Abstract
Neural oscillations at the network level synchronize activity between regions and temporal scales. Transcranial alternating current stimulation (tACS), the delivery of low-amplitude electric current to the scalp, provides a tool for investigating the causal role of neural oscillations in cognition. The parameter space for tACS is vast and optimization is required in terms of temporal and spatial targeting. We review emerging techniques and suggest novel approaches that capitalize on the non-sinusoidal and transient nature of neural oscillations and leverage the flexibility provided by a customizable electrode montage and electrical waveform. The customizability and safety profile of tACS make it a promising tool for precision intervention in psychiatric illnesses.
Keywords: Aperiodic signal, peak frequency, phase-amplitude coupling, electric field, current density, weighted phase lag index, waveform shape
1. Neural oscillations are not sine-waves
Neural oscillations are proposed to be a crucial mechanism for communication between regions of the brain [1] and between spatiotemporal scales [2, 3]. Transcranial alternating current stimulation (tACS) is the delivery of electric current to the scalp such that an electric field is generated within the brain [4–6]. Given the vast parameter space for tACS, the goal of this review is to explore the domain of spatial targeting via placement of scalp electrodes and the domain of temporal targeting via the customizable electrical waveform. While the goal of tACS is often to target neural oscillations, effective targeting requires an understanding of relevant properties of the target [7–9]. In this section, we provide a foundation for analyzing neural oscillations based on current and emerging investigations. First, genuine oscillations are defined as distinct from the background signal of the brain. Second, neural oscillations are not indefinitely sustained through time but occur as time-limited events. Third, non-sinusoidal rhythmic activity displays biologically relevant characteristics in the time domain that lend insight into their generation by neural circuits. Fourth, neural oscillations might underlie functional connectivity between regions and recently developed methods address common confounds inherent to these analyses. Finally, coupling between high frequency and low frequency oscillations may provide insight into the coordination of neural activity across spatiotemporal scales.
1.1. Aperiodic and Periodic Signal
While early recordings of neural activity via electroencephalography (EEG) have noted a prominent power law distribution in frequency domain, this signal was only recently understood to meaningfully track with biological processes such as healthy aging [10, 11]. Distribution of power of this aperiodic signal in the brain is believed to reflect the excitatory-inhibitory balance of brain activity [12], where high-frequency power reflects excitatory activity and low-frequency power reflects inhibitory activity. With the aperiodic signal, or 1/f noise, understood to represent biologically meaningful signal, neural oscillations are characterized as a Gaussian distribution superimposed above the aperiodic signal [13, 14] (Figure 1A). Thus, running a Fourier transform and deriving the amplitude of a sine wave is not the equivalent of estimating the amplitude of a genuine neural oscillations, and methods that do not explicitly check for the presence of a neural oscillations above and beyond aperiodic signal may inadvertently be studying aperiodic signal [15]. Nevertheless, most analyses in the field of neural oscillations remain valid since they avoid conflating aperiodic signal and periodic signal by utilizing baseline-correction or a contrast between two conditions subsequent to spectral analysis [16]. With a contrast, the aperiodic signal is removed except with a systematic difference in the aperiodic signal between the conditions. When analyzing a contrast, the spectra should reveal amplitude modulation within a narrow band. If spectral amplitude is modulated around a pivot point, in which the amplitude is decreased below and increased above the pivot point (or vice versa), then this pattern of amplitude modulation is most parsimoniously explained by a shift in the slope of the aperiodic signal [15].
Figure 1. Neural oscillations as a mechanism for network coordination between regions and across spatiotemporal scales.
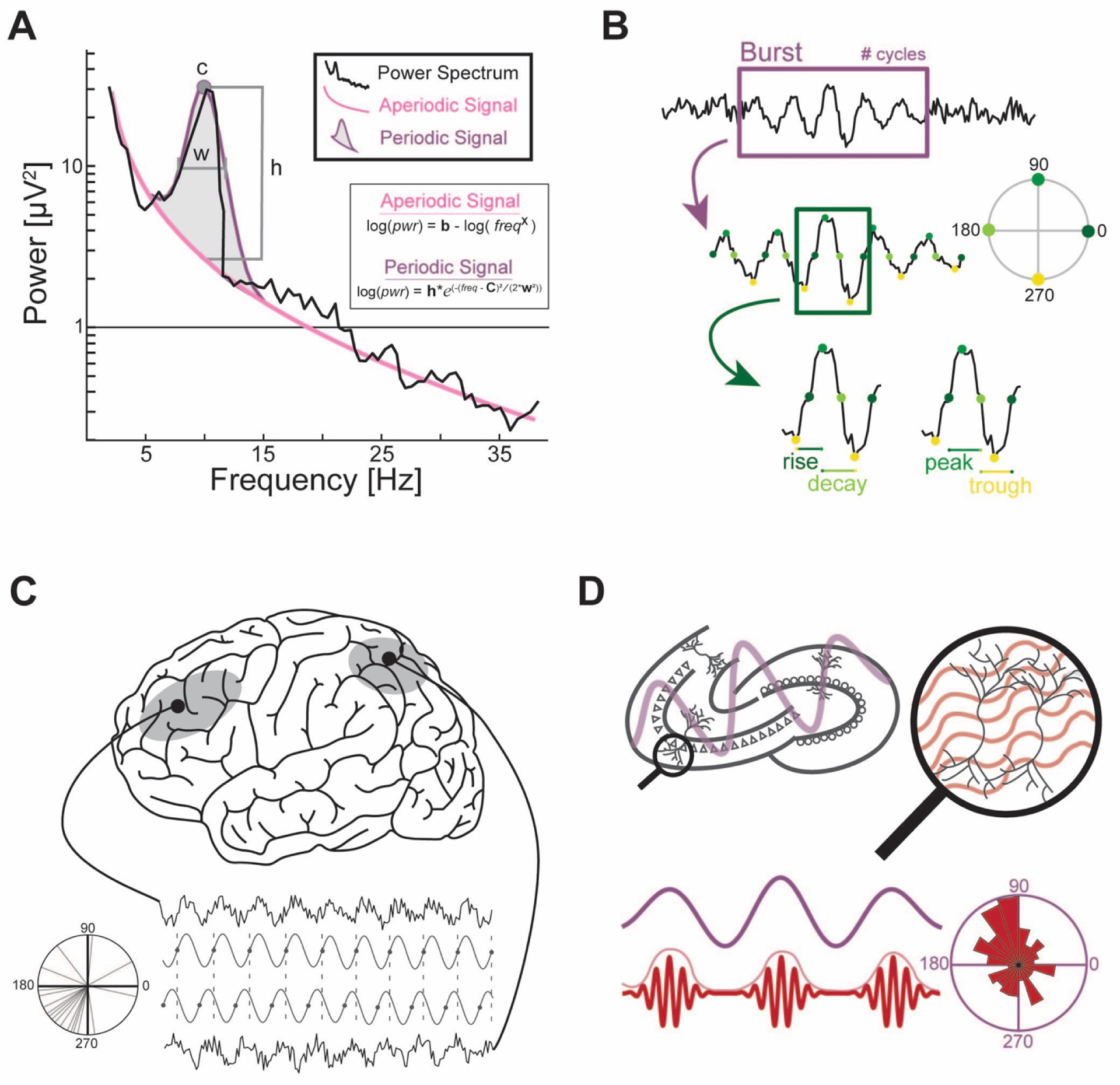
(A) Neural oscillations are defined as peaks above the aperiodic signal of the brain. By estimating and removing the aperiodic signal, the peak and amplitude of the resulting Gaussian distribution most accurately approximates the true periodic signal. (B) Neural oscillations often occur as a burst. A burst is characterized by the number of oscillation cycles which it comprises. The waveform shape of a neural oscillation in time domain is characterized by its rise-fall symmetry and peak-trough symmetry. (C) Interregional communication is estimated using functional connectivity. Genuine functional connections display a consistent phase lag. Weighted phase lag index capitalizes on this property and avoids the confound of volume conduction. (D) Phase-amplitude coupling (PAC) between two regions, or within the same region, estimates communication between a low-frequency oscillation and a high-frequency oscillation. The low-frequency oscillations represent activity at a larger spatiotemporal scale relative to the high-frequency oscillations.
By estimating the aperiodic signal explicitly, additional characteristics of a neural oscillation can be derived. The peak frequency of the neural oscillation is the apex of the Gaussian fit. The true amplitude is the difference in amplitude from the peak frequency at the apex to the amplitude of that frequency in the aperiodic signal. The bandwidth is the half-width half-mass of the Gaussian. When delivering tACS, the peak frequency and bandwidth of the targeted neural oscillations are critical [7]. By individualizing tACS to be aligned with peak frequency, entrainment of the neural oscillation to the stimulation waveform is achieved with lower amplitude, the Arnold tongue phenomenon [7] (see Section 3.1).
1.2. Neural oscillations occur in transient bursts
Neural oscillations do not typically occur as sustained oscillations, but in rhythmic bursts of activity [17–19] (Figure 1B). The sine wave is a mathematical construct in which a stable pattern is sustained infinitely. In practice, sine waves are a useful tool for identifying rhythmic activity. However, the mathematical specifications of sine waves can be misleading. Traditional analyses of neural oscillations average across many trials and multiple participants. These analyses result in clear bands of activity sustained across an entire epoch. However, trial-by-trial analysis reveals discrete burst events of rhythmic activity that only emerge as a band after trial averaging [20, 21]. Bursts of oscillations have additional properties not accessible by traditional time-frequency analysis. One interspecies analysis of beta frequency oscillations during somatosensation explicitly analyzed different burst properties: number of cycles of the oscillations, spread in frequency domain, amplitude of bursts, and number of burst events [21]. This study found that the number of burst events was most predictive of perceptual experience. To interpret the impact of tACS on neural oscillations, the goal of stimulation might be to increase the probability of a rhythmic burst event rather than the generation of a sustained neural oscillation indefinitely over time.
