Abstract
Bipolar spectrum disorders (BSDs) and substance use disorders (SUDs) are associated with neural reward dysfunction. However, it is unclear what pattern of neural reward function underlies pre-existing vulnerability to BSDs and SUDs, or whether neural reward function explains their high co-occurrence. The current paper provides an overview of the separate literatures on neural reward sensitivity in BSDs and SUDs. We provide a systematic review of 35 studies relevant to identifying neural reward function vulnerability to BSDs and SUDs. These studies include those examining neural reward processing on a monetary reward task with prospective designs predicting initial onset of SUDs, familial risk studies that examine unaffected offspring or first-degree relatives of family members with BSDs or SUDs, and studies that examine individuals with BSDs or SUDs who are not currently in an episode of the disorder. Findings from the review highlight that aberrant responding and connectivity across neural regions associated with reward and cognitive control confers risk for the development of BSDs and SUDs. Discussion focuses on limitations of the extant literature. We conclude with an integration and theoretical model for understanding how aberrant neural reward responding may constitute a vulnerability to the development of both BSDs and SUDs.
Keywords: reward processing, Monetary Incentive Delay, bipolar spectrum disorder, substance use disorder, risk
1. Introduction
Reward sensitivity, the level of one’s approach motivation and responsiveness towards goals and rewards, is associated with the onset and course of bipolar spectrum disorders (BSDs; (Alloy, Bender, et al., 2012; Alloy, Urošević, et al., 2012; Nusslock, Harmon-Jones, et al., 2012) and substance use disorders (SUDs; Alloy et al., 2009; Dawe et al., 2004; Dawe & Loxton, 2004). Thus, it is not surprising that these two disorders frequently co-occur (Conway et al., 2006); SUDs are present in over a third of people diagnosed with BSDs (Merikangas et al., 2011). Although BSDs generally are known to be associated with reward hypersensitivity (Alloy et al., 2015; Johnson et al., 2012; Nusslock & Alloy, 2017), there is debate regarding whether SUDs arise from reward hypo- or hypersensitivity (Nusslock & Alloy, 2017). Furthermore, few studies have examined reward processing as a trait level risk factor for these disorders. Identification of neural mechanisms that pre-exist and/or constitute a trait-level vulnerability would help inform our understanding of the onset and course of BSDs and SUDs, and may help clarify why they so highly co-occur.
There are several potential explanations for the high co-occurrence of BSDs and SUDs; 1) substance use (SU) occurs as part of the bipolar syndrome as individuals with BSDs engage in risky and impulsive behaviors, including SU, 2) SU may be an attempt by individuals with BSDs to self-medicate, 3) SU may cause BSDs, and 4) BSDs and SUDs share common risk factors (for review see Strakowski & DelBello, 2000). Relevant to this latter possibility, we examine the extent to which dysfunction in neural reward processing constitutes a risk factor for both BSDs and SUDs. Specifically, in one prospective study examining bipolar diagnosis and SU problems over a follow-up time period, self-reported reward sensitivity at baseline was associated with prospective BSDs and SU, and even partially explained the prospective comorbid relationship between BSDs and SUDs (Alloy et al., 2009). Although these associations were found using self-report measures of reward sensitivity, this adds to the theory that abnormalities in reward sensitivity comprise a common risk factor for both BSDs and SUDs. Identifying clinical features and personality characteristics that put individuals at risk for BSDs and/or SUDs using self-report and behavioral measures has been a common approach; however, this approach may be insufficient to fully understand risk profiles in individuals with BSDs and SUDs. Examining neural mechanisms not only would provide an important complement to other measures of risk, but identification of biomarkers also could provide important insights into possible pathophysiology of BSDs and SUDs and help inform novel targets for treatment and diagnosis. The current review investigates neural mechanisms as shared risk factors in BSDs and SUDs.
In this review, we examine the degree to which dysfunction in the neural reward circuit represents a pre-existing and/or trait-level vulnerability to BSDs and SUDs by reviewing literature that implements the following types of study designs: 1) truly prospective studies predicting initial onset of SUDs1, 2) familial risk studies that examine unaffected offspring or first-degree relatives of family members with a BSD or SUD, and 3) studies that examine neural reward processing in individuals with a BSD or SUD who are not currently in an episode of the disorder (e.g., remitted or euthymic). We conclude by offering a synthesis of the findings in both disorders, and present a potential integrated theoretical model of neural reward processing in BSDs and SUDs. Limitations of the extant literature and future directions also are discussed.
1.1. Neural Substrates of Reward Sensitivity
The fronto-striatal reward circuit is a vital component of the human brain. A range of brain regions form the reward circuit; these areas process both internal and external reward-related stimuli, predict the probability of future reward based on past events, and are associated with incentive salience, associative reward learning, and positively-valenced emotions, all of which contribute to the regulation of motivated and goal-directed behaviors (Haber & Knutson, 2010; Schultz & Dickinson, 2000). Two primary regions of this circuit are the ventral striatum (VS, which includes the nucleus accumbens or NAcc), and orbitofrontal cortex (OFC). The VS may be involved primarily in encoding and anticipating rewards (Dillon et al., 2008; Knutson, 2005), although some have found elevated VS activity in the presence of reward receipt (Seymour et al., 2007). The OFC primarily is implicated in assessing both the value and probability of reward receipt (McDannald et al., 2011). A meta-analysis of human neuroimaging studies confirmed the predominant roles of the VS and OFC in reward anticipation and reward consumption, respectively (Diekhof et al., 2012). Other regions that comprise this circuit and interact via dopaminergic pathways are the ventral tegmental area (VTA), substantia nigra, anterior cingulate cortex (ACC), amygdala, ventral pallidum, dorsal striatum (DS), raphe nuclei, lateral habenula nucleus, and more frontal regions of the prefrontal cortex including dorso- and ventrolateral regions (dlPFC/vlPFC; Haber & Knutson, 2010). Together, they form the fronto-striatal reward circuit, which is facilitated by dopamine transmission, and aids in reinforcement signaling and learning (reviewed in Haber & Knutson, 2010). Importantly, this circuit regulates anticipatory and consummatory reward processing to help drive motivation, goal-striving, and approach behavior in the presence of reward-related cues (Berridge & Robinson, 1998, 2003).
Researchers can study the reward circuit experimentally by observing brain responses during presentation (or omission) of reward stimuli. Secondary rewards are those that are not inherently valuable but are associated with pleasurable consequences, such as money, and often are used experimentally such as in the Monetary Incentive Delay (MID) task (for review see Lutz & Widmer, 2014). The MID presents the participant with an opportunity to either gain or lose rewards (e.g., $0.00, $0.50, $5.00) based on how quickly they respond to a target, and thus, can be used to study distinct phases of reward processing such as anticipation (period of the task when they are awaiting feedback of reward or loss) versus consumption (period of the task when they have received feedback of reward or loss; (Knutson et al., 2001). Another monetary reward task asks participants to guess whether the value of a card will be greater than or equal to, or less than 5, where correct guesses result in winning money on reward trials and avoiding losing money on loss trials (Delgado et al., 2000). This task also has differentiated neural activity to different phases of reward processing (e.g. anticipation vs. consumption) reliably in both healthy individuals and those with various psychopathologies (Forbes et al., 2009; Knutson et al., 2001). Thus, the MID and card guessing tasks are effective tools for capturing differences in reward processing among groups (e.g. BSDs and SUDs) with known abnormalities in neural reward processing.
1.2. Reward and Bipolar Spectrum Disorders
There is considerable theoretical and empirical support for a prominent role of dysfunction in the fronto-striatal reward circuit in BSDs. The Behavioral Approach System (BAS)/reward hypersensitivity model of BSDs posits that excessive activation or deactivation of the reward system results in the extreme mood and behavior swings that are characteristic of BSDs (for reviews see Alloy et al., 2015, 2016; Depue et al., 1987; Depue & Iacono, 1989; Johnson, 2005; Johnson et al., 2012). The reward system responds to certain triggering events (e.g., events related to excessive goal-striving or rewards that activate it, and events related to definite failures or losses that deactivate it) as well as internal (e.g. expectation of meeting a goal) or external (e.g., receiving an award) stimuli. Someone with a hypersensitive BAS/reward system would react to these stimuli more strongly than others. Reward hypersensitive individuals may experience strong positive emotions or engage in risky, yet pleasurable, behaviors when in a state of reward system activation. On the other hand, these same individuals may experience strong negative emotions and extreme anhedonia when in a state of reward system deactivation. Indeed, there is a large body of research that supports the reward hypersensitivity theory across multiple methods. People with BSDs have been shown consistently to have elevated self-reported reward sensitivity and reward-relevant personality traits (Alloy et al., 2008; Johnson et al., 2009, 2012; Meyer et al., 2001), behavioral tasks involving reward sensitivity (i.e., delayed gratification of rewards, increased positive affect after receiving rewards) differentiate people with BSDs or with manic symptoms from those without (Johnson et al., 2005; Swann et al., 2009), and reward hypersensitivity also is a key predictor of the initial onset and course of BSDs (for reviews see Alloy et al., 2015, 2016; Nusslock & Alloy, 2017).
There is additional support for the reward hypersensitivity model of BSDs in the neurobiological and neurophysiological literatures (for reviews see Alloy et al., 2015; Nusslock & Alloy, 2017; Phillips & Swartz, 2014). Elevated left frontal EEG activity has been linked to increased approach behavior and response bias towards reward-relevant stimuli, as well as greater self-reported reward sensitivity (Coan & Allen, 2004). Elevated left frontal EEG activity has been seen both in people who are prone to hypomania and people with a bipolar diagnosis (Harmon-Jones et al., 2002, 2008; Mason et al., 2012), and is associated with conversion to more severe forms of BSD (Nusslock, Harmon-Jones, et al., 2012). Structural MRI imaging has provided evidence for abnormalities in prefrontal and striatal volumes in people with or at-risk for BSDs (López-Larson et al., 2002; McDonald et al., 2004; Strakowski et al., 2002). Functional MRI (fMRI) studies using reward-based tasks also have demonstrated that consummatory and anticipatory reward processing abnormalities may be differentially associated with BPI versus BPII (Caseras et al., 2013). Additionally, mania and depression both have been associated with higher activation in reward regions (Abler et al., 2008; Bermpohl et al., 2010). In regard to functional connectivity, a recent qualitative review of the at-risk BSD literature provides support for altered fronto-limbic connectivity across studies of reward, emotion processing, resting-state, and affective cognition, and also highlights a central role of the vlPFC in these processes (Santos et al., 2017).
In parallel, the dopamine hypothesis of BSDs suggests that hyperdopaminergia, along with an elevated reward processing network, underlies the illness (Ashok et al., 2017). Functional imaging evidence supports the dopamine hypothesis, as elevated BOLD signals occur in the striatum and prefrontal cortex shortly after presentation of reward cues (Schott et al., 2008). These regions are rich in dopaminergic projections, and this BOLD activation is presumably related to dopamine transmission via projections from the VTA to the VS and prefrontal cortical areas (Schott et al., 2008). Although the dopamine hypothesis historically has focused on the manic phase of BSDs, new hypotheses suggest a differential role for increased striatal dopaminergic receptors in mania, and for increased striatal dopamine transporters during depression, providing support for hyperdopaminergia theory across mood states (for review see Ashok et al., 2017). However, this hypothesis does not fully explain dopamine functioning during remitted or euthymic phases of BSDs.
Although there is compelling evidence that BSDs are associated with elevated activity in the fronto-striatal reward circuit, conclusions regarding whether neural reward hypersensitivity is characteristic of BSDs are limited, and the literature on neural reward responses across different mood states of BSDs provides mixed support for this hypothesis. First, conflicting evidence exists regarding whether (hypo)manic individuals consistently have elevated activity during reward tasks, with some studies suggesting blunted striatal responses (Abler et al., 2008), elevated prefrontal responses (Bermpohl et al., 2010), and others reporting mixed findings of elevated and blunted responses in sub-cortical and prefrontal brain regions (O’Sullivan et al., 2011; Singh et al., 2013) relevant to processing of rewards. Similar conflicting results are found in studies of bipolar depression, where blunted VS responses to reward receipt (Redlich et al., 2015; Satterthwaite et al., 2015), mixed blunted and elevated frontal activations to reward anticipation (Chase et al., 2013), and elevated striatal responses in euthymic or mildly depressed BSD individuals (Berghorst et al., 2016) all have been reported. Even investigations in individuals who are not currently in a mood episode also have drawn conflicting conclusions, with support for reward hypersensitivity in striatal and prefrontal regions (Linke et al., 2012; Nusslock, Almeida, et al., 2012), blunted striatal responses (Trost et al., 2014), and heightened striatal but blunted prefrontal activity (Mason et al., 2014).
In the context of reward learning, this literature also has seen mixed results. In pediatric BSD studies, there is evidence for impaired reversal learning on probabilistic reinforcement tasks (Dickstein et al., 2010; Gorrindo et al., 2005), as well as in adolescent BSD studies (Urošević et al., 2018), whereas others have found no such deficits (Ernst et al., 2004). In the adult BSD literature, there is evidence that suggests heightened attention to variable rewards (Brambilla et al., 2013), delayed reward learning and influence of immediate positive feedback in euthymic individuals (Pizzagalli et al., 2008); however, others found no abnormalities in reversal learning (Lewandowski et al., 2016). Results from the studies described above note that individual differences in affective state and psychosis may contribute to performance differences on probabilistic reward learning tasks. Neuroimaging literature on reward learning in BSDs is even more scarce, however, evidence suggests deficits in probabilistic reinforcement learning are linked to aberrant dopamine signaling in the PFC (Urošević et al., 2018).
