Abstract
Background:
Access to health insurance and curative interventions [surgery/liver directed therapy (LDT)] affects survival for early-stage hepatocellular carcinoma (HCC). The aim of this multi-institutional study of high-volume safety-net hospitals (SNHs) and their tertiary-academic-centers (AC) was to identify the impact of type/lack of insurance on survival disparities across hospitals, particularly SNHs whose mission is to minimize insurance related access to care barriers for vulnerable populations.
Methods:
Early-stage HCC patients (2012-2014) from the US Safety-Net Collaborative were propensity-score matched by treatment at SNH/AC. Overall survival (OS) was the primary outcome. Multivariable Cox proportional-hazard analysis was performed accounting for sociodemographic and clinical parameters.
Results:
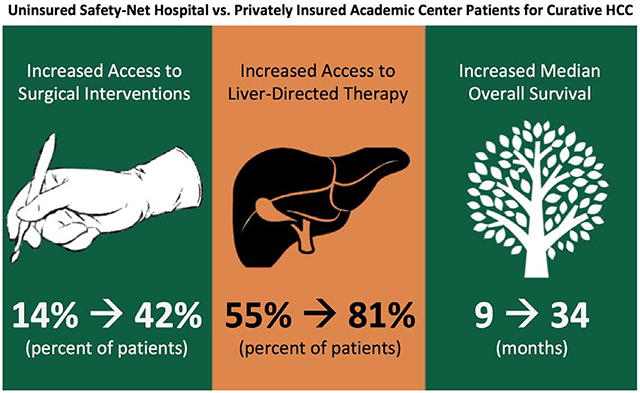
Among 925 patients, those with no insurance (NI) had decreased curative surgery, compared to those with government insurance (GI) and private insurance [PI, (PI-SNH:60.5% vs. GI-SNH:33.1% vs. NI-SNH:13.6%, p<0.001], and decreased median OS (PI-SNH:32.1 vs. GI-SNH:22.8 vs. NI-SNH:9.4 months, p=0.002). On multivariable regression controlling for sociodemographic/clinical parameters, NI-SNH (HR:2.5, 95% CI:1.3-4.9, p=0.007) was the only insurance type/hospital system combination with significantly worse OS.
Discussion:
NI-SNH patients received less curative treatment than other insurance/hospitals types suggesting that treatment barriers, beyond access to care, need to be identified and addressed to achieve survival equity in early-stage HCC for vulnerable populations (NI-SNH).
Keywords: Hepatocellular carcinoma, health insurance, uninsured, disparities
Graphical Abstract
Introduction:
Hepatocellular carcinoma (HCC) is the most common liver cancer diagnosis in the world, with an estimated 5-year survival of 31% for those with early-stage disease(1, 2). However, these survival projections are significantly lower in vulnerable patient populations(3). The cause of these disparate outcomes is multifactorial. Racial and ethnic minorities with HCC present with more advanced disease at diagnosis, and when stratified by stage, are less likely to receive curative treatment compared to non-Hispanic whites, in part due to a lack of access to healthcare(4–6). Recent studies have also shown that the quality of care delivered to patients varies by insurance type(7, 8).
With continued national scrutiny on healthcare reform and expansion, more individuals are currently covered by health insurance than individuals in 2010, likely related to implementation of the Affordable Care Act(6). However, despite this greater access to care, many patients remain without health insurance(9–11). Recent studies from the Nationwide Inpatient Sample database, comparing available treatments for HCC patients across insurance types, revealed that compared to patients with private insurance (PI), government insurance (GI), or other insurance types, those with no insurance (NI) were less likely to undergo curative surgical resection, transplantation, or liver-directed therapy(6). This inability to provide treatment to the uninsured or underinsured with potentially curable early-stage HCC could lead to preventable deaths(12–16). Additionally, it has been shown that patients with any available health insurance coverage will likely receive a greater proportion of curative interventions (e.g. chemotherapy, surgery, radiation, etc.) when compared to patients without coverage(17).
However, previous attempts to use national data sets to measure quality by payer-specific mortality rates have been unable to examine differences in the quality of care by insurance type in a predominately vulnerable population that has access to care in the safety-net hospital (SNH) system. Given our diverse multi-institutional SNH collaborative, we are perfectly poised to evaluate the impact of type or lack of insurance, as well as receipt of curative treatments, on overall survival (OS) for early-stage HCC patients treated at SNHs and tertiary academic centers (ACs). The aim of this multi-institutional study of high-volume SNHs and their ACs was to identify the impact of insurance type on survival disparities for curative HCC across hospital systems, particularly the SNH setting whose mission is to provide care to the uninsured. This allows us to uniquely study whether type or lack of insurance impacts OS for curative HCC even when vulnerable populations are afforded access to care.
Methods:
Data Source
The United States Safety-Net Collaborative Database was utilized for this retrospective study. Data is maintained and analyzed retrospectively, and no funding is utilized for maintenance. The collaborative incorporates data from five academic institutions and their adjacent SNHs. The United States Department of Health and Human Services defines a SNH as one where providers organize and deliver a significant level of both healthcare and other health-related services to the uninsured, Medicaid, and other vulnerable populations(18). Hospitals included in this consortium are New York University Langone Health, Bellevue Hospital, Baylor College of Medicine, Ben Taub Hospital, Emory University Hospital, Grady Health System, The University of Texas Southwestern Medical Center, Parkland Memorial Hospital, The University of Miami Hospital, and Jackson Memorial Hospital. ACs were included if they had an affiliated SNH. Institutional review board approval for this study was obtained at each investigative site waiving the need for individual patient consent, as this was a retrospective chart review. Only de-identified data was shared across the consortium.
