Abstract
Stress and abnormal stress response are associated with schizophrenia spectrum disorder (SSD), but the brain mechanisms linking stress to symptomatology remain unclear. In this study, we used a stress-based functional neuroimaging task, reverse-translated from preclinical studies, to test the hypothesis that abnormal corticolimbic processing of stressful threat anticipation is associated with psychosis and affective symptoms in SSD. Participants underwent an MRI-compatible ankle-shock task (AST) in which the threat of mild electrical shock was anticipated. We compared functional brain activations during anticipatory threat periods from N=18 participants with SSD (10M/8F) to those from N=12 community controls (9M/3F). After family-wise error correction, only one region, the ventral anterior cingulate cortex (vACC), showed significantly reduced activation compared with controls. vACC activation significantly correlated with clinical symptoms measured by the Brief Psychiatric Rating Scale total score (r=0.54) and the psychosis subscale (r=0.71), and inversely correlated with trait depression measured by the Maryland Trait and State Depression scale (r=−0.48). Deficient activation in vACC under stress of anticipated threat may lead to aberrant interpretation of such threat, contributing to psychosis and mood symptoms in SSD. This experimental paradigm has translational potential and may identify circuitry-level mechanisms of stress-related mental illness, leading to more targeted treatment.
Keywords: Schizophrenia, Stress, Ankle-shock, Neuroimaging, Functional Imaging
1. Introduction
The stress-diathesis model for psychosis (Corcoran et al., 2003) posits that stress is implicated in the onset and exacerbation of psychosis (Corcoran et al., 2002; Heins et al., 2011). Brain areas and cellular pathways responsible for the evaluation of and response to stress have been implicated in psychotic disorders and are themselves vulnerable to stress (Brown, 2011). In parallel, aberrant stress reactivity in schizophrenia are linked with worsened functional capacity (Jansen et al., 1998; Nugent et al., 2014) and positive symptom burden (Norman and Malla, 1994). Severity of positive symptoms has been linked to chronic cumulative stress and elevated stress sensitivity even to minor events (Berger et al., 2018; Reininghaus et al., 2016; Zhou et al., 2020). One potential mechanism by which a high cumulative burden of stress is converted to psychotic experience may be through aberrant salience representation or misattributed threat operationalized as paranoia or delusion (Holt et al., 2012; Reininghaus et al., 2016). Yet, the circuit-level mechanism of the stress-diathesis model, i.e., how stress interacts with relevant brain circuitry to lead to the psychotic psychopathology in patients with SSD, remains elusive. In comparison, stress is extensively studied at cellular and system levels from neurophysiological excitability and synaptic function to circuit-level throughput (Joëls and Baram, 2009; Thompson et al., 2015). Translating these rich neuroscience findings to fully account for how and where the stress engages the brain in individuals with schizophrenia and contributes to symptoms is critical for developing better prevention and treatment strategies.
Psychological stress comes in many forms, many of which can lead to significant health and behavioral changes through an array of biological stress mediators (Joëls and Baram, 2009). Functional imaging data in human participants has allowed study into how psychological stress exposure and resulting neuroendocrine responses associate with activity in specific brain regions and circuits and behavioral output. Acute psychological stress can alter cognitive and emotional processing, which can be reflected by functional imaging correlates. For example, psychological stress induced by a mental arithmetic task activates the hypothalamic-pituitary-adrenal (HPA) axis and leads to decreased activity in major portions of the limbic system including hypothalamus, hippocampus, amygdala, and medio-orbitofrontal cortex, with the degree of deactivation of the hippocampus directly predicting neuroendocrine cortisol response (Pruessner et al., 2008). Acute psychosocial stress alters functional connectivity in medial temporal and frontal brain areas differentially, with stress causing a bias towards processing emotional stimuli (Li et al., 2014). After chronic psychosocial stress, long-lasting but reversible impairment in PFC activation during a PFC-dependent attention-shifting task was associated with impaired attentional control in healthy volunteers (Liston et al., 2009). In healthy subjects, numerous functional imaging studies have described how tasks involving executive functions including attention, memory, and inhibition activate cortical and limbic areas that overlap with emotion and stress processing regions, and can themselves be influenced by stress (Chavanne and Robinson, 2021; Kumar et al., 2014; Mitchell and Phillips, 2007; Murphy et al., 2003; Orem et al., 2019; Wager and Smith, 2003). Psychological stress generated by unpredictable physical threat paradigms has been shown to involve not only pain perception and sensory function regions such as thalamus, periaqueductal grey, and posterior insula (Drabant et al., 2011; Grupe and Nitschke, 2013; Klumpers et al., 2017; Robinson et al., 2013), but also amygdala, ventral striatum, ventromedial and dorsolateral prefrontal cortices, anterior cingulate cortex (ACC), and anterior insula—brain regions that are related to decision-making, reward, emotional processing and stress regulation.
Limbic and frontal structures including ACC, insula, ventral striatum, hippocampus, and prefrontal cortex have been strongly implicated in schizophrenia (Heckers et al., 1998; Minzenberg et al., 2009; Wylie and Tregellas, 2010) and through their abnormal stress processing (Birur et al., 2017; Ellison-Wright and Bullmore, 2010; Vogt et al., 1992) and hypothalamic-pituitary-adrenal (HPA)-axis regulation (Diorio et al., 1993; Mizoguchi et al., 2003). Additionally, the ventral ACC and insula form the salience network which has been implicated in disrupted information processing in schizophrenia (White et al., 2010). These areas thus form a neurocircuitry crossroads by implication in both clinical symptoms in schizophrenia and their role in responses to stress. We hypothesized that dysfunctional activation of stress-regulating brain areas in response to stress contributes to psychosis symptoms in individuals with SSD. As animal models can examine stress-related cellular to circuitry mechanisms but cannot directly be used to probe psychosis symptoms, we developed a translational stress induction paradigm that is human MRI compatible and ethical to reveal the hypothesized relationship between aberrant activation during stress processing and clinical symptoms in SSD. In animal studies, the threat of electric foot shock is a classic approach to study mechanisms related to stress (Seligman and Maier, 1967). Using the unpredictable threat of electrical shocks is a valid and reliable technique to generate psychological stress or induced anxiety (Chavanne and Robinson, 2021). A similar fMRI-based task in healthy human participants has used a shock threat to engage salience network and limbic activation (McMenamin et al., 2014). Here, we developed an analogous ankle-shock threat (AST) paradigm in which the anticipation of receiving a small amount of battery-current induced uncomfortable shock to the ankle is used to simulate anticipatory stress in response to threat. In this proof-of-concept study, we show that the AST can engage dysfunctional activation in patients with SSD in brain areas relevant to both the disorder and stress regulation, and activation under such stressful conditions are highly associated with patients’ psychotic and affective symptom severities.
2. Methods
2.1. Participants
Eighteen SSD patients (10 male) and 12 adult community controls (CC, 9 male) were recruited from outpatient mental health clinics. Most patients were medicated: twelve were on a single antipsychotic medication (2 typical, 10 atypical), three patients were on two medications (two on clozapine plus another atypical, and one on a typical and an atypical), one patient was on three antipsychotic medications, and two patients were not taking an antipsychotic at the time of the study. Their current antipsychotic medication dosages were converted to chlorpromazine equivalent (CPZ) (Leucht et al., 2015). Structured Clinical Interview for DSM-IV was completed by Master’s level clinicians to establish diagnosis for all participants. Of the 18 patients recruited for SSD, 5 met the criteria for schizoaffective disorder, and 13 patients met the criteria for schizophrenia spectrum disorder. Five of these 13 patients with schizophrenia spectrum disorder had a history of a major depressive episode in full remission but did not meet criteria for schizoaffective disorder. Controls had no current DSM-IV Axis I diagnosis, including any mood or psychotic disorder, and no known family history of psychosis in two or more generations. Pregnancy, major medical illness, history of head injury with cognitive sequelae, and intellectual disability were exclusionary as well as any contraindication to MRI. Participants with substance dependence within the last 6 months, or current substance abuse (except nicotine) were excluded.
