Abstract
Objective/Hypothesis:
Glutamine inhibition has been demonstrated an antifibrotic effect in iatrogenic laryngotracheal stenosis (iLTS) scar fibroblasts in vitro. We hypothesize that broadly active glutamine antagonist, DON will reduce collagen formation and fibrosis-associated gene expression in iLTS mice.
Study Design:
Prospective controlled animal study.
Methods:
iLTS in mice were induced by chemomechanical injury of the trachea using a bleomycin-coated wire brush. PBS or DON (1.3 mg/kg) were administered by intraperitoneal injection (i.p.) every other day. Laryngotracheal complexes were harvested at days 7 and 14 after the initiation of DON treatment for the measurement of lamina propria thickness, trichrome stain, immunofluorescence staining of collagen 1, and fibrosis-associated gene expression.
Results:
The study demonstrated that DON treatment reduced lamina propria thickness (P = .025) and collagen formation in trichrome stain and immunofluorescence staining of collagen 1. In addition, DON decreased fibrosis-associated gene expression in iLTS mice. At day 7, DON inhibited Col1a1 (P < .0001), Col3a1 (P = .0046), Col5a1 (P < .0001), and Tgfβ (P = .023) expression. At day 14, DON reduced Co1a1 (P = .0076) and Tgfβ (P = .023) expression.
Conclusions:
Broadly active glutamine antagonist, DON, significantly reduces fibrosis in iLTS mice. These results suggest that the concept of glutamine inhibition may be a therapeutic option to reduce fibrosis in the laryngotracheal stenosis.
Keywords: Laryngotracheal stenosis, DON, fibrosis, glutamine, glutaminase
INTRODUCTION
Iatrogenic laryngotracheal stenosis (iLTS) is mostly caused by prolonged intubation. In iLTS patients, the endotracheal tube disrupts the laryngotracheal epithelium resulting in inflammatory cells (T lymphocytes and macrophages) and tissue disruptive enzymes overwhelming the physiologic wound-healing process.1–3 This pathologic inflammatory response leads to fibroblast hyperproliferation and extracellular matrix deposition forming scar that narrows the subglottic and/or proximal tracheal airway.4–8 The narrowing of laryngotracheal airway can cause life-threatening dyspnea; for which there are only surgical therapies at this time. Surgical procedures include serial endoscopic excision and dilation, cricotracheal resection, or sometimes permanent tracheostomy is required to relieve dyspnea in patients with iLTS.1 The lack of medical therapies for iLTS speaks to our knowledge gap in the pathologic processes driving disease. Therefore, it is critically important to better understand the pathophysiology to develop effective medical therapies for iLTS.
Our lab previously demonstrated that human iLTS-scar fibroblasts are hyperproliferative, have increased extracellular matrix deposition, and preferentially increase aerobic glycolysis when compared to normal fibroblasts.9 Utilizing aerobic glycolysis to drive cellular proliferation is known as the Warburg effect, a phenomenon identified in cancer cell physiology to generate energy for hyperproliferating cells.10 We identified a “Warburg-like effect” in iLTS-scar fibroblasts as they shifted from the physiological mechanism of oxidative phosphorylation to generate energy to the less efficient aerobic glycolysis to drive mitosis.9 This Warburg-like phenotype in iLTS may be leveraged by targeting the abnormal metabolism in scar fibroblasts to reduce or reverse fibrosis.
iLTS-scar fibroblasts’ high cellular metabolic demand is dependent on glutamine to fuel the tricarboxylic acid cycle metabolic intermediates to serve as building blocks for rapid cell proliferation.11 Glutamine is also a critical metabolic substrate for collagen production and collagen stability in human scar fibroblasts.12,13 Furthermore, our lab demonstrated increased glutaminase expression in biopsies from iLTS patients.14 Glutaminase is the primary enzyme responsible for converting glutamine to glutamate and blocking glutaminase reduces collagen production in iLTS scar fibroblasts in vitro.14 These findings support glutamine utilization as a rationale druggable target and potential therapy for iLTS.
DON (6-Diazo-5-oxo-L-norleucine) is a broadly active glutamine antagonist which blocks multiple enzymes within the glutamine pathway, including glutaminase.15 DON-mediated glutamine antagonist has successfully been utilized to suppress tumor growth in preclinical cancer models and exploratory clinical studies.16,17 In this study, we aim to translate glutamine inhibition to our preclinical mouse model to study its potential as a medical therapy for iLTS. We hypothesize that glutamine is a critical energy source for iLTS and systemic DON treatment will inhibit fibrosis in iLTS mice.