With a transient target, tACS can be optimized to target these transients using real-time feedback. Sleep spindles are a well-known example of bursts of rhythmic activity that are critical for the consolidation of new memories [22, 23]. One study utilized a real-time detection algorithm for identifying these rhythmic burst events and then delivered tACS to enhance their activity and demonstrate their causal role in memory consolidation [24]. In addition, such successful target engagement of sleep spindles offers the opportunity to design feedback stimulation paradigms for disorders such as schizophrenia that are associated with impaired sleep spindles [25]. Thus, effective targeting requires acknowledgement of the transient nature of neural oscillations.
1.3. Neural Oscillations are non-sinusoidal
Localization and analysis of rhythmic burst events has led to novel approaches to understanding neurophysiological waveform shape [26, 27]. In the example of beta burst events, averaging beta bursts in somatosensory cortex revealed a waveform characterized by a sharp singular deflection [27]. The roughly 50 millisecond deflection that comprised many of the beta events was then investigated in the laminar structure of the cerebral cortex, and was revealed to be driven by distal inputs to layer 4 pyramidal neurons hypothesized to originate in frontal cortex [27]. This insight allowed the design of a computational model that supported the role of beta oscillations in top-down control. Furthermore, these studies suggest that the characterization of waveform shape with computational models for circuit generation provide additional insights into the functional dynamics that give rise to rhythmic activity (see the Human Neocortical Neurosolver toolbox [28]).
The time domain analysis of bursts of neural oscillations has further found novel waveform shapes with several characteristic features. An analysis of pathologically elevated beta frequency oscillations in the motor cortex of patients with Parkinson’s disease revealed a “sawtooth” shaped oscillations characterized by a sharp increase followed by a more gradual decrease [29]. The authors interpreted this waveform shape as a hyper-synchronization of neural activity such that the timing of many action potentials was closely locked to a single point in phase space. Collectively, these findings suggest that convolution with sinusoidal wavelets should be the first step in identifying neural oscillations. Then, the characteristics of that activity in the time domain can be further decomposed to a few metrics: rise-decay symmetry (e.g. pathological beta oscillations in Parkinson’s disease [29]), trough-peak symmetry (e.g. beta deflection in somatosenation [27]), and number of cycles [30]. With respect to tACS, novel waveforms that approximate naturally occurring brain activity may be more effective at engaging neural populations as noted by some experimentalists [31–33] (see Section 3.4).
1.4. Communication through coherence assembles functional networks
Phase alignment of neural oscillations in multiple brain regions has been proposed as a critical mechanism for interregional communication [1]. Evidence for the role of phase alignment comes from experiments that find increased phase coherence during conditions in which the coordination of multiple brain regions is required, e.g. in attention [34]. Neural oscillations are proposed to be the mechanism by which functional connectivity patterns such as those observed in functional magnetic resonance imaging (fMRI) might be observed [35]. Functional connectivity gives neural (sub-)systems the capability of flexibly engaging and disengaging from each other. Communication between regions is limited by physical constraints such as conduction delays, i.e. the amount of time that it takes for a signal in one region to be transmitted to another region [1, 36]. Thus, when two regions are connected there will be a systematic delay between the activation of one region and the reception of that signal by the second region (Figure 1C). This property of neural signaling can be leveraged to avoid a common confound in electrophysiology recording. Volume conduction refers to the artifactual observation of an apparent zero-lag (instantaneous) synchronization between two regions driven by a conductive vibrating material such as the skull. Weighted phase lag index (wPLI) is a metric that capitalizes on the observation that genuine functional connectivity requires a consistent non-zero phase lag [37]. To calculate wPLI after wavelet convolution, the analytic signal of one region is multiplied by the complex conjugate of the other. The angle of the resulting analytic signal is the phase difference between the two. Then, the imaginary component of the unit phase difference is averaged over time. By taking the imaginary component, the metric is weighted towards phase lags at 90 or 270 degrees, whereas effects of volume conduction will be weighted towards 0 or 180 degrees. Techniques for increasing functional connectivity by tACS are detailed in Section 2.
1.5. Cross-frequency coupling across spatiotemporal scales
Cross-frequency coupling is the coordination of multiple oscillations at different frequencies. One application of cross-frequency coupling is the ability for low-frequency oscillations in prefrontal cortex to couple to higher frequency oscillations in perceptual or motor systems in cognitive control [2, 38, 39]. High-frequency oscillations are observed to originate in local circuits whereas low-frequency oscillations have been found to coordinate neural populations at the larger scale of brain networks [40]. The relationship between scale and frequency is thought to arise from anatomical constraints such as increased conduction delay between distal regions and by hierarchical organization where circuit activity is summated over time as coherent regional activity [41]. Thus, coupling between low and high frequencies may provide a mechanism whereby different spatiotemporal scales are orchestrated. For example, the sequencing of perceptual information carried at the gamma frequency (30–80 Hz) is bound into a sequence of items that are encoded into memory at the theta frequency (4–8 Hz) [42–44]. Phase-amplitude coupling is an ideal mechanism for inter-scale coordination as the amplitude envelope of high-frequency oscillations displays characteristics in a lower frequency range (Figure 1D). With rhythmic neural activity in high frequencies occurring in transient bursts, the timing of burst occurrence may be guided by the phase of low-frequency oscillations, i.e. phase-amplitude coupling. At the cellular level, such coupling can be easily conceptualized as periodic depolarization of the neuronal membrane voltage that allows synaptic interactions as input is translated into action potential output.
Currently, there are four primary means of calculating phase-amplitude coupling (PAC) [45]. To begin, the analytic signal for the frequency of interest can be extracted using Morlet wavelet convolution or by bandpass filtering and then applying the Hilbert transform. The first method for calculating PAC takes the phase of the low-frequency signal and the amplitude of the high-frequency signal and merges that information into a single hybrid signal. Then, this hybrid signal is averaged over time and the magnitude of the resulting vector is the PAC strength, referred to as mean vector length [16, 46, 47]. Another method for this calculation is to take the average amplitude of the high-frequency signal within phase bins of the low-frequency. Then, the entropy of that distribution quantifies the degree to which the distribution diverges from a uniform distribution, referred to as modulation index [48]. In the third method, the amplitude envelope of the high-frequency signal is additionally convolved with the low-frequency wavelet to extract the low-frequency phase of the amplitude envelope. The phase locking value of this signal to the phase of the low-frequency signal is the strength of PAC, referred to as phase-locking value [49, 50]. The fourth method utilizes a generalized linear model to predict the amplitude values from the phase values with the intuition that with a genuine PAC a model resembling a 3rd-order polynomial (in practice, modeled as splines around phase space) will explain more variance than a null model using a simple horizontal line, referred to as general linear model [51].For each of these methods, a null distribution is typically derived by time shifting the high-frequency amplitude signal while keeping the overall temporal structure intact. The final PAC value is a z-normalized value relative to the permutation based null distribution. Each of these methods are relatively interchangeable in the presence of a genuine phase-amplitude coupling signal with some experimentalists advocating that the modulation index is most robust with noisy and limited data, mean vector length is ideal with clean and long-epoch data, and only modulation index and general linear model are sensitive to bi-phasic PAC [45].
A confound that arises in the analysis of phase-amplitude coupling is the phenomenon that a slow oscillation with a sharp peak at its peak or trough (i.e. non-sinusoidal waveform as described in Section 1.3) will be erroneously interpreted as a phase-amplitude coupling relationship given the nature of the Fourier transform [26, 52]. Since every signal can be decomposed into a sum of sine waves of various amplitudes, the sharp edges in a time-domain signal will be filled in with high-frequency oscillations to recreate the fine temporal detail. For example, a low-frequency oscillation with sharp edges at its peaks will be represented in frequency domain as increased amplitude in the low-frequency sine waves closest to the general shape and high-frequency amplitude fluctuations where the sharp edges arise. To ensure that a phase-amplitude coupling relationship is genuine, one can provide supplemental evidence for the relationship through the fact that artificial PAC will be between a low-frequency signal and its harmonics. Some examples provided in a recent review include (1) consideration of whether each oscillation is a genuine oscillation (Section 1.1), independent of the PAC relationship, (2) inter-regional PAC avoids the problem almost entirely because the confound is generated locally, or (3) by additional associations with the signals independent of the PAC relationship [53]. In conclusion, there is mounting evidence that PAC is a critical mechanism for coordinating cognitive processes that involve neural systems at multiple spatiotemporal scales.
2. Spatial targeting to synchronize brain regions
The rational design approach is proposed as the optimal approach for the use of transcranial alternating current stimulation (tACS) [54]. First, target identification is the characterization of the relevant neural signal that is to be modulated by stimulation. In Section 1, we outlined the biologically relevant traits of neural oscillations that should be considered in identifying the target. In target identification, a brain-to-behavior or brain-to-symptom relationship should also be established as the primary end goal of stimulation is to modulate behavior or clinical symptoms. Second, target engagement requires that researchers test whether stimulation is able to boost the targeted neural activity. This step is critical as stimulation may have secondary or tertiary effects that are not specific to the identified neural target. Finally, target validation is the investigation of the impact of stimulation on cognition (behavioral assay) or clinical status (psychiatric assessment). Evidence for target identification and target engagement provide a foundation for interpreting the impact of stimulation on the higher order variable of ultimate interest. While Section 1 discussed the relevant features of neural oscillations and strategies for target identification, Sections 2 and 3 explain methods for effective spatial and temporal targeting to increase the efficacy of target engagement.
