Abstract
Endothelial cells display an extraordinary plasticity both during development and throughout adult life. During early development, endothelial cells assume arterial, venous, or lymphatic identity, while selected endothelial cells undergo additional fate changes to become hematopoietic progenitor, cardiac valve, and other cell types. Adult endothelial cells are some of the longest-lived cells in the body and their participation as stable components of the vascular wall is critical for the proper function of both the circulatory and lymphatic systems, yet these cells also display a remarkable capacity to undergo changes in their differentiated identity during injury, disease, and even normal physiological changes in the vasculature. Here, we discuss how endothelial cells become specified during development as arterial, venous, or lymphatic endothelial cells or convert into hematopoietic stem and progenitor cells or cardiac valve cells. We compare findings from in vitro and in vivo studies with a focus on the zebrafish as a valuable model for exploring the signaling pathways and environmental cues that drive these transitions. We also discuss how endothelial plasticity can aid in revascularization and repair of tissue after damage- but may have detrimental consequences under disease conditions. By better understanding endothelial plasticity and the mechanisms underlying endothelial fate transitions, we can begin to explore new therapeutic avenues.
Keywords: endothelial cell, vascular plasticity, transdifferentiation, zebrafish
INTRODUCTION
Cellular plasticity is the conversion of one cell type to another cell type through changes in gene expression, cell structure, and cell function. While cell fate changes occur throughout development, cellular plasticity has also been observed in tissue regeneration and disease progression [1–3]. Endothelial cells (ECs) line the vascular system, including blood vessels, lymphatic vessels, and the heart, which are vital for the circulation of oxygen, nutrients, and immune cells throughout an organism. These cells undergo several fate transitions throughout development until becoming arterial, venous, or lymphatic ECs, hematopoietic stem and progenitor cells (HSPCs), or cardiac valve cells, for example (Figure 1a). In addition, ECs have dynamic responses to both local signals and hemodynamic forces. Thus, changes due to injury or disease can trigger EC plasticity leading to cell conversion. Here we discuss the role that EC plasticity plays in development, repair and revascularization, and disease, emphasizing the signaling pathways and hemodynamic forces that drive these responses. We compare both in vitro and in vivo studies, with a focus on zebrafish as an important model system for understanding vascular plasticity. Finally, we explore the broad range implications of EC dynamics and how it can lead to future therapies.
Fig 1:
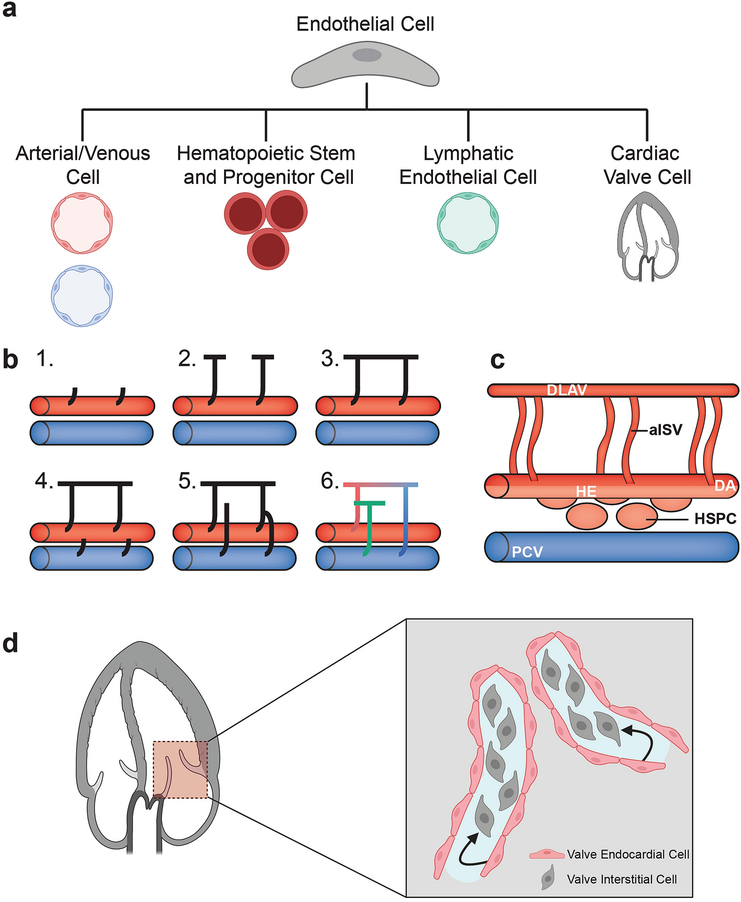
Endothelial cell plasticity during development. (a) Endothelial cells undergo several fate transitions throughout development, forming arteries and veins, hematopoietic stem and progenitor cells, lymphatic vessels, and valve interstitial cells. Endothelial plasticity is also seen during tissue regeneration and under disease conditions. (b) Intersegmental blood vessels and lymphatic vessels emerge by sprouting angiogenesis. In zebrafish, primary sprouts emerge from the DA (1), sprouts grow dorsally and branch caudally and rostrally (2), branches interconnect to form the DLAV (3), secondary sprouts emerge from the PCV (4), some secondary sprouts connect to primary sprouts and others do not (5), primary sprouts that maintain their connection to the DA remain arterial ISVs (aISVs, red), while others that connect to secondary sprout segments from the PCV become venous ISVs (vISVs, blue). Secondary sprout segments that do not connect become lymphatic vessels (green). Adapted from Isogai et al. 2003 [9]. (c) Hematopoietic stem cells emerge through an endothelial to hematopoietic transition by budding from the hemogenic endothelium (light red) in the ventral floor of the dorsal aorta. (d) Interstitial cells of the cardiac valve form via an endothelial to mesenchymal transition with endocardial cells converting into valve interstitial cells. aISV, arterial intersegmental vessel; DA, dorsal aorta; DLAV, dorsal longitudinal anastomatic vessel; HE, hemogenic endothelium; HSPC, hematopoietic stem and progenitor cell; PCV, posterior cardinal vein. Figures (a) and (d) were created using biorender.com.
Zebrafish as a model for studying vascular development and plasticity
Zebrafish have emerged as a powerful model organism to study vascular development and plasticity. The ability to maintain large numbers of adult animals and generate large numbers of progeny facilitates genetic and experimental studies, while the ex utero development and optical clarity of zebrafish embryos and larvae allows for high-resolution imaging at all stages of development. Forward genetic screens using ENU mutagenesis, gene knockdown using morpholinos, and gene editing techniques such as CRISPR have uncovered the function of genes and signaling pathways vital for many different developmental processes. Gene expression is easily assessed in fixed samples using in situ hybridization and immunohistochemistry, while a myriad of different cell types, tissues, and organs can be visualized in living embryos using the numerous available transgenic fluorescent reporter lines. Using the zebrafish, scientists have been able to build upon their current understanding of vascular development established from other model systems, and uncover additional molecular mechanisms and cellular behaviors that drive vascular morphogenesis.
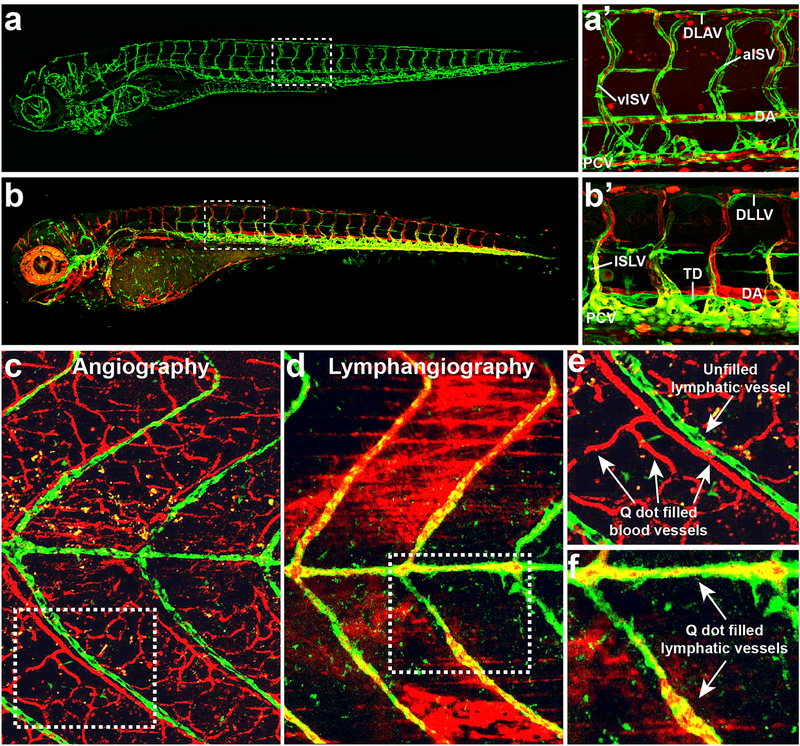
The vascular anatomy of zebrafish is highly conserved, as are the molecular mechanisms regulating vascular development [4–8]. As in other vertebrates, endothelial progenitors or angioblasts are specified in the mesoderm through the expression of ETS transcriptional regulator family members and migrate to form the axial vessels, the dorsal aorta (DA) and posterior cardinal vein (PCV). Initial formation of the major vessels during early development occurs via vasculogenesis, or the formation of vessels by the coalescence of these migratory primitive angioblasts. Subsequent vessel formation occurs via angiogenesis, or the sprouting and growth of new vessels from preexisting vessels. In the trunk, primary intersegmental vessels (ISVs) sprout from the DA and migrate dorsally along somite (future muscle blocks) boundaries to form an un-perfused network of arterially derived segments (Figure 1b) [9]. After this initial network is formed, secondary sprouts emerge from the PCV and either anastomose with the arterial ISVs to form venous ISVs or go on to form the lymphatic vasculature [9]. Since oxygenation of the early zebrafish embryo is dependent on diffusion and not blood circulation, vascular mutants that would normally be lethal early on in mammals can be assessed for several days in zebrafish [9,10]. This has helped identify mutations specifically affecting various aspects of vascular morphogenesis such as endothelial specification, migration, proliferation, and lumenization (reviewed in [6]). Vascular specific transgenic lines have also been generated that label all or subsets of endothelial cells in living animals using fluorescent markers such as GFP (Figure 2a,b). Using these lines, as well as additional vascular labeling techniques such as microangiography and lymphangiography, the formation of blood and lymphatic vessels can be visualized in real-time through confocal microscopy and time-lapse imaging (Figure 2c–f). This has helped reveal the dynamic cellular behaviors that drive vessel formation during development. Additionally, transgenic lines can be used to enrich for different endothelial cell populations via florescence activated cell sorting (FACs), allowing for genome wide cell specific gene expression analysis.
Fig 2:
Confocal images showing transgenic zebrafish with fluorescently “tagged” endothelial cells. (a) Fluorescent image of a (Tg(kdrl:eGFP), Tg(gata:dsRed)) transgenic zebrafish shows a 5 day post fertilization (dpf) larva whose blood vessels are labeled in green. (a’) A close up of the trunk area (boxed in area from a) shows the major vessels within that region including the dorsal longitudinal anastomotic vessel (DLAV), dorsal aorta (DA), intersegmental arteries (aISV) and veins (vISV), and the posterior cardinal vein (PCV). Blood cells (red) can be seen within the vessels (green). (b) Fluorescent image of a (Tg(mrc1a:eGFP), Tg(kdrl:mcherry)) transgenic zebrafish depicts the arteries (red), veins (yellow), and lymphatic vessels (green) in a 5dpf larva. (b’) A close up of the trunk area (boxed in area from b) shows the major blood vessels (DA, PCV) and lymphatic vessels within the region including the dorsal longitudinal lymphatic vessel (DLLV), intersegmental lymphatic vessel (ISLV), and thoracic duct (TD). (c-f) Confocal images depicting angiography or lymphangiography of a 28dpf (Tg(mrc1a:eGFP) zebrafish whose lymphatics are labeled in green after either intracardiac injection of Qdot705 quantum dots to label blood vasculature (red) (c,e) or intramuscular injection of Qdot705 quantum dots into the tail to label the trunk lymphatic system (red) (d,f). Close up images of boxed in areas from c and d are shown in e and f, respectively. Panels (c-f) were reprinted with permission from Jung et al. 2017 [147].
Lineage tracing techniques such as Cre/lox and Flp/FRT for “permanent” labeling or Kaede and Dendra2 for photo-conversion labeling have been utilized in zebrafish and other model organisms to track endothelial cells and monitor their cell fate decisions (a few selected examples of which are shown in Table 1) [11]. Lineage tracing permits direct visualization of endothelial fate transitions such as the specification of arterial versus venous fate, the endothelial to hematopoietic transition (EHT), formation of the lymphatic system from venous endothelium, or endothelial to mesenchymal transition (EndoMT) during cardiac valve formation [12–17]. Lineage tracing tools have also facilitated tracking of individual endothelial cells during tissue repair and regeneration after injury [18]. Zebrafish have a robust capacity to regenerate injured tissues and the vasculature that perfuses these tissues, including the heart, brain, and caudal fin. This capacity makes zebrafish ideally suited for studying endothelial cell plasticity during the regeneration process, and recent studies using fish have already begun to reveal mechanisms utilized to rebuild vasculature in damaged tissue [18–21]. Zebrafish have also been used to model many human vascular diseases (a few selected examples of these models are shown in Table 2), and combined with lineage tracing and imaging techniques, provide a powerful tool for understanding the role of endothelial cell plasticity in disease progression.
