Abstract
Objectives:
To test the hypothesis that genetic predisposition to systemic lupus erythematosus (SLE) increases the risk of cardiometabolic disorders.
Methods:
Using 41 single nucleotide polymorphisms (SNPs) associated with SLE, we calculated a weighted genetic risk score (wGRS) for SLE. In a large biobank we tested the association between this wGRS and 9 cardiometabolic phenotypes previously associated with SLE: atrial fibrillation, ischemic stroke, coronary artery disease, type 1 and type 2 diabetes, obesity, chronic kidney disease, hypertension, and hypercholesterolemia. Additionally, we performed a phenome-wide association analysis (pheWAS) to discover novel clinical associations with a genetic predisposition to SLE. Findings were replicated in the Electronic Medical Records and Genomics (eMERGE) Network. To further define the association between SLE-related risk alleles and the selected cardiometabolic phenotypes, we performed an inverse variance weighted regression (IVWR) meta-analysis.
Results:
The wGRS for SLE was calculated in 74,759 individuals of European ancestry. Among the pre-selected phenotypes, the wGRS was significantly associated with type 1 diabetes (OR [95%CI] =1.11 [1.06, 1.17], P-value=1.05x10−5). In the pheWAS, the wGRS was associated with several autoimmune phenotypes, kidney disorders, and skin neoplasm; but only the associations with autoimmune phenotypes were replicated. In the IVWR meta-analysis, SLE-related risk alleles were nominally associated with type 1 diabetes (P=0.048) but the associations were heterogeneous and did not meet the adjusted significance threshold.
Conclusion:
A weighted GRS for SLE was associated with an increased risk of several autoimmune-related phenotypes including type 1 diabetes but not with cardiometabolic disorders.
Keywords: systemic lupus erythematosus, genetic risk score, pleiotropy
INTRODUCTION
Systemic lupus erythematosus (SLE) is a complex autoimmune disorder that is associated with several cardiometabolic co-morbidities. SLE has a strong genetic component with more than a hundred risk alleles associated with disease (1). Although some risk alleles are shared with other autoimmune disorders, little is known about their association with the cardiometabolic disorders that are prevalent in SLE.
Cardiometabolic diseases contribute substantially to morbidity and mortality in SLE. For example, we and others have shown that coronary artery disease (CAD) is a prominent feature in SLE(2); also, patients with SLE have increased risk for atrial fibrillation (AF)(3), dyslipidemia (4), type 1 diabetes (T1D) and type 2 diabetes (T2D) (5), hypertension (HTN) (6), chronic kidney disease (CKD) (7), and central obesity (4) compared to the general population. Whether this increased risk for CAD and other cardiometabolic diseases and risk factors in SLE is imparted by the genetic predisposition to SLE is not known.
With the availability of large genome-wide association studies (GWAS) that have identified common single nucleotide polymorphisms (SNPs) associated with many phenotypes including SLE and cardiometabolic disorders, it is possible to study the shared genetic predisposition between various phenotypes. For example, in a previous study that used information from large GWAS, we found that genetic liability for rheumatoid arthritis (RA) was associated with increased risk of T1D and decreased risk of multiple sclerosis (MS) (8).
To define whether a genetic predisposition to SLE increases risk of cardiometabolic disorders we used two approaches: a) we examined whether a weighted genetic risk score (GRS) for SLE identified individuals in a large de-identified electronic health record (EHR) system with increased prevalence of selected prespecified cardiometabolic phenotypes; we also performed an global phenome wide association study (PheWAS) to identify potential novel clinical associations with the SLE GRS; b) we used inverse variance weighted regression (IVWR) meta-analysis to test for a causal association between predisposition to SLE and selected cardiometabolic phenotypes using publicly available genome-wide association data.
METHODS
Data Sources
We used BioVU, the Vanderbilt University Medical Center (VUMC) DNA biobank, to study the association between genetic liability for SLE and 9 cardiometabolic outcomes previously associated with SLE: AF, ischemic stroke, CAD, T2D, obesity, HTN, CKD, hypercholesterolemia, and T1D. A full description of BioVU has been published (9). Briefly, BioVU accrues DNA from blood samples obtained during routine clinical care from patients who have consented to have a DNA sample collected. DNA is extracted from blood samples that would otherwise be discarded, de-identified, and linked to a de-identified version of the EHR. Approval for the study was obtained from the Vanderbilt Institutional Review Board. For replication of findings, we use data from the electronic Medical Records and Genomics (eMERGE) Network that has been fully described elsewhere (10). Because BioVU and eMERGE participants were predominantly self-reported white, we restricted our sample to individuals of European ancestry (EA) determined by principal components in conjunction with the HapMap population as described elsewhere (11). The eMERGE network included EA individuals born prior to 1990 (n=31,773, excluding VUMC dataset) while the BioVU dataset included more than 74,000 EA individuals over 18 years old.
We selected the largest genetic meta-analysis with summary-level data available for EA individuals for SLE and the other phenotypes of interest (or proxies when the exact phenotype was not available) (Supplementary Table S1 and S2). We studied the same 9 phenotypes (or proxies) used in the GRS association analyses and 2 additional biomarkers that have been associated with increased risk of cardiometabolic disease for which there are no good phenotype equivalents in the EHR: C-reactive protein (CRP) and interleukin 6 (IL-6) concentrations in the absence of acute inflammation (12).
Genotyping
In the BioVU cohort, genotyping was performed by the Vanderbilt Technologies for Advance Genomics (VANTAGE) according to standard protocols on the Illumina Infinium Multi-Ethnic Genotyping Array (MEGAEX) platform. eMERGE participants were genotyped on multiple platforms and underwent QC analyses and imputation as previously described (13). Quality control (QC) analyses used PLINK version 1.90β3 (14) and included reconciling strand flips, verifying that allele frequencies were concordant among data sets, and identifying duplicate and related individuals (one of each pair of subjects with a pi-hat>0.05 was excluded). Data sets were standardized using the HRC-1000G-check tool v4.2.5 (http://www.well.ox.ac.uk/~wrayner/tools/) and pre-phased using SHAPEIT (15). BioVU data was imputed using IMPUTE2 (16), in conjunction with the same reference panel from which the SLE risk alleles were derived (1000 Genomes cosmopolitan reference haplotypes). All other genetic data were imputed using the Michigan Imputation Server (HRC v1.1). Imputed data were filtered for a sample missingness rate <2%, a SNP missingness rate <4% and SNP deviation from Hardy-Weinberg P<5x10−6. Principal components (PCs) were calculated using the SNPRelate package (17).
