Abstract
Zika virus (ZIKV) can infect developing fetuses in utero and cause severe congenital defects independent of route of maternal infection. Infected men can shed ZIKV RNA in semen for over six months. Whether prolonged viral RNA shedding in semen indicates a persistent infection in the male reproductive tract is unknown. We hypothesized that if ZIKV establishes a persistent infection in the male reproductive tract (MRT), then immunosuppressant treatment should stimulate ZIKV replication and seminal shedding. Male mice were infected with ZIKV and immunosuppressed when they shed viral RNA but not infectious virus in ejaculates. Following immunosuppression, we did not detect infectious virus in ejaculates. However, we did detect ZIKV positive and negative sense RNA in the epididymal lumens of mice treated with cyclophosphamide, suggesting that ZIKV persists in the epididymis. This study provides insight into the mechanisms behind ZIKV sexual transmission, which may inform public health decisions regarding ZIKV risks.
Keywords: Zika virus, sexual transmission, male reproductive tract, epididymis, semen, recrudescence, mouse model
INTRODUCTION
Zika virus (ZIKV; Flaviviridae family, flavivirus genus) can cause severe birth defects, such as microcephaly and club foot, in infants born to mothers infected with ZIKV during pregnancy. These birth defects are collectively termed congenital Zika syndrome and occur in approximately 5-15% of ZIKV-infected pregnancies (1-4). ZIKV is typically transmitted by the bite of an infected Aedes spp. mosquito (Ae. aegypti or Ae. albopictus), but sexual transmission of ZIKV was reported during the most recent epidemic (5-9). Mathematical modelling estimates that sexual transmission accounted for 3-23% of ZIKV transmission in areas with ZIKV-infected mosquitoes (10-12). Furthermore, in vivo studies in mice indicate that maternal ZIKV infection via sexual transmission resulted in higher viral titers in fetal tissue compared to maternal ZIKV infection via subcutaneous injection, a route of infection that resembles a mosquito bite (13). Therefore, it is critical that we understand the mechanisms behind ZIKV sexual transmission to reduce ZIKV transmission potential and ultimately prevent serious sequelae, such as ZIKV congenital syndrome.
ZIKV-infected men may shed infectious ZIKV in semen for weeks or even months post infection, potentially increasing the amount of time that they are infectious compared to mosquito transmission (14-18). Additionally, ZIKV RNA has been isolated from semen for over 6 months post symptom onset (18). Semen is derived from the major internal components of the male reproductive tract (MRT), which function to produce sperm (testes), mature and store sperm (epididymis) and contribute nutrients, fluids, and other non-cellular components of semen (seminal vesicles and prostate). Acute ZIKV infection of these tissues has been observed in human explant or cell culture models (testes and prostate), mouse models (testes, epididymides, and seminal vesicles), and non-human primate models (testes and prostate) (19-25). However, the tissue source of prolonged viral RNA shedding in semen or persistent ZIKV infection of any of these tissues has yet to be verified.
Persistent infections with flaviviruses are not uncommon. In fact, several of the encephalitic flaviviruses, such as West Nile virus, Japanese encephalitis virus, and tick-borne encephalitis virus, can persistently infect humans, with infectious virus and viral RNA being isolated months to years after the initial symptomatic infection (26-30). Persistent infections of flaviviruses has been evaluated using in vivo models by treating subjects with an immunosuppressant following convalescence and monitoring for viral replication (31-33). In a mouse model of West Nile virus infection, persistent infection was assessed by treating infected mice with the immunosuppressant cyclophosphamide 28 days post-infection (31). Twelve days post-immunosuppression, viral replication was observed in the central nervous system, indicating recrudescence (31).
Several mouse models of ZIKV virus sexual transmission have been developed. Mouse models in which interferon signaling is disrupted, such as the AG129 mouse (lacks IFNα and IFNγ receptors), develop high viral titers in the testes, epididymides, seminal vesicles, brain, and eye and shed infectious virus in ejaculates when infected with ZIKV (19, 34-36). The use of non-susceptible females for the collection of ejaculates ensures that all infectious virus collected via this method originates from the male mice and not from replication in female tissues. Infectious ZIKV can be detected in 51% of ejaculates, which corresponds well to the observed 50% sexual transmission rate to susceptible females (19). Kinetics of seminal shedding of ZIKV in this model is similar to what has been observed in infected humans, as infectious virus can be isolated from ejaculates for a relatively short time (approximately 20 days) following infection, whereas shedding of ZIKV RNA persists for much longer post infection (greater than 60 days) (19). A more recently developed mouse model of ZIKV infection uses wild-type C57BL/6 mice; these mice are transiently immunosuppressed via treatment with a IFNAR blocking antibody, which allows for establishment of ZIKV infection (37-39). Mortality in this model is lower than in immunosuppressed mice, while viral titers in serum and ejaculates are similar between the models (20, 40).
Our goal in this study was to determine whether ZIKV establishes a persistent infection in the MRT by testing whether immunosuppression could trigger recrudescence of seminal shedding of infectious ZIKV. We used an established wild-type mouse model of ZIKV sexual transmission that replicates the kinetics of ZIKV shedding in human semen (19, 20). Following acute infection, male mice were treated with one of a panel of immunosuppressants chosen for their varying mechanisms of action, and viral replication in the MRT was assessed. We were unable to detect infectious virus or an increase in viral RNA in ejaculates following immunosuppression. Low levels of infectious virus were detected in the testes and epididymides in some mice treated with the immunosuppressants dexamethasone and methylprednisolone acetate. Lastly, higher levels of viral RNA were detected in the epididymides of mice treated with the immunosuppressant cyclophosphamide than mice not treated with an immunosuppressant, with (+) and (−) sense RNA detected in the epididymal lumen. These results suggest that ZIKV infection does establish a persistent infection within the MRT, specifically in the epididymis.
METHODS
Virus strains and cells
Zika virus strain DakAr41524 was used for this study. This strain was isolated in Senegal in 1984 from an Aedes africanus mosquito and has since been passaged seven times (AP-61 (Aedes pseudoscutellaris) cells p1, C6/36 (Aedes albopictus) cells p2, Vero cells p3-7). This strain has been used in studies investigating sexual transmission of ZIKV and can infect mouse testes, epididymides, and seminal vesicles in vivo. Additionally, mice infected with this strain shed ZIKV in ejaculates (20). The virus stocks used in this study were previously sequenced and this sequence is identical to that found in GenBank (KX601166.2) (40).
Vero cells (for plaque assay) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 5% fetal bovine serum (FBS), 100units/mL penicillin (Gibco), and 100μg/mL streptomycin (Gibco).
Mouse inoculations and immunosuppression
Twelve-week-old male C57BL/6J were obtained from the Jackson Laboratory. Mice were allowed to acclimate in an ABSL-2 facility before infection. Mice were rendered susceptible to ZIKV infection via intraperitoneal (i.p.) injection of 2mg of α-IFNAR1 antibody (Mar1-5A3; Leinco Technologies) (20, 37-39). The following day mice were infected with either 103 (cyclophosphamide or PBS treated mice) or 104 PFU (other immunosuppressants or PBS treated mice) of ZIKV via subcutaneous (s.c.) injection in a rear footpad. On days 1 and 4 post infection, mice were given additional doses of 0.5mg of α-IFNAR1 antibody via i.p. injection (20, 39). Mice were weighed daily to monitor clinical signs of infection. Any mouse whose weight dropped below 85% of the starting weight was humanely euthanized. Blood was collected from the submandibular vein into serum collection tubes on days 3, 5, and 7 post infection. Serum was separated via centrifugation at 10,000 x g for 5 minutes and was stored at −80°C. To collect ejaculate samples, male mice were paired with 1 to 2 CD-1 female mice (Charles River) each night beginning at day 5 post-infection. Female mice were checked each morning for evidence of copulation plug. Females who successfully mated were humanely euthanized, and their uteri were dissected out and flushed with BA-1 diluent (1X M199 Hank’s Salts (Sigma), 0.005M Tris-HCL pH7.5 (Gibco), 1% Bovine Serum Albumin (v/v; Probumin; Millipore), 2mM L-glutamine (Gibco), 0.35g/L Sodium Bicarbonate (Gibco), 100 units/mL Penicillin (Gibco), 100ug/mL streptomycin (Gibco), and 1ug/mL Amphotericin B (Hyclone)) to collect the ejaculate.