Patient Selection
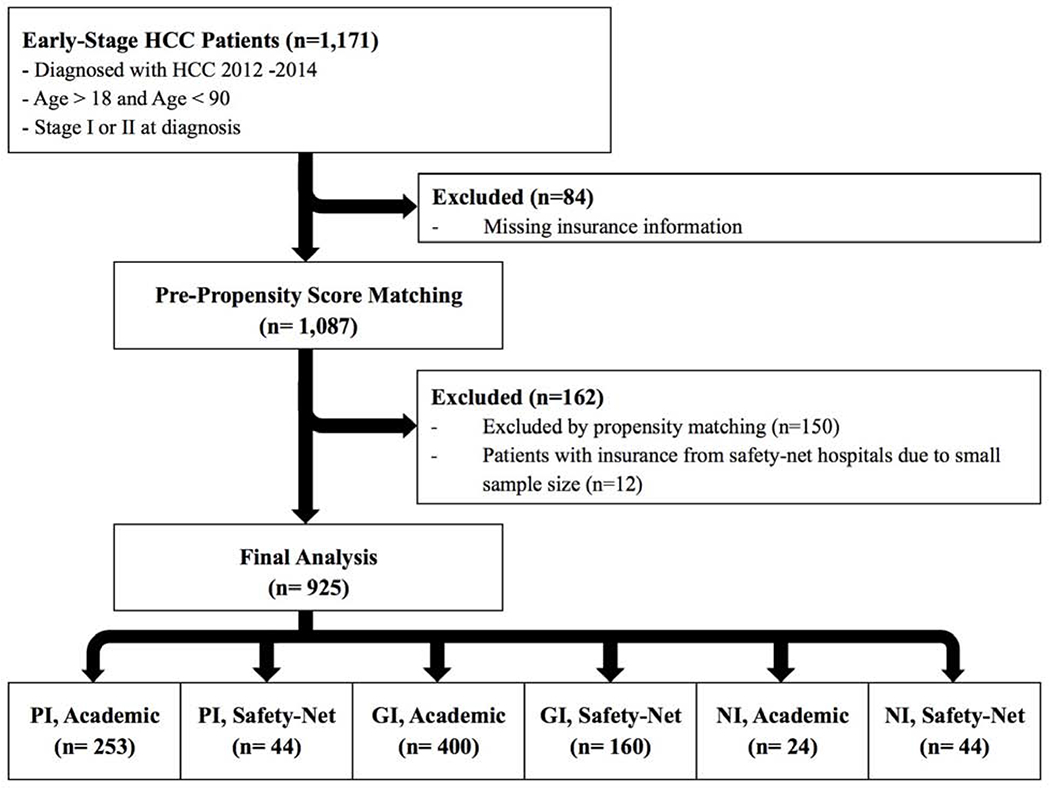
Patients were identified through the individual university cancer registry databases for those diagnosed with HCC between 2012 and 2014. International Classification of Diseases 9th edition codes were used to determine a diagnosis of HCC, and presence of HCC was confirmed through review of the medical record, imaging, or pathology. Patients were eligible for inclusion in the US Safety-Net Collaborative if they were between the ages of 18 and 90. Of the 1,171 stage I/II patients included in the collaborative, a total of 1,087 patients met inclusion criteria and were included in this study. Patients were excluded if their insurance status was unknown. AJCC 8th edition was utilized to determine clinical and pathologic stage(19).
Variables
Sociodemographic (age, gender, race, ethnicity, poverty information, housing status, and health insurance status: PI, GI, or NI), health information (primary care physician (PCP) and hepatology visit information, presence of a patient navigator, presence of cirrhosis, history of hepatitis, and treatment history of hepatitis), diagnostic information (screening imaging, screening alpha fetoprotein (AFP), model for end-stage liver disease score, and Child-Pugh Score), liver-directed or systemic therapy (radiofrequency ablation (RFA), transcatheter arterial chemoembolization (TACE), yttrium-90, chemotherapy, and radiation), surgical treatment (resection or liver transplant), and recurrence/survival analysis information was collected from electronic medical records. GI included Medicaid, Medicare, and Medicare plus supplements. Uninsured patients who can provide proof of residence within the county are eligible to receive county tax-funded care within the SNH system based on an income-adjusted fee for service scale. Patients with an income at or below 300% of the federal poverty level are eligible for full or partial coverage of services, depending on annual income(20). Patients unable to provide such documentation remain as patients with NI.
Statistical Analysis
A propensity score (PS)-matched analysis was performed to match cases by the binary predictor of treatment at a safety-net or academic hospital. Cases were matched 1:3 using the Fuzzy (Python Software Foundation, Wilmington, DE) extension and included the following confounders: gender, race, ethnicity, radiologic tumor size, and stage at presentation(21). After matching, patients receiving insurance through certain SNHs were removed due to small (n=12) sample size inadequate for analysis. Multiple group comparisons were performed, in this matched cohort and stratified by insurance type and treatment facility, using one-way analysis of variance and chi-squared tests. A Cox proportional hazards regression model was used to identify independent factors associated survival. A multivariable Cox proportional hazards regression model of OS was constructed controlling for age, gender, race/ethnicity, insurance status, Childs Class, stage at presentation, radiologic tumor size, presence of primary care physician, hepatology visit within a year of diagnosis, presence of a patient navigator, receipt of liver-directed-therapies, and receipt of surgery. Median OS was analyzed and stratified by insurance type. All P values were from 2-sided tests and results were deemed statistically significant at P <0.05. Statistical analysis was performed using SPSS version 25 (IBM Corporation, Armonk, NY, copyright 2017)(22).