Table 1:
Selected Transgenic Zebrafish Lines for Endothelial Cell Lineage Tracing
| Zebrafish Transgenic Line | Selected References |
|---|---|
| Endothelial cell driver | [148] |
| Tg(fli1ep:GAL4FF)ubs2-4 | [148] |
| TgBAC(cdh5: GAL4FF)mu101 | [149] |
| Tg(kdrl: Cre)s898 | [12] |
| Tg(flt4:Gal4FF)hu9236 | [150] |
| Hematopoietic stem cell driver | |
| TgBAC(gata2b:KalTA4)sd32 | [151] |
| Lymphatic cell driver and reporter | |
| Tg(prox1aBAC:KalTA4–4xUAS-E1b:uncTagRFP)nim5 | [150] |
| Vascular specific photoconversion | |
| Tg(kdrl: Kaede)wz3 | [152] |
| Tg(flk1:Dendra2) | [153] |
| Gal4 driven photoconversion | |
| Tg(UAS:Kaede)rk8 | [154] |
| Cre driven permanent labeling | |
| Tg(βactin2:loxP-STOP-loxP-DsRed-express)sd5 | [12] |
| Tg(−3.5ubi:loxP-EGFP-loxP-mCherry) | [155] |
| Tg(ubi:Zebrabow) | [156] |
Table 2:
Selected Zebrafish Disease Models to Study Endothelial Plasticity
| Disease | Type of Model | Selected References |
|---|---|---|
| Arteriovenous Malformations (AVMs)/ Hereditary Hemorrhagic Telangiectasia (HHT) | Alk1 mutant | [157] |
| Eng mutant | [158] | |
| Cerebral Cavernous Malformations (CCM) | CCM1 (Krit1) mutant | [159] |
| CCM2 (OSM) mutant | [159] | |
| CCM3 (PDCD10) morphant | [160] | |
| Atherosclerosis | High Cholesterol Diet (HCD) | [161] |
| Apoc2 mutant | [162] | |
| Ldlr mutant + HCD | [163] | |
| Karposi’s Sarcoma | Primary Effusion Lymphoma (PEL) Xenograft | [164] |
| Melanoma | p53 mutant with BRAFV600E expression in melanocytes | [165] |
| Pancreatic Cancer (exocrine) | Expression of KrasG12V in pancreatic progenitor cells | [166] |
| Pancreatic Cancer (endocrine) | Expression of MYCN in β cells | [167] |
VASCULAR PLASTICITY DURING DEVELOPMENT
Endothelial cell specification
Early endothelial cell fate is specified from the mesoderm through the expression of ETS transcriptional regulator family members. ETS variant 2 (Etv2) is a major driver of endothelial specific gene expression in mice and zebrafish and its loss leads to severe blood and vessel defects [22–25]. Ectopic expression of Etv2 in vivo is sufficient to drive precocious endothelial gene expression in Xenopus and zebrafish and even causes the transdifferentiation of fast skeletal muscle into functional endothelial cells [22,26]. Although Etv2 is a major regulator of angioblast specification, the transcription factor Npas4l is thought to act upstream. Npas4l was initially identified as the defective gene in cloche, a zebrafish mutant discovered over two decades ago that causes severe defects in both endothelial and hematopoietic cell lineages, suggesting these cell types derive from a similar origin [10]. The recent molecular cloning of Npas4l showed that its expression leads to the upregulation of the early endothelial and hematopoietic lineage markers Etv2 and Scl/Tal1, supporting the idea that it acts upstream as a master regulator of both lineages (Figure 3a) [27]. Although there are Npas4l-like genes in other vertebrates including mammals [27], it is still not clear whether the critical function of Npas4l is conserved in other vertebrates.
Fig 3:
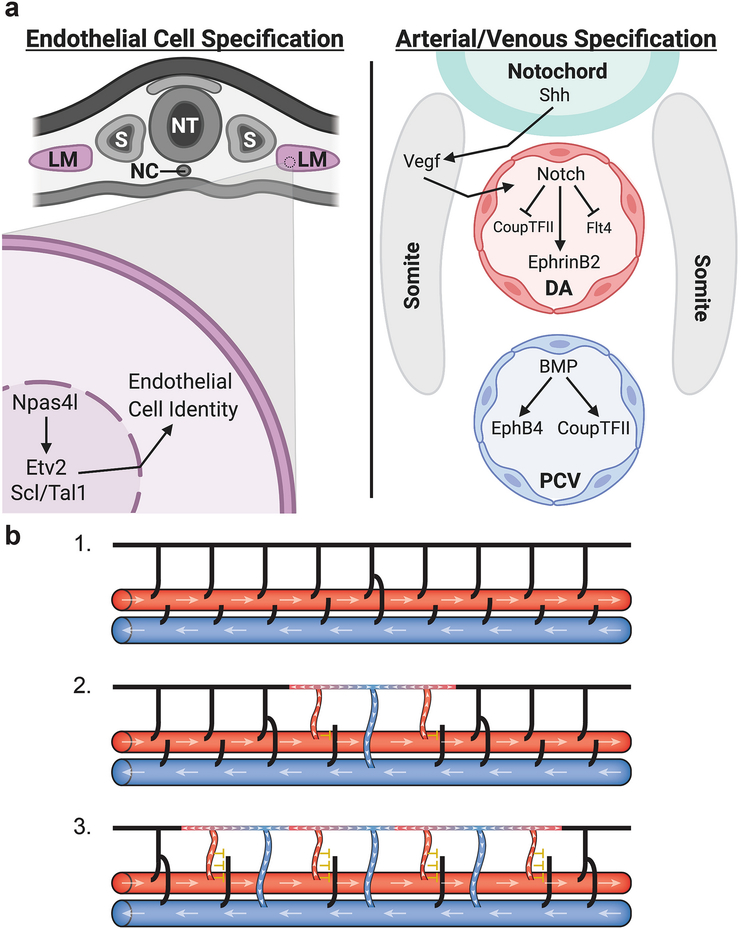
Diagrams depicting the specification of arterial and venous identity through molecular mechanisms and hemodynamic forces. (a) Endothelial cells are first specified in the lateral mesoderm (LM); in zebrafish the master regulator Npas4l activates Etv2 and Scl/Tal1 to promote endothelial identity. Once specified, endothelial cells migrate out of the lateral mesoderm to form the major axial vessels - the dorsal aorta (DA) and the posterior cardinal vein (PCV). Sonic hedgehog (Shh) signaling from the notochord activates Vegf signaling in the somites which in turn activates Notch signaling in the DA leading to the expression of arterial genes and the inhibition of venous identity. In the PCV, BMP signaling promotes the expression of venous identity genes. (b) Hemodynamic forces help drive the formation of venous intersegmental vessels in the zebrafish trunk. Initially all intersegmental vessels lack flow (black) and are connected to the DA (large red horizontal vessel) (1). Once a secondary sprout from the PCV (large blue horizontal vessel) connects to a primary sprout a circuit is created allowing blood flow (white arrowheads) to move up through adjacent primary sprouts (red) and return through the venous sprout (blue) (2). Blood flow through these primary sprouts prevents secondary sprouts from connecting (yellow inhibitory T) solidifying their arterial identity. Differences in flow then permit the next set of secondary sprouts to connect (3) leading to a largely alternating pattern of arteries and veins throughout the fish trunk and a close to 50:50 artery to vein ratio. Adapted from Isogai et al. 2003 [9]. DA, dorsal aorta; LM, lateral mesoderm, NC, notochord; NT, neural tube; PCV, posterior cardinal vein; S, somite. Figure (a) was created using biorender.com.
Arterial and venous specification
Initial arterial-venous specification in vasculogenic vessels occurs early in embryonic vessel development, prior to the initiation of blood circulation. This suggests that this initial specification event is genetically programed and not driven by blood flow, although hemodynamic forces do play a major role in endothelial cell fate once flow initiates [28]. In mammalian and avian embryos, vascular plexi show distinct patterns of arterial and venous genes before remodeling of the capillary network into morphologically distinguishable arteries and veins. It was first shown in mice that the ligand EphrinB2 is localized to arterial cells while its receptor EphB4 is expressed in venous endothelial cells [29]. Additional studies in the chick embryo have shown that the Vascular endothelial growth factor (Vegf) co-receptors Neuropilin-1 (Nrp1) and Neuropilin-2 (Nrp2) are expressed in non-overlapping endothelial arterial and venous precursor cells respectively [30]. This signaling between arterial and venous compartments is thought to be vital for the separation of their cell fates but lies downstream of other signaling pathways that first distinguish arterial and venous precursors.
Studies in zebrafish have revealed an upstream signaling cascade in which Hedgehog signaling from the notochord activates Vegf signaling in the somites which in turn induces Notch signaling in the DA leading to an arterial fate (Figure 3a) [31,32]. Embryos deficient in any of these pathways lack EphrinB2 expression in blood vessels and contain defects in vascular morphology. Furthermore, loss of Notch signaling leads to an expansion of venous markers such as EphB4 and Flt4 into the arterial domain and overexpression activates arterial markers such as EphrinB2 into the venous vessels [31]. This suggests that in early development, the loss or gain of Notch is sufficient to drive arterial/venous cell fate changes. Similar findings have been found in mammalian vessel development, where loss of Notch signaling components causes severe vascular defects and impairment of arterial cell fate [33–35].
Venous identity was initially thought to be the default vessel state since lack of Notch signaling allows the expression of the definitive venous marker EphB4. However, a knockout mouse model of the nuclear orphan receptor COUP-TFII (Nr2f2) demonstrated that loss of this receptor leads to Notch expression within veins [36]. Work in zebrafish, however, suggests that COUP-TFII does not regulate Notch signaling but instead Notch signaling suppresses COUP-TFII in the DA [37]. Recent work using both zebrafish and mouse models to study early vascular development has found that Bone morphogenetic protein (BMP) signaling is required for venous specification. Loss of the BMP receptor Alk3 or its downstream effector SMADs leads to defects in vein formation and loss of EphB4 expression without affecting arterial identity [38]. Increased BMP signaling promotes the expression of the venous identity markers EphB4 and COUP-TFII. Thus, BMP and Notch signaling play antagonistic roles in driving arterial/venous specificity and modulation of these signaling pathways is sufficient to drive arterial-venous cell fate changes (Figure 3a).
Although acquisition of arterial or venous identity is established by genetically programed pathways during early development, changes to the vessel environment have been shown to influence and even switch endothelial cell fate as demonstrated when segments of the saphenous vein are grafted to injured arteries [39]. Studies using quail-chick chimeras have shown that grafted arteries or veins from quail are capable of integrating into both vessel types in chick hosts up until embryonic day seven. Although arterial vessel segments do not graft well into veins and vice-versa at later stages of development, isolated endothelial cells separated from their vessel wall components are still able to colonize both types of host vessels regardless of their vessel origin, suggesting that vessel wall composition can impact endothelial cell specificity [40]. Changes in oxygen tension have also been found to affect artery/vein identity. A decrease in oxygen due to hypoxic conditions was found to cause a decrease in arterial marker expression in mouse retinal arteries [41]. Together these findings suggest that endothelial cells are quite plastic and can change cell fate due to perturbations in their environment.
Besides vessel wall composition and oxygen tension, endothelial cells can respond and change fate due to hemodynamic forces. Perturbing blood flow patterns in early quail and chick embryos demonstrated that arteries could convert to veins and veins could convert to arteries upon a reduction or increase in blood flow respectively [42]. Additional studies have since shown that shear stress due to laminar flow activates Notch signaling which in turn can promote an arterial fate thus connecting differences in flow to genetic regulators of identity. This has been demonstrated in both human umbilical vein cells (HUVECs) and human aortic endothelial cells (HAECs) which show an upregulation in Notch and Notch-associated genes when exposed to fluid shear stress compared to cells under static conditions [43,44]. In vivo studies in zebrafish have indicated that patterning of intersegmental vessels (ISVs) in the trunk is dictated by differences in flow in which initiation of arterial flow in an ISV leads to a high probability that neighboring ISVs will connect to secondary sprouts to become venous (Figure 3b) [9]. This ensures that the number of arterial and venous ISVs is equal allowing for circulatory blood flow to be balanced. Recent work has shown that a global reduction in blood flow disrupts this balance leading to an increase in venous ISV formation [16]. In addition, the authors found that those ISVs receiving high levels of flow increase their Notch signaling preventing them from connecting to secondary sprouts. In contrast, those vessels with weaker flow had a higher tendency of becoming venous resulting in lower Notch signaling. This suggests that Notch also helps transduce mechanosensory cues in vivo to dictate vessel identity [16]. However, unlike in quail/chick chimeras, the change in vessel identity appeared to at least partially involve replacement of arterial endothelial cells with venous endothelial cells migrating from the adjacent PCV rather than direct endothelial cell transdifferentiation. It remains unclear to what extent the effects of flow on initial ISV pattering in the zebrafish trunk are due to cellular rearrangements versus changes in cellular arterial-venous identity. Blood flow has also been shown to drive vascular remodeling in mammals through endothelial migration, proliferation, and morphogenesis but again, whether this involves endothelial cell fate changes in vivo has yet to be explored [28,45].