Phenotypes
For the 9 prespecified phenotypes, we extracted clinical diagnoses from the EHR using the 9th and 10th International Statistical Classification of Diseases and Related Health Problems (ICD) Clinical Modification (CM) codes that mapped to the phenotype and transformed these ICD9/ICD10 codes into phecodes, which aggregate one or more related ICD codes into distinct diseases or traits (18). For each phenotype, cases were defined as individuals with 2 or more instances of the specific phecode in the EHR. Controls were defined as individuals without the phecode or related phecodes (see map of phecodes at https://phewascatalog.org/phecodes). For the PheWAS analysis, we followed the same procedures and extracted information for 1162 clinical phenotypes with 100 or more cases (to assure statistical power) in the EHR. ICD9/10 codes extraction was performed on December 2019 for BioVU and October 2019 for eMERGE.
Genetic Risk Score and Statistical Analysis
To construct the GRS, we selected 41 autosomal SNPs that were associated with SLE in the largest meta-analysis performed in EA individuals (1) (Supplementary Table S1), and only included EA individuals in the analyses. Summary statistics for these 41 SNPs were included into a weighted GRS (wGRS) to calculate genetically-predicted risk for SLE using the following equation:
where β is the effect size (log odds-ratio) of the risk allele and the genotype is the number of copies of the risk allele coded as 0, 1, or 2. Only SNPs that passed quality control were included in the calculation of the wGRS. A multivariable regression analyses adjusting for the first 5 PCs, median age in the EHR, and sex was performed for the nine pre-specified phenotypes. For these 9 prespecified phenotypes, a Bonferroni-corrected P-value <0.0056 (0.05/9 phenotypes) was considered significant. In addition, we tested whether the wGRS was associated with the selected phenotypes in patients with SLE and with lupus nephropathy. SLE was defined as the presence of two or more SLE-related phecodes and lupus nephropathy as the presence of 2 or more nephropathy-related phecodes in individuals with SLE (19). A Benjamini-Hochberg false discovery rate (FDR) q<0.05 was considered significant for the global PheWAS and the replication in eMERGE. The PheWAS was adjusted by the same covariates included in the regression analysis using the PheWAS R package (20). As a secondary analysis, we performed a PheWAS that excluded patients with SLE or common autoimmune diseases (see Supplementary Table S3). All PheWAS associations were expressed as odds- ratios (OR) and 95% confidence interval (95%CI), where ORs represent the risk of disease per standard deviation (s.d.) increase in the GRS.
To further test the association between genetic liability for SLE and the selected phenotypes we performed IVWR meta-analyses. The same 41 autosomal SNPs included in the GRS were used to select a linkage disequilibrium (LD)-reduced (r2<0.05) set of SNPs with a MAF>0.05 as instrumental variables (IVs) for SLE in the IVWR meta-analysis. Heterogeneity p-values are based on the Cochran’s Q statistic, and a low p-value indicates that one or more variants in the GRS may be pleiotropic.
As a sensitivity analysis, we performed weighted median regression since this approach, while less well powered than IVWR, provides better estimates of the true effect size when less than 50% of the IVs are not valid (21). In addition, we also tested for unbalanced horizontal pleiotropy using MR-Egger regression, which provides unbiased estimates in the presence of pleiotropy (21). Analyses were performed using the Mendelian Randomization R-package and a Bonferroni-adjusted P-value<0.0045 (0.05/11 outcomes) was considered significant. A P-value<0.05 for the intercept estimate in the Egger regression indicated the presence of horizontal pleiotropy.
RESULTS
Genetic risk score analysis
All 41 autosomal SNPs passed quality control and were included in the calculation of the wGRS. We calculated the wGRS for SLE in all 74,759 individuals of European ancestry in BioVU with genotype information and clinical data available; 41,934 (56%) were women and the median value (IQR) of the average age on the EHR was 52.5 (32.7, 65.14).
Among the pre-selected phenotypes, T1D was significantly associated with the wGRS for SLE (OR [95%CI] =1.11 [1.06, 1.17], P=1.05x10−5) and a nominal association was observed for CKD (1.05 [1.01, 1.08], P=0.007) (Table 1).
Table 1:
Association of the weighted genetic risk score for systemic lupus erythematosus and selected cardiometabolic phenotypes