Results:
Sociodemographics
A total of 1,087 patients met the inclusion criteria. Median age was 61.0 (interquartile range: 49.9-72.1) and 803 (73.9%) were male (Figure 1). The majority (52%) were white (43% non-Hispanic white and 9% white Hispanic), 23% were black, and 9% were Asian. A total of 227 (21%) were Hispanic. Two percent (17) were homeless and 269 (25%) lived in a zip code with greater than 25% of the population below the poverty level. The majority (59%) had GI, 30% had PI, 2% had HI, and 9% had NI.
Figure 1.
Patient selection with inclusion and exclusion criteria
After PS-matching, median age was 61.5 (IQR: 56.3-67.7) and 683 (73.8%) were male (Figure 1). The majority (62%) were white, 27 % were black, and 10% were Asian. A total of 138 (15%) were Hispanic. When comparing sociodemographics by insurance type and treatment facility, there was no difference in gender. NI-SNH patients tended to be younger, Black, and not Hispanic (Table 1). NI-SNH patients were more likely to live in areas with greater than 25% of residents below the poverty line.
Table 1.
Sociodemographics, screening, tumor and treatment characteristics by insurance type in post-matched cohort.
| Health Insurance and Treatment Facility |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Combined | Private Insurance | Government Insurance | No Insurance | ||||||
| Academic | Safety-Net | Academic | Safety-Net | Academic | Safety-Net | ||||
| (n=925) | (n=253) | (n=44) | (n=400) | (n=160) | (n=24) | (n=44) | |||
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | p | ||
| Sociodemographics | |||||||||
| Age (median, IQR) | 61.5 (56.3-67.7) | 60.2 (56.2-63.6) | 60.2 (53.4-64.1) | 65.1 (58.4-71.7)♮ | 60.0 (55.3-65.8) | 58.3 (51.1-62.4) | 57.2 (52.4-61.7) | <0.001 | |
| Gender | Female | 242 (26.2) | 58 (22.9) | 11 (25.0) | 120 (30.0) | 38 (23.8) | 2 (8.3)* | 13 (29.5) | 0.103 |
| Male | 683 (73.8) | 195 (77.1) | 33 (75.0) | 280 (70.0) | 122 (76.3) | 22 (91.7) | 31 (70.5) | ||
| Race | Asian | 88 (10.2) | 28 (12.1) | 1 (2.3) | 30 (8.3)♮ | 20 (12.5) | 1 (4.2) | 8 (18.2) | <0.001 |
| Black | 235 (27.2) | 41 (17.7) | 14 (31.8) | 88 (24.4) | 62 (38.8) | 9 (3.7) | 21 (47.7) | ||
| White | 536 (62.0) | 159 (68.8) | 29 (65.9) | 241 (66.8) | 78 (48.8) | 14 (58.3) | 15 (34.1) | ||
| Ethnicity | Hispanic | 138 (15.1) | 42 (16.7) | 12 (27.3) | 61 (15.6) | 21 (13.1) | 1 (4.2) | 1 (2.3) | 0.015 |
| Not Hispanic | 776 (84.9) | 209 (83.3) | 32 (72.7) | 330 (84.4) | 139 (86.9) | 23 (95.8) | 43 (97.7) | ||
| >25% of Patients in Zip Code Below Poverty Level | 212 (22.9) | 33 (13.0)* | 12 (27.3) | 84 (21.0)♮ | 57 (35.6) | 10 (41.7) | 16 (36.4) | <0.001 | |
| Health Information | |||||||||
| Primary Care Physician | 595 (85.4) | 156 (89.7)* | 29 (70.7) | 246 (87.9) | 126 (86.9) | 13 (76.5) | 25 (62.5) | <0.001 | |
| Hepatology Visit in Past Year | 390 (63.2) | 129 (73.7) | 24 (61.5) | 183 (69.8)♮ | 37 (41.1) | 8 (44.4) | 9 (27.3) | <0.001 | |
| Patient Navigator | 601 (70.2) | 186 (79.1)* | 24 (60.0) | 258 (69.2) | 101 (68.2) | 11 (50.0) | 21 (55.3) | 0.001 | |
| Cirrhosis | 782 (88.2) | 211 (86.1) | 39 (88.6) | 334 (88.4) | 135 (88.2) | 23 (95.8) | 40 (93.0) | 0.650 | |
| History of Hepatitis | 674 (76.2) | 181 (74.8) | 34 (77.3) | 265 (70.9)* | 133 (84.7) | 22 (91.7) | 39 (88.6) | 0.026 | |
| Untreated Hepatitis C | 278 (55.7) | 58 (42.3)* | 11 (40.7) | 112 (56.0)♮ | 65 (68.4) | 7 (58.3)* | 25 (89.3) | <0.001 | |