MATERIALS AND METHODS
Animal Experiment
All animal experiments were approved by the Johns Hopkins University Animal Care and Use Committee (MO18M124). C57BL/6 mice underwent chemomechanical injury of the trachea using a bleomycin-coated wire brush to induce iLTS. Mice were randomized into two groups after inducing iLTS: 1) phosphate-buffered saline (PBS) control group and 2) DON treatment group. After 4 days after surgery, PBS or DON (1.3 mg/kg) were administered by intraperitoneal injection (i.p.) to mice every other day. Laryngotracheal complexes (subglottis and proximal trachea) were harvested at days 7 and 14 after the initiation of treatment in both groups for histologic analysis and fibrotic gene expression. All experiments were repeated three times. The timeline for the animal experiments was shown in Figure 1A.
Fig. 1.
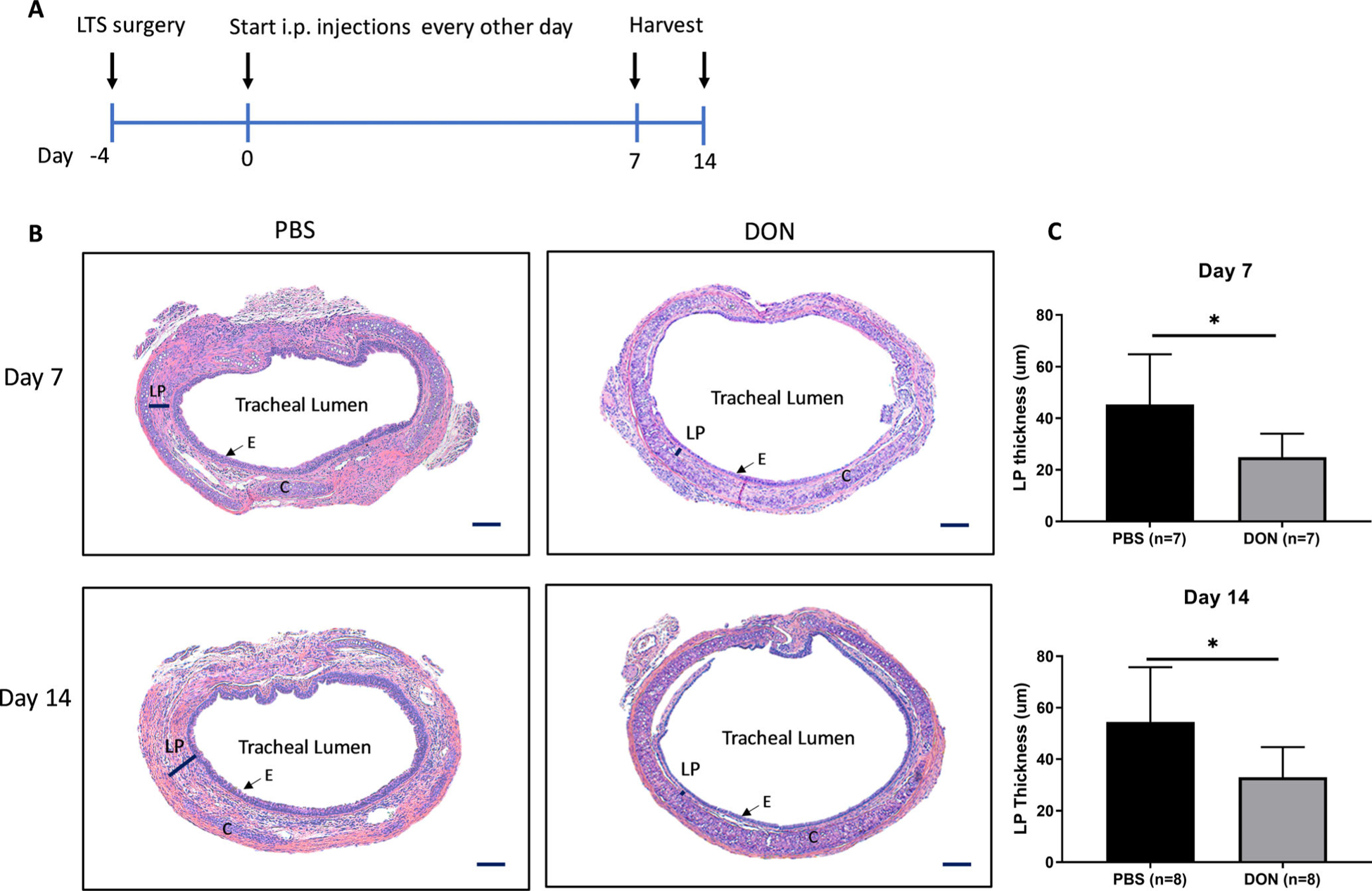
Animal experiment flow chart and histologic assessment of iLTS mice. (A) iLTS surgery was performed at day −4. PBS and DON treatments were started 4 days after surgery and were i.p. administrated to mice every other day. Laryngotracheal complexes were harvested at days 7 and 14. (B) DON treatment significantly reduced the fibrotic lamina propria thickness in iLTS mice compared with PBS group at days 7 and 14. Quantification of the lamina propria thickness measurement from the histological results is shown in (C). (*) = P < .05; i.p.: Intraperitoneal injection; DON: 6-Diazo-5-oxo-L-norleucine; LP: lamina propria; C: cartridge; E: epithelial cells. Scale bar: 100 um.
