Abstract
Autism spectrum disorder (ASD) is a neurodevelopmental disorder with a skewed sex-based diagnostic ratio. While males are at a higher risk for ASD, it is critical to understand the neurobiology of the disorder to develop better treatments for both males and females. Our prior work has demonstrated that VPA (valproic acid) treated offspring had impaired performance on an attentional set-shifting task. The current study used MRI and regions of interest analyses to measure the volumes of cerebellar subregions in VPA and controls rats that had participated in the attentional set-shifting task. VPA males had significantly more volume in lobule VI compared to male controls. VPA female rats had significantly less volume in lobules I, IV and X compared to female controls. In addition, it was revealed that decreases in volume for VPA females was associated with worse performance. Males with increases in lobule VI were also impaired on the set-shifting task. Similar volumetric differences within the cerebellum have been observed in humans with ASD, which suggests that the VPA model is capturing some of the same brain changes observed in humans with ASD, and that these changes in volume may be impacting cognition.
Keywords: sex differences, cognitive flexibility, grey matter, autism spectrum disorder, cerebellum
1.1. Introduction
Autism spectrum disorder (ASD) is characterized by deficits in social interactions, communication, and increases in repetitive behavior. Another common symptom of ASD is problems with executive functions (Demetriou et al., 2017; Geurts et al., 2009; Hughes et al., 1994; Ozonoff et al., 1991). The neurobiology of ASD is not well understood, however there are consistently alterations within the cerebellum in humans with ASD (D’Mello et al., 2015; Fatemi et al., 2012; Mosconi et al., 2015; Pierce and Courchesne, 2001) and in animal models of ASD (Chen et al., 2016; Morakotsriwan et al., 2016; Mychasiuk et al., 2012; Wang et al., 2014). Recent human studies have examined the volume of cerebellar lobules and found that decreases in volume correlate to increased ASD symptomology (D’Mello et al., 2015). In addition, it has been suggested that the cerebellum assists with cognitive regulation through its connections via the thalamus to the prefrontal cortices (Bernard et al., 2012; D’Angelo and Casali, 2012; Schmahmann, 2019). Human imaging studies have also found that cerebellar activation occurs for a wide array of cognitive tasks including working memory, language, and executive functions (Buckner et al., 2011; D’Angelo and Casali, 2013; Guell et al., 2018; Koppelmans et al., 2017). Rodent models have provided evidence of functional connectivity between posterior regions of the cerebellum and frontal cortices (Stoodley et al., 2017). Evidence from human and animal studies suggests that the cerebellum may play a role in cognitive functions.
The current study used the valproic acid (VPA) model of ASD in rodents. The VPA model was created when it was documented that pregnant women, prescribed medications containing VPA, had children that developed ASD at a higher rate than the regular population (Chomiak et al., 2013; Christensen et al., 2013; Dean et al., 2002). The VPA models has both construct and face validity (Mabunga et al., 2015), where VPA animals display altered social interactions (Schneider and Przewlocki, 2005). In addition, VPA exposure leads to a less skewed sex ratio for development of ASD, where it occurs more often in both males and females (Jeon et al., 2018; Rasalam et al., 2005). The mechanisms of VPA exposure leading to ASD symptoms are still being investigated, but VPA exposure can cause hyperacetylation in the embryonic brain (Phiel et al., 2001) and this can lead to loss of neurons in development (Mabunga et al., 2015). Other possible mechanisms include excitatory and inhibitory imbalances related to altered GABA function (Hou et al., 2018), changes in fatty acid synthase expression (Chen et al., 2016) and changes in gene expression (Weinstein-Fudim et al., 2019). Even though ASD is linked to several genes, 40-50% the variance of ASD is driven by environmental factors (Deng et al., 2015; Hallmayer et al., 2011; Modabbernia et al., 2017), therefore the VPA model is an important model to use in conjunction with knockout models.
The current study measured grey matter volumes using MRI (magnetic resonance imaging) with 3D scans of the entire cerebellum of VPA and control animals. Past research has indicated that VPA animals lose purkinje cells and that this could contribute to loss of cerebellar volume (Ingram et al., 2000; Main and Kulesza, 2017; Mychasiuk et al., 2012; Spisák et al., 2019). A recent MRI study indicated that loss of volume of vermis regions VI and VII were correlated with loss of Purkinje neurons in VPA rats (Spisák et al., 2019). One goal of the current study was to measure across many lobules to better understand how cerebellar volume may differ between anterior and posterior regions in both male and female rats. It was hypothesized that vermis lobules VI and VII, would be decreased in volume for VPA animals compared to controls. This study is unique in that 3D scans at high resolution allowed accurate segmentation across individual lobules of the cerebellum to uncover differences between groups and to better understand sex differences within the VPA model.
For the current study, the behavioral data was published (McKinnell et al., 2021), but a brief overview of the set-shifting task is as follows. Rats were trained to dig in different media types with various scented flowerpots. The digging media and the odors are cues that inform the rat where to dig for reward. After reaching criterion on one rule, the rule is changed, and rats must learn to dig to the newly rewarded cue. A switch within a category (ie., odor 1 vs 2 to odor 3 vs 4) is an intra-dimensional shift (IDS), the rat learns new odors, but it is the same rule, follow your nose. A switch between categories (ie., odor 1 vs odor 2 to media 1 vs media 2) is an extra-dimensional shift (EDS), as now the rat ignores the odor cues and attends to the digging media, see Table 1 for an example of the pairings for one rat. This task assesses the ability of an animal to learn a rule and change their behavior when the rule is changed. Accurate set-shifting performance relies on a network of frontal regions (Birrell and Brown, 2000; Chase et al., 2012; Ng et al., 2007), but recent research suggests the cerebellum may also modulate cognitive function via its connections to the frontal cortices (D’Angelo and Casali, 2013; Koppelmans et al., 2017; Schmahmann, 2019). Our prior results found a main impairment of VPA rats compared to controls on the IDS phase of the task (McKinnell et al., 2021). VPA rats completed more trials to reach criterion and made more error trials. One hypothesis related to the behavioral data was to examine if lobules impacted by VPA exposure were related to task performance. The relationship between overall task performance as measured by total trials completed across all phases of the task and the specific task phases (i.e., IDS) and cerebellar volumes impacted by VPA exposure were examined. It was predicted that for VPA rats that decreases in crus I would decrease performance accuracy on the behavioral task.
Table 1.
When the rat reaches a criterion of 6 correct trials in a row it moves to the next phase. The task phases are simple discrimination (SD), compound discrimination (CD), intra-dimensional shift (IDS), Reversal 1 (R1), extra-dimensional shift (EDS) and Reversal 2 (R2).
| Phase | SD | CD | IDS | R1 | EDS | R2 |
|---|---|---|---|---|---|---|
| Example Rat |
Manilla folder* v. Aspen |
Manilla folder*/Cinnamon v. paper/Anise |
Foam rubber*/Rum v. Felt/ Vanilla |
Felt*/Rum v. Foam Rubber/ Vanilla |
Lemon*/ Burlap v. Almond/ Ribbon |
Almond*/Burlap v. Lemon/ Ribbon |
Indicates the rewarded pot and correct choice for that trial. Note that for each phase there is another set of pots so that all combinations occur. For example, in the CD (manilla folders/ anise v. shredded paper/ cinnamon) would be offered on different trials.
3.0. Results
Sixteen pregnant dams were injected (11 VPA, 5 saline), 2 dams did not deliver. There were 25 male control pups weaned and 23 female controls weaned. There were 29 VPA male pups weaned and 29 female pups, after weaning 7 VPA rats were lost to sickness. Seventy adult rats completed the set-shifting task and were imaged in the MRI. All cerebellums were imaged but some images were not analyzable due to scanner artifacts or poor placement of tissue in the scanner, determined by a blind to condition reviewer, this excluded 1 female control, 6 male control, 5 female VPA, and 2 male VPA brains. This left 56 brains that were segmented for cerebellar volume (16 female control, 13 male control, 8 female VPA, and 19 male VPA for all regression and MRI analyses). Each lobule was color-coded. For lobules I-IV, IX and X vermis was included with entire portion of the lobule, Figure 1. For lobules V, VII and VIII the vermis was separated from the left and right sides of the lobule (each coded into 3 segments (vermis, left, right). Crus I and Crus II were separated into left and right hemispheres. Segmentation was then screened for consistency through a 3-D modeling in ITK-SNAP before evaluation of total volume.
Figure 1:
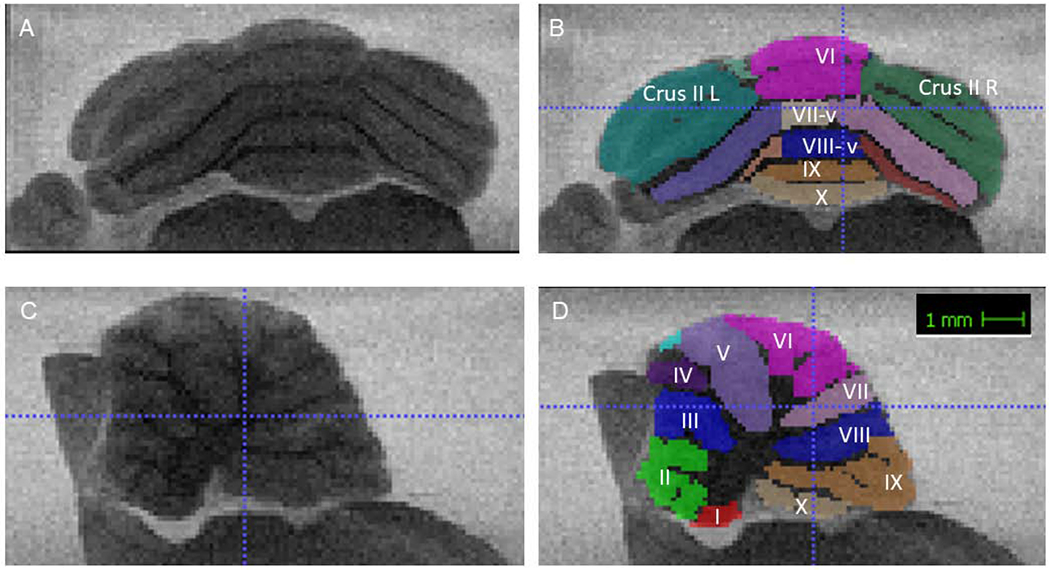
A and B are coronal sections outlining the areas of interest measured −12.84 mm from bregma. C and D are sagittal views showing the same ROIs, 1.13 mm lateral.
