Abstract
Unhealthful foods are convenient, ubiquitous, and inexpensive. Overconsumption of unhealthful foods can result in disease states such as obesity and Type 2 diabetes. In addition to the physiological consequences of unhealthful foods, research in rats has shown that diets high in processed fat and sugar induce impulsive choice behavior. Research in humans has demonstrated a link between metabolic health and impulsive choice, but most investigations have not included diet. We investigated how dietary fat intake interacts with body fat percentage, fasting glucose, insulin response, and systemic inflammation levels to predict impulsive choices in humans. Participants were split into either Control (<35% calories from fat) or High-Fat (≥40% calories from fat) groups based on self-reported dietary intake, completed an impulsive choice task, and underwent testing to determine their body fat, glucose, insulin response, and inflammation levels. High-fat diets were not predictive of impulsive choices, but added sugar was predictive. Body fat percentage was associated with impulsive choices only in the group who reported consuming high-fat diets. In addition, fasting glucose was associated with impulsive choices in the control group. Therefore, metabolic health and dietary fat intake interacted to predict impulsive choices. These findings indicate that knowledge of dietary patterns coupled with metabolic health markers may help us better understand impulsive choices, thereby improving our ability to target individuals who could benefit from interventions to reduce impulsive choice behavior, with the goal of promoting more self-controlled food choices.
Keywords: impulsive choice, diet, body fat percentage, inflammation, glucose, insulin response
Every day we make choices about the foods that we eat, including what we eat, how much we will eat, and when we will eat. The way that we eat is not just a matter of preference; it has large implications for our health. Diets high in processed fat and sugar are associated with obesity, a health condition marked by an abnormally excessive amount of body fat that is typically diagnosed using body mass index (BMI; Adab, Pallan, & Whincup, 2018; Swinburn et al., 2011; Swinburn, Sacks, & Ravussin, 2009). Diets that are high in unhealthful dietary components can lead to disrupted insulin responses and increased inflammation (Esposito & Giugliano, 2006), and these dietary components may contribute to chronic disease states such as Diabetes Mellitus and inflammatory diseases (Cordain et al., 2005; Esposito & Giugliano, 2006).
Diet also influences brain and behavioral processes, indicated by changes in cognitive functions. Mice and rats eating diets high in refined sugar and processed fats show neural inflammation as well as learning and memory deficits associated with increased inflammation in the brain (Davidson et al., 2013; Pistell et al., 2010). Additionally, consuming diets high in fat and sugar induced impulsive choice in rats (Steele, Pirkle, Davis, & Kirkpatrick, 2019; Steele, Pirkle, & Kirkpatrick, 2017).
Impulsive choices are typically measured by offering a smaller-sooner reward versus a larger-later reward. Selecting the smaller-sooner reward when the larger-later reward is a “better deal” is a more impulsive choice. It is thought that this decision-making process occurs through delay discounting. Delay discounting occurs when the subjective value of a reward is reduced as the amount of time to reward increases. Delay discounting is measured as V = A / (1 + kD), where V is the subjective value of the reward, A is the amount of the reward, D is the delay to obtain the reward, and k is an individual’s discounting rate (Mazur, 2007; Odum, 2011). The value k is a measure of delay sensitivity, and higher k values are associated with greater impulsive choices. As k increases, the same delay D has a lower subjective reward value V.
Previous studies have explored the relationship between impulsive choices, body fat, insulin signaling, and inflammation. Individuals with obesity make more impulsive choices compared to individuals of normal weight, especially when considering food choices (Barlow, Reeves, McKee, Galea, & Stuckler, 2016; Bickel et al., 2014; Hendrickson, Rasmussen, & Lawyer, 2015; Rasmussen, Lawyer, & Reilly, 2010; Schiff et al., 2015). Insulin response and impulsive choices are also related. One study found that people who had a reduced sensitivity to insulin were more impulsive (Eisenstein et al., 2015). This could be problematic for individuals with an impaired insulin response, as many convenience foods are also high in sugar. Inflammation may also be a factor affecting impulsive choice. Measuring inflammation using Interleukin-6, an inflammatory cytokine, has revealed that the level of inflammation may be used to predict impulsive choices (Gassen et al., 2019). Overall, body fat, insulin signaling, and inflammation are all affected by diet (Esposito & Giugliano, 2006) and are each associated with impulsive choice behavior (Barlow et al., 2016; Bickel et al., 2014; Eisenstein et al., 2015; Gassen et al., 2019; Hendrickson et al., 2015; Rasmussen et al., 2010; Schiff et al., 2015).
There are also associations between diet and impulsive choices. Specifically, unhealthful foods and fast food consumption have been associated with increased amounts of impulsive choices (Dassen, Houben, & Jansen, 2015; Garza, Ding, Owensby, & Zizza, 2016). Foods that are convenient and typically available at fast food restaurants are often high in fat and calories (Isganaitis & Lustig, 2005). This can create a vicious cycle because the same types of foods that induce obesity are typically more convenient. Therefore, someone with a propensity to make impulsive choices may choose unhealthful options that could further influence impulsive choice and contribute to obesity. There is a gap in the literature, however, regarding how dietary intake and metabolic health markers may interact to predict impulsive choices. The present study sought to address the lack of research in this area by gaining an understanding of how dietary fat intake interacted with body fat percentage, insulin signaling, and inflammation to predict impulsive choice behavior in humans. We hypothesized that people who reported consuming a diet high in fat would be more impulsive than people who reported consuming fat within the dietary guideline recommendations. Further, we hypothesized that body fat percentage, insulin signaling, and inflammation would interact with dietary fat intake to predict impulsive choice such that people who consume a diet high in fat and have higher body fat percentage, disrupted insulin signaling, or higher inflammation would be more impulsive.
Method
Participants
Sixty participants (68% Caucasian) were recruited through flyers and news releases in Riley County, Kansas. A power analysis was performed for sample size estimation in GPower 3.0 based on Eisenstein et al. (2015) comparing how insulin secretion was related to impulsive choice for obese and non-obese individuals in a hierarchical multiple linear regression. With effect size =.17, alpha = .05, and power = .80, the projected sample size needed was approximately 49 participants. Sixty participants were enrolled to ensure adequate power given the large amount of variance in dietary measures. One of the eligibility criteria for participation was that they enjoyed chocolate. Participants were screened for study participation based on habitual diet and physical activity as described in the procedure section. The sex, age, and physical activity characteristics of the groups are displayed in Table 1. Participants were compensated with a dual-energy X-ray Absorptiometry (DXA) scan ($250 value) plus $20 at the completion of the study.
Table 1. Demographic characteristics in humans.
Characteristics of dietary groups in humans.
| Characteristic | Control (n = 30) |
High-Fat (n = 30) |
p |
|---|---|---|---|
| Sex, male/female | 21/9 | 17/13 | .42 |
| Age, yr | 30.9 (±13.0) | 34.5 (±12.1) | .18 |
| Total MET mins/wk | 5023 (±4184) | 3580 (±3571) | .12 |
Values are presented as mean (±standard deviation). Means and standard deviations are based on the raw values. A chi-square test was used to compare frequencies of sex across groups. MET = multiples of the resting metabolic rate.
Procedure
The overall procedure is depicted in Figure 1. The study was approved by the Institutional Review Board at Kansas State University (Protocol #9418). Participants completed an online screening and participated in two in-person testing sessions. As part of the screening process, participants completed the informed consent form and the International Physical Activity Questionnaire-Short Form (IPAQ-SF) electronically (Craig et al., 2003). Following this initial online survey, participants completed the Automated Self-Administered 24-Hour (ASA24) Dietary Assessment Tool for three days (two weekdays and one weekend day). Participants were categorized into groups – Control (C) or High-Fat (HF) – based on the percentage of calories consumed from fat. After collecting 30 responses of screening data, we created an extreme groups design based on distribution of percentage of calories consumed from fat. The first quartile was used as the cut off for Group C and the third quartile was used as the cut off for Group HF. Therefore, Group HF must have consumed 40% or more of their calories from fat. Group C must have consumed less than 35% of their calorie from fat, which is the recommended amount ("2015–2020 Dietary Guidelines for Americans," 2015). A breakdown of the dietary group’s macronutrient consumption is in Table 2.
Figure 1. Procedure.
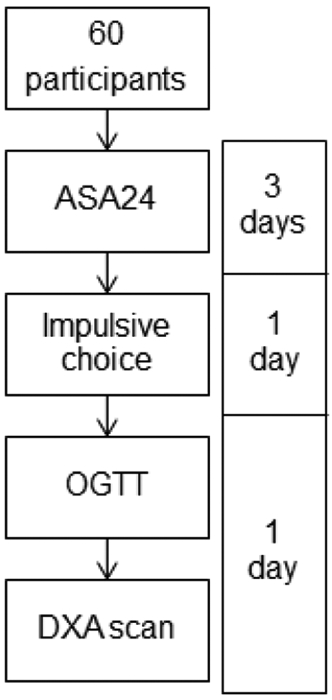
Overview of the procedure and timeline. ASA24 = 24 h dietary recall; DXA = Dual-energy X-ray Absorptiometry.
Table 2. Macronutrient composition in humans.
Macronutrient composition of the different dietary groups in humans.
| Characteristic | Control (n = 30) |
High-Fat (n = 30) |
p |
|---|---|---|---|
| Calories (kcal) | 1823 (±551) | 2102 (±516) | .03 |
| Fat (g) | 63 (±18) | 105 (±31) | |
| % of energy | 31 (±4) | 45 (±5) | <.001 |
| Protein (g) | 80 (±42) | 97 (±33) | |
| % of energy | 17 (±5) | 19 (±4) | .26 |
| Carbohydrates (g) | 230 (±78) | 196 (±57) | |
| % of energy | 51 (±8) | 37 (±7) | < .001 |
| Added sugar (g) | 13 (±8) | 10 (±6) | |
| % of energy | 3 (±2) | 2 (±1) | .011 |
| Saturated fat (g) | 20 (±8) | 36 (±16) | |
| % of energy | 5 (±1) | 7 (±2) | <.001 |
Values are presented as mean (±standard deviation). Means and standard deviations are based on the raw values.