Clinical items
Current overall psychiatric symptoms were measured by the Brief Psychiatric Rating Scale (BPRS). Psychosis was assessed using the BPRS psychosis subscale which included 4 items: conceptual disorganization, suspiciousness, hallucinatory behavior, and unusual thought content. Depression was assessed using the Maryland Trait and State Depression scale (MTSD) (Chiappelli et al., 2014), with which frequency of depression symptoms over the lifetime (“trait depression”) and symptoms of depression experienced over the previous two weeks (“state depression”) were assessed. Developmental stressors were quantified with the Childhood Trauma Questionnaire (CTQ) and recent stress level was assessed using the Perceived Stress Scale (PSS) (Cohen et al., 1983).
2.2. fMRI during the Ankle-shock task (AST)
fMRI data was collected using a 3-T Siemens Prisma scanner and 64-channel coil. Whole brain EPI was collected using the Human Connectome Project (HCP) multi-band fMRI protocol, with the following parameters: 2 mm isotropic, TR/TE=720/33.1ms, flip angle = 52 degrees, FOV=208×108 mm (RO x PE), Matrix=1–4×90 (RO x PE), slice thickness = 2 mm, multiband factor=8, AP/PA encoding, echo spacing = 0.58 ms, and 2290 Hz/Px. The AST task was presented in one run with 680 volumes collected. Images were slice timing corrected, volume registered, linearly detrended, normalized into MNI standard space, and spatially smoothed (FWHM=8mm) using SPM12. The AST was developed to generate stress for patients with SSD and controls under anticipatory threat to an ankle shock in a safe and ethical manner based on similar designs shown to engage the salience network (McMenamin et al., 2014). Prior to the day of testing, participants were tested for sensitivity outside the scanner. Two electrodes separated by 1 inch were fixed to a Velcro strap and wrapped around the ankle. The target area for placement was the sural nerve, posterior and superior to the lateral malleolus. Areas where skin was damaged or where a rash was present were avoided. The electrodes were attached to a battery-powered shock generating device (Transcutaneous Aversive Stimulator, Coulborne Instruments). The sensation of this shock can be described as similar to the uncomfortable experience of touching a surface with static electricity. Participants were advised that the purpose of this testing was to determine the level of shock found to be just on the threshold of being painful, and the participant was in charge of pressing the level to administer the shock (500 milliseconds in duration) during this sensitivity testing. Participants were exposed to increasing amperage starting at the lowest level, which was increased sequentially until the participant rated the sensation as “5 out of 10” on an analog scale to indicate “mildly painful.” The level just below that identified was considered ‘uncomfortable but not, or only mildly, painful’, the sensitivity stimulation threshold to be used for subsequent testing inside the scanner.
Participants returned on a different day for the AST inside the scanner where they were instructed to view a screen that would display either a blue or yellow circle. One would be associated with a period with the possibility of shocks and the other indicated a period of safety with no possible shocks. The determination of which color would represent shock vs. safety was randomized across participants prior to starting the task and the participant was aware of their shock vs. safety signals. Electrodes were attached to the ankle in the same manner as during sensitivity testing. The task had 3 conditions: (1) a period for a shock condition in which a shock is delivered at a pseudo-random time point (same for all participants) during the period, while a color sign on the screen indicates shocks are possible; (2) a period for a threat condition in which the same color is present indicating shocks are possible, but no shock is actually given; and (3) a period of a safe condition in which no shock is given and the color indicates safety. There was a total of 57 six-second periods (21 shock, 18 threat but no shock, 18 safe), presented in pseudorandom order, in which the threat minus safe period comparison was used for testing the primary hypothesis.
2.3. Ethical Considerations
The research protocol was approved by the University of Maryland Baltimore Institutional Review Board. Participants gave written informed consent, and patients were tested for capacity to consent prior to signing the consent form. Participants were instructed they could withdraw from the study at any time. We evaluated to ensure that they understood the voluntary nature of participation and associated risks and benefits, and how they would respond if they wished to quit or felt uncomfortable during the study. Inside the scanner, participants were given an emergency button to stop the task if at any point they felt unable or unwilling to continue.
2.4. Statistics
First level models were developed for each subject by entering all the volumes into a single analysis regressing the “shock”, “threat” and “safe” conditions. The contrast of interest was the threat - safe condition, to study brain processing the anticipatory threat of the unpleasant shock but without the interference of the actual shock. This contrast was further investigated in the second level group analysis. Statistical significance of voxel-wise whole brain analysis activations followed by family-wise error correction were computed using SPM Marsbar with a peak value of p<0.001 and cluster-wise FWE α<0.05. SPSS software version 25 (IBM Corp., Armonk NY) was used for regression analyses and for parametric statistical analysis including multiple regression and t-tests for group comparisons where appropriate. Age and sex were included as covariates and reported where significant. Means are presented ± S.D.
3. Results
3.1. Activation under anticipated experimental threat
Stimulation threshold determined during sensitivity testing was 1.18±0.66 mA in the patients and 1.21±0.95 mA in the controls (p=0.91). Comparing threat vs. safe conditions, 13 regions showed nominally significant differences between SSD and CC, at p<0.001 (t-value of activation > 3.51), most of them were located at the limbic and frontal areas (Figure 1A, Figure 2). After family-wise error cluster level correction, only one cluster at the ventral ACC showed significant group difference (274 voxels, t=4.61, FWE corrected p=0.033) (Figure 1A), where SSD showed significantly reduced activation in response to the anticipated threat compared with CC. Age (r=+0.05, p=0.84), sex (r=−0.18, p=0.47), and CPZ (r=+0.24, p=0.34) were not significantly associated with the vACC activation in the patients. Other activation clusters with a peak-level uncorrected p<0.001 that did not survive family-wise error correction are shown in Figure 2 (cluster sizes ≥14 voxels, pFWE≥0.114).
Figure 1:
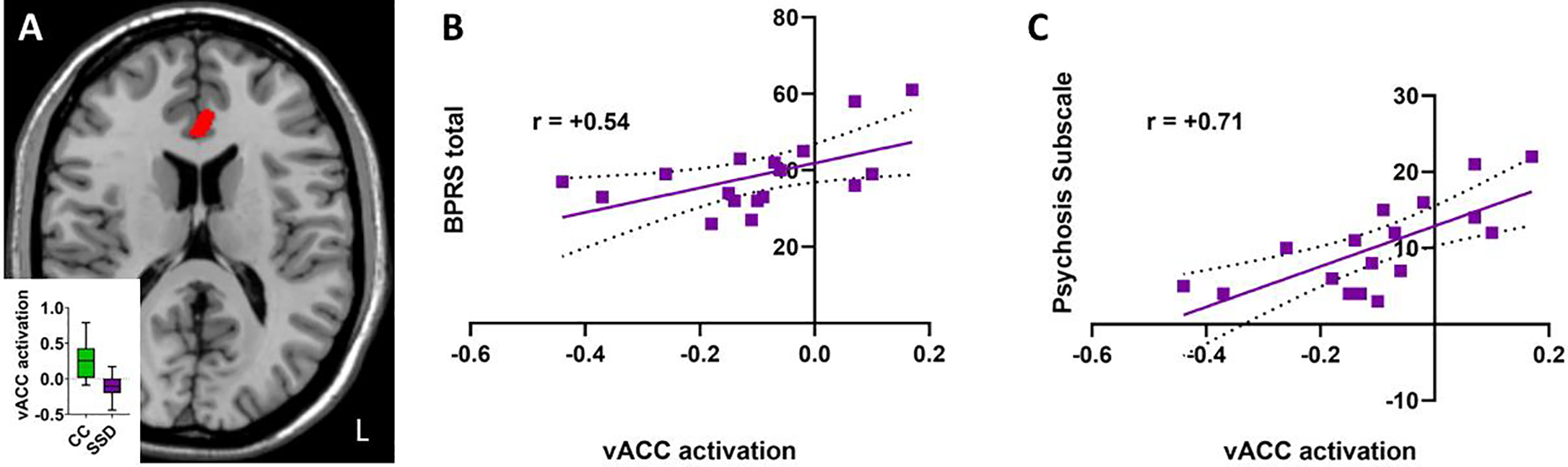
(A) Cluster activation during threat-safe comparison based on community control (CC) minus schizophrenia spectrum disorder (SSD) cases at MNI z+16. Activation shown represents t-level by voxel (red = t>3.8). Red indicates decreased peak activation of a 274-voxel cluster located in ventral ACC in SSD compared to CC. Inset with box-and-whisker plot showing group differences. (B) AST task-based vACC activation is associated with BPRS ratings (p=0.024). (C) vACC activation is strongly associated with the BPRS psychosis subscale (p=0.001). Linear regressions shown with 95% confidence interval, with r values indicated.
