Abstract
Chlamydia trachomatis injects bacterial effector proteins into human epithelial cells to facilitate the establishment of new infections. The chlamydial type III secreted effector translocated actin recruiting phosphoprotein (Tarp) has been shown to nucleate and bundle actin filaments. It is also believed to initiate new signaling pathways via an N-terminal phosphorylation domain. A comprehensive understanding of the host pathways that are controlled by Tarp to aid in the establishment of a successful infection remains incomplete. To gain further insight into the cell signaling regulated by Tarp, we generated transgenic fruit flies engineered to express the N-terminal domain of Tarp. As many signaling pathways are conserved between flies and mammals, we hypothesized that expression of the Tarp N-domain in the fruit fly might disrupt key pathways, resulting in developmental defects. Tarp N-domain expression in the fruit fly resulted in a mechanosensory bristle duplication phenotype similar to a previously characterized fly phenotype found to be a consequence of defects in the Hippo pathway. Tarp-dependent disruption of the Hippo pathway was confirmed in a C. trachomatis tissue culture infection model. The capability of Tarp to alter Hippo pathway signaling in infected epithelial cells is a previously unrecognized pathway commandeered by chlamydia and likely contributes to the establishment of chlamydia’s intracellular niche.
Keywords: Chlamydia trachomatis, Tarp, Hippo pathway, YAP, LATS1/2
Introduction:
Chlamydia trachomatis is an obligate intracellular Gram-negative bacterium which has evolved to efficiently invade human cells. Distinct biovars give rise to genital, lymphatic or ocular infections [1]. Although unique tissue tropisms result in different clinical presentations, all Chlamydia share a common biphasic developmental cycle in which the bacteria transition between two distinct forms called the elementary body (EB) and the reticulate body (RB) [2]. Reticulate bodies are characterized as the actively growing intracellular form which replicate within the protective confines of a parasitophorous vacuole called an inclusion. Once replication is complete, the intracellular RBs differentiate into the infectious EBs and are released by simultaneous cell and inclusion lysis or by an extrusion mechanism in which the intact inclusion is ejected from the infected cell [3]. A new infection results from the cumulative efforts of multiple processes initiated once the extracellular EB attaches itself to a host cell receptor. For C. trachomatis, receptors such as fibroblast growth factor 2 (FGF2) and ephrin type-A receptor 2 (EphA2) have been implicated [4,5]. Receptor engagement is followed by signaling and cytoskeletal changes inside the host that drive bacterial internalization. EB entry is facilitated in part by a type III secretion system in which prepackaged effector proteins are injected into the host cell at the time of entry [6]. One effector protein called translocated actin recruiting phosphoprotein (Tarp) is responsible for driving both cytoskeletal and cell signaling changes [7-11]. Tarp is a multifunctional chlamydial protein which harbors an N-terminal phosphorylation domain and multiple C-terminal actin binding domains. The C-terminal actin binding domains directly promote actin polymerization and actin bundle formation [10,12]. The N-terminal phosphorylation domain harbors six tyrosine rich repeats and this region is capable of being phosphorylated by multiple tyrosine kinases (Src, Abl, Syk) following injection into the host cell [9,13]. Phosphorylated Tarp is known to directly associate with SH2-domain containing proteins such as phosphatidylinositol 3-kinase (PI3K) and SHC-transforming protein 1 (SHC1) [14,15]. Furthermore, phosphorylated peptides representing individual Tarp tyrosine rich repeats have been shown to associate with a complex of host cell signaling molecules including son of sevenless homolog 1 (Sos1), Abelson interactor 1 (Abi1), epidermal growth factor receptor kinase substrate 8 (Esp8) and guanine nucleotide exchange factor VAV2 (Vav2) [14]. Collectively, the recruited host cell signaling molecules are believed to direct cytoskeletal and early anti-apoptotic signals favorable for C. trachomatis invasion and residence in the newly infected cell. Although many Tarp interacting molecules have previously been identified with certain functions implied, it is likely that additional novel pathways have yet to be characterized. Herein we used a fruit fly (Drosophila melanogaster) model system to investigate Tarp-mediated signaling. As many signaling pathways are conserved between flies and mammals, we hypothesized that expression of the N-terminal domain of Tarp in the fruit fly may reveal important cell signaling perturbations through detection of characteristic developmental defects and phenotypes. Our data revealed that expression of N-terminal Tarp in the dorsal thorax of flies resulted in duplication of the large mechanosensory bristles (macrochaetes), similar to a previously described fly phenotype due to defective Hippo pathway signaling [16]. Changes in Hippo pathway signaling was also confirmed in a C. trachomatis tissue culture infection model in a Tarp-dependent manner. The Hippo pathway is a highly conserved kinase signaling cascade that plays a role in a large number of cellular processes such as cell fate determination, proliferation, and survival [17]. Our findings indicate that the Tarp effector intersects the host cell Hippo pathway, which likely proves advantageous for chlamydial development.