The IPAQ was used to control for physical activity across the two groups using a matched groups design. People with high levels of physical activity have been shown to have lower levels of inflammation (Teeman et al., 2016), and exercise decreases impulsive choices (Sofis, Carrillo, & Jarmolowicz, 2017; Strickland, Feinstein, Lacy, & Smith, 2016), so the matched groups assignment controlled for an extraneous effect of exercise in our diet groups.
For both testing sessions, the participants reported the time of their last meal at the beginning of the session to see if they followed the required fasting time and control for subjective hunger. For the first testing session, participants were instructed not to eat anything for 4 hr before the session. Upon arrival, participants completed the experiential impulsive choice task for food followed by the hypothetical impulsive choice questionnaire. For the second testing session, participants were instructed not to eat anything for 10 h before the session. Upon arrival, an indwelling catheter was placed for the oral glucose tolerance test (OGTT), which took approximately 2.5 hr. At the end of the second testing session, the DXA scan was completed.
Measures
Dietary assessment.
Participants completed the Automated Self-Administered 24-Hour (ASA24) Dietary Assessment Tool, online dietary 24 hr recall, for three days (2 weekdays and 1 weekend day). The participants were given a unique ID and password, and they logged in to the website to report the food and drink they consumed the prior day. The average percentage of calories from protein, fat, carbohydrates, added sugar, and saturated fat across the 3 days of recall were calculated. Percentage of calories from fat was used for group assignment, added sugar was used for an exploratory analysis, and all factors were used to describe the groups.
International Physical Activity Questionnaire-Short Form (IPAQ-SF).
The IPAQ-SF was administered as part of the screening processes. The IPAQ includes 4 questions about the kinds of physical activities completed in the last 7 days (Craig et al., 2003). For example, participants were asked how many days they did vigorous activity and how long they typically spent doing that type of activity on those days. In addition, participants reported how much time they typically spent sitting during the week. Multiples of the resting metabolic rate (METS: metabolic equivalents) were obtained and multiplied by the minutes performed to obtain MET-minutes, a measure of physical activity energy expenditure.
Impulsive choice tasks.
Impulsive choices were measured using 1) an experiential choice task and 2) a hypothetical impulsive choice questionnaire. In both tasks, participants chose between smaller, more immediate (smaller-sooner, SS) and larger, delayed (larger-later, LL) rewards with SS choices characterized as impulsive. In the behavioral task, participants were presented with 25 food choices. The SS option was 1–4 mini M&Ms after a short delay or 4–5 mini M&Ms after a longer delay. The delays ranged from 5 to 30 s. Participants were presented with the choice on the screen (e.g. 2 M&Ms in 10 s or 5 M&Ms in 25 s). Participants made their choice by pressing the keyboard (F for left and J for right). After the initial press, the delay began, and participants were required to press the same key again after the delay elapsed. Following the second press, the M&Ms were dispensed into a bowl and a white screen was presented for 30 s to allow time for the participants to consume the reward. A small candy was necessary so that the dispenser could deliver the reward after the delay, consistent with impulsive choice tasks in rodent models. M&Ms were used in the current study because they contain more fat than most candy of a similar size (e.g. Skittles). In addition, mini M&Ms were used because pilot testing demonstrated that participants sated on regular sized M&Ms quickly. Details about the task including choice options are described further in Steele, Gwinner, Smith, Young, and Kirkpatrick (2019). The Kirby questionnaire (Kirby, 2009), an impulsive choice questionnaire for monetary rewards, was administered after the experiential impulsive choice task. As described in Steele, Gwinner, et al. (2019), participants answered a series of 27 questions consisting of choices between SS and LL rewards. For example, a choice could be $15 now versus $35 in 13 days. A single question appeared on the screen and advanced to the next question after the response occurred; the 27 questions were presented randomly intermixed. Instructions for the choice task were: "In the task that follows, you will have the opportunity to choose between different amounts of money available after different delays. The test consists of about 30 questions, such as the following: Would you rather have $10 in 30 days or $2 now? You will not receive any of the rewards that you choose, but you should make your decisions as though you were really going to get the rewards you choose."
Oral glucose tolerance test (OGTT).
The test was performed after a minimum 10-hr overnight fast (no food or beverages other than water). For example, if the participant came in for testing at 7:00AM, then he/she needed to finish any eating by 9:00PM the evening before. An indwelling intravenous (IV) catheter was placed in a forearm vein to collect blood at the start of the visit before consuming a 75 g glucose tolerance test beverage (Fisherbrand, 40-102-5FB). The IV catheter was used for blood collection throughout the 2-hr test to minimize participant discomfort from multiple needle pokes. Blood was drawn from the indwelling catheter before consumption and 15, 30, 45, 60, 90, and 120 min after consumption of the test beverage. A 3 mL syringe was used to flush the line of saline before drawing a whole blood sample with a 5 mL syringe that was transferred to a 6 mL K2 EDTA BD Vacutainer (BD, Franklin Lakes, NJ, USA). Blood samples were centrifuged, and plasma was aliquoted into microcentrifuge tubes, and then stored at −80° C. Glucose was measured with a Bayer contour glucose meter from a drop of blood added to the test strip from the venous sample before centrifuging. The oral glucose tolerance test allows for insulin sensitivity and insulin secretion to be parsed apart using glucose, insulin, and C-peptide to determine if there are disruptions in the insulin system (Cobelli et al., 2014). Inflammation levels were determined using the fasting blood sample collected during the oral glucose tolerance test by measuring IL-6. The samples were shipped to the Radioimmunoassay and Biomarkers Core of the University of Pennsylvania Diabetes Research Center to determine the amount of insulin (mg/ml), C-peptide (ng/ml), and IL-6 (pg/ml) in the plasma using radioimmunoassay kits (EMD Millipore, St Louis MO).
Body fat percentage.
Body fat percentage of the full body was measured by dual-energy X-ray absorptiometry (GE prodigy, Lunar-General Electric, Madison, WI). Participants were asked to remove any metal (jewelry, belts, etc) and shoes before lying down in a supine position for approximately ten minutes while the DXA scanned the individual. Participants were instructed to lay still for the duration of the scan.
Data analysis
All analyses were conducted in R version 3.5.3 (R Core Team, 2019). To confirm the effects of dietary fat intake on each of the metabolic health markers and determine whether the metabolic health markers were related, a series of regressions were performed (see Metabolic health markers). Further, moderation analyses were conducted to determine if each metabolic health variable interacted with dietary fat intake to predict impulsive choice behavior (see Metabolic health markers and impulsive choice). For all analyses, dietary fat intake was effect coded (C=1; HF=−1). Other scaling and analysis approaches are described below.
Impulsive choices.
Impulsive choice behavior was modeled through separate analyses to parse apart preference and sensitivity to delay or magnitude. Generalized mixed effects logistic regressions were conducted using the lme4 package in R. The SS delay was converted to a delay ratio and the intercept was set at the largest difference between delays (SS/LL–5/25), to measure preference for the shorter delay. The LL magnitude was converted to a magnitude ratio so that the intercept was located at the largest difference between magnitudes (SS/LL-1/5), to measure preference for the larger reward. In R, the delay or magnitude ratios were calculated using the above equation and entered as predictors in the model. Delay and magnitude ratio were included as continuous variables to test for delay and magnitude sensitivity. Intercept and delay (or magnitude) ratio were tested as possible random effects. While the model including slope as a random effect was better than the intercept only model, the slope and intercept were highly correlated, so only intercept was included as a random effect to avoid over-parameterization (Bates, Kliegl, Vasishth, & Baayen, 2015). We also tested to see whether sex and half (first vs. second half of the task) were significant predictors of choice. Half was examined to assess whether consumption of M&Ms during the real food choice task, which may have led to satiety effects, altered choices in the second half of the task. Adding half and sex did not significantly improve the model, so the intercept model without sex or half was used in subsequent analyses. Given that the Akaike information criterion (AIC) values are similar and these variables are not theoretically meaningful, sex and half were not included in the analysis. There would need to be a clear improvement in AIC value for these variables to be included in the model (Symonds & Moussalli, 2011).
Metabolic health markers.
Body fat percentage as measured by the DXA scan was used in the body fat analysis. Fasting glucose and glucose incremental area under the curve (iAUC) using the conventional trapezoidal method from the oral glucose tolerance test were used in the glucose response analyses (Matthews, Altman, Campbell, & Royston, 1990). Dietary fat intake was tested as a predictor of each of the metabolic health markers (body fat percentage, fasting glucose, insulin sensitivity, insulin secretion, and inflammation) using a linear regression. Correlations were also conducted to determine how the metabolic health factors were related. Each of the key factors was entered into each model as a continuous variable. Each of the metabolic health markers was mean-centered. Body fat was log-transformed, insulin secretion was square root-transformed, and inflammation was log-transformed to normalize the measures.
Metabolic health markers and impulsive choices.