Figure 2:
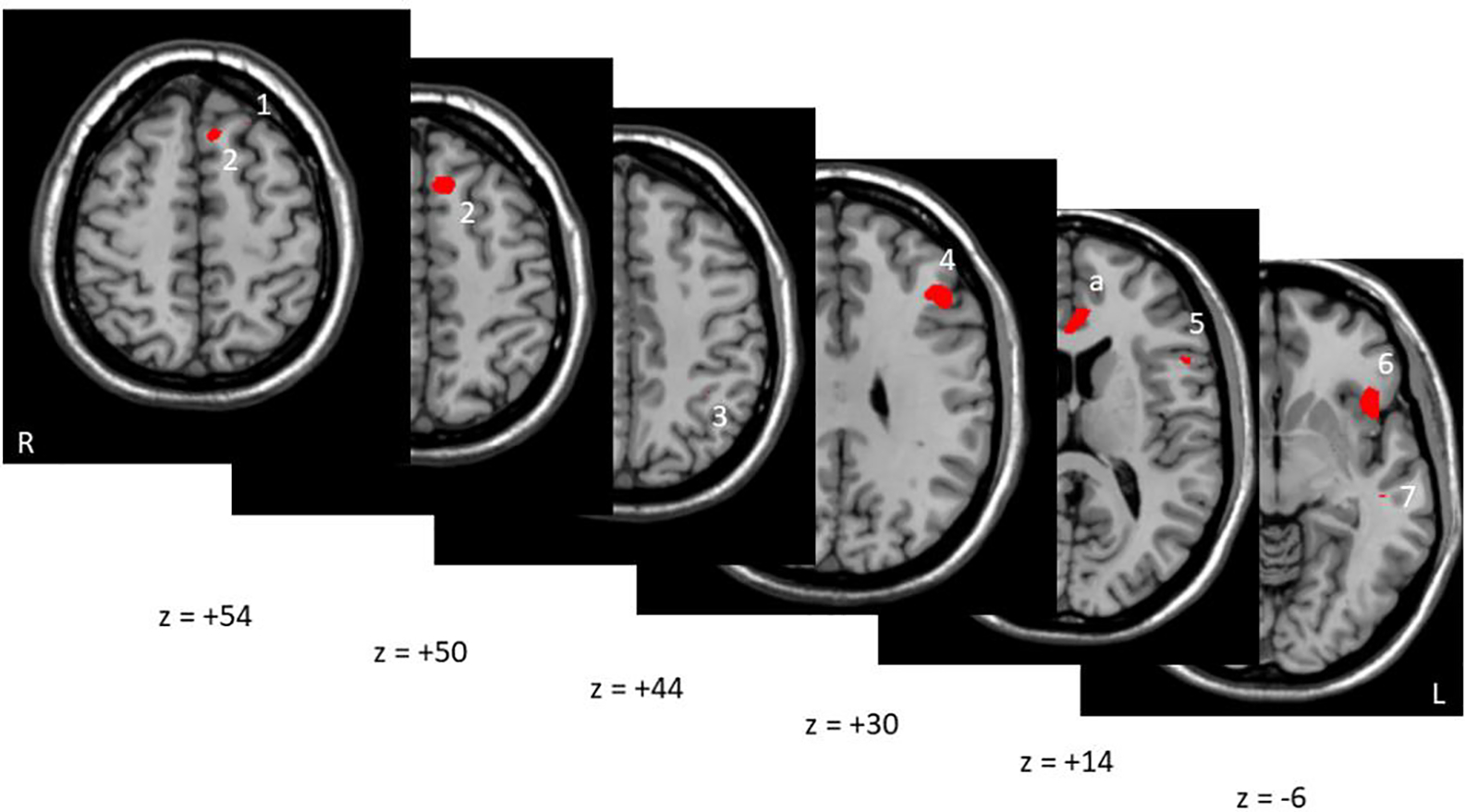
Multiple axial sections of other subthreshold areas that were at peak level of p<0.001 but did not survive cluster-level FWE correction. Red corresponds to t-values>3.73 for CC-SSD comparison of the threat-safe difference. These regions include (Brodmann’s Area – BA, voxels indicate cluster size): 1. BA8/superior frontal gyrus (21 voxels), 2. BA8/medial superior frontal gyrus (100 voxels), 3. BA 40, inferolateral parietal (27 voxels), 4. BA 9/dorsolateral PFC (179 voxels), 5. BA 44/inferior frontal gyrus (47 voxels), 6. Pars orbitalis, inferior frontal cortex (159 voxels) 7. BA 21/middle temporal gyrus (14 voxels). Five other regions were not shown due to cluster sizes <10 voxels and pFWE>0.98 for clarity, but these were located in BA 8/superior frontal gyrus (6 voxels), BA24 distinct from and lateral to the vACC activation described above (3 voxels), R insula (2 voxels), BA 10 (1 voxel), and BA 38 (1 voxel). Marker “a” indicates significant vACC activation also seen in Figure 1. No significant activations are present in the areas obscured.
3.2. Activation under actual shock condition
Three regions showed nominally and significantly reduced activation in SSD compared with CC in the shock vs. safe condition at p<0.001: bilateral insula and left pars orbitalis; one region had nominally greater activation in SSD compared with CC comparison at the right precuneus using a peak-level uncorrected p<0.001; none of these regions’ activations survived FWE (Supplementary Figure 1).
3.3. Psychosis and vACC activation during experimental threat
The beta values of the vACC activation were extracted and found to be significantly associated with the overall BPRS score (r=+0.54, p=0.024, Figure 1B), explaining about 29.2% of the overall psychiatric symptom variance. Age, sex, and CPZ equivalent were non-significant predictors in multiple regression analysis (all p>0.56). The BPRS psychosis subscale had an even greater association with vACC activation (r=+0.71, p=0.001) in patients (Figure 1C), where elevated vACC activation in response to anticipatory threat explained about 50.4% of the severity of psychosis symptoms. Examining the four individual items within the psychosis subscale, the strongest individual item associations with vACC activation were abnormal thought content (r=0.74), suspiciousness (r=0.63), and hallucinations (r=0.61), all p<0.05 after Bonferroni correction for four individual items. In controls, there was no significant association between vACC activation and BPRS total (r=0.17, p>0.6) or vACC activation and the psychosis subscale (r=0.13, p>0.7), although most controls had no or minimal symptoms on BPRS, so the variances were restricted.
Although there was no group difference in stimulation sensitivity threshold to pain perception, there was a significant inverse relationship between stimulation threshold (mA) and this ACC activation in patients (r=−0.58; p=0.014), but not in controls (r=−0.09, p =0.77) (Figure 3). There was also a significant inverse correlation between stimulation threshold and psychosis symptoms in SSD (r=−0.47, p=0.049), implying a relationship of greater burden of psychotic symptoms with lower pain threshold, despite the fact that as a group SSD patients reported similar pain threshold as the controls.