Gene Expression Analysis by Real-time Polymerase Chain Reaction
Fibrosis markers collagen 1 (Cola1), collagen 3 (Col3a1), collagen 5 (Col5a1), alpha smooth-muscle actin (αSma), transforming growth factor-beta (Tgfβ), and fibronectin (Fn) were measured with the Power SYBR® Green PCR Mastermix (Life Technologies, Carlsbad, CA) and quantified using quantitative real-time PCR (qRT-PCR) on the StepOnePlus Real-Time PCR System (Applied Biosystems, Carlsbad, CA). All samples were run in duplicate. The level of expression of each target gene was calculated as 2(−ΔΔCt) as previously described.11
Histology and Statistical Analysis
Laryngotracheal complexes were fixed in 10% formalin for 24 hours and were embedded in paraffin. The embedded tissues were cut into 5 μm sections axially through the proximal trachea and then were stained with hematoxylin–eosin (H&E), Masson’s trichrome staining or Collagen 1 immunofluorescence staining. Trichrome stain was used for identifying collagen formation in the lamina propria (LP) which was performed according to the manufacturer’s instruction of Trichrome Stain Kit (Abcam, Cambridge, MA). Collagen in the trichrome stain was identified by dense or filamentous blue staining. For the H&E and Trichrome stain, Zeiss AX10 microscope (Zeiss, Oberkochen, Germany) was used to visualize and image positive staining. Stained slides were accessed to measure lamina propria LP thickness as previously described.18
For the collagen 1 staining, tissue slides were processed with antigen retrieval buffer for 20 minutes and blocked with 10% FBS for 30 minutes. Tissue slides were incubated in a solution with rabbit anti-collagen 1 antibody (dilution: 1:400, Invitrogen, Eugene, OR) at 4°C overnight. The following day, the slides were washed three times with PBS and then incubated with goat anti-rabbit Alexa Fluor 488 antibody (dilution 1:200, Invitrogen, Eugene, OR) for 1 hour at room temperature. After washing with PBS three times, tissues were mounted with mounting media with DAPI. Immunostained samples were imaged with a LSM510 laser scanning confocal microscope (Zeiss, Oberkochen, Germany).
Statistical Analysis
Results are represented as mean ± standard error of the mean. Shapiro–Wilk test was used to test the normality of data. The student t-test was used for the analysis of the results. The significance criterion for all analyses was set at P < .05. Data analysis was performed using Prism software (GraphPad Software Inc., La Jolla, CA).
RESULTS
DON Treatment Significantly Reversed iLTS in Mice
In the induced iLTS murine model, iLTS mice that received PBS-treatment had a thicker tracheal lamina propria in H&E stained sections compared to DON-treated mice at days 7 and 14 (Fig. 1B,C) (day 7: n = 7, P = .026, 95% CI: −38.07 to −2.763; day 14: n = 8, P = .025, 95% CI: −39.99 to −3.119). Furthermore, the thickened tracheal lamina propria in PBS-treated mice showed intense collagen formation in trichome staining and collagen 1 expression in immunofluorescence staining when compared to DON-treated mice at day 14 (Fig. 2A,B).
Fig. 2.