2.1. Synchronizing multiple brain regions
The stimulation electrode montage, i.e. the placement of electrodes, chosen for tACS determines which regions receive the bulk of stimulation and which regions are placed in-phase synchrony and which are placed in anti-phase synchrony [56–58]. If behavior or neural activity is up and downregulated by these stimulation techniques respectively, then this provides strong evidence for the causal role of in-phase synchrony. With an alternating current, the electrodes alternate every oscillation cycle between acting as an anode (current source) and cathode (current sink) (Figure 2A). With direct current stimulation a constant electric current is applied and each electrode is fixed as either an electrical source or sink [59–61]. Using the simplest electrode montage of two electrodes, one electrode is the anode at the peak of the stimulation phase, and the cathode at the trough of the stimulation phase. Thus, a two-electrode stimulation montage will place the two regions under the stimulation electrodes in anti-phase synchrony. In practice, this form of stimulation can be used to target regions that are observed to be in-phase synchronous in order to disrupt performance [56–58]. Regions of the brain commonly synchronize their activity in-phase, such as in-phase theta-frequency synchrony between frontal and parietal cortex during working memory tasks [62]. When the frontal and parietal cortex were targeted with anti-phase theta-frequency tACS during a working memory task, performance was disrupted by stimulation [56, 58].
Figure 2. Spatial targeting using tACS.
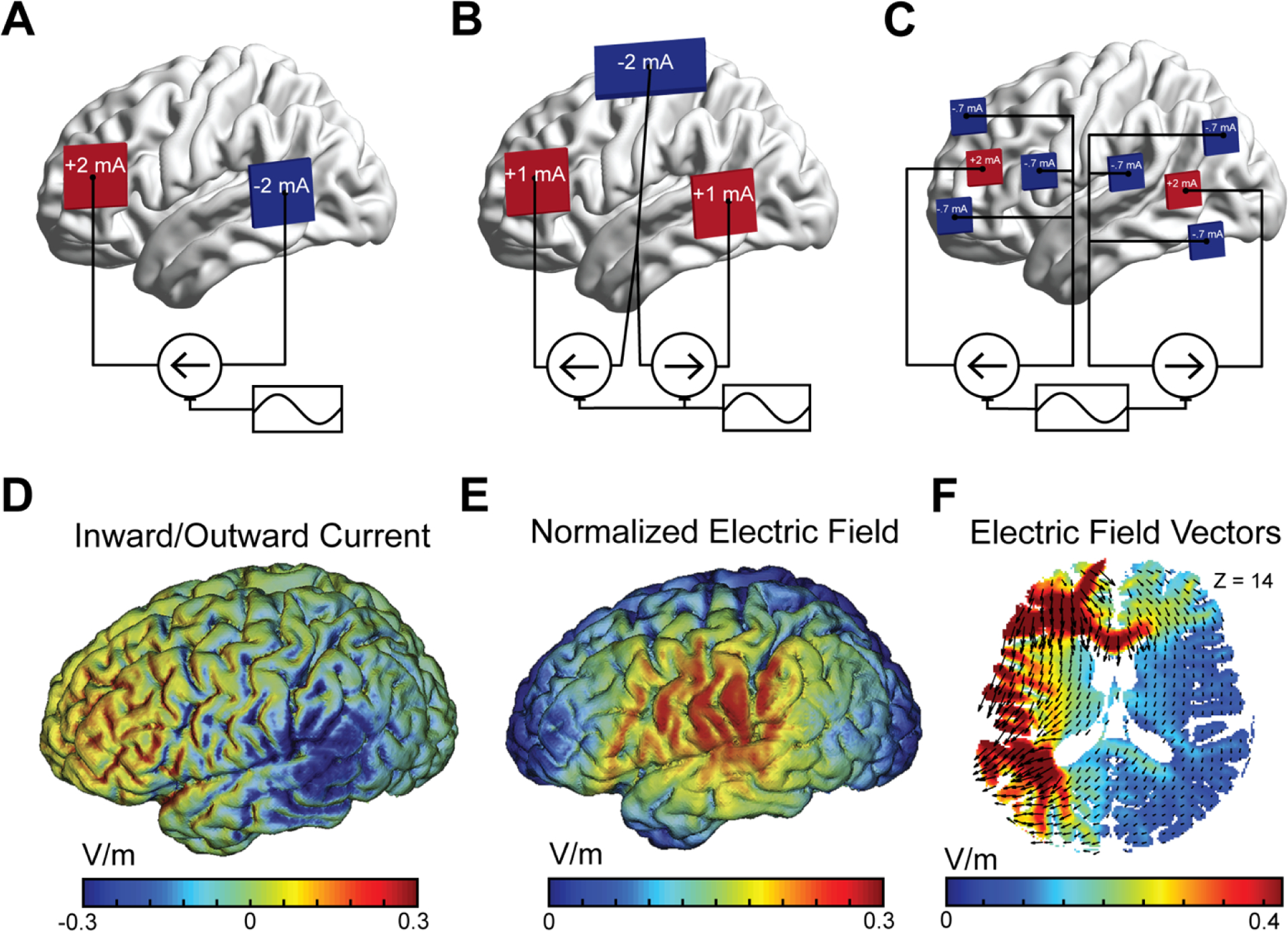
(A) With two stimulation electrodes, the current enters one electrode and is extracted from the other, then the roles are reversed. The two regions under the stimulation electrodes are theorized to be put in anti-phase synchrony. (B) With three stimulation electrodes, two stimulation electrodes are delivered identical stimulation and a third larger stimulation electrode is used to retrieve the current. The third electrode is put in anti-phase synchrony with the two target electrodes. The current density and normalized electric field for the two-electrode montage in (A) are displayed in (D) and (E). (D) Inward current density in red is greatest under the anodal electrode and outward current density is greatest under the cathodal electrode [55]. (E) The normalized electric field is at its peak where the current direction inverts between the two stimulation electrodes [55]. (F) The discrepancy between inward/outward current density and normalized electric field is resolved by considering the electric field vectors.
Using a three-electrode montage, two target electrode sites will behave as anode or cathode at the same time and a third electrode of non-interest will be placed in anti-phase synchrony (Figure 2B). In the context of frontal-parietal synchronization during working memory, a three-electrode montage with a return electrode over the central midline or a ventral-lateral center-point that delivered in-phase theta-frequency stimulation to both frontal and parietal cortex increased behavioral performance [56, 58]. These studies demonstrated a reversible dissociation between in-phase and anti-phase stimulation. Using this technique, the causal role of functional connectivity can be investigated. However, it should be noted that the two-electrode montage for in anti-phase synchronization (Figure 2A) and the three-electrode montage for in-phase synchronization (Figure 2B) generate electric fields with different strength. Some researchers estimate that the field strength for anti-phase stimulation is two to three times greater than that of in-phase stimulation and contest that this difference may account for some of the reported effects [63].
A third electrode in anti-phase synchrony is an inevitability of the three-electrode montage, and, therefore, some region must be stimulated in anti-phase to the other two regions. Furthermore, the third electrode receives twice as much current as the two target stimulation electrodes. In order to reduce the impact of the return electrode, a larger stimulation electrode is used to reduce current density. With an electrode twice as large, the current density per square centimeter is matched. In addition to increasing the size of the return electrode, the return electrode is often placed over a region of non-interest. In the domain of transcranial magnetic stimulation in which a focalized electric field is generated in the brain by electromagnetic induction from electric current in coils near the scalp, a control stimulation site in the central midline over the sensorimotor network corresponding to the legs is frequently targeted because this region is not relevant to many cognitive tasks [64–66]. Thus, the return electrode is often placed over the central-midline [55, 56, 67]. It should be noted that the effects from tACS spread over a wider spatial extent such that the selection of a control site is more difficult. Another strategy for selecting the location of the return electrode comes from the observation of anti-correlated brain networks in resting-state fMRI: the central-executive network (lateral prefrontal and dorsolateral parietal cortex) and the default-mode network (medial prefrontal, precuneus, and temporal-parietal junction) [68]. When stimulation is delivered to synchronize the central-executive network, then the return electrode can be placed over a hub in the default-mode network such as over the medial prefrontal cortex [69].
A final technique for selecting a control site is to choose a region outside of the brain such as the shoulder (see [70, 71] for example). While this technique avoids stimulation of an additional brain region that may inadvertently contribute to the stimulation effect, it introduces additional confounds such as potential stimulation of the cranial nerves in the peripheral nervous system. For example, stimulation of the vagal nerve which innervates the neck and chest is actively investigated as a treatment of epilepsy [72] and treatment-resistant major depressive disorder [73]. Stimulation that targets the vagal nerve is typically placed on the neck or ear [74, 75]. Thus, stimulation targeting the cerebral cortex that intends to control for non-specific effects should be critically aware of the research enterprise that targets the neck and shoulder with electrical stimulation.
Another technique for synchronizing brain regions is to use high-definition, multi-electrode, transcranial alternating current stimulation (HD-tACS). In HD-tACS, a stimulation electrode is typically surrounded by many return electrodes (Figure 2C) [76], although techniques to derive arbitrary configuration that maximize focal electric field strength have been proposed [77, 78], and were recently used to target the orbitofrontal cortex [78]. As discussed earlier, the return electrodes will be put in anti-phase synchrony, however, these montages use many return electrodes to reduce the current density of return electrodes. With four return electrodes of equal size to the stimulation electrode, the current delivered in these diffused sites will be four times weaker. As the name suggests, HD-tACS montages deliver current to a more focal site and can even be used to target a single site [79]. With the use of multiple HD-tACS montages, two regions can receive in-phase stimulation, anti-phase stimulation, or stimulation at an arbitrary phase-lag [57, 80]. Furthermore, researchers can use HD-tACS to analyze the impact of stimulation to each region on its own versus stimulation to both regions. A recent study found that stimulation in-phase to prefrontal and auditory cortex in theta-frequency increased behavioral performance during a working memory task, but this benefit was not observed with stimulation to either site on its own [57].