The endothelial to hematopoietic transition
Shortly after the initial primitive embryonic arteries and veins form, a subset of endothelial cells within the aorta-gonad-mesonephros (AGM) region, in particular the ventral wall of the dorsal aorta, undergo an EHT to give rise to the HSPCs [1,46]. These stem cells are necessary for the formation of all the blood lineages of the adult animal. Lineage tracing experiments in mice were the first to indicate that HSPCs originate from the endothelium and not from the underlying mesenchyme [47]. Subsequently, live imaging in zebrafish embryos provided definitive evidence that HSPCs emerge via EHT from hemogenic endothelium in the ventral floor of the dorsal aorta (Figure 1c) [12,14,15]. Retinoic acid signaling has been shown to promote the specification of the hemogenic endothelium. Loss of the enzyme Raldh2, needed to make retinoic acid, leads to a loss of hemogenic endothelial cells and transplantation of AGM lacking Raldh2 results in failure to form blood cells capable of colonizing the peripheral blood of recipient mice [48,49]. The receptor tyrosine kinase c-Kit is expressed in hemogenic endothelial cells and appears to be required for hemogenic endothelial cell formation downstream from retinoic acid signaling. Re-expression of c-Kit in Raldh2 mutant embryos is sufficient to rescue hemogenic endothelial cell development but fails to rescue in the presence of the Notch inhibitor DAPT [50]. This suggests that retinoic acid signaling drives downstream expression of c-Kit, which may in turn activate Notch signaling to promote hemogenic endothelial specification [50].
In the absence of Notch signaling, embryos fail to form HSPCs [51,52]. While part of this loss can be explained by defective arterial differentiation of the dorsal aorta upstream from HSPC formation [31], some studies suggest that Notch signaling may play a more direct role in HSPC formation independent of its role in arterial cell fate specification. Transient over-expression of the notch intracellular domain (NICD) in wild-type zebrafish embryos is sufficient to cause an expansion of HSPCs and induce ectopic expression of HSPC markers within the aortic roof and vein [51]. Furthermore, loss of the Notch ligand Jagged1 but not Jagged2 in mouse embryos leads to a decrease in hemogenic endothelial cell specification without affecting arterial cell fate [53]. More recent work has shown that Notch ligand specificity may induce different levels of Notch activation leading to different cell fates. While activation by the ligand Delta4 drives high Notch activity causing endothelial cells to remain arterial, stimulation by the ligand Jagged1 leads to low Notch activation and a hematopoietic fate [54]. However, both ligands are homogenously expressed around the aortic endothelium and thus how Notch signaling is modulated to drive these different cell fates is still not well understood.
For EHT to be complete, specified hemogenic endothelial cells must exit the DA to become HSPCs. This process requires the transcription factor Runx1 with loss of Runx1 leading to a failure of hemogenic endothelial cells to undergo EHT and thus a reduction in HSPC formation [14,55]. Expression of runx1 is regulated by Notch signaling and Runx1 in turn drives the expression of the HSPC marker cmyb [51]. However, runx1 is only transiently expressed early in development, and only required for the initial specification of HSPCs, while Cmyb is necessary for both formation and ongoing maintenance of HSPCs [55–58]. Recent work has shed some light on the mechanism of Cmyb perdurance in the absence of Runx1. The transient expression of runx1 leads to an increase in methylation of the cmyb promoter via the DNA methyltransferase 3bb.1 (Dnmt3bb.1), promoting the continued expression of cmyb [59]. Overexpression of dnmt3bb.1 in endothelial cells is also sufficient to drive ectopic cmyb expression and ectopic hematopoietic differentiation. These results demonstrate that epigenetic mechanisms also contribute to the transition of endothelial cells to the hematopoietic lineage. Whether the formation of additional HSPCs via EHT occurs later in development or in specific organs is not well explored, although recent work has suggested it does occur [60]. Future studies will be required to uncover the frequency and necessity of this form of endothelial cell plasticity throughout development or in adult life.
Lymphatic endothelial cell emergence
In addition to the blood vascular system, endothelial cells also line the lymphatic vasculature. The lymphatic system is an entirely separate and distinct vascular network from the blood vascular/circulatory system. It is required for tissue fluid homeostasis, for adsorption of lipids, and for immune cell trafficking, and plays an important role in cancer metastasis. The origins of lymphatic endothelial cells (LECs) have been debated for over a century, but recent live imaging in the larval zebrafish and lineage tracing in the mouse embryo confirmed that the earliest LECs emerge from primitive veins [17,61]. Prospero homeobox 1 (Prox1) was one of the first markers identified for LEC fate and has been shown to promote the expression of other lymphatic genes [62–64]. Loss of Prox1 in mice leads to a failure of lymphatic formation without affecting the blood vasculature suggesting its requirement is lymphatic-specific [65]. The mechanisms underlying emergence of LECs from venous endothelial cells are still being actively studied. Live imaging of the zebrafish trunk shows that precursor cells expressing low levels of Prox1 divide asymmetrically to produce daughter cells with distinct fates [66]. While one daughter becomes a LEC that upregulates Prox1 and migrates out of the PCV, the other daughter remains venous within the PCV and loses its Prox1 expression. Vegfr3/Vegfc signaling is required for this division and for secondary sprout formation, but not for initial LEC specification [66–68]. Current evidence suggests that a Prox1-Vegfr3 feedback loop forms in which Prox1 targets Vegfr3 expression which in turn upregulates Prox1 [66,68]. Prox1 is required not only for LEC specification but also to maintain lymphatic identity. Conditional knockout of Prox1 in embryonic, postnatal, or adult mice leads to a reversion of LECs back to blood endothelial cells [69]. In addition, lymphatic vessels exposed to shear stress via blood flow are reprogramed to blood vessels [70]. Together, these studies indicate that a terminal lymphatic fate must be actively maintained and that LECs retain their plasticity even into adulthood.
Although they are largely excluded from the central nervous system, lymphatics have also recently been described within the brain meninges. Mice contain a network of lymphatic vessels within the dura mater of the meninges, important for the removal of excess fluids and macromolecules from this area [71,72]. Recent work has shown that zebrafish also have a complex intracranial lymphatic vessel network which develops from the facial lymphatic vascular plexus [73]. Since the facial lymphatic system originates from the common cardinal vein with other nearby veins contributing to its formation [74], this would suggest that the intracranial lymphatic system also has a venous origin. In addition to lymphatic vessels, zebrafish also contain a population of macrophage-like individual, isolated cells in the internal meninges (leptomeninges) that express lymphatic markers but that do not form vessels and reside next to meningeal blood vessels [75–77]. These cells are also vein-derived, sprouting from the optic choroid vascular plexus, a primitive lymphovenous vascular plexus behind the eye. Although this cell population has been shown to also require Vegfc-Vegfd-Vegfr3 signaling for its development, the similarities and differences in the mechanisms leading to formation of these cells compared to vessel- forming LECs remain largely unexplored. Future studies should clarify the role of this endothelial derived meningeal cell population and their plasticity.
Endothelial-mesenchymal transition
A fourth major endothelial cell fate transition event occurs during cardiac valve development of the vertebrate heart. During this process, specialized endothelial cells that line the heart called endocardial cells undergo an EndoMT to form the interstitial cells of the cardiac valve leaflets (Figure 1d) [13,78–81]. This process is driven by many different signaling pathways including BMP, Transforming growth factor (TGF)β, and Notch, which are required for the specification, initiation, or cessation of EndoMT in the cardiac cushions, the sites of valve formation [82]. Inactivation of the ligand Bmp2 in the mouse myocardium or its receptor Bmp type 1A receptor (Bmpr1a) in the endocardium leads to a failure of EndoMT and thus a lack of mesenchymal cells in the atrioventricular (AV) cardiac cushion [83]. TGFβ signaling is also important for EndoMT initiation, with TGFβ2 deficient AV explants showing delays in this process [84]. In addition, embryos lacking TGFβ2 have enlarged cardiac cushions due to continual EndoMT suggesting that TGFβ2 is needed for EndoMT termination [84]. Notch signaling is also vital for EndoMT in both mice and zebrafish. A reduction in Notch signaling leads to a loss of mesenchymal cushion cells causing dysfunctional valves to form while constitutive activation leads to excessive EndoMT [85]. Although there are many points of crosstalk between these signaling pathways during heart valve development, all three pathways converge on regulating the expression of Snail family members, a group of transcription factors that are vital for the EndoMT process [82].
In addition to the Snail family members other transcription factors are also necessary for valve development, including Nuclear factor of activated T-cell 1 (Nfatc1). Loss of Nfatc1 in mice and zebrafish causes valve morphogenesis defects in both organisms [13,86]. In mice, Nfatc1 is required in both the myocardium to reduce Vegf signaling allowing valve progenitor cells to undergo EndoMT and the endocardium to promote valve leaflet proliferation and elongation [86,87]. In zebrafish, global loss of nfatc1 leads to a significant decrease in the mesenchymal interstitial cells that make up the valve due to a decrease in proliferation and recruitment of progenitor cells as well as a decrease in the EndoMT promoting factor twist1b [13]. More work needs to be done to better elucidate the cell specific role Nfatc1 plays in cardiac valve morphogenesis and EndoMT. Valve leaflets contain monolayers of endothelial cells surrounding the interstitial mesenchymal cells, indicating that while some endothelial cells undergo EndoMT others remain endothelial. However, the mechanisms regulating this choice are still not well understood. Since EndoMT is also required for cardiac regeneration after injury [88,89] and has been implicated in the progression of several diseases such as cerebral cavernous malformations (CCM), atherosclerosis, and cancer [1], understanding the molecular mechanisms that drive EndoMT and endothelial plasticity in general is of great therapeutic value.
PLASTICITY DURING VESSEL REPAIR AND REVASCULARIZATION
The plasticity of endothelial cells and their derivatives is not restricted to early developmental stages but also occurs in response to tissue injury due to trauma or disease. Reperfusion of damaged vessels is critical to ensure tissue survival, but vessel regrowth and reconnection depends on the tissue environment and the type of damage incurred. Amputation of the adult zebrafish caudal fin illustrates how arteriovenous fate transitions can occur after tissue damage. During tail regeneration, a vascular plexus forms that is predominately venous derived [90]. As the plexus remodels, new arteries and veins form with venous cells continuing to proliferate and contribute to the growing arteries [18,90]. Similar to arterial specification during development, this cell fate change from venous to arterial requires Notch signaling [90]. While arterial formation from venous cells does occur during later organogenesis stages in zebrafish and mice [91], the fin regeneration findings suggest that endothelial cells retain their plasticity even into adulthood. This has also been shown to be true in humans that receive vessel grafts. Saphenous vein grafts are commonly used for both venous and arterial vessel reconstruction. Recent work shows that vein grafts on the popliteal vein and artery due to an acute extremity injury are able to adapt to their environments [39]. The graft placed on the vein retained venous identity expressing markers such as EphB4 and COUP-TFII while the graft placed on the artery downregulated venous markers and started to express Ephrin-B2 and Delta-like ligand 4, markers of arterial identity [39]. This suggests that vessel identity is also plastic in human adult blood vessels. However, this plasticity may depend on the vessel the segment is grafted to or the type of injury that necessitated the need for a graft, since previous work shows vein to artery grafts losing venous identity but not gaining arterial marker expression [92]. It is also unclear to what extent the change in vessel identity results from cells moving from adjacent vessel segments into the graft, as opposed to a change in the arterial-venous identity of the grafted endothelial cells. Thus, more work needs to be done to understand what factors influence endothelial arteriovenous plasticity after injury.
The revascularization of tissue after injury is vital for its survival and proper organ function. This is especially true after cardiac injury, where lack of newly formed vessels can cause fibrotic scar formation [20,93,94]. In zebrafish, damage to the heart leads to the formation of new vessels from preexisting endothelium and studies have shown that the fast revascularization of vessels into the injured area seen after cryoinjury is due to vessel sprouting [20,95]. In mice, some studies have claimed that endocardial cells or resident cardiac fibroblasts contribute to newly forming coronary vessels after cardiac injury [96,97]. However, recent work has shown this not to be the case and similar to zebrafish, revascularization comes from preexisting endothelial cells [98,99]. Thus, unlike during the initial development of the coronary vessels from endothelial cells from the endocardium and sinus venosus, revascularization of the heart after injury is mostly from sprouting of preexisting nearby vessels [93,100].
In addition to resident tissue cells contributing to revascularization, some studies have suggested that circulating endothelial progenitor cells (EPCs) derived from the bone marrow contribute to newly forming adult organ vessels during normal physiological conditions and after myocardial ischemia, hind limb ischemia, skin wounding, or traumatic brain injury [101–105]. In contrast, other studies have shown that the contribution of EPCs to new vessels is low and that circulating bone marrow derived cells are recruited to blood vessels in order to promote angiogenesis but are not actually incorporated into the vessel wall [106,107]. The range of EPC incorporation into newly forming adult vessels seems to vary greatly from study to study. This may be dependent on the type of tissue utilized in the study, or the type of injury/disease model used to stimulate neovascularization. However, even studies using a similar injury model for the carotid artery show differing results - one study has suggested that circulating EPCs contribute to its reendothelization [108], while other studies show that EPCs do not contribute to endothelial regeneration of the carotid artery and that endothelial cells migrate from the adjacent vessel segment [109,110]. While the question of whether EPCs actually get incorporated into newly forming vessels has been widely debated, it is generally agreed that bone marrow derived cells can provide therapeutic benefit in the context of ischemic tissues by promoting angiogenesis. A variety of clinical studies on peripheral artery disease and acute myocardial infarction have shown that bone marrow cell therapy can improve patient outcomes [111,112]. New technologies in lineage tracing and in vivo imaging may help us better elucidate how bone marrow derived cells, in particular EPCs, aid in the revascularization of tissue after ischemia.