Figure 3:
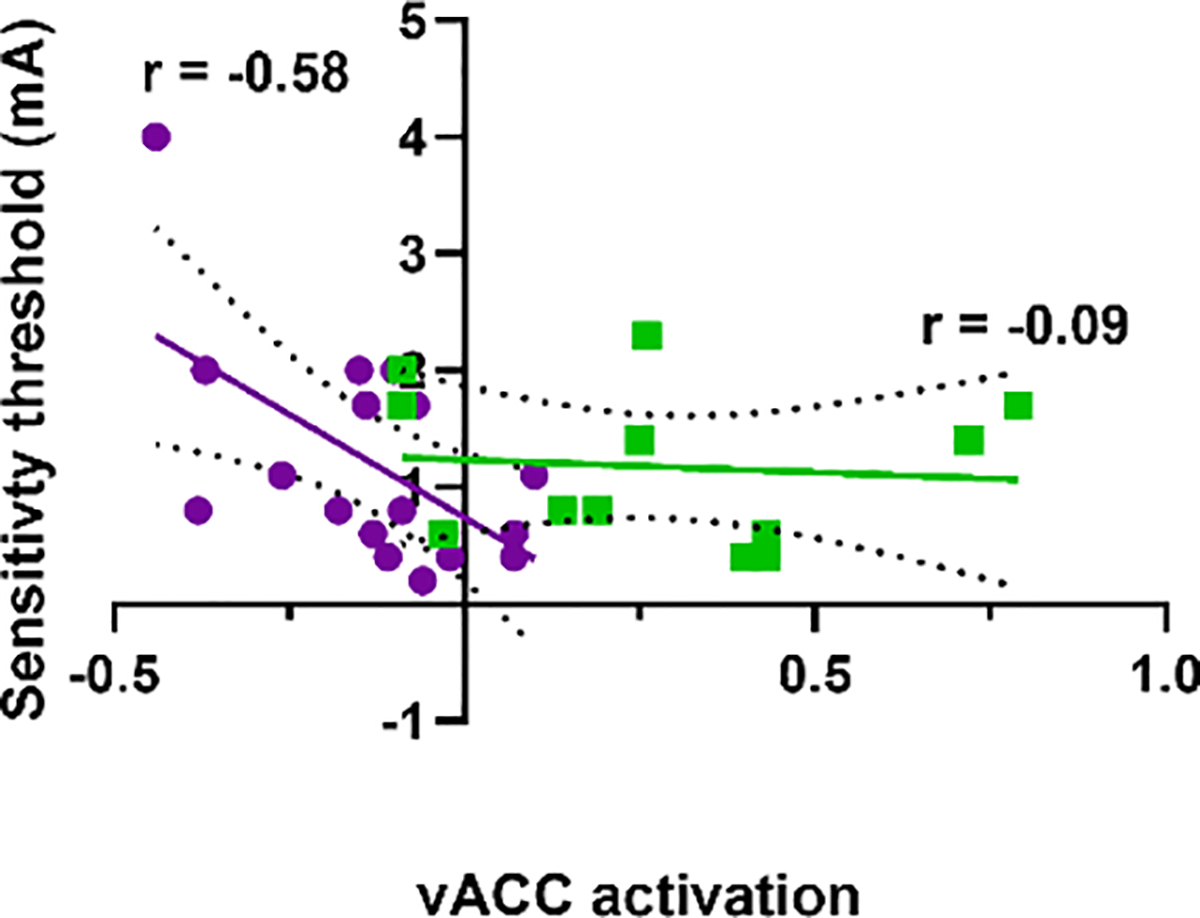
Association between AST-induced ACC activation (threat-safe condition) and stimulation amperage sensitivity threshold, with greater values indicating less sensitivity to pain. SSD patients (purple, p=0.014) and CC (green, p=0.77) shown. Linear regression shown with 95% confidence interval, with r values indicated.
3.4. No association between life stressors and vACC activation during experimental threat
Patients reported significantly greater childhood trauma than controls (p=0.012) (Table 1). Patients also had higher current perceived stress level than controls, although this was not statistically significant (p=0.13). However, there was no significant association of the vACC activation with CTQ (r=−0.20 and 0.08 for patients and controls, respectively; p=0.42, and 0.80). The vACC activation was also not significantly associated with recent stress levels (r=0.005 and 0.11 in patients and controls, respectively; both p>0.7). CTQ and PSS were not significantly associated with psychosis score in the patients (r=0.05 and 0.16 respectively; p=0.8, 0.5), suggesting that the heightened overall childhood traumatic stress experience or current stress levels are unlikely contributors to the findings on stress-induced activations and psychosis.
Table 1:
Participant demographic and clinical information
| CC n=12 | SSD n=18 | p-value | |
|---|---|---|---|
| Age [years] (SD) | 39.3 (13.6) | 40.8 (13.7) | 0.78 |
| Sex (% male) | 75% | 55.6% | 0.30 |
| BPRS (SD) | NA | 38.6 (9.4) | - |
| BPRS – Psychosis subscale (SD) | NA | 10.2 (5.9) | - |
| MTSD – State Depression | 10.4 (9.8) | 17.9 (14.9) | 0.14 |
| MTSD – Trait Depression | 13.3 (16.4) | 22.6 (19.5) | 0.18 |
| CTQ | 31.2 (4.0) | 46.9 (17.9) | 0.012 |
| PSS | 18.5 (2.6) | 1.21 (0.95) | 0.13 |
| Sensitivity Threshold (mA) | 1.18 (0.66) | 21.0 (5.1) | 0.91 |
| Medication dose (SD) † | - | 617 (793) | - |
Antipsychotic medication dose is provided in chlorpromazine equivalent dose (milligrams per day).
3.5. Depressive symptoms and vACC activation during experimental threat
Patients reported more trait depressive symptoms (p=0.14) and state depressive symptoms (p=0.18) as measured by MTSD compared with controls although, not statistically significant as the sample size is modest (Table 1). Opposite to psychosis, this anticipatory stress-induced vACC cluster activation significantly and inversely correlated with trait depression scores (r=−0.48, p=0.043) in SSD but not in CC (r=+0.14, p=0.7, Figure 4A). The effects of state depression were not significant (r=−0.28 and +0.32, p=0.25 and 0.31, SSD and CC respectively) (Figure 4B). We also tested the relationship between stimulation strength threshold and depression given the extensive literature associating pain and depressive symptoms (e.g. 29), but there was no significant relationship with either depression measure in the patients (r=0.02 state, 0.20 trait, p>0.40 for both).
Figure 4:
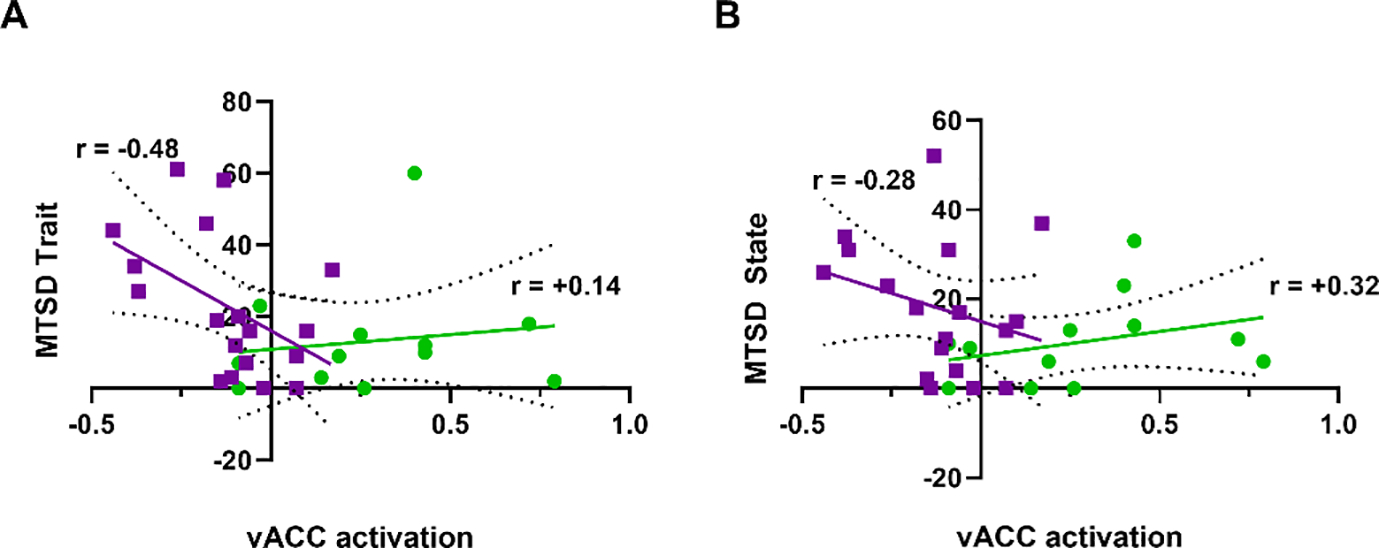
ACC activation during ankle-shock threat task (threat-safe condition) vs. trait and state depression measures in SSD patients (purple) and community controls (green). (A) Trait quantitative depression scores were significantly associated with vACC activation (p=0.043) in patients with SSD but not in controls (p=0.7) (B) State depression scores were not significantly correlated with vACC activation (SSD p>0.25, CC p>0.31). Linear regression shown with 95% confidence interval, with r values indicated.
