Abstract
Profound skeletal muscle wasting in the setting of total body hypermetabolism is a defining characteristic of massive burns, compromising the patient’s recovery and necessitating a protracted period of rehabilitation. In recent years, the prolonged use of the non-selective beta-blocker, propranolol, has gained prominence as an effective tool to assist with suppressing epinephrine-dependent burn-induced hypermetabolism and by extension, blunting muscle catabolism. However, synthetic β-adrenergic agonists, such as clenbuterol, are widely associated with the promotion of muscle growth in both animals and humans. Moreover, experimental adrenodemedullation is known to result in muscle catabolism. Therefore, the blunting of muscle β-adrenergic signaling via the use of propranolol would be expected to negatively impair muscle protein homeostasis. This review explores these paradoxical observations and identifies the manner by which propranolol is thought to exert its anti-catabolic effects in burn patients. Moreover, we identify potential avenues by which the use of beta-blocker therapy in the treatment of massive burns could potentially be further refined to promote the recovery of muscle mass in these critically ill patients while continuing to ameliorate total body hypermetabolism.
Keywords: Hypermetabolism, muscle cachexia, lipolysis, beta-adrenergic signaling, burns, muscle protein turnover
Introduction:
Over recent decades, improvements in triage and hospital protocols have resulted in remarkable increases in survival following major burns [1]. Despite this, patients continue to experience profound muscle cachexia, in large part a consequence of the hypermetabolic response to burns. This cachexia is associated with an incessant need for metabolic substrates, which is partially met by the cannibalization of skeletal muscle (see [2]). In the acute phase after injury, patients with massive burns (> 40% total body surface area (TBSA) burnt) typically experience a 40-80% increase in metabolic rate. This hypermetabolism can remain elevated for up to two years post injury [3, 4] and places a sustained metabolic burden on skeletal muscle. The loss of skeletal muscle mass has wide-reaching consequences for the patient, impairing the body’s ability to fight infections, compromising wound repair, prolonging hospitalization, and increasing overall mortality (see [5]). As a result, these patients often require protracted hospital care and intensive physical rehabilitation before a potential return to normal daily activities can be contemplated [6, 7]. In an attempt to address these challenges, efforts have been focused on developing strategies that can reduce the hypermetabolic burden experienced by patients following burns, thereby minimizing the magnitude of muscle lost. In recent years, the use of the non-selective β-blocker, propranolol, has gained prominence as an effective tool to assist with suppressing the epinephrine-induced hypermetabolism of burns and by extension, blunting the ensuing skeletal muscle catabolism [8]. However, given that synthetic β2-adrenergic agonists (e.g. clenbuterol) are widely associated with muscle growth promotion in both animals and humans, it stands to reason that the blunting of muscle β-adrenergic signaling via the use of propranolol could impair mechanisms of muscle anabolism – a counterproductive scenario for the recovering burn patient. In response to these paradoxical observations, this review attempts to rationalize the known anabolic effects of enhanced β-adrenergic signaling in skeletal muscle with reports of the total-body metabolic benefits exerted by the use of propranolol in burn patients, and identify how the use of β-blockade to aid recovery from massive burns could be further refined to simultaneously ameliorate total-body hypermetabolism while preserving the potential anabolic effects of β-adrenergic signaling at the level of skeletal muscle.
The role of β-adrenergic signaling in skeletal muscle anabolism
The potent ability of enhanced β-adrenergic signaling to stimulate muscle growth has been appreciated by researchers for several decades and is best evidenced through the effects observed following chronic administration of synthetic β-receptor agonists, such as fenoterol and clenbuterol. While prohibited in many countries [9], β-agonists are a highly effective class of repartitioning agents in livestock, increasing both feed efficiency (i.e. body mass gained per unit of feed consumed) and lean carcass weights of animals at the expense of fat marbling [10]. Likewise, despite being banned by the World Anti-Doping Agency, the above attributes of β-adrenergic agonists have made them highly attractive to unscrupulous athletes seeking a competitive advantage, with clenbuterol doping compromising many elite level sports in past years [9]. The known and potent ability of β-adrenergic agonists to increase muscle weight has also led investigators to examine their therapeutic potential in numerous diseases and conditions characterized by cachexia. To date, researchers have shown the administration of clenbuterol to be effective at minimizing, and in some cases abolishing, the loss of muscle mass and/or protein content in response to experimental fasting [11], cancer [12, 13], denervation [14, 15], and major burns [16, 17].
Three subtypes of β-adrenergic receptors are expressed in skeletal muscle (β1, β2, and β3), with the growth promoting effects of clenbuterol primarily, albeit not necessarily exclusively, mediated via the action of the β2 subtype of adrenoreceptors [18]. The majority of the adrenoreceptors expressed in skeletal muscle are of the β2 subtype, with β1-adrenoreceptors accounting for ~7-10% of the adrenoreceptors [18]. The administration of β2-specific antagonists (e.g. ICI-118,551) greatly impedes clenbuterol’s ability to rescue muscle mass under atrophying conditions, highlighting the importance of the β2 receptor in mediating the anabolic actions of β-adrenergic agonists [19]. Crucially, propranolol as a non-specific β-blocker, impedes the action of β2-adrenergic signaling, with the antagonist’s administration shown to blunt the anabolic actions of clenbuterol [19]. Given that the use of propranolol for the treatment of massive burns has become more widespread in recent years [20], understanding the metabolic events that underpin muscle hypertrophy in response to β2-adrenergic signaling is crucial in the quest to develop effective treatment modalities aimed at preserving muscle mass and function after burns.