| Diagnostic Information | |||||||||
| Screening Imaging within 1 year | 281 (33.0) | 96 (40.9)* | 7 (16.3) | 120 (33.5) | 48 (32.0) | 3 (13.0) | 7 (16.3) | <0.001 | |
| Screening AFP within 1 year | 226 (28.1) | 82 (36.9)* | 8 (19.0) | 91 (27.0) | 36 (25.5) | 0 (0.0)* | 9 (20.9) | 0.001 | |
| MELD (median, IQR) | 10 (8-14) | 10 (7-14) | 10 (7-17) | 10 (8-15)* | 9 (7-13) | 9 (8-16) | 10 (8-16) | 0.141 | |
| Childs Class | A | 488 (56.3) | 140 (59.3) | 18 (47.4) | 198 (52.4) | 93 (62.4) | 12 (54.5) | 27 (61.4) | 0.322 |
| B | 262 (30.2) | 69 (29.2) | 13 (34.2) | 126 (33.3) | 38 (25.5) | 8 (36.4) | 8 (18.2) | ||
| C | 116 (13.4) | 27 (11.4) | 7 (18.4) | 54 (14.3) | 17 (11.4) | 2 (9.1) | 9 (20.5) | ||
| Tumor Characteristics at Presentation | |||||||||
| Radiologic Tumor Size (Median, IQR) | 2.7 (1.9-4.0) | 2.6 (1.7-4.0) | 3.0 (2.1-3.8) | 2.7 (1.9-4.0) | 2.8 (2.0-4.2) | 3.0 (2.1-4.9) | 2.6 (2.0-4.1) | 0.555 | |
| Stage at Diagnosis (AJCC 8th ed.) | Ia | 215 (23.2) | 69 (27.3) | 8 (18.2) | 92 (23.0) | 36 (22.5) | 4 (16.7) | 6 (13.6) | 0.547 |
| Ib | 422 (45.6) | 111 (43.9) | 21 (47.7) | 187 (46.8) | 74 (46.3) | 9 (37.5) | 20 (45.5) | ||
| II | 288 (31.1) | 73 (28.9) | 15 (34.1) | 121 (30.3) | 50 (31.3) | 11 (45.8) | 18 (40.9) | ||
| Liver Directed and Systemic Therapy | |||||||||
| RFA | 231 (25.0) | 61 (24.2) | 6 (13.6) | 107 (26.8) | 47 (29.4) | 4 (16.7) | 6 (13.6) | 0.097 | |
| TACE | 600 (65.1) | 176 (70.1)♮ | 17 (39.5) | 291 (72.9)♮ | 76 (47.5) | 18 (75.0)* | 22 (50.0) | <0.001 | |
| Y90 | 77 (8.4) | 23 (9.2) | 5 (11.6) | 42 (10.5)* | 4 (2.5) | 3 (12.5)* | 0 (0.0) | 0.010 | |
| Receipt of Any Liver Directed Therapy | 715 (77.3) | 205 (81.0)♮ | 22 (50.0) | 340 (85.0)♮ | 104 (65.0) | 20 (83.3)* | 24 (54.5) | <0.001 | |
| Chemotherapy | 146 (15.9) | 52 (20.6) | 8 (18.2) | 51 (12.9) | 25 (15.6) | 4 (16.7) | 6 (13.6) | 0.209 | |
| Radiation | 24 (2.6) | 7 (2.8) | 1 (2.3) | 10 (2.5) | 6 (3.8) | 0 (0.0) | 0 (0.0) | 0.744 | |
| Surgical Intervention | |||||||||
| Surgery | 319 (34.7) | 105 (42.0)* | 26 (60.5) | 123 (30.8) | 53 (33.1) | 6 (25.0) | 6 (13.6) | <0.001 | |
| Type of Operation | Primary Resection | 105 (33.1) | 33 (31.4) | 6 (23.1) | 29 (24.0)♮ | 29 (54.7) | 2 (33.3)* | 6 (100.0) | <0.001 |
| Transplant | 212 (66.9) | 72 (68.6) | 20 (76.9) | 92 (76.0) | 24 (45.3) | 4 (66.7) | 0 (0.0) | ||
| Survival | |||||||||
| Overall Survival in months (median, IQR) | 28.3 (11.6-56.0) | 34.2 (12.6-59.9) | 32.1 (10.0-59.7) | 27.6 (12.6-55.6) | 22.8 (11.4-50.5) | 30.6 (10.1-63.4) | 9.4 (5.1-34.2) | 0.002 | |
AFP=Alpha Fetoprotein, MELD=Model for End-Stage Liver Disease, RFA=Radiofrequency Ablation, TACE=Trans-arterial Chemoembolization, Y90=Yttrrium-90 radioembolization Subgroup Analysis within Insurance Groups:
p<0.001;
p<0.05
Health Information
NI-SNH patients had fewer PCPs, hepatology visits in the year prior to diagnosis, and patient navigators (Table 1). Although no difference in the presence of cirrhosis or history of hepatitis, NI-SNH patients had a higher proportion of untreated Hepatitis C than PI and GI patients.
Diagnostic Information and Tumor Characteristics at Presentation
NI-SNH patients had decreased screening imaging and screening AFP levels within the year prior to diagnosis compared to PI and GI patients (Table 1). NI-SNH patients also presented with a greater proportion of stage II disease compared to PI and GI patients.