| Phenotype | Phecode | # cases | # controls | OR (95%CI) | P-value |
|---|---|---|---|---|---|
| Atrial fibrillation | 427.21 | 6601 | 32787 | 0.99 [0.96, 1.02] | 0.320 |
| Ischemic stroke | 433.21 | 1830 | 47571 | 1.04 [0.99, 1.09] | 0.935 |
| Coronary atherosclerosis | 411.4 | 10357 | 38740 | 1.01 [0.99, 1.04] | 0.370 |
| Type 2 diabetes | 250.2 | 9741 | 38763 | 1.01 [0.98, 1.03] | 0.279 |
| Essential hypertension | 401.1 | 25911 | 26027 | 0.99 [0.97, 1.01] | 0.260 |
| Chronic renal failure | 585.3 | 4742 | 42181 | 1.05 [1.01, 1.08] | 0.007 |
| Obesity | 278.1 | 6424 | 42347 | 1.01 [0.99, 1.04] | 0.355 |
| Type 1 diabetes | 250.1 | 1881 | 38647 | 1.11 [1.06,1.17] | 1.05x10−5 |
| Hyperlipidemia | 272.13 | 9448 | 33414 | 0.99 [0.97, 1.02] | 0.607 |
In addition, none of the selected phenotypes were associated with the wGRS (P>0.05) when only patients with SLE were studied. The average wGRS was higher in patients with lupus nephropathy compared to SLE patients without nephropathy (0.082 vs. 0.080, P=0.001); but the wGRS was not associated with any of the selected cardiometabolic phenotypes in patients with lupus nephropathy (P>0.05, Supplementary Table S4)
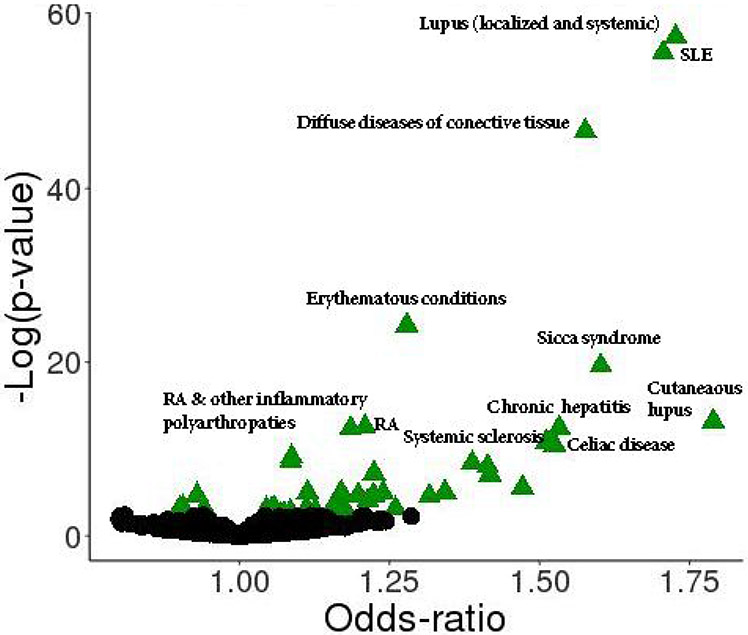
The global PheWAS in BioVU showed that the wGRS for SLE was significantly associated with 42 clinical diagnosis including several autoimmune phenotypes (FDR q<0.05) such as SLE, diffuse diseases of the connective tissue, sicca syndrome, rheumatoid arthritis (RA) related phenotypes, systemic sclerosis, celiac disease, autoimmune thyroiditis-related phenotypes, and T1D-related phenotypes among others (Table 2, Figure 1). The wGRS for SLE was also associated with non-autoimmune disorders including renal phenotypes and skin neoplasms. The replication analysis was performed in 31,773 EA individuals (55% female) from eMERGE and 24 of the 42 associated phenotypes in BioVU were also strongly associated (FDR q<0.05) in the eMERGE population; most of which were autoimmune disorders (Table 2, Supplementary Table S5).
Table 2:
Clinical diagnoses associated with a weighted genetic risk score for systemic lupus erythematosus
| BioVU | eMERGE | |||||||
|---|---|---|---|---|---|---|---|---|
| Clinical diagnoses | # Cases |
# Controls |
OR (95%CI) | FDR (q) | # Cases |
# Controls |
OR (95%CI) | FDR (q) |
| Lupus (localized and systemic) | 867 | 47037 | 1.73 [1.62, 1.85] | 6.64E-55 | 418 | 18304 | 1.82 [1.66, 2.00] | 6.62E-35 |
| Systemic lupus erythematosus | 880 | 47037 | 1.71 [1.60, 1.82] | 2.26E-53 | 393 | 18305 | 1.86 [1.69, 2.04] | 6.62E-35 |
| Diffuse diseases of connective tissue | 1034 | 47690 | 1.58 [1.48, 1.68] | 1.55E-44 | 797 | 17923 | 1.28 [1.19, 1.37] | 1.07E-10 |
| Erythematous conditions | 1916 | 48513 | 1.28 [1.22, 1.34] | 1.72E-22 | 3029 | 18445 | 1.08 [1.04, 1.13] | 1.55E-04 |
| Sicca syndrome | 388 | 47650 | 1.60 [1.45, 1.77] | 6.63E-18 | 318 | 17740 | 1.29 [1.16, 1.44] | 2.29E-05 |
| Cutaneous lupus erythematosus | 161 | 47160 | 1.79 [1.54, 2.08] | 1.31E-11 | 120 | 18302 | 2.02 [1.71, 2.40] | 5.12E-15 |
| Rheumatoid arthritis (RA) | 1623 | 48410 | 1.21 [1.15, 1.27] | 5.07E-11 | 1262 | 21595 | 1.20 [1.13, 1.26] | 4.73E-09 |
| Chronic hepatitis | 285 | 46822 | 1.53 [1.37, 1.72] | 5.41E-11 | 140 | 18968 | 1.11 [0.94, 1.31] | 0.251 |
| RA and other inflammatory polyarthropathies | 2024 | 48385 | 1.19 [1.13, 1.24] | 5.84E-11 | 1531 | 21594 | 1.18 [1.12, 1.25] | 1.33 E-09 |
| Systemic sclerosis | 269 | 47798 | 1.51 [1.34, 1.70] | 1.81E-09 | 313 | 17908 | 1.35 [1,20, 1.50] | 9.72E-07 |
| Celiac disease | 242 | 37413 | 1.53 [1.35, 1.73] | 3.69E-09 | 179 | 13860 | 1.31 [1.13, 1.51] | 5.29E-04 |
| Hypothyroidism NOS | 7095 | 44977 | 1.09 [1.06, 1.12] | 8.63E-08 | 5489 | 19992 | 1.07 [1.04, 1.10] | 5.73E-05 |
| Hypothyroidism | 6807 | 44975 | 1.09 [1.06, 1.12] | 2.43E-07 | 5229 | 20012 | 1.07 [1.04, 1.10] | 7.40E-05 |
| Nephritis and nephropathy classified elsewhere | 327 | 42085 | 1.39 [1.24, 1.55] | 3.62E-07 | 554 | 18075 | 1.17 [1.07, 1.27] | 6.46E-04 |
| Type 1 diabetes with renal manifestations | 276 | 38522 | 1.41 [1.26, 1.59] | 8.16E-07 | 165 | 17977 | 1.38 [1.19, 1.60] | 7.28E-05 |
| Other immunological findings | 768 | 51528 | 1.22 [1.14, 1.31] | 4.43E-06 | 437 | 26572 | 1.10 [1.01, 1.21] | 0.062 |
| Other specified diffuse diseases of connective tissue | 241 | 47543 | 1.42 [1.25, 1.61] | 6.28E-06 | 72 | 17792 | 1.21 [0.97, 1.53] | 0.131 |
| Unspecified diffuse connective tissue disease | 149 | 46663 | 1.47 [1.25, 1.73] | 1.84E-04 | 267 | 17937 | 1.30 [1.16, 1.47] | 5.59E-05 |
| Primary biliary cirrhosis | 230 | 42393 | 1.34 [1.18, 1.53] | 5.85E-04 | 73 | 20749 | 1.14 [0.90, 1.43] | 0.293 |
