Summary
BMP signaling plays pleiotropic roles in various tissues during embryogenesis and after birth. We have previously generated a constitutively activated Acvr1(ca-Acvr1) transgenic mouse line (line L35) through pronuclei injection to investigate impacts of enhanced BMP signaling in a tissue specific manner. However, line L35 shows a restricted expression pattern of the transgene. Here, we generated another ca-Acvr1 transgenic line, line A11, using embryonic stem (ES) transgenesis. The generated line A11 shows distinctive phenotypes from line L35, along with very limited expression levels of the transgene. When the transgene is activated in the neural crest cells in a Cre-dependent manner, line A11 exhibits cleft palate and shorter jaws, while line L35 develops ectopic cartilages and highly hypomorphic facial structures. When activated in limb buds, line A11 develops organized but smaller limb skeletal structures, while line L35 forms disorganized limbs with little mineralization. Additionally, no heterotopic ossification (HO) is identified in line A11 when bred with NFATc1-Cre mice even after induction of tissue injury, which is an established protocol for HO for line L35. Therefore, the newly generated conditional ca-Acvr1 mouse line A11 provides an additional resource to dissect highly context dependent functions of BMP signaling in development and disease.
Keywords: caALK2, BMP signaling, skeletogenesis, palatogenesis, orofacial development
Introduction
Bone Morphogenetic Proteins (BMPs) play pleiotropic roles in a context dependent manner during development and after birth (Brazil et al. 2015; Grafe et al. 2018; da Silva Madaleno et al. 2020; Sampath and Reddi 2020; Yang et al. 2020). Both loss-of and gain-of function studies for BMP signaling components revealed roles of BMPs in various types of experimental systems (Zhao 2003; Klingensmith et al. 2010; Yang and Mishina 2019). Upon ligand binding, BMP receptors form hetero-multimers consisting type I and type II BMP receptors (Wrana et al. 1994; Sanchez-Duffhues et al. 2020). Type II receptors then phosphorylate type I receptors within a GS box to activate the kinase activity of type I receptors, and allow them to phosphorylate downstream targets such as Smads (Wrana et al. 1994). A point mutation in the GS box of type I receptors that alters Gln (G) to Asp (D) results in the Ser/Thr kinase activity constitutively active without ligands, but in the presence of type II receptors (Bagarova et al. 2013).
ACVR1 (alternatively known as ALK2, ActRI, or ActRIA) is one of the BMP type I receptors. Homozygous null mutation of AVCRI in the mouse leads to early embryonic lethality around mid/late streak stage, suggesting its critical roles during gastrulation (Gu et al. 1999; Mishina et al. 1999). It has been shown that BMP signaling mediated by ACVR1 also plays a critical role in establishment of right-side identity in early mouse development (Kishigami et al. 2004) (Kishigami et al. 2004; Komatsu et al. 2011), during cardiac and cartilage development (Dudas et al. 2004; Kaartinen et al. 2004) and bone homeostasis (Kamiya et al. 2011; Shi et al. 2018).
We previously reported generation of a conditional gain-of-function mouse model for ACVR1 using a constitutively activated form of ACVR1 with a mutation in the GS box (Q207D) along with the Cre-loxP system to avoid the potential lethality caused by an overdose, and ectopic BMP signaling during embryogenesis (ca-Acvr1, line L35) (Hu et al. 2004; Fukuda et al. 2006). This mouse line exhibits embryonic lethality at the gastrulation stage when the transgene is activated in the epiblast, while develops heterotopic ossification (HO) when recombinant adenovirus that expresses Cre is injected to legs with cardiotoxin for tissue injury (Yu et al. 2008; Shimono et al. 2011; Pan et al. 2020). Meanwhile, an R206H mutation in ACVR1 was identified as a cause of Fibrodysplasia ossificans progressiva (OMIN #135100), which is a rare genetic disorder to develops HO (Shore et al. 2006).