Moderation analyses were conducted to determine if any of the metabolic health markers (body fat percentage, fasting glucose, insulin sensitivity, insulin secretion, and inflammation) altered the size or direction of the effect of dietary fat intake on impulsive choice. In the moderation analyses, each metabolic health variable was tested as a moderator using a theory-driven approach. The fixed effects included the interaction between each metabolic health variable (body fat percentage, fasting glucose, insulin sensitivity, insulin secretion, and inflammation), dietary condition, and delay or magnitude ratio. The two-way interaction (e.g., Body Fat Percentage × Diet) indicates whether body fat percentage moderated the effects of dietary fat intake on preference for shorter delay/larger magnitude. The three-way interaction (e.g., Body Fat Percentage × Diet × Delay or Magnitude Ratio) indicates whether body fat percentage moderated the effects of dietary fat intake on sensitivity to delay or magnitude. Insulin sensitivity was derived using the ogttMetrics package in R (Stubbs et al., 2017). Insulin secretion was measured using the postprandial C-peptide-to-glucose ratio (PCGR) by dividing incremental area under the curve (iAUC) for C-peptide by the iAUC for glucose (Lee et al., 2014). Post-hoc comparisons were conducted for significant interactions using the emmeans package in R. A p-value adjustment was included using the Tukey method for comparing a family of 3 estimates. Given that the metabolic health variables were treated as continuous measures in the analysis, the slopes of the delay and magnitude functions within each group were compared at three values of each physiological variable. No comparisons across groups were conducted to minimize multiple comparisons. The 1st quartile, mean, and 3rd quartile values were used to probe the slopes, which represent delay and magnitude sensitivity, within each group using the emtrends() function within the emmeans package. Therefore, the estimates are provided to demonstrate the trend in how delay/magnitude sensitivity changes as the metabolic health variable changes. However, the slope estimates were not compared to reduce multiple comparisons. The figures depict the three representative values used for each continuous measure to observe how delay/magnitude sensitivity changes as the metabolic health variable changes.
Results and Discussion
Dietary effects
High fat effects on impulsive choices
For the experiential choice delay model (Figure 2A), there was no effect of group, z = −0.67, p = .51, b = −0.11. There was a significant effect of delay ratio, such that participants made more LL choices as the delays became more similar, z = 4.54, p < .001, b = 1.30. There were no group differences in slope, z = −0.54, p = .59, b = −0.15. For the magnitude model (Figure 2B), there was no effect of group, z = −1.49, p = .14, b = −0.24. There was a significant effect of magnitude ratio, such that participants made more SS choices as the magnitudes became more similar, z = −4.34, p < .001, b = −1.24. There were no group differences in slope, z = 0.99, p = .32, b = 0.32. Therefore, dietary fat consumption as measured by a 24-hr recall did not predict impulsive choice. Group, t = 1.30, p = .2, did not predict performance on the Kirby monetary questionnaire. Overall, dietary fat intake was not associated with impulsive choices. These findings are in contrast to previous demonstrations in rats (Steele, Pirkle, et al., 2019; Steele et al., 2017).
Figure 2. Dietary effects in humans.
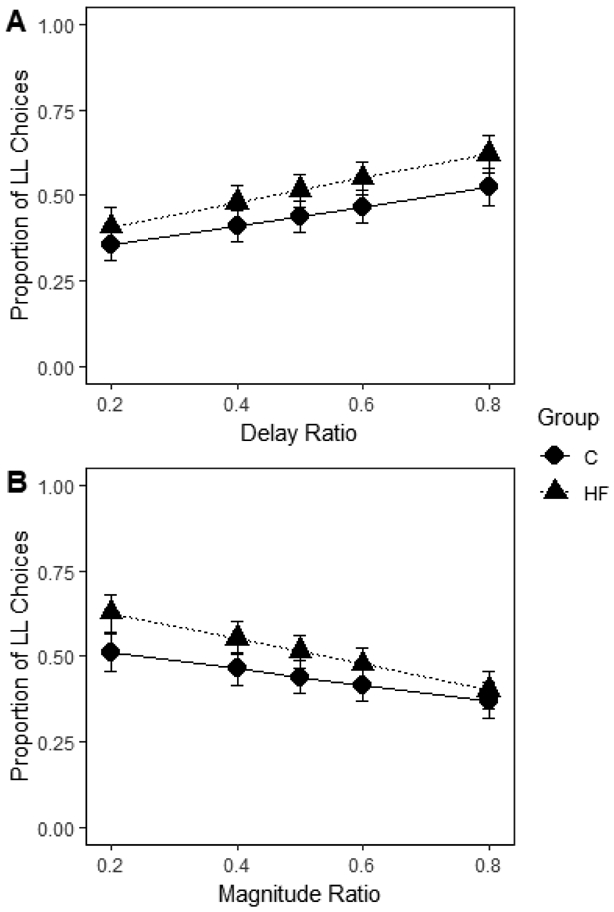
Mean proportion of larger-later (LL) choices for each group as a function of delay (A) and magnitude (B) ratio in humans. The delay and magnitude ratios were the SS / LL delay or magnitude, respectively. Smaller ratios indicate a larger difference in the delays or magnitudes. HF = high-fat; C = control. Error bars (+/− SEM) were computed with respect to the estimated marginal means of the fitted generalized linear mixed-effects model. There were no group difference in intercept or slope for delay (A) or magnitude (B) ratio.
It is likely that the type and amount of fat are important for predicting impulsive choices. In rodent studies, diets are typically high in processed, saturated fat, mainly from lard or hydrogenated vegetable oil. In contrast, the high-fat group in the current study only consumed 6.7% of their total calories from saturated fat. There is evidence in rodents that a diet consisting of 60% of the calories from fat that include lard produced cognitive impairments, while a diet consisting of 41% of the calories from fat did not disrupt cognition (Pistell et al., 2010). The diets utilized in Pistell et al. (2010) also had different types of fat (e.g. lard versus butter fat and corn oil), which may influence how dietary fat affects behavior. It has been proposed that processed saturated fats (e.g., lard) result in neuroinflammation, which is a key factor in producing cognitive deficits (Pistell et al., 2010). The people in the high-fat group in the current study consumed 45% of their calories from fat on average, but only a small proportion was from saturated fat. Based on the rodent literature, this diet would not be expected to result in cognitive impairments. Future research should investigate the complexities of a high-fat diet to better understand how the quality of fat consumed may affect impulsive choices.
It is also important to note that the sample used in the current study may have more healthful diets than the typical population because Riley County is fairly affluent and well educated, with many of the participants being staff, students, and faculty at Kansas State University. Indeed, more than 50% of the participants reported consuming between 1500 and 2500 calories, suggesting that most of the participants were consuming calories within the dietary guidelines, and consistent with their metabolic rates. With a broader community sample, it may be possible to obtain better differentiation of the high-fat and control groups. Further, the participants may have reported more healthful diets given that they knew dietary intake was being assessed.
In addition, it is possible that the short-term nature of the dietary assessment did not fully capture consumption of dietary fat. Research investigating the dietary effects on impulsive choice and cognition in rodents typically examine the effects of long-term diet on behavior (Pistell et al., 2010; Steele, Pirkle, et al., 2019; Steele et al., 2017). Investigating diet over the past year may better approximate the results found in rats. However, research suggests that people have stable dietary patterns over a two-year period (Borland, Robinson, Crozier, & Inskip, 2008), so three days of the ASA-24 may be assessing habitual dietary intake.
Added sugar effects on impulsive choices
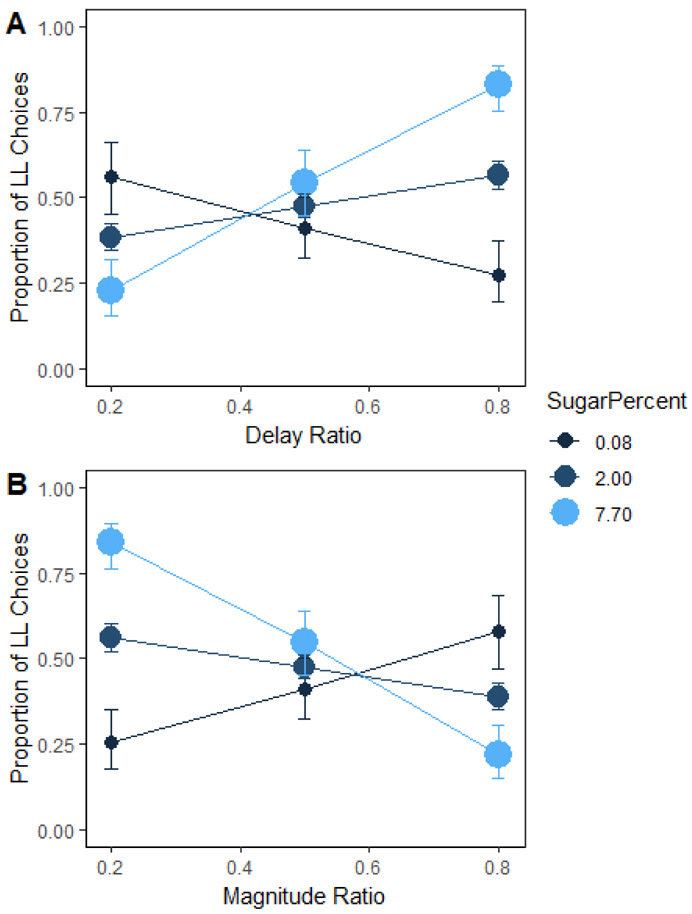
We conducted an exploratory analysis on the percentage of calories from added sugar, based on our previous research in rats that has shown that diets high in added sugar induced a greater preference for the shorter delay (Steele, Pirkle, et al., 2019; Steele et al., 2017). There was a significant interaction between percentage of calories from added sugar and delay ratio (Figure 3A), such that the slope of the delay sensitivity function shifted from negative to positive for lower, b = −2.01, to higher, b = 4.68, amounts of added sugar, z = 4.21, p < .001, b = 2.66. For magnitude ratio (Figure 3B), there was a main effect of percentage of calories from added sugar, z = 3.11, p = .002, b = 1.08. This indicates that people who consumed more added sugar had a greater preference for the larger magnitude when the magnitudes were maximally different (ratio = .2). There was a significant Added Sugar × Magnitude Ratio interaction such that the slope of the magnitude sensitivity function shifted from positive to negative as percentage of calories from added sugar increased from lower, b = 2.31, to higher, b = −4.86, amounts of sugar , z = −4.49, p < .001, b = −2.86.