Because muscle proteins exist in a state of dynamic equilibrium, any change in muscle mass occurs as a consequence of a sustained imbalance in the rate at which muscle proteins are synthesized and degraded (lost) within the muscle cell (see [21]). Anabolic and catabolic events are thus underpinned by chronic changes in muscle protein kinetics that fail to maintain protein homeostasis. Studies investigating the impact of epinephrine (a non-specific β-agonist) or β2-adrenergic agonists on muscle protein turnover have revealed that both impart an anabolic action on skeletal muscle [19, 22] and modify multiple parameters governing protein turnover [22-26]. In particular, multiple reports have demonstrated that enhanced β-adrenergic agonism is highly effective at blunting muscle proteolysis, both under normal [25, 27-29] and cachectic conditions [15]. Moreover, 3-methylhistidine release, a byproduct of myofibrillar degradation, is reduced in skeletal muscle of clenbuterol treated animals [26], demonstrating that clenbuterol acts to spare the degradation of the muscle contractile apparatus.
In concordance with the ability of epinephrine to stimulate β2-adrenergic signaling, administration of the catecholamine to incubated chick muscle likewise reduces muscle proteolysis [25]. Moreover, the infusion of epinephrine in humans, so as to mimic circulating concentrations seen during times of stress such as after burns, further reinforces the anti-catabolic nature of the hormone. Epinephrine administration results in a rapid and dose-dependent fall in blood amino acid (AA) concentrations [23, 30], due in large part to a decreased efflux of AAs from bodily tissues [24], demonstrating that amino acids are likely being sequestered in cells due to reduced proteolytic rates. However, examination of AA kinetics across either the leg or forearm of participants infused epinephrine has revealed inconsistent results concerning the specific effects of hyperepinephrinemia on muscle protein metabolism. In contrast to the majority of experiments performed in isolated muscles of small animals, Kraenzlin and colleagues noted that net leucine release (i.e. proteolysis) from the forearm was increased with epinephrine infusion, an effect that was blocked by propranolol administration [23]. A direct catabolic effect of epinephrine on human muscle metabolism has not been replicated by other groups. Fryberg and colleagues, using similar experimental approaches as described by Kraenzlin et al., observed that phenylalanine appearance from the forearm is suppressed with epinephrine infusion [31], consistent with a decline in blood AA concentrations and an anti-catabolic effect of the hormone. Nonetheless, the dearth of reports on the impact of epinephrine on human muscle protein kinetics in healthy humans hinders our ability to confirm whether the anabolic properties of enhanced muscle β2-adrenergic signaling pertain to humans as they do for small animals and livestock where the anabolic effects of the β2-agonists are firmly established.
While the impact of exogenous epinephrine on human muscle proteolysis remains unclear, the notion that epinephrine, and by extension β-adrenergic signaling, can impart an important role in the inhibition of muscle proteolysis, is evidenced by the effects of chemical and surgical adrenodemedullation [32]. Both methods of adrenodemedullation rapidly and dramatically reduce circulating catecholamine concentrations and, in parallel, lead to the prompt increase in markers of muscle proteolysis. These findings underlie the fundamental control that β-adrenergic signaling plays in the regulation of muscle protein breakdown, and by extension muscle protein homeostasis. Moreover, over the past twenty years, a series of reports have identified specific components of the cellular proteolytic machinery that are modulated by β-adrenergic signaling, explaining the ability of the pathway to exert an anti-catabolic effect.
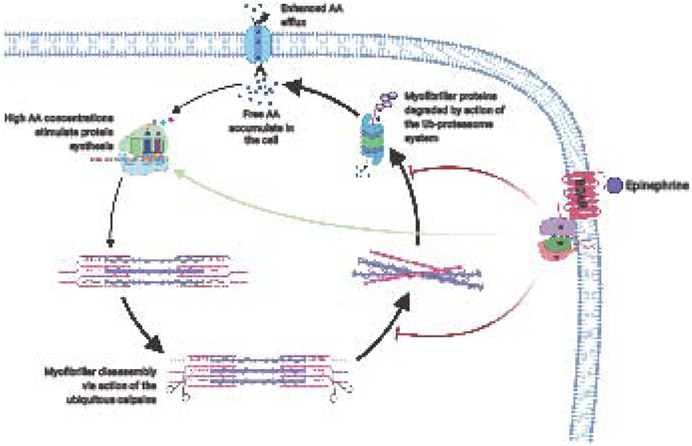
During muscle cachexia, the bulk of the proteolysis that takes place is typically due to the action of the ubiquitin-proteasome system, a tightly regulated proteolytic event where proteins are “marked” for degradation via the attachment of ubiquitin chains (see [33]). Consequently, polyubiquitinated proteins are recognized by the 26S proteasome as a target for proteolysis and are subsequently fed into the barrel-like structure of the proteasome (Figure 1). Multiple proteolytic enzymes reside in the inner core of the proteasome [33], resulting in sequential degradation of the earmarked protein as it is passes through the protein complex. Notably, the proteolytic activity of the 26S proteasome and enzymes involved in the attachment of ubiquitin to target proteins appear blunted by β-adrenergic agonists [15, 29], in a complex known as the ubiquitin-proteosome system (UPS). This complex represents one mechanism by which β-adrenergic signaling can impede muscle proteolysis – albeit as highlighted below, other possible mechanisms also appear to exist.
Figure 1: The role of beta2-adrenergic signaling in the control of muscle protein turnover.
Massive burns result in the net loss of muscle protein content, while simultaneously increasing the intracellular recycling of free amino acids. Under normal conditions, beta2-adrenergic signaling exerts a positive influence on muscle protein anabolism (shown with colored arrows). The impact of the pathway on the control of muscle protein turnover following burns is unclear. AA: Amino acids; B2AR: beta2-adrenergic receptor; Ub: Ubiquitin.