The multi-level gamma regression had a sex effect, (B = 0.04, SE = 0.003, t = 11.69, p < .001; Figure 2) and a condition difference (Bdiff = −0.02, SE = 0.01, t = −2.21, p = .03; Figure 3).
Figure 2.
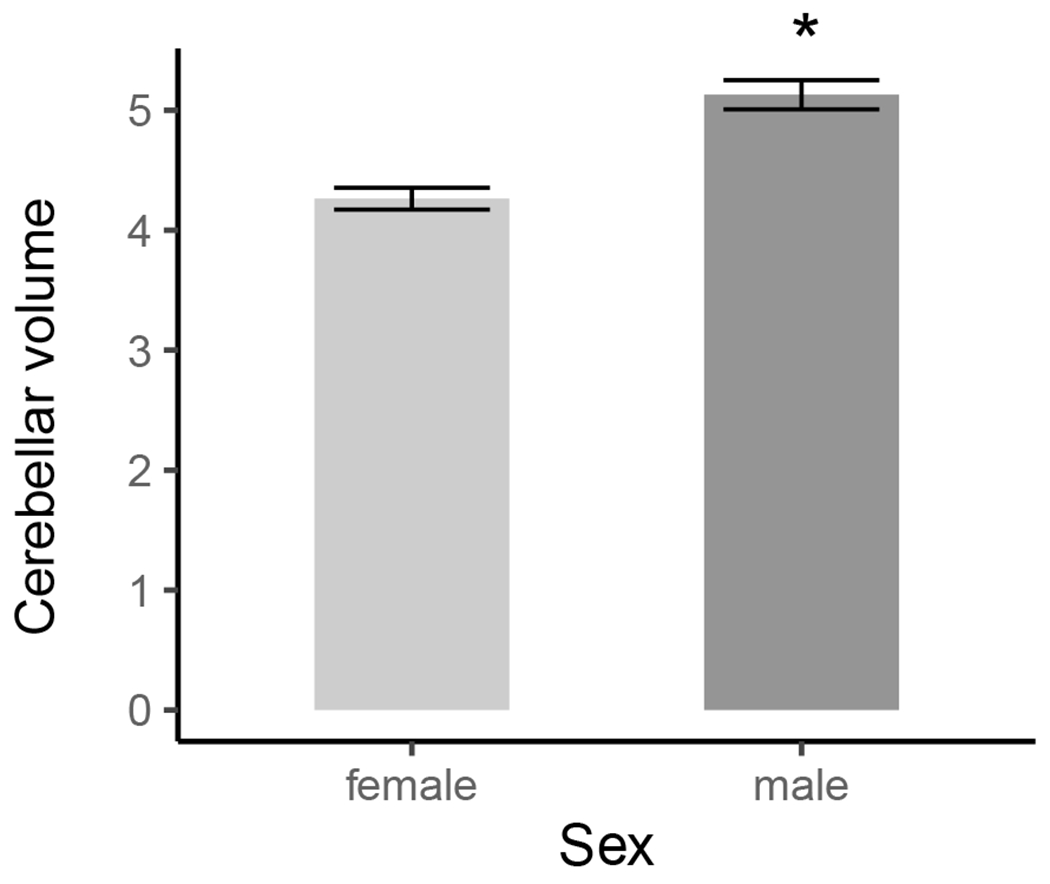
Sex differences in total cerebellar volume (mm3). Error bars represent ± 1SE. Females had significantly smaller cerebellar volumes compared to male rats.
Figure 3.
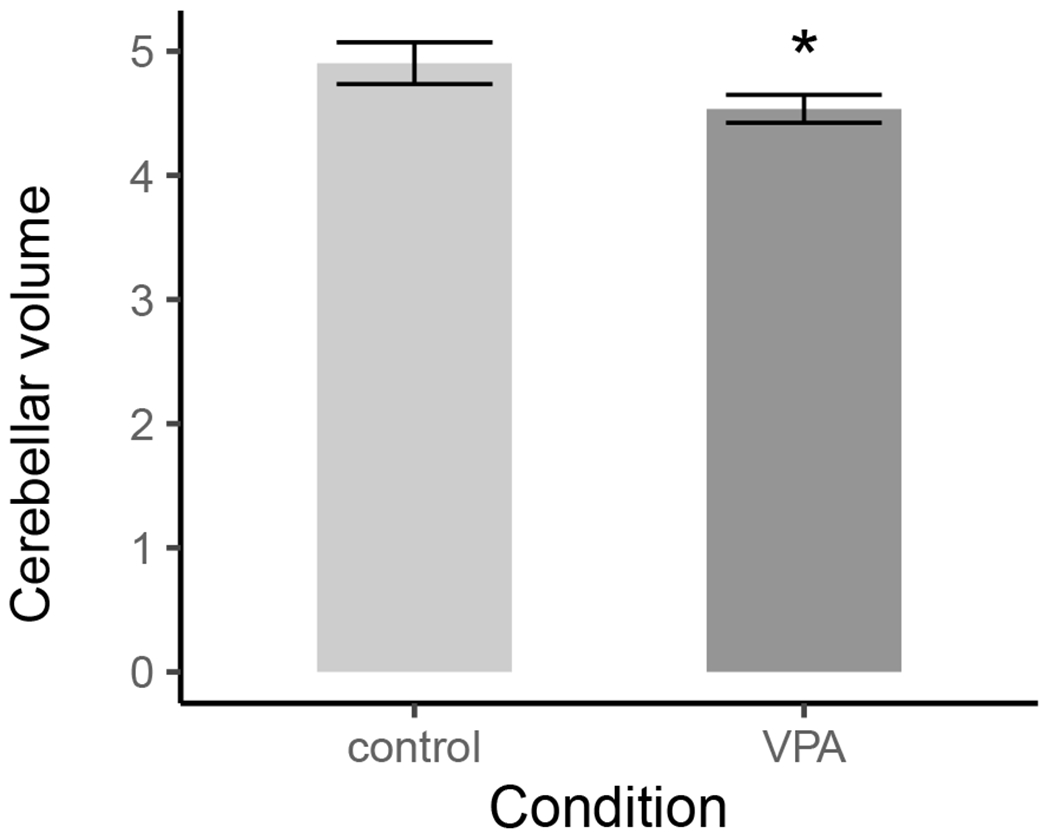
Condition differences in total cerebellar volume (mm3). Error bars represent ± 1SE. VPA rats had smaller cerebellar volumes than control rats.
These outcomes were driven by female VPA rats which had smaller cerebellar volumes than did their control counterparts (Bdiff = −0.04, SE = 0.01, z = −4.14, p < .001); whereas this decrease was not present among male rats (Bdiff = 0.002, SE = 0.01, z = 0.22, p = .99), Figure 4.
Figure 4.
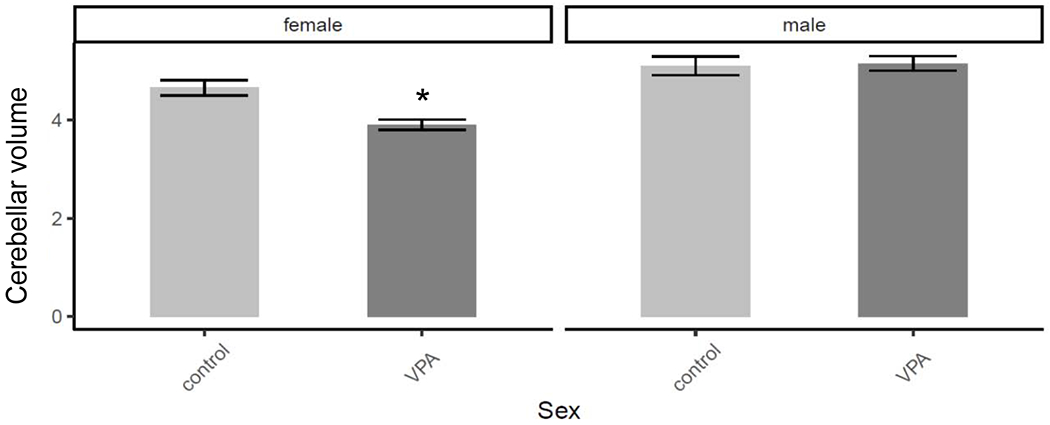
The female VPA rats had the smallest overall cerebellar volume (mm3) compared to female control rats. Error bars represent ± 1SE.
Planned comparisons, with Tukey corrections, of specific brain regions supported these findings and indicated that VPA females had reduced cerebellar volume of lobules I, IV, and X compared to control females (Table 2 and Figure 5). VPA males had increased cerebellar volume of lobule VI compared to control males (Table 3 and Figure 5).
Table 2.