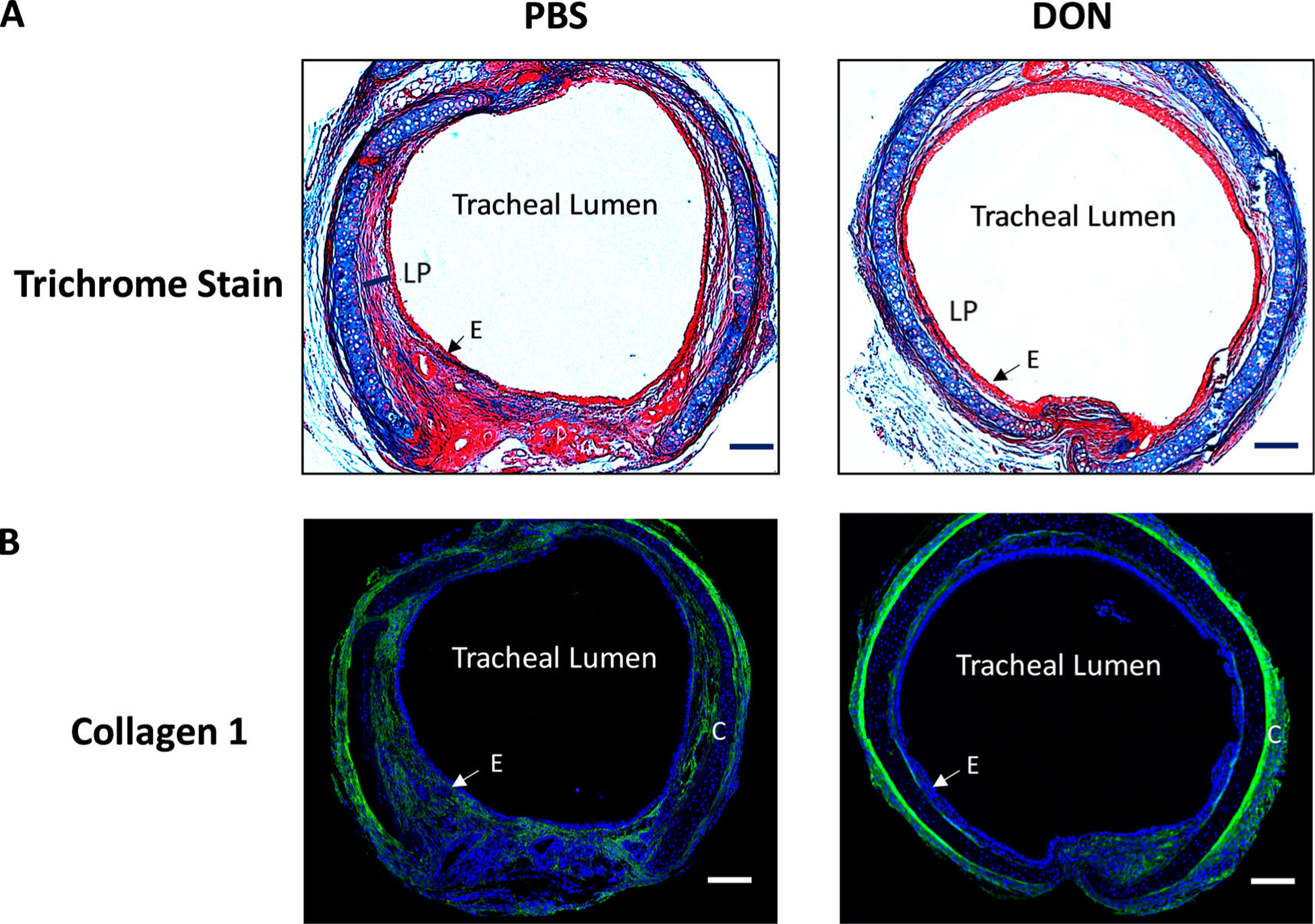
DON treatment reduced collagen expression in iLTS mice. (A) representative trichrome-stained tissue sections and (B) immunofluorescent staining of collagen 1 tissue sections demonstrated that DON treatment significantly reduced collagen formation in iLTS mice compared to PBS-treated group at day 14. DON: 6-Diazo-5-oxo-L-norleucine; LP: lamina propria; C: cartridge; E: epithelial cells. Scale bar: 100 um.