The 26S proteasome, by virtue of its structure, is unable to degrade intact myofibrils [34], necessitating some form of pre-processing of the contractile apparatus by an alternative proteolytic process before degradation by the proteasome can proceed. The ubiquitous calpains, a class of calcium-sensitive proteases, are thought to perform the prerequisite myofibril cleavage required for bulk proteolysis of the myofibrils [35]. This notion is supported by evidence that calpain-specific inhibitors can block muscle atrophy [36], underlying the essential role of the calpains for muscle protein degradation to occur. Furthermore, the ubiquitous calpains are often found accumulated around the z-disk of muscle fibers [37], a crucial junction where adjacent sarcomeres are crosslinked. While a role for β-adrenergic signaling in the activity of the UPS has been previously demonstrated [15, 22, 29], it is permissible that muscle proteolysis is further regulated by catecholamines and β2-agonists at the level of calpain-mediated proteolytic cleavage. The administration of clenbuterol to the incubation media of chick muscle has been shown to reduce overall muscle proteolysis by 20% and Ca2+-dependent proteolysis by >40%, effects that were prevented by the administration of the β2-selective antagonist ICI 118.551 [25]. Likewise, the enhanced muscle proteolysis that occurs with chemical and surgical adrenodemedullation, occurs in concert with increased Ca2+-dependent proteolytic activity [32], highlighting the potential regulatory role that β-adrenergic signaling may play in calpain-mediated proteolysis and skeletal muscle protein breakdown per se. Moreover, the inhibition of calpain activity may prove beneficial in avoiding the loss of contractile force generation, an otherwise inevitable consequence of myofibrillar disassembly and a common occurrence in burn patients (see [38]).
The beneficial impact of β-adrenergic signaling on muscle protein anabolism may also extend to processes governing the synthesis of muscle proteins. It has previously been demonstrated that clenbuterol added to the media of muscle incubated ex vivo encourages the synthesis of new muscle proteins [15]. Similarly, decreases in muscle protein synthesis rates as seen with chemical adrenodemedullation, fasting, or denervation are largely prevented via the administration of either a β-agonist or epinephrine [39]. However, while these results demonstrate that β-adrenergic signaling may play a prominent role in muscle protein anabolism, including under conditions of cachexia, it is permissible that these effects are transient. Epinephrine infusion in humans results in a robust but short-lived increase in the rate of disappearance of circulating leucine and phenylalanine [30], a phenomenon indicative of increased whole-body protein synthesis and/or oxidation of the AAs. Similarly, the fractional synthesis rates of muscle proteins of rats treated with clenbuterol are significantly increased, but this effect begins to wane over time, and by seven days of treatment, protein synthetic rates have returned to baseline levels [40]. The reasons for the transient nature of epinephrine and β2-agonists on muscle protein synthesis is currently unclear and could be evidence of tachyphylaxis to the agonists during prolonged periods of β-adrenergic signaling stimulation. However, this seems unlikely in light of reports that chronic (3 month) clenbuterol administration results in lean tissue accretion in humans [41]. Regardless, strategies that can prolong the ability of these compounds to stimulate the synthesis of muscle proteins will likely further enhance the anabolic potential of β2 agonists.
While therapeutic strategies aimed at protecting muscle mass with massive burns should be principally focused on blunting muscle proteolysis as it occurs unabated [42], the targeting of muscle protein synthesis in any future therapy remains of pivotal importance. Currently, the loss of muscle protein content in burn patients is offset by an inappropriate rise in muscle protein synthesis rates, caused by the intracellular recycling of AAs liberated from degraded muscle proteins [43]. Impeding muscle proteolysis following burns would be expected to diminish the supply of free AAs from which replacement proteins are generated, thereby eliminating the purported benefits of elevated muscle protein synthesis on net protein content. Of concern, we have shown that the ability of exogenous AAs to stimulate muscle protein synthesis is diminished following massive burns and persists for many months after injury [44]. This suggests that the mere act of providing replacement AAs via dietary means would be insufficient to compensate for the loss of muscle protein synthesis rates due to diminished intracellular AA recycling. Thus, the fact that epinephrine and β2 agonists appear capable, at least transiently, of stimulating the synthesis of new muscle proteins and improving feeding efficiency, their use could diminish post-burn proteolysis, thereby avoiding unnecessarily compromising muscle protein synthetic rates. However, the impact of muscle β-adrenergic signaling on muscle anabolic processes after burns remains to be established.
Impact of beta-blockade on muscle protein turnover following massive burns
Chronic β-adrenergic stress is a defining feature of major burns. Circulating concentrations of the catecholamines epinephrine and norepinephrine are rapidly and profoundly increased and remain elevated for many months following injury [45]. Based on the purported anabolic potential of β-adrenergic agonists, it would be reasonable to speculate that in skeletal muscle, the catecholamine surge post-injury acts as a beneficial countermeasure against other potential catabolic stimuli that are also increased after burns, such as pro-inflammatory cytokines [46] and glucocorticoids [45]. However, the wide-spread adoption of non-specific β-blockade in the treatment of the hypermetabolic response to massive burns has shown that the indiscriminate inhibition of β-adrenergic signaling is overall advantageous in impeding the loss of lean tissue in burn patients [47]. Viewed in conjunction with the known negative impact of adrenodemedullation on muscle protein accretion [22], it stands to reason that the negative impact of burn-induced catecholamine production on muscle protein turnover likely occurs via indirect mechanisms. Below we discuss known metabolic consequences of epinephrine release following massive burns and how they could individually contribute to the loss of muscle mass. These findings highlight likely mechanisms by which propranolol exerts a beneficial impact on burn-induced cachexia.
Futile fatty acid-triglyceride cycling after burns
After severe burns, epinephrine-induced lipolysis occurs unabated within adipose tissue. Even under conditions of hyperinsulinemia [48], the rate of hydrolysis of stored triglycerides into their glycerol and free fatty acid (FFA) constituent parts far exceeds the rate at which they are utilized as a fuel source by peripheral tissues [49]. The majority of liberated FFAs are re-esterified within hepatic tissue and secreted back into the circulation as very-low density lipoprotein triglycerides, before being stored in adipose tissue [49]. The combination of these metabolic pathways occurring in parallel but opposing directions, results in a futile and ATP-consuming cycle [48]. This wasted energy is compounded by the continual hydrolysis and re-esterification of triglycerides within the adipocyte itself via intracellular cycling, which further increases ATP usage [48]. Collectively, triglyceride-fatty acid cycling in burn patients is elevated both extracellularly and intracellularly by approximately 1650% and 200%, respectively, contributing >10% towards the hypermetabolic response to burns [48]. Notably, the infusion of propranolol in patients suffering from major burns dramatically reduces the rate of both intracellular and extracellular fatty acid-triglyceride cycle activity [48], demonstrating that catecholamine-induced β-adrenergic stimulation is primarily responsible for promoting enhanced substrate cycling in burn patients. The reduced activity of ATP-consuming fatty acid cycles with administration of the β-adrenergic antagonist decreases demand for gluconeogenic substrates and, by extension, the requirement for alanine of muscle protein origin. Under normal conditions, pyruvate oxidation is increased 300% with burns, with high-dose glucose infusion able to prevent the liberation of the gluconeogenic amino acid alanine [50], demonstrating carbohydrate oxidation, and by extension energy demand, as a key determinant of muscle proteolysis with burns.