Planned contrasts compare female rats’ regional cerebellar volume (mm3) across control and VPA conditions.
| Region | Bdiff | SE | z | p |
|---|---|---|---|---|
| Crus I L | −0.03 | 0.01 | −2.18 | .13 |
| Crus I R | −0.01 | 0.01 | −1.11 | .68 |
| Crus II L | −0.02 | 0.01 | −1.40 | .50 |
| Crus II R | −0.01 | 0.01 | −1.05 | .72 |
| Lobule I | −0.19 | 0.07 | −2.85 | .02 |
| Lobule II | −0.02 | 0.01 | −1.50 | .44 |
| Lobule III | −0.02 | 0.01 | −1.69 | .33 |
| Lobule IV | −0.07 | 0.02 | −2.86 | .02 |
| Lobule V-vermis | −0.03 | 0.01 | −2.52 | .06 |
| Lobule V L | −0.03 | 0.02 | −1.39 | .51 |
| Lobule V R | −0.02 | 0.02 | −0.75 | .88 |
| Lobule VI | −0.002 | 0.01 | −0.18 | .99 |
| Lobule VII-vermis | −0.02 | 0.03 | −0.51 | .96 |
| Lobule VII L | −0.03 | 0.02 | −1.87 | .24 |
| Lobule VII R | −0.03 | 0.02 | −1.78 | .28 |
| Lobule VIII-vermis | −0.04 | 0.02 | −1.60 | .38 |
| Lobule VIII L | −0.08 | 0.05 | −1.72 | .32 |
| Lobule VIII R | −0.08 | 0.04 | −1.87 | .24 |
| Lobule IX | −0.02 | 0.01 | −1.14 | .66 |
| Lobule X | −0.10 | 0.03 | −3.03 | .01 |
Figure 5.
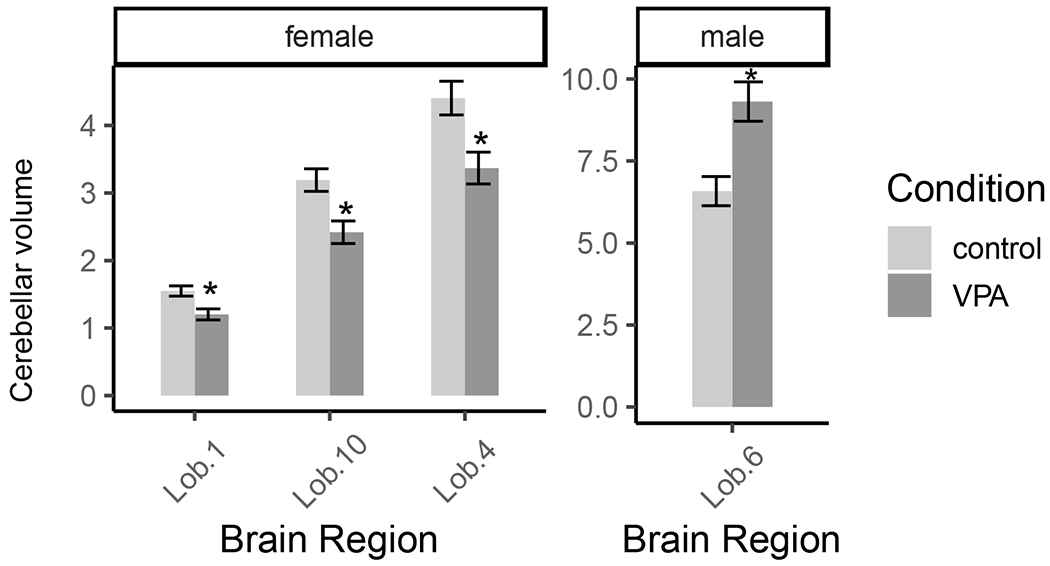
Prenatal exposure to VPA reduced cerebellar volume (mm3) of lobules I, IV, and X in female rats and increased cerebellar volume of lobule VI in male rats. Error bars represent ± 1SE.
Table 3.
Planned contrasts compare male rats’ regional cerebellar volume (mm3) across control and VPA conditions.
| region | Bdiff | SE | z | p |
|---|---|---|---|---|
| Crus I L | 0.03 | 0.01 | 2.48 | .06 |
| Crus I R | 0.02 | 0.01 | 2.16 | .14 |
| Crus II L | 0.02 | 0.01 | 2.03 | .18 |
| Crus II R | 0.02 | 0.01 | 1.70 | .32 |
| Lobule I | −0.06 | 0.04 | −1.41 | .49 |
| Lobule II | −0.01 | 0.01 | −1.01 | .74 |
| Lobule III | −0.01 | 0.01 | −0.52 | .95 |
| Lobule IV | −0.02 | 0.01 | −1.08 | .70 |
| Lobule V-vermis | 0.01 | 0.01 | 0.57 | .94 |
| Lobule V L | −0.002 | 0.01 | −0.14 | .99 |
| Lobule V R | −0.01 | 0.01 | −0.88 | .82 |
| Lobule VI | 0.04 | 0.01 | 3.61 | .001 |
| Lobule VII-vermis | −0.03 | 0.03 | −1.32 | .55 |
| Lobule VII L | −0.003 | 0.01 | −0.20 | .99 |
| Lobule VII R | −0.002 | 0.01 | −0.19 | .99 |
| Lobule VIII-vermis | −0.02 | 0.02 | −1.39 | .51 |
| Lobule VIII L | 0.04 | 0.03 | 1.42 | .49 |
| Lobule VIII R | 0.06 | 0.03 | 2.09 | .16 |
| Lobule IX | 0.005 | 0.01 | 0.42 | .97 |
| Lobule X | −0.04 | 0.02 | −1.86 | .25 |
2.1. Effect of Cerebellar Volume and set-shifting performance
Complete behavioral results examining the set-shifting task were reported in (McKinnell et al., 2021), but each phase of the task requires rats to reach criterion before moving to the next phase of the task. Therefore, the more trials required to complete the entire task the worse rats’ overall performance. Total trials were used as a proxy of overall task performance. In addition, VPA rats performed the worst at the IDS phase of the task. The models examined if sex, condition, and the volume of specific cerebellar regions were predictive of behavior as measured by total trials and IDS trials. Cerebellar regions were selected based on significant differences between VPA and control groups (see prior analysis) or in the case of crus I L because of its connections to the frontal cortices and homology with human crus region (Luo et al., 2017), this also preserved statistical power. The model included these important regions of interest for female (Lobules I, IV, and X; Crus I L) and male (Lobule VI, Crus I L) rats. These multi-level Poisson regressions included the main effects of condition and cerebellar region volume, as well as their two-way interactions. The model also nested by litter and allowed the model intercept to vary as a random effect. The condition predictor was dummy-coded, and the region predictors were means-centered to ensure that the estimate of the model’s intercept represented the average cerebellar volume of rats in the control condition.
2.1.2. IDS Trials – Male Rats
The model indicated a significant interaction between Condition × Lobule VI (B = 0.18, SE = 0.09, z = 2.03, p = .04). VPA males with enlarged volumes of lobule VI performed worse at the IDS phase of the task compared to male controls, Figure 6. For further visualization purposes trials to criterion were plotted for male rats as defined by a median split, to compare rats with larger volumes versus rats with smaller volumes. This emphasized the observed behavioral impairment on trials to criterion for the IDS phase of the task for VPA rats with enlarged volumes, Figure 7.
Figure 6.
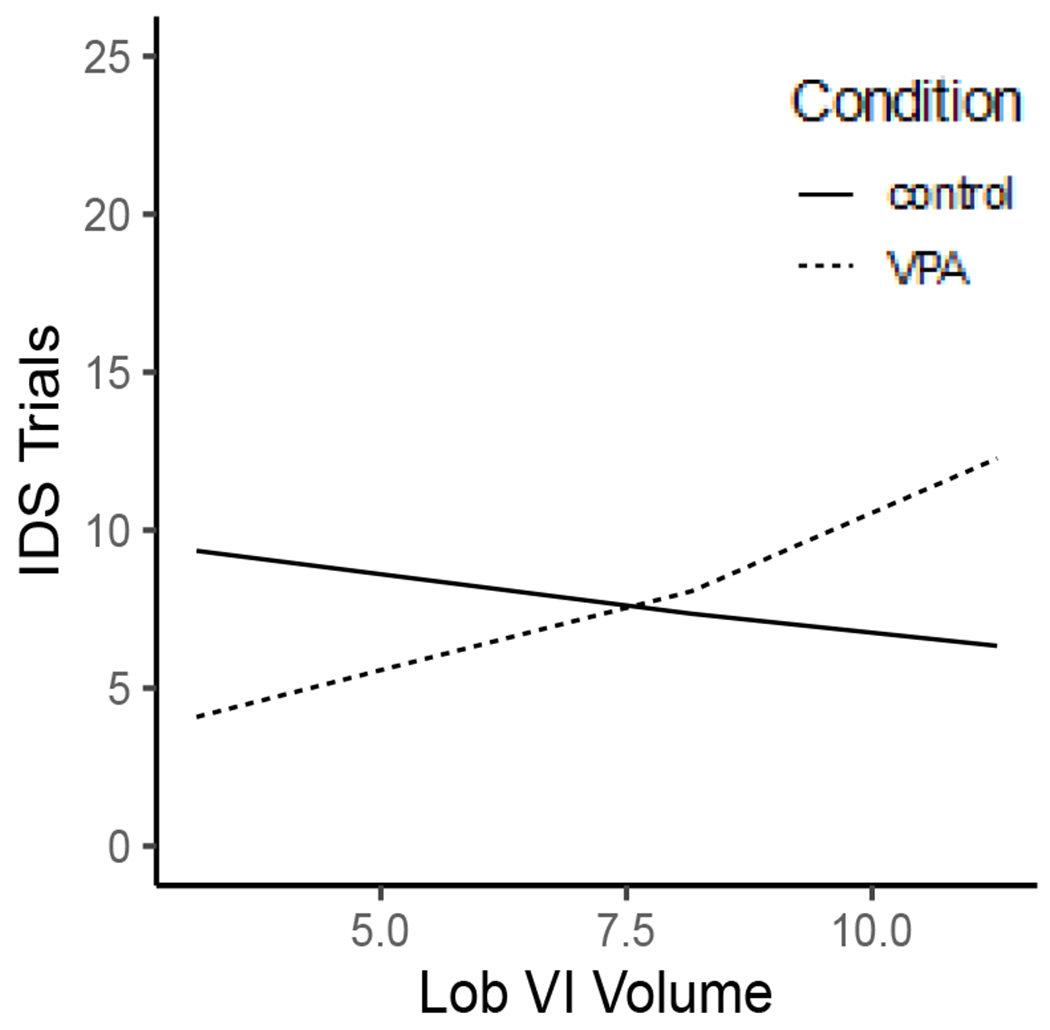
Male VPA rats with larger Lobule VI volumes (mm3) were worse at the IDS phase of the task.