In addition to blood endothelial revascularization after injury, lymphatic vessels also respond to tissue damage. In both mice and zebrafish, the cardiac lymphatic system is reactivated after injury in response to Vegfc signaling and expands into the wounded region [113–116]. In zebrafish, sprouting from pre-existing ventricular lymphatics does contribute to the newly forming lymphatic vessels, but the majority of new vessels seem to originate from the coalescence of LECs either from isolated LEC clusters normally found in the zebrafish heart or possibly from LECs detaching and migrating away from lymphatic vessels into the injured area [113]. Since isolated LEC clusters are only found transiently in mouse hearts until P23 it would be interesting to ascertain if lymphatic sprouting or LEC coalescence is utilized to form new lymphatic vessels following cardiac injury in mice. Furthermore, whether the endocardium or the cardiac blood endothelium can contribute to lymphatic revascularization after cardiac injury has not been explored. As noted above, the zebrafish meninges also contain a population of individual perivascular cells expressing lymphatic markers [75–77]. Although they do not contribute to lymphatic vessels under normal circumstances, one recent report suggests that these cells (variably called, fluorescent granular perithelial (FGP) cells, mural (mu)LECs, or brain (B)LECs) have the capacity to migrate into the brain parenchyma after brain injury to form lumenized lymphatic vessels that drain interstitial fluid and act as “tracks” for the growth of new blood vessels [117], suggesting they may be more plastic in the injury setting. This study was carried out in zebrafish larvae, however, and it remains to be seen whether these cells have the same plasticity after injury in the adult brain, which contains both FGPs/muLECs/BLECs and bona fide lymphatic vessels in the meninges [73]. In mice, photothrombosis induced stroke but not transient middle cerebral artery occlusion led to lymphatic vessel growth from the sagittal sinus into the alymphatic zone where the stroke occurred [118]. These findings suggest that lymphatic neovascularization can occur after mammalian brain injury but may depend on the severity of the injury incurred. In addition, whether these newly forming lymphatic vessels emerge from preexisting lymphatics in the meninges or derive from other cell types within the brain still needs to be investigated, but better understanding how new lymphatics form after injury could greatly aid in treating several diseases such as stroke, traumatic brain injury, and neurodegenerative disorders.
Similar to its development, the cardiac valve also undergoes EndoMT after injury in zebrafish. Genetic ablation of the valve interstitial cells leads to the recruitment of endothelial cells that transdifferentiate into interstitial cells to build a new valve [88]. Furthermore, this regeneration process is promoted by TGFβ signaling. In sheep, tethering of the mitral valve leaflets to restrict valve closure led to an increase in endothelial cells undergoing EndoMT and an increase in leaflet area and thickness [119]. This suggests that changes in mechanical stress can induce endothelial cell plasticity to drive mitral valve growth, perhaps to help improve valve closure. EndoMT is also seen in the mitral valves of patients with ischemic mitral regurgitation which causes a reversal of blood flow [119]. Therefore, a compensatory mechanism seems to be initiated in these diseased hearts but it is not sufficient to prevent the backflow of blood. Better understanding how EndoMT is induced during injury or disease conditions could lead to new therapeutic approaches to further promote compensatory mechanisms.
ENDOTHELIAL PLASTICITY IN DISEASE
While endothelial plasticity can be beneficial when it comes to the repair and revascularization of ischemic tissue and/or damaged vessels, it can also be detrimental under pathological conditions, leading to severe outcomes. Arteriovenous malformations (AVMs) are aberrant shunts with high blood flow that bypass capillary beds and may cause hemorrhage or hypoxia in the surrounding tissue [120]. These malformations lack well defined arterial-venous identity and many animal studies have shown that loss or activation of genes that regulate arterial-venous specification, such as Notch, EphrinB2, and EphB4, can lead to the formation of AVMs [121,122]. AVMs can form throughout the body and occur in various human disorders such as capillary malformation-AVM (CM-AVM) and hereditary hemorrhagic telangiectasia (HHT). Recently, loss of function mutations in EPHB4 have been linked to a type of CM-AVM suggesting that loss of vessel identity can contribute to AVM formation [123]. HHT results from mutations in the BMP receptor ALK1/ACVRL1, the accessory type III receptor endoglin (ENG), or the downstream BMP transcription factor SMAD4, leading to downregulation of BMP signaling in endothelial cells [124]. Expression of EphrinB2, a marker of arterial identity, has been shown to be reduced in the arterial domain of AVMs in homozygous Alk1 mutant mice, although it appears unaffected in Eng mutants [125]. Smad4 has been recently implicated in the acquisition of venous identity in mice and fish [38], thus loss of any of these genes may affect the arterial or venous identity of endothelial cells leading to AVM formation. However, it remains unclear whether the formation of AVMs is the consequence of a failure in arterial-venous specification, or whether loss of arterial-venous identity is a secondary consequence of increased shear stress and enlargement of the malformed shunt vessels. Future studies using CM-AVM and HHT disease models in mice and fish will help us better ascertain whether loss of endothelial identity is the root cause of AVMs.
EndoMT is frequently seen in a variety of pathologies including tissue fibrosis, CCM, and atherosclerosis, to name a few [1]. In many fibrotic diseases there is an uncontrolled accumulation of fibrotic tissue due to activated fibroblasts or myofibroblasts that can lead to organ dysfunction [126]. While tissue resident fibroblasts are most likely the major cellular source contributing to fibrotic disorders, other cell types including endothelial cells may also contribute to disease progression by transdifferentiating into fibroblasts. EndoMT has been observed in patient samples and/or mouse models of systemic sclerosis, pulmonary fibrosis, cardiac fibrosis, and fibrotic kidney disease, making its role in disease progression an interesting avenue to explore [126–128]. In CCM, vascular lesions form from enlarged or irregular vessels within the central nervous system that can lead to hemorrhage [1,126]. This disease is caused by loss of function mutations in either CCM1 (KRIT1), CCM2 (OSM), or CCM3 (PDCD10). Mouse models for CCM1 have shown that endothelial cells lining the brain lesion had an increase in TGFβ signaling and mesenchymal marker expression compared to non-lesion controls suggesting that these cells may be undergoing EndoMT [129]. Similar results have since been shown in samples of human brain lesions from CCM patients [130,129]. This suggests that the same pathway that drives EndoMT during development could contribute to lesion formation in CCM mutants. In support of this idea, inhibiting TGFβ signaling in endothelial CCM1-deficient mice was sufficient to reduce the size and number of lesions and decrease vessel leakage [129]. Modeling CCM in other organisms such as zebrafish has revealed additional downstream signaling pathways [131] and further work in these model organisms can help us better understand if EndoMT contributes to disease progression as well as allow us to test a vast array of pharmacological therapies [132]. Future studies are needed to determine if TGFβ inhibition or other treatments would be effective in human patients.
Atherosclerosis is the build-up of plaque in artery walls leading to the obstruction of blood flow. Rupturing of plaques can have severe consequences, causing blood clots that lead to heart attack, stroke, or even death. While the pathology of atherosclerosis has been studied for quite some time, the role of EndoMT in its progression has only recently been considered [126]. Using ApoE-deficient mice on high fat diets which form atherosclerotic plaques, studies have shown that lineage-traced endothelial cells near the plaque undergo EndoMT by downregulating endothelial markers and upregulating mesenchymal markers [133,134]. Specifically, these lineage-traced endothelial cells become fibroblast-like and incorporate into the plaque [134], although whether they become bona fide fibroblasts remains unclear. Plaques within human patients have also been shown to contain cells that express both endothelial and mesenchymal markers suggesting that these cells may be undergoing a fate transition. Furthermore, the proportion of cells undergoing EndoMT seems to increase with an increase in disease severity indicating that EndoMT may help drive disease progression [133,134]. While this is just correlative, in vitro work has shown that endothelial derived fibroblasts express higher levels of matrix metalloproteinases and lower levels of collagen compared to normal fibroblasts, which may contribute to plaque rupture [134]. More work still needs to be done to fully elucidate the role EndoMT plays in atherosclerosis pathology.
Cellular plasticity is a major hallmark of cancer progression. While many tumor cells undergo some form of epithelial to mesenchymal transition, there are also many examples of endothelial plasticity within cancer [3]. In Kaposi’s sarcoma, highly vascularized tumors form from spindle cells which are thought to be endothelial and/or mesenchymal in origin [135,136]. These cells undergo reprogramming, forming aberrant phenotypes and expressing both blood and lymphatic cell markers [137,138]. Bone marrow-derived EPCs contribute to the vascularization of tumors in many cancers by secreting pro-angiogenic factors as well as incorporating into the newly forming vessels [139]. This has been shown to be the case for tumor angiogenesis in mouse models and within tissue samples of patient tumors [140,141]. In addition, cancer cells can take on endothelial-like characteristics forming vascular channels that carry blood and plasma from host blood vessels to the tumor [142]. Known as vascular mimicry, this phenomenon was first described in melanoma and has now been identified in other cancers such as sarcomas, carcinomas, and breast, prostate, and ovarian tumors [142,143]. EndoMT also occurs in cancer, with endothelial cells becoming fibroblast-like, apparently contributing to the cancer-associated fibroblast (CAF) population through cell conversion. Endothelial cells within tumors have been shown to express fibroblast markers such as fibroblast specific protein 1 and smooth muscle actin in mouse models of melanoma and pancreatic cancer, suggesting they are undergoing EndoMT [144], although again whether these cells fully lose their endothelial identity and assume a fibroblast identity still needs to be explored. CAFs are thought to facilitate tumor progression through depositing extracellular matrix and secreting paracrine factors that affect tumor cells as well as the surrounding microenvironment [145]. While human endothelial cells are capable of undergoing EndoMT in vitro when treated with different stimuli such as TGFβ, the prevalence and importance of EndoMT in human tumors needs to be further elucidated [146]. Since many types of cancer contain some form of endothelial plasticity which usually coincides with a more severe prognosis, better understanding the mechanisms that maintain or dysregulate endothelial identity could have great therapeutic value in preventing tumor metastasis.
CONCLUDING REMARKS
The ability of endothelial cells to undergo cell fate changes has been seen throughout development, during injury repair, and within different disease pathologies. This indicates that endothelial cells are not only plastic during embryonic stages but also into adulthood where they can respond to changes in local signals and hemodynamic forces. Zebrafish have emerged as a powerful model organism to study vascular plasticity due to their experimental accessibility and optical transparency that allows endothelial fate transitions to be lineage traced and observed in real time. Work in zebrafish has already furthered our knowledge of the molecular mechanisms that contribute to these changes in fate. As in mammals, endothelial cells in zebrafish undergo a number of major fate transitions during development, including specification of arteries and veins, formation of hematopoietic stem cells from the dorsal aorta, emergence of lymphatic endothelial cells from venous endothelial cells, and conversion of endocardium to cardiac mesenchyme to generate the heart valve. Endothelial plasticity also contributes to the repair and revascularization of tissue after injury. While there are various examples seen in zebrafish, chick, and mammals, the ability of the zebrafish to regenerate many organs after injury allows for the discovery of new examples of plasticity during repair. Many studies of endothelial plasticity in disease have been done using human tissue samples or mouse models. However, with the development of many new zebrafish disease models such as HHT, CCM, atherosclerosis, and cancer (Table 2), zebrafish can become a useful tool in understanding just how prevalent and important endothelial plasticity is in disease progression. Altogether this review highlights the occurrences of endothelial plasticity during development, injury repair, and disease progression as well as the molecular mechanisms that drive these processes. Better understanding the causes and consequences of this plasticity could open up new approaches to regenerative therapies and disease treatments.
ACKNOWLEDGEMENTS
The authors thank members of the Weinstein lab for their help and support. We apologize to authors whose work we could not cite due to space limitations.
SOURCES OF FUNDING
This paper was supported by the intramural program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (ZIA-HD008915, ZIA-HD008808, and ZIA-HD001011, to BMW).