Figure 3.
Mean proportion of larger-later (LL) choices as a function of percentage of calories consumed from sugar and delay (A) and magnitude (B) ratio in humans. The delay and magnitude ratios were the SS / LL delay or magnitude, respectively. Smaller ratios indicate a larger difference in the delays or magnitudes. Error bars (+/− SEM) were computed with respect to the estimated marginal means of the fitted generalized linear mixed-effects model. There was a significant interaction between percentage of calories from added sugar and delay ratio, such that delay sensitivity increased from lower to higher amounts of added sugar. People who consume more added sugar had a greater preference for the larger magnitude when the magnitudes were maximally different. Magnitude sensitivity increased as percentage of calories from added sugar went from lower to higher amounts of sugar.
In summary, the percentage of calories from added sugar predicted greater delay and magnitude sensitivity on the impulsive choice task for food. Individuals with higher added sugar in their diets preferred to wait for the larger reward when the smaller-sooner magnitude was 1 M&M (Magnitude ratio = .2) and they increasingly preferred the shorter delays when those delays were associated with larger magnitudes (e.g., magnitude ratio = .8 and delay ratio = .2). Ultimately, these results suggest that people who consume more sugar prefer not to wait for rewards, but also want larger rewards.
The type of food reward received during the task may explain why sugar intake predicted impulsive choice while dietary fat intake did not. We may have seen more sensitivity in the high-fat condition if we had used a reinforcer type that is typically thought of as “high fat.” While M&Ms do have fat, we typically think of candy as sugar. It is also important to note that people who consumed low amounts of sugar did not perform as would be expected on the impulsive choice task as indicated by the negative slope for delay ratio. It is possible that people who consumed lower amounts of sugar in their regular diet were not motivated by the parameters of the task. Participants indicated that they enjoyed M&Ms to participate in the study, but they may not have enjoyed consuming the rewards during the task. Or, these individuals may have been controlling their diets using decision biases or heuristics such as “avoid sugar” or “choose lower amounts of sugar” and this could have affected their willingness to choose to consume more than the minimum number of M&Ms. Together, these results suggest that people who consume low amounts of sugar may not value the reinforcers in this task. Future research should investigate how different types of reinforcers alter the relationship between dietary intake and impulsive choice.
Metabolic health markers
There were no group differences in body fat percentage, body mass index (BMI), fasting glucose, insulin sensitivity, insulin secretion, or inflammation (Table 3). We predicted that there would be differences between the two dietary groups, such that the high-fat group would have higher body fat percentages, higher BMIs, higher fasting glucose levels, lower insulin sensitivity, higher insulin secretion, and higher inflammation given previous research connecting diet to these variables (Esposito & Giugliano, 2006; Posey et al., 2009). The fact that we did not find group differences in the metabolic health markers for humans may explain why we did not find the dietary effects on impulsive choice and confirms the proposal that our participants were generally eating a healthful diet, even in the higher-fat condition. Importantly, high-fat groups in the human literature typically contain 60-80% of calories from fat. Therefore, the differences in the groups in this study may not have been large enough to detect differences in metabolic health or impulsive choice.
Table 3. Dietary effects on metabolic health markers in humans.
Group differences in metabolic health markers for humans.
| Characteristic | Control (n = 30) |
High-Fat (n = 30) |
p |
|---|---|---|---|
| PBF (%) | 29 (±12) | 28 (±10) | .79 |
| BMI (kg/m2) | 25.8 (±4.5) | 26.5 (±6.1) | .77 |
| FG (mg/dl) | 82 (±8) | 86 (±11) | .16 |
| SI (min−1/μU·ml−1 ) | 0.003 (±0.007) | 0.001 (±0.001) | .33 |
| PCGR (c-peptide iAUC/glucose iAUC) | 0.09 (±0.29) | 0.09 (±0.21) | .72 |
| IL-6 (pg/ml) | 1.25 (±0.79) | 1.36 (±1.41) | .77 |
Values are presented as mean (±standard deviation). Means and standard deviations are based on the raw values. PBF = body fat percentage; BMI = body mass index; FG = fasting glucose; SI = insulin sensitivity; PCGR = insulin secretion; IL-6 = inflammation.
Individual differences
The correlation for all participants between pairs of metabolic health markers is shown in Supplementary Figure 1. Body fat percentage and BMI (inverse transformed) were negatively correlated, r = −0.57, p < .001 (Supplementary Figure 1, A), such that higher body fat percentages were associated with higher BMIs. Note that the negative relationship is a result of the inverse transformation of BMI for analysis purposes. This is consistent with previous research showing that BMI is correlated with body fat percentage as measured by a DXA scan (Adab et al., 2018).
Body fat percentage was positively correlated with inflammation levels, r = .54, p < .001 (Supplementary Figure 1, E), consistent with the hypothesis that body fat may be a source of inflammation (Esposito & Giugliano, 2006). Fasting glucose levels were negatively associated with insulin sensitivity indicating that those with higher fasting glucose had worse insulin sensitivity, r = −0.32, p = .02 (Supplementary Figure 1, F). While fasting glucose and low insulin sensitivity often co-occur, they are independent of each other, consistent with the weak correlation (Nathan et al., 2007). No other metabolic health markers were correlated, ps > .08. Given the Esposito and Giugliano (2006) model, we would have expected to see a relationship between body fat percentage and insulin signaling as well as a relationship between inflammation and insulin signaling. This relationship may not have been present given that the participants in the current study were fairly young and relatively healthy.
Impulsive choice as measured by the real food impulsive choice task and the Kirby questionnaire were not related to any of the metabolic health markers, rs < .22, ps > .11. This is inconsistent with some previous research (Amlung, Petker, Jackson, Balodis, & MacKillop, 2016; Barlow et al., 2016; Eisenstein et al., 2015; Gassen et al., 2019), and highlights the need to investigate dietary intake in addition to metabolic health markers. In addition, performance on the real food impulsive choice task was not related to impulsive choice as measured by the Kirby questionnaire, rs < .05, ps > .69. This is consistent with previous research (Reynolds, Richards, & De Wit, 2006; Reynolds & Schiffbauer, 2004; Smits, Stein, Johnson, Odum, & Madden, 2013; Steele, Gwinner, et al., 2019). Altogether, this suggests that the experiential impulsive choice task for food and the monetary hypothetical impulsive choice questionnaire are not measuring the same construct, possibly as a result of the reward durations, amounts, or type (food vs. money).
Metabolic health moderators
All metabolic health markers were analyzed in separate models to determine how they interacted with dietary fat intake to predict impulsive choices within the real food experiential impulsive choice task and the monetary hypothetical impulsive choice task. The models included the fixed effects of Group (Control vs. High-Fat), Delay (or Magnitude) Ratio, and Body Fat Percentage (or Fasting Glucose or Insulin Sensitivity or Insulin Secretion or Inflammation). Intercept was included as a random effect. The full model outputs for body fat percentage (Supplementary Table 1), fasting glucose (Supplementary Table 2), insulin sensitivity (Supplementary Table 3), insulin secretion (Supplementary Table 4), and inflammation (Supplementary Table 5) are included in tables with SE values, and significant findings are discussed below.
Group did not interact with any of the metabolic health markers to predict impulsive choice as measured by the Kirby questionnaire, ts < 1.80, ps > .08. This is consistent with previous research indicating that the most robust relationship between impulsive choice, diet and body fat percentage is seen on impulsive choice tasks for food (Amlung et al., 2016; Barlow et al., 2016).
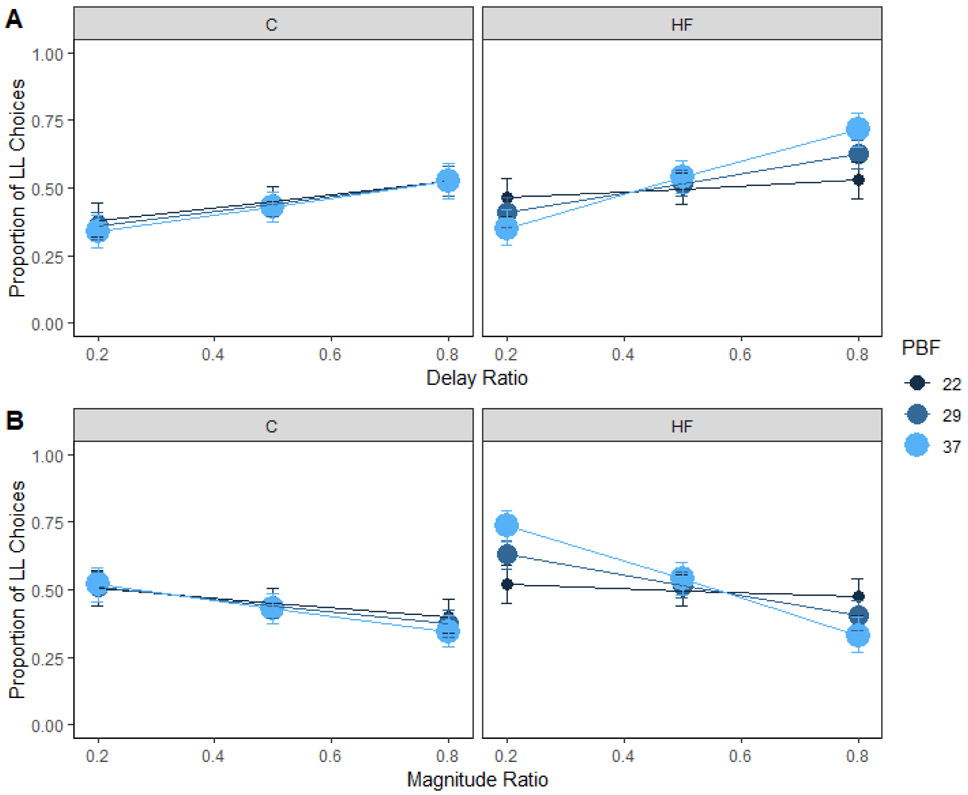
Body fat percentage
Body fat percentage interacted with dietary fat intake to predict delay sensitivity (Figure 4A). Post-hoc comparisons for the three-way interaction reported in Supplementary Table 1 indicated that sensitivity to delay increased from lower, b = 0.43, to higher body fat percentages, b = 2.59, for the high-fat group, z = −3.33, p = .003 (Figure 4A, right panel). This is consistent with previous literature demonstrating that obesity is associated with steeper impulsive choice functions, or higher k-values (Barlow et al., 2016). However, sensitivity to delay did not change significantly from lower, b = 0.98, to higher body fat percentages, b = 1.30, for the control group, z = −0.61, p = .82 (Figure 4A, left panel). Therefore, body fat percentage interacted with dietary fat intake to affect delay sensitivity.