While HD-tACS provides the ability to stimulate individual regions or multiple regions simultaneously, the electric field generated by HD-tACS is roughly twice as weak as the equivalent current delivered with a two or three-electrode montage. Furthermore, the flow of current between regions within a functional connected network, e.g. from lateral prefrontal to superior parietal lobule, via two or three-electrode montages might activate white matter tracts between the targeted regions further mimicking the endogenous activity that is targeted by stimulation. Early modeling work that incorporated the anisotropy (direction) of white matter tracts found reduced resistance when current flowed along fibers relative to perpendicular to fibers suggesting that applied current may flow preferentially along established white matter tracts [81]. Thus, montages in which current flows between stimulation electrodes may impact brain activity via grey matter as well as white matter capitalizing on distributed network activity, whereas HD-tACS is designed to impact brain activity via grey matter under the stimulation electrode(s) capitalizing on experimental control over the precision of effects. HD-tACS is a nascent technique and there are several unresolved, fundamental questions. For example, having electrodes in close proximity biases the resulting current towards staying superficial and not reaching cortex. Also, at a more fundamental level, computer simulations suggests that tACS entrains networks by boosting hotspots of near-threshold subnetworks. Targeting larger areas of cortex (with “conventional” tACS electrodes) increases the number of enhanced hotspots and may thus increase the efficacy of tACS [82]. Finally, the conduction delays between different brain network nodes appears to shape the response to tACS. Computer simulations of two interconnected cortical networks with physiological propagation delays showed the occurrence of complex response patterns, including a paradoxical desynchronization of two oscillating network [83]. More computational simulations and experimental direct comparisons of tACS and HD-tACS will be needed to provide clarity.
2.2. Consideration of electric field strength and current density
Modeling of the electrical properties of stimulation in the brain is commonly used to estimate the spread of effects [84–86]. When modeling the effects of transcranial direct current stimulation (tDCS) on the brain, the current density of stimulation is often displayed [55, 61, 87, 88]. For example, when an anodal stimulation electrode over the prefrontal cortex and a cathodal stimulation electrode over the temporal-parietal junction, the inward current density is seen to be highest directly under the anode and lowest directly under the cathode (Figure 2D). With tACS using this montage, the current density would alternate from inward to outward with every cycle of the stimulation. In addition to current density, the normalized electric field depicts the location where the current direction switches and the field inverts (Figure 2E) [87, 88]. While inward current density is often depicted with tDCS, the normalized electric field is often reported in tACS studies. However, there is a clear discrepancy between the prediction of peak neural effects based purely on visual inspection of inward/outward current density and normalized electric field strength. The apparent discrepancy is resolved when considering that electric fields comprise directed vectors of electric force of various amplitude in space (Figure 2F).
The geometry of neurons may be the best predictor of stimulation efficacy. The effect of electrical stimulation on neurons is presumed to arise from depolarization of the somatic compartment of neurons with somato-dendritic axis that is oriented in parallel to the vectors of stimulation current [89, 90]. With consistent laminar profiles across the cerebral cortex, electric field models often treat pyramidal neurons oriented from deep to superficial layers as the primary target for stimulation [91]. Thus, gyral geometry of individual brains can be used to predict the efficacy of stimulation on specific anatomical targets in the cerebral cortex [91]. In vivo recordings from the human and non-human primate brain during electrical stimulation have verified the accuracy of electric field models [92, 93].
In conclusion, tACS typically targets network-scale activity with spatial targeting on the order of centimeters. For example, an experimenter could investigate the causal role of theta oscillations in a working memory task. Functional magnetic resonance imaging (fMRI) could be run to understand which subregions of the frontal-parietal network are activated during the task. Then, tACS could be delivered to the frontal-parietal network such that the generated electric field encompasses the targets derived from fMRI. As discussed previously, computational models of the impact of tACS suggest that hot-spots will respond primarily to the weak perturbation of tACS [7]. Often times, placing target electrodes over F3 and P3 will encompass the relevant regions in the frontal-parietal network, but additional electric field modeling could be run to ensure sufficient field strength in the targeted regions [84]. Alternatively, tACS could be conceptualized as enhancing an entire network of regions, and the stimulation montage could be selected to deliver the maximal electric field strength summated across the targeted network [94–96]. Finally, stimulation montage could be tailored to maximize electric field strength on an individual basis using electric field modeling with an anatomical target or a target derived from functional MRI [84].
3. Temporal targeting that mimics endogenous neural activity
In the previous section, we presented approaches for effective spatial targeting. In this section, we explore temporal targeting by customizing the tACS waveform. By generating a customized waveform to be delivered, the precision of temporal targeting is significantly increased as stimulation can be tailored to the particular neural target under investigation. We present four different optimization methods to target rhythmic neural activity: individualizing stimulation frequency to peak frequency of the participant based on task or rest data, adding noise to the stimulation waveform to better approximate endogenous activity, targeting phase-amplitude coupling, and stimulating using non-sinusoidal waveforms.
3.1. Individual frequency localization and the Arnold tongue
TACS is typically delivered as a pure sine wave of a single frequency. The frequency of stimulation usually is chosen as the canonical peak frequency of an oscillatory band. For example, 10 Hz tACS is delivered as “alpha-frequency tACS” since 10 Hz is the most commonly found peak frequency of the alpha band during the resting state [67, 97–99]. Alternatively, stimulation can be delivered at the peak frequency of the individual [57, 100–102] (Figure 3A). Computational models suggest that stimulation will be most effective at entraining neural oscillations when delivered at the frequency of the endogenous neural oscillation, i.e. the Arnold tongue [7, 103–105]. When explicitly tested using rhythmic transcranial magnetic stimulation in the beta frequency, one investigation found that the individualized frequency of stimulation produced greater neural entrainment when delivered at peak beta frequency relative to stimulation plus or minus 3 and 6 Hertz [106]. Thus, experimenters can use a baseline EEG analysis to isolate the peak frequency of the neural activity of interest, e.g. contrast of task conditions [57, 69], resting-state [101], motor-evoked activity from transcranial magnetic stimulation [106].
Figure 3. Temporal targeting that mimics endogenous neural activity.
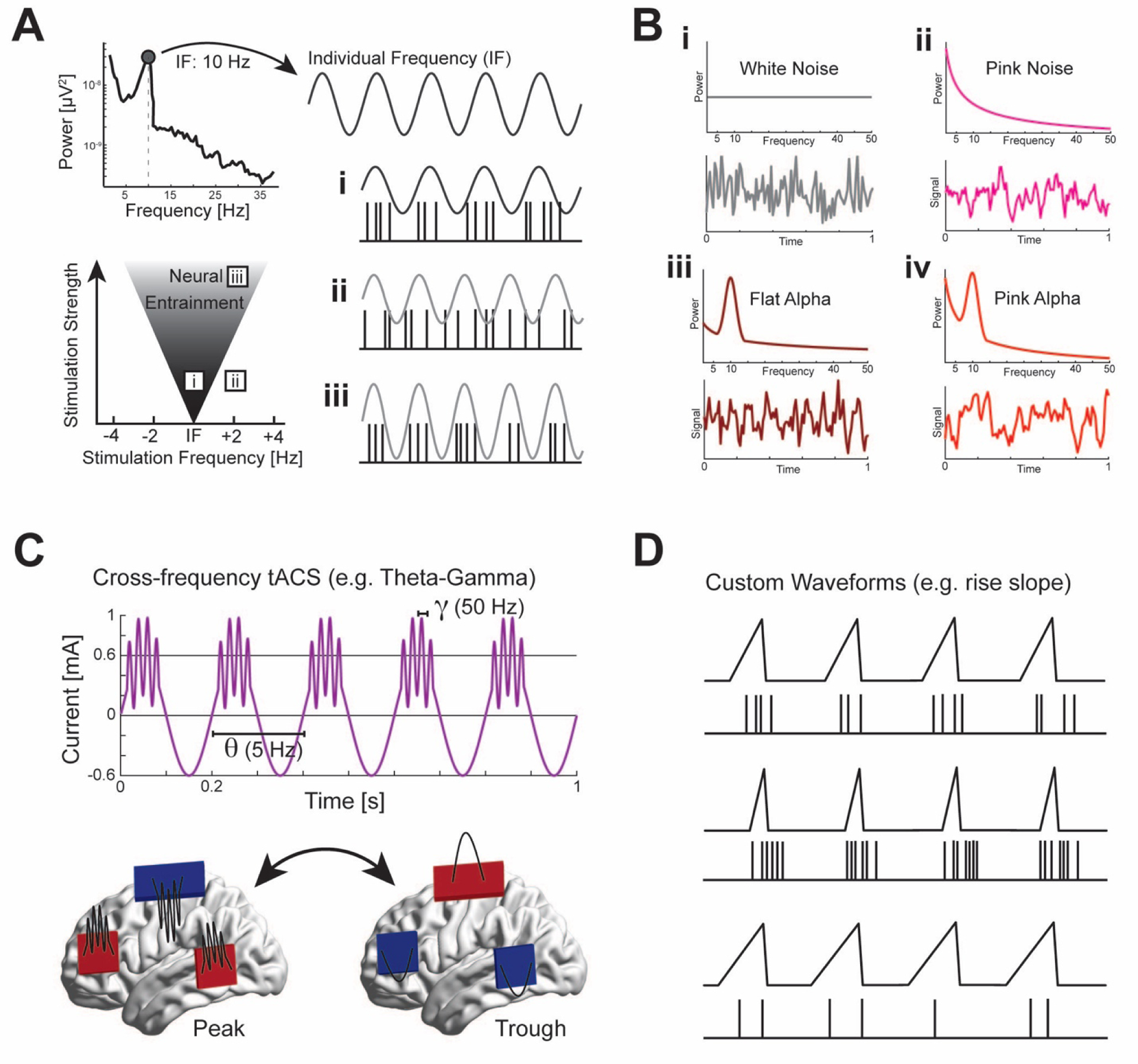
(A) According to the Arnold tongue phenomenon, weaker stimulation is required to entrain neural oscillations when delivered at the same frequency as the endogenous oscillation (i). Whereas stimulation off the peak frequency is ineffective at a low amplitude (ii) or requires greater stimulation strength to entrain oscillations at the stimulation frequency (iii). Individual frequency targeting can be used to maximize entrainment or to shift the peak frequency of neural oscillations. (B) Stimulation can be delivered with a selected power spectrum (top) to generate unique waveforms (example in bottom). Transcranial random noise stimulation delivers a random waveform with equal power at all frequencies (i), or with a power distribution that approximates the aperiodic signal of the brain (ii). A random phase is used to generate unique waveforms. Stimulation can also be delivered with a chosen periodic and aperiodic signal. For example, an alpha frequency periodic waveform is mixed with an aperiodic signal that is flat in slope (iii) or steep in slope (iv). A random phase for the power distribution with a uniform phase for the bandwidth of the periodic signal is used to generate unique waveforms. (C) Cross-frequency tACS delivers a waveform that mimics phase-amplitude coupling with a high-frequency amplitude modulation at a particular phase of a low-frequency component. (D) Custom waveforms such as manipulating rise-decay symmetry can probe time-domain characteristics of neural oscillations. A previous study found that a steeper slope of direct electric stimulation increased spike rate [31].