Footnotes
DISCLOSURES
None
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- 1.Dejana E, Hirschi KK, Simons M (2017) The molecular basis of endothelial cell plasticity. Nat Commun 8:14361. doi: 10.1038/ncomms14361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tata PR, Rajagopal J (2016) Cellular plasticity: 1712 to the present day. Curr Opin Cell Biol 43:46–54. doi: 10.1016/j.ceb.2016.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yuan S, Norgard RJ, Stanger BZ (2019) Cellular Plasticity in Cancer. Cancer Discov 9 (7):837–851. doi: 10.1158/2159-8290.CD-19-0015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ellertsdottir E, Lenard A, Blum Y, Krudewig A, Herwig L, Affolter M, Belting HG (2010) Vascular morphogenesis in the zebrafish embryo. Dev Biol 341 (1):56–65. doi: 10.1016/j.ydbio.2009.10.035 [DOI] [PubMed] [Google Scholar]
- 5.Gore AV, Monzo K, Cha YR, Pan W, Weinstein BM (2012) Vascular development in the zebrafish. Cold Spring Harb Perspect Med 2 (5):a006684. doi: 10.1101/cshperspect.a006684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hogan BM, Schulte-Merker S (2017) How to Plumb a Pisces: Understanding Vascular Development and Disease Using Zebrafish Embryos. Dev Cell 42 (6):567–583. doi: 10.1016/j.devcel.2017.08.015 [DOI] [PubMed] [Google Scholar]
- 7.Isogai S, Horiguchi M, Weinstein BM (2001) The vascular anatomy of the developing zebrafish: an atlas of embryonic and early larval development. Dev Biol 230 (2):278–301. doi: 10.1006/dbio.2000.9995 [DOI] [PubMed] [Google Scholar]
- 8.Lawson ND, Weinstein BM (2002) In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev Biol 248 (2):307–318. doi: 10.1006/dbio.2002.0711 [DOI] [PubMed] [Google Scholar]
- 9.Isogai S, Lawson ND, Torrealday S, Horiguchi M, Weinstein BM (2003) Angiogenic network formation in the developing vertebrate trunk. Development 130 (21):5281–5290. doi: 10.1242/dev.00733 [DOI] [PubMed] [Google Scholar]
- 10.Stainier DY, Weinstein BM, Detrich HW 3rd, Zon LI, Fishman MC (1995) Cloche, an early acting zebrafish gene, is required by both the endothelial and hematopoietic lineages. Development 121 (10):3141–3150 [DOI] [PubMed] [Google Scholar]
- 11.Das RN, Yaniv K (2020) Discovering New Progenitor Cell Populations through Lineage Tracing and In Vivo Imaging. Cold Spring Harb Perspect Biol. doi: 10.1101/cshperspect.a035618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bertrand JY, Chi NC, Santoso B, Teng S, Stainier DY, Traver D (2010) Haematopoietic stem cells derive directly from aortic endothelium during development. Nature 464 (7285):108–111. doi: 10.1038/nature08738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gunawan F, Gentile A, Gauvrit S, Stainier DYR, Bensimon-Brito A (2020) Nfatc1 Promotes Interstitial Cell Formation During Cardiac Valve Development in Zebrafish. Circ Res 126 (8):968–984. doi: 10.1161/CIRCRESAHA.119.315992 [DOI] [PubMed] [Google Scholar]
- 14.Kissa K, Herbomel P (2010) Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature 464 (7285):112–115. doi: 10.1038/nature08761 [DOI] [PubMed] [Google Scholar]
- 15.Lam EY, Hall CJ, Crosier PS, Crosier KE, Flores MV (2010) Live imaging of Runx1 expression in the dorsal aorta tracks the emergence of blood progenitors from endothelial cells. Blood 116 (6):909–914. doi: 10.1182/blood-2010-01-264382 [DOI] [PubMed] [Google Scholar]
- 16.Weijts B, Gutierrez E, Saikin SK, Ablooglu AJ, Traver D, Groisman A, Tkachenko E (2018) Blood flow-induced Notch activation and endothelial migration enable vascular remodeling in zebrafish embryos. Nat Commun 9 (1):5314. doi: 10.1038/s41467-018-07732-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yaniv K, Isogai S, Castranova D, Dye L, Hitomi J, Weinstein BM (2006) Live imaging of lymphatic development in the zebrafish. Nat Med 12 (6):711–716. doi: 10.1038/nm1427 [DOI] [PubMed] [Google Scholar]
- 18.Xu C, Hasan SS, Schmidt I, Rocha SF, Pitulescu ME, Bussmann J, Meyen D, Raz E, Adams RH, Siekmann AF (2014) Arteries are formed by vein-derived endothelial tip cells. Nat Commun 5:5758. doi: 10.1038/ncomms6758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gurevich DB, Severn CE, Twomey C, Greenhough A, Cash J, Toye AM, Mellor H, Martin P (2018) Live imaging of wound angiogenesis reveals macrophage orchestrated vessel sprouting and regression. EMBO J 37 (13). doi: 10.15252/embj.201797786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marin-Juez R, Marass M, Gauvrit S, Rossi A, Lai SL, Materna SC, Black BL, Stainier DY (2016) Fast revascularization of the injured area is essential to support zebrafish heart regeneration. Proc Natl Acad Sci U S A 113 (40):11237–11242. doi: 10.1073/pnas.1605431113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noishiki C, Yuge S, Ando K, Wakayama Y, Mochizuki N, Ogawa R, Fukuhara S (2019) Live imaging of angiogenesis during cutaneous wound healing in adult zebrafish. Angiogenesis 22 (2):341–354. doi: 10.1007/s10456-018-09660-y [DOI] [PubMed] [Google Scholar]
- 22.De Val S, Chi NC, Meadows SM, Minovitsky S, Anderson JP, Harris IS, Ehlers ML, Agarwal P, Visel A, Xu SM, Pennacchio LA, Dubchak I, Krieg PA, Stainier DY, Black BL (2008) Combinatorial regulation of endothelial gene expression by ets and forkhead transcription factors. Cell 135 (6):1053–1064. doi: 10.1016/j.cell.2008.10.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee D, Park C, Lee H, Lugus JJ, Kim SH, Arentson E, Chung YS, Gomez G, Kyba M, Lin S, Janknecht R, Lim DS, Choi K (2008) ER71 acts downstream of BMP, Notch, and Wnt signaling in blood and vessel progenitor specification. Cell Stem Cell 2 (5):497–507. doi: 10.1016/j.stem.2008.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pham VN, Lawson ND, Mugford JW, Dye L, Castranova D, Lo B, Weinstein BM (2007) Combinatorial function of ETS transcription factors in the developing vasculature. Dev Biol 303 (2):772–783. doi: 10.1016/j.ydbio.2006.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sumanas S, Lin S (2006) Ets1-related protein is a key regulator of vasculogenesis in zebrafish. PLoS Biol 4 (1):e10. doi: 10.1371/journal.pbio.0040010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Veldman MB, Zhao C, Gomez GA, Lindgren AG, Huang H, Yang H, Yao S, Martin BL, Kimelman D, Lin S (2013) Transdifferentiation of fast skeletal muscle into functional endothelium in vivo by transcription factor Etv2. PLoS Biol 11 (6):e1001590. doi: 10.1371/journal.pbio.1001590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reischauer S, Stone OA, Villasenor A, Chi N, Jin SW, Martin M, Lee MT, Fukuda N, Marass M, Witty A, Fiddes I, Kuo T, Chung WS, Salek S, Lerrigo R, Alsio J, Luo S, Tworus D, Augustine SM, Mucenieks S, Nystedt B, Giraldez AJ, Schroth GP, Andersson O, Stainier DY (2016) Cloche is a bHLH-PAS transcription factor that drives haemato-vascular specification. Nature 535 (7611):294–298. doi: 10.1038/nature18614 [DOI] [PubMed] [Google Scholar]
- 28.Garcia-Cardena G, Slegtenhorst BR (2016) Hemodynamic Control of Endothelial Cell Fates in Development. Annu Rev Cell Dev Biol 32:633–648. doi: 10.1146/annurev-cellbio-100814-125610 [DOI] [PubMed] [Google Scholar]
- 29.Wang HU, Chen ZF, Anderson DJ (1998) Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell 93 (5):741–753. doi: 10.1016/s0092-8674(00)81436-1 [DOI] [PubMed] [Google Scholar]
- 30.Herzog Y, Guttmann-Raviv N, Neufeld G (2005) Segregation of arterial and venous markers in subpopulations of blood islands before vessel formation. Dev Dyn 232 (4):1047–1055. doi: 10.1002/dvdy.20257 [DOI] [PubMed] [Google Scholar]
- 31.Lawson ND, Scheer N, Pham VN, Kim CH, Chitnis AB, Campos-Ortega JA, Weinstein BM (2001) Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development 128 (19):3675–3683 [DOI] [PubMed] [Google Scholar]
- 32.Lawson ND, Vogel AM, Weinstein BM (2002) sonic hedgehog and vascular endothelial growth factor act upstream of the Notch pathway during arterial endothelial differentiation. Dev Cell 3 (1):127–136. doi: 10.1016/s1534-5807(02)00198-3 [DOI] [PubMed] [Google Scholar]
- 33.Fischer A, Schumacher N, Maier M, Sendtner M, Gessler M (2004) The Notch target genes Hey1 and Hey2 are required for embryonic vascular development. Genes Dev 18 (8):901–911. doi: 10.1101/gad.291004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gale NW, Dominguez MG, Noguera I, Pan L, Hughes V, Valenzuela DM, Murphy AJ, Adams NC, Lin HC, Holash J, Thurston G, Yancopoulos GD (2004) Haploinsufficiency of delta-like 4 ligand results in embryonic lethality due to major defects in arterial and vascular development. Proc Natl Acad Sci U S A 101 (45):15949–15954. doi: 10.1073/pnas.0407290101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krebs LT, Xue Y, Norton CR, Shutter JR, Maguire M, Sundberg JP, Gallahan D, Closson V, Kitajewski J, Callahan R, Smith GH, Stark KL, Gridley T (2000) Notch signaling is essential for vascular morphogenesis in mice. Genes Dev 14 (11):1343–1352 [PMC free article] [PubMed] [Google Scholar]
- 36.You LR, Lin FJ, Lee CT, DeMayo FJ, Tsai MJ, Tsai SY (2005) Suppression of Notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature 435 (7038):98–104. doi: 10.1038/nature03511 [DOI] [PubMed] [Google Scholar]
- 37.Swift MR, Pham VN, Castranova D, Bell K, Poole RJ, Weinstein BM (2014) SoxF factors and Notch regulate nr2f2 gene expression during venous differentiation in zebrafish. Dev Biol 390 (2):116–125. doi: 10.1016/j.ydbio.2014.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neal A, Nornes S, Payne S, Wallace MD, Fritzsche M, Louphrasitthiphol P, Wilkinson RN, Chouliaras KM, Liu K, Plant K, Sholapurkar R, Ratnayaka I, Herzog W, Bond G, Chico T, Bou-Gharios G, De Val S (2019) Venous identity requires BMP signalling through ALK3. Nat Commun 10 (1):453. doi: 10.1038/s41467-019-08315-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bai H, Wang Z, Li M, Sun P, Wei S, Wang Z, Xing Y, Dardik A (2020) Adult Human Vein Grafts Retain Plasticity of Vessel Identity. Ann Vasc Surg. doi: 10.1016/j.avsg.2020.04.046 [DOI] [PubMed] [Google Scholar]
- 40.Moyon D, Pardanaud L, Yuan L, Breant C, Eichmann A (2001) Plasticity of endothelial cells during arterial-venous differentiation in the avian embryo. Development 128 (17):3359–3370 [DOI] [PubMed] [Google Scholar]
- 41.Claxton S, Fruttiger M (2005) Oxygen modifies artery differentiation and network morphogenesis in the retinal vasculature. Dev Dyn 233 (3):822–828. doi: 10.1002/dvdy.20407 [DOI] [PubMed] [Google Scholar]
- 42.le Noble F, Moyon D, Pardanaud L, Yuan L, Djonov V, Matthijsen R, Breant C, Fleury V, Eichmann A (2004) Flow regulates arterial-venous differentiation in the chick embryo yolk sac. Development 131 (2):361–375. doi: 10.1242/dev.00929 [DOI] [PubMed] [Google Scholar]
- 43.Fang JS, Coon BG, Gillis N, Chen Z, Qiu J, Chittenden TW, Burt JM, Schwartz MA, Hirschi KK (2017) Shear-induced Notch-Cx37-p27 axis arrests endothelial cell cycle to enable arterial specification. Nat Commun 8 (1):2149. doi: 10.1038/s41467-017-01742-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mack JJ, Mosqueiro TS, Archer BJ, Jones WM, Sunshine H, Faas GC, Briot A, Aragon RL, Su T, Romay MC, McDonald AI, Kuo CH, Lizama CO, Lane TF, Zovein AC, Fang Y, Tarling EJ, de Aguiar Vallim TQ, Navab M, Fogelman AM, Bouchard LS, Iruela-Arispe ML (2017) NOTCH1 is a mechanosensor in adult arteries. Nat Commun 8 (1):1620. doi: 10.1038/s41467-017-01741-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garcia MD, Larina IV (2014) Vascular development and hemodynamic force in the mouse yolk sac. Front Physiol 5:308. doi: 10.3389/fphys.2014.00308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gore AV, Pillay LM, Venero Galanternik M, Weinstein BM (2018) The zebrafish: A fintastic model for hematopoietic development and disease. Wiley Interdiscip Rev Dev Biol 7 (3):e312. doi: 10.1002/wdev.312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zovein AC, Hofmann JJ, Lynch M, French WJ, Turlo KA, Yang Y, Becker MS, Zanetta L, Dejana E, Gasson JC, Tallquist MD, Iruela-Arispe ML (2008) Fate tracing reveals the endothelial origin of hematopoietic stem cells. Cell Stem Cell 3 (6):625–636. doi: 10.1016/j.stem.2008.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chanda B, Ditadi A, Iscove NN, Keller G (2013) Retinoic acid signaling is essential for embryonic hematopoietic stem cell development. Cell 155 (1):215–227. doi: 10.1016/j.cell.2013.08.055 [DOI] [PubMed] [Google Scholar]