Figure 4. Body fat percentage and choice in humans.
Mean proportion of larger-later (LL) choices for each group as a function of body fat percentage and delay (A) and magnitude (B) ratio in humans. Sensitivity to delay or magnitude did not change significantly from lower to higher body fat percentages for the control group. However, sensitivity to delay and magnitude increased from lower to higher body fat percentages for the high-fat group. The delay and magnitude ratios were the SS / LL delay or magnitude, respectively. Smaller ratios indicate a larger difference in the delays or magnitudes. HF = high-fat; C = control; PBF = percent body fat. Error bars (+/− SEM) were computed with respect to the estimated marginal means of the fitted generalized linear mixed-effects model.
Body fat percentage predicted preference for the larger magnitude such that people with high body fat percentages had a higher preference for the larger reward, b = 0.55, compared to people with lower body fat percentages, b = 0.05. Body fat percentage interacted with dietary fat intake to predict magnitude sensitivity (Figure 4B). Post-hoc comparisons for the three-way interaction reported in Supplementary Table 1 indicated that sensitivity to magnitude increased from lower, b = −0.32, to higher body fat percentages, b = −2.90, for the high-fat group, z = 3.89, p < .001 (Figure 4B, right panel). However, sensitivity to magnitude did not significantly change from lower, b = −0.71, to higher body fat percentages, b = −1.20, for the control group, z = 0.94, p = .61 (Figure 4B, left panel).
These results indicate that body fat percentage is associated with increased delay and magnitude sensitivity for people who consumed a high-fat diet, consistent with previous research showing that long-term high-fat consumption in rats contributes to obesity and impulsive choices (Steele, Pirkle, et al., 2019). This expands our current knowledge of body fat percentage and impulsive choices. The current evidence in the field suggests that sensitivity to delay is related to body fat percentage especially for food reward (Barlow et al., 2016; Rasmussen et al., 2010). Our analysis technique further suggests that people consuming a high-fat diet, who also have higher body fat percentages, have a greater preference for the larger reward and greater magnitude sensitivity compared to those with lower body fat percentages. The inconsistent findings regarding the relationship between impulsive choice and obesity in the literature may stem from the dietary patterns of the participants, where the relationships are more likely to be observed in studies where the participants consume higher amounts of dietary fat (Amlung et al., 2016; Barlow et al., 2016). Knowledge of both body fat percentage and dietary fat intake can better predict impulsive choices than either alone.
Together the findings from the current study suggest that people who consume a high-fat diet and who have higher body fat percentages are less willing to wait for rewards and prefer larger rewards, similar to what was observed in the exploratory analysis with added sugar. Within the context of the impulsive choice tasks, an individual with the combination of high delay sensitivity and high magnitude sensitivity would not be characterized as impulsive (Wileyto, Audrain-McGover, Epstein, & Lerman, 2004; Young, 2018). In part, this is because it is usually not possible to have “more now” within the construction of these tasks. However, when considering food choices people make every day, the fast food culture along with highly processed foods make it possible to have large amounts of highly palatable, energy dense foods immediately. It is important to determine how behavior on the real food impulsive choice task predicts actual food choices, as preferences for more-now may be important indicators of obesity that are not typically measured in impulsive choice tasks. Adding choices that measure this preference, especially within experiential tasks, would be an interesting extension that may be a more sensitive indicator of problematic food choice patterns.
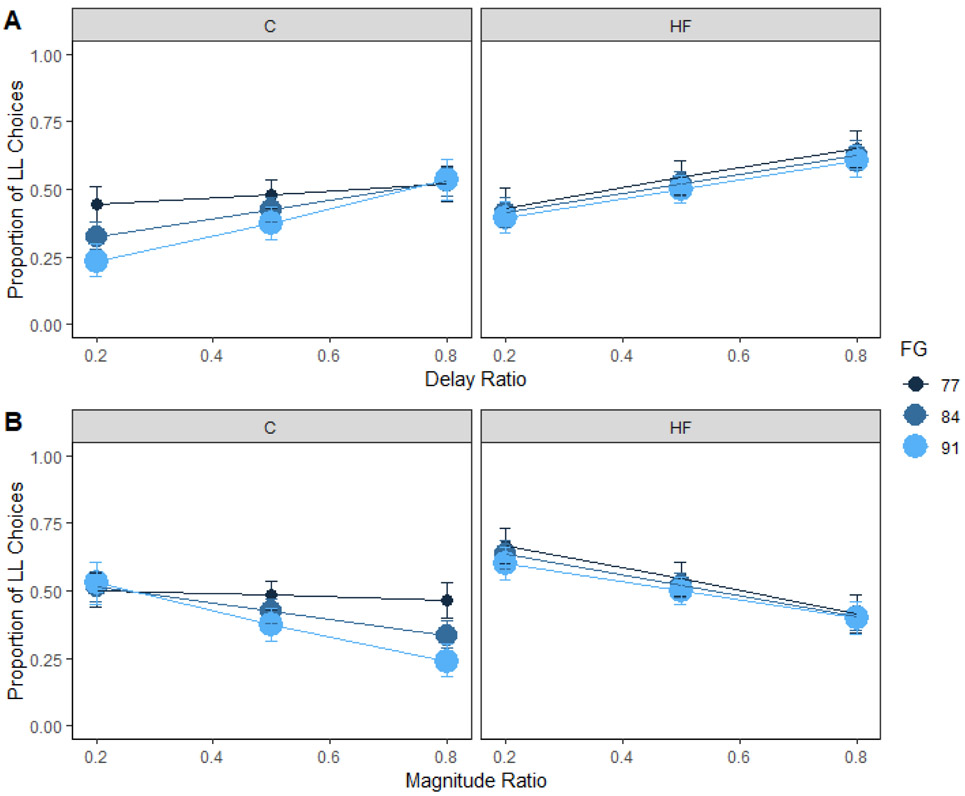
Fasting glucose
Fasting glucose predicted preference for the shorter delay such that people with high fasting glucose levels had a higher preference for the shorter delay, b = −0.81, compared to people with lower fasting glucose levels, b = −0.25. Fasting glucose levels interacted with dietary fat intake to predict delay sensitivity (Figure 5A). Post-hoc comparisons for the three-way interaction reported in Supplementary Table 2 indicated that sensitivity to delay increased from lower, b = 0.15, to higher fasting glucose levels, b = 2.22, for the control group, z = −2.46, p = .04 (Figure 5A, left panel). However, sensitivity to delay did not change significantly from lower, b = 1.50, to higher fasting glucose levels, b = 1.42, for the high-fat group, z = 0.13, p = .99 (Figure 5A, right panel). Fasting glucose also interacted with dietary fat intake to predict magnitude sensitivity (Figure 5B). Post-hoc comparisons for the three-way interaction reported in Supplementary Table 2 indicated that sensitivity to magnitude increased from lower, b = −0.27, to higher fasting glucose levels, b = −2.13, for the control group, z = 2.68, p = .02 (Figure 5B, left panel). However, sensitivity to magnitude did not change significantly from lower, b = −1.75, to higher fasting glucose levels, b = −1.40, for the high-fat group, z = −0.64, p = .80 (Figure 5B, right panel).
Figure 5. Fasting glucose and choice in humans.
Mean proportion of larger-later (LL) choices for each group as a function of fasting glucose and delay (A) and magnitude (B) ratio in humans. Sensitivity to delay and magnitude increased from lower to higher fasting glucose levels for the control group. However, sensitivity to delay and magnitude did not change significantly from lower to higher fasting glucose levels for the high-fat group. The delay and magnitude ratios were the SS / LL delay or magnitude, respectively. Smaller ratios indicate a larger difference in the delays or magnitudes. HF = high-fat; C = control; FG = fasting glucose. Error bars (+/− SEM) were computed with respect to the estimated marginal means of the fitted generalized linear mixed-effects model.
Altogether, people in the control group with higher fasting glucose levels were more sensitive to changes in delay and magnitude. This suggests that higher fasting glucose levels are associated with an unwillingness to wait for a reward and a desire for larger amounts of the reward in people who are consuming lower amounts of fat. These findings complement and extend on previous research that has shown that higher glucose levels following a 2-h fast are associated with greater delay sensitivity on a hypothetical, monetary impulsive choice task (Hendrickson et al., 2015). The results indicate that high levels of fasting glucose may predict impulsive food choices in people who consume dietary fat within the recommended ranges. Importantly, the control group consumed significantly more added sugar, which could contribute to higher glucose levels.