Emerging evidence suggests that the peak frequency of a neural oscillation may be critical for cognition. For example, the peak frequency of the alpha and theta oscillation has been shown to shift higher or lower depending on task condition [107, 108]. In other instances, peak frequency is predictive of disease state such as a slower alpha oscillation in patients with chronic pain and a slower alpha oscillation correlates with increased pain sensitivity in health individuals [109, 110]. The example of chronic pain and pain sensitivity highlights an instance where enhancing the endogenous neural activity may not be therapeutic, but instead stimulation that could shift the peak of the oscillation to a higher frequency is desirable. One study that attempted to alter the peak frequency of a neural oscillation targeted the theta oscillation. The peak frequency of theta oscillations has been correlated with working memory capacity such that participants with a slower peak theta frequency have greater working memory capacity [44]. Therefore, investigators delivered tACS at a low theta-frequency versus a high theta-frequency and found that stimulation in low-theta increased working memory capacity relative to high-theta [111].
3.2. Aperiodic signal filtered oscillations
As discussed in Section 1.1, the aperiodic signal of the brain meaningfully tracks the excitatory-inhibitory balance of neural activity wherein relatively greater power in the low-frequency range is inhibitory and relatively greater power in the high-frequency range is excitatory [12]. Furthermore, the aperiodic signal of the brain has recently been shown to track with the 1/f noise profile of visual and auditory stimuli [112] suggesting that the aperiodic signal is a mutable neural phenomenon. In parallel, tACS has been used to deliver random noise to increase neural excitability [113, 114] (Figure 3Bi). Transcranial random noise stimulation (tRNS) is similar to tACS except that instead of delivering current in a sine-wave, a randomly fluctuating waveform is used [115–117]. Therefore, tRNS is characterized by the power-spectral properties of the electrical waveform, e.g. equal relative power across a frequency range and has been demonstrated to increase neural excitability based on a probe of motor cortex excitability via motor evoked potentials from single pulse transcranial magnetic stimulation and decreased reaction time in an implicit motor learning task [113]. One proposed mechanism for tRNS is stochastic resonance, in which all frequencies are boosted such that the endogenous activity regardless of its frequency band is amplified [117, 118]. In addition, tRNS may flatten the slope of the aperiodic signal towards relatively greater high-frequency power by virtue of its uniform power distribution.
Alternatively, transcranial pink noise stimulation (tPNS) could be delivered that is similar to tRNS but the power distribution of the waveform is matched to the 1/f characteristics of the endogenous activity of the brain (Figure 3Bii). While appreciation of the importance of the aperiodic signal in the brain is a new development [10, 11], a recent study delivered high-density tPNS targeted to the anterior cingulate cortex and found that repeated stimulation produced a reduction in food craving in obese women determined by the authors to have a food addiction [119]. This study found a reduction in low-frequency beta oscillations over the targeted region suggesting that the relatively higher power in low-frequencies from tPNS may have had a suppressive effect on activity in higher frequencies. Critically, this landmark study did not individualize stimulation to the participant, nor did they investigate the impact of HD-tPNS on the aperiodic signal directly.
A recent study that found that brain activity tracks with the 1/f signal properties of visual and auditory stimuli [112]. Thus, tPNS could be individualized similar to individual frequency localization except that the slope of the aperiodic signal is estimated and used for stimulation (Section 3.1), or the slope of tPNS could be selected to push the aperiodic signal of the brain towards a steepened or flattened slope. Similar to tRNS, the electrical waveform of tPNS is created using a random phase for each frequency but with a different amplitude for each frequency based on 1/f parameters. Furthermore, an aperiodic signal superimposed on a periodic component (the periodic component could be individualized or use canonical characteristics) could generate a unique waveform that approximates endogenous neural signals (Figure 3B iii,iv). Here, a uniform phase is used for the bandwidth of the periodic signal, and a random phase for the other frequencies. This approach mimics the endogenous activity of the brain in order to increase the ecological validity of the stimulation waveform. While currently unknown, we speculate that tPNS with a superposed periodic component may be more effective at driving endogenous neural activity than a simple sine-wave as in traditional tACS. Finally, this approach provides additional dissociable dimensions of the signal that could be investigated, e.g. peak-frequency, oscillatory bandwidth, aperiodic signal slope. With emerging evidence for the role of aperiodic signal in cognition, tACS could be used to causally dissociate the role that periodic and aperiodic signals play in cognition.
3.3. Waveforms to target cross-frequency coupling
As discussed in Section 1.5, multiple neural oscillations display phase-amplitude coupling as a mechanism for coordination across spatiotemporal scales. As with any proposed neural mechanism, causal evidence is required to substantiate correlational findings. In a landmark study, investigators used a custom waveform of tACS to mimic the theta-gamma phase-amplitude coupling pattern that is found during working memory tasks [79] (Figure 3C) (also see [69, 120–123]). Stimulation was delivered in theta-frequency alone (6 Hz), theta phase coupled to gamma amplitude at the peak of theta phase and at the trough of theta phase, bursts of gamma stimulation alone, and theta phase coupled to various frequencies of gamma amplitude. The authors found that theta-frequency tACS during a working memory task was sufficient to benefit behavioral performance. The behavioral benefit was increased even greater when the peak of the phase of theta oscillations was coupled to high-frequency gamma amplitude from 80 to 140 Hz. This study demonstrates the potential for tACS to probe the specific frequencies that are critical to phase-amplitude coupling. Furthermore, this study suggests that bursts of gamma occurring at the peak of the theta phase was critical. Indeed, a follow-up study by this research group found that gamma bursts at the trough of theta phase was disruptive to performance during a cognitive control task when delivered to anterior frontal pole [122]. However, neither of these studies provided direct evidence of increased phase-amplitude coupling from CF-tACS. Future studies could further optimize stimulation by measuring individual phase-amplitude coupling strength and then targeting stimulation to the peak frequencies of endogenous activity [69].
Additionally, some studies have found interregional phase-amplitude coupling whereby the phase of low-frequency oscillations typically thought to originate in prefrontal cortex modulate the amplitude of neural oscillations in motor or perceptual regions [39, 121, 124]. HD-tACS could be tailored to target interregional phase-amplitude coupling by delivering low-frequency stimulation to prefrontal cortex in one montage and bursts of stimulation in a high-frequency in a second HD-tACS montage over motor/perceptual cortex that are phase locked to the peak/trough of low-frequency stimulation [120]. Alternatively, stimulation could be delivered to both regions using dual HD-tACS or a three-electrode montage with the full cross-frequency waveform delivered to both sites of interest [69, 123] (Figure 3C). In this scenario, both regions receive low and high frequency stimulation. Thus, neural population with an intrinsic oscillation in either frequency should be entrained and functional connectivity and/or interregional phase-amplitude coupling should increase. As discussed in section 2.1, due to the close proximity of electrodes in HD-tACS montages the induced electric fields are weaker than two or three-electrode montages. Also, traditional electrode montages capitalize on the flow of current along white matter tracts and may more closely replicate the diffuse nature of traveling electrical potentials [125].
3.4. Novel waveform shapes for particular targets
In Section 1.3, we discussed how the sine wave is a mathematical construct and that neural oscillations in practice will display non-sinusoidal properties. These properties are sometimes regular and, thus, can be characterized [27, 30] and related to biological function [21, 29]. A landmark investigation that delivered electric current directly to neural tissue found that a sharper slope when ramping up current was most effective at increasing neural spiking [31] (Figure 3D). While this study did not investigate specific non-sinusoidal waveform shapes such as rise-decay symmetry and peak-trough symmetry within the context of neural oscillations, the findings suggest that greater asymmetry in rise-decay may more effectively drive neural activity. One potential neurophysiological explanation for this phenomenon may be that faster depolarizations transiently overcome inactivation of sodium channels, which is slower than activation. TACS can be used to approximate arbitrary waveform shapes. Therefore, non-sinusoidal properties of rhythmic neural activity can be systematically investigated using customized waveforms. For example, the sawtooth shape of pathological beta oscillations could be causally investigated by parametrically altering the rise-decay symmetry of a custom waveform delivered with tACS.