| Type 1 diabetes | 1881 | 38647 | 1.11 [1.06, 1.17] | 6.43E-04 | 1156 | 18035 | 1.11 [1.05, 1.18] | 9.55E-04 |
| Nephritis; nephrosis; renal sclerosis | 836 | 42105 | 1.17 [1.09, 1.26] | 6.66E-04 | 1263 | 18185 | 1.13 [1.06, 1.19] | 1.10E-04 |
| Raynaud's syndrome | 438 | 46322 | 1.24 [1.13, 1.36] | 6.72E-04 | 687 | 17499 | 1.17 [1.09, 1.27] | 1.10E-04 |
| Renal failure NOS | 834 | 42085 | 1.17 [1.09, 1.25] | 8.92E-04 | 824 | 18003 | 1.06 [0.99, 1.13] | 0.156 |
| Nephritis & nephropathy without glomerulonephritis | 471 | 42066 | 1.22 [1.12, 1.34] | 9.73E-04 | 851 | 18186 | 1.12 [1.04, 1.20] | 0.003 |
| Chronic lymphocytic thyroiditis | 592 | 44562 | 1.20 [1.10, 1.30] | 9.73E-04 | 437 | 20049 | 1.09 [0.99, 1.29] | 0.128 |
| Skin cancer | 4321 | 46382 | 0.93 [0.90, 0.96] | 9.73E-04 | 4570 | 20651 | 0.98[0.94, 1.01] | 0.188 |
| Type 1 diabetes with ophthalmic manifestations | 240 | 38262 | 1.32 [1.16, 1.50] | 1.18E-03 | 230 | 18024 | 1.34 [1.18, 1.52] | 2.85E-05 |
| Graves' disease | 425 | 44697 | 1.21 [1.10, 1.34] | 4.42E-03 | 293 | 19949 | 1.26 [1.12, 1.41] | 2.02E-04 |
| Thyroiditis | 710 | 44885 | 1.16 [1.08, 1.25] | 5.57E-03 | 483 | 20027 | 1.07 [0.98, 1.18] | 0.160 |
| Melanomas of skin, diagnosed or personal history | 1463 | 46873 | 0.91 [0.86, 0.96] | 0.020 | 783 | 21163 | 1.01 [0.94, 1.09] | 0.790 |
| Vitamin deficiency | 4596 | 41765 | 1.06 [1.02, 1.09] | 0.024 | 3359 | 20746 | 1.02 [1.99, 1.06] | 0.268 |
| Melanomas of skin | 1226 | 47151 | 0.90 [0.85, 0.96] | 0.025 | 666 | 21175 | 1.03 [0.95, 1.11] | 0.469 |
| Other non-epithelial cancer of skin | 3968 | 44957 | 0.94 [0.91, 0.97] | 0.025 | 4428 | 20232 | 0.96 [0.93, 1.00] | 0.064 |
| Anemia in chronic kidney disease | 1136 | 36565 | 1.11 [1.05, 1.18] | 0.025 | 915 | 16227 | 1.07 [1.00, 1.15] | 0.067 |
| Glomerulonephritis | 222 | 41878 | 1.26 [1.10, 1.44] | 0.025 | 510 | 18124 | 1.17 [1.07, 1.28] | 8.03E-04 |
| Osteoarthrosis | 8248 | 41162 | 1.04 [1.02, 1.07] | 0.029 | 11092 | 13738 | 0.99 [0.97, 1.02] | 0.668 |
| Chronic airway obstruction | 4931 | 44980 | 1.05 [1.02,1.09] | 0.034 | 3843 | 17874 | 1.03 [0.99, 1.06] | 0.188 |
| Primary hypercoagulable state | 426 | 43779 | 1.18 [1.07, 1.30] | 0.036 | 466 | 21377 | 1.14 [1.04, 1.25] | 0.010 |
| Diabetic retinopathy | 790 | 51138 | 1.13 [1.05, 1.21] | 0.039 | 913 | 20135 | 1.11 [1.04, 1.18] | 0.006 |
| Type 1 diabetes with neurological manifestations | 475 | 38597 | 1.16 [1.06, 1.28] | 0.044 | 218 | 17911 | 1.13 [0.99, 1.29] | 0.114 |
| Other retinal disorders | 1727 | 51681 | 1.08 [1.03, 1.14] | 0.045 | 3987 | 19275 | 1.04 [1.00, 1.08] | 0.059 |
| Chronic thyroiditis | 411 | 44294 | 1.17 [1.06, 1.30] | 0.050 | 369 | 19996 | 1.08 [0.97, 1.20] | 0.188 |
OR (95%CI): Odds ratio (95% confidence interval); FDR: False discovery rate (significant associations FDR q<0.05)
Figure 1:
Clinical diagnoses associated with a weighted genetic risk score (wGRS) for SLE in individuals of European ancestry in BioVU. Green triangles represent significant associations at FDR q<0.05. Black dots represent non-significant associations. Table 2 shows the complete list of significant associations arranged by FDR
When patients with SLE were excluded from the PheWAS analysis in BioVU, most of the autoimmune phenotypes (e.g.: rheumatoid arthritis-related phenotypes, sicca syndrome, celiac disease, systemic sclerosis, autoimmune thyroiditis-related phenotypes, and T1D related phenotypes among others), renal failure, and skin neoplasms remained significantly associated with the wGRS (all FDR q<0.05, Supplementary Table S6); but when we additionally excluded patients with other common autoimmune diseases from the analysis none of the phenotypes were associated with the wGRS for SLE (FDR>0.05). (Supplementary Table S7)
Inverse variance weighted regression meta-analyses
Genetic predisposition to SLE was not significantly associated with any of the pre-selected outcomes (all P>0.0045, Table 3) using the IVWR method. Nominal associations were observed for T1D and LDL cholesterol, with a positive association for T1D (estimate= 0.249, P=0.048), and a negative association for LDL cholesterol (estimate = −0.015, P=0.018). Although the MR-Egger analysis did not show evidence of horizontal pleiotropy (Egger intercept p-value >0.05) for both phenotypes (Supplementary Table S8), we observed that rs2476601 was the SLE-associated SNP with the strongest association with T1D (effect size= 0.636, P-value=1.10x10−122), and that exclusion of this SNP from the IVWR attenuated the association with T1D (P=0.093).