- 49.Goldie LC, Lucitti JL, Dickinson ME, Hirschi KK (2008) Cell signaling directing the formation and function of hemogenic endothelium during murine embryogenesis. Blood 112 (8):3194–3204. doi: 10.1182/blood-2008-02-139055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marcelo KL, Sills TM, Coskun S, Vasavada H, Sanglikar S, Goldie LC, Hirschi KK (2013) Hemogenic endothelial cell specification requires c-Kit, Notch signaling, and p27-mediated cell-cycle control. Dev Cell 27 (5):504–515. doi: 10.1016/j.devcel.2013.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burns CE, Traver D, Mayhall E, Shepard JL, Zon LI (2005) Hematopoietic stem cell fate is established by the Notch-Runx pathway. Genes Dev 19 (19):2331–2342. doi: 10.1101/gad.1337005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kumano K, Chiba S, Kunisato A, Sata M, Saito T, Nakagami-Yamaguchi E, Yamaguchi T, Masuda S, Shimizu K, Takahashi T, Ogawa S, Hamada Y, Hirai H (2003) Notch1 but not Notch2 is essential for generating hematopoietic stem cells from endothelial cells. Immunity 18 (5):699–711. doi: 10.1016/s1074-7613(03)00117-1 [DOI] [PubMed] [Google Scholar]
- 53.Robert-Moreno A, Guiu J, Ruiz-Herguido C, Lopez ME, Ingles-Esteve J, Riera L, Tipping A, Enver T, Dzierzak E, Gridley T, Espinosa L, Bigas A (2008) Impaired embryonic haematopoiesis yet normal arterial development in the absence of the Notch ligand Jagged1. EMBO J 27 (13):1886–1895. doi: 10.1038/emboj.2008.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gama-Norton L, Ferrando E, Ruiz-Herguido C, Liu Z, Guiu J, Islam AB, Lee SU, Yan M, Guidos CJ, Lopez-Bigas N, Maeda T, Espinosa L, Kopan R, Bigas A (2015) Notch signal strength controls cell fate in the haemogenic endothelium. Nat Commun 6:8510. doi: 10.1038/ncomms9510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen MJ, Yokomizo T, Zeigler BM, Dzierzak E, Speck NA (2009) Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature 457 (7231):887–891. doi: 10.1038/nature07619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lieu YK, Reddy EP (2009) Conditional c-myb knockout in adult hematopoietic stem cells leads to loss of self-renewal due to impaired proliferation and accelerated differentiation. Proc Natl Acad Sci U S A 106 (51):21689–21694. doi: 10.1073/pnas.0907623106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Soza-Ried C, Hess I, Netuschil N, Schorpp M, Boehm T (2010) Essential role of c-myb in definitive hematopoiesis is evolutionarily conserved. Proc Natl Acad Sci U S A 107 (40):17304–17308. doi: 10.1073/pnas.1004640107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Y, Jin H, Li L, Qin FX, Wen Z (2011) cMyb regulates hematopoietic stem/progenitor cell mobilization during zebrafish hematopoiesis. Blood 118 (15):4093–4101. doi: 10.1182/blood-2011-03-342501 [DOI] [PubMed] [Google Scholar]
- 59.Gore AV, Athans B, Iben JR, Johnson K, Russanova V, Castranova D, Pham VN, Butler MG, Williams-Simons L, Nichols JT, Bresciani E, Feldman B, Kimmel CB, Liu PP, Weinstein BM (2016) Epigenetic regulation of hematopoiesis by DNA methylation. Elife 5:e11813. doi: 10.7554/eLife.11813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yvernogeau L, Gautier R, Petit L, Khoury H, Relaix F, Ribes V, Sang H, Charbord P, Souyri M, Robin C, Jaffredo T (2019) In vivo generation of haematopoietic stem/progenitor cells from bone marrow-derived haemogenic endothelium. Nat Cell Biol 21 (11):1334–1345. doi: 10.1038/s41556-019-0410-6 [DOI] [PubMed] [Google Scholar]
- 61.Srinivasan RS, Dillard ME, Lagutin OV, Lin FJ, Tsai S, Tsai MJ, Samokhvalov IM, Oliver G (2007) Lineage tracing demonstrates the venous origin of the mammalian lymphatic vasculature. Genes Dev 21 (19):2422–2432. doi: 10.1101/gad.1588407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hong YK, Harvey N, Noh YH, Schacht V, Hirakawa S, Detmar M, Oliver G (2002) Prox1 is a master control gene in the program specifying lymphatic endothelial cell fate. Dev Dyn 225 (3):351–357. doi: 10.1002/dvdy.10163 [DOI] [PubMed] [Google Scholar]
- 63.Petrova TV, Makinen T, Makela TP, Saarela J, Virtanen I, Ferrell RE, Finegold DN, Kerjaschki D, Yla-Herttuala S, Alitalo K (2002) Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. EMBO J 21 (17):4593–4599. doi: 10.1093/emboj/cdf470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wigle JT, Harvey N, Detmar M, Lagutina I, Grosveld G, Gunn MD, Jackson DG, Oliver G (2002) An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J 21 (7):1505–1513. doi: 10.1093/emboj/21.7.1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wigle JT, Oliver G (1999) Prox1 function is required for the development of the murine lymphatic system. Cell 98 (6):769–778. doi: 10.1016/s0092-8674(00)81511-1 [DOI] [PubMed] [Google Scholar]
- 66.Koltowska K, Lagendijk AK, Pichol-Thievend C, Fischer JC, Francois M, Ober EA, Yap AS, Hogan BM (2015) Vegfc Regulates Bipotential Precursor Division and Prox1 Expression to Promote Lymphatic Identity in Zebrafish. Cell Rep 13 (9):1828–1841. doi: 10.1016/j.celrep.2015.10.055 [DOI] [PubMed] [Google Scholar]
- 67.Karkkainen MJ, Haiko P, Sainio K, Partanen J, Taipale J, Petrova TV, Jeltsch M, Jackson DG, Talikka M, Rauvala H, Betsholtz C, Alitalo K (2004) Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol 5 (1):74–80. doi: 10.1038/ni1013 [DOI] [PubMed] [Google Scholar]
- 68.Srinivasan RS, Escobedo N, Yang Y, Interiano A, Dillard ME, Finkelstein D, Mukatira S, Gil HJ, Nurmi H, Alitalo K, Oliver G (2014) The Prox1-Vegfr3 feedback loop maintains the identity and the number of lymphatic endothelial cell progenitors. Genes Dev 28 (19):2175–2187. doi: 10.1101/gad.216226.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Johnson NC, Dillard ME, Baluk P, McDonald DM, Harvey NL, Frase SL, Oliver G (2008) Lymphatic endothelial cell identity is reversible and its maintenance requires Prox1 activity. Genes Dev 22 (23):3282–3291. doi: 10.1101/gad.1727208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen CY, Bertozzi C, Zou Z, Yuan L, Lee JS, Lu M, Stachelek SJ, Srinivasan S, Guo L, Vicente A, Mericko P, Levy RJ, Makinen T, Oliver G, Kahn ML (2012) Blood flow reprograms lymphatic vessels to blood vessels. J Clin Invest 122 (6):2006–2017. doi: 10.1172/JCI57513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, Wiig H, Alitalo K (2015) A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med 212 (7):991–999. doi: 10.1084/jem.20142290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J (2015) Structural and functional features of central nervous system lymphatic vessels. Nature 523 (7560):337–341. doi: 10.1038/nature14432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Castranova D, Samasa B, Venero Galanternik M, Jung HM, Pham VN, Weinstein BM (2020) Live Imaging of Intracranial Lymphatics in the Zebrafish. Circ Res. doi: 10.1161/CIRCRESAHA.120.317372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Okuda KS, Astin JW, Misa JP, Flores MV, Crosier KE, Crosier PS (2012) lyve1 expression reveals novel lymphatic vessels and new mechanisms for lymphatic vessel development in zebrafish. Development 139 (13):2381–2391. doi: 10.1242/dev.077701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bower NI, Koltowska K, Pichol-Thievend C, Virshup I, Paterson S, Lagendijk AK, Wang W, Lindsey BW, Bent SJ, Baek S, Rondon-Galeano M, Hurley DG, Mochizuki N, Simons C, Francois M, Wells CA, Kaslin J, Hogan BM (2017) Mural lymphatic endothelial cells regulate meningeal angiogenesis in the zebrafish. Nat Neurosci 20 (6):774–783. doi: 10.1038/nn.4558 [DOI] [PubMed] [Google Scholar]
- 76.van Lessen M, Shibata-Germanos S, van Impel A, Hawkins TA, Rihel J, Schulte-Merker S (2017) Intracellular uptake of macromolecules by brain lymphatic endothelial cells during zebrafish embryonic development. Elife 6. doi: 10.7554/eLife.25932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Venero Galanternik M, Castranova D, Gore AV, Blewett NH, Jung HM, Stratman AN, Kirby MR, Iben J, Miller MF, Kawakami K, Maraia RJ, Weinstein BM (2017) A novel perivascular cell population in the zebrafish brain. Elife 6. doi: 10.7554/eLife.24369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Beis D, Bartman T, Jin SW, Scott IC, D’Amico LA, Ober EA, Verkade H, Frantsve J, Field HA, Wehman A, Baier H, Tallafuss A, Bally-Cuif L, Chen JN, Stainier DY, Jungblut B (2005) Genetic and cellular analyses of zebrafish atrioventricular cushion and valve development. Development 132 (18):4193–4204. doi: 10.1242/dev.01970 [DOI] [PubMed] [Google Scholar]
- 79.Chen IH, Wang HH, Hsieh YS, Huang WC, Yeh HI, Chuang YJ (2013) PRSS23 is essential for the Snail-dependent endothelial-to-mesenchymal transition during valvulogenesis in zebrafish. Cardiovasc Res 97 (3):443–453. doi: 10.1093/cvr/cvs355 [DOI] [PubMed] [Google Scholar]
- 80.de Lange FJ, Moorman AF, Anderson RH, Manner J, Soufan AT, de Gier-de Vries C, Schneider MD, Webb S, van den Hoff MJ, Christoffels VM (2004) Lineage and morphogenetic analysis of the cardiac valves. Circ Res 95 (6):645–654. doi: 10.1161/01.RES.0000141429.13560.cb [DOI] [PubMed] [Google Scholar]
- 81.Lincoln J, Alfieri CM, Yutzey KE (2004) Development of heart valve leaflets and supporting apparatus in chicken and mouse embryos. Dev Dyn 230 (2):239–250. doi: 10.1002/dvdy.20051 [DOI] [PubMed] [Google Scholar]
- 82.Garside VC, Chang AC, Karsan A, Hoodless PA (2013) Co-ordinating Notch, BMP, and TGF-beta signaling during heart valve development. Cell Mol Life Sci 70 (16):2899–2917. doi: 10.1007/s00018-012-1197-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ma L, Lu MF, Schwartz RJ, Martin JF (2005) Bmp2 is essential for cardiac cushion epithelial-mesenchymal transition and myocardial patterning. Development 132 (24):5601–5611. doi: 10.1242/dev.02156 [DOI] [PubMed] [Google Scholar]
- 84.Azhar M, Runyan RB, Gard C, Sanford LP, Miller ML, Andringa A, Pawlowski S, Rajan S, Doetschman T (2009) Ligand-specific function of transforming growth factor beta in epithelial-mesenchymal transition in heart development. Dev Dyn 238 (2):431–442. doi: 10.1002/dvdy.21854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Timmerman LA, Grego-Bessa J, Raya A, Bertran E, Perez-Pomares JM, Diez J, Aranda S, Palomo S, McCormick F, Izpisua-Belmonte JC, de la Pompa JL (2004) Notch promotes epithelial-mesenchymal transition during cardiac development and oncogenic transformation. Genes Dev 18 (1):99–115. doi: 10.1101/gad.276304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chang CP, Neilson JR, Bayle JH, Gestwicki JE, Kuo A, Stankunas K, Graef IA, Crabtree GR (2004) A field of myocardial-endocardial NFAT signaling underlies heart valve morphogenesis. Cell 118 (5):649–663. doi: 10.1016/j.cell.2004.08.010 [DOI] [PubMed] [Google Scholar]
- 87.Wu B, Wang Y, Lui W, Langworthy M, Tompkins KL, Hatzopoulos AK, Baldwin HS, Zhou B (2011) Nfatc1 coordinates valve endocardial cell lineage development required for heart valve formation. Circ Res 109 (2):183–192. doi: 10.1161/CIRCRESAHA.111.245035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bensimon-Brito A, Ramkumar S, Boezio GLM, Guenther S, Kuenne C, Helker CSM, Sanchez-Iranzo H, Iloska D, Piesker J, Pullamsetti S, Mercader N, Beis D, Stainier DYR (2020) TGF-beta Signaling Promotes Tissue Formation during Cardiac Valve Regeneration in Adult Zebrafish. Dev Cell 52 (1):9–20 e27. doi: 10.1016/j.devcel.2019.10.027 [DOI] [PubMed] [Google Scholar]
- 89.Bischoff J, Aikawa E (2011) Progenitor cells confer plasticity to cardiac valve endothelium. J Cardiovasc Transl Res 4 (6):710–719. doi: 10.1007/s12265-011-9312-0 [DOI] [PubMed] [Google Scholar]