Figure 7.
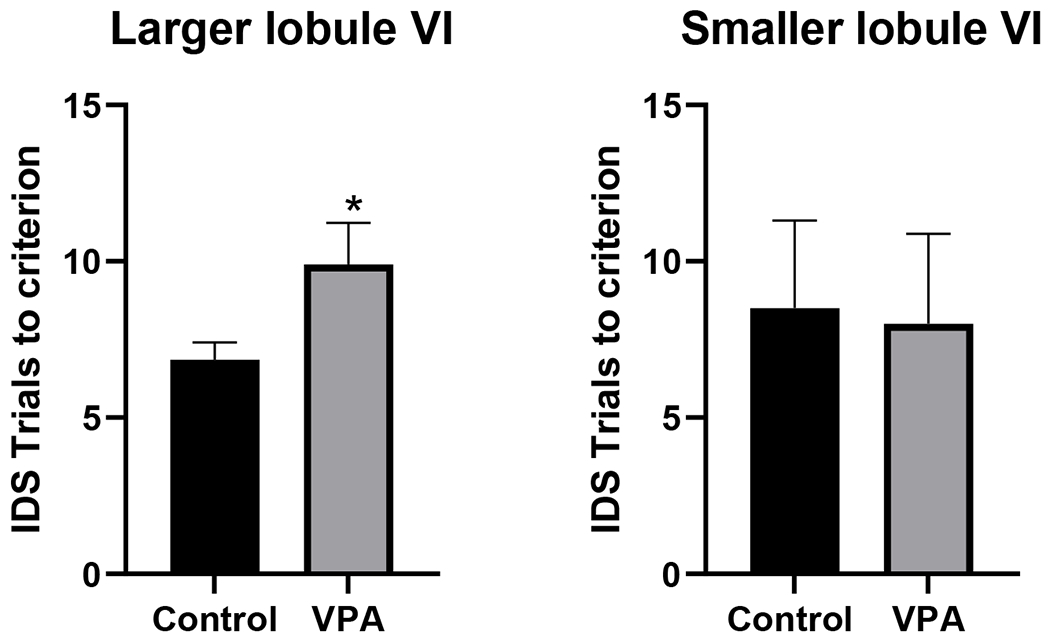
Left panel) VPA rats with enlarged lobule VI volumes (greater than the median volume) were significanlty worse compared to control rats (*p<0.05, unpaired t-test). Right panel) VPA rats with smaller volumes were not impaired on the IDS phase of the task.
2.1.3. Total Trials – Female Rats
The model indicated that condition (B = −0.34, SE = 0.17, z = −2.01, p = .04) and Crus I L volume (B = −0.06, SE = 0.03, z = −2.17, p = .03) were significant predictors of total trials in female rats. These main effects were qualified by a significant interaction (B = −0.14, SE = 0.05, z = −2.58, p = .01). Rats with smaller Crus I L volumes completed more total task trials, regardless of condition. At larger volumes, the VPA animals perform better than controls (Figure 8), which suggests that if volumes in VPA rats could be restored it may improve cognitive performance.
Figure 8.
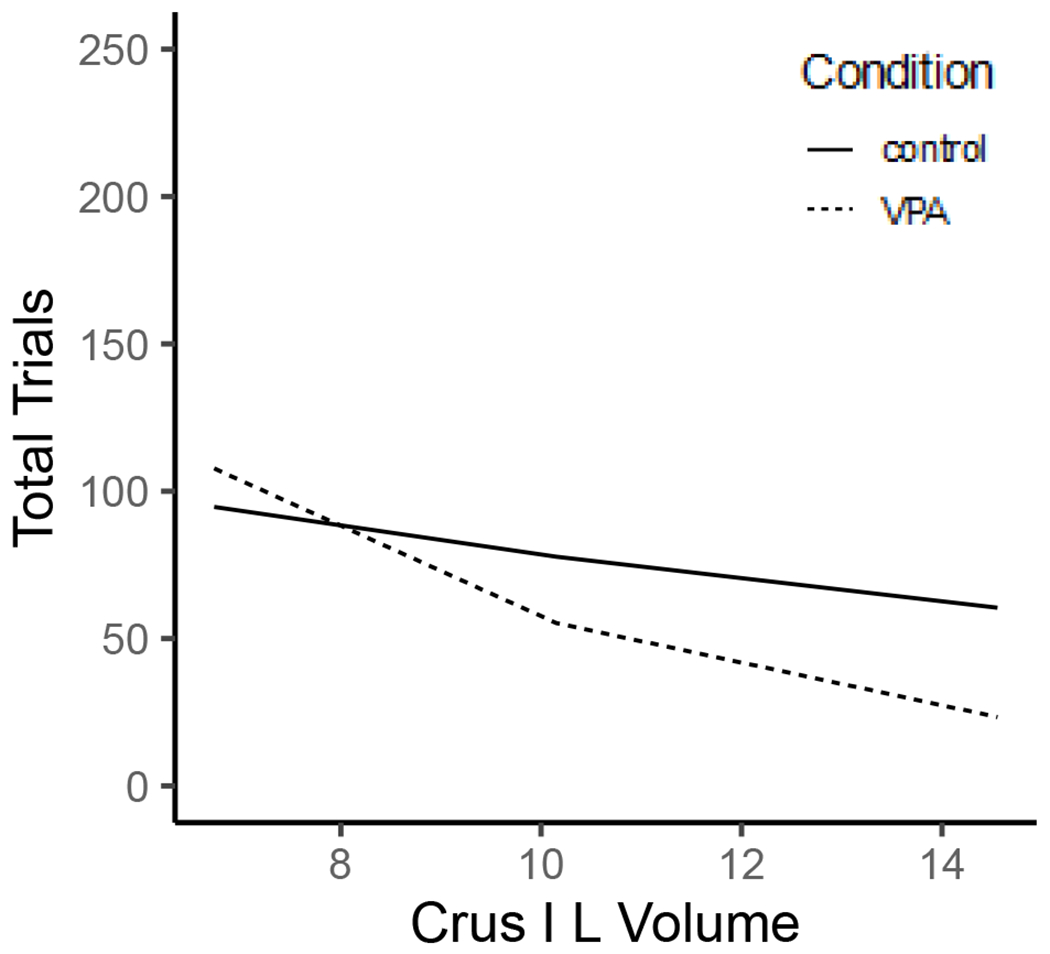
All female rats were worse across the task as measured by total trials for smaller Crus I L brain volumes (mm3).
2.1.4. IDS Trials – Female Rats
The model contained one significant predictor: the Condition × Lobule X interaction. The interaction demonstrated that control rats with larger lobule X volumes were worse at the IDS phase of the task (B = −0.47, SE = 0.23, z = −2.03, p = .04; Figure 9).
Figure 9.
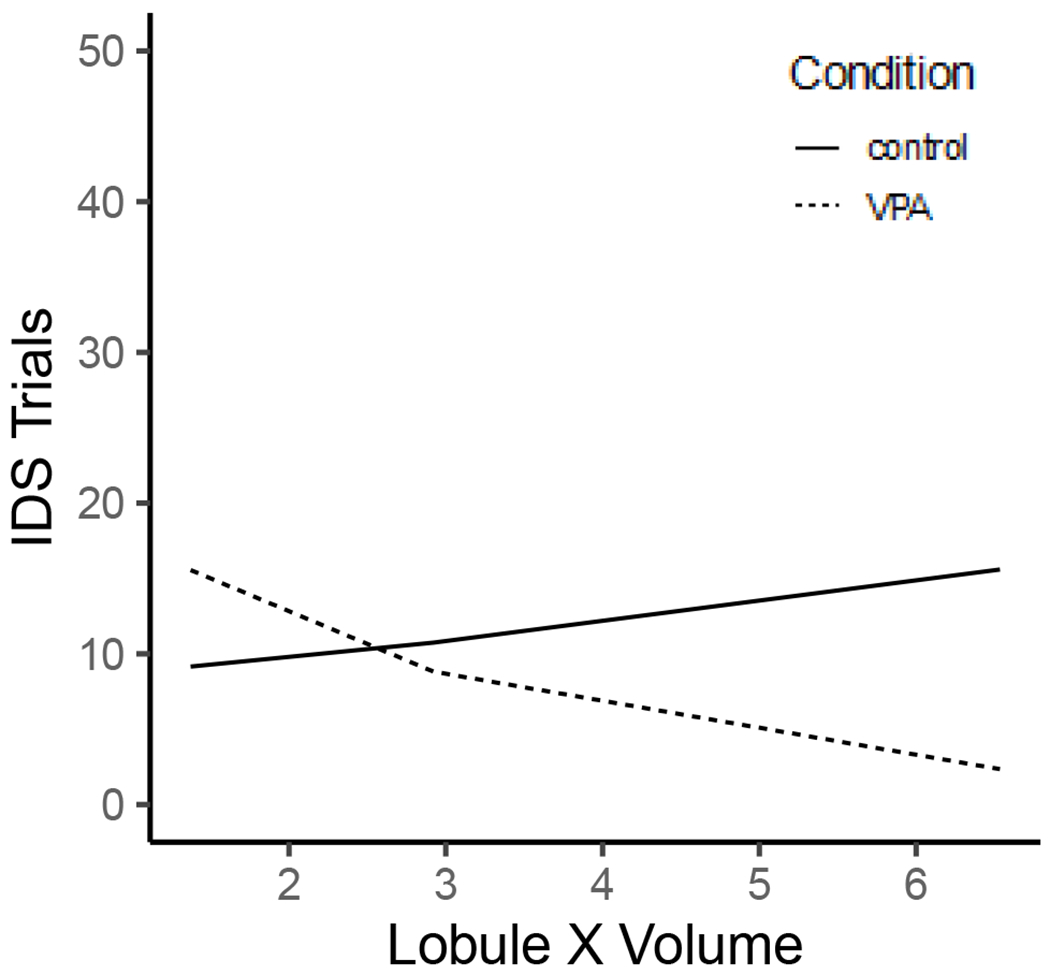
Prenatal VPA exposure was a predictor of IDS task trials in female rats at larger Lobule X brain volumes (mm3).