4. Discussion
The present proof-of-concept study shows that this translational stress-based task to generate anticipatory threat to a shock in human subjects reliably engaged brain areas involved in emotional and stress regulation and revealed deficient activation in the ventral anterior cingulate cortex of individuals with schizophrenia spectrum disorder. This aberrant cingulate activation significantly and robustly correlated with clinical symptoms in patients with SSD, particularly psychosis symptom severity.
Previous fMRI studies have used instructed threat, without actual threat exposure during fMRI, to identify associations between paranoid delusions in patients with schizophrenia, and showed activations of salience network among a large number of other brain regions (Perez et al., 2015). While those findings are broadly consistent with ours, the voxelwise p< 0.01 used to identify significant regions in that study is usually considered too liberal in fMRI studies (Cox et al., 2017; Eklund et al., 2016), which may in part be due to the use of an instructed shock threat without actual shock exposure during fMRI (Perez et al., 2015). Another study using similar shock-induced anticipatory threat in healthy participants have also shown threat transiently alters salience network efficiency (McMenamin et al., 2014). Building on these previous studies, we developed an anticipatory threat stress task designed to translate decades of animal studies using foot shocks as stressors to generating stress with both physical and emotional components (Bali and Jaggi, 2015; Maier and Seligman, 2016) for ethical human research. Our main finding of the group difference is localized to the ventral anterior cingulate. Amongst its many anatomical connections, the ACC receives nociceptive afferents from the thalamic nuclei and also has connections to periaqueductal grey and amygdala, while functionally it is involved in specifying the affective content of noxious stimuli and learning the prediction and avoidance of noxious stimuli (Devinsky et al., 1995). Accordingly, cingulate lesions decrease pain sensitivity (Brown and Schäfer, 1888). Therefore, our finding that vACC activation in patients was significantly associated with lower threshold of sensitivity to the applied shock is consistent with this region’s role in detecting physical and emotional aspects of noxious stimuli (Price, 2000).
The ACC also functions in several complex processes including error detection and evaluation (Bush et al., 2000; Gehring et al., 1993; Van Veen and Carter, 2002), processing of affective responses to emotional cues (Lane et al., 1998), and social evaluation especially involving detection of situations that may cause social/emotional pain (Dedovic et al., 2016; Eisenberger et al., 2003). Previous studies have suggested that stress sensitivity, aberrant salience, and threat anticipation are key psychological processes in the development of psychotic experiences in daily life (Reininghaus et al., 2016). Cingulate lesions in animal studies lead to inappropriately constrained expression of behavioral responses to stressful stimuli generated by aversive anticipated foot shock (Frysztak and Neafsey, 1991), which may be analogous to aspects of the reduced ACC activation found in the participants with SSD in this ankle shock paradigm. Dysfunction in this region could lead to abnormal threat salience evaluation and thus over-represent the otherwise normal stress from the threat anticipation in everyday activities, contributing to paranoia or other psychotic symptoms. Alternatively, SSD patients with more psychotic symptoms may be more sensitized to stressful events, leading to greater vACC activation as compared to patients with less psychotic symptoms and thus the positive correlation we observed. However, the latter interpretation may not explain why depression symptoms, which should also sensitize SSD patients to more stressful events, showed inverse correlation with vACC activation; and it also does not explain why SSD patients in general have lower vACC activation compared with controls. Still another possibility is that improper stress response to the anticipated threat may reinforce the already established abnormal thought patterns due to abnormal autonomic responses to stress (Castro et al., 2008) and altered glucocorticoid receptor expression in schizophrenia (Sinclair et al., 2011). While the causal direction between vACC and psychosis can be difficult to decipher by cross-sectional studies with human fMRI, our findings provide a link between abnormal vACC activation under the stressful condition of anticipating threat and psychosis.
Despite lower overall vACC activation in SSD participants compared to controls, individuals with higher activation had more severe psychotic symptoms. Interestingly, while ACC has been frequently associated with reduced activations in fMRI and PET activation studies (Carter et al., 2001, 1997), surgical cingulumotomy has led to improvement in psychosis and disordered affect (Ballantine et al., 1967), suggesting that there is no unidimensional explanation to the contributions ACC plays towards symptoms. In animal studies, lesions to ACC lead to poor discrimination between tones that positively or negatively anticipate a foot shock (Gabriel et al., 1989). Thus, although the results may seem paradoxical, reduced vACC activation in patients with SSD may represent poor discrimination of threat signals in general as part of the underlying disease state, while the strong positive correlation with psychosis could indicate that more severe psychosis is linked to inability to resolve, and thus over-interpretation of, anticipated fear or stress. Overall, the ACC may function in a more nuanced manner, combining its roles as an evaluator of anticipated threat, appropriate response selection, and conflict monitor (Devinsky et al., 1995). Reduced activation in patients broadly may represent underlying genetic to cellular level abnormalities, with higher level of activation representing over-interpretation of threat and greater distress and paranoia, thus the strong positive correlation with psychosis symptoms severity. In comparison, healthy controls without the underlying abnormalities in the ACC in the context of stress would have appropriate threat appraisal and are not vulnerable to the generation of psychosis symptoms as a result.
Opposite to the relationship with psychosis severity, the AST-induced vACC activation inversely correlated with more severe trait depression symptoms as measured by MTSD (r=−0.48, p=0.043) in SSD (Figure 4A). Depressive symptoms are common but often underappreciated in patients with SSD. The estimate for lifetime prevalence of depressive episodes in individuals with schizophrenia range from 20% to 80%, and these symptoms are potentially devastating through added morbidity and mortality (Brown, 1997). These depressive symptoms have been hypothesized to be an independent, core component of the disorder, or alternatively secondary to severe psychotic symptoms or medications (Zisook et al., 1999). The opposite relationship between vACC and psychosis vs. depression severities in the same group of patients may suggest that they are independent components but also anchored on a common abnormal stress-processing nexus within the vACC. Trait depressive symptoms are prominent in schizophrenia, are distinct from negative symptoms (Carpenter et al., 1985; Chiappelli et al., 2014), and are not restricted to periods following acute psychosis exacerbation (Bartels and Drake, 1988). Our results highlighting vACC in depressive symptoms in SSD is consistent with previous findings implicating reduced ACC activation overall and in predicting poor treatment response (Auer et al., 2000; Godlewska et al., 2018) and in reduced reward anticipation and stress reactivity (Vidal-Ribas et al., 2019) in depression.
There were several frontal, parietal, and temporal regions that had activations at p<0.001 although they did not meet the cluster level criterion during the anticipatory threat condition compared to safety (Figure 2). For example these regions include: the superior frontal gyrus cluster including BA 8 and 9, which has been implicated in managing uncertainty (Volz et al., 2005); the inferior frontal cortex cluster, which participates in selective response suppression (Forstmann et al., 2008); the somatosensory association cortex cluster which should be involved in this sensory based task; and the cluster at the pars orbitalis of the inferior frontal cortex, which is activated in response to recognition of negative emotions including fear (Sprengelmeyer et al., 1998). It is thus logical that the AST, which involves exposure to a visuospatial image to generate uncertainty about a potential noxious sensory stimulus, would engage these areas. Each of these regions exhibited decreased activation in SSD compared to controls, potentially suggesting a broader pattern of dysfunctional activation, although the lack of statistical significance limited our ability to further interpret these findings.