Liver Directed, Systemic, and Surgical Therapy
NI-SNH patients were less likely to receive LDTs or surgical intervention compared to PI and GI patients (Table 1). Of the patients who received a surgical intervention, fewer transplantations were performed for NI-SNH patients.
Survival Analysis
On univariable analysis, NI-SNH patients had decreased OS in months compared to all other insurance and treatment facilities (PI-AC: 34.2 vs. PI-SNH: 32.1 vs. GI-AC: 27.6 vs. GI-SNH: 22.8 vs. NI-AC: 30.6 vs. NI-SNH: 9.4 months, p=0.002, Table 1).
Cox proportional hazard regression analysis was performed controlling for age, gender, race/ethnicity, insurance status, Childs Class, stage at presentation, radiologic tumor size, presence of primary care physician, hepatology visit within a year of diagnosis, presence of a patient navigator, and receipt of surgery/liver-directed therapies (Table 2). Independent predictors of decreased OS were Childs Class C at diagnosis, increasing tumor size, not having health insurance while being treated at a SNH, not receiving LDTs, and not receiving surgery (Figure 2).
Table 2.
Multivariable Cox Proportional Hazards Regression Model of Overall Survival
| Variable | HR (95% CI) | p Value | |
|---|---|---|---|
| Age at Diagnosis | |||
| Increasing Age | 1.01 (0.99-1.02) | 0.272 | |
| Gender | |||
| Female Gender | 1 [Reference] | ||
| Male Gender | 0.71 (0.51-0.99) | 0.043 | |
| Race | |||
| White | 1 [Reference] | ||
| Black Race | 0.66 (0.46-0.95) | 0.024 | |
| Asian | 0.39 (0.19-0.79) | 0.009 | |
| Ethnicity (Hispanic) | |||
| No | 1 [Reference] | ||
| Yes | 0.61 (0.38-0.97) | 0.038 | |
| Insurance and Treatment Facility | |||
| Private Insurance, Academic | 1 [Reference] | ||
| Private Insurance, Safety-Net | 1.17 (0.56-2.42) | 0.677 | |
| Government Insurance, Academic | 1.15 (0.76-1.73) | 0.514 | |
| Government Insurance, Safety-Net | 1.09 (0.65-1.83) | 0.747 | |
| No Insurance, Academic | 1.36 (0.59-3.17) | 0.473 | |
| No Insurance, Safety-Net | 2.50 (1.29-4.85) | 0.007 | |
| Childs Class | |||
| Childs Class A | 1 [Reference] | ||
| Childs Class B | 1.21 (0.87-1.73) | 0.257 | |
| Childs Class C | 2.51 (1.58-3.98) | <0.001 | |
| Stage at Diagnosis | |||
| Stage Ia Disease | 1 [Reference] | ||
| Stage Ib Disease | 0.87 (0.52-1.47) | 0.606 | |
| Stage II Disease | 1.41 (0.84-2.37) | 0.200 | |
| Radiologic Tumor Size | |||
| Increasing Tumor Size | 1.14 (1.07-1.20) | <0.001 | |
| Primary Care Physician | |||
| Yes | 1 [Reference] | ||
| No | 1.27 (0.86-1.90) | 0.226 | |
| Hepatologist within last Year | |||
| Yes | 1 [Reference] | ||
| No | 0.92 (0.63-1.34) | 0.660 | |
| Patient Navigator | |||
| Yes | 1 [Reference] | ||
| No | 1.28 (0.93-1.76) | 0.127 | |
| Liver-Directed Therapy | |||
| Yes | 1 [Reference] | ||
| No | 3.40 (2.27-5.08) | <0.001 | |
| Surgical Intervention | |||
| Yes | 1 [Reference] | ||
| No | 11.63 (7.05-19.20) | <0.001 |
Figure 2.

Overall survival stratified by insurance type and institution where care was delivered.
Discussion:
Although the incidence of HCC has been increasing for years, incidence-based mortality has plateaued, partly due to earlier detection and improved treatment options such as surgical and procedural interventions(23). However, survival outcomes continue to differ significantly in vulnerable patient populations based on access to care(24). Given our diverse multi-institutional SNH collaborative, we are perfectly positioned to identify the impact of insurance type on survival disparities for curative HCC across hospital systems, particularly the SNH setting whose mission is to provide care to the uninsured. This allowed us to uniquely study whether type or lack of insurance impacts OS for curative HCC even when vulnerable populations are afforded access to care. We discovered that NI-SNH patients were most vulnerable patients and that OS was more than three times higher in PI-AC patients when compared to those with NI-SNH. More striking, the type of treatment received also differed by type of insurance, with more definitive curative surgical or interventional treatments given to those with PI or GI. Furthermore, in this national cohort of SNHs, we show that when uninsured SNH patients have insurance (including GI such as Medicaid), they may overcome other unmeasured barriers and achieve survival comparable to patients with PI, a promising finding in an era where healthcare expansion is highly debated(25–27).