Materials and methods:
Drosophila melanogaster genetics, rearing, bristle scoring and imaging.
The open reading frame that encodes for the N-terminal region of Chlamydia trachomatis serovar L2 strain 434/Bu (ATCC VR-902B) Tarp (amino acids 1-431) (GenBank: AAT47185.1) was PCR amplified from C. trachomatis genomic DNA using primers 5’-GTTTGCGGCCGCATGACGAATTCTATATCAGGTGATC-3’ and 5’-GCCCTCTAGACTACTCGTTACGAGGCCCTGTTGTG-3’ and cloned into the Drosophila transformation and expression vector (pUAST) using the Not1 and Xba1 restriction sites. The sequence-verified pUAST-N-terminal Tarp plasmid was used for P-element-mediated germline transformation to generate transgenic flies [18]. The GAL4 driver stocks used are,: w[*]: ubi-GAL4/CyO (BDSC_32551), expresses GAL4 in all cells; y[1] w[1118]: pnr-GAL4/TM3,UAS-y,Ser (BSDC_3039), expresses GAL4 in tissues along the dorsal length of the fly; hs-GAL4/CyO (a generous gift from J. McDonald, Kansas State University) is a heat-shock-inducible ubiquitous GAL4. Viability experiments were performed at 22°C. Fly stocks and crosses were maintained at 25°C. Duplication in at least one of the dorsocentral or scutellar macrochaets was considered a bristle duplication phenotype. Fly notum (dorsal thorax) images were captured on a Zeiss Stemi 508 with an Axiocam 208 color camera.
Chlamydia trachomatis and host cells.
C. trachomatis L2 (LGV 434) clones [8] were propagated in HeLa 229 cells (ATCC CCL-2.1) and purified by diatrizoate meglumine and diatrizoate sodium density gradient centrifugation [19]. HeLa 229 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% L-glutamine.
Infections and Immunoblots.
Clones of C. trachomatis L2 (wild type, Δtarp and Δtarp+pTarp) [8] were used to infect HeLa cells at an MOI of 100, and the cultures were incubated at 37°C for the indicated time points. Cells were harvested either using sample buffer for immunoblot analysis or washed and frozen for RNA extraction. Proteins were separated by SDS-PAGE and immunoblot performed using antibodies for the Hippo pathway (Hippo antibody sampler kit) (Cell signaling), the anti-actin C4 monoclonal antibody (Chemicon International), the anti-chlamydial Hsp60 A57-B9 monoclonal antibody (Thermo Fisher Scientific), and the anti-Tarp rabbit polyclonal antibody [7].
RNA extraction, reverse transcription, and qPCR
Host cell RNA was isolated using the RNeasy plus kit (Qiagen) and cDNA synthesized using the Quantitect reverse transcription kit (Qiagen). Quantitative PCR was performed with iQ SYBR Green Supermix (Bio-Rad) and run on a CFX96 C1000 RThermal Cycler (Bio-Rad). The primers used for amplification are: human TEAD4: forward, 5′-CTGGACAAGCCCATCGACAA-3′; reverse, 5′-AGCTTGATGTAGCGGGCAAT-3′; human XIAP: forward, 5′-TGGGGTTCAGTTTCAAGGACA-3′; reverse, 5′-CGCCTTAGCTGCTCTTCAGT −3′; and human GAPDH, forward, 5′-AGCCACATCGCTCAGACAC-3′; reverse, 5′-GCCCAATACGACCAAATCC-3′.
Statistical analysis
Statistical analyses were performed using GraphPad Prism (version 9.0.0, GraphPad) and the one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparisons test to the uninfected control.
Results
Transgenic fruit flies expressing the N-terminal domain of Tarp present with dorsal bristle duplication.