DON Reduced Col1a1, Collagen3a1, Collagen5a1, and Tgfβ Gene Expression in iLTS Mice
Quantitative RT-PCR was used to evaluate the expression of fibrosis-associated genes, such as Col1a1, Col3a1, Col5a1, αSma, and Tgfβ. The results showed that DON-treated mice resulted in a 72% reduction in Col1a1 expression at days 7 and 14 (n = 5, P < .0001, 95% −0.9224 to −0.5835 and n = 4, P = .0076, 95% −1.207 to −0.2819, respectively) compared to PBS-treated mice (Fig. 3A,B). In addition, Col3a1 expression was reduced by 75% at days 7 when compared to PBS-treated mice (n = 5, P = .0046, 95% CI: −1.289 to −0.3296) but displayed no difference at day 14 (Fig. 3A,B). In DON-treated mice, there was a 75% decrease in Col5a1 expression when compared to PBS-treated mice at day 7 (n = 5, P < .0001, 95% CI: −0.9091 to −0.5970) but there was no difference at day 14 between two groups (Fig. 3A,B). There was a significant increase in αSma expression at day 7 in DON-treated group compared to PBS-treated group (n = 5, P = .0170.01, 95% CI: 0.2005–1.530), but there was no difference in αSma expression at day 14 between two groups. Furthermore, DON treatment significantly reduced Tgfβ expression at days 7 and 14 (n = 5, P = .023, 95% CI: −0.6618 to −0.06373 and n = 4, P = .0310, 95% CI: −1.006 to −0.06863) compared to PBS-treated mice (Fig. 2A,B). In addition, we observed no significant differences in Fn expression between DON and PBS-treated groups at days 7 and 14 (Fig. 3A,B).
Fig. 3.
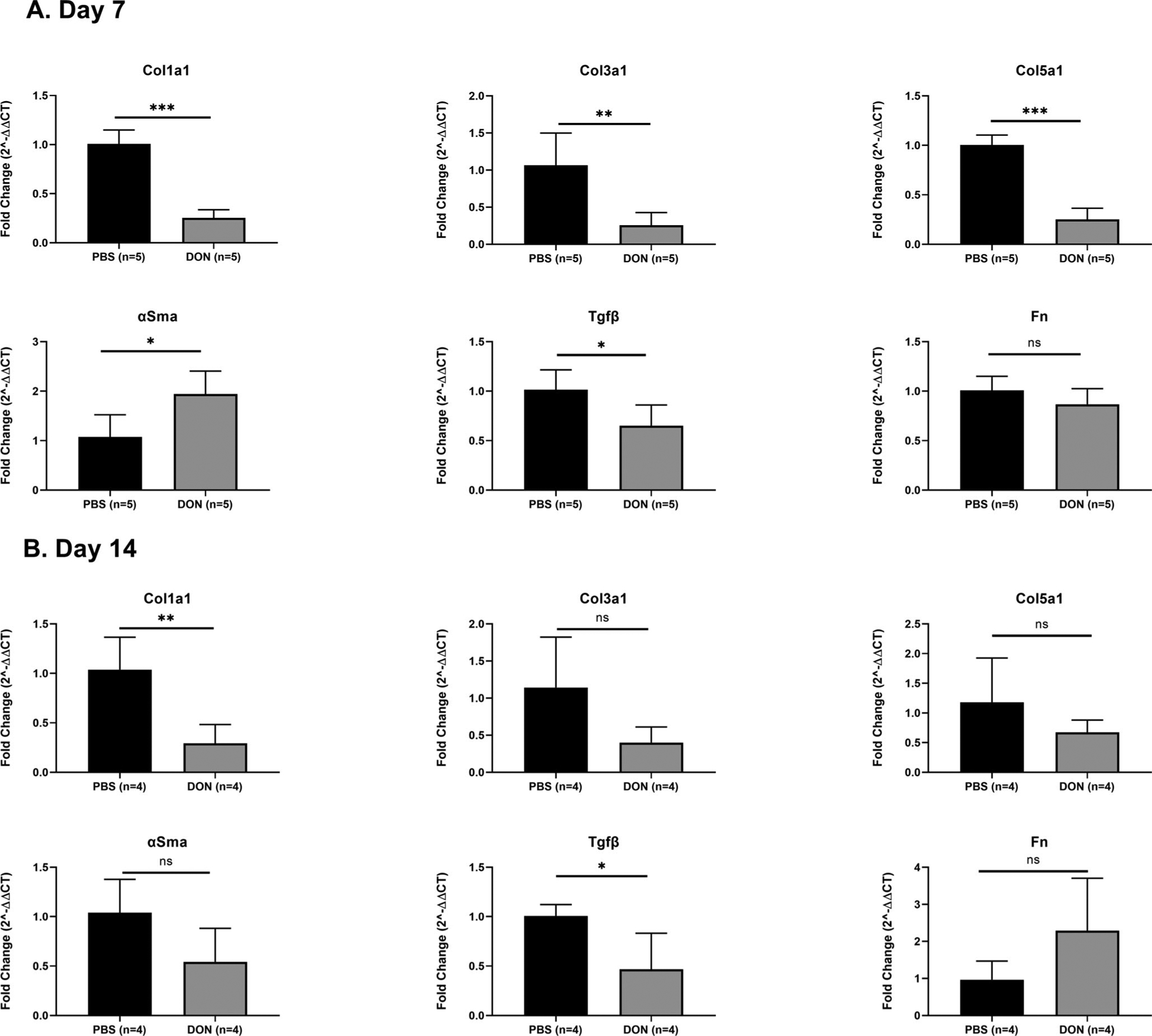
DON treatment reduced Col1a1, Col3a1, Col5a1, and Tgfβ expression in iLTS mice. (A). At day 7, DON treatment significantly decreased the expression of Cola1, Col3a1, Col5a1 and Tgfβ and increased αSma expression in iLTS mice compared to PBS group. Fn expression did not change after DON treatment at day 7. (B) At day 14, expression of Cola1, Col3a1, and Tgfβ were decreased after DON treatment in iLTS mice. Col5a1 and αSma expression did not change. (*) = P < .05; (**) = P < .01. DON: 6-Diazo-5-oxo-L-norleucine; Colla1: collagen 1; Col3a1: collagen 3; Col5a1 collagen 5; αSma: alpha-smooth muscle actin; Tgfβ: transforming growth factor-beta; Fn: fibronectin.
DISCUSSION
In this study, we demonstrated systemic DON treatment reduced fibrosis in an iLTS mouse model. DON-induced glutamine inhibition increased tracheal airspace in mice, reduced lamina propria thickness, collagen expression, and fibrosis gene expression at both 7 and 14 days after initiation of therapy. These results build off previous studies showing glutamine inhibition suppresses the metabolism of hyperproliferative fibroblasts in iLTS in vivo.