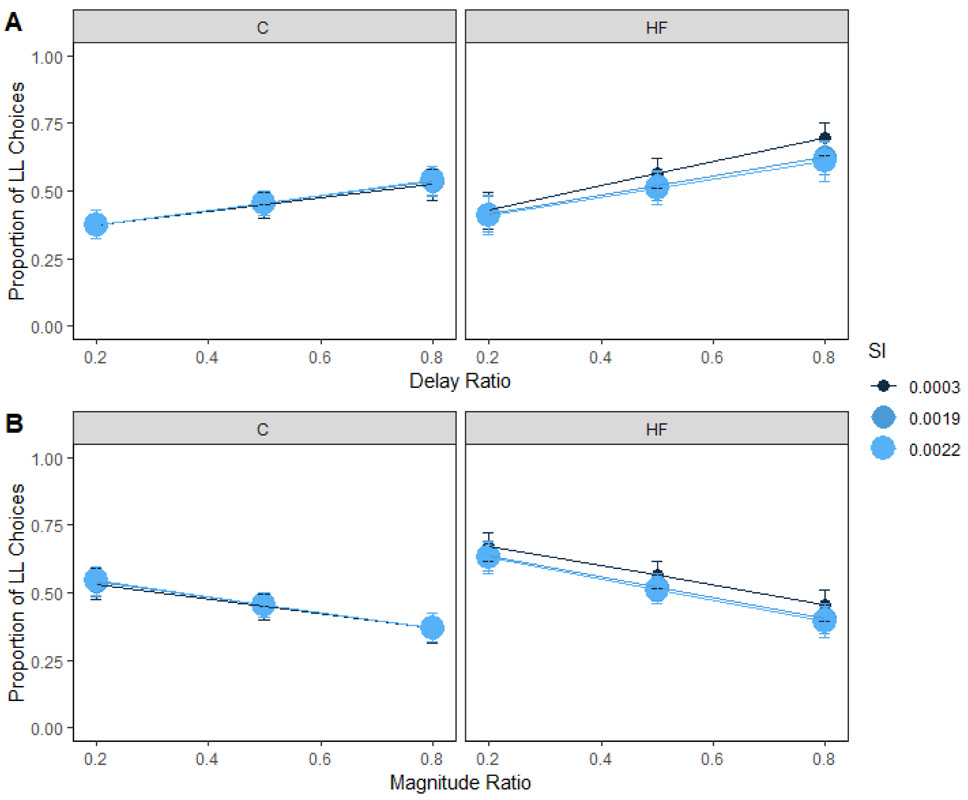
Insulin sensitivity and secretion
Insulin sensitivity refers to how well insulin reduces blood glucose levels; those who are insulin sensitive would need less insulin to dispose of glucose in the blood than someone who is less insulin sensitive. While we expected to see that people who consumed a high-fat diet and who had poorer insulin sensitivity would be more sensitive to delay, insulin sensitivity did not interact with dietary fat intake to predict delay sensitivity (Figure 6A) or magnitude sensitivity (Figure 6B). Previous research has found that, for people with obesity, lower levels of insulin sensitivity were associated with higher delay sensitivity (Eisenstein et al., 2015). This discrepancy in results may be a result of the population assessed. The population in the current study was relatively healthy whereas the population in the previous study exhibited higher body fat percentages in the obese (PBF = 49%) and non-obese (PBF = 33%) groups.
Figure 6. Insulin sensitivity and choice in humans.
Mean proportion of larger-later (LL) choices for each group as a function of insulin sensitivity and delay (A) and magnitude (B) ratio in humans. Insulin sensitivity did not interact with diet to predict delay sensitivity or magnitude sensitivity. The delay and magnitude ratios were the SS / LL delay or magnitude, respectively. Smaller ratios indicate a larger difference in the delays or magnitudes. HF = high-fat; C = control; SI = insulin sensitivity. Error bars (+/− SEM) were computed with respect to the estimated marginal means of the fitted generalized linear mixed-effects model.
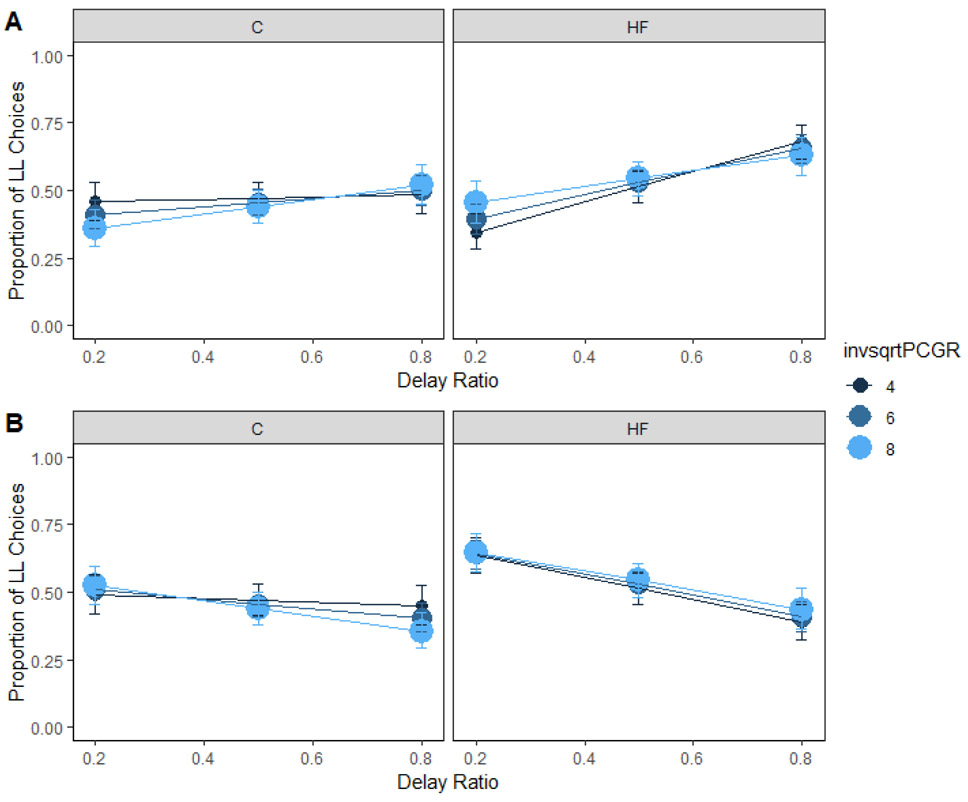
Insulin is secreted in response to blood glucose. If an individual is not insulin sensitive, the body would need to secrete more insulin to deal with the glucose load. Insulin secretion interacted with dietary fat intake to predict delay sensitivity (Figure 7A). However, post-hoc comparisons for the three-way interaction reported in Supplementary Table 4 were unable to localize the effect, ps > .25. Previous literature did not find a relationship between insulin secretion and delay sensitivity (Eisenstein et al., 2015).
Figure 7. Insulin secretion and choice in humans.
Mean proportion of larger-later (LL) choices for each group as a function of insulin secretion and delay (A) and magnitude (B) ratio in humans. Insulin secretion interacted with diet to predict delay sensitivity. However, post-hoc comparisons were unable to localize the source of the effect. Insulin secretion did not interact with diet to predict magnitude sensitivity. The delay and magnitude ratios were the SS / LL delay or magnitude, respectively. Smaller ratios indicate a larger difference in the delays or magnitudes. HF = high-fat; C = control; invsqrtPCGR = insulin secretion-inverse square root transformed. Error bars (+/− SEM) were computed with respect to the estimated marginal means of the fitted generalized linear mixed-effects model.
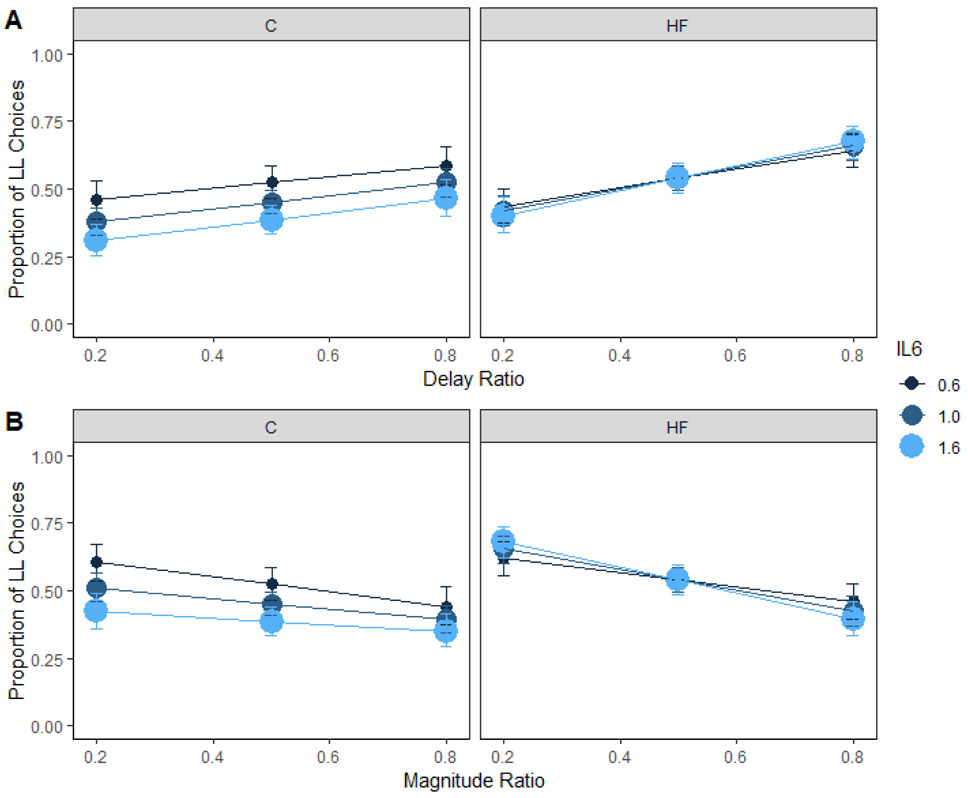
Inflammation
IL-6 levels measured in the plasma interacted with dietary group to predict preference for the larger magnitude (Supplementary Table 5; Figure 8B). While there is a trend for people with higher levels of inflammation to make fewer LL choices in Group C, this effect was not significant, z = 1.89, p = .14. Gassen et al. (2019) found that current inflammation levels (IL-6 and TNF-α), as measured in plasma, were predictive of impulsivity in a sub-clinical population. They tightly controlled for other sources of inflammation by excluding participants who were sick recently and by asking participants to avoid inflammation altering activities such as exercise and alcohol for 48 hrs prior to testing. In addition, that study included 159 participants. Therefore, future research investigating the relationship between inflammation and impulsive choice should consider a larger sample size and methods to control for outside sources of inflammation, as well as other markers of inflammation associated with adiposity. IL-6 is considered a proinflammatory cytokine that is related to adiposity as demonstrated in this study, but it is also affected by other factors. It is important to consider these other factors when trying to uncover how inflammation may alter behavior.