After male mice cleared the initial infection, as evidenced by weight gain and lack of infectious ZIKV in serum and ejaculate samples (via plaque assay), mice were immunosuppressed via cyclophosphamide, dexamethasone, ketoconazole/cyclosporine, methylprednisolone acetate, or α-IFNAR1 antibody. Cyclophosphamide (Sigma), dissolved in PBS, was administered at 5mg/mouse via i.p. injection on days 31 and 36 post infection. Water-soluble dexamethasone (Sigma), dissolved in PBS, was administered at 1mg/kg via oral gavage daily from dpi 32-42. Ketoconazole (Sigma), dissolved in peanut oil (Sigma), was administered at 10 mg/kg via oral gavage daily from dpi 32-42. Cyclosporine (Sigma), dissolved in DMSO (ATCC) and diluted in PBS, was administered at 30 mg/kg via i.p. injection daily from dpi 32-42. Methylprednisolone acetate (Zoetis) was administered at 600 mg/kg via s.c. injection on the back on day 32 post infection. α-IFNAR1 antibody, diluted in PBS, was administered at 2 mg per mouse via i.p injection on day 32 post infection and 0.5 mg per mouse via i.p. injection on days 34 and 37 post infection. Mice that received PBS via i.p. injection on days 32, 34, and 37 post infection served as controls.
Male mice were humanely euthanized ten days after immunosuppression. Blood was collected via submandibular vein or intracardiac bleed. Serum was separated and stored as described above. Testes, epididymides, and seminal vesicles were dissected out of each mouse. One set of reproductive tissues from each mouse was preserved in neutral buffered formalin for later ISH and H&E analysis, while remaining tissues were frozen at −80°C for later virus quantification.
Quantification of infectious virus
Infectious virus was quantified via Vero cell plaque assay. Briefly, tissues from mice were weighed, and an equal volume of BA-1 diluent was added to each sample. One 5mm stainless steel bead was added to each sample, and tissues were homogenized using a TissueLyserLT (Qiagen) set to 5 oscillations/s for 2 minutes. Tissue samples were clarified via centrifugation at 19,000g for 3 minutes.
Serum, ejaculate, and clarified tissue samples were serially diluted in BA-1 diluent. These dilutions were plated on confluent Vero cells and incubated at 37°C, 5% CO2 for 1 hour, with gentle rocking every 15 minutes. Following incubation, Vero cells were overlaid with Miller’s Ye-Lah agarose overlay (2X Ye-Lah media (0.132% yeast extract (w/v), 0.66% lactalbumin hydrolysate (w/v), 10X Earle’s Balanced Salt Solution, 2% Fetal Bovine Serum (v/v), Amphotericin B, Gentamycin), 1.6% agarose (w/v), and 0.225% sodium bicarbonate (v/v)). A second overlay containing neutral red (1:300) was added four days later. Plaques were counted the following day. The limit of detection is 2 log10 PFU/mL serum, 0.4 log10 PFU/ejaculate, and 0.4 log10 PFU/organ.
Quantification of viral RNA
Viral RNA was extracted from ejaculates and homogenized tissue samples using the QIAamp viral RNA mini kit (Qiagen). Briefly, 70μL sample was diluted 1:2 in 10μM dithiothreitol (DTT; Pierce) to denature seminal proteins. Samples were lysed in 560μL buffer AVL with linear acrylamide added (1μg per sample). The extraction was continued following the manufacturer’s protocol. Viral RNA was eluted in 60μL nuclease-free water (Qiagen) and stored at −80C.
Viral RNA was quantified via a one-step qRT-PCR using the iTaq universal probes one-step kit (Bio-Rad) per the manufacturer’s instructions for a 20μL reaction, with the exception that the quantity of reverse transcriptase per reaction was halved. Five microliters of RNA were used per reaction. ZIKV specific primers and probes were synthesized by IDT using 6-Fam as the reporter dye and Zen/Iowa Black as the quencher. Primer and probe sequences and cycling conditions are as previously described (41). The amplification product is an approximately 75bp region of the envelope protein. Viral RNA concentration was determined via an absolute standard curve of in vitro transcribed RNA standards from a plasmid containing a segment of the ZIKV envelope gene (20, 41). The limit of detection for this assay was 2 log10 RNA copies per ejaculate or 1.5 log10 RNA copies per organ.
Visualization of viral RNA within tissues via In Situ Hybridization (ISH)
Testes, epididymides, and seminal vesicles were collected from male mice upon euthanasia and fixed and stored in 10% buffered formalin. Tissues were paraffin-embedded, and 5μM slices were attached to charged, glass slides. ISH was performed using the view RNA ISH Tissue Assay (Invitrogen) per the manufacturer’s instructions. Tissues underwent pretreatment and protease treatment for 10 minutes each (42). Proprietary but publicly available probe sets specific for positive sense (African and Asian lineage) or negative sense (Asian lineage) ZIKV RNA was used (Thermofisher). RNA (red) was visualized via alkaline phosphatase labeled probes. Nuclei (blue) were counterstained via Gill’s hematoxylin (American Master Tech Scientific) for 3 minutes. Tissues from uninfected and infected mice euthanized during acute infection served as negative and positive controls, respectively. Contrast, brightness, and sharpness were adjusted equally across all images using Photoshop Elements 2021 (Adobe).
Histological analysis
Tissues were collected and processed as for ISH. Slides were stained for Hematoxylin and Eosin (H&E) following normal procedures. Slides from infected mice euthanized before immunosuppression (dpi 31) and from uninfected mice treated with cyclophosphamide or PBS served as controls. Slides were analyzed by Sheryl Coutermarsh-Ott, DVM, PhD, Diplomate of the American College of Veterinary Pathologists (DACVP), who was blinded throughout the analysis. Testes were assessed for degradation of tubule architecture, inflammation of interstitial spaces, and Leydig cell loss. Epididymides were assessed for epithelial damage and interstitial inflammation. Each of these factors were scored from 0 (no pathology) to 3 (severe pathology), and the scores from each of these subcategories were added together to achieve a total organ score. Contrast, brightness, and sharpness were adjusted equally across all images using Photoshop Elements 2021 (Adobe).
Statistics
Statistical analyses were performed using GraphPad Prism (v8.4.1). Weight data were analyzed using repeated measures analysis of variance (ANOVA) with multiple comparisons t-tests using Tukey correction. Infectious virus and viral RNA concentrations in ejaculates were assessed via multiple comparisons t-tests using the Holm-Sidak correction. Correlations were assessed via Spearman correlation coefficient (ZIKV RNAc in epididymis vs. peak ZIKV RNAc in the ejaculates or number of matings; total testis or epididymis histology score vs. ZIKV RNAc in testis or epididymis), Mann-Whitney rank sum test (ISH results vs. ZIKV RNAc in epididymis, number of matings, viremia at 3 dpi, or viremia at 5 dpi).
Ethics statement
All animal experiments were approved by the Institutional Animal Care and Use Committee at Virginia Polytechnic Institute and State University (IACUC protocol 18-085) and followed the recommendations in the Guide for the Care and Use of Laboratory Animals, 8th edition (Institute for Laboratory Animal Research, National Research Council, National Academy of Sciences, 2011).