The Chlamydia trachomatis Tarp effector can be divided into two parts that possess unique biochemical activities (Fig. 1A). The N-terminal portion of Tarp contains 6 tyrosine-rich repeats located between amino acids 125 and 424 and is implicated in redirecting host cell signaling (Fig. 1A). In order to focus on Drosophila melanogaster phenotypes associated with Tarp’s tyrosine-rich N-terminal domain and possible cell signaling pathways influenced by Tarp, transgenic flies were developed which expressed the Tarp N-terminal domain only. The exclusion of the C-terminal domain of Tarp was purposeful as we were interested in Tarp-mediated phenotypes unrelated to actin dynamics. Multiple fly clones containing the N-terminal Tarp transgene were successfully generated. The N-terminal Tarp transgene is under the control of the UAS promoter and depends on the presence of the GAL4 protein to drive efficient expression [20]. Crossing the UAS-N-terminal Tarp fly stocks to a ubiquitous or tissue-specific GAL4 fly stock gave rise to progeny expressing N-terminal Tarp in the tissues that expressed GAL4.
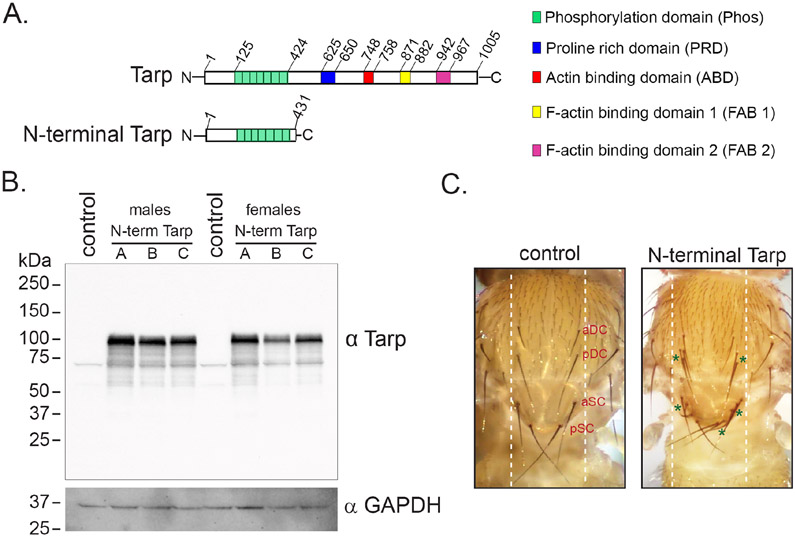
Figure 1. The N-terminal fragment of C. trachomatis Tarp induces mechanosensory bristle duplication in Drosophila melanogaster.
(A) Schematic diagram of full-length Tarp (top) and the N-terminal fragment (amino acids 1-431) of Tarp (bottom). The functional domains of Tarp are indicated. (B) Expression of the N-terminal Tarp protein in transgenic flies is confirmed by immunoblot. Immunoblot analysis using anti-Tarp antibodies (α Tarp) was performed on whole fly lysates following a 2-hour 37°C heat shock of hs-GAL4/UAS-N-terminal Tarp fly lines (N-term Tarp A, B and C) and hs-GAL4/UAS-mCD8:GFP (control) flies. GAPDH was used as a loading control (α GAPDH). (C) Representative images of the Drosophila notum of pnr-GAL4/UAS-mCD8:GFP (control) flies and pnr-GAL4/UAS-N-terminal Tarp (N-terminal Tarp) flies. The normal pattern and number of dorsocentral bristles, anterior (aDC) and posterior (pDC), as well as scutellar bristles, anterior (aSC) and posterior (pDC) is shown in the control. Transgene expression occurs between the white dashed lines. Excess dorsocentral and scutellar bristles in the N-terminal Tarp image are indicated with green asterisks.
We first set out to confirm the expression of N-terminal Tarp in transgenic Drosophila. Ubiquitous expression of N-terminal Tarp, using ubi-GAL4, was found to be 100% lethal. Two independent trials using three unique transgenic stocks of N-terminal Tarp failed to generate viable ubi-GAL4/UAS-N-terminal Tarp adults out of 101-158 adult progeny scored per cross (Table S1). Ubiquitous expression of a control GFP transgene did not impact adult viability, producing all adult flies at the correct Mendelian ratio in both trials (Table S1). To circumvent the lethal phenotype of constitutive ubiquitous N-terminal Tarp expression, ubiquitous expression of N-terminal Tarp was transiently induced using a heat-shock-inducible GAL4. hs-GAL4/UAS-N-terminal Tarp adults were generated, and the flies were subjected to 2 hours of heat shock at 37°C to induce N-terminal Tarp expression. Following transgene induction, whole flies were homogenized in SDS-PAGE sample buffer. Immunoblot analysis of the whole fly lysates using anti-Tarp antibodies revealed a strong band at ~100 kDa in all hs-GAL4/UAS-N-terminal Tarp samples that was absent in the control hs-GAL4/UAS-GFP samples (Fig 1B). The N-Terminal Tarp protein size observed in the immunoblot was consistent with that of recombinant N-terminal Tarp fragments previously characterized [9].