Increased mitochondrial uncoupling with burns
An additional mechanism by which catecholamines may contribute to hypermetabolism is via their abilities to uncouple mitochondrial respiration from ATP synthesis. Under typical conditions, electrons are transferred across a series of complexes embedded in the inner membrane of the mitochondria, simultaneously generating an electrochemical proton gradient used to drive ATP synthesis via the action of ATP synthase (see [51]). In burn patients, β-adrenergic stimulation is thought to be responsible for the induced expression of uncoupling protein (UCP) in thermogenic tissues [52], allowing protons to freely traverse the inner mitochondrial membrane without contributing to the synthesis of ATP. As a consequence, the electrochemical proton gradient across the inner mitochondrial membrane is dissipated, impairing the organelle’s ATP synthetic capacity [51]. Under such circumstances, mitochondrial O2 utilization is no longer coupled to ATP production, with the energy of respiration instead dissipated as heat, giving rise to the thermogenic properties of the tissue [51]. It appears increasingly evident that humans have functional brown adipose tissue which is thermogenic in nature [53], and becomes activated in burns [54]. However, the total mass of brown adipose tissue in humans appears small [55] and its contribution to whole-body metabolism likely minimal. In contrast, burn patients display a greater abundance of UCP1 positive mitochondria in subcutaneous white adipose tissue relative to that of healthy individuals, suggesting that the latter tissue may become more thermogenic in nature following injury [52, 56]. Given that the typical adult has several kilograms of white adipose tissue, an increase in the thermogenic capacity of this abundant tissue could theoretically contribute significantly to the overall hypermetabolic response seen with burns. Notably, pharmacological inhibition of hormone-sensitive lipase has been reported to suppress lipolysis via non β-blocker means, thereby preventing the “browning” of white adipose tissue after burns, suggesting that FFA mobilization may be central to this catabolic process [57]. However, it should be noted that while UCP1 induction has been noted in burn patients [52], whether white adipose tissue of burn survivors can express functional UCP1 remains unproven. Thus, whether propranolol exerts an impact on whole-body energy expenditure following burns by altering UCP1 dependent thermogenesis in adipose tissue remains unclear and requires further investigation.
Rapid ectopic lipid deposition post-injury
The release of FFA from adipose stores following massive burns may also have additional consequences. The liberation of FFA from adipose tissue far exceeds the rate at which they are utilized as a fuel source [49]. With reports that muscle lipid oxidation following burns is depressed [58] lipids then begin to accumulate in ectopic tissues. The use of magnetic resonance imaging has revealed that the intramyocellular lipid (IMCL) content of muscle is three times greater in pediatric burn patients compared to healthy adults [59], an effect that is mirrored in experimental animal models of burns [60].
Many lipid species are now known to be biologically active, and their enhanced deposition in muscle after burns is unlikely to be benign. We’ve shown that acute lipid infusion, in part recapitulating the effect of burn-induced lipolysis, completely blunts the ability of exogenous AAs to stimulate the synthesis of new muscle proteins in humans [61]. Likewise, in animals provided high-fat diets where IMCL are increased, intracellular signaling events associated with the synthesis of muscle proteins are diminished [62]. Given that AA infusion is unable to stimulate muscle protein synthesis for at least a year after burns, the accumulation of intramuscular lipids could underpin the failure of nutritional interventions to counter the cachexia seen following burns [63]. However, the exact mechanism by which lipid oversupply impairs muscle protein synthesis remains unclear.
Alternative treatment strategies
The known ability of adrenodemedullation to reduce muscle protein synthesis rates [39] in conjunction with stimulating muscle proteolysis [22] highlights the crucial role that β-adrenergic signaling plays in the maintenance of muscle protein homeostasis under normal conditions. The benefits from propranolol in burn patients are unequivocal, helping to reduce both the hypermetabolic response to injury and tachycardia [64]. However, the administration of non-selective β-blockers eliminates any potential advantage increased catecholamine concentrations have on governing muscle protein anabolism. Moreover, the use of non-specific adrenergic blockers eliminates the ability to incorporate β2 agonists into the treatment regime to help recover muscle mass and build strength, a strategy that has proven effective in experimental animal models of burns [16, 17]. Thus, an advantageous strategy would be one where β2-signalling in skeletal muscle is not inhibited, but the deleterious effects of the catecholamines on other tissues are prevented. We propose that utilizing β1 specific antagonists coupled with drug(s) that directly prevent lipolysis could represent one potential solution.
Unlike adipocytes and skeletal muscle cells, the dominant β-adrenergic receptor present in cardiomyocytes are of the β1 subtype [65]. While β2 and β3 subtypes represent 30% and 20% of the total β-adrenergic receptors found in whole heart tissue, they are mainly expressed in nonmyocytes [65]. In contrast, myocytes are largely devoid of β2- and β3-adrenergic receptors [65]. As a consequence, β1 specific antagonists are highly effective at modulating cardiac function, including reducing tachycardia [66]. However, while β1-adrenoreceptors are prevalent in adipocytes [67], and β1-antagonists such as atenolol can lower the rate of lipolysis, they are not effective as propranolol under conditions of stress [68], suggesting involvement of the β2-adrenoreceptor. Likewise, a comparison of the effects of propranolol and atenolol in burn patients reveal both drugs to be equally effective at reducing tachycardia, but only the former with its β2-blocking action attenuates lipolysis [69]. Collectively, these findings suggest that the use of β1 specific antagonism performed in isolation is inadequate in the treatment of burns.