RESULTS
Immunosuppression following ZIKV infection does not lead to systemic recrudescence
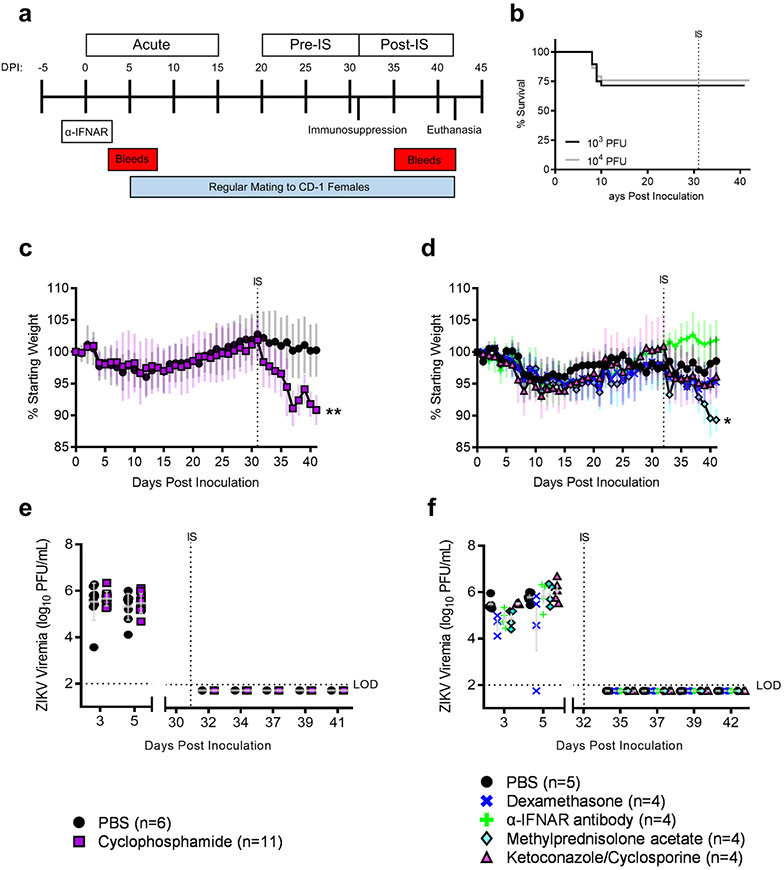
To assess whether ZIKV can recrudescence following acute infection, C57BL/6J male mice pre-treated with an IFNAR blocking antibody were infected subcutaneously with ZIKV strain Dakar41524. Serum and ejaculates were monitored regularly for presence of infectious virus. When infectious virus was no longer shed in ejaculates (~30 dpi; see Figures 2a and 2b), male mice were treated with one of the following immunosuppressants chosen for their differing mechanisms of action: cyclophosphamide, IFNAR blocking antibody, methylprednisolone acetate, dexamethasone, or ketoconazole/cyclosporine. This was performed in two separate studies; the cyclophosphamide study was performed first and was followed by a study with the other immunosuppressants to investigate whether the mechanism of immunosuppression impacted ZIKV persistence or recrudescence. In both studies, PBS treated mice served as a control. Post-immunosuppression, serum and ejaculates were collected regularly to assess for infectious virus. Mice were euthanized ten days post immunosuppression (Figure 1a).
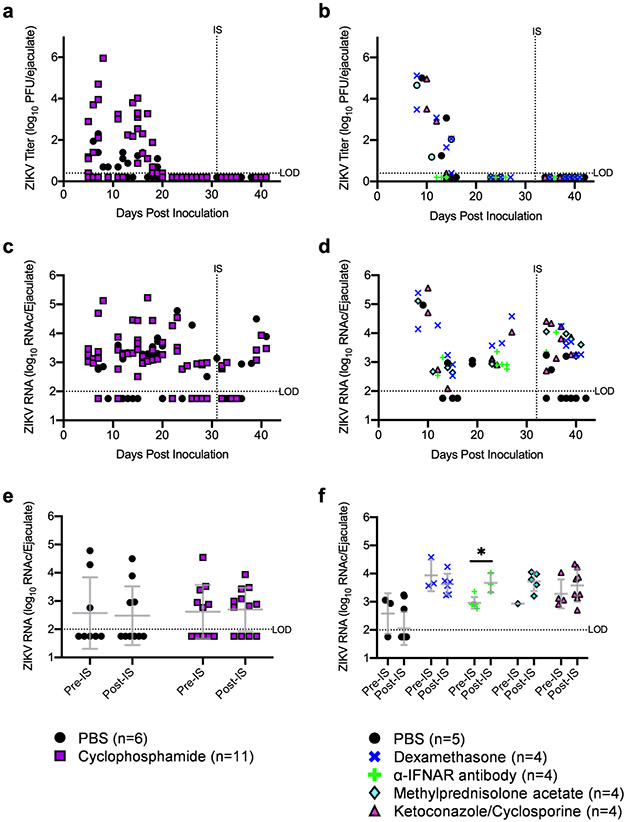
Figure 2: Immunosuppression did not significantly increase ZIKV in ejaculates.
Infectious ZIKV in ejaculates (a,b) and ZIKV RNA copies in ejaculates (c,d) were quantified via qRT-PCR. For further analysis of ejaculate qRT-PCR results, ejaculate samples were grouped based on the stage of infection in which they were collected (e,f): pre-immunosuppression (days 22-27) and post-immunosuppression (days 33-42). Each data point represents an individual sample, with lines and error bars representing the mean and standard deviation, respectively. Data were analyzed via two-way ANOVA followed by multiple comparisons t-tests using the Holm-Sidak correction.
* p<0.05; ** p<0.01; *** p<0.005; **** p<0.001.
Abbreviations: RNAc, RNA copies; LOD, limit of detection; IS, immunosuppression; ns, not significant.
Figure 1: Treatment with immunosuppressants affects morbidity but not mortality or viremia in ZIKV-infected male mice.
Study design (a). Survival curve of mice grouped by inoculum dose; 103 PFU: n = 28 (cyclophosphamide or PBS treated mice); 104 PFU: n=28 (other immunosuppressants or PBS treated mice) (b). Mouse weights were recorded daily (mean ± standard deviation) PBS: n=6; Cyclophosphamide: n = 11 (c). Mouse weights PBS: n=5; Dexamethasone: n=4; α-IFNAR antibody: n=4; Methylprednisolone acetate: n=4; Ketoconazole/Cyclosporine: n=4 (d). Infectious ZIKV in serum was quantified via plaque assay (e,f). Data points represent individual mice, horizontal lines represent group mean, and error bars represent standard deviation. Data were analyzed via repeated measures ANOVA.
* p<0.05; ** p<0.01; *** p<0.005; **** p<0.001.
Abbreviations: DPI, days post inoculation; IS, immunosuppression; LOD, limit of detection.
Mortality, morbidity, and viremia were monitored throughout the course of the study. During acute infection, there was a 29% mortality rate in mice infected with 103 PFUs of ZIKV (cyclophosphamide or PBS treated mice) and a 24% mortality rate in mice infected with 104 PFUs of ZIKV (other immunosuppressants or PBS treated mice), with mortalities occurring between dpi 8 and 10 (Figure 1b). No mortalities occurred post-immunosuppression, regardless of the immunosuppressant used. On average, mice lost approximately 5% of their starting weight during acute infection and gained that weight back during the pre-immunosuppression phase (Figures 1c and 1d). Post-immunosuppression, there was significant weight loss in mice treated with cyclophosphamide (10% weight loss; p = 0.003) and methylprednisolone acetate (9% weight loss; p=0.03) compared to their respective PBS treated controls (2% weight loss); however, it is likely that this weight loss was due to the immunosuppressant agents themselves and not due to ZIKV recrudescence, since no infectious virus was detected in serum post-immunosuppression, regardless of immunosuppressant treatment (Figures 1e and 1f). In contrast, infectious virus was present in serum at concentrations ranging from 3.6 to 6.7 log10 PFUs/mL during acute infection. Taken together, these results confirm that mice were infected with ZIKV experienced acute disease. Systemic ZIKV recrudescence following immunosuppression was unlikely because viremia was not detected post immunosuppression.
Immunosuppression does not promote ZIKV recrudescence in ejaculates
To assess whether ZIKV recrudescence occurred in ejaculates following immunosuppression, infectious virus was quantified in ejaculates via plaque assay (Figures 2a and 2b). During acute infection, male mice shed up to 6 log10 PFU in ejaculates, with infectious virus cleared by twenty days post inoculation. No infectious virus was detected in ejaculates post immunosuppression regardless of immunosuppressant treatment. In human males infected with ZIKV, ZIKV RNA is shed in the semen long after shedding of infectious virus ceases (14-18). Therefore, we also investigated whether immunosuppression impacted ZIKV RNA levels in ejaculates. ZIKV RNA levels were quantified in ejaculates via qRT-PCR (Figures 2c and 2d). ZIKV RNA was detected in ejaculates throughout the course of the entire study with the highest concentrations (5 to 6 log10) detected in samples collected during acute infection. To determine whether ZIKV RNA concentrations increased in ejaculates post-immunosuppression, samples were grouped based on collection time: 10 days pre-immunosuppression and 10 days post-immunosuppression (Figures 2e and 2f). ZIKV RNA levels in ejaculates from mice immunosuppressed with the α-IFNAR antibody were significantly higher post-immunosuppression than those from pre-immunosuppression samples (p=0.03); however, it is unclear whether this approximately 0.5log10 increase in ZIKV RNA is biologically relevant. No other immunosuppressant treatments increased ZIKV RNA levels in ejaculates. There were no significant changes in ZIKV RNA levels in ejaculates pre- and post-immunosuppression in PBS treated mice. Taken together, these data indicate that immunosuppression did not significantly increase infectious ZIKV in mouse ejaculates.