Table 3:
Association between genetic predictors for SLE and genetic predictors of selected cardiometabolic phenotypes in the IVWR
| Cardiometabolic phenotypes | #SNPs | Estimate | [95%CI] | P-value |
|---|---|---|---|---|
| Atrial fibrillation | 30 | 0.006 | [−0.007, 0.019] | 0.381 |
| Ischemic stroke | 30 | 0.009 | [−0.010, 0.029] | 0.342 |
| Coronary atherosclerosis | 30 | 0.021 | [−0.004, 0.046] | 0.096* |
| Type 2 diabetes | 30 | 0.027 | [−0.002, 0.056] | 0.070* |
| Systolic blood pressure | 30 | 0.034 | [−0.101, 0.170] | 0.620* |
| Chronic renal failure | 30 | 0.014 | [−0.006, 0.035] | 0.171* |
| Waist circumference | 27 | 0.003 | [−0.007, 0.013] | 0.598* |
| Type 1 diabetes | 18 | 0.249 | [0.002, 0.496] | 0.048* |
| LDL cholesterol | 26 | −0.015 | [−0.027, −0.003] | 0.018* |
| C-reactive protein | 30 | 0.004 | [−0.007, 0.014] | 0.467* |
| Interleukin 6 | 30 | 0.023 | [−0.002, 0.049] | 0.111 |
estimates represent change in risk for the outcome per unit of change in the exposure
heterogeneity P-value <0.05
DISCUSSION
The main finding of this study was that a genetic predisposition to SLE based on common SNPs is not associated with an increased risk of cardiometabolic phenotypes but is associated with increased risk of other autoimmune disorders. In a similar study in RA, we found that genetic predisposition to RA was not associated with an increased risk of cardiometabolic phenotypes but was associated with increased risk for T1D (8).
The finding that genetic susceptibility to SLE is associated with increased risk of other autoimmune diseases in the PheWAS is not surprising, since autoimmune diseases share clinical and immunological characteristics as well as risk susceptibility loci (22). For example, a cross phenotype meta-analysis found that 44% of risk alleles were shared across seven common autoimmune diseases (SLE, T1D, RA, multiple sclerosis, psoriasis, Crohn’s and coeliac disease) although not across all autoimmune disorders (23). The same study found that risk variants that are common to a subset of autoimmune diseases aggregate in discrete pathways such as the tumor necrosis factor (TNF) pathway for shared SNPs in RA and SLE (23). Another study reported only a modest genetic overlap between SLE and 17 common autoimmune diseases with no apparent association between several individual SLE risk loci with these autoimmune diseases (24). In our study, we estimated the aggregated the effect of individual SNPs using a wGRS and found that the GRS is associated with modest increases in risk for several autoimmune diseases.
Because SLE is a heterogenous disease, we also performed a PheWAS that excluded patients with SLE to determine if the associations with autoimmune disorders were independent SLE and found that most of the autoimmune phenotypes remained significantly associated with the wGRS for SLE, supporting the hypothesis of shared immunogenetic mechanisms among autoimmune diseases.
Although several established SLE risk loci have been associated with susceptibility for our pre-selected cardiometabolic phenotypes (e.g. cardiac arrythmias with BANK1 (25); CAD with FCGR2A (26), TNFSF4 (27), IL10 (28), WDFY4 (29), and SH2B3 (30); HTN with TNFSF4 (31), NCF2 (32), and SH2B3 (33); obesity with IL10 (34); T2D with JAZF1 (35); and T1D with TYK2 (36), IFIH1 (37), IRF7 (38), SOCS1(39), IKZF1 (40), TNFAIP3 (41), and SH2B3 (39)), to our knowledge this is the first study that examined the genetic sharing between SLE and cardiometabolic comorbidities that are prevalent among individuals with SLE. Consistent with our findings in the PheWAS analysis, the IVWR analysis did not show significant associations between genetic liability for SLE with the selected cardiometabolic phenotypes, which suggests that genetic liability for SLE is not associated with these disorders. However, we did not examine subpopulations of SLE, and we only studied EA individuals (42).
Previous studies have focused on the identification of risk alleles that may increase the risk of sub-phenotypes of SLE, mainly cardiovascular (CVD) and renal disease (43, 44). The largest study for CVD performed in SLE patients of EA (2088 SLE patients) found that variants at two loci, IL19 and SRP54-AS, were associated with increased risk of stroke and myocardial infarction in patients with SLE (45). Interestingly, none of these loci have been associated with SLE susceptibility or CVD risk in the general population, suggesting a different mechanism for CVD in SLE (45). Likewise, a cross-phenotype meta-analysis of 6 common autoimmune diseases (including SLE) found no association between CVD risk and any SLE risk loci. However, the same study identified 8 genetic clusters strongly associated with CVD in SLE, two of which were enriched for genes in the TNFα and INFγ response, suggesting that genetic variations in these immune pathways could contributed to the increased risk of CVD in SLE (46).
Genetic studies of kidney disorders in SLE have focused on defining the genetic basis lupus nephritis (LN) and have shown that some, but not all, established SLE risk loci are also associated with LN (44). More recent studies have identified genes that are specifically associated with LN (but not with SLE susceptibility), which suggests that genetic liability for LN is a combination of general SLE risk genes and disease specific genes (44). In our study, lupus patients with nephropathy had a higher wGRS than those without nephropathy and the wGRS for SLE was associated with renal phenotypes only when patients with SLE were included in the PheWAS analysis, suggesting that renal disorders were common complications in SLE patients and associated with the wGRS for SLE, which has been previously described (47, 48). Concordant with that interpretation, a genetic predisposition to SLE was not associated with CKD in the IVWR analysis.
The observed association between the wGRS for SLE and T1D-related phenotypes in BioVU and eMERGE, along with the nominal association in the IVWR, suggest shared genetic risk between these phenotypes. Genetic studies have not only shown that SLE and T1D share risk loci (IRF7 (38), SOCS1 (39), IKZF1 (40), TNFAIP3 (41), IL10 (24), TCF7 (49), and BANK1(50)), but they also have common risk alleles (e.g.: rs2476601 in PTPN22, rs2304256 in TYK2 (36), rs2111485 in IFIH1(51), and rs1801274 in FCGR2A (52)) or risk alleles in close LD (e.g. rs10774625 with rs3184504 in SH2B3 (39), rs11889341 with rs7574865 in STAT4 (41), rs12785878 with rs3794060 in DHRC7 (53)). Also, a study using hierarchical clustering of 47 pleiotropic SNPs across different autoimmune diseases (including SLE and T1D) found that both phenotypes shared cluster patterns that represent distinct molecular mechanisms affected by these variants (23).