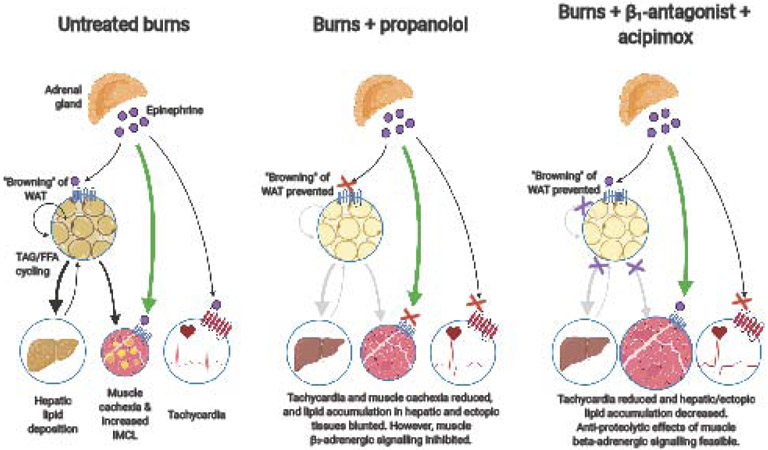
A potential strategy by which the limitations of β1-antagonists could be overcome is to combine their use with drugs that inhibit lipolysis independent of β2-adrenergic signaling. For example, niacin and its derivatives (e.g. acipimox) potently inhibit hormone sensitive lipase, a crucial step for lipolysis and are used clinically in the treatment of hyperlipidemia ([70]; Figure 2). In humans, the use of acipimox reverses ectopic lipid accumulation and is proven effective at reducing the accumulation of intramuscular lipids [71]. Moreover, in experimental animal models of burns, acipimox has been shown to reduce hepatic lipid accumulation and eliminate “browning” of adipose tissue [57], effects that mirror benefits seen with propranolol that would be advantageous for burn patients [72, 73]. Whether similar effects are observed in patient populations is currently unknown, but if found to be effective, it could open up future avenues allowing for the exploitation of the catecholamine response following burns to reduce cachexia and accelerate the patient’s recovery.
Figure 2: Diagrammatic representation of the key impacts of burn-induced catecholamine surge on tissue metabolism, and the beneficial effects of beta-blockers.
The hypothesized benefits of beta1-specific blockade in combination with the inhibition of lipolysis via acipimox is presented. FFA: free fatty acids; IMCL: intramyocellular lipid; TAG: triacylglycerols; WAT: white adipose tissue.
Conclusions
Undoubtedly the use of the non-specific β-blocker propranolol for the treatment of burns has proven highly advantageous, reducing tachycardia and the hypermetabolic response to injury. However, given that β2 agonists have been used (at times illegitimately) for decades to promote muscle growth in athletes and livestock, coupled with knowledge that the inhibition of endogenous catecholamine production results in rapid muscle protein catabolism, the maintenance of β2-adrenergic signaling in muscle after burns could be advantageous for the recovery of muscle mass and function. We suggest that a targeted approach involving β1 blockers in combination with drugs that inhibit lipolysis may bring additional benefits over current treatments and warrants further consideration.
Highlights.
Burns-induced catecholamine surge contributes to hypermetabolism and muscle cachexia
β -blockade appears beneficial at reducing hypermetabolism and muscle catabolism
In contrast, in healthy individuals, β2-agonists induce muscle hypertrophy
Benefits of β-blockade on muscle protein turnover appear due to preventing lipolysis
Use of β1-antagonists while inhibiting lipolysis may aid muscle recovery after burns
Acknowledgements
The authors’ work was supported by an endowment provided to The University of Texas Medical Branch in memory of the 15 individuals who died in the 2005 Texas City refinery explosion. EB and ER were additionally supported by a training grant from the National Institute of Health (T32 GM008256). The funders had no involvement in the preparation of the manuscript or the decision to publish. The authors would like to acknowledge Anthony Nguyen and Zhihao Zhu for initial drafting of figures.
Abbreviations
- AA
Amino acids
- FFA
Free fatty acids
- IMCL
Intramyocellular lipid
- TBSA
Total body surface area burned
- UCP
Uncoupling protein
- UPS
Ubiquitin proteasome system
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Capek KD, Sousse LE, Hundeshagen G, Voigt CD, Suman OE, Finnerty CC, et al. Contemporary Burn Survival. J Am Coll Surg. 2018;226:453–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Porter C, Tompkins RG, Finnerty CC, Sidossis LS, Suman OE, Herndon DN. The metabolic stress response to burn trauma: current understanding and therapies. Lancet. 2016;388:1417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Porter C, Herndon DN, Borsheim E, Bhattarai N, Chao T, Reidy PT, et al. Long-Term Skeletal Muscle Mitochondrial Dysfunction is Associated with Hypermetabolism in Severely Burned Children. J Burn Care Res. 2016;37:53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hart DW, Wolf SE, Mlcak R, Chinkes DL, Ramzy PI, Obeng MK, et al. Persistence of muscle catabolism after severe burn. Surgery. 2000;128:312–9. [DOI] [PubMed] [Google Scholar]
- [5].Polychronopoulou E, Herndon DN, Porter C. The Long-Term Impact of Severe Burn Trauma on Musculoskeletal Health. J Burn Care Res. 2018;39:869–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Benjamin NC, Andersen CR, Herndon DN, Suman OE. The effect of lower body burns on physical function. Burns. 2015;41:1653–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Suman OE, Spies RJ, Celis MM, Mlcak RP, Herndon DN. Effects of a 12-wk resistance exercise program on skeletal muscle strength in children with burn injuries. J Appl Physiol (1985). 2001;91:1168–75. [DOI] [PubMed] [Google Scholar]
- [8].Pereira C, Murphy K, Jeschke M, Herndon DN. Post burn muscle wasting and the effects of treatments. Int J Biochem Cell Biol. 2005;37:1948–61. [DOI] [PubMed] [Google Scholar]
- [9].Prezelj A, Obreza A, Pecar S. Abuse of clenbuterol and its detection. Curr Med Chem. 2003;10:281–90. [DOI] [PubMed] [Google Scholar]