- 90.Kametani Y, Chi NC, Stainier DY, Takada S (2015) Notch signaling regulates venous arterialization during zebrafish fin regeneration. Genes Cells 20 (5):427–438. doi: 10.1111/gtc.12234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Red-Horse K, Siekmann AF (2019) Veins and Arteries Build Hierarchical Branching Patterns Differently: Bottom-Up versus Top-Down. Bioessays 41 (3):e1800198. doi: 10.1002/bies.201800198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kudo FA, Muto A, Maloney SP, Pimiento JM, Bergaya S, Fitzgerald TN, Westvik TS, Frattini JC, Breuer CK, Cha CH, Nishibe T, Tellides G, Sessa WC, Dardik A (2007) Venous identity is lost but arterial identity is not gained during vein graft adaptation. Arterioscler Thromb Vasc Biol 27 (7):1562–1571. doi: 10.1161/ATVBAHA.107.143032 [DOI] [PubMed] [Google Scholar]
- 93.Harrison MR, Bussmann J, Huang Y, Zhao L, Osorio A, Burns CG, Burns CE, Sucov HM, Siekmann AF, Lien CL (2015) Chemokine-guided angiogenesis directs coronary vasculature formation in zebrafish. Dev Cell 33 (4):442–454. doi: 10.1016/j.devcel.2015.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Marin-Juez R, El-Sammak H, Helker CSM, Kamezaki A, Mullapuli ST, Bibli SI, Foglia MJ, Fleming I, Poss KD, Stainier DYR (2019) Coronary Revascularization During Heart Regeneration Is Regulated by Epicardial and Endocardial Cues and Forms a Scaffold for Cardiomyocyte Repopulation. Dev Cell 51 (4):503–515 e504. doi: 10.1016/j.devcel.2019.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhao L, Borikova AL, Ben-Yair R, Guner-Ataman B, MacRae CA, Lee RT, Burns CG, Burns CE (2014) Notch signaling regulates cardiomyocyte proliferation during zebrafish heart regeneration. Proc Natl Acad Sci U S A 111 (4):1403–1408. doi: 10.1073/pnas.1311705111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Miquerol L, Thireau J, Bideaux P, Sturny R, Richard S, Kelly RG (2015) Endothelial plasticity drives arterial remodeling within the endocardium after myocardial infarction. Circ Res 116 (11):1765–1771. doi: 10.1161/CIRCRESAHA.116.306476 [DOI] [PubMed] [Google Scholar]
- 97.Ubil E, Duan J, Pillai IC, Rosa-Garrido M, Wu Y, Bargiacchi F, Lu Y, Stanbouly S, Huang J, Rojas M, Vondriska TM, Stefani E, Deb A (2014) Mesenchymal-endothelial transition contributes to cardiac neovascularization. Nature 514 (7524):585–590. doi: 10.1038/nature13839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.He L, Huang X, Kanisicak O, Li Y, Wang Y, Li Y, Pu W, Liu Q, Zhang H, Tian X, Zhao H, Liu X, Zhang S, Nie Y, Hu S, Miao X, Wang QD, Wang F, Chen T, Xu Q, Lui KO, Molkentin JD, Zhou B (2017) Preexisting endothelial cells mediate cardiac neovascularization after injury. J Clin Invest 127 (8):2968–2981. doi: 10.1172/JCI93868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tang J, Zhang H, He L, Huang X, Li Y, Pu W, Yu W, Zhang L, Cai D, Lui KO, Zhou B (2018) Genetic Fate Mapping Defines the Vascular Potential of Endocardial Cells in the Adult Heart. Circ Res 122 (7):984–993. doi: 10.1161/CIRCRESAHA.117.312354 [DOI] [PubMed] [Google Scholar]
- 100.Chen HI, Sharma B, Akerberg BN, Numi HJ, Kivela R, Saharinen P, Aghajanian H, McKay AS, Bogard PE, Chang AH, Jacobs AH, Epstein JA, Stankunas K, Alitalo K, Red-Horse K (2014) The sinus venosus contributes to coronary vasculature through VEGFC-stimulated angiogenesis. Development 141 (23):4500–4512. doi: 10.1242/dev.113639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM (1999) Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res 85 (3):221–228. doi: 10.1161/01.res.85.3.221 [DOI] [PubMed] [Google Scholar]
- 102.Crosby JR, Kaminski WE, Schatteman G, Martin PJ, Raines EW, Seifert RA, Bowen-Pope DF (2000) Endothelial cells of hematopoietic origin make a significant contribution to adult blood vessel formation. Circ Res 87 (9):728–730. doi: 10.1161/01.res.87.9.728 [DOI] [PubMed] [Google Scholar]
- 103.Guo X, Liu L, Zhang M, Bergeron A, Cui Z, Dong JF, Zhang J (2009) Correlation of CD34+ cells with tissue angiogenesis after traumatic brain injury in a rat model. J Neurotrauma 26 (8):1337–1344. doi: 10.1089/neu.2008-073310.1089/neu.2008.0733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, Homma S, Edwards NM, Itescu S (2001) Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med 7 (4):430–436. doi: 10.1038/86498 [DOI] [PubMed] [Google Scholar]
- 105.Urbich C, Dimmeler S (2004) Endothelial progenitor cells: characterization and role in vascular biology. Circ Res 95 (4):343–353. doi: 10.1161/01.RES.0000137877.89448.78 [DOI] [PubMed] [Google Scholar]
- 106.Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Jung S, Chimenti S, Landsman L, Abramovitch R, Keshet E (2006) VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell 124 (1):175–189. doi: 10.1016/j.cell.2005.10.036 [DOI] [PubMed] [Google Scholar]
- 107.Ziegelhoeffer T, Fernandez B, Kostin S, Heil M, Voswinckel R, Helisch A, Schaper W (2004) Bone marrow-derived cells do not incorporate into the adult growing vasculature. Circ Res 94 (2):230–238. doi: 10.1161/01.RES.0000110419.50982.1C [DOI] [PubMed] [Google Scholar]
- 108.Strehlow K, Werner N, Berweiler J, Link A, Dirnagl U, Priller J, Laufs K, Ghaeni L, Milosevic M, Bohm M, Nickenig G (2003) Estrogen increases bone marrow-derived endothelial progenitor cell production and diminishes neointima formation. Circulation 107 (24):3059–3065. doi: 10.1161/01.CIR.0000077911.81151.30 [DOI] [PubMed] [Google Scholar]
- 109.Hagensen MK, Raarup MK, Mortensen MB, Thim T, Nyengaard JR, Falk E, Bentzon JF (2012) Circulating endothelial progenitor cells do not contribute to regeneration of endothelium after murine arterial injury. Cardiovasc Res 93 (2):223–231. doi: 10.1093/cvr/cvr278 [DOI] [PubMed] [Google Scholar]
- 110.Tsuzuki M (2009) Bone marrow-derived cells are not involved in reendothelialized endothelium as endothelial cells after simple endothelial denudation in mice. Basic Res Cardiol 104 (5):601–611. doi: 10.1007/s00395-009-0021-7 [DOI] [PubMed] [Google Scholar]
- 111.Fadini GP, Agostini C, Avogaro A (2010) Autologous stem cell therapy for peripheral arterial disease meta-analysis and systematic review of the literature. Atherosclerosis 209 (1):10–17. doi: 10.1016/j.atherosclerosis.2009.08.033 [DOI] [PubMed] [Google Scholar]
- 112.Gyongyosi M, Haller PM, Blake DJ, Martin Rendon E (2018) Meta-Analysis of Cell Therapy Studies in Heart Failure and Acute Myocardial Infarction. Circ Res 123 (2):301–308. doi: 10.1161/CIRCRESAHA.117.311302 [DOI] [PubMed] [Google Scholar]
- 113.Gancz D, Raftrey BC, Perlmoter G, Marin-Juez R, Semo J, Matsuoka RL, Karra R, Raviv H, Moshe N, Addadi Y, Golani O, Poss KD, Red-Horse K, Stainier DY, Yaniv K (2019) Distinct origins and molecular mechanisms contribute to lymphatic formation during cardiac growth and regeneration. Elife 8. doi: 10.7554/eLife.44153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Harrison MR, Feng X, Mo G, Aguayo A, Villafuerte J, Yoshida T, Pearson CA, Schulte-Merker S, Lien CL (2019) Late developing cardiac lymphatic vasculature supports adult zebrafish heart function and regeneration. Elife 8. doi: 10.7554/eLife.42762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Henri O, Pouehe C, Houssari M, Galas L, Nicol L, Edwards-Levy F, Henry JP, Dumesnil A, Boukhalfa I, Banquet S, Schapman D, Thuillez C, Richard V, Mulder P, Brakenhielm E (2016) Selective Stimulation of Cardiac Lymphangiogenesis Reduces Myocardial Edema and Fibrosis Leading to Improved Cardiac Function Following Myocardial Infarction. Circulation 133 (15):1484–1497; discussion 1497. doi: 10.1161/CIRCULATIONAHA.115.020143 [DOI] [PubMed] [Google Scholar]
- 116.Klotz L, Norman S, Vieira JM, Masters M, Rohling M, Dube KN, Bollini S, Matsuzaki F, Carr CA, Riley PR (2015) Cardiac lymphatics are heterogeneous in origin and respond to injury. Nature 522 (7554):62–67. doi: 10.1038/nature14483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen J, He J, Ni R, Yang Q, Zhang Y, Luo L (2019) Cerebrovascular Injuries Induce Lymphatic Invasion into Brain Parenchyma to Guide Vascular Regeneration in Zebrafish. Dev Cell 49 (5):697–710 e695. doi: 10.1016/j.devcel.2019.03.022 [DOI] [PubMed] [Google Scholar]
- 118.Yanev P, Poinsatte K, Hominick D, Khurana N, Zuurbier KR, Berndt M, Plautz EJ, Dellinger MT, Stowe AM (2020) Impaired meningeal lymphatic vessel development worsens stroke outcome. J Cereb Blood Flow Metab 40 (2):263–275. doi: 10.1177/0271678X18822921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dal-Bianco JP, Aikawa E, Bischoff J, Guerrero JL, Handschumacher MD, Sullivan S, Johnson B, Titus JS, Iwamoto Y, Wylie-Sears J, Levine RA, Carpentier A (2009) Active adaptation of the tethered mitral valve: insights into a compensatory mechanism for functional mitral regurgitation. Circulation 120 (4):334–342. doi: 10.1161/CIRCULATIONAHA.108.846782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fang J, Hirschi K (2019) Molecular regulation of arteriovenous endothelial cell specification. F1000Res 8. doi: 10.12688/f1000research.16701.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Krebs LT, Starling C, Chervonsky AV, Gridley T (2010) Notch1 activation in mice causes arteriovenous malformations phenocopied by ephrinB2 and EphB4 mutants. Genesis 48 (3):146–150. doi: 10.1002/dvg.20599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Murphy PA, Kim TN, Huang L, Nielsen CM, Lawton MT, Adams RH, Schaffer CB, Wang RA (2014) Constitutively active Notch4 receptor elicits brain arteriovenous malformations through enlargement of capillary-like vessels. Proc Natl Acad Sci U S A 111 (50):18007–18012. doi: 10.1073/pnas.1415316111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Amyere M, Revencu N, Helaers R, Pairet E, Baselga E, Cordisco M, Chung W, Dubois J, Lacour JP, Martorell L, Mazereeuw-Hautier J, Pyeritz RE, Amor DJ, Bisdorff A, Blei F, Bombei H, Dompmartin A, Brooks D, Dupont J, Gonzalez-Ensenat MA, Frieden I, Gerard M, Kvarnung M, Hanson-Kahn AK, Hudgins L, Leaute-Labreze C, McCuaig C, Metry D, Parent P, Paul C, Petit F, Phan A, Quere I, Salhi A, Turner A, Vabres P, Vicente A, Wargon O, Watanabe S, Weibel L, Wilson A, Willing M, Mulliken JB, Boon LM, Vikkula M (2017) Germline Loss-of-Function Mutations in EPHB4 Cause a Second Form of Capillary Malformation-Arteriovenous Malformation (CM-AVM2) Deregulating RAS-MAPK Signaling. Circulation 136 (11):1037–1048. doi: 10.1161/CIRCULATIONAHA.116.026886 [DOI] [PubMed] [Google Scholar]
- 124.Whitehead KJ, Smith MC, Li DY (2013) Arteriovenous malformations and other vascular malformation syndromes. Cold Spring Harb Perspect Med 3 (2):a006635. doi: 10.1101/cshperspect.a006635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sorensen LK, Brooke BS, Li DY, Urness LD (2003) Loss of distinct arterial and venous boundaries in mice lacking endoglin, a vascular-specific TGFbeta coreceptor. Dev Biol 261 (1):235–250. doi: 10.1016/s0012-1606(03)00158-1 [DOI] [PubMed] [Google Scholar]
- 126.Piera-Velazquez S, Jimenez SA (2019) Endothelial to Mesenchymal Transition: Role in Physiology and in the Pathogenesis of Human Diseases. Physiol Rev 99 (2):1281–1324. doi: 10.1152/physrev.00021.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Manetti M, Romano E, Rosa I, Guiducci S, Bellando-Randone S, De Paulis A, Ibba-Manneschi L, Matucci-Cerinic M (2017) Endothelial-to-mesenchymal transition contributes to endothelial dysfunction and dermal fibrosis in systemic sclerosis. Ann Rheum Dis 76 (5):924–934. doi: 10.1136/annrheumdis-2016-210229 [DOI] [PubMed] [Google Scholar]
- 128.Mendoza FA, Piera-Velazquez S, Farber JL, Feghali-Bostwick C, Jimenez SA (2016) Endothelial Cells Expressing Endothelial and Mesenchymal Cell Gene Products in Lung Tissue From Patients With Systemic Sclerosis-Associated Interstitial Lung Disease. Arthritis Rheumatol 68 (1):210–217. doi: 10.1002/art.39421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Maddaluno L, Rudini N, Cuttano R, Bravi L, Giampietro C, Corada M, Ferrarini L, Orsenigo F, Papa E, Boulday G, Tournier-Lasserve E, Chapon F, Richichi C, Retta SF, Lampugnani MG, Dejana E (2013) EndMT contributes to the onset and progression of cerebral cavernous malformations. Nature 498 (7455):492–496. doi: 10.1038/nature12207 [DOI] [PubMed] [Google Scholar]
