimaging Aspects of Cleft Lip and Palate. In: Losee J, Kirschner RE, eds. Comprehensive Cleft Care, 2nd Edition: Two Volume Set. Vol 1. CRC Press; 2015. [Google Scholar]
- 23.Nopoulos P, Berg S, Canady J, Richman L, Van Demark D, Andreasen NC. Abnormal brain morphology in patients with isolated cleft lip, cleft palate, or both: a preliminary analysis. The Cleft palate-craniofacial journal : official publication of the American Cleft Palate-Craniofacial Association. 2000;37(5):441–446. [DOI] [PubMed] [Google Scholar]
- 24.Nopoulos P, Langbehn DR, Canady J, Magnotta V, Richman L. Abnormal brain structure in children with isolated clefts of the lip or palate. Arch Pediatr Adolesc Med. 2007;161(8):753–758. [DOI] [PubMed] [Google Scholar]
- 25.Bodoni PSB, Leoni RF, do Vale AB, et al. Neuropsychological functioning and its relationship with brain anatomical measures of children and adolescents with non-syndromic cleft lip and palate. Child Neuropsychol. 2020:1–15. [DOI] [PubMed] [Google Scholar]
- 26.Yang FF, McPherson B, Shu H, Xie N, Xiang K. Structural abnormalities of the central auditory pathway in infants with non-syndromic cleft lip and/or palate. The Cleft palate-craniofacial journal : official publication of the American Cleft Palate-Craniofacial Association. 2011. [DOI] [PubMed] [Google Scholar]
- 27.Conrad AL, Wermke K, Eisenmann M, et al. Preliminary Evaluation of Pre-Speech and Neurodevelopmental Measures in 7–11 week Old Infants with Isolated Oral Clefts. Pediatric Research. 2020;Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nopoulos P, Boes AD, Jabines A, et al. Hyperactivity, impulsivity, and inattention in boys with cleft lip and palate: relationship to ventromedial prefrontal cortex morphology. J Neurodev Disord. 2010;2(4):235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shriver AS, Canady J, Richman L, Andreasen NC, Nopoulos P. Structure and function of the superior temporal plane in adult males with cleft lip and palate: pathologic enlargement with no relationship to childhood hearing deficits. Journal of Child Psychology and Psychiatry. 2006;47(10):994–1002. [DOI] [PubMed] [Google Scholar]
- 30.Conrad AL, Dailey S, Richman L, et al. Cerebellum Structure Differences and Relationship to Speech in Boys and Girls With Nonsyndromic Cleft of the Lip and/or Palate. The Cleft palate-craniofacial journal : official publication of the American Cleft Palate-Craniofacial Association. 2010;47(5):469–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nopoulos P, Choe I, Berg S, Van Demark D, Canady J, Richman L. Ventral frontal cortex morphology in adult males with isolated orofacial clefts: relationship to abnormalities in social function. Cleft Palate Craniofacial Journal. 2005;42(2):138–144. [DOI] [PubMed] [Google Scholar]
- 32.Goldsberry G, OĽeary D, Hichwa R, Nopoulos P. Functional abnormalities in the neural circuitry of reading in men with nonsyndromic clefts of the lip or palate. Cleft Palate Craniofacial Journal. 2006;43(6):683–690. [DOI] [PubMed] [Google Scholar]
- 33.Zhang W, Li C, Chen L, et al. Increased activation of the hippocampus during a Chinese character subvocalization task in adults with cleft lip and palate palatoplasty and speech therapy. Neuroreport. 2017;28(12):739–744. [DOI] [PubMed] [Google Scholar]
- 34.Conrad AL, Richman L, Nopoulos P. Reading Achievement in Boys With Non-Syndromic Cleft Palate Only: Relationship to Neuropsychological Skill and Neurocircuitry. Developmental neuropsychology. 2015;40(7–8):395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wehby GL, Collet B, Barron S, Romitti PA, Ansley TN, Speltz M. Academic achievement of children and adolescents with oral clefts. Pediatrics. 2014;133(5):785–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eicher JD, Montgomery AM, Akshoomoff N, et al. Dyslexia and language impairment associated genetic markers influence cortical thickness and white matter in typically developing children. Brain Imaging Behav. 2016;10(1):272–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skeide MA, Bazin PL, Trampel R, et al. Hypermyelination of the left auditory cortex in developmental dyslexia. Neurology. 2018. [DOI] [PubMed] [Google Scholar]
- 38.Williams VJ, Juranek J, Cirino P, Fletcher JM. Cortical Thickness and Local Gyrification in Children with Developmental Dyslexia. Cereb Cortex. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Conrad AL. Are predictors of reading impairment in isolated cleft similar to those in idiopathic dyslexia? Annals of dyslexia. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hollingshead AB. Four Factor Index of Social Status. New Haven: Department of Sociology, Yale University; 1975. [Google Scholar]
- 41.Wechsler. Wechsler Intelligence Scale for Children, Fifth Edition. United States of America: PsychCorp; 2014. [Google Scholar]
- 42.Woodcock RW. Woodcock Reading Mastery Tests, Third Edition Manual. Bloomington, MN: Pearson; 2011. [Google Scholar]
- 43.Fortin JP, Cullen N, Sheline YI, et al. Harmonization of cortical thickness measurements across scanners and sites. Neuroimage. 2018;167:104–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fortin JP, Parker D, Tunc B, et al. Harmonization of multi-site diffusion tensor imaging data. Neuroimage. 2017;161:149–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shaywitz BA, Skudlarski P, Holahan JM, et al. Age-related changes in reading systems of dyslexic children. Ann Neurol. 2007;61(4):363–370. [DOI] [PubMed] [Google Scholar]
- 46.Fonov V, Evans AC, Botteron K, et al. Unbiased average age-appropriate atlases for pediatric studies. Neuroimage. 2011;54(1):313–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. [DOI] [PubMed] [Google Scholar]
- 48.Tustison NJ, Avants BB, Cook PA, et al. N4ITK: improved N3 bias correction. IEEE Trans Med Imaging. 2010;29(6):1310–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pierson R, Johnson H, Harris G, et al. Fully automated analysis using BRAINS: AutoWorkup. Neuroimage. 2011;54(1):328–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang H, Suh JW, Das SR, Pluta JB, Craige C, Yushkevich PA. Multi-Atlas Segmentation with Joint Label Fusion. IEEE Trans Pattern Anal Mach Intell. 2013;35(3):611–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Desikan RS, Segonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. [DOI] [PubMed] [Google Scholar]
- 52.Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics. 2007;8(1):118–127. [DOI] [PubMed] [Google Scholar]
- 53.Koscik TR. ez.combat toolbox in R. In:2018. [Google Scholar]
- 54.Brown J Statistics Corner. JALT Testing & Evaluation SIG Newsletter. April 2008: 38–43. [Google Scholar]
- 55.Adamson CL, Anderson VA, Nopoulos P, Seal ML, Da Costa AC. Regional brain morphometric characteristics of nonsyndromic cleft lip and palate. Dev Neurosci. 2014;36(6):490–498. [DOI] [PubMed] [Google Scholar]
- 56.Conrad AL, Richman LC, Nopoulos P. Reading Achievement in Boys With Non-Syndromic Cleft Palate Only: Relationship to Neuropsychological Skill and Neurocircuitry. Developmental neuropsychology. 2015;40(7–8):395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chapman KL. The relationship between early reading skills and speech and language performance in young children with cleft lip and palate. The Cleft palate-craniofacial journal : official publication of the American Cleft Palate-Craniofacial Association. 2011;48(3):301–311. [DOI] [PubMed] [Google Scholar]