Due to the high demand for glutamine in hyperproliferating cells, targeted therapies have been developed against glutamine uptake and glutamine-catalyzed enzymes for the treatment of cancer and idiopathic pulmonary fibrosis.19–22 For example, the glutamine antagonists CB-839 and BPTES (bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl) ethyl sulfide) blocked the production of glutamate from glutamine through a process called glutaminolysis and reduced tumor growth in colorectal cancer and lung cancer, as well as reversed pulmonary fibrosis.23–26 Previous studies in our lab demonstrated global inhibition of glutamine with DON reversed fibrosis with decreased Col1a1 and Col3a1 expression in vitro, and specific inhibition of glutaminase with BPTES reduced Col1a1, Col3a1, and α-Sma in iLTS scar fibroblasts.11,14 In the present in vivo study, DON treatment significantly reduced tracheal lamina propria thickness, collagen formation, and fibrotic gene expression, including Col1a1, Col3a1, Col5a1, and Tgfβ in iLTS mice, thereby demonstrating glutamine to be a critical building block for the development of fibrosis in iLTS.
These preclinical results suggest DON therapy has the potential to translate to human clinical trials. Here we showed DON had a significant antifibrotic effect in vivo with a relatively small sample size, ranging from n = 4 to n = 8 mice in the various experiments. However, systemic DON has been shown to have significant side effects. Previous clinical studies using systemic DON as primary and/or adjuvant cancer therapy demonstrated significant unintended effects on proliferating cells in the gastrointestinal (GI) mucosa, resulting in high dropout rates and in some cases, prematurely ending the trial.17,27 Therefore, it is important to identify strategies to deliver DON to the site of disease while avoiding systemic side effects. One option would be topical treatments, such as intralesional injection or a drug-eluting stent, to effectively deliver glutamine inhibition to scar tissue in iLTS patients.28,29 Alternatively, systemic administration of selective glutaminase inhibitor, CB-839, has potential in iLTS as it showed a robust antitumor effect in preclinical trials without significant toxicity.30
Although the in vivo results for the DON treatment in iLTS are encouraging, there are certain limitations in the present study. First, the measurements of fibrosis in iLTS were only evaluated up to 2 weeks which was 1 week shorter than our previous studies. However, in the present study, we initiated DON treatment 4 days after iLTS induction and then accessed the fibrosis measurements 2 weeks later. The actual timing for the fibrosis measurements was 18 days which was close to our previous studies of 21 days. Furthermore, the myofibroblast marker, αSma, did not show an expected downregulation with DON treatment, differing from the other extracellular matrix genes, including Tgfβ, that were analyzed. Tgfβ has played a key role in the transformation of fibroblasts into myofibroblasts.31,32 One possible explanation for the lack of correlation between Tgfβ and αSma results is the short study period in which DON does not have an effect on αSma expression at early time points but might demonstrate at later time points. Day 14 reveals a trend toward decreased αSma expression. Further αSma protein expression studies are warranted to confirm this.