Finally, bilateral insula and the left pars orbitalis exhibited reduced activation in SSD compared with CC in the shock-safe comparison at voxelwise p<0.001 but did not survive FWE. These regions have important functions in pain, interoception, processing of aversive stimulation and the corresponding bodily and emotional responses (Lu et al., 2016; Ray and Zald, 2012; Sprengelmeyer et al., 1998). Patients also showed higher activation in the precuneus than controls in the shock-safe comparison, part of the default-mode network that has been implicated in schizophrenia (Kühn and Gallinat, 2013), and heightened activation during the aversive shock stimuli may signal an overrepresentation of negative experience. However, further study is required to evaluate the contributions to symptoms in these regions that did not survive FWE.
Study limitations
The current study has several limitations. First, the sample size is modest, although the main findings were based on properly corrected statistical thresholds. However, this may leave the study underpowered for identifying some other relevant brain regions that are still important but less robust. Second, patients reported higher early lifetime traumatic stress exposure and a nonsignificant trend for higher current stress perception, which may confound the stress-induced activations. However, these experiences were not significantly related to vACC activation, suggesting that the abnormal vACC activations are not directly impacted by past stress exposure or current subjective stress levels. Third, although there were no effects based on current CPZ dose equivalent, it is possible that cumulative medication effects may be important. Fourth, participants were not asked their subjective assessment of the probability of receiving a shock, leaving open the possibility that the overall hypoactivation of the vACC in patients reflects an under-estimation of threat materializing. Future studies should include such assessments. Finally, as a cross-sectional study our results do not give direction of causality between associated measures.
Conclusion
This anticipatory threat task can reliably engage stress-induced brain activation in individuals with SSD and our results suggest that ventral ACC appears a fronto-limbic crossroad for the increased vulnerability to more severe psychotic symptoms during stress in SSD. Our findings also indicate a potential stress-related node for affective symptoms in SSD within the ventral ACC. Further study is required to better understand how stress interacts with the multiple known ACC functions including error detection, social and emotional responsiveness, threat evaluation, and reward-based decision making in promoting psychosis formation. Understanding how aberrant ventral anterior cingulate signaling contributes to psychosis under stress may be important for developing treatments that prevent and treat psychosis formation under stressful conditions.
Supplementary Material
Highlights.
A stressful anticipatory threat task using ankle-shock can be used during fMRI to interrogate brain areas responsible for stress and threat processing.
Patients with schizophrenia spectrum disorder have deficient activation in the ventral anterior cingulate cortex during anticipated threat periods.
This vACC activation significantly and robustly correlates with psychosis symptoms, and inversely correlates with trait depression measures.
The association between clinical symptoms and abnormal threat-induced brain activation may represent a key biological substrate linking the deficiencies in threat anticipation and stress regulation in schizophrenia.
Acknowledgments:
We thank all the volunteer participants for their patience and participation. We thank the efforts of all our staff to make this research possible. Support was received from NIH grants K23MH112010, R01MH116948, R01MH112180, and University of Maryland/Sheppard-Pratt Psychiatry Residency Physician-Scientist Training Program. We would also like to thank Dr. Luiz Pessoa for his expert insight and suggestions.
Footnotes
Disclosures: The authors declare no conflicts of interest that influence the collection, interpretation, or reporting of the results herein. LEH has received or plans to receive research funding or consulting fees on research projects from Mitsubishi, Your Energy Systems LLC, Neuralstem, Taisho, Heptares, Pfizer, Luye Pharma, Sound Pharma, Takeda, and Regeneron. MDK is co-inventor of a patent pending for the use of GABA-related compounds as fast-acting antidepressants, with rights assigned to the University of Maryland Baltimore.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Auer DP, Pütz B, Kraft E, Lipinski B, Schill J, Holsboer F, 2000. Reduced glutamate in the anterior cingulate cortex in depression: an in vivo proton magnetic resonance spectroscopy study. Biol. Psychiatry 47, 305–13. 10.1016/s0006-3223(99)00159-6 [DOI] [PubMed] [Google Scholar]
- Bair MJ, Robinson RL, Katon W, Kroenke K, 2003. Depression and Pain Comorbidity. Arch. Intern. Med 163, 2433. 10.1001/archinte.163.20.2433 [DOI] [PubMed] [Google Scholar]
- Bali A, Jaggi AS, 2015. Electric foot shock stress: a useful tool in neuropsychiatric studies. Rev. Neurosci. 26, 655–77. 10.1515/revneuro-2015-0015 [DOI] [PubMed] [Google Scholar]
- Ballantine HT, Cassidy WL, Flanagan NB, Marino R, 1967. Stereotaxic Anterior Cingulotomy for Neuropsychiatric Illness and Intractable Pain. J. Neurosurg 26, 488–495. 10.3171/jns.1967.26.5.0488 [DOI] [PubMed] [Google Scholar]
- Bartels SJ, Drake RE, 1988. Depressive symptoms in schizophrenia: comprehensive differential diagnosis. Compr. Psychiatry 29, 467–83. 10.1016/0010-440x(88)90062-4 [DOI] [PubMed] [Google Scholar]
- Berger M, Juster R-P, Westphal S, Amminger GP, Bogerts B, Schiltz K, Bahn S, Steiner J, Sarnyai Z, 2018. Allostatic load is associated with psychotic symptoms and decreases with antipsychotic treatment in patients with schizophrenia and first-episode psychosis. Psychoneuroendocrinology 90, 35–42. 10.1016/j.psyneuen.2018.02.001 [DOI] [PubMed] [Google Scholar]
- Birur B, Kraguljac NV, Shelton RC, Lahti AC, 2017. Brain structure, function, and neurochemistry in schizophrenia and bipolar disorder—a systematic review of the magnetic resonance neuroimaging literature. npj Schizophr. 3, 15. 10.1038/s41537-017-0013-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, 2011. The environment and susceptibility to schizophrenia. Prog. Neurobiol. 93, 23–58. 10.1016/j.pneurobio.2010.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S, 1997. Excess mortality of schizophrenia. Br. J. Psychiatry 171, 502–508. 10.1192/bjp.171.6.502 [DOI] [PubMed] [Google Scholar]
- Brown S, Schäfer E, 1888. An investigation into the functions of the occipital and temporal lobes of the monkey’s brain. Philos. Trans. R. Soc. London 179, 303–27. [Google Scholar]
- Bush G, Luu P, Posner MI, 2000. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. 4, 215–222. 10.1016/S1364-6613(00)01483-2 [DOI] [PubMed] [Google Scholar]
- Carpenter WT, Heinrichs DW, Alphs LD, 1985. Treatment of negative symptoms. Schizophr. Bull. 11, 440–52. 10.1093/schbul/11.3.440 [DOI] [PubMed] [Google Scholar]
- Carter CS, MacDonald AW, Ross LL, Stenger VA, 2001. Anterior cingulate cortex activity and impaired self-monitoring of performance in patients with schizophrenia: an event-related fMRI study. Am. J. Psychiatry 158, 1423–8. 10.1176/appi.ajp.158.9.1423 [DOI] [PubMed] [Google Scholar]