3.1. Methods and Materials
3.2. Subjects
Sixteen pregnant dams (Long-Evans) were injected on gestational day 12 with a single dose of saline or VPA (sodium valproate (Sigma), 250 mg/ml, mixed in saline, 600 mg/kg). Dams were briefly anesthetized on isoflurane gas in an induction chamber to administer a less stressful I.P. injection, required by the IACUC. This brief exposure to isoflurane is not associated with negative developmental outcomes (Andropoulos, 2018). Pups were reared with litter mates until weaning at PND 21, then were pair housed with a same-sex littermate.
3.3. Set-shifting task
Apparatus, stimuli and training.
A Plexiglass box (50 x 37.5 x 25 cm) with a black divider was used as the testing arena. The sliding door allowed access to the flowerpots (which were held to the table with Velcro to prevent tipping). All stimuli were mixed and recycled between rats to allow any odor cues to be disseminated. After a dig (successful or unsuccessful the opposite pot was removed to prevent digging in the other flowerpot, after the first 4 discovery trials).
Adult rats were trained in the set shifting task with 6 stages (simple discrimination (SD), compound discrimination (CD), intra-dimensional shift (IDS), reversal 1 (R1), extra-dimensional shift (EDS) and reversal 2 (R2) to test cognitive flexibility. Rats underwent food restriction for between 7-10 days and then underwent training. Honey Nut Cheerios (General Mills, MN) and a flowerpot were introduced into the home cage the night before training began. Basic training consisted of shaping digging behavior. Rats were allowed to obtain cereal pieces on top of the bedding of the flowerpots. During successive trials the cereal was buried deeper until the rat was successfully digging to retrieve it at about 3.5 cm deep. Day 2 of training consisted of an odor discrimination (tea tree oil vs lavender) with plain pine bedding, and a media discrimination (shredded paper vs pine shavings) on unscented pots. Pots themselves were not scented rather blotting paper was taped to the inner lip of the ceramic pot and scented. The paper was re-scented before every training session. Crushed Cheerio powder was applied to all digging media to prevent reward odor from serving as a digging cue. In all sessions the first four trials were discovery trials and were not scored, the rat could sample the other pot in these trials only. After these trials the undug pot was lifted out immediately after the rat initiated a dig (defined as vigorously moving the media with nose or paw). Training criteria for basic training (day 1) was 10 consecutive digs, whereas for all other training phases it was 6 consecutive trials to move to next phase of training or next phase of the task. On the day of the set shifting task the SD, CD, IDS, R1, EDS and R2 were administered. All stimuli were counterbalanced with a Latin square design and half of the rats were trained odor to media shifts, half media to odor shifts. Medias were shredded manila folders, aspen shavings, foam rubber, felt, burlap ribbon, and silk ribbon. Odors were rum, vanilla, lemon, almond, cinnamon, and anise. A pilot study demonstrated that rats could discriminate these odors. Behavioral data are reported in (McKinnell et al., 2021). All procedures were approved by Kansas State University IACUC.
3.4. Brain Processing
Adult rats were perfused, and brains were extracted. 4% Paraformaldehyde at a pH of 7.2 was used and a rate of 50mL/min for 8-10 minutes was utilized in the perfusion process. The cerebellums were removed and left in paraformaldehyde overnight then moved to a 30% sucrose solution until the brain sunk. Once sunk they were brought to the MRI for imaging. Cerebellums were placed in sucrose because they are also being processed for immunohistochemistry analysis to assess Purkinje cells, which is an ongoing project.
3.5. MRI Volumetric Analyses
Magnetic resonance images were obtained with a Bruker 600 MHz and a bottom loading probe. They were placed on an agar platform within an approximately 40 mm long glass tube and covered with a sucrose solution. Imaging was performed through Paravision 6.0.1 software with scan parameters: 27.52489 ms TE, 1575 TR, and a 90° flip angle for RARE imaging with a RARE factor of 16. A three-dimensional scan was obtained with a 100 μm3 voxel size, 66 slices in the axial orientation, and 150 slices for both sagittal and coronal. The field of view was limited to 15 x 15 x 6.6 mm and slices were obtained with an interlaced acquisition pattern. Segmentation was completed using the open source ITK-SNAP platform. Initially images were retrieved from the instrumentation in dicom format, then converted to NIfTI to condense data sets for easier file transfer using dcm2nii. Following the rat atlas (Paxinos, G, Watson, 2007) the cerebellums was segmented according to different lobule identification in all three views. Blind to condition reviewers were trained to use anatomical landmarks and the atlas to manually segment the different lobules of the cerebellum.
3.6. Data analysis
3.6.1. Volumes and Set-shifting data
Imaging data were analyzed with ITK-SNAP and all statistics were conducted in R or JMP analysis software (Statistical Discovery. From SAS, NC, USA). To determine how cerebellum volume was affected by VPA exposure multi-level gamma regressions were conducted (Bates, D, Maechler, M, Bolker, B, Walker, S, Christensen, R, H, B, Singmann H, Krivitsky, P, 2020). The regression included the main effects of condition, cerebellar region, and sex as well as their higher-order interactions. Additionally, the model nested by litter and allowed the model intercept to vary as a random effect. This model structure controlled for differences in cerebellar volume being driven by litter (Gelman, A, Hill, 2006; Lazic and Essioux, 2013), while still identifying sex-specific changes in cerebellar volume due to VPA exposure. The sex, region, and condition predictors were effect coded to ensure that the estimate of the model’s intercept represented the sample grand mean. Tukey-corrected planned comparisons were conducted using the emmeans package (Lenth, R, Singmann, H, Love, J, Buerkner, P, Herve, 2019); graphs were created with emmeans (Lenth, R, Singmann, H, Love, J, Buerkner, P, Herve, 2019) and ggplot2 (Wickham, H; Chang, W; Henry, L; Pedersen, T, L; Takahashi, K; Wilke, C…Dunnington, 2020).
4.0. Discussion
4.1. Male Rats
Lobule VI increases in male VPA rat volume aligns with some results found in humans with ASD (Chen and Van Horn, 2017; Courchesne et al., 1994). Lobule VI also had altered functional connectivity to frontal network regions in humans with ASD (D’Mello et al., 2015). In addition, lobule VI is connected to the anterior cingulate gyrus (ACC) (Coffman et al., 2011) as well as infralimbic and prelimbic regions (Badura et al., 2018) and also is active during cognitive tasks such as the tower of London, which test similar executive functions (Stoodley and Schmahmann, 2010). In humans with ASD, the anterior cingulate is also associated with reduced cognitive control (Agam et al., 2010; Charman et al., 2011), and lobule VI is associated with working memory cognitive tasks (Stoodley et al., 2012). In rodents, lesions or inactivation of the ACC lead to deficits on IDS performance (Bubb et al., 2020; Ng et al., 2007). Therefore, it is interesting that lobule VI’s enlargement is associated with impaired cognitive performance on the set-shifting task. It is possible that overgrowth of this structure modified connectivity to the frontal regions that are important for IDS performance. In our previous paper the male VPA rats were worse at the IDS compared to male controls but it did not reach statistical significance. Here, sorting rats by lobule VI volume, a region altered in ASD and connected to the critical area for IDS performance, revealed that male VPA rats with enlarged lobule VI were in fact significantly impaired at the IDS. This result is novel and suggests that the cerebellum could in part be modulating frontal cognitive function in an animal model of ASD. This hypothesis needs to be tested to verify the role of the cerebellum within this specific cognitive task. The cerebellum could be interacting with the thalamus to decrease performance as well. Future research should examine this relationship more closely by manipulating lobule VI directly during task performance. Prior groups have used MRI to measure cerebellum volumes and found decreases in overall cerebellar volumes of VPA male rats (Spisák et al., 2019). The discrepancies in volume measurements between their study and this study could be due to several factors including scanning parameters and differences in rat strains. The current study is unique in that the 3D scans were performed at ultra-high fields of 14 Tesla in a 25 mm probe, compared to the 9.4 Tesla instrumentation utilized in previous studies. While the benefits and deficits of ultra-high field MRI are always a topic of debate, one factor remains consistent, as the strength of the instrumentation increases, the signal to noise ratio increases as well (Ladd et al., 2018). This was further improved by use of smaller probe which minimized free space around samples, enhancing contrast to noise ratios as well. Ultra-high field, and smaller probe size allowed for crisp images that were delineated without the same complications seen in lower field magnets as the result of low signal to noise ratios. Combining these benefits with imaging without motion, high signal to noise ratio, and high resolution pushed the boundaries of what is possible in lower fields. The images were not exposed to rigorous post processing techniques that can attribute to artificial smoothing which can potentially obscure lobule folds within the cerebellum. Images acquired in 3D allowed for complete segmentation at each anatomical orientation which resulted in complete and thorough encapsulation of each voxel, which is difficult to perform correctly when restricted to two dimensional views. Additionally, the three-dimensional imaging allowed for tight voxel dimensions with high signal, thus tissue types were more accurately represented within the images. Within MRI, signals were averaged for each voxel which encloses them, by imaging at high resolutions it is possible to more accurately record the signal of limited tissue types which results in more accurate representation of true morphology. Through a combination of ultra-high resolution and careful manual segmentation by blind to condition observers, it exposed volume changes that may be difficult to see through other methods. In addition, prior work has noted that decreases in lobules VI were associated with decreased purkinje cell numbers (Spisák et al., 2019). It is possible that the rats in this study also lost purkinje neurons, but that other cells compensated resulting in larger volume. For instance, glial cells have been found to increase in VPA rats (Bronzuoli et al., 2018; Kazlauskas et al., 2016). To address this issue, the cerebellums from this project are being processed for histology to count purkinje neurons and will be presented in a different manuscript.