Most of the extant literature includes BSD samples that have been medicated with antidopaminergic medications. Dopamine transmission plays a key role in reward processes in the brain, and thus, medications that regulate this system may lead to lasting neural adaptations within the reward circuit. Thus, in order to draw conclusions regarding profiles of reward processing as pre-existing vulnerabilities to BSDs, there is a need to study reward processing using longitudinal approaches, prior to the onset of BSDs and before the effects of medication have set in. Additionally, examining these processes in euthymic or remitted individuals may also help in understanding if these processes are a mood-independent characteristic of BSDs.
1.3. Reward and Substance Use Disorders
Whereas most of the literature suggests that BSDs are associated with fronto-striatal hypersensitivity (Alloy et al., 2015, 2016; Nusslock & Alloy, 2017), the literature concerning reward function and SUDs is guided by two opposing theoretical models. The Reward Deficiency Model of addiction (Blum et al., 2000; Bowirrat & Oscar-Berman, 2005; Volkow et al., 2003) posits that all addictive drugs activate reward regions through increasing dopamine, but that once addicted, drugs trigger smaller increases in dopamine. This system becomes less stimulated by both drug and non-drug related cues (e.g., everyday stimuli). This effect also can be seen in neural circuits involved in emotion regulation (e.g., amygdala), as people try to cope with the negative emotions and dysphoria related to withdrawal by increasing approach behaviors towards drugs (e.g., increased substance-seeking; (Volkow et al., 2016). For example, people who have resumed cocaine use show significantly lower activation in the bilateral striatum across trials on a reward learning task, compared to their abstinent counterparts (Stewart et al., 2014). Additionally, findings from positron emission tomography (PET) studies fairly consistently demonstrate down-regulation of dopamine in people with substance addictions (for review see Volkow et al., 2003). Thus, according to the Reward Deficiency Model, substance-seeking behaviors arise from an individual’s attempts to compensate for the lack of recruitment in the reward circuit and inability to experience pleasure from rewards (Blum et al., 2000; Bowirrat & Oscar-Berman, 2005; Volkow et al., 2003).
On the other hand, the Reward Hypersensitivity Model postulates that people with high reward sensitivity engage in excessive approach behavior to attain rewards that can lead to risky behaviors with pleasurable consequences like SU (Alloy et al., 2009; Dawe et al., 2004; Dawe & Loxton, 2004; Kambouropoulos & Staiger, 2004). In line with this perspective, researchers have found that high reward sensitivity as assessed by self-report and behavioral measures predict SUDs, and distinguish between heavy and light drinkers (for review see Nusslock & Alloy, 2017). Additionally, the inability to delay gratification is associated with increased risk for addiction, and evidence from imaging studies suggests hyperactivity in the VS underlies a preference for immediate over delayed rewards (Hariri et al., 2006). Similar to neurophysiological findings in BSDs, there also is support for elevated left frontal EEG cortical activity in SUDs. This pattern was found in nicotine-dependent individuals when presented with a reward cue to smoke (Zinser et al., 1999). Substances of abuse are themselves rewarding, and studies examining neural response to drug cues consistently have demonstrated hyperactivation in the reward circuit (for review see Leyton & Vezina, 2013). Furthermore, drugs stimulate reward regions (e.g., VS), which over time become hypersensitized, leading to increased approach motivation towards substances (Baskin-Sommers & Foti, 2015; Di Chiara et al., 2004). This hyper-responsivity in the reward circuit may underlie a propensity to be motivated towards rewarding and pleasurable stimuli such as drugs (McClure, 2004).
Deficits in reward learning and decision-making also are present in individuals with SUDs (Bechara, 2003; De Bellis et al., 2013). Studies have found that individuals with SUDs will perseverate on once-rewarded stimuli and fail to adaptively learn new associations (Wilson et al., 2004). These reward learning deficits may be linked to abnormal activation in the PFC, and altered connectivity between the PFC and subcortical regions (Motzkin et al., 2014; Volkow et al., 2003). Deficits in reward learning neural circuitry also are associated with increased impulsive choices and delayed discounting, particularly in the presence of drug cues, and this is thought to reflect how salient drug cues seize attentional resources that then trigger lasting changes in these neural circuits (for review see Baskin-Sommers & Foti, 2015).
A possible explanation for these inconsistencies in the literature on reward and SUDs is that reward processing may be different depending on phase of addiction. For example, it is thought that attenuated responses in the VS and DS during monetary reward processing may be particularly prevalent during remission, whereas more robust responses in these regions reflect active addiction. However, current addiction phase did not seem to impact findings from a recent meta-analysis demonstrating that SUDs are associated with an overall blunted response during monetary reward processing (Luijten et al., 2017). Furthermore, evidence from the neuroimaging and neuropsychological literature suggests there are important underlying neural vulnerabilities (including smaller brain volumes and hyper responses in reward regions), that put adolescents with a family history of substance use at greater risk (Squeglia & Cservenka, 2017). Given possible predictive factors in at-risk youth, and the neural adaptations that substances of abuse create in the reward circuit, it is important to consider substance-naïve samples when addressing questions related to neural reward functioning and SUD risk (Hommer et al., 2011). Given the considerable research on underlying reward processes once addiction has set in, there is a substantial gap in our knowledge of how reward processing impacts the initial onset of SUDs, and thus, we still do not fully understand whether reward hyper- or hyposensitivity comprises a pre-existing risk factor for SUDs.
2. Methods
This review focused on functional activation in reward-related neural circuits during processing of monetary rewards during the MID or card-guessing tasks. We chose to focus on monetary rewards because they have been shown consistently to be dysregulated in individuals with SUDs and BSDs, and the MID and card-guessing tasks are reliable in analyzing discrete reward anticipation and consumption processes (Knutson et al., 2000; Lutz & Widmer, 2014). These tasks also were selected as the literature supports the predominant role of the anticipation phase of reward processing in both BSDs and SUDs, as well as in primary reward fronto-striatal regions’ involvement in processes related to incentive salience (i.e., NAcc; Cooper & Knutson, 2008; Zink et al., 2004). Additionally, reward anticipation is particularly relevant to the development of SUDs, because drug expectancy plays an important role in craving and attentional bias towards drug cues (Jedras et al., 2013). As the aim of the current review was to focus solely on whether reward circuit dysfunction during reward processing constitutes a vulnerability or risk factor for SUDs and BSDs, articles reporting on current in-episode samples were excluded. Given the small body of literature, we included a variety of study designs that could assess trait-level risk for SUDs and BSDs. Studies were longitudinal and prospective (e.g., using fMRI to prospectively predict first onset of SUD or BSD), or cross-sectional (e.g., remitted/euthymic samples compared to healthy controls or to in-episode individuals). Additionally, to expand our review of pre-existing reward-related risk factors, we also included studies that examined samples of “at-risk” individuals based on having a first-degree relative with either a SUD or BSD, but no current diagnosis themselves. Articles related to remitted/euthymic samples were excluded if they were part of a treatment trial as the treatment is considered a confounding variable. Additionally, in order for the remitted/euthymic samples to be included, any report of mood symptoms had to be in the non-clinical range. We searched for peer-reviewed journal articles through February 14, 2021 using the following search terms in PsycInfo and PubMed databases: (MRI OR fMRI OR imaging) and (reward OR reward processing OR reward sensitivity OR monetary reward OR MID OR monetary incentive delay OR card-guessing OR card guessing) AND (bipolar OR substance* OR drug* OR addiction) AND (predict OR longitudinal OR prospective OR follow up OR follow-up OR familial OR risk OR offspring OR onset OR euthymic OR vulnerability OR remitted OR remission). Additional articles were collected by manual searches of the reference sections of the retrieved articles.
3. Results
Figure 1 depicts a flow diagram of the number of studies identified for the current review, the number excluded and reasons for exclusion, as well as numbers included. The remaining 34 articles were read in full to determine their eligibility. There were no articles that used fMRI measures of monetary reward processing to prospectively predict to onset of BSDs. Thus, a total of 34 distinct articles met eligibility for this systematic review: 7 on familial risk for BSDs, 8 on euthymic BSDs, 9 prospective studies of SUDs, 8 on familial risk for SUDs, and 3 on remitted SUDs (see Appendix Tables 1, 2, and 3). Note that one article (Kollmann et al., 2017) included results from two separate studies on euthymic and familial risk samples, thus 35 separate studies were included in the review, from 34 separate articles.
Figure 1.
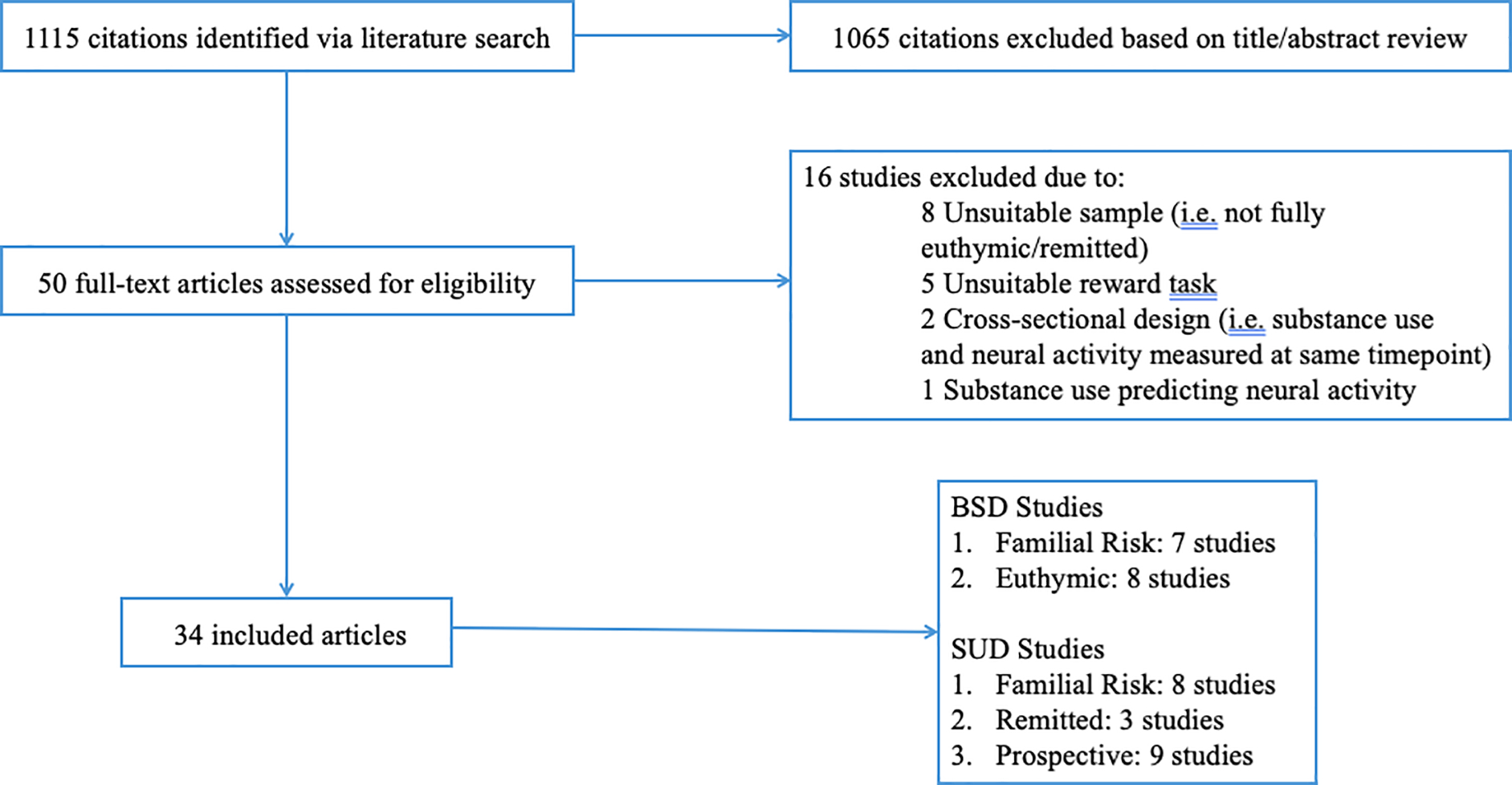
Flowchart of study selection.
Note. Thirty-four distinct articles were identified, consisting of 35 separate studies (Kollman et al., 2017 included results from two separate studies on familial risk and euthymic samples).
3.1. Neural Reward Dysfunction as a Familial Risk Factor for BSDs
The literature search revealed no prospective studies examining neural reward function as a predictor of first onset of BSDs/(hypo)mania, although one study using two samples at high genetic risk for BSDs attempted to identify and validate markers of future affective lability (Bertocci et al., 2019). Given the high genetic risk associated with BSDs, studies that utilize a high-risk design by examining neural reward function in unaffected offspring or first degree relatives of people with BSDs can inform our understanding of the neural reward dysfunction that may mediate genetic and/or familial risk for the development of BSDs. The literature search revealed seven studies that utilized functional MRI during the MID or card-guessing task to examine reward processing in offspring of individuals with BSDs.