Specifically, this finding that NI-SNH patients are less likely to complete curative treatment, even after they access the healthcare system which offers equivalent treatment regardless of a patients ability to pay, brings to light that other external factors might be driving differential OS outcomes in these most vulnerable patients. For example, education, employment status, income levels, and other individual level barriers may contribute to the disparities seen between NI patients and those with various forms of insurance coverage. Further studies are needed to investigate these external barriers and their implications on disparate HCC outcomes. However, other studies have identified that childcare, transportation, and even navigating clinic appointments are critical barriers to receipt of treatment even when access is available(28, 29). Additionally, language barriers, educational disparities, and poor health literacy may also serve as barriers that must be overcome in order to receive curative treatments(30, 31). This highlights the importance of equity over equality. Although patients may have access to equal care at the SNH and AC, there are likely individual barriers that lead to decreased ability to receive the available treatments. It is therefore critical to identify and tackle these barriers to achieve health equity, an active area of research at our institution.
In addition to identifying predictors of OS, this study also critically evaluated access to screening, a key component of identifying early stage HCC(32–34). We discovered that patients with any type of insurance had increased access to a PCP, hepatologist, and patient navigator, unlike those with NI. This led to improved screening with ultrasound imaging and/or serum AFP levels. On the other hand, the NI-SNH group remained at a persistent disadvantage even compared to other vulnerable populations, such as GI-SNH or NI-AC patients. In particular, those with NI-SNH compared to those with GI-SNH were less likely to have a PCP, prior hepatology visits, and screening either with ultrasounds or AFP levels within the year prior to diagnosis. These findings suggest that access to any type of insurance (including Medicaid) can improve HCC screening. This suggests that having insurance, particularly plans that cover LDTs or surgical interventions, independently provides a significant survival benefit to patients, a trend that has been noted in other malignancies including breast and melanoma(35, 36). A recent study by Sabik et al. also found that in Massachusetts, healthcare reform by expanding public coverage and providing lower-cost health insurance options to those demonstrating a need for financial assistance, resulted in a lower percentage of late-stage colorectal cancer ultimately improved survival(37).
Studies evaluating the impact of the Affordable Care Act have also shown enhanced self-perceived health, improved testing for and treatment of chronic diseases, expanded screening for curable malignancies, and increased identification of cancers at early-stages, thus supporting the importance of access to healthcare(38–42). Expanded insurance coverage, largely due to the Affordable Care Act, has also demonstrated improved survival in several malignancies such as those affecting the colon, rectum, head/neck, and other areas(42–44). As a growing number of malignancies and other health conditions show improved treatment and survival outcomes with insurance coverage, it becomes increasingly vital to ensure expanded health care access. An encouraging finding from this study is that vulnerable patients with any type of insurance, including low-cost GI, had improved survival compared to those with NI. This suggests that any insurance may overcome other well-described barriers such as assistance with transportation or taking time of work to potentially overcome barriers that mutually coexist with lack of access to insurance(45).
Overall, this study uncovers important disparities in HCC survival based on type of insurance or lack of insurance across a large, socioeconomically diverse patient population. This is the first, multi-institutional study to show that any type of insurance may improve HCC survival in vulnerable patients with curative HCC as it improves access to screening and access to treatment, both of which are independent predictors of improved HCC OS. However, this study is limited by its retrospective nature in that unmeasured factors may influence treatment decision-making and the records for patients who received care at outside facilities were not available. Another potential limitation is the use of the AJCC 8th edition for staging patients as certain institutions prefer the Barcelona Clinic Liver Cancer staging system, however, studies have proposed better validity through the AJCC system(46, 47). Despite these limitations, unlike large national databases, this study has granular detail on whether patients saw their PCP or hepatologist within the last year and could directly evaluate the impact of type or lack of insurance on OS for patients receiving care at SNHs, a hospital network whose mission is to provide care to all patients regardless of the patients ability to pay.
Conclusion:
This study demonstrates that early-stage HCC patients with PI and GI had increased median OS compared to those with NI treated at SNHs. In fact, those with PI lived more than three times longer than uninsured patients, a striking disparity. This improvement appears to be related to increased receipt of surgical treatment or LDT in the PI and GI patients compared to NI-SNH patients, even after they have access to care within the SNH system, suggesting other potential barriers to receiving treatment. More promising is the finding that when vulnerable SNH patients have any insurance, including low-cost GI, they had significantly improved OS, suggesting that insurance somehow overcomes other potential barriers besides access to care. Insurance might serve as a proxy for employment, which may come with other benefits such as transportation or childcare enabling one to receive recommended treatments. In other words, lack of insurance may serve as a marker of additional individual barriers to receiving care even when access is available to vulnerable patients through the SNH system. Overall, to achieve equitable survival for early-stage HCC, is it critical to improve access to insurance, particularly plans that cover surgical treatment and LDT treatments. Further research is needed to unravel additional individual level barriers beyond access to care that might be preventing uninsured patients in the SNH population from receiving recommended treatments.
Acknowledgements:
This research was supported by the National Institute of Health under a training grant, T32CA211034 and a K12CA226330-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meeting Presentation: Presented at the American College of Surgeons, Chicago, IL, October 2020
References:
- 1.American Cancer Society. Survival Rates for Pancreatic Cancer Atlana, GA 2019. [Available from: https://www.cancer.org/cancer/pancreatic-cancer/detection-diagnosis-staging/survival-rates.html.
- 2.Ghouri YA, Mian I, Rowe JH. Review of hepatocellular carcinoma: Epidemiology, etiology, and carcinogenesis. J Carcinog. 2017; 16:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Society AC. Cancer Facts & Figures 2019: American Cancer Society; 2019. [Available from: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2019/cancer-facts-and-figures-2019.pdf.