Mice treated with cyclophosphamide have higher levels of ZIKV RNA in epididymal lumens
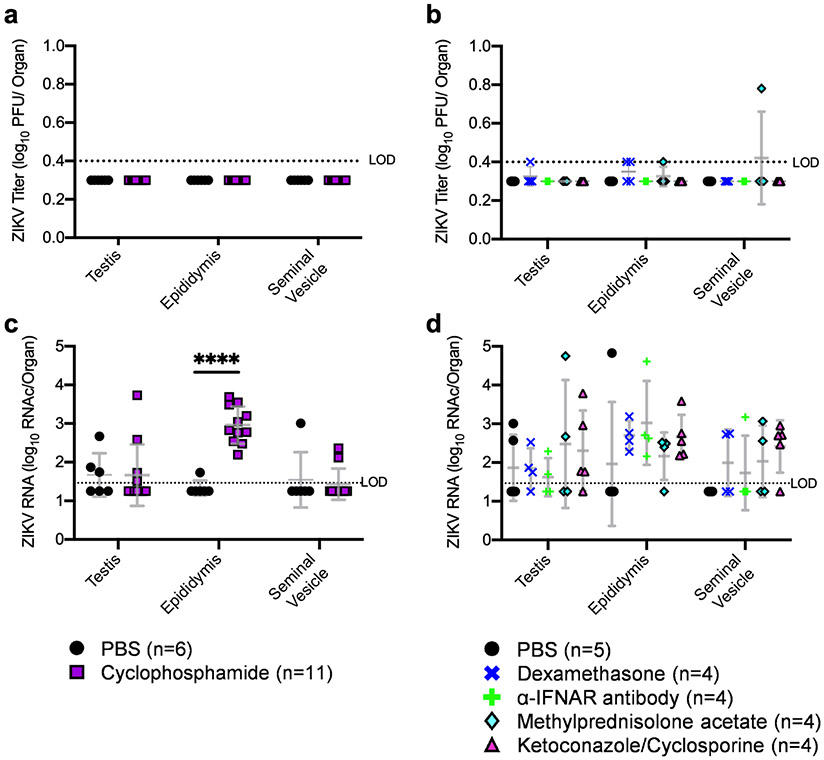
We next assessed whether infectious virus was present in MRT tissues following immunosuppression. Testes, epididymides, and seminal vesicles were collected from mice upon euthanasia, and infectious virus was quantified via plaque assay. Infectious virus was detected in reproductive tract tissues of mice that succumbed to acute ZIKV infection (data not shown). Infectious virus was not detected in tissues from any of the mice treated with cyclophosphamide or PBS (Figures 3a). Infectious virus was detected at or near the limit of detection in the testes of one mouse treated with dexamethasone, the epididymides of two mice treated with dexamethasone and one mouse treated with methylprednisolone acetate, and the seminal vesicle of one mouse treated with methylprednisolone acetate (Figures 3b). Whether the low level of infectious virus in the MRT following immunosuppression indicates recrudescence in the MRT is unclear; thus, we also evaluated viral RNA in tissues. ZIKV RNA was significantly higher in epididymides from mice treated with cyclophosphamide than from those treated with PBS (Figure 3c; p <0.001). There were no significant changes in ZIKV RNA levels in tissues of mice treated with any of the other immunosuppressants compared to PBS controls (Figure 3d).
Figure 3: ZIKV RNA concentration increased in the epididymis following cyclophosphamide treatment.
Infectious ZIKV levels in MRT tissues (a,b) was quantified via plaque assay. ZIKV RNA was quantified in MRT tissues via qRT-PCR (c,d). Each data point represents an individual sample, with lines and error bars representing the mean and standard deviation, respectively. Data were analyzed via two-way ANOVA followed by multiple comparisons t-tests using the Holm-Sidak correction.
* p<0.05; ** p<0.01; *** p<0.005; **** p<0.001.
Abbreviations: RNAc, RNA copies; LOD, limit of detection; ns, not significant.
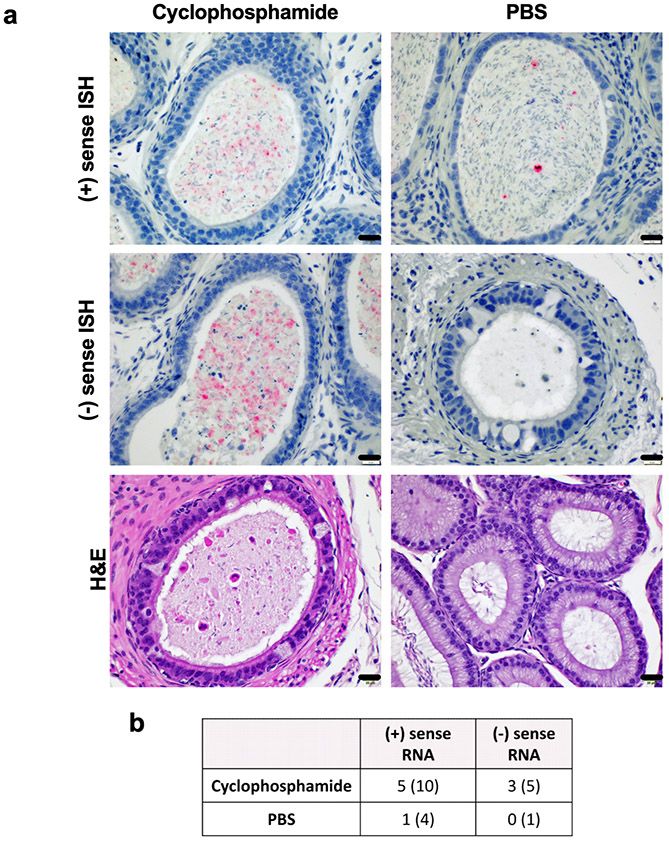
To validate our results of higher ZIKV RNA in the epididymides of cyclophosphamide treated mice, we performed in situ hybridization (ISH) against ZIKV (+) sense RNA in the epididymides of cyclophosphamide and PBS treated mice. We detected ZIKV (+) sense RNA via ISH in the epididymal lumen of 5 of the cyclophosphamide treated mice (n=10) but in only one of the epididymides from the PBS treated mice (n=4) (Figure 4a and b). In human males, duration of ZIKV RNA in ejaculates is inversely correlated ejaculation frequency, suggesting a role for viral clearance rate in the long-term detection of ZIKV RNA in semen (18). To help ascertain whether our qRT-PCR and ISH results indicate ZIKV persistence as opposed to delayed RNA clearance, we performed correlation analyses between the positive ISH results and mating frequency or magnitude of the initial infection. There were no significant correlations between ZIKV RNAc in the epididymis and number of matings (p=0.33), ISH results (p=0.23), or ZIKV RNAc in the ejaculates (p=0.15), as well as no significant correlation between ISH results and number of matings (p=0.94), viremia at 3 dpi (p=0.27), or viremia at 5 dpi (p=0.072) (data not shown). These results suggest that the viral RNA in the epididymides might be due to persistent infection, rather than delayed RNA clearance.
Figure 4: ZIKV RNA is present within the lumen of epididymides of cyclophosphamide treated mice.
Epididymides from cyclophosphamide and PBS treated mice were assessed for ZIKV (+) or (−) sense RNA via ISH. ZIKV RNA is stained red and nuclei are stained blue. Scale bars represent 20uL (a). Number of positive samples and number of samples tested are listed in (b).
To determine whether ZIKV actively replicated in the epididymides following immunosuppression, we performed ISH specific for ZIKV (−) sense RNA, which is only present during viral replication, on epididymis samples from mice that stained for (+) sense RNA via ISH. We detected (−) sense RNA in the epididymal lumens of 3 of the cyclophosphamide treated mice that had (+) sense RNA in the epididymis (n=5) and did not detect (−) sense RNA in the one PBS treated mouse that had (+) sense RNA in the epididymis (Figure 4a and b).