We expressed N-terminal Tarp in a tissue-specific manner as another means to generate surviving adults. For this, we used pnr-GAL4, which expresses in the tissues along the dorsal midline of the adult fly [21]. This includes the dorsal portion of the fly thorax, called the notum, which contains an array of small and large mechanosensory bristles, whose pattern and number is developmentally controlled (Fig 1C, left, white dashed lines). Within this region, there are two pairs of dorsocentral macrochaetes (aDC and pDC) and 2 pair of scutellar macrochaetes (aSC and pSC). Expression of N-terminal Tarp using pnr-GAL4 resulted in dramatic duplication of dorsocentral and scutellar macrochaetes (Fig 1C, right, green asterisks). Bristle duplication was rare in control GFP-expressing flies (~1%) but was highly prevalent in N-terminal Tarp-expressing flies (67.5%-100%) (Table S2). Interestingly, our findings strongly phenocopied the macrochaete duplication observed upon perturbation of the Hippo pathway in the notum [16]. This suggested that Tarp alone, through its N-terminal domain, may be sufficient to impact Hippo pathway signaling.
The chlamydial Tarp effector intersects Hippo pathway signaling.
The Hippo pathway is a conserved signaling pathway first identified in Drosophila melanogaster but subsequently identified in mammals and is associated with many mammalian genes that function as oncogenes or tumor suppressors [17]. The bristle duplication observed in fruit flies expressing N-terminal Tarp is similar to fly phenotypes that result from mutations in the Hippo pathway, which regulates cell proliferation and apoptosis [16]. We therefore investigated whether Hippo pathway signaling was altered in chlamydia-infected cells using a panel of antibodies targeting key Hippo pathway proteins. We compared the kinetics of the Hippo pathway core signaling components in wild type C. trachomatis infected cells to cells infected with a mutant clone lacking the Tarp effector (Δtarp). At 6- and 8-hours post-infection, lower levels of yes-associated protein 1 (YAP), phosphorylated YAP (serine residues 127 and 397), and large tumor suppressor kinase (LATS1) were detected in the Δtarp-infected cells compared to the wild type-infected cells (Fig. 2A). In both infections YAP and WW domain-containing transcription regulator protein 1 (WWTR1 also known as TAZ) protein levels decreased between 2- and 8- hours post infection, although YAP levels decreased to a greater extent in the mutant infected cells. Interestingly, no difference in the protein levels of the mammalian homolog of the Hippo kinase, sterile-20-type kinase (STK4/3) also known as MST1 or MST 2 were found between the wild type- and Δtarp-infected cells (Fig. 2A). Because time points 4-, 6-, and 8-hours post-infection revealed Tarp-dependent alterations to YAP, phosphorylated YAP and LATS1, we expanded the study to include uninfected control cells and the Tarp complement clone for each of these time points. The results indicated that at 6- and 8-hours post-infection, YAP, phosphorylated YAP and LATS1 were reduced in the host cells infected with the Tarp mutant compared to cells infected with the wild type and complement clones. Together, these results support a role for Tarp in the modulation of the Hippo pathway during chlamydia infection.
Figure 2. C. trachomatis affects the Hippo pathway in a Tarp-dependent manner.