Figure 8. Inflammation and choice in humans.
Mean proportion of larger-later (LL) choices for each group as a function of inflammation and delay (A) and magnitude (B) ratio in humans. Inflammation did not interact with diet to predict delay or magnitude sensitivity. The delay and magnitude ratios were the SS / LL delay or magnitude, respectively. Smaller ratios indicate a larger difference in the delays or magnitudes. HF = high-fat; C = control; IL6 = inflammation. Error bars (+/− SEM) were computed with respect to the estimated marginal means of the fitted generalized linear mixed-effects model.
Conclusions
The current study investigated how dietary fat intake and metabolic health markers (body fat percentage, glucose, insulin sensitivity, insulin response, and inflammation) interacted to predict impulsive choices in humans. Previous research indicates that a high-fat diet induced impulsive choice in rats (Steele, Pirkle, et al., 2019; Steele et al., 2017), unhealthy diets and fast food consumption are associated with impulsive choices in humans (Dassen et al., 2015; Garza et al., 2016), and metabolic health is associated with impulsive choice in humans (Amlung et al., 2016; Barlow et al., 2016; Eisenstein et al., 2015; Gassen et al., 2019). Given the effects that diet can have on metabolic health (Esposito & Giugliano, 2006), it is important to understand how diet and metabolic health markers might interact to affect impulsive choices. However, to our knowledge, dietary fat and sugar intake, metabolic health, and impulsive choice for food have not been investigated together in humans.
The current results demonstrated that self-reported dietary fat intake was not predictive of impulsive choice on its own, inconsistent with the previous research in rats (Steele, Pirkle, et al., 2019; Steele et al., 2017). Interestingly, an exploratory analysis of added sugar found that people who consumed higher amounts of sugar had greater delay sensitivity, greater preference for the larger magnitude, and increased magnitude sensitivity, consistent with previous research in rats (Steele, Pirkle, et al., 2019; Steele et al., 2017).
While previous research has found that body fat percentage, insulin sensitivity, and inflammation are associated with impulsive choice (Amlung et al., 2016; Barlow et al., 2016; Eisenstein et al., 2015; Gassen et al., 2019), the current study expanded on the current literature by investigating the moderating effects of dietary fat intake on these relationships. Our results suggest that body fat percentage was associated with impulsive choice for people that were consuming a diet high in fat. Therefore, the relationship between body fat percentage and impulsive choice (Amlung et al., 2016; Barlow et al., 2016) may be affected by the amount of fat contained in the diet. This suggests that diet should be included as a factor when trying to predict impulsive choice, particularly the percentage of the diet that is made up of fat. We also found that fasting glucose was predictive of a greater preference for the shorter delay regardless of dietary fat intake, and fasting glucose was predictive of greater sensitivity to delay and magnitude in the control group. The interaction between dietary intake and metabolic health markers may highlight individuals who are currently eating a high fat diet and have likely been eating that diet chronically. Chronic dietary intake in rats affects metabolic health and impulsive choice (Steele, Pirkle, et al., 2019), so the combination of the factors may be demonstrating how chronic dietary effects on health are a predictor of impulsive choices.
Therefore, dietary fat intake and metabolic health (body fat percentage and fasting glucose) interacted to predict impulsive choices, suggesting that the combination of metabolic health and high levels of dietary fat and added sugar could be risk factors for impulsive food choices (Dassen et al., 2015; Garza et al., 2016; Garza, Harris, & Bolding, 2013; Guerrieri et al., 2007). Dietary intake, body fat percentage, and fasting glucose are all feasible measures to obtain, and these measures could be signs of early disease risk. Therefore, obtaining these measures could give researchers or practitioners a quick assessment of risk of impulsive choices that could ultimately lead to diet-induced obesity and related chronic diseases. Dietary and metabolic health markers, in combination, could be used to identify individuals most at risk for impulsive choice in clinical settings. Behavioral interventions that promote self-control could then be used as a primary preventative measure or in conjunction with traditional obesity treatment programs (Peterson, Hill, Marshall, Stuebing, & Kirkpatrick, 2015; Rung & Madden, 2018; Smith, Panfil, Bailey, & Kirkpatrick, 2019). Together, these factors could inform treatment and possibly predict treatment success (Nederkoorn, Jansen, Mulkens, & Jansen, 2006). Intervening to improve metabolic health markers and impulsive choices at an early stage could reduce the risk of developing diet-related diseases such as obesity and Type-2 diabetes.
Supplementary Material
Acknowledgements
This research was supported by the National Science Foundation Graduate Research Fellowship Program, R01 grant MH085739 from the National Institutes of Mental Health, and the University Distinguished Professors Graduate Student Award from Kansas State University. CCS, TJS, SKR, and KK designed research; CCS, MG, and TJS performed research; CCS analyzed the data; CCS, TJS, MG, and KK wrote the paper; and CCS, TJS, MG, SKR, and KK edited the manuscript. Thanks to Carrie Bailey, Cassi Friday, and Julia Duran for assisting with the development of the impulsive choice task. Parts of this manuscript were presented at Obesity Week in 2019.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none
Ethical Statement
The study was approved by the Institutional Review Board at Kansas State University (Protocol #9418). Participants completed an online screening and participated in two in-person testing sessions. As part of the screening process, participants completed the informed consent form and the International Physical Activity Questionnaire-Short Form (IPAQ-SF) electronically (Craig et al., 2003).