Finally, closed-loop stimulation can be used to amplify or suppress endogenous electrical activity in real time when that activity is non-sinusoidal (arbitrary) [31] or when it is characteristic rhythmic activity [24, 126]. Additionally, electrical activity either in time domain or band-filtered can be recorded and then delivered to the brain via electrical stimulation. These techniques can focus on variables related to task condition or cognitive state, and utilize naturally-occurring electrical activity patterns, but do not attempt to interpret particular aspects of the electrical activity. For example, investigations into the neural mechanisms of episodic memory have found that similar patterns of neural activity are present both at encoding and retrieval of memories [127], but these similarity metrics do not attempt to interpret the pattern of neural activity itself. An analogous approach could be employed using tACS in which the pattern of electrical activity in time-domain during memory encoding is recorded and that pattern of electrical activity is delivered via tACS during memory retrieval. Here, the causal role of pattern similarity is addressed by tACS without an interpretation of the periodic or aperiodic characteristics of the electrical activity. Presumably, this stimulation paradigm would approximate traditional tACS when the memory trace includes a prominent rhythmic component, e.g. the theta rhythm, or theta-nested-gamma rhythm, but allows for increased flexibility to account for personal variability and dynamic changes in the dominant frequency of activity.
In a recent study, synchrony between students and a teacher in a naturalistic classroom setting was calculated for the frequency range of 1–20 Hz [128]. Higher synchrony was associated with greater classroom engagement. Theoretically, the preprocessed signal derived from the EEG of the teacher could be delivered via tACS in real-time to the students in the classroom in an effort to increase brain-to-brain synchrony. In this context, this type of tACS with a data-driven waveform would undoubtedly contain periods of stimulation similar to traditional tACS when the brain of the teacher generated a prominent rhythmic signal; but also, this stimulation paradigm allows for the waveform to dynamically change with an evolving real-world environment. This technique could be constrained to focus on low-frequency network activity, for example network oscillations in the frontal-parietal network with stimulation applied to said network. Alternatively, when attempting to reinstate a specific memory trace as in the previous example, the signal in visual electrodes across a broad frequency spectrum at encoding could be calculated and delivered via tACS to visual cortex upon probe. This latter form of stimulation might more closely resemble tPNS than a traditional tACS waveform, but provides flexibility for prominent rhythmic components. To our knowledge, there is yet to be an experiment that utilizes tACS to enhance inter-brain synchrony or to enhance an arbitrary memory trace.
4. Concluding remarks
Transcranial alternating current stimulation (tACS) offers a vast parameter space for systematically investigating the spatiotemporal dynamics of neural activity. By optimizing stimulation parameters to the spatial and temporal dynamics of rhythmic brain activity, tACS provides a powerful means to test for the causal role of neural oscillations in cognition and to boost or alter neural targets (but see Outstanding Questions). With emerging evidence that tACS can be used to restore cognitive function in healthy aging [57] and reduce symptoms of depression [67, 129], auditory hallucinations [55, 130], chronic pain [131], inhibitory control in substance use disorder [132], and obsessive-compulsive disorder [78], the efficacy of tACS can undoubtedly be increased with further optimization using the techniques described here. There is a push towards personalized medicine in psychiatry exemplified by a recent study that found that the symptom profile of major depressive disorder was predictive of which brain region targeted by TMS produced the greatest treatment response [133]. Thus, spatial and temporal targeting is a vital next step in the application of personalized non-invasive brain stimulation for the treatment of psychiatric illness.
Highlights.
Rational approach to causality: target identification, engagement, and validation
Neural oscillations are best characterized as non-sinusoidal rhythmic bursts events
Effective tACS mimics spatial and temporal features of endogenous neural activity
TACS can enhance connectivity between regions or across spatiotemporal scales
Waveform can target peak frequency, aperiodic signal, or non-sinusoidal activity
Acknowledgements
This review was supported in part by the National Institute of Mental Health of the National Institutes of Health under Award Numbers R01MH101547 and R01MH111889 awarded to FF and the postdoctoral training program T32MH09331502 (JR). FF is the lead inventor of IP filed by UNC. FF is founder, shareholder, and chief science officer of Pulvinar Neuro, which did not play any role in the writing of this article. FF has received honoraria from the following entities in the last twelve months: Sage Therapeutics, Academic Press, Insel Spital, Pulvinar Neuro, and Strategic Innovation. JR has no conflict of interest. Figure 1A was created in collaboration with Dillan Cellier, Kai Hwang, and Issac Peterson (University of Iowa). Figure 1D was created in collaboration with Jonathan Schooler (University of California, Santa Barbara).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fries P, Rhythms for cognition: communication through coherence. Neuron, 2015. 88(1): p. 220–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canolty RT and Knight RT, The functional role of cross-frequency coupling. Trends in cognitive sciences, 2010. 14(11): p. 506–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palva JM and Palva S, Functional integration across oscillation frequencies by cross-frequency phase synchronization. European Journal of Neuroscience, 2018. 48(7): p. 2399–2406. [DOI] [PubMed] [Google Scholar]
- 4.Antal A and Paulus W, Transcranial alternating current stimulation (tACS). Frontiers in human neuroscience, 2013. 7: p. 317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herrmann CS, et al. , Transcranial alternating current stimulation: a review of the underlying mechanisms and modulation of cognitive processes. Frontiers in human neuroscience, 2013. 7: p. 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fröhlich F, Experiments and models of cortical oscillations as a target for noninvasive brain stimulation. Progress in brain research, 2015. 222: p. 41–73. [DOI] [PubMed] [Google Scholar]
- 7.Ali MM, Sellers KK, and Fröhlich F, Transcranial alternating current stimulation modulates large-scale cortical network activity by network resonance. Journal of Neuroscience, 2013. 33(27): p. 11262–11275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt SL, et al. , Endogenous cortical oscillations constrain neuromodulation by weak electric fields. Brain Stimul, 2014. 7(6): p. 878–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurmann R, et al. , Rational design of transcranial alternating current stimulation:Identification, engagement, and validation of network oscillations as treatment targets. 2018. 2(2): p. 2514183X18793515. [Google Scholar]
- 10.Voytek B, et al. , Age-related changes in 1/f neural electrophysiological noise. Journal of Neuroscience, 2015. 35(38): p. 13257–13265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donoghue T, et al. , Parameterizing neural power spectra into periodic and aperiodic components. Nature neuroscience, 2020. 23(12): p. 1655–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao R, Peterson EJ, and Voytek B, Inferring synaptic excitation/inhibition balance from field potentials. Neuroimage, 2017. 158: p. 70–78. [DOI] [PubMed] [Google Scholar]
- 13.He BJ, et al. , The temporal structures and functional significance of scale-free brain activity. Neuron, 2010. 66(3): p. 353–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haller M, et al. , Parameterizing neural power spectra. BioRxiv, 2018: p. 299859. [Google Scholar]
- 15.Donoghue T, Dominguez J, and Voytek B, Electrophysiological Frequency Band Ratio Measures Conflate Periodic and Aperiodic Neural Activity. eneuro, 2020: p. ENEURO.0192–20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen MX, Analyzing neural time series data: theory and practice. 2014: MIT press. [Google Scholar]
- 17.van Ede F, et al. , Neural oscillations: sustained rhythms or transient burst-events? Trends in Neurosciences, 2018. 41(7): p. 415–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones SR, When brain rhythms aren’t ‘rhythmic’: implication for their mechanisms and meaning. Current Opinion in Neurobiology, 2016. 40: p. 72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allen JJ and Cohen MX, Deconstructing the “resting” state: exploring the temporal dynamics of frontal alpha asymmetry as an endophenotype for depression. Frontiers in human neuroscience, 2010. 4: p. 232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lundqvist M, et al. , Gamma and beta bursts underlie working memory. Neuron, 2016. 90(1): p. 152–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shin H, et al. , The rate of transient beta frequency events predicts behavior across tasks and species. Elife, 2017. 6: p. e29086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schabus M, et al. , Sleep spindles and their significance for declarative memory consolidation. Sleep, 2004. 27(8): p. 1479–1485. [DOI] [PubMed] [Google Scholar]
- 23.Warby SC, et al. , Sleep-spindle detection: crowdsourcing and evaluating performance of experts, non-experts and automated methods. Nature methods, 2014. 11(4): p. 385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lustenberger C, et al. , Feedback-controlled transcranial alternating current stimulation reveals a functional role of sleep spindles in motor memory consolidation. Current Biology, 2016. 26(16): p. 2127–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fröhlich F and Lustenberger C, Neuromodulation of sleep rhythms in schizophrenia: Towards the rational design of non-invasive brain stimulation. Schizophrenia Research, 2020. 221: p. 71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cole SR and Voytek B, Brain oscillations and the importance of waveform shape. Trends in cognitive sciences, 2017. 21(2): p. 137–149. [DOI] [PubMed] [Google Scholar]
- 27.Sherman MA, et al. , Neural mechanisms of transient neocortical beta rhythms: Converging evidence from humans, computational modeling, monkeys, and mice. Proceedings of the National Academy of Sciences, 2016. 113(33): p. E4885–E4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neymotin SA, et al. , Human Neocortical Neurosolver (HNN), a new software tool for interpreting the cellular and network origin of human MEG/EEG data. Elife, 2020. 9: p. e51214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cole SR, et al. , Nonsinusoidal beta oscillations reflect cortical pathophysiology in Parkinson’s disease. Journal of Neuroscience, 2017. 37(18): p. 4830–4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cole S and Voytek B, Cycle-by-cycle analysis of neural oscillations. Journal of neurophysiology, 2019. 122(2): p. 849–861. [DOI] [PubMed] [Google Scholar]