Despite our intention to achieve ubiquitous expression of the transgene upon Cre-recombination, an expression of the line L35 is somehow restricted and augmentation of signaling levels is within several folds (Fukuda et al. 2006; Yang et al. 2021). Here, we employed a different approach to screen mouse embryonic stem (ES) cells to select clones that express high levels of the transgenic construct. Resulted mouse line showed, however, very limited expression levels of the transgene, but demonstrated distinctive phenotypes from ones developed in line L35. The conditional gain-of-function system described here provides additional resources to dissect highly context dependent functions of BMP signaling.
Results and Discussion
To generate conditional constitutively active (ca) Acvr1 transgenic mice, we used CAG-Z-EGFP vector that is the same transgenic construct previously used to generate a transgenic line through pronuclei injection (line L35) (Fig. 1A) (Fukuda et al. 2005; Fukuda et al. 2006). A CAG promoter was used for ubiquitous expression and a lacZ with a nuclear translocation signal was used for detection of transgene expression. The lacZ cassette followed by a triple polyA signal site was floxed to allow Cre-dependent production of ACVR1 (aka Activin receptor like kinase 2, ALK2) with a Q207D gain-of-function mutation. An IRES-EGFP cassette was used to monitor Cre-dependent activation of the transgene. Here, we took an advantage of ES transgenesis to screen ES cell clones that highly express the transgene before generation of chimeric mice. The linearized transgenic vector was electroporated to AB2.2 ES cells with a Pgk-neo-bpA selection cassette and G418 resistant colonies were picked up to detect their beta-galactosidase activity (Fig 1B, C). We selected two clones (A11 and D9) that showed strong X-gal staining and injected to blastocysts. Both clones underwent germline, however, unexpectedly, neither line showed any X-gal staining. Because the line A11 developed craniofacial phenotypes as described below, we further analyzed line A11. In this report, we also compared phenotypes with line L35 ca-Acvr1 transgenic mouse line we generated some years ago through pronuclei injection using the same transgenic vector (Fukuda et al. 2006).