Singh and colleagues (2014) examined activation and connectivity in the reward circuit during the MID in young offspring of at least one biological parent with BPI, compared to offspring with no first- or second-degree relative with Axis I psychopathology. Differential patterns emerged related to anticipation vs consumption phases. Specifically, compared to children of healthy parents, offspring of a BPI parent exhibited lower activation in medial cortical regions (e.g., pregenual cingulate cortex) during loss anticipation, as well as decreased connectivity between these regions and higher order cortical regions (e.g., vlPFC) during reward anticipation. During reward consumption, offspring of BP had greater activation in the OFC. Overall, high-risk children exhibited decreased activation in and connectivity between regions associated with reward-related decision-making during loss anticipation, and greater prefrontal (e.g., OFC) activation to reward consumption.
Results from three studies using the card-guessing task and drawing from the same high-risk sample (Acuff et al., 2019; Manelis et al., 2016; Soehner et al., 2016) provide conflicting results. High-risk offspring exhibited increased connectivity between striatal (e.g., VS) and prefrontal (e.g., vlPFC) regions to control conditions compared to reward or loss consumption (Manelis et al., 2016), decreased connectivity between striatal and prefrontal regions during loss, but increased connectivity to rewards (Acuff et al., 2019), and high-risk status (based on genetic and environmental factors) was associated with a more positive relationship between adverse events and activation in both striatal and prefrontal regions during reward processing (Hanford et al., 2019). An additional study utilizing the card-guessing paradigm revealed significantly greater connectivity between the striatum and prefrontal areas (e.g., insula and vlPFC) in unaffected offspring during reward consumption (Soehner et al., 2016).
Two final studies revealed no significant results relevant to reward processing in unaffected offspring. Kollman et al. (2017) found no significant differences in activation during reward or loss anticipation in the striatum (VS) or prefrontal areas (OFC, insula, ACC) between unaffected offspring and healthy controls. Bertocci et al. (2019) used several methods to determine whether reward and emotion processing indices predicted affective lability in individuals at high genetic risk for BSD; however, none of their results were specific to reward processing as indicated on the card guessing task. Thus, in the offspring literature, there are contradictory findings regarding patterns of reward processing as possible genetic/familial risk biomarkers to BSDs, although overall neural deficits in regions important for processes related to decision-making, delaying gratification, and regulating emotion may comprise an endophenotype. The current state of the literature is such that larger studies are needed to replicate and reconcile these disparate findings. The Bipolar Illness Onset study is a large longitudinal study currently underway, which will use the MID to study reward function in offspring of parents with BSDs (Kessing et al., 2017). Thus, these findings may be replicated in the future; however, currently there are limited conclusions regarding neural reward processing in familial high-risk samples.
It is worth noting a few important limitations to these studies. People who have a familial history of BSDs also are at higher risk for developing non-BSD psychopathology as well as BSD (Birmaher et al., 2009; Goldstein et al., 2010), Thus, this may be a confounding factor when designing high-risk offspring studies. Manelis et al. (2016) attempted to address this issue by comparing offspring of parents with a history of BPI or BPII, offspring of parents with non-BSD psychopathology (e.g., unipolar depression), and offspring of parents with no psychopathology, and identifying unique risk factors for BSDs versus other psychopathology. Additionally, many of the offspring had a history of other psychopathology and either were on medication or had a history of taking psychiatric medication. These factors all can impact reward-related brain function, and thus, it is important to study these processes in offspring without psychopathology who are medication naive. Additionally, these few offspring studies had modest sample sizes and cross-sectional designs, which do not allow for an examination of developmental trajectories of reward processing. Indeed, the offspring in these studies included a wide age range (e.g., 8 to 15 years) during a time period when rapid brain changes are occurring in the fronto-striatal circuit. Additionally, because we do not know which offspring go on to develop BSDs, our understanding of reward function profiles that constitute a risk factor for BSDs is limited.
3.2. Neural Reward Dysfunction as a Trait Risk Factor for BSDs
Examining neural responses to rewards in individuals who have a BSD, but are euthymic or are in a period of “remission”, may reveal whether reward-related brain function constitutes a more persistent, trait-like factor for BSDs, rather than a specific profile that is present only during mood states (e.g. depression vs. mania). Eight studies examined reward processing during an fMRI MID task in remitted BSD patients. A review of these studies, however, reveals mixed findings regarding whether euthymic individuals have hyper- or hypo-sensitivity in reward regions during monetary reward processing.
Two studies provide evidence that individuals with BSDs, who are not currently in a mood episode, exhibit increased activation in the reward circuit when processing rewards in a card-guessing task (Caseras et al., 2013; Nusslock, Almeida, et al., 2012). Specifically, euthymic BDI participants exhibited greater activation in the VS and OFC during reward anticipation compared to loss anticipation and to healthy controls (Nusslock, Almeida, et al., 2012). Similarly, Caseras et al. (2013) found that reward processing in both the anticipation and outcome phases of the task are characterized by neural reward circuit hypersensitivity. Specifically, there was increased VS activity during reward anticipation in euthymic BPII individuals, and increased VS activity during positive outcomes in euthymic BPI individuals. Additional whole-brain analyses suggest increased activation in areas of the PFC (vlPFC and dlPFC) during reward anticipation were characteristic of euthymic BPII individuals. Finally, although Kollman et al. (2017) did not show any group differences between BSDs and a healthy comparison group in VS and OFC, they did find greater activation in the ACC, a region important to emotion regulation, in their BSD group, thus providing some support for the reward hypersensitivity theory.
In contrast, others have found that reduced activation in reward regions distinguished euthymic individuals. In a sample entirely psychiatric medication naïve and without prolonged BSD illness, Yip et al. (2015) found that euthymic BSD patients had decreased activation in the DS during reward and loss anticipation on the MID; however, no findings were found for the VS. In contrast, Schreiter et al. (2016) did find blunted activation in the VS during reward anticipation in BSD patients in remission from a mood episode for at least five months. Additional psychophysiological interaction (PPI) analyses revealed that the BSD group also had decreased connectivity between the left VS and anterior PFC during reward anticipation. Finally, in their study of remitted BPI individuals, Johnson et al. (2019) found that the BSD group showed significantly lower activation to reward anticipation in mesolimbic regions, specifically in the VS, but increased activation to reward outcomes in frontal regions (i.e. medial PFC). This also was qualified by the finding that BSD individuals with greater positive urgency scores had more pronounced blunted NAcc activation during reward anticipation.
The final two studies from this systematic review utilizing the same sample include evidence for both heightened and blunted activity in the reward circuit during the processing of monetary rewards, and thus, do not provide strong support for either perspective (Dutra et al., 2015, 2017). Using combined monetary and social incentive delay tasks, comparing euthymic BPI individuals (defined as having not experienced a mood episode in last month) to a healthy comparison group, Dutra et al. (2015) found increased activity in the NAcc during reward consumption in the BSD group, and whole brain analyses revealed that healthy controls had greater OFC activity during reward anticipation than the BSD group. In a follow up study of the same sample, the authors probed the significant findings for elevated VS activation during reward consumption with functional connectivity analyses (Dutra et al., 2017). They found increased connectivity between the left VS and left OFC and between the bilateral VS and left amygdala to reward consumption, but decreased connectivity between the right VS and medial PFC when expected rewards were omitted in BSD participants. Thus, Dutra and colleagues’ (Dutra et al., 2015, 2017) findings provide mixed evidence for both reward hypo- and hyperactivation during different stages of reward anticipation and consumption.
It is important to interpret these findings in the context of their limitations. First and foremost, all of the studies discussed above have small to modest sample sizes, and thus, are lacking in sufficient power to detect smaller effects. Second, the majority of these studies included individuals who either were currently on mood stabilizers or other psychiatric medications, or had a history of being on medication. Many psychiatric medications, particularly antipsychotics, act on the dopaminergic system and can impact functioning in the fronto-striatal reward circuit. Although some studies attempted to limit the possible influence of these medications by either including only participants who were free from medications for a specified period of time, or by conducting additional analyses that excluded participants on current medication, a history of psychiatric medication use still may impact findings (Caseras et al., 2013; Kollmann et al., 2017). Indeed, the one study that recruited mood stabilizer and antipsychotic naïve patients is not without limitations (Yip et al., 2015). Although a major strength was limiting potential medication confounds by using a medication naïve sample, this also meant that their sample was young and early in the course of their BSD illness, and tended to have less severe forms of BSDs (i.e. BSD NOS). Therefore, the sample from this study may not be directly comparable to those with relatively more severe illnesses.
Although studying euthymic BSD individuals helps examine questions about whether reward dysfunction is a state-independent vulnerability, this group is not ideal for determining what actually underlies a pre-existing risk factor for BSDs. Indeed, inherent in the description of euthymic individuals is that many have residual symptoms of depression. All of the studies had cut-offs greater than zero for their euthymic participants on measures of current depression and mania. Thus, we cannot rule out current residual mood symptoms as confounding the results, particularly those that found BSDs were associated with reward hypoactivity, as this profile is also seen in individuals with depression (Hägele et al., 2015). Furthermore, mood instability in a wide array of disorders persists in periods of “remission” and evidence suggests that this pervasive mood instability is linked to connectivity in ventromedial regions in BSDs (Broome et al., 2015). Thus, given the presence of pervasive mood instability in BSD, one can argue that individuals who are not currently in a mood episode are not truly “euthymic.” In fact, it is theorized that trait mood instability in BSDs may be explained by a stable “mood bias” parameter that interacts with and influences either hypo (e.g., when mood bias is low) or hyper (e.g., when mood bias is high) responses to rewards (Mason et al., 2017). Thus, our conflicting results (some reporting hyposensitivity to rewards, and others reporting hypersensitivity) in the “euthymic” literature may be better explained by individuals in relatively elevated vs. relatively depressed mood states. This highlights the importance of study designs in addressing mood instability as a potential factor confounding reward-based measures.
3.3. Summary of Findings from Neuroimaging Literature on BSD
In sum, there are conflicting findings that support both reward hyper- and hypo-sensitivity in BSDs across both offspring and remitted studies, and conclusions from previous meta-analyses and reviews suggesting that BSD constitutes a predominantly reward hypersensitive profile may be premature given the current review. However, trends seem to emerge when one considers the results in the context of processing type (anticipation vs. consumption) and valence (reward vs. loss). For example, the majority of studies suggest that decreased fronto-striatal connectivity is associated with loss consumption, whereas increased fronto-striatal connectivity is associated with reward consumption. The most contradictory findings occur in the reward and loss anticipation analyses. For example, whereas it appears that unaffected offspring of parents with BSDs exhibit blunted activation in the pregenual cingulate (a region that has important connections to both frontal and striatal regions in the reward circuit), and decreased connectivity between this region and frontal regions during anticipation trials, in studies examining euthymic individuals, we see both blunted and heightened activation in the striatum and frontal regions during anticipation. These discrepancies point to the importance of other factors that may confound results. For example, prolonged illness itself, psychotropic medication, and comorbidities all may be confounding variables and a reason why the results are mixed. Additionally, it may not be possible to directly compare bipolar offspring and euthymic studies because of the differences in developmental periods between samples (offspring studies include youth, whereas euthymic studies include adults). Regardless, it appears that differences in connectivity between striatal regions responsible for motivation, emotion regulation, and reward value and frontal areas responsible for inhibitory control may underlie a vulnerability to BSDs, and that this connectivity may undergo normative and disease-specific (e.g., medication use, illness course, comorbidities) changes across development.
3.4. Neural Reward Dysfunction as a Familial Risk Factor for SUDs
Epidemiological studies suggest that individuals with a family history (FH+) of SUDs have an 8-fold risk of developing a SUD compared to individuals without a family history (FH−; (Merikangas et al., 1998). Multiple factors may contribute to this heightened risk, including family conflict, increased stress, and inherited traits such as increased impulsivity and higher incidence of externalizing behaviors (Smith et al., 2016). Neurobiological factors also may exist, particularly related to neural reward dysfunction. Our literature search yielded eight studies examining reward processing on the MID task in individuals at high familial risk for SUDs.
The vast majority of these studies used samples at elevated familial risk for alcohol use disorder/alcoholism (seven out of eight). Three studies from the Michigan Longitudinal Study (MLS; Zucker et al., 2000) examined a relatively young sample (aged 18 to mid-late 20s) of offspring of parents with an alcohol use disorder (AUD) (Martz et al., 2018; Weiland et al., 2013, 2017). Taken together, the results from the MLS studies support the reward hypersensitivity theory of SU. An initial study (Weiland et al., 2013) identified increased coupling between the striatum (NAcc) and motor areas, and that the degree of connectivity between these regions during reward anticipation mediated the relationship between sensation seeking and alcohol consumption in the FH+ group. These findings may illustrate that among offspring at genetic risk for AUD, those with higher personality traits associated with SU (e.g., sensation seeking) exhibit increased communication between regions associated with hedonic value or wanting (e.g., the VS and NAcc) and regions important for motor actions (e.g., the SSMA). In a follow-up study using PET data in conjunction with the MID, Weiland et al. (2017) found that being in the FH+ group (regardless of “high” or “low” risk status defined as experiencing drunkenness by age 15) was associated with increased dopamine release during monetary reward, although no group differences were found with the fMRI. A final study found that higher VS activation during the reward anticipation phase of the MID was positively correlated with frequency of marijuana use and binge drinking between ages 17 and 18 among the FH+ group in general, but that this did not distinguish high and low risk FH+ groups (Martz et al., 2018). Taken together, these three studies suggest that having a family history of AUD results in similar underlying neurobiology for reward hypersensitivity, regardless of whether the FH+ individuals develop later SU problems.