- [10].Trotta RJ, Maddock Carlin KR, Swanson KC. Effects of ractopamine hydrochloride supplementation on feeding behavior, growth performance, and carcass characteristics of finishing steers(). Transl Anim Sci. 2019;3:1143–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Choo JJ, Horan MA, Little RA, Rothwell NJ. Effects of the beta 2-adrenoceptor agonist, clenbuterol, on muscle atrophy due to food deprivation in the rat. Metabolism. 1990;39:647–50. [DOI] [PubMed] [Google Scholar]
- [12].Costelli P, Garcia-Martinez C, Llovera M, Carbo N, Lopez-Soriano FJ, Agell N, et al. Muscle protein waste in tumor-bearing rats is effectively antagonized by a beta 2-adrenergic agonist (clenbuterol). Role of the ATP-ubiquitin-dependent proteolytic pathway. J Clin Invest. 1995;95:2367–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Costelli P, Llovera M, Garcia-Martinez C, Carbo N, Lopez-Soriano FJ, Argiles JM. Enhanced leucine oxidation in rats bearing an ascites hepatoma (Yoshida AH-130) and its reversal by clenbuterol. Cancer Lett. 1995;91:73–8. [DOI] [PubMed] [Google Scholar]
- [14].Agrawal S, Thakur P, Katoch SS. Beta adrenoceptor agonists, clenbuterol, and isoproterenol retard denervation atrophy in rat gastrocnemius muscle: use of 3-methylhistidine as a marker of myofibrillar degeneration. Jpn J Physiol. 2003;53:229–37. [DOI] [PubMed] [Google Scholar]
- [15].Goncalves DA, Silveira WA, Lira EC, Graca FA, Paula-Gomes S, Zanon NM, et al. Clenbuterol suppresses proteasomal and lysosomal proteolysis and atrophy-related genes in denervated rat soleus muscles independently of Akt. Am J Physiol Endocrinol Metab. 2012;302:E123–33. [DOI] [PubMed] [Google Scholar]
- [16].Martineau L, Little RA, Rothwell NJ, Fisher MI. Clenbuterol, a beta 2-adrenergic agonist, reverses muscle wasting due to scald injury in the rat. Burns. 1993;19:26–34. [DOI] [PubMed] [Google Scholar]
- [17].Hollyoak MA, Muller MJ, Meyers NA, Williams WG, Barrow RE, Herndon DN. Beneficial wound healing and metabolic effects of clenbuterol in burned and nonburned rats. J Burn Care Rehabil. 1995;16:233–40. [DOI] [PubMed] [Google Scholar]
- [18].Sato S, Shirato K, Tachiyashiki K, Imaizumi K. Muscle plasticity and beta(2)-adrenergic receptors: adaptive responses of beta(2)-adrenergic receptor expression to muscle hypertrophy and atrophy. J Biomed Biotechnol. 2011;2011:729598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Choo JJ, Horan MA, Little RA, Rothwell NJ. Anabolic effects of clenbuterol on skeletal muscle are mediated by beta 2-adrenoceptor activation. Am J Physiol. 1992;263:E50–6. [DOI] [PubMed] [Google Scholar]
- [20].Flores O, Stockton K, Roberts JA, Muller MJ, Paratz JD. The efficacy and safety of adrenergic blockade after burn injury: A systematic review and meta-analysis. J Trauma Acute Care Surg. 2016;80:146–55. [DOI] [PubMed] [Google Scholar]
- [21].Murton AJ, Greenhaff PL. Physiological control of muscle mass in humans during resistance exercise, disuse and rehabilitation. Curr Opin Clin Nutr Metab Care. 2010;13:249–54. [DOI] [PubMed] [Google Scholar]
- [22].Graca FA, Goncalves DA, Silveira WA, Lira EC, Chaves VE, Zanon NM, et al. Epinephrine depletion exacerbates the fasting-induced protein breakdown in fast-twitch skeletal muscles. Am J Physiol Endocrinol Metab. 2013;305:E1483–94. [DOI] [PubMed] [Google Scholar]
- [23].Kraenzlin ME, Keller U, Keller A, Thelin A, Arnaud MJ, Stauffacher W. Elevation of plasma epinephrine concentrations inhibits proteolysis and leucine oxidation in man via beta-adrenergic mechanisms. J Clin Invest. 1989;84:388–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Miles JM, Nissen SL, Gerich JE, Haymond MW. Effects of epinephrine infusion on leucine and alanine kinetics in humans. Am J Physiol. 1984;247:E166–72. [DOI] [PubMed] [Google Scholar]
- [25].Navegantes LC, Machado CR, Resano NM, Migliorini RH, Kettelhut IC. Beta2-agonists and cAMP inhibit protein degradation in isolated chick (Gallus domesticus) skeletal muscle. Br Poult Sci. 2003;44:149–54. [DOI] [PubMed] [Google Scholar]
- [26].Benson DW, Foley-Nelson T, Chance WT, Zhang FS, James JH, Fischer JE. Decreased myofibrillar protein breakdown following treatment with clenbuterol. J Surg Res. 1991;50:1–5. [DOI] [PubMed] [Google Scholar]
- [27].Navegantes LC, Resano NM, Migliorini RH, Kettelhut IC. Role of adrenoceptors and cAMP on the catecholamine-induced inhibition of proteolysis in rat skeletal muscle. Am J Physiol Endocrinol Metab. 2000;279:E663–8. [DOI] [PubMed] [Google Scholar]
- [28].Navegantes LC, Resano NM, Migliorini RH, Kettelhut IC. Catecholamines inhibit Ca(2+)-dependent proteolysis in rat skeletal muscle through beta(2)-adrenoceptors and cAMP. Am J Physiol Endocrinol Metab. 2001;281:E449–54. [DOI] [PubMed] [Google Scholar]
- [29].Yimlamai T, Dodd SL, Borst SE, Park S. Clenbuterol induces muscle-specific attenuation of atrophy through effects on the ubiquitin-proteasome pathway. J Appl Physiol (1985). 2005;99:71–80. [DOI] [PubMed] [Google Scholar]