Our study has limitations. First, the findings may not generalize to all patients but rather to those of European ancestry seeking care at a tertiary care hospital. Second, because billing codes were aggregated to assemble clinical phenotypes into phecodes and the quality of case-control discrimination varies across phenotypes, there is potential misclassification bias, which can bias the results towards the null, resulting in false negative associations. Third, many unmeasured factors (e.g., diet, smoking, exercise, medications, and other interventions) may modulate the risk for some of the phenotypes examined in the PheWAS and thus obscure a genotype-phenotype relationship. However, the consistency of the findings between the BioVU and the eMERGE populations support the validity of the findings.
In conclusion, we found that a weighted GRS for SLE was associated with an increased risk of several autoimmune-related phenotypes but not with cardiometabolic disorders.
Supplementary Material
ACKNOWLEDGMENTS
The authors have no conflict of interest to report. The study was supported by American Heart Association (AHA) grant 18SFRN34230089. The dataset(s) used for the analyses described were obtained from Vanderbilt University Medical Center’s BioVU which is supported by numerous sources: institutional funding, private agencies, and federal grants. These include the NIH funded Shared Instrumentation Grant S10RR025141; and CTSA grants UL1TR002243, UL1TR000445, and UL1RR024975. Genomic data are also supported by investigator-led projects that include U01HG004798, R01NS032830, RC2GM092618, P50GM115305, U01HG006378, U19HL065962, R01HD074711; and additional funding sources listed at https://victr.vanderbilt.edu/pub/biovu/. VKK was supported by K23GM117395, R01AR076516, and the Arthritis National Research Foundation – All Arthritis Grant Program Award. JDM is funded by the AHA 16FTF30130005 and R01GM130791. QF is funded by R01GM120523, and CPC by R01AR073764. The eMERGE Network was initiated and funded by NHGRI through the following grants for phase II: U01HG006828 (Cincinnati Children’s Hospital Medical Center/Boston Children’s Hospital); U01HG006830 (Children’s Hospital of Philadelphia); U01HG006389 (Essentia Institute of Rural Health, Marshfield Clinic Research Foundation and Pennsylvania State University); U01HG006382 (Geisinger Clinic); U01HG006375 (Group Health Cooperative/University of Washington); U01HG006379 (Mayo Clinic); U01HG006380 (Icahn School of Medicine at Mount Sinai); U01HG006388 (Northwestern University); U01HG006378 (Vanderbilt University Medical Center); U01HG006385 (Vanderbilt University Medical Center serving as the Coordinating Center); U01HG004438 (CIDR) and U01HG004424 (the Broad Institute) serving as Genotyping Centers. For phase I: U01-HG-004610 (Group Health Cooperative/University of Washington); U01-HG-004608 (Marshfield Clinic Research Foundation and Vanderbilt University Medical Center); U01-HG-04599 (Mayo Clinic); U01HG004609 (Northwestern University); U01-HG-04603 (Vanderbilt University Medical Center, also serving as the Administrative Coordinating Center); U01HG004438 (CIDR) and U01HG004424 (the Broad Institute) serving as Genotyping Centers.
We acknowledge the following Consortiums/studies for summary statistics: (1) on SLE from Bentham et al, in Nature Genetics (2015) publication and available in the GWAS catalog; (2) on AF contributed by AFGen Consortium investigators and available in the GWAS catalog; (3) on IS contributed by MEGASTROKE Consortium project, which received funding from sources specified at http://www.megastroke.org/acknowledgements.html and a list of MEGASTROKE Consortium investigators are available at http://www.megastroke.org/authors.html; (4) on CAD contributed by CARDIoGRAMplusC4D investigators and available at www.CARDIOGRAMPLUSC4D.ORG; (5) on CKD contributed by CKDGEN Consortium and available at https://ckdgen.imbi.uni-freiburg.de/; (6) for LDL cholesterol contributed by the GLGC and available at http://csg.sph.umich.edu/willer/public/lipids2013/; (7) for waist circumference contributed by the GIANT consortium and available at https://portals.broadinstitute.org/collaboration/giant/index.php/GIANT_consortium_data_files; (8) for SBP from Evangelou et al, in Nat Genet (2018) publication and available in the GWAS catalog; (9) for T1D contributed by the T1D Genetics Consortium, a collaborative clinical study sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institute of Allergy and Infectious Diseases (NIAID), National Human Genome Research Institute (NHGRI), National Institute of Child Health and Human Development (NICHD), and Juvenile Diabetes Research Foundation International (JDRF); (10) for T2D contributed by the DIAGRAM Consortium and available at http://diagram-consortium.org/downloads.html; (11) for C-reactive protein from Ligthart et al, in Am J Hum Genetics (2018) publication and available by contacting the investigator (s.ligthart@erasmusmc.nl); (12) for interleukin 6 from Ahola-Olli et al, in Am J Hum Genetics (2017) publication and available at http://computationalmedicine.fi/data#Cytokine_GWAS.