- 4.Ha J, Yan M, Aguilar M, Bhuket T, Tana MM, Liu B, et al. Race/ethnicity-specific disparities in cancer incidence, burden of disease, and overall survival among patients with hepatocellular carcinoma in the United States. Cancer. 2016;122(16):2512–23. [DOI] [PubMed] [Google Scholar]
- 5.Sayiner M, Golabi P, Younossi ZM. Disease Burden of Hepatocellular Carcinoma: A Global Perspective. Dig Dis Sci. 2019;64(4):910–7. [DOI] [PubMed] [Google Scholar]
- 6.Sobotka LA, Hinton A, Conteh LF. Insurance status impacts treatment for hepatocellular carcinoma. Ann Hepatol. 2019; 18(3):461–5. [DOI] [PubMed] [Google Scholar]
- 7.Adler Jaffe S, Myers O, Meisner ALW, Wiggins CL, Hill DA, McDougall JA. Relationship between Insurance Type at Diagnosis and Hepatocellular Carcinoma Survival. Cancer Epidemiol Biomarkers Prev. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones PD, Scheinberg AR, Muenyi V, Gonzalez-Diaz J, Martin PM, Kobetz E. Socioeconomic And Survival Differences Among Minorities With Hepatocellular Carcinoma In Florida. J Hepatocell Carcinoma. 2019;6:167–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith J, Medalia C. Health Insurance Coverage in the United States: 2014. US Government Printing Office. 2015(U.S. Census Bureau, Current Population Reports):60–253. [Google Scholar]
- 10.Berchick E, Barnett J, and Upton R Health Insurance Coverage in the United States: 2018. The United States Census Bureau. 2019:60–267. [Google Scholar]
- 11.Hoehn RS, Hanseman DJ, Jernigan PL, Wima K, Ertel AE, Abbott DE, et al. Disparities in care for patients with curable hepatocellular carcinoma. HPB (Oxford). 2015;17(9):747–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harlan LC, Parsons HM, Wiggins CL, Stevens JL, Patt YZ. Treatment of hepatocellular carcinoma in the community: disparities in standard therapy. Liver Cancer. 2015;4(1):70–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdelsattar ZM, Hendren S, Wong SL. The impact of health insurance on cancer care in disadvantaged communities. Cancer. 2017;123(7):1219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldberg DS, Valderrama A, Kamalakar R, Sansgiry SS, Babajanyan S, Lewis JD. Hepatocellular carcinoma surveillance rates in commercially insured patients with noncirrhotic chronic hepatitis B. J Viral Hepat. 2015;22(9):727–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zaydfudim V, Whiteside MA, Griffin MR, Feurer ID, Wright JK, Pinson CW. Health insurance status affects staging and influences treatment strategies in patients with hepatocellular carcinoma. Ann Surg Oncol. 2010;17(12):3104–11. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Ha J, Lopez A, Bhuket T, Liu B, Wong RJ. Medicaid and Uninsured Hepatocellular Carcinoma Patients Have More Advanced Tumor Stage and Are Less Likely to Receive Treatment. J Clin Gastroenterol. 2018;52(5):437–43. [DOI] [PubMed] [Google Scholar]
- 17.Eguia E, Cobb AN, Kothari AN, Molefe A, Afshar M, Aranha GV, et al. Impact of the Affordable Care Act (ACA) Medicaid Expansion on Cancer Admissions and Surgeries. Ann Surg. 2018;268(4):584–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ASPE. Definition of Safety Net Hospitals: United States Department of Health and Human Services; 2013. [Available from: https://aspe.hhs.gov/report/environmental-scan-identify-major-research-questions-and-metrics-monitoring-effects-affordable-care-act-safety-net-hospitals/c-definition-safety-net-hospitals.
- 19.Park S, Choi S, Cho YA, Sinn DH, Kim JM, Park CK, et al. Evaluation of the American Joint Committee on Cancer (AJCC) 8th Edition Staging System for Hepatocellular Carcinoma in 1,008 Patients with Curative Resection. Cancer Res Treat. 2020;52(4):1145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seeking Financial Assistance at Jackson: Jackson Health System; 2020. [Available from: https://jacksonhealth.org/patients/financial-assistance/.
- 21.Glover NM, Altenhoff A, Dessimoz C. Assigning confidence scores to homoeologs using fuzzy logic. PeerJ. 2019;6:e6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.IBM. IBM SPSS Statistics 25 2019. [Available from: https://www.ibm.com/support/pages/downloading-ibm-spss-statistics-25.