- [30].Ratheiser KM, Pesola GR, Campbell RG, Matthews DE. Epinephrine transiently increases amino acid disappearance to lower amino acid levels in humans. JPEN J Parenter Enteral Nutr. 1999;23:279–87. [DOI] [PubMed] [Google Scholar]
- [31].Fryburg DA, Gelfand RA, Jahn LA, Oliveras D, Sherwin RS, Sacca L, et al. Effects of epinephrine on human muscle glucose and protein metabolism. Am J Physiol. 1995;268:E55–9. [DOI] [PubMed] [Google Scholar]
- [32].Navegantes LC, Baviera AM, Kettelhut IC. The inhibitory role of sympathetic nervous system in the Ca2+-dependent proteolysis of skeletal muscle. Braz J Med Biol Res. 2009;42:21–8. [DOI] [PubMed] [Google Scholar]
- [33].Murton AJ, Constantin D, Greenhaff PL. The involvement of the ubiquitin proteasome system in human skeletal muscle remodelling and atrophy. Biochim Biophys Acta. 2008;1782:730–43. [DOI] [PubMed] [Google Scholar]
- [34].Solomon V, Goldberg AL. Importance of the ATP-ubiquitin-proteasome pathway in the degradation of soluble and myofibrillar proteins in rabbit muscle extracts. J Biol Chem. 1996;271:26690–7. [DOI] [PubMed] [Google Scholar]
- [35].Hasselgren PO, Fischer JE. Muscle cachexia: current concepts of intracellular mechanisms and molecular regulation. Ann Surg. 2001;233:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Fareed MU, Evenson AR, Wei W, Menconi M, Poylin V, Petkova V, et al. Treatment of rats with calpain inhibitors prevents sepsis-induced muscle proteolysis independent of atrogin-1/MAFbx and MuRF1 expression. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1589–97. [DOI] [PubMed] [Google Scholar]
- [37].Kumamoto T, Kleese WC, Cong JY, Goll DE, Pierce PR, Allen RE. Localization of the Ca(2+)-dependent proteinases and their inhibitor in normal, fasted, and denervated rat skeletal muscle. Anat Rec. 1992;232:60–77. [DOI] [PubMed] [Google Scholar]
- [38].Porter C, Hardee JP, Herndon DN, Suman OE. The role of exercise in the rehabilitation of patients with severe burns. Exerc Sport Sci Rev. 2015;43:34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Navegantes LC, Resano NM, Baviera AM, Migliorini RH, Kettelhut IC. Effect of sympathetic denervation on the rate of protein synthesis in rat skeletal muscle. Am J Physiol Endocrinol Metab. 2004;286:E642–7. [DOI] [PubMed] [Google Scholar]
- [40].Sneddon AA, Delday MI, Steven J, Maltin CA. Elevated IGF-II mRNA and phosphorylation of 4E-BP1 and p70(S6k) in muscle showing clenbuterol-induced anabolism. Am J Physiol Endocrinol Metab. 2001;281:E676–82. [DOI] [PubMed] [Google Scholar]
- [41].Kamalakkannan G, Petrilli CM, George I, LaManca J, McLaughlin BT, Shane E, et al. Clenbuterol increases lean muscle mass but not endurance in patients with chronic heart failure. J Heart Lung Transplant. 2008;27:457–61. [DOI] [PubMed] [Google Scholar]
- [42].Chao T, Herndon DN, Porter C, Chondronikola M, Chaidemenou A, Abdelrahman DR, et al. Skeletal Muscle Protein Breakdown Remains Elevated in Pediatric Burn Survivors up to One-Year Post-Injury. Shock. 2015;44:397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Biolo G, Fleming RY, Maggi SP, Nguyen TT, Herndon DN, Wolfe RR. Inverse regulation of protein turnover and amino acid transport in skeletal muscle of hypercatabolic patients. J Clin Endocrinol Metab. 2002;87:3378–84. [DOI] [PubMed] [Google Scholar]
- [44].Porter C, Cotter M, Diaz EC, Jennings K, Herndon DN, Borsheim E. Amino acid infusion fails to stimulate skeletal muscle protein synthesis up to 1 year after injury in children with severe burns. J Trauma Acute Care Surg. 2013;74:1480–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Jeschke MG, Gauglitz GG, Kulp GA, Finnerty CC, Williams FN, Kraft R, et al. Long-term persistance of the pathophysiologic response to severe burn injury. PLoS One. 2011;6:e21245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Finnerty CC, Przkora R, Herndon DN, Jeschke MG. Cytokine expression profile over time in burned mice. Cytokine. 2009;45:20–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Pereira CT, Jeschke MG, Herndon DN. Beta-blockade in burns. Novartis Found Symp. 2007;280:238–48; discussion 48-51. [DOI] [PubMed] [Google Scholar]
- [48].Wolfe RR, Herndon DN, Jahoor F, Miyoshi H, Wolfe M. Effect of severe burn injury on substrate cycling by glucose and fatty acids. N Engl J Med. 1987;317:403–8. [DOI] [PubMed] [Google Scholar]
- [49].Aarsland A, Chinkes D, Wolfe RR, Barrow RE, Nelson SO, Pierre E, et al. Beta-blockade lowers peripheral lipolysis in burn patients receiving growth hormone. Rate of hepatic very low density lipoprotein triglyceride secretion remains unchanged. Ann Surg. 1996;223:777–87; discussion 87-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Wolfe RR, Jahoor F, Herndon DN, Miyoshi H. Isotopic evaluation of the metabolism of pyruvate and related substrates in normal adult volunteers and severely burned children: effect of dichloroacetate and glucose infusion. Surgery. 1991;110:54–67. [PubMed] [Google Scholar]