- Carter CS, Mintun M, Nichols T, Cohen JD, 1997. Anterior cingulate gyrus dysfunction and selective attention deficits in schizophrenia: [15O]H2O PET study during single-trial Stroop task performance. Am. J. Psychiatry 154, 1670–5. 10.1176/ajp.154.12.1670 [DOI] [PubMed] [Google Scholar]
- Castro MN, Vigo DE, Weidema H, Fahrer RD, Chu EM, de Achával D, Nogués M, Leiguarda RC, Cardinali DP, Guinjoan SM, 2008. Heart rate variability response to mental arithmetic stress in patients with schizophrenia. Schizophr. Res. 99, 294–303. 10.1016/j.schres.2007.08.025 [DOI] [PubMed] [Google Scholar]
- Chavanne AV, Robinson OJ, 2021. The overlapping neurobiology of induced and pathological anxiety: A meta-analysis of functional neural activation. Am. J. Psychiatry 178, 156–164. 10.1176/appi.ajp.2020.19111153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiappelli J, Nugent KL, Thangavelu K, Searcy K, Hong LE, 2014. Assessment of trait and state aspects of depression in schizophrenia. Schizophr. Bull. 40, 132–42. 10.1093/schbul/sbt069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R, 1983. A global measure of perceived stress. J. Health Soc. Behav. 24, 385–96. [PubMed] [Google Scholar]
- Corcoran C, Mujica-Parodi L, Yale S, Leitman D, Malaspina D, 2002. Could stress cause psychosis in individuals vulnerable to schizophrenia? CNS Spectr. 7, 33–42. 10.1017/S1092852900022240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran C, Walker E, Huot R, Mittal V, Tessner K, Kestler L, Malaspina D, 2003. The stress cascade and schizophrenia: etiology and onset. Schizophr. Bull. 29, 671–92. 10.1093/oxfordjournals.schbul.a007038 [DOI] [PubMed] [Google Scholar]
- Cox RW, Chen G, Glen DR, Reynolds RC, Taylor PA, 2017. FMRI Clustering in AFNI: False-Positive Rates Redux. Brain Connect. 7, 152–171. 10.1089/brain.2016.0475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedovic K, Slavich GM, Muscatell KA, Irwin MR, Eisenberger NI, 2016. Dorsal Anterior Cingulate Cortex Responses to Repeated Social Evaluative Feedback in Young Women with and without a History of Depression. Front. Behav. Neurosci 10, 64. 10.3389/fnbeh.2016.00064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA, 1995. Review article: Contributions of anterior cingulate cortex to behaviour. Brain 118, 279–306. 10.1093/brain/118.1.279 [DOI] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney MJ, 1993. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J. Neurosci 13, 3839–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drabant EM, Kuo JR, Ramel W, Blechert J, Edge MD, Cooper JR, Goldin PR, Hariri AR, Gross JJ, 2011. Experiential, autonomic, and neural responses during threat anticipation vary as a function of threat intensity and neuroticism. Neuroimage 55, 401–10. 10.1016/j.neuroimage.2010.11.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD, 2003. Does rejection hurt? An FMRI study of social exclusion. Science 302, 290–2. 10.1126/science.1089134 [DOI] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H, 2016. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc. Natl. Acad. Sci. U. S. A. 113, 7900–5. 10.1073/pnas.1602413113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison-Wright I, Bullmore E, 2010. Anatomy of bipolar disorder and schizophrenia: A meta-analysis. Schizophr. Res 117, 1–12. 10.1016/j.schres.2009.12.022 [DOI] [PubMed] [Google Scholar]
- Forstmann BU, van den Wildenberg WPM, Ridderinkhof KR, 2008. Neural mechanisms, temporal dynamics, and individual differences in interference control. J. Cogn. Neurosci. 20, 1854–65. 10.1162/jocn.2008.20122 [DOI] [PubMed] [Google Scholar]
- Frysztak RJ, Neafsey EJ, 1991. The effect of medial frontal cortex lesions on respiration, “freezing,” and ultrasonic vocalizations during conditioned emotional responses in rats. Cereb. cortex 1, 418–25. 10.1093/cercor/1.5.418 [DOI] [PubMed] [Google Scholar]
- Gabriel M, Sparenborg S, Kubota Y, 1989. Anterior and medial thalamic lesions, discriminative avoidance learning, and cingulate cortical neuronal activity in rabbits. Exp. brain Res. 76, 441–57. 10.1007/BF00247901 [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E, 1993. A Neural System for Error Detection and Compensation. Psychol. Sci. 4, 385–390. 10.1111/j.1467-9280.1993.tb00586.x [DOI] [Google Scholar]
- Godlewska BR, Browning M, Norbury R, Igoumenou A, Cowen PJ, Harmer CJ, 2018. Predicting Treatment Response in Depression: The Role of Anterior Cingulate Cortex. Int. J. Neuropsychopharmacol. 21, 988–996. 10.1093/ijnp/pyy069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupe DW, Nitschke JB, 2013. Uncertainty and anticipation in anxiety: An integrated neurobiological and psychological perspective. Nat. Rev. Neurosci. 14, 488–501. 10.1038/nrn3524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckers S, Rauch SL, Goff D, Savage CR, Schacter DL, Fischman AJ, Alpert NM, 1998. Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nat. Neurosci. 1, 318–23. 10.1038/1137 [DOI] [PubMed] [Google Scholar]
- Heins M, Simons C, Lataster T, Pfeifer S, Versmissen D, Lardinois M, Marcelis M, Delespaul P, Krabbendam L, van Os J, Myin-Germeys I, 2011. Childhood trauma and psychosis: a case-control and case-sibling comparison across different levels of genetic liability, psychopathology, and type of trauma. Am. J. Psychiatry 168, 1286–94. 10.1176/appi.ajp.2011.10101531 [DOI] [PubMed] [Google Scholar]
- Holt DJ, Coombs G, Zeidan MA, Goff DC, Milad MR, 2012. Failure of neural responses to safety cues in schizophrenia. Arch. Gen. Psychiatry 69, 893–903. 10.1001/archgenpsychiatry.2011.2310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen LM, Gispen-de Wied CC, Gademan PJ, De Jonge RC, van der Linden JA, Kahn RS, 1998. Blunted cortisol response to a psychosocial stressor in schizophrenia. Schizophr. Res 33, 87–94. 10.1016/s0920-9964(98)00066-8 [DOI] [PubMed] [Google Scholar]
- Joëls M, Baram TZ, 2009. The neuro-symphony of stress. Nat. Rev. Neurosci 10, 459–66. 10.1038/nrn2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpers F, Kroes MCW, Baas JMP, Fernández G, 2017. How Human Amygdala and Bed Nucleus of the Stria Terminalis May Drive Distinct Defensive Responses. J. Neurosci 37, 9645–9656. 10.1523/JNEUROSCI.3830-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn S, Gallinat J, 2013. Resting-state brain activity in schizophrenia and major depression: a quantitative meta-analysis. Schizophr. Bull 39, 358–65. 10.1093/schbul/sbr151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Berghorst LH, Nickerson LD, Dutra SJ, Goer FK, Greve DN, Pizzagalli DA, 2014. Differential effects of acute stress on anticipatory and consummatory phases of reward processing. Neuroscience 266, 1–12. 10.1016/j.neuroscience.2014.01.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane RD, Reiman EM, Axelrod B, Yun LS, Holmes A, Schwartz GE, 1998. Neural correlates of levels of emotional awareness. Evidence of an interaction between emotion and attention in the anterior cingulate cortex. J. Cogn. Neurosci 10, 525–35. 10.1162/089892998562924 [DOI] [PubMed] [Google Scholar]
- Leucht S, Samara M, Heres S, Patel MX, Furukawa T, Cipriani A, Geddes J, Davis JM, 2015. Dose Equivalents for Second-Generation Antipsychotic Drugs: The Classical Mean Dose Method. Schizophr. Bull 41, 1397–402. 10.1093/schbul/sbv037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Weerda R, Milde C, Wolf OT, Thiel CM, 2014. Effects of acute psychosocial stress on neural activity to emotional and neutral faces in a face recognition memory paradigm. Brain Imaging Behav. 8, 598–610. 10.1007/s11682-013-9287-3 [DOI] [PubMed] [Google Scholar]