4.2. Female Rats
In general, female VPA rats had reduced total cerebellum volumes compared to control animals, both females and males. In addition, in alignment with prior groups (Sumiyoshi et al., 2017) there was a sex effect demonstrating smaller volumes for female rats, regardless of condition. However, the VPA females had the smallest overall volumes, which is interesting because it coincides with the prior behavioral results from these same animals where VPA females performed the worst at the set-shifting task. Although the cerebellum is not the essential structure for the set-shifting task, it is connected to the frontal areas (Kelly and Strick, 2003; Sugihara, 2018) that are necessary for accurate task performance (Birrell and Brown, 2000; Chase et al., 2012; Ng et al., 2007). Therefore, it is interesting that VPA females with loss of volume across the entire cerebellum, performed worse across the set-shifting task. The set-shifting task is a digging task and therefore requires motor planning and execution. However, any VPA rats that failed to complete the task were not included in this data set, because only rats that completed all stages of task were scanned in the MRI. VPA rats were not observed to have difficulty with digging, sniffing, or investigating the odor or media cues. This suggests that female rats with reduced overall volumes (rats that completed the task) of the cerebellum were impaired in a way that impacted cognition and not motor function.
Decreased volumes were found for lobule I, IV, and X of the VPA females compared to female control rats. The regression analysis did not find lobule I or IV to be a significant predictor for set-shifting behavior. Lobule IV is part of the anterior cerebellum and linked to the motor cortex (Stoodley and Schmahmann, 2010). The human literature has found correlations of decreased volume of lobule IV is associated with poorer scores on ASD diagnostic scales measuring social and repetitive behavior (D’Mello et al., 2015). Future studies could evaluate this relationship by testing repetitive and social behaviors, where it would be hypothesized that decreases in lobule IV would lead to exacerbated repetitive behaviors in female VPA rats.
The result that all female rats, regardless of condition, with decreased volumes of left crus I were also worse at the overall task suggests that left crus I may be important for modulating cognitive performance. Crus I volumes were not found to be significantly different between conditions although it appears there was a trend toward the VPAs being smaller (p = .13 in table 2). Due to the fact that some VPA female brains were not analyzable, this could be an important factor to evaluate in a larger study. Crus I in rodents is homologous to crus I in humans (Luo et al., 2017; Sugihara, 2018) and is connected to the prefrontal cortices (Badura et al., 2018), to the regions which are critical for this task (Birrell and Brown, 2000; Ng et al., 2007). Crus I in humans is active during set-shifting tasks for non-motor functions in fMRI studies (Ide and Li, 2011; Le et al., 1998). This relationship could be studied more directly in future work, such as manipulating crus I directly and measuring behavioral outcomes. Other groups have found that modulation of right crus I is related to social behaviors in a mouse model of ASD (Stoodley et al., 2017). Perhaps, left crus I is important for cognitive functions future studies should evaluate this hypothesis as well.
Larger lobule X volumes for female control rats were associated with worse IDS performance. In this case, having smaller volumes of lobule X could have been advantageous for VPA rats. Lobule X has been shown to have decreased GABA receptors in VPA mice (Varman et al., 2018). In humans with ASD, alterations within lobule X have been associated with eye-gaze behaviors, although this was for males and not females (Skefos et al., 2014). Lobule X has mostly been associated with vestibular functions (Xiong and Matsushita, 2000) therefore, understanding the role of lobule X as it relates to these issues needs more investigation.
4.3. Sex differences
These data also had robust sex differences. The behavioral data indicated that female control rats are worse at the set-shifting task compared to control males (McKinnell et al., 2021), however the MRI data highlights sex differences within the VPA model. The female VPA rats had the largest decreases in cerebellar volumes as a whole and were the most impaired on the set-shifting task. In general, VPA males did not have decreases in volume or general impairments in the set-shifting task. However, VPA males with larger lobule VI volumes were impaired at the IDS phase of the task, suggesting that the cerebellum’s role for this task may be different based on sex. Female VPA rats were worse when they had decreased left Crus I volumes. Within the VPA model sex differences have been found in communication behaviors, where VPA males exhibited fewer vocalizations than VPA females (Melancia et al., 2018). Additional tests demonstrated that male VPA rats were more impaired on social behaviors, but both male and female VPA rats exhibited stereotypic behaviors (Melancia et al., 2018). Additional sex differences have been observed for purkinje cells in the posterior regions of the cerebellum of male mice, with greater loss of purkinje cells occurring in males compared to females (Roux et al., 2019). This suggests that based on the specific domain of interest whether, social, cognitive, or repetitive behaviors, and/or brain region, the VPA impact may vary by sex, highlighting the need to test both VPA males and females.
Sex differences in humans with ASD have also been documented. In humans with ASD there is a bias of more males diagnosed with ASD (Loomes et al., 2017), however this is in part due to diagnostic criteria that were built around male patients. There is a push in the clinical community to acknowledge that different symptoms may occur in females with ASD and that there may be an under diagnosis of females with ASD (Lai et al., 2011). This bias is mirrored in the MRI human literature, where most of the volumetric work did not examine sex differences or did not include females with ASD (Stoodley, 2014). Therefore, it is possible the manifestation of the disorder is different between the sexes and that there are changes in the cerebellum of females. Additional models of ASD would also be beneficial in examining these issues.
However, sex differences in executive functions have been examined. For example, females with ASD perform worse at executive function tasks compared to males with ASD (Hjelmquist, 2000; Lemon et al., 2011; Memari et al., 2013). Clinicians have posited that diagnosis is marred by gender bias (Loomes et al., 2017) and that females with ASD may have a different phenotype compared to males (Lai et al., 2011). Whereas males with ASD are impaired in response latency for specific executive function tasks (Bölte et al., 2011). The current results suggest that it is important to evaluate behavioral and brain differences to understand possible sex differences related to the disorder. It is possible that decreased cerebellar volumes contribute to cognitive flexibility issues for females with ASD, whereas enlarged cerebellar volumes impact male behavior, additional studies are needed to verify this hypothesis.
Altered cerebellar structure and function impacts ASD behaviors including motor behavior but also extends to working memory deficits (Tsai, 2016). The IDS impairment in the male VPA rats suggests that the cerebellum may be modulating set-shifting behaviors in VPA rodents. An important caveat is that early developmental changes to the cerebellum have lifelong impacts on behavior (Badura et al., 2018), therefore future work should examine if volumes are altered earlier in development within the VPA model.
4.4. Limitations
Although all female VPA brains that completed the task were scanned, a number of them were unanalyzable due to scanner artifact or poor alignment in the holder, which was determined by a reviewer that was blind to condition for all scans. Future studies will need to use a larger sample size to verify the results found here for the female VPA rats. In addition, the volumetric measurements occurred in adult rats, the sex differences observed here could be driven by different developmental mechanisms. Neural sex differences can be impacted by hormones and even differences in micro RNA functions during development (McCarthy et al., 2015). Future research could measure volumes and behavior at critical developmental time points such as during adolescence, when changes in synaptic pruning are occurring.
One other concern with the version of this task was that there may not have been enough phases before the EDS to see an impairment in the VPA animals on the EDS. The EDS is viewed as being more difficult compared to the IDS, but rats may not show impairments on EDS if they have not formed an attentional set (Tait et al., 2018). This may be the case for these rats, and future experiments are using a variant of the set-shifting task with multiple IDS phases, which should further drive set formation, and which may induce an EDS deficit.
4.5. Conclusions
Overall, the results support the hypothesis that changes in cerebellar volume in VPA rats impacts performance of the set-shifting task. Decreased volume was associated with impaired female performance, whereas increased volume of lobule VI was associated with impaired male performance. Recent work has provided evidence that the cerebellum can modulate behavior through frontal cortex connections (Stoodley et al., 2017). These results support this interpretation and provide a platform for future hypothesis testing to further investigate the role of the cerebellum in cognitive functions within ASD.
Highlights:
VPA male rats had larger lobule VI volume compared to control males
Valproic acid (VPA) female rats had smaller volumes across lobules of the cerebellum compared to control females
Decreases in crus I volume for female rats was associated with reduced accuracy in an attentional set-shifting task
Increased volume of lobule VI in VPA male rats was associated with worse intra-dimensional shift performance in the set-shifting task
Acknowledgments.
We would like to thank Adam Fox and William DeCoteau for their mentorship and guidance.