Independent from the MLS, Yau et al. (Yau et al., 2012) also compared FH+ and FH− for alcoholism groups, but also measured the FH+ group’s current alcohol involvement based on the frequency and problems associated with how much alcohol the participants currently drank. Thus, they divided their FH+ group into those with “low” versus “high” alcohol involvement. During the reward anticipation phase of the MID, they found that only the FH+ with low current alcohol involvement had significantly lower activation in the NAcc compared to high involvement FH+ and healthy controls (Yau et al., 2012). Because this blunted activation was seen only in the low alcohol involvement FH+ group, these findings suggest that reward hyposensitivity may be a resilience factor for SU in individuals who are at genetic risk for developing SUDs. Thus, this latter study may provide support for a reward hypersensitivity perspective of SUDs.
Results from the literature search revealed one study that supports the reward hyposensitive perspective on SUDs. Andrews et al. (2011) found that FH+ individuals had lower activation in both striatal and frontal reward regions in response to both reward anticipation and loss consumption compared to the FH− group, suggesting that reward hyposensitivity during both anticipatory and consummatory processing phases may constitute an inherited predisposition to SUDs. The final two AUD studies utilizing familial risk samples obtained no group differences during reward anticipation or consumption in the striatum (Bjork et al., 2008; Müller et al., 2015). However, Bjork et al. (2008) did find a positive correlation between striatal activation and personality traits like sensation seeking, traits that underlie risk taking behaviors, particularly among adolescents (Byck et al., 2015). Thus, although family history of SUD did not seem to be directly related to differences in neural reward processing, personality traits that have a high correlation with substance use behaviors were associated with hyperactivation in striatal regions during the anticipation of rewards.
Finally, one study compared four groups (individuals with family history for a SUD who also were stimulant-dependent, their siblings who did not have a SUD, another group who had no familial history of SUD but were non-dependent stimulant users, and a final group who had neither family nor personal history of SUD; Just et al., 2019). This design allowed the authors to examine the interactive effect of familial risk and stimulant drug use on reward processing. Familial risk was associated with altered functional connectivity within corticostriatal regions. Specifically, during reward anticipation, those with familial history of SUD had decreased connectivity between putamen (located in the dorsal striatum) and cortical reward regions (e.g., ACC), and increased connectivity between the putamen and an array of different brain regions including both the frontal and temporal pole and brainstem (Just et al., 2019b). These findings suggest altered fronto-striatal connectivity between regions involved in inhibitory control and reward processing is most important for conferring risk for SUDs.
Contradictory findings within the unaffected offspring literature in SUDs may be explained in the context of the limitations across these studies. First and foremost, most of these studies included very small sample sizes. Second, the age range of participants varied between studies, with some examining reward responsivity in early to mid-adolescence (Bjork et al., 2008; Müller et al., 2015), whereas others included young to middle-age adults (Andrews et al., 2011; Yau et al., 2012). Developmental differences in the maturation of the reward circuit across adolescence into adulthood may explain why these studies yielded such different and opposite results (Van Leijenhorst et al., 2010). Additionally, different versions of the MID task were used across studies, and thus, results may not be directly comparable. Despite mixed findings, there does seem to be some continuity, particularly across functional connectivity analyses, suggesting that an impaired ability to recruit cortico-limbic motivational circuitry is implicated in SUDs.
3.5. Neural Reward Dysfunction as a Trait Risk Factor for SUDs
The majority of studies that examine reward function in remitted SUDs take place in clinical settings, and thus, are part of trials aimed at predicting treatment response. Most of these treatment studies find that blunted reward processing in key fronto-striatal regions in response to non-drug related stimuli is associated with worse clinical outcomes (e.g., shorter time to relapse; Moeller & Paulus, 2018). Because interventions themselves may result in neuroplastic changes in the brain, treatment is a confounding variable when examining trait vulnerabilities to SUDs in the reward circuit. Therefore, we excluded any study related to treatment outcomes from our review, and instead, focused on naturalistic studies incorporating remitted SUD groups. However, many of these studies also included groups of substance users who were in “initial abstinence,” which typically refers to the first couple of weeks abstaining from a substance. Thus, initial abstinence does not reflect a state of true remission as defined in the DSM (American Psychiatric Association, 2013), and we excluded these types of studies from our review. We found three studies that used the MID during fMRI to study brain function in groups of individuals in remission from or in prolonged abstinence from substances. One study was specific to former cocaine users and two studies used a sample of former nicotine smokers.
Among cocaine users, Patel et al. (Patel et al., 2013) found that compared to current cocaine users, former users (in remission for at least 6 months) had less activation to loss anticipation in the PFC (e.g., BA10), but greater activation in the striatum (e.g., VTA) to loss consumption. Compared to healthy controls without a substance history, former cocaine users had less activation to loss anticipation in the right parahippocampal gyrus, right insula, and BA10, but greater activation to loss consumption in the hippocampus. There were no significant differences between groups during reward anticipation or consumption. Conclusions regarding the robustness of these effects are limited given small sample sizes. Both former and current cocaine users differed equally from healthy controls, with generally less robust activation in key fronto-striatal rewards regions, which supports a reward deficiency hypothesis.
Two additional studies of the same sample examined reward processing on the MID in current cigarette smokers, former cigarette smokers (in remission for at least one year), and controls (Nestor et al., 2018a). Both former and current smokers showed greater activation in the PFC (e.g., OFC and anterior insular cortex) during reward and loss anticipation. Additionally, in the dorsal striatum (e.g., putamen and caudate), former smokers had greater change in activation during loss anticipation than current smokers and controls. Together, these findings suggest that in smokers, neural substrates underlying motivation and incentive salience (i.e., OFC), as well as regions underlying a reward-motor network (i.e. caudate, putamen) may be sensitized to loss avoidance (Nestor et al., 2018a). Expanding on these initial findings, Nestor et al. (2018b) aimed to identify activation to operant responses (e.g., collapsing reward/loss/neutral across “hit” or “miss” trials). Here, they found that there was less activation change to both negative and positive outcomes in corticolimbic regions in both current and former smokers compared to controls, but only former smokers showed greater activation change in the amygdala during negative outcomes (Nestor et al., 2018b). Taken together, these results suggest that smoking may permanently sensitize regions of the corticolimbic reward pathway, but that ex-smokers may compensate to promote smoking abstinence through heightened signaling in negative valence outcome monitoring.
Conclusions from the remitted SUD literature are difficult to draw given the limitations of these three studies. First, they examined different substances (nicotine vs cocaine) that may act on different regions/receptors and to varying degrees. Additionally, sample sizes differed greatly, and the findings must be interpreted with caution as the analyses were likely underpowered. Nonetheless, it appears that drugs may sensitize a fronto-striatal reward circuit that subserves motivational processes involved in both attaining non-drug rewards and avoiding losses. However, it is important to note that we cannot conclude whether these differences in reward processing in former users reflect features related to pre-existing brain function, exposure to a substance of abuse, or recovery.
3.6. Neural Reward Dysfunction as a Pre-existing Risk Factor for SUDs
Cross-sectional research cannot clarify whether aberrant neural responses to rewards precede or result from SU. Thus, to fully understand neural reward function as a pre-existing risk factor for SUDs, longitudinal research is needed to prospectively predict initiation of substance use and onset of SUDs. Our literature search yielded a handful of studies examining neural reward processing on the MID task in adolescents prior to problematic SU. In fact, all these studies used samples in early to mid-adolescence, which is necessary to capture brain function before exposure to drugs. We review the findings from nine eligible studies below.
Four studies used data from the IMAGEN consortium, which is one of the largest studies aimed at increasing our understanding of the neurobiological mechanisms related to behavior and adolescent brain development worldwide (Schumann et al., 2010). As such, there is a large sample of youth in mid-adolescence who have completed fMRI scans and measures of SU at multiple time points. Of note, the MID task in the IMAGEN study only featured reward anticipation and outcome phases. Specifically, these studies from the IMAGEN cohort identified prospective predictors at age 14 of subsequent binge drinking at age 16 (Whelan et al., 2014) found that future binge drinkers had reduced activation in occipito-temporal and posterior cingulate regions to reward anticipation, and reduced activation in the left temporal pole and increased activation in the bilateral superior frontal gyrus to reward consumption at age 14. Nees et al. (2015) found that adolescents who carried the Met allele of the BDNF gene (a polymorphism commonly known to modulate neurotransmitter activity in the reward system) and who had high alcohol consumption at age 14 showed lower activation in the putamen to reward anticipation, and results did not differ based on sex. Additionally, among Met carriers, those with lower activation in the putamen to reward consumption also were more likely to drink alcohol at the two-year follow-up. Identifying activation in more specific reward regions, Baker et al. (Baker et al., 2019) extended the previous findings by examining interactions between the VS and OFC. Although neither of these regions uniquely predicted alcohol use, significant interactions between the OFC and VS during anticipation of high rewards were associated with current alcohol use at age 14. These findings suggest that the degree of connectivity between OFC and VS regions may be a correlate of current early alcohol use, but does not predict future alcohol use in adolescence. Heinrich et al. (2016) used the IMAGEN dataset to examine what factors were most important in explaining increased alcohol use across adolescence. In their four-factor model, they found that personality traits and genetic factors contributed more than reward-related brain function to predict future alcohol use. They also did not find sex differences in their results. Thus, even though there are differing brain reward profiles that may differentiate and predict problematic alcohol use, it appears that other factors such as personality traits may be more important in conferring risk for SU.
Indeed, genetic factors may play an important role in reward circuit sensitivity in general. In one study from the Michigan Longitudinal study (described above), the authors attempted to examine reward processing as well as genetic factors and their association with alcohol use across time in 175 adolescents (Heitzeg et al., 2014). Participants had anywhere between one and four scans in which they completed a MID. In addition, most of the sample was at high risk due to having a parent with an AUD. They found that heightened NAcc activation to reward anticipation was associated with alcohol use across adolescence. Additionally, variations in the GABRA2 gene (which is known to be associated with alcoholism in adulthood as well as with impulsivity and externalizing behaviors) were associated with differences in NAcc activation (Heitzeg et al., 2014). Specifically, NAcc activation to reward anticipation mediated the relationship between genotype and future problematic alcohol use, suggesting an interplay between genes, reward-related brain function, and future SU. Although they did not find significant effects of gender, findings were more robust in males, suggesting the possibility of sex difference in GABRA2 effects on neural reward responses.
In a final recent study examining neural reward predictors of alcohol use specifically, Swartz et al. (2020) identified separate pathways to drinking behavior in boys and girls. On the MID, they found that whereas higher VS activity to reward anticipation and average mPFC activity to reward consumption predicted increased alcohol use from age 16 to 18 in boys, this was opposite in girls. Girls who had increased drinking from age 16 to 18 had lower VS and higher mPFC activity to reward anticipation. These findings highlight potential sex differences in neural risk pathways, which will have important implications for intervention efforts.
There also are mixed findings from studies examining prospective predictors of substance use more generally (e.g., defined by engagement in alcohol or illicit drug use). Büchel and colleagues (2017) examined brain function in adolescents who were characterized as being high novelty seekers via personality questionnaire at age 14, and whether it predicted who developed problematic SU at age 16. They found that participants who went on to develop problematic drug use had both decreased striatal (e.g., VS) and frontal (e.g., dlPFC) activation to large reward anticipation at age 14. These findings suggest that among high novelty seeking adolescents, reward hyposensitivity underlies risk for developing problematic SU later in adolescence. In contrast, Cope et al. (2019), who examined brain activation in 8–12 year-olds at high risk for SUD (majority had a FH+ for AUD), found that the only significant predictor of SU initiation at age 16 was higher NAcc activation during anticipation of large rewards. This supports the reward hypersensitivity theory of SUDs. Despite this study’s strength in that no participants had tried substances prior to scanning, the sample also had other diagnoses (e.g., ADHD, depression, externalizing disorders), which is characteristic of children at high familial risk for SUDs.
Another study examined 73 youth (average age 14) during a card-guessing paradigm, and categorized the youth into two groups: substance users (defined as drinking more than a few sips of alcohol or any illicit drug use) and those who were not, about 24 months after scanning (Bertocci et al., 2017). Thirty-six reported SU post-scan. The authors found that increased activation in the left middle PFC to reward consumption and decreased left ventral anterior insula activation to loss consumption predicted membership in the substance user group at 24 months. When removing 15 youth who had SU at the time of scan, only increased left middle PFC activation to reward consumption predicted SU, which suggests that left ventral anterior insula activity to loss may be driven by prior SU. One major limitation of this study was that the substance analyses were post-hoc, and thus, the authors could not control for SU at time of scanning. Additionally, some participants were taking psychotropic medication.