FIGURE 1.
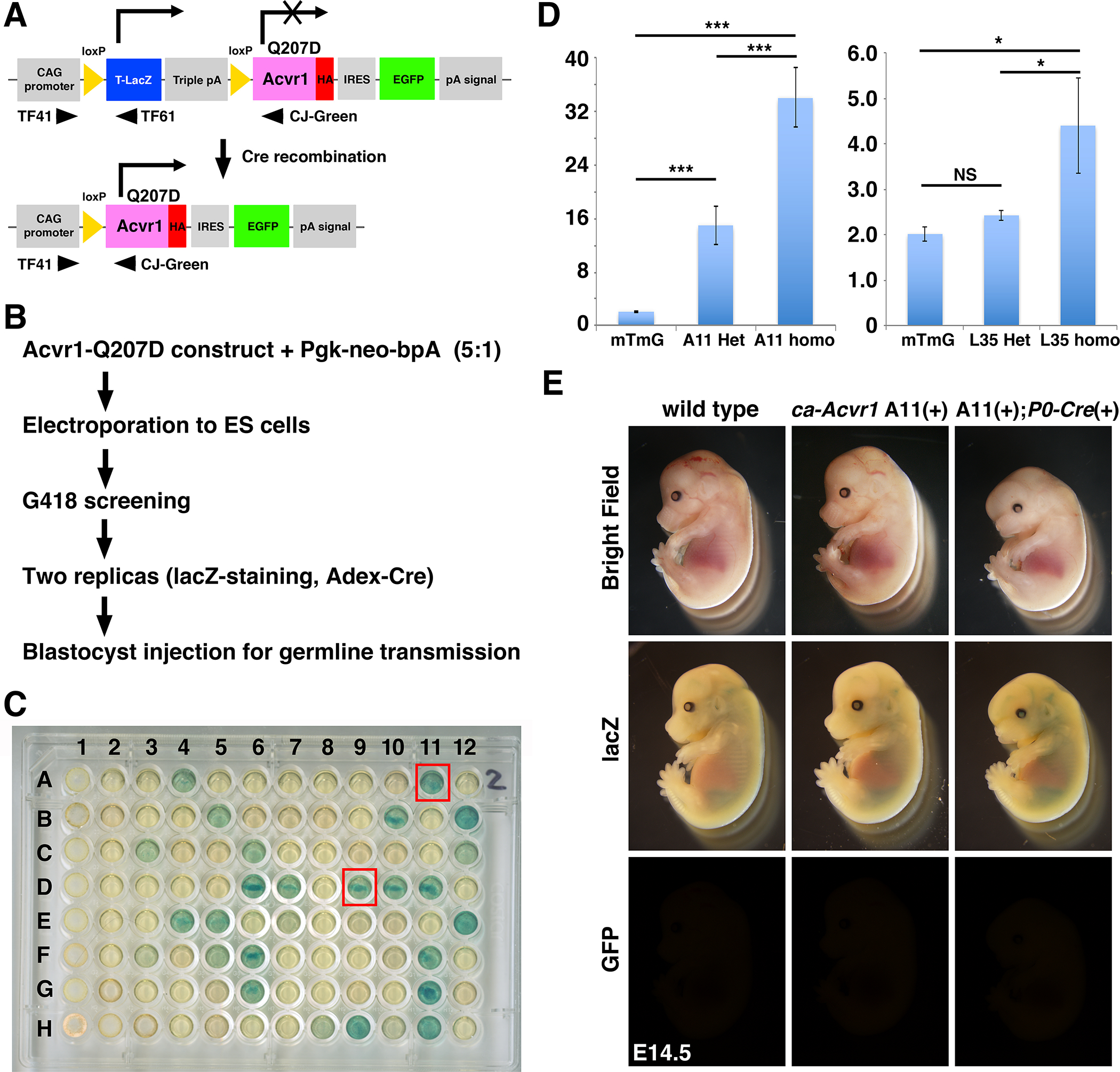
Generation of constitutively activated ACVR1 mouse line and initial characterization. A: A concept of the transgenic construct. Human ACVR1 cDNA with the Q207D mutation was ligated with IRES-EGFP and also ligated with the CAG promoter but intercepted by a floxed lacZ cassette with triple polyA sites. Cells with this transgenic construct are expected to generate beta-galactosidase from the lacZ cassette. Cre-dependent DNA recombination removed triple polyA sites to generate ACVR1 with Q207D protein (constitutively activated ACVR1, ca-ACVR1) and to produce GFP fluorescence. Approximate positions of three PCR primers for genotyping are shown. Sequence information of those primers are shown in Supplemental Table 1. B: Screening strategy. The linearized transgenic construct was electroporated into ES cells with a Pgk-neo selection vector. Ninety eight G418-resistance clones were picked up and examined their beta-galactosidase activity and capability to recombination capability by Cre. C: lacZ staining results. Replicated cells were stained with X-gal. Two clones that showed strong signal, A11 and D9, were further propagated and injected to blastocysts for germline transmission. D: Quantitative genomic PCR for copy number estimation. Homozygous mice for mTmG are used as a control (1 copy of EGFP per haploid). n=3. Sequence information of TaqMan primers are shown in Supplementary Table 2. E: Initial characterization of ca-Acvr1 line A11. After breeding with P0-Cre mice, embryos were harvested at E14.5. After observation of EGFP, embryos were stained with X-gal. Littermates who did not have both transgenes were used as wild type.