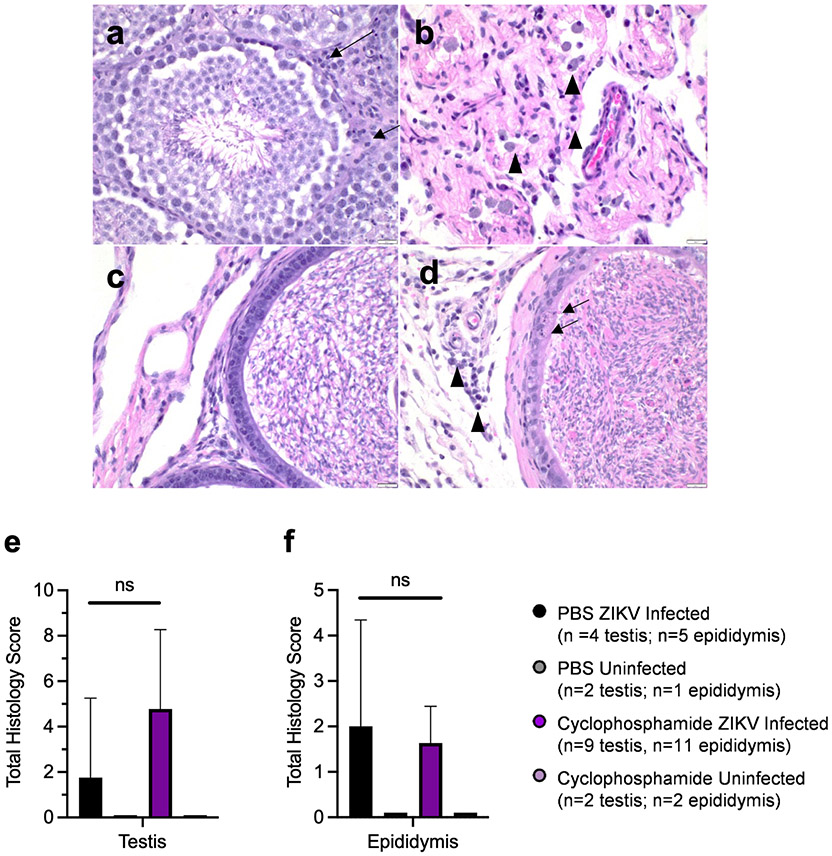
Lastly, we wanted to determine whether immunosuppression altered tissue pathology in ZIKV-infected mice. Sections of testis and epididymis were evaluated histologically for evidence of tissue damage and inflammation. In general, all ZIKV mice exhibited some degree of tissue pathology. In the testis, these changes ranged from moderate increases in interstitial lymphocytes and plasma cells to massive loss of seminiferous tubules with collapse of normal architecture (Figures 5a, 5b), which is consistent with other studies of ZIKV infection of the testes (43-45). In the epididymis, these changes were less severe with mild infiltration of inflammatory cells and mild to moderate degeneration and loss of epithelial cells (Figures 5c, 5d). These changes were graded semi-quantitatively to produce a total histologic score. There were no significant differences in histologic scores of testes (p=0.082) or epididymides (p=0.86) between cyclophosphamide and PBS treated ZIKV infected mice (Figures 5e, 5f). Additionally, there were no correlations between ZIKV RNAc in tissues and the total histology score for either testes (p=0.7665) or epididymides (p=0.7996). No pathology was observed in testes or epididymides of uninfected mice treated with PBS or cyclophosphamide, indicating that cyclophosphamide treatment alone does not cause MRT pathologies. Severe testicular damage and mild to moderate epididymal damage were observed in tissues from mice euthanized immediately before immunosuppressant treatment, indicating that MRT pathology likely manifested before immunosuppressant treatment due to acute ZIKV infection. Taken together, these results indicate that cyclophosphamide treatment increased ZIKV replication in the epididymides, specifically in non-sperm cells within the lumen, but not MRT tissue pathology.
Figure 5: Pathologies in testes and epididymides were observed following ZIKV infection independent of immunosuppressant treatment.
Testes and epididymides from cyclophosphamide and PBS treated mice were stained via H&E and evaluated by a board certified pathologist. Sections of testis were scored according to the degree of seminiferous tubule loss, presence and degree of interstitial inflammation, and degree of Leydig cell loss. Tissues with low scores exhibited normal seminiferous tubules, lack of interstitial inflammation, and normal Leydig cells (arrows) (a). Tissues with high scores exhibited severe loss of seminiferous tubules, prominent interstitial inflammation that was most often composed of macrophages, lymphocytes, and plasma cells (arrowheads), and prominent loss of Leydig cells (b). Sections of epididymis were scored according to the degree of epithelial damage and presence and degree of interstitial inflammation. Scores were then summed to provide a total histology score for each tissue. Tissues with low scores had minimal epithelial damage and minimal to no observable interstitial inflammation (c). Tissues with high scores exhibited moderate epithelial damage characterized by cell swelling and/or the presence of cellular debris (arrow) as well as moderate amounts of interstitial inflammation characterized primarily by lymphocytes, plasma cells and macrophages. (arrowhead) (d). Scores were then summed to provide a total histology score for each tissue (e,f). Data are displayed as mean with error bars representing standard deviation. Statistical analyses were assessed using the Mann-Whitney ranked sum test.
* p<0.05; ** p<0.01; *** p<0.005; **** p<0.001.
DISCUSSION
In utero ZIKV transmission can occur if the mother is infected via sexual transmission or mosquito bite during pregnancy, which can lead to severe congenital defects in the developing fetus (46). Since ZIKV RNA has been detected in human semen for six months post symptom onset (14, 15, 17, 18), we investigated whether ZIKV persists in the MRT and could recrudesce upon immunosuppressant treatment in a mouse model. We observed that immunosuppression did not stimulate systemic ZIKV recrudescence or resumption of shedding of infectious ZIKV in ejaculates. However, ZIKV RNA levels as detected by qRT-PCR were significantly higher in the epididymides of mice treated with the immunosuppressant cyclophosphamide compared to PBS-treated controls. Additionally, ZIKV (+) and (−) sense RNA was visualized via ISH in in the extracellular, luminal contents of the epididymis of mice treated with cyclophosphamide. Rarely, we also identified them within degenerate cells within epididymis of these mice as well. Collectively, these results suggest that ZIKV persistently infects the epididymis. While our study used an African lineage ZIKV strain, which in mouse models is more virulent than the Asian lineage strains isolated during the recent ZIKV pandemic, the two lineages have similar sexual transmission potential in mouse models (47). Therefore, our results are likely applicable to the more contemporary, Asian genotype strains.
In humans, infectious ZIKV and ZIKV RNA have been isolated from ejaculates up to 38 days and 370 days post symptom onset, respectively, in otherwise healthy individuals (17, 18, 48). In a ZIKV-infected male on immunosuppressant therapy for an autoimmune condition, ZIKV RNA was shed in semen for over 900 days post infection (49). The potential for long-term sexual transmission is unknown. We hypothesized that immunosuppression after the acute phase of ZIKV infection would stimulate ZIKV replication in reservoirs within the MRT, if any exist. While our results indicate that immunosuppression is unlikely to result in the recurrence of infectious ZIKV in ejaculates, this study allowed us to identify the epididymis as a potential site for persistent ZIKV infection. We may not have observed infectious virus in ejaculates because we only assessed ejaculates for ten days post-immunosuppression. In a study of persistence of West Nile virus in a mouse model, recrudescence was detected in tissues fifteen days post cyclophosphamide treatment (31).