Even among prospective findings, there are still conflicting results about whether problematic SU is driven by reward hypo- or hypersensitivity. However, these studies suggest that differences in activations in both striatal and cortical regions, aberrant functioning in the mesolimbic reward motivation system, as well as impaired prefrontal control may confer risk for future SU. Even though methodologically strong, these longitudinal studies also have their limitations. For example, given the young samples, the majority of studies measured “problematic” SU through questionnaires, and not by actual DSM criteria for SUDs. Thus, there is a question of how pathological this initiation of problematic SU is in adolescence, as most participants did not reach clinical threshold (Cope et al., 2019). Moreover, most of the substances used by adolescents were legal drugs (e.g., nicotine, marijuana, alcohol), and thus, may not capture the severity of those who engage in illicit drug use (Büchel et al., 2017). These types of prospective studies need to be extended into later adolescence and adulthood to ascertain the extent to which these findings contribute to the development of actual SUDs. Additionally, only one study (Cope et al., 2019) used a completely substance naïve sample at baseline, and even this study had a small sample size. Thus, longitudinal studies need to be refined to target adolescents prior to any substance initiation and to capture developmental effects of reward processing on normative initiation of drug use in adolescence to habitual drug seeking behaviors.
3.7. Summary of Findings from Neuroimaging Literature of SUDs
An interesting pattern that emerged in the SUDs literature is that a substantial number of studies did not find significant differences in neural activation across family history, remitted, and prospective studies. Of the studies that did yield significant results, the majority focused on the reward anticipation phase. It appears that the literature on high familial risk and remitted SUDs is mixed, with some reporting hypo- and some reporting hyper-activations particularly in striatal regions. Unfortunately, the prospective literature does not clarify these mixed findings much. Although the majority of the studies report blunted activations in striatal and frontal regions during reward processing, a couple of methodologically strong studies (e.g., with samples who never took any substances) suggest that heighted NAcc activation, particularly during reward anticipation, may underlie a vulnerability to initiate SU. Furthermore, some suggest that reward function may not be as important for the development of SU, and that genetics and personality traits drive this vulnerability for SU (Heinrich et al., 2016; Heitzeg et al., 2014). What is lacking in the SUDs literature are analyses related to fronto-striatal connectivity; thus, future research should focus on the extent to which communication within the reward circuit predicts SUDs.
4. Summary and Integration
This literature review yielded a limited number of studies that examined reward processing on the fMRI MID or card-guessing tasks in individuals at risk for developing BSDs or SUDs. Furthermore, the results were mixed, making it difficult to draw conclusions regarding whether reward circuit hypo- or hypersensitivity is a risk factor for both BSDs and SUDs. Additionally, given the variety of study designs included in this review, it is difficult to compare results across the extant literature. We elaborate on the reasons for these discrepancies, address important limitations, and provide suggestions for future directions and improved methodology.
First, during adolescence, the fronto-striatal reward circuit goes through rapid and drastic changes, maturing relatively quickly and prior to other prefrontal regions associated with the cognitive control network (McClure, 2004). This maturational imbalance between the reward and cognitive control networks is hypothesized to be a reason why adolescence is a period of increased risk for engaging in risky behaviors such as SU (Steinberg, 2010). Thus, even normative reward processing during adolescence differs from reward processing during adulthood; consequently, from a developmental perspective, one cannot directly compare brain processes between these age epochs. Additionally, most of the research conducted has been cross-sectional, and thus, does not capture these developmental nuances. In order to capture changes in brain development and their contributions to the development of SUDs and BSDs, it is important to conduct larger, longitudinal studies utilizing within person designs to assess trajectories throughout adolescence into adulthood. Indeed, key regions associated with reward and cognitive control, are involved in BSDs and SUDs, and also are implicated in the development of behavioral control in adolescence. Specifically, behavioral control is associated with increased vmPFC and dlPFC connectivity (Steinbeis et al., 2016), where the vmPFC is an important “relay” region in which signals converge from the VS and dlPFC (Leon & Shadlen, 1998). Particularly as it relates to interpretation of the findings in this review, there is evidence to suggest there are differential developmental trajectories of different components of reward processing, such that from childhood to young adulthood, activation to reward anticipation increases, whereas activation to reward outcome decreases in both striatal and cortical regions of the reward circuit (Hoogendam et al., 2013).
A better understanding of precise mechanisms underlying the complex normative neurodevelopmental changes that occur in adolescence will inform our understanding of factors that increase vulnerability to SUDs and BSDs starting in adolescence. For example, we know that social interactions and pubertal hormones significantly influence reward circuitry maturation, and that sensitization of particularly the PFC by substances and social stress can have lasting effects on the reward circuit (for review see Walker et al., 2017). Expanding on this important consideration is the effect of sex differences on neurodevelopment. In adolescence, females reach peak structural development in both the striatum and PFC quicker than boys (Raznahan et al., 2010, 2014), and these changes may be why we see sex differences in different risk pathways to SUDs (for review see Heitzeg et al., 2018). Despite this evidence, sex differences have not been systematically studied and only a small portion of the studies identified in this review specifically examined sex differences (Heinrich et al., 2016; Heitzeg et al., 2014; Nees et al., 2015; Swartz et al., 2020) or highlighted this as an important limitation to interpreting their findings (Cope et al., 2019). Thus, further investigations into individual difference factors, including sex, during both typical and atypical reward functioning is important to identify risk factors for the development of SUDs and BSDs.
Second, the effects of prior treatment with psychotropic medication and prior SU confounds studies that aim to identify pre-existing neural markers, because these substances change brain structure and function. To resolve this issue, some researchers included only people who were never treated (e.g., Yip et al., 2015) or who never initiated SU (e.g., Cope et al., 2019). However, in so doing, these studies also restricted the variance of disorder severity in their samples. For example, individuals who do not require intervention have less severe forms of BSDs (e.g., BDNOS), and thus, may not be directly comparable to those with more severe forms. Indeed, a similar issue exists for studies examining the onset of SUDs in that they recruit very young children, who never meet clinically significant cut-offs for DSM disorders. Furthermore, in examining such young children, one is unable to draw conclusions regarding normative risk-taking versus more problematic SU that may develop later in adolescence or in adulthood.
Third, except for the small number of studies that actively sought to exclude participants with comorbidities, most of the studies included participants who had more than one psychiatric diagnosis. In including people with multiple diagnoses, it is not possible to draw conclusions about what mechanisms are unique to a single disorder; however, this approach is more reflective of the actual BSD and SUD populations. Perhaps such samples are most relevant translationally as comorbidity accurately reflects patient populations and the high incidence of different diagnoses among people with BSDs and SUDs. It also may be prudent to move away from approaches in which one compares distinct groups of participants to approaches that are more in line with an RDoC perspective, which considers how brain-behavior relationships and mechanisms influence specific symptoms. Using an RDoC approach in identifying common domains may be a better way to operationalize the high co-occurrence of BSDs and SUDs.
A final important limitation across the reviewed studies relates to studying reward processing with the MID or card-guessing tasks. First, researchers differed in the version of the tasks that they used. Some utilized a card-guessing paradigm that included a “decision-making” component, whereas others required only a button press to a target. Whether an active response to attain rewards and/or avoid losses is required or not in the task has an important impact on which regions of the neural reward circuitry are likely to be activated and involved in functional connectivity patterns (Haber & Knutson, 2010). Whereas some tasks displayed actual dollar amounts of rewards and losses, others used tokens representative of a monetary reward. Furthermore, different studies used different magnitudes of money (e.g., $1 versus $5). Thus, the heterogeneity across monetary reward tasks may confound findings. Additionally, different task durations may impact neural responses because activation in certain regions, particularly the OFC, only may occur after lengthy anticipation phases (Diekhof et al., 2012). There also may be individual differences in salience and motivation for monetary rewards during reward processing. Even though meta-analyses demonstrate the validity of a number of different monetary reward tasks in activating neural reward regions, the above limitations still should be taken into consideration (Oldham et al., 2018).
4.1. Proposed Theoretical Integration
In line with the newer RDoC perspective, it is important to identify common mechanisms of vulnerability across psychiatric disorders, and this approach may be particularly useful in understanding the high co-occurrence of BSDs and SUDs. Indeed, one key theory of why BSDs and SUDs have such high co-occurrence suggests that it is a result of their shared common mechanisms related to modulating motivation and reward responsivity (Swann, 2010). It is abundantly clear that both disorders are characterized by dysfunction in the neural reward circuit; however, the direction and precise nature of these abnormalities remains unclear given the discrepancies in the extant literature. Nonetheless, we suggest the multifinality/equifinality perspective described first by Whitton et al. (2015) and expanded upon by Nusslock and Alloy (2017) is an important conceptual framework for interpreting these results. Multifinality suggests that a common pathway may lead to different outcomes/symptoms across different disorders (e.g., BSDs and SUDs), and equifinality suggests that a similar outcome (e.g., SUD) may arise from dissimilar pathways (e.g., hyper- vs hypo- reward processing; Whitton et al., 2015). Thus, we propose that it is possible that both hypo- and hyper-sensitivity in the reward circuit may contribute to the development of BSDs and SUDs via different mechanisms.
For example, individuals with a blunted reward system may engage in SU to attenuate dysphoria and increase their positive affect. In other words, they seek exogenously (in substances of abuse), what they lack endogenously. This same blunted reward profile may underlie a vulnerability to develop BSDs as well. For example, some researchers found that reduced activation in the striatum (both dorsal and ventral), as well as blunted activation in regions associated with affect regulation, was observed in at-risk individuals (children with familial history of BSD), and in euthymic individuals with BSDs (Schreiter et al., 2016; Singh et al., 2014; Yip et al., 2015). Thus, this is an example of the multifinality perspective – blunted striatal responses may lead to dissimilar symptoms associated with BSDs and SUDs. On the other hand, initiation of SU (and over time SUDs) may be driven by reward hypersensitivity, instantiated in strong approach behavior toward rewards (including substances of abuse), as also evidenced by the current literature review (Cope et al., 2019). The reward processing abnormalities that contribute to the development of BSDs and SUDs also can reflect equifinality processes because both reward hypo- and hyper-sensitivity may explain initial vulnerability to these disorders depending on the target of these different pathways (e.g., increased sensation-seeking via hypersensitivity versus attenuating dysphoria via hyposensitivity). Nusslock and Alloy (2017), argue that an equifinality perspective explains SUD onset, in that it can be explained by both the reward deficiency and hypersensitivity models, and that this also may explain distinct pathways for SUD comorbidity with unipolar depression (via reward hypo-sensitivity) and BSDs (via reward hyper-sensitivity). However, given evidence outlined in this review that BSDs may arise from both hyper- and hypo- reward responses, we expand on Nusslock and Alloy’s (2017) perspective, and argue that reward hypo- and hyper-sensitivity may underlie a vulnerability to the initial onset and course of both BSDs and SUDs.
Understanding differential neural reward risk pathways to BSDs and SUDs also has important clinical implications related to prevention and integrated treatment. First, identifying cognitive and behavioral strategies that target reward and related processes (e.g., impulsive behaviors, risk-taking) may be particularly important when treating co-occurring SUDs and BSDs. Individuals with BSDs and SUDs (compared to those without co-occurring SUDs) are significantly more likely to drop out of treatment (Manwani et al., 2007), which contributes to poorer prognosis and overall worse outcomes (Salloum & Thase, 2000), and reward-relevant processes including risk-taking and impulsivity were found to be predictive of treatment dropout in individuals with BSDs and co-occurring alcohol and stimulant use disorder, respectively (Akingbala et al., 2006; Prisciandaro et al., 2011). Thus, psychosocial interventions targeting BAS-relevant themes (e.g., incentive motivation) may be particularly important for individuals with BSDs and SUDs (for review see Nusslock et al., 2009). In terms of pharmacological interventions, treatment trials historically have focused on medications designed to treat either BSD or SUD while assuming that these treatments also would prove effective in individuals with both disorders. However, understanding differential neurobiological reward mechanisms may help inform more precise treatments targeting specific neurotransmitter systems or pathways. Indeed, there is evidence to suggest that there are unique patterns of prefrontal GABA and glutamate in individuals with BSD and AUD, compared to individuals with either disorder alone (Prisciandaro et al., 2017), and the current perspective may help inform future endeavors to understand precise neural reward mechanisms underlying BSD and SUD co-occurrence.
4.2. Conclusions and Future Directions
In this review, we identified studies from the BSD and SUD literatures in an attempt to understand the neural substrates underlying vulnerability to developing BSDs and SUDs with an aim to shed light on potentially common mechanisms responsible for their high co-occurrence. We propose that aberrant responding and connectivity across neural regions associated with reward and cognitive control confer risk for the development of BSDs and SUDs; however, given the heterogeneity in study designs and samples, we cannot definitively state the exact nature of this dysfunction. Instead, we propose an equifinality/multifinality perspective in that similar neural reward processing dysfunctions can lead to both BSDs and SUDs and different neural reward processing abnormalities can lead to a single outcome (e.g., SUDs).
Finally, the review revealed a few important areas for future research. First and foremost, there is an important need for large-scale longitudinal studies starting in youth to fully assess neurodevelopmental trajectories of the reward system and their influence on the onset and course of BSDs and SUDs. Second, there is a need for further examination of how sex differences may confer differential risk for these neurobiological pathways. Finally, neuroimaging analyses that can better account for the complexity of reward processing and more fully characterize the nature of neural representations of reward within brain regions would more fully inform this area of research. The majority of the studies in the review examined reward-related activity across whole ROIs. In addition, the heterogeneity of findings further highlights the need for further research in a multifinality/ equifinality approach, as this perspective is better able to address the nuances and complexities of neural reward processes. Using more sensitive approaches, such as multi-voxel pattern analysis (MVPA), could better elucidate the relationships between voxels within a given region, and thus, more fully ascertain how different components of reward processing relate to different outcomes. Indeed, researchers have used MVPA and other pattern-based approaches to identify distinct neural processes despite similar fMRI activity within the same neuroanatomical regions (Kahnt et al., 2014; Woo et al., 2014). Thus, developing and utilizing more sensitive approaches could help tease apart more nuanced components of reward processing, help inform the multifinality/equifinality perspective on reward processing, and better elucidate the heterogeneity of results in the literature.