- 31.Fröhlich F and McCormick DA, Endogenous electric fields may guide neocortical network activity. Neuron, 2010. 67(1): p. 129–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foutz TJ and McIntyre CC, Evaluation of novel stimulus waveforms for deep brain stimulation. Journal of neural engineering, 2010. 7(6): p. 066008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peterchev AV, et al. , Fundamentals of transcranial electric and magnetic stimulation dose: definition, selection, and reporting practices. Brain stimulation, 2012. 5(4): p. 435–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fries P, et al. , Modulation of oscillatory neuronal synchronization by selective visual attention. Science, 2001. 291(5508): p. 1560–1563. [DOI] [PubMed] [Google Scholar]
- 35.Cabral J, et al. , Role of local network oscillations in resting-state functional connectivity. Neuroimage, 2011. 57(1): p. 130–139. [DOI] [PubMed] [Google Scholar]
- 36.Pajevic S, Basser PJ, and Fields RD, Role of myelin plasticity in oscillations and synchrony of neuronal activity. Neuroscience, 2014. 276: p. 135–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vinck M, et al. , An improved index of phase-synchronization for electrophysiological data in the presence of volume-conduction, noise and sample-size bias. Neuroimage, 2011. 55(4): p. 1548–1565. [DOI] [PubMed] [Google Scholar]
- 38.Riddle J, et al. , Distinct Oscillatory Dynamics Underlie Different Components of Hierarchical Cognitive Control. The Journal of Neuroscience, 2020. 40(25): p. 4945–4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Helfrich RF, et al. , Prefrontal cortex modulates posterior alpha oscillations during top-down guided visual perception. Proceedings of the National Academy of Sciences, 2017. 114(35): p. 9457–9462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Von Stein A and Sarnthein J, Different frequencies for different scales of cortical integration: from local gamma to long range alpha/theta synchronization. International journal of psychophysiology, 2000. 38(3): p. 301–313. [DOI] [PubMed] [Google Scholar]
- 41.Nunez PL, Toward a quantitative description of large-scale neocortical dynamic function and EEG. Behavioral and Brain Sciences, 2000. 23(3): p. 371–398. [DOI] [PubMed] [Google Scholar]
- 42.Lisman JE and Jensen O, The theta-gamma neural code. Neuron, 2013. 77(6): p. 1002–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heusser AC, et al. , Episodic sequence memory is supported by a theta–gamma phase code. Nature neuroscience, 2016. 19(10): p. 1374–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Axmacher N, et al. , Cross-frequency coupling supports multi-item working memory in the human hippocampus. Proceedings of the National Academy of Sciences, 2010. 107(7): p. 3228–3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hülsemann MJ, Naumann E, and Rasch B, Quantification of Phase-Amplitude Coupling in Neuronal Oscillations: Comparison of Phase-Locking Value, Mean Vector Length, Modulation Index, and Generalized Linear Modeling Cross-Frequency Coupling. Frontiers in neuroscience, 2019. 13: p. 573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tort AB, et al. , Measuring phase-amplitude coupling between neuronal oscillations of different frequencies. Journal of neurophysiology, 2010. 104(2): p. 1195–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Canolty RT, et al. , High gamma power is phase-locked to theta oscillations in human neocortex. science, 2006. 313(5793): p. 1626–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tort AB, et al. , Dynamic cross-frequency couplings of local field potential oscillations in rat striatum and hippocampus during performance of a T-maze task. Proceedings of the National Academy of Sciences, 2008. 105(51): p. 20517–20522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Voytek B, et al. , Shifts in gamma phase–amplitude coupling frequency from theta to alpha over posterior cortex during visual tasks. Frontiers in human neuroscience, 2010. 4: p. 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mormann F, et al. , Phase/amplitude reset and theta–gamma interaction in the human medial temporal lobe during a continuous word recognition memory task. Hippocampus, 2005. 15(7): p. 890–900. [DOI] [PubMed] [Google Scholar]
- 51.Kramer M and Eden U, Assessment of cross-frequency coupling with confidence using generalized linear models. Journal of Neuroscience Methods, 2013. 220(1): p. 64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Giehl J, Noury N, and Siegel M, Dissociating harmonic and non-harmonic phase-amplitude coupling in the human brain. NeuroImage, 2021. 227: p. 117648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jensen O, Spaak E, and Park H, Discriminating valid from spurious indices of phase-amplitude coupling. Eneuro, 2016. 3(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kurmann R, et al. , Rational design of transcranial alternating current stimulation: Identification, engagement, and validation of network oscillations as treatment targets. Clinical and translational neuroscience, 2018. 2(2): p. 2514183X18793515. [Google Scholar]
- 55.Ahn S, et al. , Targeting reduced neural oscillations in patients with schizophrenia by transcranial alternating current stimulation. Neuroimage, 2019. 186: p. 126–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Polanía R, et al. , The importance of timing in segregated theta phase-coupling for cognitive performance. Current Biology, 2012. 22(14): p. 1314–1318. [DOI] [PubMed] [Google Scholar]
- 57.Reinhart RM and Nguyen JA, Working memory revived in older adults by synchronizing rhythmic brain circuits. Nature neuroscience, 2019. 22(5): p. 820–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Violante IR, et al. , Externally induced frontoparietal synchronization modulates network dynamics and enhances working memory performance. Elife, 2017. 6: p. e22001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nitsche MA and Paulus W, Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology, 2001. 57(10): p. 1899–1901. [DOI] [PubMed] [Google Scholar]
- 60.Lauro LJR, et al. , TDCS increases cortical excitability: direct evidence from TMS–EEG. cortex, 2014. 58: p. 99–111. [DOI] [PubMed] [Google Scholar]
- 61.Ahn S and Fröhlich F, Pinging the brain with transcranial magnetic stimulation reveals cortical reactivity in time and space. Brain Stimulation, 2021. 14(2): p. 304–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fell J and Axmacher N, The role of phase synchronization in memory processes. Nature reviews neuroscience, 2011. 12(2): p. 105–118. [DOI] [PubMed] [Google Scholar]
- 63.Alekseichuk I, et al. , Electric field dynamics in the brain during multi-electrode transcranial electric stimulation. Nature communications, 2019. 10(1): p. 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tambini A, Nee DE, and D’Esposito M, Hippocampal-targeted theta-burst stimulation enhances associative memory formation. Journal of cognitive neuroscience, 2018. 30(10): p. 1452–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gratton C, et al. , The effect of theta-burst TMS on cognitive control networks measured with resting state fMRI. Frontiers in systems neuroscience, 2013. 7: p. 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rahnev D, et al. , Causal evidence for frontal cortex organization for perceptual decision making. Proceedings of the National Academy of Sciences, 2016. 113(21): p. 6059–6064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Alexander ML, et al. , Double-blind, randomized pilot clinical trial targeting alpha oscillations with transcranial alternating current stimulation (tACS) for the treatment of major depressive disorder (MDD). Translational psychiatry, 2019. 9(1): p. 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Raichle ME, Two views of brain function. Trends in cognitive sciences, 2010. 14(4): p. 180–190. [DOI] [PubMed] [Google Scholar]
- 69.Riddle J, McFerren A, and Frohlich F, Causal role of cross-frequency coupling in distinct components of cognitive control. Progress in Neurobiology, 2021: p. 102033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Miyaguchi S, et al. , Gamma tACS over M1 and cerebellar hemisphere improves motor performance in a phase-specific manner. Neuroscience Letters, 2019. 694: p. 64–68. [DOI] [PubMed] [Google Scholar]
- 71.Lorenz R, et al. , Efficiently searching through large tACS parameter spaces using closed-loop Bayesian optimization. Brain stimulation, 2019. 12(6): p. 1484–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Connor DE, et al. , Vagal nerve stimulation for the treatment of medically refractory epilepsy: a review of the current literature. Neurosurgical focus, 2012. 32(3): p. E12. [DOI] [PubMed] [Google Scholar]
- 73.Cimpianu C-L, et al. , Vagus nerve stimulation in psychiatry: a systematic review of the available evidence. Journal of neural transmission, 2017. 124(1): p. 145–158. [DOI] [PubMed] [Google Scholar]
- 74.Groves DA and Brown VJ, Vagal nerve stimulation: a review of its applications and potential mechanisms that mediate its clinical effects. Neuroscience & Biobehavioral Reviews, 2005. 29(3): p. 493–500. [DOI] [PubMed] [Google Scholar]
- 75.Johnson RL and Wilson CG, A review of vagus nerve stimulation as a therapeutic intervention. Journal of inflammation research, 2018. 11: p. 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alam M, et al. , Spatial and polarity precision of concentric high-definition transcranial direct current stimulation (HD-tDCS). Physics in Medicine & Biology, 2016. 61(12): p. 4506. [DOI] [PubMed] [Google Scholar]
- 77.Dmochowski JP, et al. , Optimized multi-electrode stimulation increases focality and intensity at target. Journal of neural engineering, 2011. 8(4): p. 046011. [DOI] [PubMed] [Google Scholar]
- 78.Grover S, et al. , High-frequency neuromodulation improves obsessive–compulsive behavior. Nature Medicine, 2021. 27(2): p. 232–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Alekseichuk I, et al. , Spatial working memory in humans depends on theta and high gamma synchronization in the prefrontal cortex. Current Biology, 2016. 26(12): p. 1513–1521. [DOI] [PubMed] [Google Scholar]
- 80.Helfrich RF, et al. , Selective modulation of interhemispheric functional connectivity by HD-tACS shapes perception. PLoS Biol, 2014. 12(12): p. e1002031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Holdefer R, Sadleir R, and Russell M, Predicted current densities in the brain during transcranial electrical stimulation. Clinical neurophysiology, 2006. 117(6): p. 1388–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ali MM, Sellers KK, and Frohlich F, Transcranial alternating current stimulation modulates large-scale cortical network activity by network resonance. The Journal of neuroscience : the official journal of the Society for Neuroscience, 2013. 33(27): p. 11262–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kutchko KM and Fröhlich F, Emergence of metastable state dynamics in interconnected cortical networks with propagation delays. PLoS Comput. Biol, 2013. 9(10): p. e1003304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kasten FH, et al. , Integrating electric field modeling and neuroimaging to explain inter-individual variability of tACS effects. Nature communications, 2019. 10(1): p. 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Evans C, et al. , Dose-controlled tDCS reduces electric field intensity variability at a cortical target site. Brain stimulation, 2020. 13(1): p. 125–136. [DOI] [PubMed] [Google Scholar]