First, we quantified the copy number of the transgene in line A11 ca-Acvr1 transgenic mice using genomic quantitative real-time PCR. Both lines were maintained for several years in our colony and bred more than 20 generations, suggesting that each mouse line has one integrated locus per haploid. We used homozygous mT/mG mice (Muzumdar et al. 2007) as a standard (2 copies per animal) to quantify copy number of Egfp. Results showed that heterozygous mice have 16 copies per animal while homozygous mice have 32 copies per animal suggesting that 16 copies of the transgenic cassette were integrated into one locus in the genome (Fig. 1D). Similar analyses were done for line L35 suggesting that 2 copies of the transgenic cassette were integrated per locus in this line (Fig. 1D). The mice used for quantitative PCR were subsequently bred with wild type mates to genetically confirm their genotypes as either heterozygous or homozygous for the transgene (data not shown). Homozygous mice either for line L35 or line A11 caAcvr1 mice are normal, healthy and fertile with a normal litter size.
We next bred the line A11 mice with P0-Cre transgenic mice that expresses Cre in a neural crest-specific manner (Yamauchi et al. 1999; Chen et al. 2017; Suzawa et al. 2020). Unlike expected embryos that carry the ca-Acvr1 transgenic construct were negative for beta-galactosidase activity (Fig. 1E, middle row). Embryos that carry both the transgenic construct and P0-Cre transgene were negative for green fluorescence either (Fig 1E, bottom row). These results suggest that even when we selected ES cell clones that highly expressed beta-galactosidase, expression of the transgene was down regulated during ES transgenesis. However, embryos carrying both ca-Acvr1 and P0-Cre transgenes showed abnormal craniofacial development (Fig. 1E, right column) suggesting that very limited expression of ca-Acvr1 from the transgenic locus is sufficient to increase BMP signaling leading to developmental abnormalities.