Previous studies using human testicular explants or cells and primary prostate organoids suggest ZIKV may infect or persists in these tissues in men (21-23). There are few to no studies investigating the infection of the epididymis in ZIKV-infected men. However, the infection of the epididymis during acute ZIKV infection has been well characterized in a mouse model. Infection begins in the head of epididymis and quickly spreads to the tail of the epididymis. Epididymal epithelial cells and luminal leukocytes were found to be the targets of ZIKV infection during acute infection (42). In our study, we did find ZIKV (+) sense RNA in the epididymis, but it was primarily extracellular and only within luminal contents. While the epididymal epithelium was damaged, there was no evidence of epididymal epithelium infection post-immunosuppression. Given that the epididymis functions, in part, as a storage for mature sperm before ejaculation, it is possible that the infected luminal cells we observed were not a result of persistent ZIKV infection but rather residual infected cells that had yet to clear the MRT. In humans, duration of ZIKV shedding in semen is inversely correlated with the frequency of ejaculation (18). In our study, we found that there was no correlation between mating frequency and epididymal ZIKV RNA levels by qRT-PCR or ISH staining. Additionally, there were no correlations between ZIKV ISH staining and viremia titers, peak ejaculate RNA copies, or epididymal RNA copies. These results indicate that ZIKV RNA in the epididymis is more likely a result of persistent ZIKV infection as opposed to residual infected cells in the epididymis. Furthermore, via ISH, we showed that ZIKV (−) sense RNA was present in epididymal lumens in some of the cyclophosphamide treated mice, suggesting ZIKV was replicating within those tissues. Since the (−) sense RNA probe was designed using Asian lineage ZIKV RNA sequences (as opposed to the African lineage strain used in this study), it is possible that additional samples contained (−) sense RNA that were not identified. Nonetheless, our results indicate that ZIKV replicates in the MRT after cessation of infectious viral shedding in ejaculates.
Interestingly, we did identify ZIKV RNA rarely within cells within the lumen of epididymis of immunosuppressed mice. Since these cells are located in the lumen, as opposed to epithelial or interstitial spaces, it is possible that these cells originated in the testes and transited to the epididymis; however, there was little evidence in cyclophosphamide treated mice that testes were infected upon euthanasia. These cells were often degenerate and thus unable to be definitively identified solely on morphology. It is suspected, however, that these cells are likely macrophages or degenerate epithelial cells. Macrophages are present throughout the MRT in both the interstitial spaces of the testes and epididymis; however, macrophages rarely cross the blood testes barrier or the blood epididymis barrier (which serve to maintain immune-privileged sites for sperm development and maturation) in healthy individuals (50-52). ZIKV infection may disrupt these barriers in the MRT, allowing for macrophages to enter the seminiferous tubules of either the testes or the epididymis (53, 54); however, it is unknown whether macrophages are infected before or after entrance into these tubules. Degenerate epithelial cells are derived from epididymal epithelial cells that are sloughed into the lumen upon epithelial damage. Since ZIKV does infect the epithelium of the epididymis and causes epithelial damage (42, 55), infected degenerate epithelial cells within the epididymal lumen were likely infected before being sloughed off into the lumen. Additionally, we did not detect ZIKV RNA within sperm cells present in the epididymis, indicating that it may be safe for men with persistent shedding of ZIKV RNA in their ejaculates to conceive children via in vitro fertilization or similar assistive reproductive technologies. These results are consistent with a recent report of long-term ZIKV infection in non-sperm cells in human semen (56). Future studies will determine the specific cell types harboring ZIKV RNA in the epididymis during persistent infection.
The mechanisms of action of the various immunosuppressants used in this study may provide insights into how ZIKV establishes a persistent infection in the MRT and what immune responses are necessary to clear ZIKV from the MRT. The immunosuppressants used in this study were chosen for their differing mechanisms of action, which are as follows: cyclophosphamide induces apoptosis of rapidly dividing cells (31, 57); α-IFNAR1 antibody inhibits the interferon response by preventing type 1 interferons from binding to their cognate receptors (37); the combination of ketoconazole and cyclosporine inhibits T cell activation (58-60); dexamethasone induces apoptosis of peripheral T cells and induction of an anti-inflammatory response (61-63); and methylprednisolone acetate decreases T cell and monocyte populations and induces an anti-inflammatory response (64). In our study, we observed higher levels of ZIKV RNA only in the epididymides of mice treated with cyclophosphamide. This implies that rapidly dividing cells (such as developing monocytes or macrophages or expanding T or B cell clonal populations) are important in the immune response to ZIKV in the MRT. This information may be useful to healthcare providers in assessing risk of immunosuppressant treatments in men who are shedding infectious ZIKV or ZIKV RNA in their ejaculates. Future studies will delve into the immune response to ZIKV in the MRT, specifically the epididymis.
The long-term impacts of ZIKV on the MRT are still largely unknown. The study presented here provides insights into the role of the epididymis in ZIKV infection and the mechanism of ZIKV persistence in the MRT. Additionally, this study provides the foundation for studies to harness immune responses to decrease viral infection in the MRT, particularly within the epididymis. Understanding how ZIKV infects and persists within the MRT will help to explain the mechanisms behind ZIKV sexual transmission, allowing for increased knowledge of ZIKV transmission risk and reduced incidence of ZIKV congenital syndrome.
Research Highlights.
Zika virus persists in the epididymal lumen after virus is no longer shed in ejaculates.
Infectious Zika virus does not recrudesce following acute infection and immunosuppression.
Immunosuppression resulted in higher levels of ZIKV RNA in the epidiymides.
ACKNOWLEDGEMENTS
Funding for this project was provided by the NIAID (R21A142504). We thank VT Laboratory Animal Research staff for contributions to animal husbandry and training for mouse procedures. We thank the VT ViTALS lab for processing tissues for microscopy and performing H&E staining.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Honein MA, Dawson AL, Petersen EE, Jones AM, Lee EH, Yazdy MM, Ahmad N, Macdonald J, Evert N, Bingham A, Ellington SR, Shapiro-Mendoza CK, Oduyebo T, Fine AD, Brown CM, Sommer JN, Gupta J, Cavicchia P, Slavinski S, White JL, Owen SM, Petersen LR, Boyle C, Meaney-Delman D, Jamieson DJ, Collaboration USZPR. 2017. Birth Defects Among Fetuses and Infants of US Women With Evidence of Possible Zika Virus Infection During Pregnancy. JAMA 317:59–68. [DOI] [PubMed] [Google Scholar]