CONCLUSION
Systemic DON treatment demonstrated anti-fibrotic effects in iLTS in vivo. Glutamine inhibition with DON reduced lamina propria thickness and extracellular matrix deposition in iLTS mice. These results indicate that iLTS fibroblasts have high demands for glutamine to generate energy and building blocks during mitosis. In conclusion, this preclinical animal study suggests glutamine inhibition to be a promising treatment strategy to reverse fibrosis in iLTS by targeting pathologic fibroblasts.
Acknowledgments
Research reported in this publication was supported by the National Institute on Deafness and Other Communication Disorders of the National Institutes of Health under award numbers 1R01DC018567, 1K23DC014082, and R21DC017225. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This study was also financially supported by the Triological Society and American College of Surgeons (Alexander Hillel).
Footnotes
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
BIBLIOGRAPHY
- 1.Dankle SK, Schuller DE, McClead RE. Risk factors for neonatal acquired subglottic stenosis. Ann Otol Rhinol Laryngol 1986;95:626–630. [DOI] [PubMed] [Google Scholar]
- 2.Gelbard A, Francis DO, Sandulache VC, Simmons JC, Donovan DT, Ongkasuwan J. Causes and consequences of adult laryngotracheal stenosis. Laryngoscope 2015;125:1137–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esteller-More E, Ibanez J, Matino E, Adema JM, Nolla M, Quer IM. Prognostic factors in laryngotracheal injury following intubation and/or tracheotomy in ICU patients. Eur Arch Otorhinolaryngol 2005;262:880–883. [DOI] [PubMed] [Google Scholar]
- 4.Rockey DC, Bell PD, Hill JA. Fibrosis–a common pathway to organ injury and failure. N Engl J Med 2015;373:96. [DOI] [PubMed] [Google Scholar]
- 5.Hillel AT, Samad I, Ma G, et al. Dysregulated macrophages are present in bleomycin-induced murine Laryngotracheal stenosis. Otolaryngol Head Neck Surg 2015;153:244–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minnigerode B, Richter HG. Pathophysiology of subglottic tracheal stenosis in childhood. Prog Pediatr Surg 1987;21:1–7. [DOI] [PubMed] [Google Scholar]
- 7.Motz KM, Yin LX, Samad I, et al. Quantification of inflammatory markers in laryngotracheal stenosis. Otolaryngol Head Neck Surg 2017;157: 466–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hillel AT, Ding D, Samad I, Murphy MK, Motz K. T-helper 2 lymphocyte immunophenotype is associated with iatrogenic laryngotracheal stenosis. Laryngoscope 2019;129:177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma G, Samad I, Motz K, et al. Metabolic variations in normal and fibrotic human laryngotracheal-derived fibroblasts: a Warburg-like effect. Laryngoscope 2017;127:E107–E113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 2009; 324:1029–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphy MK, Motz KM, Ding D, et al. Targeting metabolic abnormalities to reverse fibrosis in iatrogenic laryngotracheal stenosis. Laryngoscope 2018; 128:E59–E67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamanaka RB, O’Leary EM, Witt LJ, et al. Glutamine metabolism is required for collagen protein synthesis in lung fibroblasts. Am J Respir Cell Mol Biol 2019;61:597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ge J, Cui H, Xie N, et al. Glutaminolysis promotes collagen translation and stability via alpha-ketoglutarate-mediated mTOR activation and Proline hydroxylation. Am J Respir Cell Mol Biol 2018;58:378–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsai HW, Motz KM, Ding D, et al. Inhibition of glutaminase to reverse fibrosis in iatrogenic laryngotracheal stenosis. Laryngoscope 2020;130: E773–E781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinkus LM. Glutamine binding sites. Methods Enzymol 1977;46:414–427. [DOI] [PubMed] [Google Scholar]