- [51].Demine S, Renard P, Arnould T. Mitochondrial Uncoupling: A Key Controller of Biological Processes in Physiology and Diseases. Cells. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Sidossis LS, Porter C, Saraf MK, Borsheim E, Radhakrishnan RS, Chao T, et al. Browning of Subcutaneous White Adipose Tissue in Humans after Severe Adrenergic Stress. Cell Metab. 2015;22:219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Porter C, Herndon DN, Chondronikola M, Chao T, Annamalai P, Bhattarai N, et al. Human and Mouse Brown Adipose Tissue Mitochondria Have Comparable UCP1 Function. Cell Metab. 2016;24:246–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Bhattarai N, Rontoyanni VG, Ross E, Ogunbileje JO, Murton AJ, Porter C. Brown adipose tissue recruitment in a rodent model of severe burns. Burns. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Yoneshiro T, Aita S, Matsushita M, Kayahara T, Kameya T, Kawai Y, et al. Recruited brown adipose tissue as an antiobesity agent in humans. J Clin Invest. 2013;123:3404–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Patsouris D, Qi P, Abdullahi A, Stanojcic M, Chen P, Parousis A, et al. Burn Induces Browning of the Subcutaneous White Adipose Tissue in Mice and Humans. Cell Rep. 2015;13:1538–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Barayan D, Vinaik R, Auger C, Knuth CM, Abdullahi A, Jeschke MG. Inhibition of Lipolysis With Acipimox Attenuates Postburn White Adipose Tissue Browning and Hepatic Fat Infiltration. Shock. 2020;53:137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Galster AD, Bier DM, Cryer PE, Monafo WW. Plasma palmitate turnover in subjects with thermal injury. J Trauma. 1984;24:938–45. [DOI] [PubMed] [Google Scholar]
- [59].Cree MG, Zwetsloot JJ, Herndon DN, Newcomer BR, Fram RY, Angel C, et al. Insulin sensitivity is related to fat oxidation and protein kinase C activity in children with acute burn injury. J Burn Care Res. 2008;29:585–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Tzika AA, Astrakas LG, Cao H, Mintzopoulos D, Zhang Q, Padfield K, et al. Murine intramyocellular lipids quantified by NMR act as metabolic biomarkers in burn trauma. Int J Mol Med. 2008;21:825–32. [PubMed] [Google Scholar]
- [61].Stephens FB, Chee C, Wall BT, Murton AJ, Shannon CE, van Loon LJ, et al. Lipid-induced insulin resistance is associated with an impaired skeletal muscle protein synthetic response to amino acid ingestion in healthy young men. Diabetes. 2015;64:1615–20. [DOI] [PubMed] [Google Scholar]
- [62].Rivas DA, McDonald DJ, Rice NP, Haran PH, Dolnikowski GG, Fielding RA. Diminished anabolic signaling response to insulin induced by intramuscular lipid accumulation is associated with inflammation in aging but not obesity. Am J Physiol Regul Integr Comp Physiol. 2016;310:R561–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Patterson BW, Nguyen T, Pierre E, Herndon DN, Wolfe RR. Urea and protein metabolism in burned children: effect of dietary protein intake. Metabolism. 1997;46:573–8. [DOI] [PubMed] [Google Scholar]
- [64].Ojeda S, Blumenthal E, Stevens P, Andersen CR, Robles L, Herndon DN, et al. The Safety and Efficacy of Propranolol in Reducing the Hypermetabolic Response in the Pediatric Burn Population. J Burn Care Res. 2018;39:963–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Myagmar BE, Flynn JM, Cowley PM, Swigart PM, Montgomery MD, Thai K, et al. Adrenergic Receptors in Individual Ventricular Myocytes: The Beta-1 and Alpha-1B Are in All Cells, the Alpha-1A Is in a Subpopulation, and the Beta-2 and Beta-3 Are Mostly Absent. Circ Res. 2017;120:1103–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Wong GW, Boyda HN, Wright JM. Blood pressure lowering efficacy of beta-1 selective beta blockers for primary hypertension. Cochrane Database Syst Rev. 2016;3:CD007451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Deng C, Paoloni-Giacobino A, Kuehne F, Boss O, Revelli JP, Moinat M, et al. Respective degree of expression of beta 1-, beta 2- and beta 3-adrenoceptors in human brown and white adipose tissues. Br J Pharmacol. 1996;118:929–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Deacon SP. The effects of atenolol and propranolol upon lipolysis. Br J Clin Pharmacol. 1978;5:123–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Herndon DN, Nguyen TT, Wolfe RR, Maggi SP, Biolo G, Muller M, et al. Lipolysis in burned patients is stimulated by the beta 2-receptor for catecholamines. Arch Surg. 1994;129:1301–4; discussion 4-5. [DOI] [PubMed] [Google Scholar]
- [70].Robinson AW, Sloan HL, Arnold G. Use of niacin in the prevention and management of hyperlipidemia. Prog Cardiovasc Nurs. 2001;16:14–20. [DOI] [PubMed] [Google Scholar]
- [71].Bajaj M, Suraamornkul S, Romanelli A, Cline GW, Mandarino LJ, Shulman GI, et al. Effect of a sustained reduction in plasma free fatty acid concentration on intramuscular long-chain fatty Acyl-CoAs and insulin action in type 2 diabetic patients. Diabetes. 2005;54:3148–53. [DOI] [PubMed] [Google Scholar]
- [72].Barrow RE, Wolfe RR, Dasu MR, Barrow LN, Herndon DN. The use of beta-adrenergic blockade in preventing trauma-induced hepatomegaly. Ann Surg. 2006;243:115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Morio B, Irtun O, Herndon DN, Wolfe RR. Propranolol decreases splanchnic triacylglycerol storage in burn patients receiving a high-carbohydrate diet. Ann Surg. 2002;236:218–25. [DOI] [PMC free article] [PubMed] [Google Scholar]