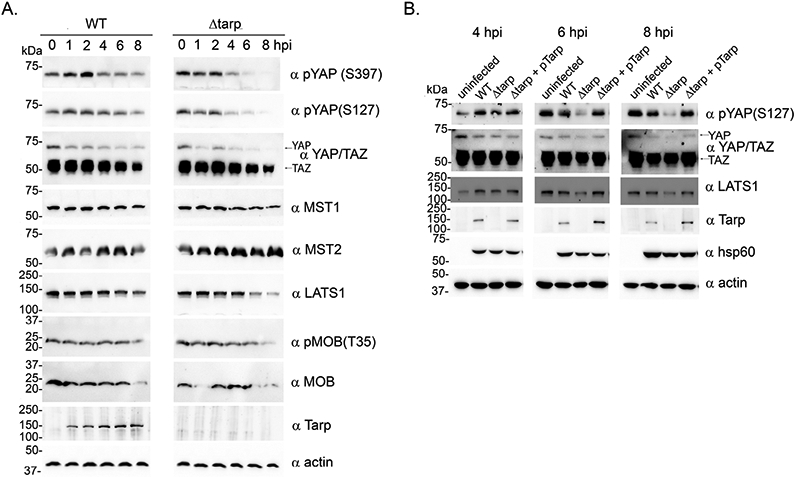
(A) HeLa cells were infected with either wild-type C. trachomatis L2 (WT), or a tarP deletion mutant (Δtarp) for 0-, 1-, 2-, 4-, 6- or 8-hours. Culture material was harvested, and protein lysates were generated. Proteins were resolved by SDS-PAGE and immunoblots were performed with actin (α actin), Tarp (α Tarp), large tumor suppressor (α LATS), Yes-associated protein (YAP) and WW domain-containing transcription regulator protein1 (WWTR1 also known as TAZ) are both recognized by the same polyclonal sera (α YAP/TAZ), mammalian sterile-20-like 1 (α MST1), mammalian sterile-20-like 2 (α MST2), phosphorylated YAP (α pYAPS397 and α pYAPS127), mps one binder kinase activator-like 1A and 1B (α MOB) and phosphorylated MOB (α pMOBT35) (B) Similar to (A) with the addition of uninfected control cells (uninfected) and HeLa cells infected with C. trachomatis mutant complemented with pTarp (Δtarp + pTarp) at 4-, 6- and 8- hours. Antisera specific for C. trachomatis heat shock protein 60 (α hsp60) served as an additional control.
C. trachomatis increases the mRNA levels of YAP-dependent genes in a Tarp-dependent manner.
In order to investigate potential downstream consequences of Tarp-dependent alterations in Hippo pathway signaling, the mRNA levels of YAP-dependent genes were measured. Total RNA was isolated from uninfected and C. trachomatis wild type, Tarp mutant and Tarp complement infected cells after an 8-hour incubation. The levels of expression of well characterized Hippo pathway genes encoding TEAD4 [22], a transcription factor that associates with YAP to regulate gene expression and XIAP [23], a protein that inhibits programmed cell death were determined by quantitative PCR. Our results demonstrated that TEAD4 and XIAP transcripts were significantly elevated in wild type C. trachomatis and complement infected cells compared to the uninfected control. Interestingly, the levels of these transcripts in the Δtarp -infected cells were similar to or slightly lower compared to those of the uninfected cells (Fig. 3), further supporting the finding that C. trachomatis modulation of the Hippo pathway is Tarp-dependent.
Figure 3. YAP regulated transcripts TEAD4 and XIAP are induced during C. trachomatis infection in a Tarp-dependent manner.
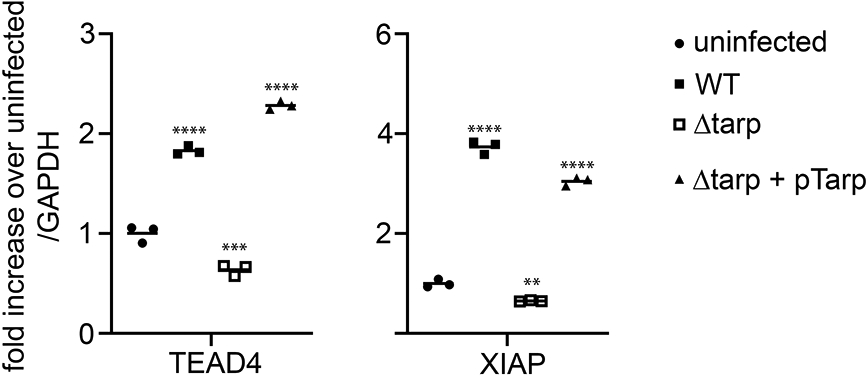
HeLa cells were infected with C. trachomatis L2 (WT), a tarP deletion mutant (Δtarp), or the mutant complemented with pTarp (Δtarp + pTarp). Mock infected cells (uninfected) served as the negative control. RNA was harvested from uninfected or infected cells 8 hours post infection. Data are presented as the relative fold change in transcriptional enhancer factor TEF 4 (TEAD4) and X-linked inhibitor of apoptosis protein (XIAP) mRNA levels in infected versus uninfected cells normalized to GAPDH transcripts. Statistical analysis was performed using a one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparisons test to the uninfected control (** p<0.01, ***p<0.001, **** p<0.0001).