- 23.Njei B, Rotman Y, Ditah I, Lim JK. Emerging trends in hepatocellular carcinoma incidence and mortality. Hepatology. 2015;61(1):191–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vaccaro AR, Getz CL, Cohen BE, Cole BJ, Donnally CJ, 3rd. Practice Management During the COVID-19 Pandemic. J Am Acad Orthop Surg. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roetzheim RG, Pal N, Tennant C, Voti L, Ayanian JZ, Schwabe A, et al. Effects of health insurance and race on early detection of cancer. J Natl Cancer Inst. 1999;91(16):1409–15. [DOI] [PubMed] [Google Scholar]
- 26.Franco RA, Fan Y, Jarosek S, Bae S, Galbraith J. Racial and Geographic Disparities in Hepatocellular Carcinoma Outcomes. Am J Prev Med. 2018;55(5 Suppl 1):S40–S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ward E, Halpern M, Schrag N, Cokkinides V, DeSantis C, Bandi P, et al. Association of insurance with cancer care utilization and outcomes. CA Cancer J Clin. 2008;58(1):9–31. [DOI] [PubMed] [Google Scholar]
- 28.Del Rio M, Hargrove WL, Tomaka J, Korc M. Transportation Matters: A Health Impact Assessment in Rural New Mexico. Int J Environ Res Public Health. 2017;14(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bellettiere J, Chuang E, Hughes SC, Quintanilla I, Hofstetter CR, Hovell MF. Association Between Parental Barriers to Accessing a Usual Source of Care and Children’s Receipt of Preventive Services. Public Health Rep. 2017;132(3):316–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levy H, Janke A. Health Literacy and Access to Care. J Health Commun. 2016;21 Suppl 1:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meuter RF, Gallois C, Segalowitz NS, Ryder AG, Hocking J. Overcoming language barriers in healthcare: A protocol for investigating safe and effective communication when patients or clinicians use a second language. BMC Health Serv Res. 2015;15:371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loomba R, Lim JK, Patton H, El-Serag HB. AGA Clinical Practice Update on Screening and Surveillance for Hepatocellular Carcinoma in Patients with Nonalcoholic Fatty Liver Disease: Expert Review. Gastroenterology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar A, Acharya SK, Singh SP, Arora A, Dhiman RK, Aggarwal R, et al. 2019 Update of Indian National Association for Study of the Liver Consensus on Prevention, Diagnosis, and Management of Hepatocellular Carcinoma in India: The Puri II Recommendations. J Clin Exp Hepatol. 2020;10(1):43–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldberg DS, Valderrama A, Kamalakar R, Sansgiry SS, Babajanyan S, Lewis JD. Hepatocellular Carcinoma Surveillance Among Cirrhotic Patients With Commercial Health Insurance. J Clin Gastroenterol. 2016;50(3):258–65. [DOI] [PubMed] [Google Scholar]
- 35.Ko NY, Hong S, Winn RA, Calip GS. Association of Insurance Status and Racial Disparities With the Detection of Early-Stage Breast Cancer. JAMA Oncol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abudu B, Cook KA, Gershenwald JE, Cohen PR, Geller AC. Quantitative associations between health insurance and stage of melanoma at diagnosis among nonelderly adults in the United States. Cancer. 2020;126(4):775–81. [DOI] [PubMed] [Google Scholar]
- 37.Sabik LM, Eom KY, Dahman B, Li J, Yao N, van Londen GJ, et al. The Impact of Massachusetts Health Reform on Colorectal and Breast Cancer Stage at Diagnosis. Med Care. 2020;58(2):183–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Courtemanche C, Marton J, Ukert B, Yelowitz A, Zapata D. Effects of the Affordable Care Act on Health Care Access and Self-Assessed Health After 3 Years. Inquiry. 2018;55:46958018796361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Angier HE, Marino M, Springer RJ, Schmidt TD, Huguet N, DeVoe JE. The Affordable Care Act improved health insurance coverage and cardiovascular-related screening rates for cancer survivors seen in community health centers. Cancer. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toyoda Y, Oh EJ, Premaratne ID, Chiuzan C, Rohde CH. Affordable Care Act State-Specific Medicaid Expansion: Impact on Health Insurance Coverage and Breast Cancer Screening Rates. J Am Coll Surg. 2020. [DOI] [PubMed] [Google Scholar]
- 41.Takvorian SU, Oganisian A, Mamtani R, Mitra N, Shulman LN, Bekelman JE, et al. Association of Medicaid Expansion Under the Affordable Care Act With Insurance Status, Cancer Stage, and Timely Treatment Among Patients With Breast, Colon, and Lung Cancer. JAMA Netw Open. 2020;3(2):e1921653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gan T, Sinner HF, Walling SC, Chen Q, Huang B, Tucker TC, et al. Impact of the Affordable Care Act on Colorectal Cancer Screening, Incidence, and Survival in Kentucky. J Am Coll Surg. 2019;228(4):342–53 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cannon RB, Shepherd HM, McCrary H, Carpenter PS, Buchmann LO, Hunt JP, et al. Association of the Patient Protection and Affordable Care Act With Insurance Coverage for Head and Neck Cancer in the SEER Database. JAMA Otolaryngol Head Neck Surg. 2018;144(11):1052–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hsu CD, Wang X, Habif DV Jr., Ma CX, Johnson KJ. Breast cancer stage variation and survival in association with insurance status and sociodemographic factors in US women 18 to 64 years old. Cancer. 2017;123(16):3125–31. [DOI] [PubMed] [Google Scholar]
- 45.Robinson MR, Daniel LC, O’Hara EA, Szabo MM, Barakat LP. Insurance status as a sociodemographic risk factor for functional outcomes and health-related quality of life among youth with sickle cell disease. J Pediatr Hematol Oncol. 2014;36(1):51–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sirivatanauksorn Y, Tovikkai C. Comparison of staging systems of hepatocellular carcinoma. HPB Surg. 2011. ;2011:818217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kinoshita A, Onoda H, Fushiya N, Koike K, Nishino H, Tajiri H. Staging systems for hepatocellular carcinoma: Current status and future perspectives. World J Hepatol. 2015;7(3):406–24. [DOI] [PMC free article] [PubMed] [Google Scholar]