- 86.Opitz A, et al. , Determinants of the electric field during transcranial direct current stimulation. Neuroimage, 2015. 109: p. 140–150. [DOI] [PubMed] [Google Scholar]
- 87.Saturnino GB, et al. , SimNIBS 2.1: a comprehensive pipeline for individualized electric field modelling for transcranial brain stimulation. Brain and Human Body Modeling, 2019: p. 3–25. [PubMed] [Google Scholar]
- 88.Huang Y, et al. , Realistic volumetric-approach to simulate transcranial electric stimulation—ROAST—a fully automated open-source pipeline. Journal of neural engineering, 2019. 16(5): p. 056006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bikson M, et al. , Effects of uniform extracellular DC electric fields on excitability in rat hippocampal slices in vitro. The Journal of Physiology, 2004. 557(1): p. 175–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Radman T, et al. , Spike Timing Amplifies the Effect of Electric Fields on Neurons: Implications for Endogenous Field Effects. The Journal of Neuroscience, 2007. 27(11): p. 3030–3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Thielscher A, Opitz A, and Windhoff M, Impact of the gyral geometry on the electric field induced by transcranial magnetic stimulation. Neuroimage, 2011. 54(1): p. 234–243. [DOI] [PubMed] [Google Scholar]
- 92.Huang Y, et al. , Measurements and models of electric fields in the in vivo human brain during transcranial electric stimulation. Elife, 2017. 6: p. e18834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Opitz A, et al. , Spatiotemporal structure of intracranial electric fields induced by transcranial electric stimulation in humans and nonhuman primates. Scientific reports, 2016. 6(1): p. 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ruffini G, et al. , Targeting brain networks with multichannel transcranial current stimulation (tCS). Current Opinion in Biomedical Engineering, 2018. 8: p. 70–77. [Google Scholar]
- 95.Fischer DB, et al. , Multifocal tDCS targeting the resting state motor network increases cortical excitability beyond traditional tDCS targeting unilateral motor cortex. Neuroimage, 2017. 157: p. 34–44. [DOI] [PubMed] [Google Scholar]
- 96.Ruffini G, et al. , Optimization of multifocal transcranial current stimulation for weighted cortical pattern targeting from realistic modeling of electric fields. Neuroimage, 2014. 89: p. 216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wach C, et al. , Effects of 10 Hz and 20 Hz transcranial alternating current stimulation (tACS) on motor functions and motor cortical excitability. Behavioural brain research, 2013. 241: p. 1–6. [DOI] [PubMed] [Google Scholar]
- 98.Hopfinger JB, Parsons J, and Fröhlich F, Differential effects of 10-Hz and 40-Hz transcranial alternating current stimulation (tACS) on endogenous versus exogenous attention. Cognitive neuroscience, 2017. 8(2): p. 102–111. [DOI] [PubMed] [Google Scholar]
- 99.Clayton MS, Yeung N, and Cohen Kadosh R, The effects of 10 Hz transcranial alternating current stimulation on audiovisual task switching. Frontiers in neuroscience, 2018. 12: p. 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zaehle T, Rach S, and Herrmann CS, Transcranial alternating current stimulation enhances individual alpha activity in human EEG. PloS one, 2010. 5(11): p. e13766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vossen A, Gross J, and Thut G, Alpha Power Increase After Transcranial Alternating Current Stimulation at Alpha Frequency (α-tACS) Reflects Plastic Changes Rather Than Entrainment. Brain Stimulation, 2015. 8(3): p. 499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stecher HI and Herrmann CS, Absence of alpha-tACS aftereffects in darkness reveals importance of taking derivations of stimulation frequency and individual alpha variability into account. Frontiers in psychology, 2018. 9: p. 984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Notbohm A, Kurths J, and Herrmann CS, Modification of brain oscillations via rhythmic light stimulation provides evidence for entrainment but not for superposition of event-related responses. Frontiers in human neuroscience, 2016. 10: p. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Negahbani E, et al. , Targeting alpha-band oscillations in a cortical model with amplitude-modulated high-frequency transcranial electric stimulation. Neuroimage, 2018. 173: p. 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Negahbani E, et al. , Transcranial Alternating Current Stimulation (tACS) Entrains Alpha Oscillations by Preferential Phase Synchronization of Fast-Spiking Cortical Neurons to Stimulation Waveform. bioRxiv, 2019: p. 563163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Romei V, et al. , Causal evidence that intrinsic beta-frequency is relevant for enhanced signal propagation in the motor system as shown through rhythmic TMS. Neuroimage, 2016. 126: p. 120–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wutz A, Melcher D, and Samaha J, Frequency modulation of neural oscillations according to visual task demands. Proceedings of the National Academy of Sciences, 2018. 115(6): p. 1346–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Senoussi M, et al. , Theta oscillations shift towards optimal frequency for cognitive control. bioRxiv, 2020. [DOI] [PubMed] [Google Scholar]
- 109.Furman AJ, et al. , Cerebral peak alpha frequency predicts individual differences in pain sensitivity. Neuroimage, 2018. 167: p. 203–210. [DOI] [PubMed] [Google Scholar]
- 110.Furman AJ, et al. , Sensorimotor Peak Alpha Frequency Is a Reliable Biomarker of Prolonged Pain Sensitivity. Cerebral Cortex, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wolinski N, et al. , The speed of parietal theta frequency drives visuospatial working memory capacity. PLoS biology, 2018. 16(3): p. e2005348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Waschke L, et al. Aperiodic EEG activity tracks 1/f stimulus characteristics and the allocation of cognitive resources. 2019. Poster session presented at the Conference on Cognitive Computational …. [Google Scholar]
- 113.Terney D, et al. , Increasing human brain excitability by transcranial high-frequency random noise stimulation. Journal of Neuroscience, 2008. 28(52): p. 14147–14155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chaieb L, Paulus W, and Antal A, Evaluating aftereffects of short-duration transcranial random noise stimulation on cortical excitability. Neural plasticity, 2011. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mulquiney PG, et al. , Improving working memory: exploring the effect of transcranial random noise stimulation and transcranial direct current stimulation on the dorsolateral prefrontal cortex. Clinical Neurophysiology, 2011. 122(12): p. 2384–2389. [DOI] [PubMed] [Google Scholar]
- 116.van der Groen O and Wenderoth N, Transcranial random noise stimulation of visual cortex: stochastic resonance enhances central mechanisms of perception. Journal of Neuroscience, 2016. 36(19): p. 5289–5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Antal A and Herrmann CS, Transcranial alternating current and random noise stimulation: possible mechanisms. Neural plasticity, 2016. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Stacey WC and Durand DM, Stochastic resonance improves signal detection in hippocampal CA1 neurons. Journal of Neurophysiology, 2000. 83(3): p. 1394–1402. [DOI] [PubMed] [Google Scholar]
- 119.Leong SL, et al. , High definition transcranial pink noise stimulation of anterior cingulate cortex on food craving: An explorative study. Appetite, 2018. 120: p. 673–678. [DOI] [PubMed] [Google Scholar]
- 120.Bramson B, et al. , Improving emotional-action control by targeting long-range phase-amplitude neuronal coupling. eLife, 2020. 9: p. e59600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Berger B, et al. , Dynamic regulation of interregional cortical communication by slow brain oscillations during working memory. Nature communications, 2019. 10(1): p. 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Turi Z, et al. , ϑ-γ Cross-Frequency Transcranial Alternating Current Stimulation over the Trough Impairs Cognitive Control. eneuro, 2020. 7(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.de Lara GA, et al. , Perturbation of theta-gamma coupling at the temporal lobe hinders verbal declarative memory. Brain stimulation, 2018. 11(3): p. 509–517. [DOI] [PubMed] [Google Scholar]
- 124.Voytek B, et al. , Oscillatory dynamics coordinating human frontal networks in support of goal maintenance. Nature neuroscience, 2015. 18(9): p. 1318–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang H, et al. , Theta and alpha oscillations are traveling waves in the human neocortex. Neuron, 2018. 98(6): p. 1269–1281. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Berényi A, et al. , Closed-loop control of epilepsy by transcranial electrical stimulation. Science, 2012. 337(6095): p. 735–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tompary A and Davachi L, Consolidation promotes the emergence of representational overlap in the hippocampus and medial prefrontal cortex. Neuron, 2017. 96(1): p. 228–241. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dikker S, et al. , Brain-to-brain synchrony tracks real-world dynamic group interactions in the classroom. Current biology, 2017. 27(9): p. 1375–1380. [DOI] [PubMed] [Google Scholar]
- 129.Riddle J, Rubinow DR, and Frohlich F, A case study of weekly tACS for the treatment of major depressive disorder. Brain Stimulation: Basic, Translational, and Clinical Research in Neuromodulation, 2020. 13(3): p. 576–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Force RB, et al. , A case study of the feasibility of weekly tACS for the treatment of auditory hallucinations in schizophrenia. Brain Stimul, 2021. 14(2): p. 361–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ahn S, et al. , Identifying and engaging neuronal oscillations by transcranial alternating current stimulation in patients with chronic low back pain: a randomized, crossover, double-blind, sham-controlled pilot study. The Journal of Pain, 2019. 20(3): p. 277. e1–277. e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Daughters SB, et al. , Alpha-tACS effect on inhibitory control and feasibility of administration in community outpatient substance use treatment. Drug and Alcohol Dependence, 2020. 213: p. 108132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Siddiqi SH, et al. , Distinct symptom-specific treatment targets for circuit-based neuromodulation. American Journal of Psychiatry, 2020. 177(5): p. 435–446. [DOI] [PMC free article] [PubMed] [Google Scholar]