References
- 2015–2020 Dietary Guidelines for Americans. (2015). U.S. Department of Health and Human Services and U.S. Department of Agriculture, 8th Edition. [Google Scholar]
- Adab P, Pallan M, & Whincup PH (2018). Is BMI the best measure of obesity? BMJ, 360, 1–2. [DOI] [PubMed] [Google Scholar]
- Amlung M, Petker T, Jackson J, Balodis I, & MacKillop J (2016). Steep discounting of delayed monetary and food rewards in obesity: a meta-analysis. Psychological medicine, 46. doi: 10.1017/S0033291716000866 [DOI] [PubMed] [Google Scholar]
- Barlow P, Reeves A, McKee M, Galea G, & Stuckler D (2016). Unhealthy diets, obesity and time discounting: a systematic literature review and network analysis. Obesity Reviews, 17, 810–819. doi: 10.1111/obr.12431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Kliegl R, Vasishth S, & Baayen H (2015). Parsimonious mixed models. arXiv preprint arXiv:1506.04967. [Google Scholar]
- Bickel WK, George Wilson A, Franck CT, Terry Mueller E, Jarmolowicz DP, Koffarnus MN, & Fede SJ (2014). Using crowdsourcing to compare temporal, social temporal, and probability discounting among obese and non-obese individuals. Appetite, 75, 82–89. doi: 10.1016/j.appet.2013.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borland SE, Robinson SM, Crozier SR, & Inskip HM (2008). Stability of dietary patterns in young women over a 2-year period. European Journal of Clinical Nutrition, 62, 119–126. doi: 10.1038/sj.ejcn.1602684 [DOI] [PubMed] [Google Scholar]
- Cobelli C, Dalla Man C, Toffolo G, Basu R, Vella A, & Rizza R (2014). The oral minimal model. Diabetes, 63, 1203–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, Watkins B. a., & Keefe JHO (2005). Origins and evolution of the Western diet: Health implications for the 21st century. American Journal of Clinical Nutrition, 81, 341–354. [DOI] [PubMed] [Google Scholar]
- Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, … Oja P (2003). International physical activity questionnaire: 12-Country reliability and validity. Medicine and Science in Sports and Exercise, 35(8), 1381–1395. [DOI] [PubMed] [Google Scholar]
- Dassen FC, Houben K, & Jansen A (2015). Time orientation and eating behavior: Unhealthy eaters consider immediate consequences, while healthy eaters focus on future health. Appetite, 91, 13–19. doi: 10.1016/j.appet.2015.03.020 [DOI] [PubMed] [Google Scholar]
- Davidson TL, Hargrave SL, Swithers SE, Sample CH, Fu X, Kinzig KP, & Zheng W (2013). Inter-relationships among diet, obesity and hippocampal-dependent cognitive function. Neuroscience, 253, 110–122. doi: 10.1016/j.neuroscience.2013.08.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein SA, Gredysa DM, Antenor-Dorsey JA, Green L, Arbeláez AM, Roller JM, … Hershey T (2015). Insulin, central dopamine D2 receptors, and monetary reward discounting in obesity. PLoS ONE, 10, e0133621. doi: 10.1371/journal.pone.0133621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito K, & Giugliano D (2006). Diet and inflammation: A link to metabolic and cardiovascular diseases. European Heart Journal, 27, 15–20. [DOI] [PubMed] [Google Scholar]
- Garza KB, Ding M, Owensby JK, & Zizza CA (2016). Impulsivity and fast-food consumption: A cross-sectional study among working adults. Journal of the Academy of Nutrition and Dietetics, 116(1), 61–68. doi: 10.1016/j.jand.2015.05.003 [DOI] [PubMed] [Google Scholar]
- Garza KB, Harris CV, & Bolding MS (2013). Examination of value of the future and health beliefs to explain dietary and physical activity behaviors. Research in Social and Administrative Pharmacy, 9, 851–862. doi: 10.1016/j.sapharm.2012.12.001 [DOI] [PubMed] [Google Scholar]
- Gassen J, Prokosch ML, Eimerbrink MJ, Proffitt Leyva RP, White JD, Peterman JL, … Hill SE (2019). Inflammation predicts decision-making characterized by impulsivity, present focus, and an inability to delay gratification. Scientific Reports, 9, 4928. doi: 10.1038/s41598-019-41437-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrieri R, Nederkoorn C, Stankiewicz K, Alberts H, Geschwind N, Martijn C, & Jansen A (2007). The influence of trait and induced state impulsivity on food intake in normal-weight healthy women. Appetite, 49(1), 66–73. doi: 10.1016/j.appet.2006.11.008 [DOI] [PubMed] [Google Scholar]
- Hendrickson KL, Rasmussen EB, & Lawyer SR (2015). Measurement and validation of measures for impulsive food choice across obese and healthy-weight individuals. Appetite, 90, 254–263. doi: 10.1016/j.appet.2015.03.015 [DOI] [PubMed] [Google Scholar]
- Isganaitis E, & Lustig RH (2005). Fast food, central nervous system insulin resistance, and obesity. Arteriosclerosis, Thrombosis, and Vascular Biology, 25, 2451–2462. doi: 10.1161/01.ATV.0000186208.06964.91 [DOI] [PubMed] [Google Scholar]
- Lee EY, Hwang S, Lee SH, Lee YH, Choi AR, Lee Y, … Lee HC (2014). Postprandial C-peptide to glucose ratio as a predictor of β-cell function and its usefulness for staged management of type 2 diabetes. Journal of Diabetes Investigation, 5, 517–524. doi: 10.1111/jdi.12187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews JNS, Altman DG, Campbell MJ, & Royston P (1990). Analysis of serial measurements in medical research. Br Med J, 300, 230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur JE (2007). Rats' choices between one and two delayed reinforcers. Learning & Behavior, 35(3), 169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan DM, Davidson MB, DeFronzo RA, Heine RJ, Henry RR, Pratley R, … Kahn R (2007). Impaired fasting glucose and impaired glucose tolerance: Implications for care. Diabetes Care, 30, 753–759. doi: 10.2337/dc07-9920 [DOI] [PubMed] [Google Scholar]
- Nederkoorn C, Jansen E, Mulkens S, & Jansen A (2006). Impulsivity predicts treatment outcome in obese children. Behaviour Research and Therapy, 45(5), 1071–1075. doi: 10.1038/sj.ijo.0802460 [DOI] [PubMed] [Google Scholar]
- Odum AL (2011). Delay discounting: I'm a k, you're a k. Journal of Experimental Analysis of Behavior, 96(3), 427–439. doi: 10.1901/jeab.2011.96-423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JR, Hill CC, Marshall AT, Stuebing SL, & Kirkpatrick K (2015). I can't wait: methods for measuring and moderating individual differences in impulsive choice. Journal of Agricultural and Food Industrial Organization, 13(1), 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistell PJ, Morrison CD, Gupta S, Knight AG, Keller JN, Ingram DK, & Bruce-Keller AJ (2010). Cognitive impairment following high fat diet consumption is associated with brain inflammation. Journal of Neuroimmunology, 36, 25–32. doi: 10.1016/j.cgh.2008.07.016.Cytokeratin [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posey K. a., Clegg DJ, Printz RL, Byun J, Morton GJ, Vivekanandan-giri A, … Niswender KD (2009). Hypothalamic proinflammatory lipid accumulation , inflammation , and insulin resistance in rats fed a high-fat diet. American Journal of Physiology: Endocrinology and Metabolism, 296, 1003–1012. doi: 10.1152/ajpendo.90377.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. (2019). R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Rasmussen EB, Lawyer SR, & Reilly W (2010). Percent body fat is related to delay and probability discounting for food in humans. Behavioural Processes, 83(1), 23–30. doi: 10.1016/j.beproc.2009.09.001 [DOI] [PubMed] [Google Scholar]
- Reynolds B, Richards JB, & De Wit H (2006). Acute-alcohol effects on the Experiential Discounting Task (EDT) and a question-based measure of delay discounting. Pharmacology, Biochemistry and Behavior, 83, 194–202. doi: 10.1016/j.pbb.2006.01.007 [DOI] [PubMed] [Google Scholar]
- Reynolds B, & Schiffbauer R (2004). Measuring state changes in human delay discounting: an experiential discounting task. Behavioural Processes, 67, 343–356. doi: 10.1016/j.beproc.2004.06.003 [DOI] [PubMed] [Google Scholar]
- Rung JM, & Madden GJ (2018). Experimental reductions of delay discounting and impulsive choice: A systematic review and meta-analysis. Journal of Experimental Psychology: General, 147(9), 1349–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff S, Amodio P, Testa G, Nardi M, Montagnese S, Caregaro L, & Sellitto M (2015). Brain and Cognition Impulsivity toward food reward is related to BMI: Evidence from intertemporal choice in obese and normal-weight individuals. Brain and Cognition, 1–8. doi: 10.1016/j.bandc.2015.10.001 [DOI] [PubMed] [Google Scholar]
- Smith T, Panfil K, Bailey C, & Kirkpatrick K (2019). Cognitive and behavioral training interventions to promote self-control. J Exp Psychol Anim Learn Cogn, 45(3), 259–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits RR, Stein JS, Johnson PS, Odum AL, & Madden GJ (2013). Test–retest reliability and construct validity of the Experiential Discounting Task. Experimental and Clinical Psychopharmacology, 21(2), 155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofis MJ, Carrillo A, & Jarmolowicz DP (2017). Maintained Physical Activity Induced Changes in Delay Discounting. Behav Modif 41(4), 499–528. doi: 10.1177/0145445516685047 [DOI] [PubMed] [Google Scholar]
- Steele CC, Gwinner M, Smith T, Young ME, & Kirkpatrick K (2019). Experience matters: The effects of hypothetical versus experiential delays and magnitudes on impulsive choice in delay discounting tasks. Brain Sciences, 9(379), 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele CC, Pirkle JRA, Davis IR, & Kirkpatrick K (2019). Dietary effects on the determinants of food choice: Impulsive choice, discrimination, incentive motivation, preference, and liking in male rats. Appetite, 136, 160–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele CC, Pirkle JRA, & Kirkpatrick K (2017). Diet-induced impulsivity: Effects of a high-fat and a high-sugar diet on impulsive choice in rats. PLoS One, 12(6), e0180510. doi: 10.1371/journal.pone.0180510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland JC, Feinstein MA, Lacy RT, & Smith MA (2016). The effects of physical activity on impulsive choice: Infleunce of sensitivity to reinforcement amount and delay. Behavioural Processes, 126, 36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs BJ, Frankston K, Ramos M, Laranjo N, Sacks FM, & Carey VJ (2017). ogttMetrics: Data structures and algorithms for oral glucose tolerance tests. F1000Research, 6, 1–10. doi: 10.12688/f1000research.11317.1 [DOI] [Google Scholar]
- Swinburn BA, Sacks G, Hall KD, McPherson K, Finegood DT, Moodie ML, & Gortmaker SL (2011). The global obesity pandemic: shaped by global drivers and local environments. The Lancet, 378(9793), 804–814. doi: 10.1016/s0140-6736(11)60813-1 [DOI] [PubMed] [Google Scholar]
- Swinburn BA, Sacks G, & Ravussin E (2009). Increased food energy supply is more than sufficient to explain the US epidemic of obesity. American Journal of Clinical Nutrition, 90(6), 1453–1456. doi: 10.3945/ajcn.2009.28595 [DOI] [PubMed] [Google Scholar]
- Symonds MRE, & Moussalli A (2011). A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike's information criterion. Behavioral Ecology and Sociobiology, 65, 13–21. doi: 10.1007/s00265-010-1037-6 [DOI] [Google Scholar]
- Teeman CS, Kurti SP, Cull BJ, Emerson SR, Haub MD, & Rosenkranz SK (2016). Postprandial lipemic and inflammatory responses to high-fat meals: A review of the roles of acute and chronic exercise. Nutrition and Metabolism, 13, 1–14. doi: 10.1186/s12986-016-0142-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wileyto EP, Audrain-McGover J, Epstein LH, & Lerman C (2004). Using logistic regression to estimate delay-discounting functions. Behavior Research Methods, Instruments, & Computers, 36(1), 41–51. [DOI] [PubMed] [Google Scholar]
- Young ME (2018). Discounting: A practical guide to multilevel analysis of choice data. Journal of the Experimental Analysis of Behavior, 109, 293–312. doi: 10.1002/jeab.316 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.