Supplementary Material
Highlights.
Bipolar disorders and substance use disorders are associated with dysfunction in neural reward circuitry and are highly comorbid.
This systematic review examined the literature on neural reward processing to secondary rewards in both disorders to elucidate common risk factors.
We extend the equifinality/multifinality perspective as a possible framework for explaining how reward processing abnormalities confer risk for both bipolar and substance use disorders.
Role of Funding Sources
Manuscript preparation for this article was supported by National Institute of Mental Health (NIMH) Grant MH077908 to Lauren B. Alloy. NIMH had no role in the development of the current manuscript, or in the decision to submit the paper for publication.
Footnotes
Conflict of Interests
All authors declare that they have no conflicts of interest, financial or otherwise.
The literature review yielded no prospective studies of neural reward function as measured by the MID task predicting to BSD onset.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abler B, Greenhouse I, Ongur D, Walter H, & Heckers S (2008). Abnormal Reward System Activation in Mania. Neuropsychopharmacology, 33(9), 2217–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acuff HE, Versace A, Bertocci MA, Ladouceur CD, Hanford LC, Manelis A, … Phillips ML (2019). Baseline and follow-up activity and functional connectivity in reward neural circuitries in offspring at risk for bipolar disorder. Neuropsychopharmacology, 44(9), 1570–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akingbala F, Dhanani N, & Brown ES (2006). Impulsivity in Patients with Bipolar Disorder and Cocaine or Amphetamine Dependence Given Lamotrigine. Journal of Dual Diagnosis, 2(3), 73–83. [Google Scholar]
- Alloy LB, Abramson LY, Walshaw PD, Cogswell A, Grandin LD, … Hogan ME (2008). Behavioral Approach System and Behavioral Inhibition System sensitivities and bipolar spectrum disorders: Prospective prediction of bipolar mood episodes. Bipolar Disorders, 10(2), 310–322. [DOI] [PubMed] [Google Scholar]
- Alloy LB, Bender RE, Wagner CA, Whitehouse WG, Abramson LY, Hogan ME, … Harmon-Jones E (2009). Bipolar spectrum–substance use co-occurrence: Behavioral approach system (BAS) sensitivity and impulsiveness as shared personality vulnerabilities. Journal of Personality and Social Psychology, 97(3), 549–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy LB, Bender RE, Whitehouse WG, Wagner CA, Liu RT, Grant DA, … Abramson LY (2012). High behavioral approach system (BAS) sensitivity, reward responsiveness, and goal-striving predict first onset of bipolar spectrum disorders: A prospective behavioral high-risk design. Journal of Abnormal Psychology, 121(2), 339–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy LB, Nusslock R, & Boland EM (2015). The Development and Course of Bipolar Spectrum Disorders: An Integrated Reward and Circadian Rhythm Dysregulation Model. Annual Review of Clinical Psychology, 11(1), 213–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy LB, Olino T, Freed RD, & Nusslock R (2016). Role of Reward Sensitivity and Processing in Major Depressive and Bipolar Spectrum Disorders. Behavior Therapy, 47(5), 600–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy LB, Urošević S, Abramson LY, Jager-Hyman S, Nusslock R, Whitehouse WG, & Hogan M (2012). Progression along the bipolar spectrum: A longitudinal study of predictors of conversion from bipolar spectrum conditions to bipolar I and II disorders. Journal of Abnormal Psychology, 121(1), 16–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association, & American Psychiatric Association (Eds.). (2013). Diagnostic and statistical manual of mental disorders: DSM-5 (5th ed). American Psychiatric Association. [Google Scholar]
- Andrews MM, Meda SA, Thomas AD, Potenza MN, Krystal JH, Worhunsky P, … Pearlson GD (2011). Individuals Family History Positive for Alcoholism Show Functional Magnetic Resonance Imaging Differences in Reward Sensitivity That Are Related to Impulsivity Factors. Biological Psychiatry, 69(7), 675–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashok AH, Marques TR, Jauhar S, Nour MM, Goodwin GM, Young AH, & Howes OD (2017). The dopamine hypothesis of bipolar affective disorder: The state of the art and implications for treatment. Molecular Psychiatry, 22(5), 666–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TE, Castellanos-Ryan N, Schumann G, Cattrell A, Flor H, Nees F, Banaschewski T, Bokde A, Whelan R, Buechel C, Bromberg U, Papadopoulos Orfanos D, Gallinat J, Garavan H, Heinz A, Walter H, Brühl R, Gowland P, Paus T, … the IMAGEN consortium. (2019). Modulation of orbitofrontal-striatal reward activity by dopaminergic functional polymorphisms contributes to a predisposition to alcohol misuse in early adolescence. Psychological Medicine, 49(5), 801–810. [DOI] [PubMed] [Google Scholar]
- Baskin-Sommers AR, & Foti D (2015). Abnormal reward functioning across substance use disorders and major depressive disorder: Considering reward as a transdiagnostic mechanism. International Journal of Psychophysiology, 98(2), 227–239. [DOI] [PubMed] [Google Scholar]
- Bechara A (2003). Risky business: Emotion, decision-making, and addiction. Journal of Gambling Studies, 19(1), 23–51. [DOI] [PubMed] [Google Scholar]
- Berghorst LH, Kumar P, Greve DN, Deckersbach T, Ongur D, Dutra SJ, & Pizzagalli DA (2016). Stress and reward processing in bipolar disorder: A functional magnetic resonance imaging study. Bipolar Disorders, 18(7), 602–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermpohl F, Kahnt T, Dalanay U, Hägele C, Sajonz B, Wegner T, … Heinz A (2010). Altered representation of expected value in the orbitofrontal cortex in mania. Human Brain Mapping, 31(7), 958–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, & Robinson TE (1998). What is the role of dopamine in reward: Hedonic impact, reward learning, or incentive salience? Brain Research Reviews, 28(3), 309–369. [DOI] [PubMed] [Google Scholar]
- Berridge KC, & Robinson TE (2003). Parsing reward. Trends in Neurosciences, 26(9), 507–513. [DOI] [PubMed] [Google Scholar]
- Bertocci MA, Bebko G, Versace A, Iyengar S, Bonar L, Forbes EE, … Phillips ML (2017). Reward-related neural activity and structure predict future substance use in dysregulated youth. Psychological Medicine, 47(8), 1357–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertocci MA, Hanford L, Manelis A, Iyengar S, Youngstrom EA, Gill MK, … Phillips ML (2019). Clinical, cortical thickness and neural activity predictors of future affective lability in youth at risk for bipolar disorder: Initial discovery and independent sample replication. Molecular Psychiatry, 24(12), 1856–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B, Axelson D, Monk K, Kalas C, Goldstein B, Hickey MB, … Brent D (2009). Lifetime Psychiatric Disorders in School-aged Offspring of Parents With Bipolar Disorder: The Pittsburgh Bipolar Offspring Study. Archives of General Psychiatry, 66(3), 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, & Hommer DW (2008). Incentive-elicited striatal activation in adolescent children of alcoholics. Addiction, 103(8), 1308–1319. [DOI] [PubMed] [Google Scholar]
- Blum K, Braverman ER, Holder JM, Lubar JF, Monastra VJ, Miller D, … Comings DE (2000). The Reward Deficiency Syndrome: A Biogenetic Model for the Diagnosis and Treatment of Impulsive, Addictive and Compulsive Behaviors. Journal of Psychoactive Drugs, 32(sup1), 1–112. [DOI] [PubMed] [Google Scholar]
- Bowirrat A, & Oscar-Berman M (2005). Relationship between dopaminergic neurotransmission, alcoholism, and reward deficiency syndrome. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 132B(1), 29–37. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Perlini C, Bellani M, Tomelleri L, Ferro A, Cerruti S, … Frangou S (2013). Increased salience of gains versus decreased associative learning differentiate bipolar disorder from schizophrenia during incentive decision making. Psychological Medicine, 43(3), 571–580. [DOI] [PubMed] [Google Scholar]
- Broome MR, He Z, Iftikhar M, Eyden J, & Marwaha S (2015). Neurobiological and behavioural studies of affective instability in clinical populations: A systematic review. Neuroscience & Biobehavioral Reviews, 51, 243–254. [DOI] [PubMed] [Google Scholar]
- Büchel C, Peters J, Banaschewski T, Bokde ALW, Bromberg U, Conrod PJ, … Knutson B (2017). Blunted ventral striatal responses to anticipated rewards foreshadow problematic drug use in novelty-seeking adolescents. Nature Communications, 8(1), 14140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byck GR, Swann G, Schalet B, Bolland J, & Mustanski B (2015). Sensation Seeking Predicting Growth in Adolescent Problem Behaviors. Child Psychiatry & Human Development, 46(3), 466–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caseras X, Lawrence NS, Murphy K, Wise RG, & Phillips ML (2013). Ventral Striatum Activity in Response to Reward: Differences Between Bipolar I and II Disorders. American Journal of Psychiatry, 170(5), 533–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase HW, Nusslock R, Almeida JR, Forbes EE, LaBarbara EJ, & Phillips ML (2013). Dissociable patterns of abnormal frontal cortical activation during anticipation of an uncertain reward or loss in bipolar versus major depression. Bipolar Disorders, 15(8), 839–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coan JA, & Allen JJB (2004). Frontal EEG asymmetry as a moderator and mediator of emotion. Biological Psychology, 67(1–2), 7–50. [DOI] [PubMed] [Google Scholar]
- Conway KP, Compton W, Stinson FS, & Grant BF (2006). Lifetime Comorbidity of DSM-IV Mood and Anxiety Disorders and Specific Drug Use Disorders: Results From the National Epidemiologic Survey on Alcohol and Related Conditions. The Journal of Clinical Psychiatry, 67(02), 247–258. [DOI] [PubMed] [Google Scholar]
- Cooper JC, & Knutson B (2008). Valence and salience contribute to nucleus accumbens activation. NeuroImage, 39(1), 538–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope LM, Martz ME, Hardee JE, Zucker RA, & Heitzeg MM (2019). Reward activation in childhood predicts adolescent substance use initiation in a high-risk sample. Drug and Alcohol Dependence, 194, 318–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawe S, Gullo MJ, & Loxton NJ (2004). Reward drive and rash impulsiveness as dimensions of impulsivity: Implications for substance misuse. Addictive Behaviors, 29(7), 1389–1405. [DOI] [PubMed] [Google Scholar]
- Dawe S, & Loxton NJ (2004). The role of impulsivity in the development of substance use and eating disorders. Neuroscience & Biobehavioral Reviews, 28(3), 343–351. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Wang L, Bergman SR, Yaxley RH, Hooper SR, & Huettel SA (2013). Neural mechanisms of risky decision-making and reward response in adolescent onset cannabis use disorder. Drug and Alcohol Dependence, 133(1), 134–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Nystrom LE, Fissell C, Noll DC, & Fiez JA (2000). Tracking the Hemodynamic Responses to Reward and Punishment in the Striatum. Journal of Neurophysiology, 84(6), 3072–3077. [DOI] [PubMed] [Google Scholar]
- Depue RA, & Iacono WG (1989). Neurobehavioral Aspects of Affective Disorders. Annual Review of Psychology, 40(1), 457–492. [DOI] [PubMed] [Google Scholar]
- Depue RA, Krauss SP, & Spoont MR (1987). A two-dimensional threshold model of seasonal bipolar affective disorder. In Personality, psychopathology, and psychotherapy. Psychopathology: An interactional perspective (pp. 95–123). Academic Press. [Google Scholar]
- Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, … Lecca D (2004). Dopamine and drug addiction: The nucleus accumbens shell connection. Neuropharmacology, 47, 227–241. [DOI] [PubMed] [Google Scholar]
- Dickstein DP, Finger EC, Brotman MA, Rich BA, Pine DS, Blair JR, & Leibenluft E (2010). Impaired probabilistic reversal learning in youths with mood and anxiety disorders. Psychological Medicine, 40(7), 1089–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekhof EK, Kaps L, Falkai P, & Gruber O (2012). The role of the human ventral striatum and the medial orbitofrontal cortex in the representation of reward magnitude – An activation likelihood estimation meta-analysis of neuroimaging studies of passive reward expectancy and outcome processing. Neuropsychologia, 50(7), 1252–1266. [DOI] [PubMed] [Google Scholar]