Discussion
The tyrosine-rich repeat domain of Tarp is thought to redirect host cell signaling once delivered into the host cell. Once phosphorylated, SH2-domain containing proteins are capable of directly binding to Tarp [14,15]. The possible consequence of Tarp binding may lead to enhancement or sequestration of specific host signaling pathways. We hypothesized that additional yet undefined signaling pathways might be disrupted once Tarp is injected into a host cell. In order to explore this possibility, we leveraged a Drosophila melanogaster model and generated fruit flies that express the N-terminal tyrosine-rich repeat domain of Tarp. Previous studies have highlighted the utility of Drosophila in identifying novel functions for bacterial effectors [24]. Drosophila melanogaster is an established model organism with an abundance of genetic tools, reagents, and approaches available for study. More importantly, the molecular and cellular mechanisms that govern the various aspects important for Drosophila development are well defined. Therefore, perturbations in the proper development of easily scorable adult fly structures can often be traced back to the relevant molecular pathways. Using transgenic expression, Drosophila becomes a powerful in vivo platform to investigate the impact of the C. trachomatis Tarp effector on host cell biology without the confounding influence of active infection. Herein, we focused on elucidation of molecular events specifically associated with the N-terminal portion of Tarp. Interestingly, N-terminal Tarp expression in the tissues that give rise to the dorsal thorax, or notum, of flies produced a striking and highly penetrant duplication of the large mechanosensory bristles. This phenotype has been described previously for flies in which Hippo pathway signaling has been disrupted [16]. The Hippo pathway was first characterized in Drosophila and is associated with organ development [25]. Subsequent studies revealed that the Hippo pathway is conserved in mammals albeit with additional levels of homeostatic regulation [26]. In order to verify that Chlamydia trachomatis infection affects the Hippo pathway of host cells in a Tarp-dependent manner, mammalian Hippo pathway homologs were assessed by immunoblot analysis of uninfected and C. trachomatis infected cells. Interestingly, YAP (the mammalian homolog of Drosophila’s Yorkie transcriptional coactivator), phosphorylated YAP and LATS1 (the mammalian homolog to Drosophila’s Warts kinase), were found to be reduced in Δtarp -infected cells compared to wild type and complement controls. Unphosphorylated YAP is the active form of YAP, which can promote expression of transcriptional targets that regulate cell growth, proliferation and apoptosis (anti-apoptotic factors). Our data indicate that the absence of Tarp results in lower overall amounts of YAP (and TAZ) and LATS1 in infected host cells, suggesting that C. trachomatis infection intersection of the Hippo pathway is Tarp-dependent. Furthermore, expression of YAP/TAZ-TEAD regulated target genes, XIAP and the co-transcription factor TEA-domain family member (TEAD) itself [22] was significantly increased in chlamydia infected cells compared to uninfected control cells. Induction of XIAP and TEAD transcripts by chlamydia infection required Tarp. Although the reduced signal for phosphorylated YAP in the Tarp mutant might imply the potential for greater expression of target genes, we believe the reduced phosphorylation level of YAP is likely a consequence of reduced total levels of YAP in the Tarp mutant infected cells. Therefore, we propose that the Tarp effector slows down YAP and LATS1 degradation by an unknown mechanism, leading to expression of YAP/TAZ-TEAD target genes.
Other pathogens have developed ways to hijack the Hippo pathway. Infections of human umbilical vein endothelial cells (HUVEC) by the intracellular apicomplexan parasite Toxoplasma gondii results in increased phosphorylation of LATS1 and cytosolic retention of YAP leading to reduced YAP target gene expression [27]. Helicobacter pylori, a bacterium associated with gastric and duodenal ulcers and gastric cancer delivers CagA to gastric epithelial cells via a type IV secretion system (T4SS). A recent study revealed that deletion of cagA results in reduced expression of YAP in H. pylori infections [28]. Furthermore, lower levels of YAP in the absence of cagA suppressed H. pylori induced epithelial-mesenchymal transition (EMT) drawing a mechanistic link between H. pylori infections and gastric cancer [28]. The intracellular pathogen Legionella pneumophila employs the effector LegK7 to hijack the Hippo signaling pathway in mammalian macrophages [29]. LegK7 acts as a Hippo/MST1 kinase imposter to phosphorylate the scaffolding protein Mps one binder kinase activator 1A (MOB1A). MOB1A phosphorylation consequentially leads to the degradation of TAZ and YAP and a transcriptional profile more advantageous for bacterial intracellular growth and survival [29].