Funding: This work was supported by the National Institute of General Medical Science GM113109 which supports the Cognitive and Neurobiological Approaches to Plasticity (CNAP) Center, which awarded a Pilot grant to BP and provided core lab support. Start-up funds from Kanas State University and a USRG from KSU to BP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none
References
- Agam Y, Joseph RM, Barton JJ, Manoach DS, 2010. Reduced cognitive control of response inhibition by the anterior cingulate cortex in autism spectrum disorders. Neuroimage 52, 336–348. 10.1016/j.neuroimage.2010.04.010 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andropoulos DB, 2018. Effect of Anesthesia on the Developing Brain : Infant and Fetus 77030, 1–11. 10.1159/000475928 [DOI] [PubMed] [Google Scholar]
- Badura A, Verpeut JL, Metzger JW, Pereira TD, Pisano TJ, Deverett B, Bakshinskaya DE, Wang SSH, 2018. Normal cognitive and social development require posterior cerebellar activity. Elife 7, 1–36. 10.7554/eLife.36401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B, Walker S, Christensen R,H,B, Singmann H, Krivitsky P,N, 2020. Package Ime4 (1.1-25). R. [Google Scholar]
- Bernard JA, Seidler RD, Hassevoort KM, Benson BL, Welsh RC, Lee Wiggins J, Jaeggi SM, Buschkuehl M, Monk CS, Jonides J, Peltier SJ, 2012. Resting state cortico-cerebellar functional connectivity networks: A comparison of anatomical and self-organizing map approaches. Front. Neuroanat 6, 1–19. 10.3389/fnana.2012.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ, 2000. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J. Neurosci 20, 4320–4325. https://doi.org/20/11/4320 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bölte S, Duketis E, Poustka F, Holtmann M, 2011. Sex differences in cognitive domains and their clinical correlates in higher-functioning autism spectrum disorders. Autism 15, 497–511. 10.1177/1362361310391116 [DOI] [PubMed] [Google Scholar]
- Bronzuoli MR, Facchinetti R, Ingrassia D, Sarvadio M, Schiavi S, Steardo L, Verkhratsky A, Trezza V, Scuderi C, 2018. Neuroglia in the autistic brain: evidence from a preclinical model. Mol. Autism 9, 1–17. 10.1186/s13229-018-0254-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubb EJ, Aggleton JP, Mara SMO, Nelson AJD, 2020. ORIGINAL ARTICLE Chemogenetics Reveal an Anterior Cingulate-Thalamic Pathway for Attending to Task-Relevant Information. Cereb. Cortex 1–18. 10.1093/cercor/bhaa353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BT, 2011. The organization of the human cerebellum estimated by intrinsic functional connectivity. J. Neurophysiol 106, 2322–2346. 10.1152/jn.00339.2011 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charman T, Pickles A, Simonoff E, Chandler S, Loucas T, Baird G, 2011. IQ in children with autism spectrum disorders: data from the Special Needs and Autism Project (SNAP). Psychol. Med 41, 619–628. 10.1017/S0033291710000991 [doi] [DOI] [PubMed] [Google Scholar]
- Chase EA, Tait DS, Brown VJ, 2012. Lesions of the orbital prefrontal cortex impair the formation of attentional set in rats. Eur. J. Neurosci 36, 2368–2376. 10.1111/j.1460-9568.2012.08141.x [doi] [DOI] [PubMed] [Google Scholar]
- Chen C, Van Horn JD, 2017. Developmental neurogenetics and multimodal neuroimaging of sex differences in autism. Brain Imaging Behav. 11, 38–61. 10.1007/s11682-015-9504-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Wu W, Fu Y, Yu S, Cui D, Zhao M, Du Y, Li I, Li X, 2016. Increased expression of fatty acid synthase and acetyl-CoA carboxylase in the prefrontal cortex and cerebellum in the valproic acid model of autism. Exp. Ther. Med 12, 1293–1298. 10.3892/etm.2016.3508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomiak T, Turner N, Hu B, 2013. What We Have Learned about Autism Spectrum Disorder from Valproic Acid. Patholog. Res. Int 2013, 712758. 10.1155/2013/712758 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen J, Gronborg TK, Sorensen MJ, Schendel D, Parner ET, Pedersen LH, Vestergaard M, 2013. Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. Jama 309, 1696–1704. 10.1001/jama.2013.2270 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman KA, Dum RP, Strick PL, 2011. Cerebellar vermis is a target of projections from the motor areas in the cerebral cortex. Proc. Natl. Acad. Sci. U. S. A 108, 16068–16073. 10.1073/pnas.1107904108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Saitoh O, Yeung-Courchesne R, Press GA, Lincoln AJ, Haas RH, Schreibman L, 1994. Abnormality of cerebellar vermian lobules VI and VII in patients with infantile autism: identification of hypoplastic and hyperplastic subgroups with MR imaging. AJR. American J. Roentgenol 162, 123–131. 10.2214/ajr.162.18273650 [doi] [DOI] [PubMed] [Google Scholar]
- D’Angelo E, Casali S, 2013. Seeking a unified framework for cerebellar function and dysfunction: from circuit operations to cognition. Front. Neural Circuits 6. https://doi.org/116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Angelo E, Casali S, 2012. Seeking a unified framework for cerebellar function and dysfunction: From circuit operations to cognition. Front. Neural Circuits 6, 1–23. 10.3389/fncir.2012.00116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Mello AM, Crocetti D, Mostofsky SH, Stoodley CJ, 2015. Cerebellar gray matter and lobular volumes correlate with core autism symptoms. NeuroImage Clin. 7, 631–639. 10.1016/j.nicl.2015.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean JC, Hailey H, Moore SJ, Lloyd DJ, Turnpenny PD, Little J, 2002. Long term health and neurodevelopment in children exposed to antiepileptic drugs before birth. J. Med. Genet 39, 251–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demetriou EA, Lampit A, Quintana DS, Naismith SL, Song YJC, Pye JE, Hickie I, Guastella AJ, 2017. Autism spectrum disorders: a meta-analysis of executive function. Mol. Psychiatry 10.1038/mp.2017.75 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Zou X, Deng H, Li J, Tang C, Wang X, Guo X, 2015. The Relationship among Genetic Heritability, Environmental Effects, and Autism Spectrum Disorders. J. Child Neurol 30, 1794–1799. 10.1177/0883073815580645 [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Aldinger KA, Ashwood P, Bauman ML, Blaha CD, Blatt GJ, Chauhan A, Chauhan V, Dager SR, Dickson PE, Estes AM, Goldowitz D, Heck DH, Kemper TL, King BH, Martin LA, Millen KJ, Mittleman G, Mosconi MW, Persico AM, Sweeney JA, Webb SJ, Welsh JP, 2012. Consensus paper: pathological role of the cerebellum in autism. Cerebellum 11, 777–808. 10.1007/s12311-012-0355-9 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman A, Hill J, 2006. Multilevel linear models: the basics. In Data analysis using regression and multilevel/hierarchical models. Cambridge University Press, Cambridge. [Google Scholar]
- Geurts HM, Corbett B, Solomon M, 2009. The paradox of cognitive flexibility in autism. Trends Cogn. Sci 13, 74–82. 10.1016/j.tics.2008.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guell X, Schmahmann JD, Gabrieli JDE, Ghosh SS, 2018. Functional gradients of the cerebellum. Elife 7, 1–22. 10.7554/eLife.36652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, Miller J, Fedele A, Collins J, Smith K, Lotspeich L, Croen LA, Ozonoff S, Lajonchere C, Grether JK, Risch N, 2011. Genetic heritability and shared environmental factors among twin pairs with autism. Arch. Gen. Psychiatry 68, 1095–1102. 10.1001/archgenpsychiatry.2011.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelmquist E, 2000. Nydn2000 185, 180–185. [Google Scholar]
- Hou Q, Wang Y, Li Y, Chen D, Yang F, Wang S, 2018. A developmental study of abnormal behaviors and altered GABAergic signaling in the VPA-treated rat model of autism. Front. Behav. Neurosci 12, 1–15. 10.3389/fnbeh.2018.00182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes C, Russell J, Robbins TW, 1994. Evidence for executive dysfunction in autism. Neuropsychologia 32, 477–493. https://doi.org/0028-3932(94)90092-2 [pii] [DOI] [PubMed] [Google Scholar]
- Ide JS, Li C. shan R., 2011. A cerebellar thalamic cortical circuit for error-related cognitive control. Neuroimage 54, 455–464. 10.1016/j.neuroimage.2010.07.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram JL, Peckham SM, Tisdale B, Rodier PM, 2000. Prenatal exposure of rats to valproic acid reproduces the cerebellar anomalies associated with autism. Neurotoxicol. Teratol 22, 319–325. https://doi.org/S0892-0362(99)00083-5 [pii] [DOI] [PubMed] [Google Scholar]
- Jeon SJ, Gonzales EL, Mabunga DFN, Valencia ST, Kim DG, Kim Y, Adil KJL, Shin D, Park D, Shin CY, 2018. Sex-specific behavioral features of rodent models of autism spectrum disorder. Exp. Neurobiol 27, 321–343. 10.5607/en.2018.27.5.321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazlauskas N, Campolongo M, Lucchina L, Zappala C, Depino AM, 2016. Postnatal behavioral and inflammatory alterations in female pups prenatally exposed to valproic acid. Psychoneuroendocrinology 72, 11–21. 10.1016/j.psyneuen.2016.06.001 [DOI] [PubMed] [Google Scholar]
- Kelly RM, Strick PL, 2003. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J. Neurosci 23, 8432–8445. https://doi.org/23/23/8432 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppelmans V, Hoogendam YY, Hirsiger S, Merillat S, Jancke L, Seidler RD, 2017. Regional cerebellar volumetric correlates of manual motor and cognitive function. Brain Struct. Funct 222, 1929–1945. 10.1007/s00429-016-1317-7 [doi] [DOI] [PubMed] [Google Scholar]
- Ladd ME, Bachert P, Meyerspeer M, Moser E, Nagel AM, Norris DG, Schmitter S, Speck O, Straub S, Zaiss M, 2018. Pros and cons of ultra-high-field MRI/MRS for human application. Prog. Nucl. Magn. Reson. Spectrosc 109, 1–50. 10.1016/j.pnmrs.2018.06.001 [DOI] [PubMed] [Google Scholar]