- Dillon DG, Holmes AJ, Jahn AL, Bogdan R, Wald LL, & Pizzagalli DA (2008). Dissociation of neural regions associated with anticipatory versus consummatory phases of incentive processing. Psychophysiology, 45(1), 36–49. 10.1111/j.1469-8986.2007.00594.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutra SJ, Cunningham WA, Kober H, & Gruber J (2015). Elevated striatal reactivity across monetary and social rewards in bipolar I disorder. Journal of Abnormal Psychology, 124(4), 890–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutra SJ, Man V, Kober H, Cunningham WA, & Gruber J (2017). Disrupted cortico-limbic connectivity during reward processing in remitted bipolar I disorder. Bipolar Disorders, 19(8), 661–675. 10.1111/bdi.12560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Dickstein DP, Munson S, Eshel N, Pradella A, Jazbec S, Pine DS, & Leibenluft E (2004). Reward-related processes in pediatric bipolar disorder: A pilot study. Journal of Affective Disorders, 82, S89–S101. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Hariri AR, Martin SL, Silk JS, Moyles DL, Fisher PM, … Dahl RE (2009). Altered Striatal Activation Predicting Real-World Positive Affect in Adolescent Major Depressive Disorder. American Journal of Psychiatry, 166(1), 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein BI, Shamseddeen W, Axelson DA, Kalas C, Monk K, Brent DA, … Birmaher B (2010). Clinical, Demographic, and Familial Correlates of Bipolar Spectrum Disorders Among Offspring of Parents With Bipolar Disorder: Journal of the American Academy of Child & Adolescent Psychiatry, 49(4), 388–396. [PMC free article] [PubMed] [Google Scholar]
- Gorrindo T, Blair RJR, Budhani S, Dickstein DP, Pine DS, & Leibenluft E (2005). Deficits on a Probabilistic Response-Reversal Task in Patients With Pediatric Bipolar Disorder. American Journal of Psychiatry, 162(10), 1975–1977. [DOI] [PubMed] [Google Scholar]
- Haber SN, & Knutson B (2010). The Reward Circuit: Linking Primate Anatomy and Human Imaging. Neuropsychopharmacology, 35(1), 4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hägele C, Schlagenhauf F, Rapp M, Sterzer P, Beck A, Bermpohl F, … Heinz A (2015). Dimensional psychiatry: Reward dysfunction and depressive mood across psychiatric disorders. Psychopharmacology, 232(2), 331–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanford LC, Eckstrand K, Manelis A, Hafeman DM, Merranko J, Ladouceur CD, … Phillips ML (2019). The impact of familial risk and early life adversity on emotion and reward processing networks in youth at-risk for bipolar disorder. PLOS ONE, 14(12), e0226135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Brown SM, Williamson DE, Flory JD, de Wit H, & Manuck SB (2006). Preference for Immediate over Delayed Rewards Is Associated with Magnitude of Ventral Striatal Activity. Journal of Neuroscience, 26(51), 13213–13217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon-Jones E, Abramson LY, Nusslock R, Sigelman JD, Urosevic S, Turonie LD, … Fearn M (2008). Effect of Bipolar Disorder on Left Frontal Cortical Responses to Goals Differing in Valence and Task Difficulty. Biological Psychiatry, 63(7), 693–698. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Abramson LY, Sigelman J, Bohlig A, Hogan ME, & Harmon-Jones C (2002). Proneness to hypomania/mania symptoms or depression symptoms and asymmetrical frontal cortical responses to an anger-evoking event. Journal of Personality and Social Psychology, 82(4), 610–618. [PubMed] [Google Scholar]
- Heinrich A, Müller KU, Banaschewski T, Barker GJ, Bokde ALW, Bromberg U, … Nees F (2016). Prediction of alcohol drinking in adolescents: Personality-traits, behavior, brain responses, and genetic variations in the context of reward sensitivity. Biological Psychology, 118, 79–87. [DOI] [PubMed] [Google Scholar]
- Heitzeg MM, Hardee JE, & Beltz AM (2018). Sex differences in the developmental neuroscience of adolescent substance use risk. Current Opinion in Behavioral Sciences, 23, 21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzeg MM, Nigg JT, Hardee JE, Soules M, Steinberg D, Zubieta J-K, & Zucker RA (2014). Left middle frontal gyrus response to inhibitory errors in children prospectively predicts early problem substance use. Drug and Alcohol Dependence, 141, 51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommer DW, Bjork JM, & Gilman JM (2011). Imaging brain response to reward in addictive disorders: Imaging brain response to reward in addictive disorders. Annals of the New York Academy of Sciences, 1216(1), 50–61. [DOI] [PubMed] [Google Scholar]
- Hoogendam JM, Kahn RS, Hillegers MHJ, van Buuren M, & Vink M (2013). Different developmental trajectories for anticipation and receipt of reward during adolescence. Developmental Cognitive Neuroscience, 6, 113–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedras P, Jones A, & Field M (2013). The role of anticipation in drug addiction and reward. Neuroscience and Neuroeconomics, 1. [Google Scholar]
- Johnson SL (2005). Life events in bipolar disorder: Towards more specific models. Clinical Psychology Review, 25(8), 1008–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Edge MD, Holmes MK, & Carver CS (2012). The Behavioral Activation System and Mania. Annual Review of Clinical Psychology, 8(1), 243–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Eisner LR, & Carver CS (2009). Elevated expectancies among persons diagnosed with bipolar disorder. British Journal of Clinical Psychology, 48(2), 217–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Mehta H, Ketter TA, Gotlib IH, & Knutson B (2019). Neural responses to monetary incentives in bipolar disorder. NeuroImage: Clinical, 24, 102018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Ruggero CJ, & Carver CS (2005). Cognitive, Behavioral, and Affective Responses to Reward: Links with Hypomanic Symptoms. Journal of Social and Clinical Psychology, 24(6), 894–906. [Google Scholar]
- Just AL, Meng C, Smith DG, Bullmore ET, Robbins TW, & Ersche KD (2019a). Effects of familial risk and stimulant drug use on the anticipation of monetary reward: An fMRI study. Translational Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just AL, Meng C, Smith DG, Bullmore ET, Robbins TW, & Ersche KD (2019b). Effects of familial risk and stimulant drug use on the anticipation of monetary reward: An fMRI study. Translational Psychiatry, 9(1), 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahnt T, Park SQ, Haynes J-D, & Tobler PN (2014). Disentangling neural representations of value and salience in the human brain. Proceedings of the National Academy of Sciences, 111(13), 5000–5005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambouropoulos N, & Staiger PK (2004). Reactivity to Alcohol-Related Cues: Relationship Among Cue Type, Motivational Processes, and Personality. Psychology of Addictive Behaviors, 18(3), 275–283. [DOI] [PubMed] [Google Scholar]
- Kessing LV, Munkholm K, Faurholt-Jepsen M, Miskowiak KW, Nielsen LB, Frikke-Schmidt R, … Vinberg M (2017). The Bipolar Illness Onset study: Research protocol for the BIO cohort study. BMJ Open, 7(6), e015462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B (2005). Distributed Neural Representation of Expected Value. Journal of Neuroscience, 25(19), 4806–4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson, Adams CM, Fong GW, & Hommer D (2001). Anticipation of Increasing Monetary Reward Selectively Recruits Nucleus Accumbens. The Journal of Neuroscience, 21(16), RC159–RC159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Westdorp A, Kaiser E, & Hommer D (2000). FMRI Visualization of Brain Activity during a Monetary Incentive Delay Task. NeuroImage, 12(1), 20–27. [DOI] [PubMed] [Google Scholar]
- Kollmann B, Scholz V, Linke J, Kirsch P, & Wessa M (2017). Reward anticipation revisited- evidence from an fMRI study in euthymic bipolar I patients and healthy first-degree relatives. Journal of Affective Disorders, 219, 178–186. [DOI] [PubMed] [Google Scholar]
- Leon MI, & Shadlen MN (1998). Exploring the Neurophysiology of Decisions. Neuron, 21(4), 669–672. [DOI] [PubMed] [Google Scholar]
- Lewandowski KE, Whitton AE, Pizzagalli DA, Norris LA, Ongur D, & Hall M-H (2016). Reward Learning, Neurocognition, Social Cognition, and Symptomatology in Psychosis. Frontiers in Psychiatry, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyton M, & Vezina P (2013). Striatal ups and downs: Their roles in vulnerability to addictions in humans. Neuroscience & Biobehavioral Reviews, 37(9), 1999–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke J, King AV, Rietschel M, Strohmaier J, Hennerici M, Gass A, … Wessa M (2012). Increased Medial Orbitofrontal and Amygdala Activation: Evidence for a Systems-Level Endophenotype of Bipolar I Disorder. American Journal of Psychiatry, 169(3), 316–325. [DOI] [PubMed] [Google Scholar]
- López-Larson MP, DelBello MP, Zimmerman ME, Schwiers ML, & Strakowski SM (2002). Regional prefrontal gray and white matter abnormalities in bipolar disorder. Biological Psychiatry, 52(2), 93–100. [DOI] [PubMed] [Google Scholar]
- Luijten M, Schellekens AF, Kühn S, Machielse MWJ, & Sescousse G (2017). Disruption of Reward Processing in Addiction: An Image-Based Meta-analysis of Functional Magnetic Resonance Imaging Studies. JAMA Psychiatry, 74(4), 387. [DOI] [PubMed] [Google Scholar]
- Lutz K, & Widmer M (2014). What can the monetary incentive delay task tell us about the neural processing of reward and punishment? Neuroscience and Neuroeconomics, 33. [Google Scholar]
- Manelis A, Ladouceur CD, Graur S, Monk K, Bonar LK, Hickey MB, … Phillips ML (2016). Altered functioning of reward circuitry in youth offspring of parents with bipolar disorder. Psychological Medicine, 46(1), 197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manwani SG, Szilagyi KA, Zablotsky B, Hennen J, Griffin ML, & Weiss RD (2007). Adherence to Pharmacotherapy in Bipolar Disorder Patients With and Without Co-Occurring Substance Use Disorders. The Journal of Clinical Psychiatry, 68(08), 1172–1176. [DOI] [PubMed] [Google Scholar]
- Martz ME, Zucker RA, Schulenberg JE, & Heitzeg MM (2018). Psychosocial and neural indicators of resilience among youth with a family history of substance use disorder. Drug and Alcohol Dependence, 185, 198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason L, Eldar E, & Rutledge RB (2017). Mood Instability and Reward Dysregulation—A Neurocomputational Model of Bipolar Disorder. JAMA Psychiatry, 74(12), 1275. [DOI] [PubMed] [Google Scholar]
- Mason L, O’Sullivan N, Bentall RP, & El-Deredy W (2012). Better Than I Thought: Positive Evaluation Bias in Hypomania. PLoS ONE, 7(10), e47754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason L, O’Sullivan N, Montaldi D, Bentall RP, & El-Deredy W (2014). Decision-making and trait impulsivity in bipolar disorder are associated with reduced prefrontal regulation of striatal reward valuation. Brain, 137(8), 2346–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure SM (2004). Separate Neural Systems Value Immediate and Delayed Monetary Rewards. Science, 306(5695), 503–507. [DOI] [PubMed] [Google Scholar]
- McDannald MA, Lucantonio F, Burke KA, Niv Y, & Schoenbaum G (2011). Ventral Striatum and Orbitofrontal Cortex Are Both Required for Model-Based, But Not Model-Free, Reinforcement Learning. Journal of Neuroscience, 31(7), 2700–2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald C, Zanelli J, Rabe-Hesketh S, Ellison-Wright I, Sham P, Kalidindi S, Murray RM, & Kennedy N (2004). Meta-analysis of magnetic resonance imaging brain morphometry studies in bipolar disorder. Biological Psychiatry, 56(6), 411–417. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Jin R, He J-P, Kessler RC, Lee S, Sampson NA, … Zarkov Z (2011). Prevalence and Correlates of Bipolar Spectrum Disorder in the World Mental Health Survey Initiative. Archives of General Psychiatry, 68(3), 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, Stolar M, Stevens DE, Goulet J, Preisig MA, Fenton B, … Rounsaville BJ (1998). Familial Transmission of Substance Use Disorders. Archives of General Psychiatry, 55(11), 973. [DOI] [PubMed] [Google Scholar]
- Meyer B, Johnson SL, & Winters R (2001). Responsiveness to threat and incentive in bipolar disorder: Relations of the BIS/BAS scales with symptoms. Journal of Psychopathology and Behavioral Assessment, 23(3), 133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, & Paulus MP (2018). Toward biomarkers of the addicted human brain: Using neuroimaging to predict relapse and sustained abstinence in substance use disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 80, 143–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzkin JC, Baskin-Sommers A, Newman JP, Kiehl KA, & Koenigs M (2014). Neural correlates of substance abuse: Reduced functional connectivity between areas underlying reward and cognitive control: Neuropsychological Correlates of SUD. Human Brain Mapping, 35(9), 4282–4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller KU, Gan G, Banaschewski T, Barker GJ, Bokde ALW, Büchel C, Conrod P, Fauth-Bühler M, Flor H, Gallinat J, Garavan H, Gowland P, Heinz A, Ittermann B, Lawrence C, Loth E, Mann K, Martinot J-L, Nees F, … the IMAGEN Consortium. (2015). No differences in ventral striatum responsivity between adolescents with a positive family history of alcoholism and controls: MID and family history alcohol. Addiction Biology, 20(3), 534–545. [DOI] [PubMed] [Google Scholar]
- Nees F, Witt SH, Dinu-Biringer R, Lourdusamy A, Tzschoppe J, Vollstädt-Klein S, … Flor H (2015). BDNF Val66Met and reward-related brain function in adolescents: Role for early alcohol consumption. Alcohol, S074183291420200X. [DOI] [PubMed] [Google Scholar]
- Nestor LJ, McCabe E, Jones J, Clancy L, & Garavan H (2018a). Smokers and ex-smokers have shared differences in the neural substrates for potential monetary gains and losses: Reward processing in smokers. Addiction Biology, 23(1), 369–378. [DOI] [PubMed] [Google Scholar]