Whether the chlamydial Tarp effector can disrupt YAP and TAZ through one or more of the mechanisms used by other pathogens is unknown. The precise interplay between the mammalian homologs in the pathway that lead to reduced levels of YAP and TAZ remain to be determined but is a promising avenue for future research. Collectively, our work provides evidence that the C. trachomatis effector Tarp is able to alter the Hippo pathway in infected host cells and this potentially contributes to the mechanisms that chlamydia uses to hijack host cell functions to promote infection and pathogenesis.
Supplementary Material
Supplemental Table 1. N-terminal Tarp and GFP control transgenic fly viability scores.
The viability of transgenic flies ubiquitously expressing N-terminal Tarp (N-term Tarp A, B or C) or GFP control using the ubi-GAL4 driver presented in two trials with male and female data provided separately.
Supplemental Table 2. N-terminal Tarp and GFP control transgenic fly bristle duplication scores.
The duplication of dorsocentral and scutellar macrochaete bristles scores for transgenic flies following heat shock induced expression of N-terminal Tarp (N-term Tarp A, B or C) or GFP control using the pnr-GAL4 driver presented in two trials with male and female data provided separately.
The N-domain of Tarp expressed in the fruit fly results in bristle duplications.
The chlamydial effector Tarp intersects the Hippo signaling pathway.
Chlamydia trachomatis infected cells demonstrate altered Hippo signaling
Acknowledgments
The authors thank members of the Mollie W. Jewett Laboratory for helpful discussions as well as acknowledge the assistance of Kayli Rohal. The authors would like to thank a Dr. Jocelyn McDonald of Kansas State University for fly stocks used in this study. This work is supported by Public Health Service grants from the National Institutes of Health, NIAID, R01AI139242 and R21AI148999 awarded to T.J.J.
Footnotes
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Elwell C, Mirrashidi K, Engel J, Chlamydia cell biology and pathogenesis, Nat Rev Microbiol 14 (2016) 385–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Moulder JW, Interaction of chlamydiae and host cells in vitro, Microbiol Rev 55 (1991) 143–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hybiske K, Stephens RS, Mechanisms of host cell exit by the intracellular bacterium Chlamydia, Proc Natl Acad Sci U S A 104 (2007) 11430–11435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Subbarayal P, Karunakaran K, Winkler AC, Rother M, Gonzalez E, Meyer TF, Rudel T, EphrinA2 receptor (EphA2) is an invasion and intracellular signaling receptor for Chlamydia trachomatis, PLoS Pathog 11 (2015) e1004846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kim JH, Jiang S, Elwell CA, Engel JN, Chlamydia trachomatis Co-opts the FGF2 signaling pathway to enhance infection, PLoS Pathog 7 e1002285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ferrell JC, Fields KA, A working model for the type III secretion mechanism in Chlamydia, Microbes Infect (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Clifton DR, Fields KA, Grieshaber SS, Dooley CA, Fischer ER, Mead DJ, Carabeo RA, Hackstadt T, A chlamydial type III translocated protein is tyrosine-phosphorylated at the site of entry and associated with recruitment of actin, Proc Natl Acad Sci U S A 101 (2004) 10166–10171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ghosh S, Ruelke EA, Ferrell JC, Bodero MD, Fields KA, Jewett TJ, Fluorescence-Reported Allelic Exchange Mutagenesis-Mediated Gene Deletion Indicates a Requirement for Chlamydia trachomatis Tarp during In Vivo Infectivity and Reveals a Specific Role for the C Terminus during Cellular Invasion, Infect Immun 88 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Jewett TJ, Dooley CA, Mead DJ, Hackstadt T, Chlamydia trachomatis tarp is phosphorylated by src family tyrosine kinases, Biochem Biophys Res Commun 371 (2008) 339–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jewett TJ, Fischer ER, Mead DJ, Hackstadt T, Chlamydial TARP is a bacterial nucleator of actin, Proc Natl Acad Sci U S A 103 (2006) 15599–15604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jewett TJ, Miller NJ, Dooley CA, Hackstadt T, The conserved Tarp actin binding domain is important for chlamydial invasion, PLoS Pathog 6 (2010) e1000997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Jiwani S, Alvarado S, Ohr RJ, Romero A, Nguyen B, Jewett TJ, Chlamydia trachomatis Tarp harbors distinct G and F actin binding domains that bundle actin filaments, J Bacteriol 195 (2013) 708–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mehlitz A, Banhart S, Hess S, Selbach M, Meyer TF, Complex kinase requirements for Chlamydia trachomatis Tarp phosphorylation, FEMS Microbiol Lett 289 (2008) 233–240. [DOI] [PubMed] [Google Scholar]