- 2.Reynolds MR, Jones AM, Petersen EE, Lee EH, Rice ME, Bingham A, Ellington SR, Evert N, Reagan-Steiner S, Oduyebo T, Brown CM, Martin S, Ahmad N, Bhatnagar J, Macdonald J, Gould C, Fine AD, Polen KD, Lake-Burger H, Hillard CL, Hall N, Yazdy MM, Slaughter K, Sommer JN, Adamski A, Raycraft M, Fleck-Derderian S, Gupta J, Newsome K, Baez-Santiago M, Slavinski S, White JL, Moore CA, Shapiro-Mendoza CK, Petersen L, Boyle C, Jamieson DJ, Meaney-Delman D, Honein MA, Collaboration USZPR. 2017. Vital Signs: Update on Zika Virus-Associated Birth Defects and Evaluation of All U.S. Infants with Congenital Zika Virus Exposure - U.S. Zika Pregnancy Registry, 2016. MMWR Morb Mortal Wkly Rep 66:366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shapiro-Mendoza CK, Rice ME, Galang RR, Fulton AC, VanMaldeghem K, Prado MV, Ellis E, Anesi MS, Simeone RM, Petersen EE, Ellington SR, Jones AM, Williams T, Reagan-Steiner S, Perez-Padilla J, Deseda CC, Beron A, Tufa AJ, Rosinger A, Roth NM, Green C, Martin S, Lopez CD, deWilde L, Goodwin M, Pagano HP, Mai CT, Gould C, Zaki S, Ferrer LN, Davis MS, Lathrop E, Polen K, Cragan JD, Reynolds M, Newsome KB, Huertas MM, Bhatangar J, Quinones AM, Nahabedian JF, Adams L, Sharp TM, Hancock WT, Rasmussen SA, Moore CA, Jamieson DJ, Munoz-Jordan JL, Garstang H, Kambui A, Masao C, et al. 2017. Pregnancy Outcomes After Maternal Zika Virus Infection During Pregnancy - U.S. Territories, January 1, 2016-April 25, 2017. MMWR Morb Mortal Wkly Rep 66:615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smoots AN, Olson SM, Cragan J, Delaney A, Roth NM, Godfred-Cato S, Jones AM, Nahabedian JF 3rd, Fornoff J, Sandidge T, Yazdy MM, Higgins C, Olney RS, Eckert V, Forkner A, Fox DJ, Stolz A, Crawford K, Cho SJ, Knapp M, Ahmed MF, Lake-Burger H, Elmore AL, Langlois P, Breidenbach R, Nance A, Denson L, Caton L, Forestieri N, Bergman K, Humphries BK, Leedom VO, Tran T, Johnston J, Valencia-Prado M, Perez-Gonzalez S, Romitti PA, Fall C, Bryan JM, Barton J, Arias W, St John K, Mann S, Kimura J, Orantes L, Martin B, de Wilde L, Ellis EM, Song Z, Akosa A, et al. 2020. Population-Based Surveillance for Birth Defects Potentially Related to Zika Virus Infection - 22 States and Territories, January 2016-June 2017. MMWR Morb Mortal Wkly Rep 69:67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foy BD, Kobylinski KC, Chilson Foy JL, Blitvich BJ, Travassos da Rosa A, Haddow AD, Lanciotti RS, Tesh RB. 2011. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis 17:880–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Musso D, Roche C, Robin E, Nhan T, Teissier A, Cao-Lormeau VM. 2015. Potential sexual transmission of Zika virus. Emerg Infect Dis 21:359–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hills SL, Russell K, Hennessey M, Williams C, Oster AM, Fischer M, Mead P. 2016. Transmission of Zika Virus Through Sexual Contact with Travelers to Areas of Ongoing Transmission - Continental United States, 2016. MMWR Morb Mortal Wkly Rep 65:215–6. [DOI] [PubMed] [Google Scholar]
- 8.D'Ortenzio E, Matheron S, Yazdanpanah Y, de Lamballerie X, Hubert B, Piorkowski G, Maquart M, Descamps D, Damond F, Leparc-Goffart I. 2016. Evidence of Sexual Transmission of Zika Virus. N Engl J Med 374:2195–8. [DOI] [PubMed] [Google Scholar]
- 9.Freour T, Mirallie S, Hubert B, Splingart C, Barriere P, Maquart M, Leparc-Goffart I. 2016. Sexual transmission of Zika virus in an entirely asymptomatic couple returning from a Zika epidemic area, France, April 2016. Euro Surveill 21. [DOI] [PubMed] [Google Scholar]
- 10.Towers S, Brauer F, Castillo-Chavez C, Falconar AKI, Mubayi A, Romero-Vivas CME. 2016. Estimate of the reproduction number of the 2015 Zika virus outbreak in Barranquilla, Colombia, and estimation of the relative role of sexual transmission. Epidemics 17:50–55. [DOI] [PubMed] [Google Scholar]
- 11.Gao D, Lou Y, He D, Porco TC, Kuang Y, Chowell G, Ruan S. 2016. Prevention and Control of Zika as a Mosquito-Borne and Sexually Transmitted Disease: A Mathematical Modeling Analysis. Sci Rep 6:28070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Counotte MJ, Kim CR, Wang J, Bernstein K, Deal CD, Broutet NJN, Low N. 2018. Sexual transmission of Zika virus and other flaviviruses: A living systematic review. PLoS Med 15:e1002611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duggal NK, McDonald EM, Ritter JM, Brault AC. 2018. Sexual transmission of Zika virus enhances in utero transmission in a mouse model. Sci Rep 8:4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fontaine A, de Laval F, Belleoud D, Briolant S, Matheus S. 2018. Duration of Zika Viremia in Serum. Clin Infect Dis 67:1143–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mansuy JM, Mengelle C, Pasquier C, Chapuy-Regaud S, Delobel P, Martin-Blondel G, Izopet J. 2017. Zika Virus Infection and Prolonged Viremia in Whole-Blood Specimens. Emerg Infect Dis 23:863–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tesla B, Demakovsky LR, Packiam HS, Mordecai EA, Rodriguez AD, Bonds MH, Brindley MA, Murdock CC. 2018. Estimating the effects of variation in viremia on mosquito susceptibility, infectiousness, and R0 of Zika in Aedes aegypti. PLoS Negl Trop Dis 12:e0006733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medina FA, Torres G, Acevedo J, Fonseca S, Casiano L, De Leon-Rodriguez CM, Santiago GA, Doyle K, Sharp TM, Alvarado LI, Paz-Bailey G, Munoz-Jordan JL. 2019. Duration of the Presence of Infectious Zika Virus in Semen and Serum. J Infect Dis 219:31–40. [DOI] [PubMed] [Google Scholar]
- 18.Mead PS, Duggal NK, Hook SA, Delorey M, Fischer M, Olzenak McGuire D, Becksted H, Max RJ, Anishchenko M, Schwartz AM, Tzeng WP, Nelson CA, McDonald EM, Brooks JT, Brault AC, Hinckley AF. 2018. Zika Virus Shedding in Semen of Symptomatic Infected Men. N Engl J Med 378:1377–1385. [DOI] [PubMed] [Google Scholar]
- 19.Duggal NK, Ritter JM, Pestorius SE, Zaki SR, Davis BS, Chang GJ, Bowen RA, Brault AC. 2017. Frequent Zika Virus Sexual Transmission and Prolonged Viral RNA Shedding in an Immunodeficient Mouse Model. Cell Rep 18:1751–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McDonald EM, Duggal NK, Delorey MJ, Oksanish J, Ritter JM, Brault AC. 2019. Duration of seminal Zika viral RNA shedding in immunocompetent mice inoculated with Asian and African genotype viruses. Virology 535:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matusali G, Houzet L, Satie AP, Mahé D, Aubry F, Couderc T, Frouard J, Bourgeau S, Bensalah K, Lavoué S, Joguet G, Bujan L, Cabié A, Avelar G, Lecuit M, Le Tortorec A, Dejucq-Rainsford N. 2018. Zika virus infects human testicular tissue and germ cells. J Clin Invest 128:4697–4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar A, Jovel J, Lopez-Orozco J, Limonta D, Airo AM, Hou S, Stryapunina I, Fibke C, Moore RB, Hobman TC. 2018. Human Sertoli cells support high levels of Zika virus replication and persistence. Sci Rep 8:5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spencer JL, Lahon A, Tran LL, Arya RP, Kneubehl AR, Vogt MB, Xavier D, Rowley DR, Kimata JT, Rico-Hesse RR. 2018. Replication of Zika Virus in Human Prostate Cells: A Potential Source of Sexually Transmitted Virus. J Infect Dis 217:538–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halabi J, Jagger BW, Salazar V, Winkler ES, White JP, Humphrey PA, Hirsch AJ, Streblow DN, Diamond MS, Moley K. 2020. Zika Virus Causes Acute and Chronic Prostatitis in Mice and Macaques. J Infect Dis 221:1506–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peregrine J, Gurung S, Lindgren MC, Husain S, Zavy MT, Myers DA, Papin JF. 2019. Zika Virus Infection, Reproductive Organ Targeting, and Semen Transmission in the Male Olive Baboon. J Virol 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mlera L, Melik W, Bloom ME. 2014. The role of viral persistence in flavivirus biology. Pathog Dis 71:137–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baty SA, Gibney KB, Staples JE, Patterson AB, Levy C, Lehman J, Wadleigh T, Feld J, Lanciotti R, Nugent CT, Fischer M. 2012. Evaluation for West Nile Virus (WNV) RNA in urine of patients within 5 months of WNV infection. J Infect Dis 205:1476–7. [DOI] [PubMed] [Google Scholar]
- 28.Murray K, Walker C, Herrington E, Lewis JA, McCormick J, Beasley DW, Tesh RB, Fisher-Hoch S. 2010. Persistent infection with West Nile virus years after initial infection. J Infect Dis 201:2–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gritsun TS, Frolova TV, Zhankov AI, Armesto M, Turner SL, Frolova MP, Pogodina VV, Lashkevich VA, Gould EA. 2003. Characterization of a siberian virus isolated from a patient with progressive chronic tick-borne encephalitis. J Virol 77:25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharma S, Mathur A, Prakash V, Kulshreshtha R, Kumar R, Chaturvedi UC. 1991. Japanese encephalitis virus latency in peripheral blood lymphocytes and recurrence of infection in children. Clin Exp Immunol 85:85–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Appler KK, Brown AN, Stewart BS, Behr MJ, Demarest VL, Wong SJ, Bernard KA. 2010. Persistence of West Nile virus in the central nervous system and periphery of mice. PLoS One 5:e10649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pogodina VV, Frolova MP, Malenko GV, Fokina GI, Koreshkova GV, Kiseleva LL, Bochkova NG, Ralph NM. 1983. Study on West Nile virus persistence in monkeys. Arch Virol 75:71–86. [DOI] [PubMed] [Google Scholar]