- Liston C, McEwen BS, Casey BJ, 2009. Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proc. Natl. Acad. Sci. U. S. A 106, 912–7. 10.1073/pnas.0807041106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Yang T, Zhao H, Zhang M, Meng F, Fu H, Xie Y, Xu H, 2016. Insular Cortex is Critical for the Perception, Modulation, and Chronification of Pain. Neurosci. Bull 32, 191–201. 10.1007/s12264-016-0016-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SF, Seligman MEP, 2016. Learned helplessness at fifty: Insights from neuroscience. Psychol. Rev. 123, 349–67. 10.1037/rev0000033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMenamin BW, Langeslag SJE, Sirbu M, Padmala S, Pessoa L, 2014. Network organization unfolds over time during periods of anxious anticipation. J. Neurosci. 34, 11261–11273. 10.1523/JNEUROSCI.1579-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC, 2009. Meta-analysis of 41 Functional Neuroimaging Studies of Executive Function in Schizophrenia. Arch. Gen. Psychiatry 66, 811. 10.1001/archgenpsychiatry.2009.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell RLC, Phillips LH, 2007. The psychological, neurochemical and functional neuroanatomical mediators of the effects of positive and negative mood on executive functions. Neuropsychologia 45, 617–29. 10.1016/j.neuropsychologia.2006.06.030 [DOI] [PubMed] [Google Scholar]
- Mizoguchi K, Ishige A, Aburada M, Tabira T, 2003. Chronic stress attenuates glucocorticoid negative feedback: involvement of the prefrontal cortex and hippocampus. Neuroscience 119, 887–97. 10.1016/s0306-4522(03)00105-2 [DOI] [PubMed] [Google Scholar]
- Murphy FC, Nimmo-Smith I, Lawrence AD, 2003. Functional neuroanatomy of emotions: a meta-analysis. Cogn. Affect. Behav. Neurosci. 3, 207–33. 10.3758/cabn.3.3.207 [DOI] [PubMed] [Google Scholar]
- Norman RM, Malla AK, 1994. A prospective study of daily stressors and symptomatology in schizophrenic patients. Soc. Psychiatry Psychiatr. Epidemiol. 29, 244–9. 10.1007/BF00802047 [DOI] [PubMed] [Google Scholar]
- Nugent KL, Chiappelli J, Rowland LM, Daughters SB, Hong LE, 2014. Distress intolerance and clinical functioning in persons with schizophrenia. Psychiatry Res. 220, 31–6. 10.1016/j.psychres.2014.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orem TR, Wheelock MD, Goodman AM, Harnett NG, Wood KH, Gossett EW, Granger DA, Mrug S, Knight DC, 2019. Amygdala and prefrontal cortex activity varies with individual differences in the emotional response to psychosocial stress. Behav. Neurosci 133, 203–211. 10.1037/bne0000305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez DL, Pan H, Weisholtz DS, Root JC, Tuescher O, Fischer DB, Butler T, Vago DR, Isenberg N, Epstein J, Landa Y, Smith TE, Savitz AJ, Silbersweig DA, Stern E, 2015. Altered threat and safety neural processing linked to persecutory delusions in schizophrenia: A two-task fMRI study. Psychiatry Res. - Neuroimaging 233, 352–366. 10.1016/j.pscychresns.2015.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price D, 2000. Psychological and neural mechanisms of the affective dimension of pain. Science 288, 1769–72. 10.1126/science.288.5472.1769 [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Dedovic K, Khalili-Mahani N, Engert V, Pruessner M, Buss C, Renwick R, Dagher A, Meaney MJ, Lupien S, 2008. Deactivation of the limbic system during acute psychosocial stress: evidence from positron emission tomography and functional magnetic resonance imaging studies. Biol. Psychiatry 63, 234–40. 10.1016/j.biopsych.2007.04.041 [DOI] [PubMed] [Google Scholar]
- Ray RD, Zald DH, 2012. Anatomical insights into the interaction of emotion and cognition in the prefrontal cortex. Neurosci. Biobehav. Rev. 36, 479–501. 10.1016/j.neubiorev.2011.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reininghaus U, Kempton MJ, Valmaggia L, Craig TKJ, Garety P, Onyejiaka A, Gayer-Anderson C, So SH, Hubbard K, Beards S, Dazzan P, Pariante C, Mondelli V, Fisher HL, Mills JG, Viechtbauer W, McGuire P, van Os J, Murray RM, Wykes T, Myin-Germeys I, Morgan C, 2016. Stress Sensitivity, Aberrant Salience, and Threat Anticipation in Early Psychosis: An Experience Sampling Study. Schizophr. Bull. 42, 712–22. 10.1093/schbul/sbv190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson OJ, Overstreet C, Charney DR, Vytal K, Grillon C, 2013. Stress increases aversive prediction error signal in the ventral striatum. Proc. Natl. Acad. Sci. U. S. A 110, 4129–33. 10.1073/pnas.1213923110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligman ME, Maier SF, 1967. Failure to escape traumatic shock. J. Exp. Psychol 74, 1–9. 10.1037/h0024514 [DOI] [PubMed] [Google Scholar]
- Sinclair D, Tsai SY, Woon HG, Weickert CS, 2011. Abnormal glucocorticoid receptor mRNA and protein isoform expression in the prefrontal cortex in psychiatric illness. Neuropsychopharmacology 36, 2698–709. 10.1038/npp.2011.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprengelmeyer R, Rausch M, Eysel UT, Przuntek H, 1998. Neural structures associated with recognition of facial expressions of basic emotions. Proceedings. Biol. Sci 265, 1927–31. 10.1098/rspb.1998.0522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SM, Kallarackal AJ, Kvarta MD, Van Dyke AM, LeGates TA, Cai X, 2015. An excitatory synapse hypothesis of depression. Trends Neurosci. 38, 279–94. 10.1016/j.tins.2015.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Veen V, Carter CS, 2002. The anterior cingulate as a conflict monitor: FMRI and ERP studies. Physiol. Behav. 77, 477–482. 10.1016/S0031-9384(02)00930-7 [DOI] [PubMed] [Google Scholar]
- Vidal-Ribas P, Benson B, Vitale AD, Keren H, Harrewijn A, Fox NA, Pine DS, Stringaris A, 2019. Bidirectional Associations Between Stress and Reward Processing in Children and Adolescents: A Longitudinal Neuroimaging Study. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 1–9. 10.1016/j.bpsc.2019.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Finch DM, Olson CR, 1992. Functional Heterogeneity in Cingulate Cortex: The Anterior Executive and Posterior Evaluative Regions. Cereb. Cortex 2, 435–443. 10.1093/cercor/2.6.435-a [DOI] [PubMed] [Google Scholar]
- Volz KG, Schubotz RI, von Cramon DY, 2005. Variants of uncertainty in decision-making and their neural correlates. Brain Res. Bull. 67, 403–12. 10.1016/j.brainresbull.2005.06.011 [DOI] [PubMed] [Google Scholar]
- Wager TD, Smith EE, 2003. Neuroimaging studies of working memory: a meta-analysis. Cogn. Affect. Behav. Neurosci 3, 255–74. 10.3758/cabn.3.4.255 [DOI] [PubMed] [Google Scholar]
- White TP, Joseph V, Francis ST, Liddle PF, 2010. Aberrant salience network (bilateral insula and anterior cingulate cortex) connectivity during information processing in schizophrenia. Schizophr. Res 123, 105–115. 10.1016/j.schres.2010.07.020 [DOI] [PubMed] [Google Scholar]
- Wylie KP, Tregellas JR, 2010. The role of the insula in schizophrenia. Schizophr. Res 123, 93–104. 10.1016/j.schres.2010.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y-F, Huang J-C, Zhang P, Fan F-M, Chen S, Fan H-Z, Cui Y-M, Luo X-G, Tan S-P, Wang Z-R, Feng W, Yuan Y, Yang F-D, Savransky A, Ryan M, Goldwaser E, Chiappelli J, Rowland LM, Kochunov P, Tan Y-L, Hong LE, 2020. Choroid Plexus Enlargement and Allostatic Load in Schizophrenia. Schizophr. Bull. 46, 722–731. 10.1093/schbul/sbz100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zisook S, McAdams LA, Kuck J, Harris MJ, Bailey A, Patterson TL, Judd LL, Jeste DV, 1999. Depressive symptoms in schizophrenia. Am. J. Psychiatry 156, 1736–1743. 10.1176/ajp.156.11.1736 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