- [14].Lane BJ, Mutchler C, Al Khodor S, Grieshaber SS, Carabeo RA, Chlamydial entry involves TARP binding of guanine nucleotide exchange factors, PLoS Pathog 4 (2008) e1000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mehlitz A, Banhart S, Maurer AP, Kaushansky A, Gordus AG, Zielecki J, Macbeath G, Meyer TF, Tarp regulates early Chlamydia-induced host cell survival through interactions with the human adaptor protein SHC1, J Cell Biol 190 (2010) 143–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wang LH, Baker NE, Salvador-Warts-Hippo pathway regulates sensory organ development via caspase-dependent nonapoptotic signaling, Cell Death Dis 10 (2019) 669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zheng Y, Pan D, The Hippo Signaling Pathway in Development and Disease, Dev Cell 50 (2019) 264–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Brand AH, Perrimon N, Targeted gene expression as a means of altering cell fates and generating dominant phenotypes, Development 118 (1993) 401–415. [DOI] [PubMed] [Google Scholar]
- [19].Scidmore MA, Cultivation and Laboratory Maintenance of Chlamydia trachomatis, Curr Protoc Microbiol Chapter 11 (2005) Unit 11A 11. [DOI] [PubMed] [Google Scholar]
- [20].Southall TD, Elliott DA, Brand AH, The GAL4 System: A Versatile Toolkit for Gene Expression in Drosophila, CSH Protoc 2008 (2008) pdb top49. [DOI] [PubMed] [Google Scholar]
- [21].Calleja M, Herranz H, Estella C, Casal J, Lawrence P, Simpson P, Morata G, Generation of medial and lateral dorsal body domains by the pannier gene of Drosophila, Development 127 (2000) 3971–3980. [DOI] [PubMed] [Google Scholar]
- [22].Lin KC, Park HW, Guan KL, Regulation of the Hippo Pathway Transcription Factor TEAD, Trends Biochem Sci 42 (2017) 862–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Holcik M, Gibson H, Korneluk RG, XIAP: apoptotic brake and promising therapeutic target, Apoptosis 6 (2001) 253–261. [DOI] [PubMed] [Google Scholar]
- [24].Boyer L, Paquette N, Silverman N, Stuart LM, Bacterial effectors: learning on the fly, Adv Exp Med Biol 710 (2012) 29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Pan D, The hippo signaling pathway in development and cancer, Dev Cell 19 (2010) 491–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Moroishi T, Park HW, Qin B, Chen Q, Meng Z, Plouffe SW, Taniguchi K, Yu FX, Karin M, Pan D, Guan KL, A YAP/TAZ-induced feedback mechanism regulates Hippo pathway homeostasis, Genes Dev 29 (2015) 1271–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Franklin-Murray AL, Mallya S, Jankeel A, Sureshchandra S, Messaoudi I, Lodoen MB, Toxoplasma gondii Dysregulates Barrier Function and Mechanotransduction Signaling in Human Endothelial Cells, mSphere 5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Li N, Feng Y, Hu Y, He C, Xie C, Ouyang Y, Artim SC, Huang D, Zhu Y, Luo Z, Ge Z, Lu N, Helicobacter pylori CagA promotes epithelial mesenchymal transition in gastric carcinogenesis via triggering oncogenic YAP pathway, J Exp Clin Cancer Res 37 (2018) 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lee PC, Machner MP, The Legionella Effector Kinase LegK7 Hijacks the Host Hippo Pathway to Promote Infection, Cell Host Microbe 24 (2018) 429–438 e426. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. N-terminal Tarp and GFP control transgenic fly viability scores.
The viability of transgenic flies ubiquitously expressing N-terminal Tarp (N-term Tarp A, B or C) or GFP control using the ubi-GAL4 driver presented in two trials with male and female data provided separately.
Supplemental Table 2. N-terminal Tarp and GFP control transgenic fly bristle duplication scores.
The duplication of dorsocentral and scutellar macrochaete bristles scores for transgenic flies following heat shock induced expression of N-terminal Tarp (N-term Tarp A, B or C) or GFP control using the pnr-GAL4 driver presented in two trials with male and female data provided separately.