- 33.Mathur A, Arora KL, Rawat S, Chaturvedi UC. 1986. Persistence, latency and reactivation of Japanese encephalitis virus infection in mice. J Gen Virol 67 ( Pt 2):381–5. [DOI] [PubMed] [Google Scholar]
- 34.Aliota MT, Caine EA, Walker EC, Larkin KE, Camacho E, Osorio JE. 2016. Characterization of Lethal Zika Virus Infection in AG129 Mice. PLoS Negl Trop Dis 10:e0004682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rossi SL, Tesh RB, Azar SR, Muruato AE, Hanley KA, Auguste AJ, Langsjoen RM, Paessler S, Vasilakis N, Weaver SC. 2016. Characterization of a Novel Murine Model to Study Zika Virus. Am J Trop Med Hyg 94:1362–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dowall SD, Graham VA, Rayner E, Atkinson B, Hall G, Watson RJ, Bosworth A, Bonney LC, Kitchen S, Hewson R. 2016. A Susceptible Mouse Model for Zika Virus Infection. PLoS Negl Trop Dis 10:e0004658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sheehan KC, Lai KS, Dunn GP, Bruce AT, Diamond MS, Heutel JD, Dungo-Arthur C, Carrero JA, White JM, Hertzog PJ, Schreiber RD. 2006. Blocking monoclonal antibodies specific for mouse IFN-alpha/beta receptor subunit 1 (IFNAR-1) from mice immunized by in vivo hydrodynamic transfection. J Interferon Cytokine Res 26:804–19. [DOI] [PubMed] [Google Scholar]
- 38.Lazear HM, Govero J, Smith AM, Platt DJ, Fernandez E, Miner JJ, Diamond MS. 2016. A Mouse Model of Zika Virus Pathogenesis. Cell Host Microbe 19:720–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith DR, Hollidge B, Daye S, Zeng X, Blancett C, Kuszpit K, Bocan T, Koehler JW, Coyne S, Minogue T, Kenny T, Chi X, Yim S, Miller L, Schmaljohn C, Bavari S, Golden JW. 2017. Neuropathogenesis of Zika Virus in a Highly Susceptible Immunocompetent Mouse Model after Antibody Blockade of Type I Interferon. PLoS Negl Trop Dis 11:e0005296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duggal NK, Ritter JM, McDonald EM, Romo H, Guirakhoo F, Davis BS, Chang GJ, Brault AC. 2017. Differential Neurovirulence of African and Asian Genotype Zika Virus Isolates in Outbred Immunocompetent Mice. Am J Trop Med Hyg 97:1410–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert AJ, Johnson AJ, Stanfield SM, Duffy MR. 2008. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis 14:1232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McDonald EM, Duggal NK, Ritter JM, Brault AC. 2018. Infection of epididymal epithelial cells and leukocytes drives seminal shedding of Zika virus in a mouse model. PLoS Negl Trop Dis 12:e0006691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Govero J, Esakky P, Scheaffer SM, Fernandez E, Drury A, Platt DJ, Gorman MJ, Richner JM, Caine EA, Salazar V, Moley KH, Diamond MS. 2016. Zika virus infection damages the testes in mice. Nature 540:438–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma W, Li S, Ma S, Jia L, Zhang F, Zhang Y, Zhang J, Wong G, Zhang S, Lu X, Liu M, Yan J, Li W, Qin C, Han D, Qin C, Wang N, Li X, Gao GF. 2016. Zika Virus Causes Testis Damage and Leads to Male Infertility in Mice. Cell 167:1511–1524 e10. [DOI] [PubMed] [Google Scholar]
- 45.Uraki R, Hwang J, Jurado KA, Householder S, Yockey LJ, Hastings AK, Homer RJ, Iwasaki A, Fikrig E. 2017. Zika virus causes testicular atrophy. Sci Adv 3:e1602899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yarrington CD, Hamer DH, Kuohung W, Lee-Parritz A. 2019. Congenital Zika syndrome arising from sexual transmission of Zika virus, a case report. Fertil Res Pract 5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McDonald EM, Duggal NK, Brault AC. 2017. Pathogenesis and sexual transmission of Spondweni and Zika viruses. PLoS Negl Trop Dis 11:e0005990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barzon L, Percivalle E, Pacenti M, Rovida F, Zavattoni M, Del Bravo P, Cattelan AM, Palu G, Baldanti F. 2018. Virus and Antibody Dynamics in Travelers With Acute Zika Virus Infection. Clin Infect Dis 66:1173–1180. [DOI] [PubMed] [Google Scholar]
- 49.Petridou C, Bonsall D, Ahmed A, Roberts M, Bell C, de Cesare M, Bowden R, Graham V, Bailey D, Simpson A, Aarons E. 2019. Prolonged Zika Virus RNA Detection in Semen of Immunosuppressed Patient. Emerg Infect Dis 25:1598–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mital P, Hinton BT, Dufour JM. 2011. The blood-testis and blood-epididymis barriers are more than just their tight junctions. Biol Reprod 84:851–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao S, Zhu W, Xue S, Han D. 2014. Testicular defense systems: immune privilege and innate immunity. Cell Mol Immunol 11:428–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li N, Wang T, Han D. 2012. Structural, cellular and molecular aspects of immune privilege in the testis. Front Immunol 3:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hui L, Nie Y, Li S, Guo M, Yang W, Huang R, Chen J, Liu Y, Lu X, Chen Z, Yang Q, Wu Y. 2020. Matrix metalloproteinase 9 facilitates Zika virus invasion of the testis by modulating the integrity of the blood-testis barrier. PLoS Pathog 16:e1008509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Siemann DN, Strange DP, Maharaj PN, Shi PY, Verma S. 2017. Zika Virus Infects Human Sertoli Cells and Modulates the Integrity of the In Vitro Blood-Testis Barrier Model. J Virol 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clancy CS, Van Wettere AJ, Siddharthan V, Morrey JD, Julander JG. 2018. Comparative Histopathologic Lesions of the Male Reproductive Tract during Acute Infection of Zika Virus in AG129 and Ifnar(−/−) Mice. Am J Pathol 188:904–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mahe D, Bourgeau S, Frouard J, Joguet G, Pasquier C, Bujan L, Dejucq-Rainsford N. 2020. Long-term Zika virus infection of non-sperm cells in semen. Lancet Infect Dis 20:1371. [DOI] [PubMed] [Google Scholar]
- 57.Halford WP, Schaffer PA. 2000. Optimized viral dose and transient immunosuppression enable herpes simplex virus ICP0-null mutants To establish wild-type levels of latency in vivo. J Virol 74:5957–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jivrajani M, Shaikh MV, Shrivastava N, Nivsarkar M. 2014. An improved and versatile immunosuppression protocol for the development of tumor xenograft in mice. Anticancer Res 34:7177–83. [PubMed] [Google Scholar]
- 59.Matsuda S, Koyasu S. 2000. Mechanisms of action of cyclosporine. Immunopharmacology 47:119–25. [DOI] [PubMed] [Google Scholar]
- 60.Russell G, Graveley R, Seid J, al-Humidan AK, Skjodt H. 1992. Mechanisms of action of cyclosporine and effects on connective tissues. Semin Arthritis Rheum 21:16–22. [DOI] [PubMed] [Google Scholar]
- 61.Miller TA, Schaefer FW 3rd. 2007. Changes in mouse circulating leukocyte numbers in C57BL/6 mice immunosuppressed with dexamethasone for Cryptosporidium parvum oocyst production. Vet Parasitol 149:147–57. [DOI] [PubMed] [Google Scholar]
- 62.Coutinho AE, Chapman KE. 2011. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol 335:2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Giles AJ, Hutchinson MND, Sonnemann HM, Jung J, Fecci PE, Ratnam NM, Zhang W, Song H, Bailey R, Davis D, Reid CM, Park DM, Gilbert MR. 2018. Dexamethasone-induced immunosuppression: mechanisms and implications for immunotherapy. J Immunother Cancer 6:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miller TA, Schaefer FW 3rd. 2006. Characterization of a single dose methylprednisolone acetate immune suppression model using Cryptosporidium muris and Cryptosporidium parvum. Vet Parasitol 141:66–83. [DOI] [PubMed] [Google Scholar]