References
- 1.Bentham J, Morris DL, Graham DSC, Pinder CL, Tombleson P, Behrens TW, et al. Genetic association analyses implicate aberrant regulation of innate and adaptive immunity genes in the pathogenesis of systemic lupus erythematosus. Nat Genet. 2015;47(12):1457–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asanuma Y, Oeser A, Shintani AK, Turner E, Olsen N, Fazio S, et al. Premature coronary-artery atherosclerosis in systemic lupus erythematosus. N Engl J Med. 2003;349(25):2407–15. [DOI] [PubMed] [Google Scholar]
- 3.Chen SK, Barbhaiya M, Solomon DH, Guan H, Yoshida K, Feldman CH, et al. Atrial Fibrillation/Flutter Hospitalizations among U.S. Medicaid Recipients with and without Systemic Lupus Erythematosus. J Rheumatol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung CP, Oeser A, Solus JF, Avalos I, Gebretsadik T, Shintani A, et al. Prevalence of the metabolic syndrome is increased in rheumatoid arthritis and is associated with coronary atherosclerosis. Atherosclerosis. 2008;196(2):756–63. [DOI] [PubMed] [Google Scholar]
- 5.Bruce IN, Urowitz MB, Gladman DD, Ibanez D, Steiner G. Risk factors for coronary heart disease in women with systemic lupus erythematosus: the Toronto Risk Factor Study. Arthritis Rheum. 2003;48(11):3159–67. [DOI] [PubMed] [Google Scholar]
- 6.Gandelman JS, Khan OA, Shuey MM, Neal JE, McNeer E, Dickson A, et al. Increased Incidence of Resistant Hypertension in Patients with Systemic Lupus Erythematosus: A Retrospective Cohort Study. Arthritis Care Res (Hoboken). 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mageau A, Timsit JF, Perrozziello A, Ruckly S, Dupuis C, Bouadma L, et al. The burden of chronic kidney disease in systemic lupus erythematosus: A nationwide epidemiologic study. Autoimmun Rev. 2019;18(7):733–7. [DOI] [PubMed] [Google Scholar]
- 8.Kawai VK, Shi M, Feng Q, Chung CP, Liu G, Cox NJ, et al. Pleiotropy in the Genetic Predisposition to Rheumatoid Arthritis - a phenome-wide association study and inverse-variance weighted meta-analysis. Arthritis Rheumatol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roden DM, Pulley JM, Basford MA, Bernard GR, Clayton EW, Balser JR, et al. Development of a large-scale de-identified DNA biobank to enable personalized medicine. Clin Pharmacol Ther. 2008;84(3):362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCarty CA, Chisholm RL, Chute CG, Kullo IJ, Jarvik GP, Larson EB, et al. The eMERGE Network: a consortium of biorepositories linked to electronic medical records data for conducting genomic studies. BMC Med Genomics. 2011;4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mosley JD, Shoemaker MB, Wells QS, Darbar D, Shaffer CM, Edwards TL, et al. Investigating the Genetic Architecture of the PR Interval Using Clinical Phenotypes. Circ Cardiovasc Genet 2017;10(2):e001482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Held C, White HD, Stewart RAH, Budaj A, Cannon CP, Hochman JS, et al. Inflammatory Biomarkers Interleukin-6 and C-Reactive Protein and Outcomes in Stable Coronary Heart Disease: Experiences From the STABILITY (Stabilization of Atherosclerotic Plaque by Initiation of Darapladib Therapy) Trial. J Am Heart Assoc. 2017;6(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zuvich RL, Armstrong LL, Bielinski SJ, Bradford Y, Carlson CS, Crawford DC, et al. Pitfalls of merging GWAS data: lessons learned in the eMERGE network and quality control procedures to maintain high data quality. Genet Epidemiol. 2011;35(8):887–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, Lee JJ. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience. 2015;4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delaneau O, Zagury JF, Marchini J. Improved whole-chromosome phasing for disease and population genetic studies. Nat Methods. 2013;10(1):5–6. [DOI] [PubMed] [Google Scholar]
- 16.Howie B, Fuchsberger C, Stephens M, Marchini J, Abecasis GR. Fast and accurate genotype imputation in genome-wide association studies through pre-phasing. Nat Genet. 2012;44(8):955–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng X, Levine D, Shen J, Gogarten SM, Laurie C, Weir BS. A high-performance computing toolset for relatedness and principal component analysis of SNP data. Bioinformatics. 2012;28(24):3326–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu P, Gifford A, Meng X, Li X, Campbell H, Varley T, et al. Mapping ICD-10 and ICD-10-CM Codes to Phecodes: Workflow Development and Initial Evaluation. JMIR Med Inform. 2019;7(4):e14325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pelletier EM, Ogale S, Yu E, Brunetta P, Garg J. Economic outcomes in patients diagnosed with systemic lupus erythematosus with versus without nephritis: results from an analysis of data from a US claims database. Clin Ther. 2009;31(11):2653–64. [DOI] [PubMed] [Google Scholar]
- 20.Carroll RJ, Bastarache L, Denny JC. R PheWAS: data analysis and plotting tools for phenome-wide association studies in the R environment. Bioinformatics. 2014;30(16):2375–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burgess S, Bowden J, Fall T, Ingelsson E, Thompson SG. Sensitivity Analyses for Robust Causal Inference from Mendelian Randomization Analyses with Multiple Genetic Variants. Epidemiology. 2017;28(1):30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barturen G, Beretta L, Cervera R, Van Vollenhoven R, Alarcon-Riquelme ME. Moving towards a molecular taxonomy of autoimmune rheumatic diseases. Nat Rev Rheumatol. 2018;14(2):75–93. [DOI] [PubMed] [Google Scholar]
- 23.Cotsapas C, Voight BF, Rossin E, Lage K, Neale BM, Wallace C, et al. Pervasive sharing of genetic effects in autoimmune disease. PLoS Genet. 2011;7(8):e1002254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramos PS, Criswell LA, Moser KL, Comeau ME, Williams AH, Pajewski NM, et al. A comprehensive analysis of shared loci between systemic lupus erythematosus (SLE) and sixteen autoimmune diseases reveals limited genetic overlap. PLoS Genet. 2011;7(12):e1002406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohler PJ, Schott JJ, Gramolini AO, Dilly KW, Guatimosim S, duBell WH, et al. Ankyrin-B mutation causes type 4 long-QT cardiac arrhythmia and sudden cardiac death. Nature. 2003;421(6923):634–9. [DOI] [PubMed] [Google Scholar]
- 26.Kroupis C, Theodorou M, Chaidaroglou A, Dalamaga M, Oliveira SC, Cokkinos DV, et al. The association between a common FCGR2A polymorphism and C-reactive protein and coronary artery disease revisited. Genet Test Mol Biomarkers. 2010;14(6):839–46. [DOI] [PubMed] [Google Scholar]