- 16.Rais R, Jancarik A, Tenora L, et al. Discovery of 6-diazo-5-oxo-l-norleucine (DON) prodrugs with enhanced CSF delivery in monkeys: a potential treatment for glioblastoma. J Med Chem 2016;59:8621–8633. [DOI] [PubMed] [Google Scholar]
- 17.Lemberg KM, Vornov JJ, Rais R, Slusher BS. We’re not “DON” yet: optimal dosing and prodrug delivery of 6-diazo-5-oxo-L-norleucine. Mol Cancer Ther 2018;17:1824–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hillel AT, Namba D, Ding D, Pandian V, Elisseeff JH, Horton MR. An in situ, in vivo murine model for the study of laryngotracheal stenosis. JAMA Otolaryngol Head Neck Surg 2014;140:961–966. [DOI] [PubMed] [Google Scholar]
- 19.Leone RD, Zhao L, Englert JM, et al. Glutamine blockade induces divergent metabolic programs to overcome tumor immune evasion. Science 2019; 366:1013–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vigeland CL, Chan-Li Y, Collins SL, et al. Inhibition of glutamine metabolism arrests the development of pulmonary fibrosis. In ATS Conference 2016, C37 Pulmonary Fibrosis. Am J Respir Crit Care Med 2016;193: A4935. [Google Scholar]
- 21.Pavlova NN, Thompson CB. The emerging hallmarks of cancer metabolism. Cell Metab 2016;23:27–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang J, Mao S, Guo Y, Wu Y, Yao X, Huang Y. Inhibition of GLS suppresses proliferation and promotes apoptosis in prostate cancer. Biosci Rep 2019;39(6):BSR20181826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gross MI, Demo SD, Dennison JB, et al. Antitumor activity of the glutaminase inhibitor CB-839 in triple-negative breast cancer. Mol Cancer Ther 2014;13:890–901. [DOI] [PubMed] [Google Scholar]
- 24.Cui H, Xie N, Jiang D, et al. Inhibition of glutaminase 1 attenuates experimental pulmonary fibrosis. Am J Respir Cell Mol Biol 2019;61:492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song Z, Wei B, Lu C, Li P, Chen L. Glutaminase sustains cell survival via the regulation of glycolysis and glutaminolysis in colorectal cancer. Oncol Lett 2017;14:3117–3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee JS, Kang JH, Lee SH, Lee CH, Son J, Kim SY. Glutaminase 1 inhibition reduces thymidine synthesis in NSCLC. Biochem Biophys Res Commun 2016;477:374–382. [DOI] [PubMed] [Google Scholar]
- 27.Lynch G, Kemeny N, Casper E. Phase II evaluation of DON (6-diazo-5-oxo-L-norleucine) in patients with advanced colorectal carcinoma. Am J Clin Oncol 1982;5:541–543. [PubMed] [Google Scholar]
- 28.Duvvuri M, Motz K, Murphy M, et al. Engineering an immunomodulatory drug-eluting stent to treat laryngotracheal stenosis. Biomater Sci 2019;7: 1863–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duvvuri M, Motz K, Tsai HW, et al. Design of a biocompatible drug-eluting tracheal stent in mice with laryngotracheal stenosis. J Vis Exp 2020;155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harding J, Telli M, Munster P, Le M, Molineaux C. Safety and tolerability of increasing doses of CB-839, a first-in-class, orally administered small molecule inhibitor of glutaminase, in solid tumors. J Clin Oncol 2015;33: 2512. [Google Scholar]
- 31.Akst LM, Haque OJ, Clarke JO, Hillel AT, Best SR, Altman KW. The changing impact of gastroesophageal reflux disease in clinical practice. Ann Otol Rhinol Laryngol 2017;126:229–235. [DOI] [PubMed] [Google Scholar]
- 32.Ronnov-Jessen L, Petersen OW. Induction of alpha-smooth muscle actin by transforming growth factor-beta 1 in quiescent human breast gland fibroblasts. Implications for myofibroblast generation in breast neoplasia. Lab Invest 1993;68:696–707. [PubMed] [Google Scholar]