- 130.Bravi L, Malinverno M, Pisati F, Rudini N, Cuttano R, Pallini R, Martini M, Larocca LM, Locatelli M, Levi V, Bertani GA, Dejana E, Lampugnani MG (2016) Endothelial Cells Lining Sporadic Cerebral Cavernous Malformation Cavernomas Undergo Endothelial-to-Mesenchymal Transition. Stroke 47 (3):886–890. doi: 10.1161/STROKEAHA.115.011867 [DOI] [PubMed] [Google Scholar]
- 131.Zheng X, Xu C, Di Lorenzo A, Kleaveland B, Zou Z, Seiler C, Chen M, Cheng L, Xiao J, He J, Pack MA, Sessa WC, Kahn ML (2010) CCM3 signaling through sterile 20-like kinases plays an essential role during zebrafish cardiovascular development and cerebral cavernous malformations. J Clin Invest 120 (8):2795–2804. doi: 10.1172/JCI39679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Otten C, Knox J, Boulday G, Eymery M, Haniszewski M, Neuenschwander M, Radetzki S, Vogt I, Hahn K, De Luca C, Cardoso C, Hamad S, Igual Gil C, Roy P, Albiges-Rizo C, Faurobert E, von Kries JP, Campillos M, Tournier-Lasserve E, Derry WB, Abdelilah-Seyfried S (2018) Systematic pharmacological screens uncover novel pathways involved in cerebral cavernous malformations. EMBO Mol Med 10 (10). doi: 10.15252/emmm.201809155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chen PY, Qin L, Baeyens N, Li G, Afolabi T, Budatha M, Tellides G, Schwartz MA, Simons M (2015) Endothelial-to-mesenchymal transition drives atherosclerosis progression. J Clin Invest 125 (12):4514–4528. doi: 10.1172/JCI82719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Evrard SM, Lecce L, Michelis KC, Nomura-Kitabayashi A, Pandey G, Purushothaman KR, d’Escamard V, Li JR, Hadri L, Fujitani K, Moreno PR, Benard L, Rimmele P, Cohain A, Mecham B, Randolph GJ, Nabel EG, Hajjar R, Fuster V, Boehm M, Kovacic JC (2016) Endothelial to mesenchymal transition is common in atherosclerotic lesions and is associated with plaque instability. Nat Commun 7:11853. doi: 10.1038/ncomms11853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Li Y, Zhong C, Liu D, Yu W, Chen W, Wang Y, Shi S, Yuan Y (2018) Evidence for Kaposi Sarcoma Originating from Mesenchymal Stem Cell through KSHV-induced Mesenchymal-to-Endothelial Transition. Cancer Res 78 (1):230–245. doi: 10.1158/0008-5472.CAN-17-1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang HW, Trotter MW, Lagos D, Bourboulia D, Henderson S, Makinen T, Elliman S, Flanagan AM, Alitalo K, Boshoff C (2004) Kaposi sarcoma herpesvirus-induced cellular reprogramming contributes to the lymphatic endothelial gene expression in Kaposi sarcoma. Nat Genet 36 (7):687–693. doi: 10.1038/ng1384 [DOI] [PubMed] [Google Scholar]
- 137.Breiteneder-Geleff S, Soleiman A, Kowalski H, Horvat R, Amann G, Kriehuber E, Diem K, Weninger W, Tschachler E, Alitalo K, Kerjaschki D (1999) Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am J Pathol 154 (2):385–394. doi: 10.1016/S0002-9440(10)65285-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Morris VA, Punjabi AS, Lagunoff M (2008) Activation of Akt through gp130 receptor signaling is required for Kaposi’s sarcoma-associated herpesvirus-induced lymphatic reprogramming of endothelial cells. J Virol 82 (17):8771–8779. doi: 10.1128/JVI.00766-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Flamini V, Jiang WG, Lane J, Cui YX (2016) Significance and therapeutic implications of endothelial progenitor cells in angiogenic-mediated tumour metastasis. Crit Rev Oncol Hematol 100:177–189. doi: 10.1016/j.critrevonc.2016.02.010 [DOI] [PubMed] [Google Scholar]
- 140.Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, Wu Y, Hicklin D, Zhu Z, Hackett NR, Crystal RG, Moore MA, Hajjar KA, Manova K, Benezra R, Rafii S (2001) Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med 7 (11):1194–1201. doi: 10.1038/nm1101-1194 [DOI] [PubMed] [Google Scholar]
- 141.Yu D, Sun X, Qiu Y, Zhou J, Wu Y, Zhuang L, Chen J, Ding Y (2007) Identification and clinical significance of mobilized endothelial progenitor cells in tumor vasculogenesis of hepatocellular carcinoma. Clin Cancer Res 13 (13):3814–3824. doi: 10.1158/1078-0432.CCR-06-2594 [DOI] [PubMed] [Google Scholar]
- 142.Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, Pe’er J, Trent JM, Meltzer PS, Hendrix MJ (1999) Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol 155 (3):739–752. doi: 10.1016/S0002-9440(10)65173-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sun B, Zhang D, Zhao N, Zhao X (2017) Epithelial-to-endothelial transition and cancer stem cells: two cornerstones of vasculogenic mimicry in malignant tumors. Oncotarget 8 (18):30502–30510. doi: 10.18632/oncotarget.8461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zeisberg EM, Potenta S, Xie L, Zeisberg M, Kalluri R (2007) Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res 67 (21):10123–10128. doi: 10.1158/0008-5472.CAN-07-3127 [DOI] [PubMed] [Google Scholar]
- 145.Madar S, Goldstein I, Rotter V (2013) ‘Cancer associated fibroblasts’--more than meets the eye. Trends Mol Med 19 (8):447–453. doi: 10.1016/j.molmed.2013.05.004 [DOI] [PubMed] [Google Scholar]
- 146.Platel V, Faure S, Corre I, Clere N (2019) Endothelial-to-Mesenchymal Transition (EndoMT): Roles in Tumorigenesis, Metastatic Extravasation and Therapy Resistance. J Oncol 2019:8361945. doi: 10.1155/2019/8361945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jung HM, Castranova D, Swift MR, Pham VN, Venero Galanternik M, Isogai S, Butler MG, Mulligan TS, Weinstein BM (2017) Development of the larval lymphatic system in zebrafish. Development 144 (11):2070–2081. doi: 10.1242/dev.145755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Herwig L, Blum Y, Krudewig A, Ellertsdottir E, Lenard A, Belting HG, Affolter M (2011) Distinct cellular mechanisms of blood vessel fusion in the zebrafish embryo. Curr Biol 21 (22):1942–1948. doi: 10.1016/j.cub.2011.10.016 [DOI] [PubMed] [Google Scholar]
- 149.Bussmann J, Wolfe SA, Siekmann AF (2011) Arterial-venous network formation during brain vascularization involves hemodynamic regulation of chemokine signaling. Development 138 (9):1717–1726. doi: 10.1242/dev.059881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.van Impel A, Zhao Z, Hermkens DM, Roukens MG, Fischer JC, Peterson-Maduro J, Duckers H, Ober EA, Ingham PW, Schulte-Merker S (2014) Divergence of zebrafish and mouse lymphatic cell fate specification pathways. Development 141 (6):1228–1238. doi: 10.1242/dev.105031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Butko E, Distel M, Pouget C, Weijts B, Kobayashi I, Ng K, Mosimann C, Poulain FE, McPherson A, Ni CW, Stachura DL, Del Cid N, Espin-Palazon R, Lawson ND, Dorsky R, Clements WK, Traver D (2015) Gata2b is a restricted early regulator of hemogenic endothelium in the zebrafish embryo. Development 142 (6):1050–1061. doi: 10.1242/dev.119180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Nicenboim J, Malkinson G, Lupo T, Asaf L, Sela Y, Mayseless O, Gibbs-Bar L, Senderovich N, Hashimshony T, Shin M, Jerafi-Vider A, Avraham-Davidi I, Krupalnik V, Hofi R, Almog G, Astin JW, Golani O, Ben-Dor S, Crosier PS, Herzog W, Lawson ND, Hanna JH, Yanai I, Yaniv K (2015) Lymphatic vessels arise from specialized angioblasts within a venous niche. Nature 522 (7554):56–61. doi: 10.1038/nature14425 [DOI] [PubMed] [Google Scholar]
- 153.Tian Y, Xu J, Feng S, He S, Zhao S, Zhu L, Jin W, Dai Y, Luo L, Qu JY, Wen Z (2017) The first wave of T lymphopoiesis in zebrafish arises from aorta endothelium independent of hematopoietic stem cells. J Exp Med 214 (11):3347–3360. doi: 10.1084/jem.20170488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hatta K, Tsujii H, Omura T (2006) Cell tracking using a photoconvertible fluorescent protein. Nat Protoc 1 (2):960–967. doi: 10.1038/nprot.2006.96 [DOI] [PubMed] [Google Scholar]
- 155.Mosimann C, Kaufman CK, Li P, Pugach EK, Tamplin OJ, Zon LI (2011) Ubiquitous transgene expression and Cre-based recombination driven by the ubiquitin promoter in zebrafish. Development 138 (1):169–177. doi: 10.1242/dev.059345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pan YA, Freundlich T, Weissman TA, Schoppik D, Wang XC, Zimmerman S, Ciruna B, Sanes JR, Lichtman JW, Schier AF (2013) Zebrabow: multispectral cell labeling for cell tracing and lineage analysis in zebrafish. Development 140 (13):2835–2846. doi: 10.1242/dev.094631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Corti P, Young S, Chen CY, Patrick MJ, Rochon ER, Pekkan K, Roman BL (2011) Interaction between alk1 and blood flow in the development of arteriovenous malformations. Development 138 (8):1573–1582. doi: 10.1242/dev.060467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sugden WW, Meissner R, Aegerter-Wilmsen T, Tsaryk R, Leonard EV, Bussmann J, Hamm MJ, Herzog W, Jin Y, Jakobsson L, Denz C, Siekmann AF (2017) Endoglin controls blood vessel diameter through endothelial cell shape changes in response to haemodynamic cues. Nat Cell Biol 19 (6):653–665. doi: 10.1038/ncb3528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mably JD, Chuang LP, Serluca FC, Mohideen MA, Chen JN, Fishman MC (2006) santa and valentine pattern concentric growth of cardiac myocardium in the zebrafish. Development 133 (16):3139–3146. doi: 10.1242/dev.02469 [DOI] [PubMed] [Google Scholar]
- 160.Yoruk B, Gillers BS, Chi NC, Scott IC (2012) Ccm3 functions in a manner distinct from Ccm1 and Ccm2 in a zebrafish model of CCM vascular disease. Dev Biol 362 (2):121–131. doi: 10.1016/j.ydbio.2011.12.006 [DOI] [PubMed] [Google Scholar]
- 161.Stoletov K, Fang L, Choi SH, Hartvigsen K, Hansen LF, Hall C, Pattison J, Juliano J, Miller ER, Almazan F, Crosier P, Witztum JL, Klemke RL, Miller YI (2009) Vascular lipid accumulation, lipoprotein oxidation, and macrophage lipid uptake in hypercholesterolemic zebrafish. Circ Res 104 (8):952–960. doi: 10.1161/CIRCRESAHA.108.189803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Liu C, Gates KP, Fang L, Amar MJ, Schneider DA, Geng H, Huang W, Kim J, Pattison J, Zhang J, Witztum JL, Remaley AT, Dong PD, Miller YI (2015) Apoc2 loss-of-function zebrafish mutant as a genetic model of hyperlipidemia. Dis Model Mech 8 (8):989–998. doi: 10.1242/dmm.019836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Liu C, Kim YS, Kim J, Pattison J, Kamaid A, Miller YI (2018) Modeling hypercholesterolemia and vascular lipid accumulation in LDL receptor mutant zebrafish. J Lipid Res 59 (2):391–399. doi: 10.1194/jlr.D081521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Pringle ES, Wertman J, Melong N, Coombs AJ, Young AL, O’Leary D, Veinotte C, Robinson CA, Ha MN, Dellaire G, Druley TE, McCormick C, Berman JN (2019) The Zebrafish Xenograft Platform-A Novel Tool for Modeling KSHV-Associated Diseases. Viruses 12 (1). doi: 10.3390/v12010012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Patton EE, Widlund HR, Kutok JL, Kopani KR, Amatruda JF, Murphey RD, Berghmans S, Mayhall EA, Traver D, Fletcher CD, Aster JC, Granter SR, Look AT, Lee C, Fisher DE, Zon LI (2005) BRAF mutations are sufficient to promote nevi formation and cooperate with p53 in the genesis of melanoma. Curr Biol 15 (3):249–254. doi: 10.1016/j.cub.2005.01.031 [DOI] [PubMed] [Google Scholar]
- 166.Park SW, Davison JM, Rhee J, Hruban RH, Maitra A, Leach SD (2008) Oncogenic KRAS induces progenitor cell expansion and malignant transformation in zebrafish exocrine pancreas. Gastroenterology 134 (7):2080–2090. doi: 10.1053/j.gastro.2008.02.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yang HW, Kutok JL, Lee NH, Piao HY, Fletcher CD, Kanki JP, Look AT (2004) Targeted expression of human MYCN selectively causes pancreatic neuroendocrine tumors in transgenic zebrafish. Cancer Res 64 (20):7256–7262. doi: 10.1158/0008-5472.CAN-04-0931 [DOI] [PubMed] [Google Scholar]