- 27.Ria M, Lagercrantz J, Samnegard A, Boquist S, Hamsten A, Eriksson P. A common polymorphism in the promoter region of the TNFSF4 gene is associated with lower allele-specific expression and risk of myocardial infarction. PLoS One. 2011;6(3):e17652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heiskanen M, Kahonen M, Hurme M, Lehtimaki T, Mononen N, Juonala M, et al. Polymorphism in the IL10 promoter region and early markers of atherosclerosis: the Cardiovascular Risk in Young Finns Study. Atherosclerosis. 2010;208(1):190–6. [DOI] [PubMed] [Google Scholar]
- 29.Carty CL, Keene KL, Cheng YC, Meschia JF, Chen WM, Nalls M, et al. Meta-Analysis of Genome-Wide Association Studies Identifies Genetic Risk Factors for Stroke in African Americans. Stroke. 2015;46(8):2063–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gudbjartsson DF, Bjornsdottir US, Halapi E, Helgadottir A, Sulem P, Jonsdottir GM, et al. Sequence variants affecting eosinophil numbers associate with asthma and myocardial infarction. Nat Genet. 2009;41(3):342–7. [DOI] [PubMed] [Google Scholar]
- 31.Mashimo Y, Suzuki Y, Hatori K, Tabara Y, Miki T, Tokunaga K, et al. Association of TNFRSF4 gene polymorphisms with essential hypertension. J Hypertens. 2008;26(5):902–13. [DOI] [PubMed] [Google Scholar]
- 32.Li H, Han X, Hu Z, Huang J, Chen J, Hixson JE, et al. Associations of NADPH oxidase-related genes with blood pressure changes and incident hypertension: The GenSalt Study. J Hum Hypertens. 2018;32(4):287–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levy D, Ehret GB, Rice K, Verwoert GC, Launer LJ, Dehghan A, et al. Genome-wide association study of blood pressure and hypertension. Nat Genet. 2009;41(6):677–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scarpelli D, Cardellini M, Andreozzi F, Laratta E, Hribal ML, Marini MA, et al. Variants of the interleukin-10 promoter gene are associated with obesity and insulin resistance but not type 2 diabetes in caucasian italian subjects. Diabetes. 2006;55(5):1529–33. [DOI] [PubMed] [Google Scholar]
- 35.Zhou T, Hu Z, Yang S, Sun L, Yu Z, Wang G. Role of Adaptive and Innate Immunity in Type 2 Diabetes Mellitus. J Diabetes Res. 2018;2018:7457269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagafuchi S, Kamada-Hibio Y, Hirakawa K, Tsutsu N, Minami M, Okada A, et al. TYK2 Promoter Variant and Diabetes Mellitus in the Japanese. EBioMedicine. 2015;2(7):744–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nejentsev S, Walker N, Riches D, Egholm M, Todd JA. Rare variants of IFIH1, a gene implicated in antiviral responses, protect against type 1 diabetes. Science. 2009;324(5925):387–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heinig M, Petretto E, Wallace C, Bottolo L, Rotival M, Lu H, et al. A trans-acting locus regulates an anti-viral expression network and type 1 diabetes risk. Nature. 2010;467(7314):460–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Todd JA, Walker NM, Cooper JD, Smyth DJ, Downes K, Plagnol V, et al. Robust associations of four new chromosome regions from genome-wide analyses of type 1 diabetes. Nat Genet. 2007;39(7):857–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Swafford AD, Howson JM, Davison LJ, Wallace C, Smyth DJ, Schuilenburg H, et al. An allele of IKZF1 (Ikaros) conferring susceptibility to childhood acute lymphoblastic leukemia protects against type 1 diabetes. Diabetes. 2011;60(3):1041–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fung EY, Smyth DJ, Howson JM, Cooper JD, Walker NM, Stevens H, et al. Analysis of 17 autoimmune disease-associated variants in type 1 diabetes identifies 6q23/TNFAIP3 as a susceptibility locus. Genes Immun. 2009;10(2):188–91. [DOI] [PubMed] [Google Scholar]
- 42.Fernandez-Rhodes L, Young KL, Lilly AG, Raffield LM, Highland HM, Wojcik GL, et al. Importance of Genetic Studies of Cardiometabolic Disease in Diverse Populations. Circ Res. 2020;126(12):1816–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Svenungsson E, Gustafsson J, Leonard D, Sandling J, Gunnarsson I, Nordmark G, et al. A STAT4 risk allele is associated with ischaemic cerebrovascular events and anti-phospholipid antibodies in systemic lupus erythematosus. Ann Rheum Dis. 2010;69(5):834–40. [DOI] [PubMed] [Google Scholar]
- 44.Iwamoto T, Niewold TB. Genetics of human lupus nephritis. Clin Immunol. 2017;185:32–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leonard D, Svenungsson E, Dahlqvist J, Alexsson A, Arlestig L, Taylor KE, et al. Novel gene variants associated with cardiovascular disease in systemic lupus erythematosus and rheumatoid arthritis. Ann Rheum Dis. 2018;77(7):1063–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perrotti PP, Aterido A, Fernandez-Nebro A, Canete JD, Ferrandiz C, Tornero J, et al. Genetic variation associated with cardiovascular risk in autoimmune diseases. PLoS One. 2017;12(10):e0185889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen L, Wang YF, Liu L, Bielowka A, Ahmed R, Zhang H, et al. Genome-wide assessment of genetic risk for systemic lupus erythematosus and disease severity. Hum Mol Genet. 2020;29(10):1745–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reid S, Alexsson A, Frodlund M, Morris D, Sandling JK, Bolin K, et al. High genetic risk score is associated with early disease onset, damage accrual and decreased survival in systemic lupus erythematosus. Ann Rheum Dis. 2020;79(3):363–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Erlich HA, Valdes AM, Julier C, Mirel D, Noble JA, Type IDGC. Evidence for association of the TCF7 locus with type I diabetes. Genes Immun. 2009;10 Suppl 1:S54–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zouidi F, Stayoussef M, Bouzid D, Fourati H, Abida O, Joao C, et al. Association of BANK1 and cytokine gene polymorphisms with type 1 diabetes in Tunisia. Gene. 2014;536(2):296–301. [DOI] [PubMed] [Google Scholar]
- 51.Winkler C, Lauber C, Adler K, Grallert H, Illig T, Ziegler AG, et al. An interferon-induced helicase (IFIH1) gene polymorphism associates with different rates of progression from autoimmunity to type 1 diabetes. Diabetes. 2011;60(2):685–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alizadeh BZ, Valdigem G, Coenen MJ, Zhernakova A, Franke B, Monsuur A, et al. Association analysis of functional variants of the FcgRIIa and FcgRIIIa genes with type 1 diabetes, celiac disease and rheumatoid arthritis. Hum Mol Genet. 2007;16(21):2552–9. [DOI] [PubMed] [Google Scholar]
- 53.Cooper JD, Smyth DJ, Walker NM, Stevens H, Burren OS, Wallace C, et al. Inherited variation in vitamin D genes is associated with predisposition to autoimmune disease type 1 diabetes. Diabetes. 2011;60(5):1624–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.