- Nestor LJ, McCabe E, Jones J, Clancy L, & Garavan H (2018b). Shared and divergent neural reactivity to non-drug operant response outcomes in current smokers and ex-smokers. Brain Research, 1680, 54–61. [DOI] [PubMed] [Google Scholar]
- Nusslock R, Abramson L, Harmon-Jones E, Alloy L, & Coan J (2009). Psychosocial interventions for bipolar disorder: Perspective from the behavioral approach system (BAS) dysregulation theory. Clinical Psychology: Science and Practice, 16(4), 449–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, & Alloy LB (2017). Reward processing and mood-related symptoms: An RDoC and translational neuroscience perspective. Journal of Affective Disorders, 216, 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, Almeida JR, Forbes EE, Versace A, Frank E, LaBarbara EJ, …Phillips ML (2012). Waiting to win: Elevated striatal and orbitofrontal cortical activity during reward anticipation in euthymic bipolar disorder adults: Nusslock et al. Bipolar Disorders, 14(3), 249–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, Harmon-Jones E, Alloy LB, Urosevic S, Goldstein K, & Abramson LY (2012). Elevated left mid-frontal cortical activity prospectively predicts conversion to bipolar I disorder. Journal of Abnormal Psychology, 121(3), 592–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldham S, Murawski C, Fornito A, Youssef G, Yücel M, & Lorenzetti V (2018). The anticipation and outcome phases of reward and loss processing: A neuroimaging meta-analysis of the monetary incentive delay task. Human Brain Mapping, 39(8), 3398–3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan N, Szczepanowski R, El-Deredy W, Mason L, & Bentall RP (2011). FMRI evidence of a relationship between hypomania and both increased goal-sensitivity and positive outcome-expectancy bias. Neuropsychologia, 49(10), 2825–2835. [DOI] [PubMed] [Google Scholar]
- Patel KT, Stevens MC, Meda SA, Muska C, Thomas AD, Potenza MN, & Pearlson GD (2013). Robust Changes in Reward Circuitry During Reward Loss in Current and Former Cocaine Users During Performance of a Monetary Incentive Delay Task. Biological Psychiatry, 74(7), 529–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, & Swartz HA (2014). A Critical Appraisal of Neuroimaging Studies of Bipolar Disorder: Toward a New Conceptualization of Underlying Neural Circuitry and a Road Map for Future Research. American Journal of Psychiatry, 171(8), 829–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Goetz E, Ostacher M, Iosifescu DV, & Perlis RH (2008). Euthymic Patients with Bipolar Disorder Show Decreased Reward Learning in a Probabilistic Reward Task. Biological Psychiatry, 64(2), 162–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prisciandaro JJ, Tolliver BK, Prescot AP, Brenner HM, Renshaw PF, Brown TR, & Anton RF (2017). Unique prefrontal GABA and glutamate disturbances in co-occurring bipolar disorder and alcohol dependence. Translational Psychiatry, 7(7), e1163–e1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prisciandaro James J., Rembold J, Brown DG, Brady KT, & Tolliver BK (2011). Predictors of clinical trial dropout in individuals with co-occurring bipolar disorder and alcohol dependence. Drug and Alcohol Dependence, 118(2–3), 493–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Lee Y, Stidd R, Long R, Greenstein D, Clasen L, … Giedd JN (2010). Longitudinally mapping the influence of sex and androgen signaling on the dynamics of human cortical maturation in adolescence. Proceedings of the National Academy of Sciences, 107(39), 16988–16993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Shaw PW, Lerch JP, Clasen LS, Greenstein D, Berman R, … Giedd JN (2014). Longitudinal four-dimensional mapping of subcortical anatomy in human development. Proceedings of the National Academy of Sciences, 111(4), 1592–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redlich R, Dohm K, Grotegerd D, Opel N, Zwitserlood P, Heindel W, … Dannlowski U (2015). Reward Processing in Unipolar and Bipolar Depression: A Functional MRI Study. Neuropsychopharmacology, 40(11), 2623–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salloum IM, & Thase ME (2000). Impact of substance abuse on the course and treatment of bipolar disorder: Impact of substance abuse. Bipolar Disorders, 2(3p2), 269–280. [DOI] [PubMed] [Google Scholar]
- Santos V, Coroa M, Caldeira S, Bajouco M, & Madeira N (2017). Neural connectivity in youth at-risk for bipolar disorder: A review of functional magnetic resonance imaging studies. International Journal of Clinical Neurosciences and Mental Health, 4(Suppl. 3), S02. [Google Scholar]
- Satterthwaite TD, Kable JW, Vandekar L, Katchmar N, Bassett DS, Baldassano CF, … Wolf DH (2015). Common and Dissociable Dysfunction of the Reward System in Bipolar and Unipolar Depression. Neuropsychopharmacology, 40(9), 2258–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott BH, Minuzzi L, Krebs RM, Elmenhorst D, Lang M, Winz OH, … Bauer A (2008). Mesolimbic Functional Magnetic Resonance Imaging Activations during Reward Anticipation Correlate with Reward-Related Ventral Striatal Dopamine Release. Journal of Neuroscience, 28(52), 14311–14319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiter S, Spengler S, Willert A, Mohnke S, Herold D, Erk S, … Bermpohl F (2016). Neural alterations of fronto-striatal circuitry during reward anticipation in euthymic bipolar disorder. Psychological Medicine, 46(15), 3187–3198. [DOI] [PubMed] [Google Scholar]
- Schultz W, & Dickinson A (2000). Neuronal Coding of Prediction Errors. Annual Review of Neuroscience, 23(1), 473–500. [DOI] [PubMed] [Google Scholar]
- Schumann G, Loth E, Banaschewski T, Barbot A, Barker G, Büchel C, … Struve M (2010). The IMAGEN study: Reinforcement-related behaviour in normal brain function and psychopathology. Molecular Psychiatry, 15(12), 1128–1139. [DOI] [PubMed] [Google Scholar]
- Seymour B, Daw N, Dayan P, Singer T, & Dolan R (2007). Differential Encoding of Losses and Gains in the Human Striatum. Journal of Neuroscience, 27(18), 4826–4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh MK, Chang KD, Kelley RG, Cui X, Sherdell L, Howe ME, … Reiss AL (2013). Reward Processing in Adolescents With Bipolar I Disorder. Journal of the American Academy of Child & Adolescent Psychiatry, 52(1), 68–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh MK, Kelley RG, Howe ME, Reiss AL, Gotlib IH, & Chang KD (2014). Reward Processing in Healthy Offspring of Parents With Bipolar Disorder. JAMA Psychiatry, 71(10), 1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith VC, Wilson CR, & COMMITTEE ON SUBSTANCE USE AND PREVENTION. (2016). Families Affected by Parental Substance Use. Pediatrics, 138(2), e20161575. [DOI] [PubMed] [Google Scholar]
- Soehner AM, Bertocci MA, Manelis A, Bebko G, Ladouceur CD, Graur S, … Phillips ML (2016). Preliminary investigation of the relationships between sleep duration, reward circuitry function, and mood dysregulation in youth offspring of parents with bipolar disorder. Journal of Affective Disorders, 205, 144–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, & Cservenka A (2017). Adolescence and drug use vulnerability: Findings from neuroimaging. Current Opinion in Behavioral Sciences, 13, 164–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbeis N, Haushofer J, Fehr E, & Singer T (2016). Development of Behavioral Control and Associated vmPFC-DLPFC Connectivity Explains Children’s Increased Resistance to Temptation in Intertemporal Choice. Cerebral Cortex, 26(1), 32–42. [DOI] [PubMed] [Google Scholar]
- Steinberg L (2010). A dual systems model of adolescent risk-taking. Developmental Psychobiology, 52, 216–224. [DOI] [PubMed] [Google Scholar]
- Stewart JL, Connolly CG, May AC, Tapert SF, Wittmann M, & Paulus MP (2014). Cocaine dependent individuals with attenuated striatal activation during reinforcement learning are more susceptible to relapse. Psychiatry Research: Neuroimaging, 223(2), 129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakowski S, & DelBello MP (2000). The co-occurrence of bipolar and substance use disorders. Clinical Psychology Review, 20(2), 191–206. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, Adler CM, & DelBello MP (2002). Volumetric MRI studies of mood disorders: Do they distinguish unipolar and bipolar disorder?: Volumetric MRI studies of mood disorders. Bipolar Disorders, 4(2), 80–88. [DOI] [PubMed] [Google Scholar]
- Swann AC (2010). The strong relationship between bipolar and substance-use disorder: Mechanisms and treatment implications. Annals of the New York Academy of Sciences, 1187(1), 276–293. [DOI] [PubMed] [Google Scholar]
- Swann AC, Lijffijt M, Lane SD, Steinberg JL, & Moeller FG (2009). Increased trait-like impulsivity and course of illness in bipolar disorder. Bipolar Disorders, 11(3), 280–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz JR, Weissman DG, Ferrer E, Beard SJ, Fassbender C, Robins RW, … Guyer AE (2020). Reward-Related Brain Activity Prospectively Predicts Increases in Alcohol Use in Adolescents. Journal of the American Academy of Child & Adolescent Psychiatry, 59(3), 391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trost S, Diekhof EK, Zvonik K, Lewandowski M, Usher J, Keil M, … Gruber O (2014). Disturbed Anterior Prefrontal Control of the Mesolimbic Reward System and Increased Impulsivity in Bipolar Disorder. Neuropsychopharmacology, 39(8), 1914–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urošević S, Halverson T, Youngstrom EA, & Luciana M (2018). Probabilistic reinforcement learning abnormalities and their correlates in adolescent bipolar disorders. Journal of Abnormal Psychology, 127(8), 807–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leijenhorst L, Zanolie K, Van Meel CS, Westenberg PM, Rombouts SARB, & Crone EA (2010). What Motivates the Adolescent? Brain Regions Mediating Reward Sensitivity across Adolescence. Cerebral Cortex, 20(1), 61–69. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, & Wang G-J (2003). The addicted human brain: Insights from imaging studies. Journal of Clinical Investigation, 111(10), 1444–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Koob GF, & McLellan AT (2016). Neurobiologic Advances from the Brain Disease Model of Addiction. New England Journal of Medicine, 374(4), 363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DM, Bell MR, Flores C, Gulley JM, Willing J, & Paul MJ (2017). Adolescence and Reward: Making Sense of Neural and Behavioral Changes Amid the Chaos. The Journal of Neuroscience, 37(45), 10855–10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland BJ, Welsh RC, Yau W-YW, Zucker RA, Zubieta J-K, & Heitzeg MM (2013). Accumbens functional connectivity during reward mediates sensation-seeking and alcohol use in high-risk youth. Drug and Alcohol Dependence, 128(1–2), 130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland BJ, Zucker RA, Zubieta J-K, & Heitzeg MM (2017). Striatal dopaminergic reward response relates to age of first drunkenness and feedback response in at-risk youth: Risk and NAcc reward response. Addiction Biology, 22(2), 502–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan R, Watts R, Orr CA, Althoff RR, Artiges E, Banaschewski T, … Garavan H (2014). Neuropsychosocial profiles of current and future adolescent alcohol misusers. Nature, 512(7513), 185–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitton AE, Treadway MT, & Pizzagalli DA (2015). Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Current Opinion in Psychiatry, 28(1), 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, & Fiez JA (2004). Prefrontal responses to drug cues: A neurocognitive analysis. Nature Neuroscience, 7(3), 211–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo C-W, Krishnan A, & Wager TD (2014). Cluster-extent based thresholding in fMRI analyses: Pitfalls and recommendations. NeuroImage, 91, 412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau W-YW, Zubieta J-K, Weiland BJ, Samudra PG, Zucker RA, & Heitzeg MM (2012). Nucleus Accumbens Response to Incentive Stimuli Anticipation in Children of Alcoholics: Relationships with Precursive Behavioral Risk and Lifetime Alcohol Use. Journal of Neuroscience, 32(7), 2544–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip SW, Worhunsky PD, Rogers RD, & Goodwin GM (2015). Hypoactivation of the Ventral and Dorsal Striatum During Reward and Loss Anticipation in Antipsychotic and Mood Stabilizer-Naive Bipolar Disorder. Neuropsychopharmacology, 40(3), 658–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink CF, Pagnoni G, Martin-Skurski ME, Chappelow JC, & Berns GS (2004). Human Striatal Responses to Monetary Reward Depend On Saliency. Neuron, 42(3), 509–517. [DOI] [PubMed] [Google Scholar]
- Zinser MC, Fiore MC, Davidson RJ, & Baker TB (1999). Manipulating smoking motivation: Impact on an electrophysiological index of approach motivation. Journal of Abnormal Psychology, 108(2), 240–254. [DOI] [PubMed] [Google Scholar]
- Zucker RA, Fitzgerald HE, Refior SK, Puttler LI, Pallas DM, & Ellis DA (2000). The clinical and social ecology of childhood for children of alcoholics: Description of a study and implications for a differentiated social policy. In Children of Addiction: Research, Health and Policy Issues (pp. 109–141). Routledge Falmer. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