- Lai MC, Lombardo MV, Pasco G, Ruigrok ANV, Wheelwright SJ, Sadek SA, Chakrabarti B, Baron-Cohen S, 2011. A behavioral comparison of male and female adults with high functioning autism spectrum conditions. PLoS One 6. 10.1371/journal.pone.0020835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazic SE, Essioux L, 2013. Improving basic and translational science by accounting for litter-to-litter variation in animal models. BMC Neurosci. 14. 10.1186/1471-2202-14-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le TH, Pardo JV, Hu X, 1998. 4 T-fMRI study of nonspatial shifting of selective attention: Cerebellar and parietal contributions. J. Neurophysiol 79, 1535–1548. 10.1152/jn.1998.79.3.1535 [DOI] [PubMed] [Google Scholar]
- Lemon JM, Gargaro B, Enticott PG, Rinehart NJ, 2011. Brief report: Executive functioning in autism spectrum disorders: A gender comparison of response inhibition. J. Autism Dev. Disord 41, 352–356. 10.1007/s10803-010-1039-2 [DOI] [PubMed] [Google Scholar]
- Lenth R, Singmann H, Love J, Buerkner P, Herve M, 2019. Package emmeans (1.3.4). R. [Google Scholar]
- Loomes R, Hull L, Mandy WPL, 2017. What Is the Male-to-Female Ratio in Autism Spectrum Disorder? A Systematic Review and Meta-Analysis. J. Am. Acad. Child Adolesc. Psychiatry 56, 466–474. 10.1016/jjaac.2017.03.013 [DOI] [PubMed] [Google Scholar]
- Luo Y, Fujita H, Nedelescu H, Biswas MS, Sato C, Ying S, Takahashi M, Akita K, Higashi T, Aoki I, Sugihara I, 2017. Lobular homology in cerebellar hemispheres of humans, non-human primates and rodents: a structural, axonal tracing and molecular expression analysis. Brain Struct. Funct 222, 2449–2472. 10.1007/s00429-017-1436-9 [DOI] [PubMed] [Google Scholar]
- Mabunga DF, Gonzales EL, Kim JW, Kim KC, Shin CY, 2015. Exploring the Validity of Valproic Acid Animal Model of Autism. Exp. Neurobiol 24, 285–301. 10.5607/en.2015.24A285 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Main SL, Kulesza RJ, 2017. Repeated prenatal exposure to valproic acid results in cerebellar hypoplasia and ataxia. Neuroscience 340, 34–48. https://doi.org/S0306-4522(16)30592-9 [pii] [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Pickett LA, VanRyzin JW, Kight KE, 2015. Surprising origins of sex differences in the brain. Horm. Behav 76, 3–10. 10.1016/j.yhbeh.2015.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnell ZE, Maze T, Ramos A, Challans B, Plakke B, 2021. Valproic acid treated female Long-Evans rats are impaired on attentional set-shifting. Behav. Brain Res 397, 112966. 10.1016/j.bbr.2020.112966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melancia F, Schiavi S, Servadio M, Cartocci V, Campolongo P, Palmery M, Pallottini V, Trezza V, 2018. Sex-specific autistic endophenotypes induced by prenatal exposure to valproic acid involve anandamide signalling. Br. J. Pharmacol 175, 3699–3712. 10.1111/bph.14435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memari AH, Ziaee V, Shayestehfar M, Ghanouni P, Mansournia MA, Moshayedi P, 2013. Cognitive flexibility impairments in children with autism spectrum disorders: Links to age, gender and child outcomes. Res. Dev. Disabil 34, 3218–3225. 10.1016/j.ridd.2013.06.033 [DOI] [PubMed] [Google Scholar]
- Modabbernia A, Velthorst E, Reichenberg A, 2017. Environmental risk factors for autism: an evidence-based review of systematic reviews and meta-analyses. Mol. Autism 8, 1–16. 10.1186/s13229-017-0121-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morakotsriwan N, Wattanathorn J, Kirisattayakul W, Chaisiwamongkol K, 2016. Autistic-Like Behaviors, Oxidative Stress Status, and Histopathological Changes in Cerebellum of Valproic Acid Rat Model of Autism Are Improved by the Combined Extract of Purple Rice and Silkworm Pupae. Oxid. Med. Cell. Longev 2016. 10.1155/2016/3206561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi MW, Wang Z, Schmitt LM, Tsai P, Sweeney JA, 2015. The role of cerebellar circuitry alterations in the pathophysiology of autism spectrum disorders. Front. Neurosci 9, 296. 10.3389/fnins.2015.00296 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mychasiuk R, Richards S, Nakahashi A, Kolb B, Gibb R, 2012. Effects of rat prenatal exposure to valproic acid on behaviour and neuro-anatomy. Dev. Neurosci 34, 268–277. https://doi.org/000341786 [pii] [DOI] [PubMed] [Google Scholar]
- Ng CW, Noblejas MI, Rodefer JS, Smith CB, Poremba A, 2007. Double dissociation of attentional resources: prefrontal versus cingulate cortices. J. Neurosci 27, 12123–12132. https://doi.org/27/45/12123 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Pennington BF, Rogers SJ, 1991. Executive function deficits in high-functioning autistic individuals: relationship to theory of mind. J. Child Psychol. Psychiatry 32, 1081–1106. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C, 2007. The rat brain in stereotaxic coordinates, 6th ed. Elsevier. [DOI] [PubMed] [Google Scholar]
- Phiel CJ, Zhang F, Huang EY, Guenther MG, Lazar MA, Klein PS, 2001. Histone Deacetylase is a Direct Target of Valproic Acid, a Potent Anticonvulsant, Mood Stabilizer, and Teratogen. J. Biol. Chem 276, 36734–36741. 10.1074/jbc.M101287200 [DOI] [PubMed] [Google Scholar]
- Pierce K, Courchesne E, 2001. Evidence for a cerebellar role in reduced exploration and stereotyped behavior in autism. Biol. Psychiatry 49, 655–665. https://doi.org/S0006-3223(00)01008-8 [pii] [DOI] [PubMed] [Google Scholar]
- Rasalam AD, Hailey H, Williams JH, Moore SJ, Turnpenny PD, Lloyd DJ, Dean JC, 2005. Characteristics of fetal anticonvulsant syndrome associated autistic disorder. Dev. Med. Child Neurol 47, 551–556. [DOI] [PubMed] [Google Scholar]
- Roux S, Bailly Y, Bossu JL, 2019. Regional and sex-dependent alterations in Purkinje cell density in the valproate mouse model of autism. Neuroreport 30, 82–88. 10.1097/WNR.0000000000001164 [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, 2019. The cerebellum and cognition. Neurosci. Lett 688, 62–75. 10.1016/j.neulet.2018.07.005 [DOI] [PubMed] [Google Scholar]
- Schneider T, Przewlocki R, 2005. Behavioral alterations in rats prenatally exposed to valproic acid: animal model of autism. Neuropsychopharmacology 30, 80–90. 10.1038/sj.npp.1300518 [doi] [DOI] [PubMed] [Google Scholar]
- Skefos J, Cummings C, Enzer K, Holiday J, Weed K, Levy E, Yuce T, Kemper T, Bauman M, 2014. Regional alterations in Purkinje cell density in patients with autism. PLoS One 9, 1–12. 10.1371/journal.pone.0081255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spisák T, Román V, Papp E, Kedves R, Sághy K, Csölle CK, Varga A, Gajári D, Nyitrai G, Spisák Z, Kincses ZT, Lévay G, Lendvai B, Czurkó A, 2019. Purkinje cell number-correlated cerebrocerebellar circuit anomaly in the valproate model of autism. Sci. Rep 9, 1–15. 10.1038/s41598-019-45667-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ, 2014. Distinct regions of the cerebellum show gray matter decreases in autism, ADHD, and developmental dyslexia. Front. Syst. Neurosci 8, 1–17. 10.3389/fnsys.2014.00092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ, D’Mello AM, Ellegood J, Jakkamsetti V, Liu P, Nebel MB, Gibson JM, Kelly E, Meng F, Cano CA, Pascual JM, Mostofsky SH, Lerch JP, Tsai PT, 2017. Altered cerebellar connectivity in autism and cerebellar-mediated rescue of autism-related behaviors in mice. Nat. Neurosci 20, 1744–1751. 10.1038/s41593-017-0004-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD, 2010. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 46, 831–844. 10.1016/j.cortex.2009.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ, Valera EM, Schmahmann JD, 2012. Functional topography of the cerebellum for motor and cognitive tasks: An fMRI study. Neuroimage 59, 1560–1570. 10.1016/j.neuroimage.2011.08.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugihara I, 2018. Crus I in the Rodent Cerebellum: Its Homology to Crus I and II in the Primate Cerebellum and Its Anatomical Uniqueness Among Neighboring Lobules. Cerebellum 17, 49–55. 10.1007/s12311-017-0911-4 [DOI] [PubMed] [Google Scholar]
- Sumiyoshi A, Nonaka H, Kawashima R, 2017. Sexual differentiation of the adolescent rat brain: A longitudinal voxel-based morphometry study. Neurosci. Lett 642, 168–173. 10.1016/j.neulet.2016.12.023 [DOI] [PubMed] [Google Scholar]
- Tait DS, Bowman EM, Neuwirth LS, Brown VJ, 2018. Assessment of intradimensional/extradimensional attentional set-shifting in rats. Neurosci. Biobehav. Rev 89, 72–85. https://doi.org/S0149-7634(17)30751-0 [pii] [DOI] [PubMed] [Google Scholar]
- Tsai PT, 2016. Autism and cerebellar dysfunction: Evidence from animal models. Semin. Fetal Neonatal Med 21, 349–355. 10.1016/j.siny.2016.04.009 [DOI] [PubMed] [Google Scholar]
- Varman DR, Soria-Ortíz MB, Martínez-Torres A, Reyes-Haro D, 2018. GABAρ3 expression in lobule X of the cerebellum is reduced in the valproate model of autism. Neurosci. Lett 687, 158–163. 10.1016/j.neulet.2018.09.042 [DOI] [PubMed] [Google Scholar]
- Wang SS, Kloth AD, Badura A, 2014. The cerebellum, sensitive periods, and autism. Neuron 83, 518–533. 10.1016/j.neuron.2014.07.016 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein-Fudim L, Ergaz Z, Turgeman G, Yanai J, Szyf M, Ornoy A, 2019. Gender related changes in gene expression induced by valproic acid in a mouse model of autism and the correction by s-adenosyl methionine. Does it explain the gender differences in autistic like behavior? Int. J. Mol. Sci 20, 1–24. 10.3390/ijms20215278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H; Chang W; Henry L; Pedersen T,L; Takahashi K;Wilke C … Dunnington D, 2020. Package ggplot2 (3.3.2). R. [Google Scholar]
- Xiong G, Matsushita M, 2000. Connections of Purkinje cell axons of lobule X with vestibulocerebellar neurons projecting to lobule X or IX in the rat. Exp. Brain Res 133, 219–228. 10.1007/S002210000372 [DOI] [PubMed] [Google Scholar]