We then set up timed mating to follow morphological changes of line A11 ca-Acvr1 embryos along with line L35 ca-Acvr1 embryos. For line L35 caAcvr1 mice bred with P0-Cre mouse line (caAcvr1(+):P0-Cre(+), L35 mut hereafter), they were relatively normal while showing a smaller nasal processes at embryonic day 10.5 (E10.5) (Fig. 2A). The deformed maxillary and nasal regions were observed as early as at E13.5 (Fig. 2A, middle row). A bulge-like structure in the forehead became obvious from E15.5. The mutant embryos also developed deformed lips. None of the L35 mut was able to survive after birth (n>40). For line A11 caAcvr1 mice bred with P0-Cre mouse line, (caAcvr1(+):P0-Cre(+), A11 mut hereafter), there were no overt morphological changes at E10.5 except showing a smaller nasal processes (Fig. 2A). Like L35 mut, hypomorphic facial processes were recognized as early as at E13.5 (Fig. 2A, bottom row). However, contrasting to L35 mut, the size of the frontal region of A11 mut is comparable with that of control (E17.5 lateral view). Approximately 90% of the A11 mut was not able to survive after birth (n>40).
FIGURE 2.
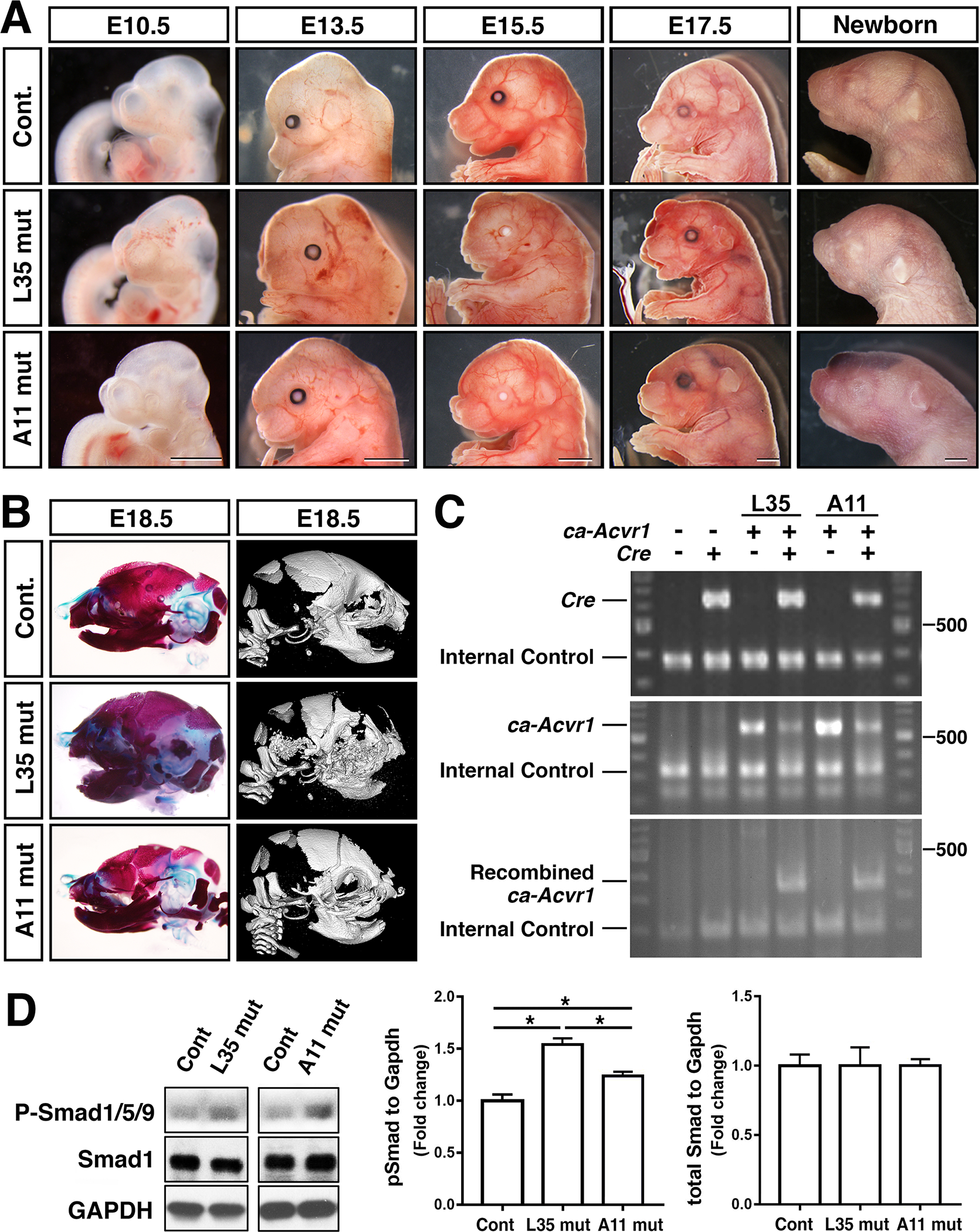
Neural-crest specific augmentation of BMP signaling via ACVR1-Q207D develops distinctive phenotypes in two transgenic lines. A: Lateral views of the head region of control and two transgenic lines during mid to late gestation, and newborn stages. Bar =1 mm. B: Assessment of skeletal abnormalities at E18.5 through Alcian blue/alizarin red staining (left) and microCT (right). C: Genomic PCR to confirm genotypes of each embryos. Primers used; Cre F and Cre R (top), TF41 and TF61 (middle), and TF41 and CJ-Green (bottom) to detect presence of Cre, ca-Acvr1, recombined ca-Acvr1, respectively. D: Protein lysates were prepared from E10.5 embryos and BMP-Smad levels were measured by western blots using antibodies against total Smad1 and pSmad1/5/9. Levels of signals were normalized by GAPDH. *, p<0.05. L35 mut, ca-Avcr1 line 35 embryos (Fukuda, et al., 2006) also carrying P0-Cre transgene. A11 mut, ca-Avcr1 line A11 (this study) embryos also carrying P0-Cre transgene.
Next, we examined morphological changes in cranial skeletogenesis. Alcian blue - alizarin red staining as well as microCT images revealed that L35 mut developed a shorter skull and highly hypomorphic nasal bones and mandibles. Frontal bones and maxilla were hypo-mineralized (Fig. 2B). For A11 mut, overall skeletal structure was comparable to that of controls, but they developed shorter maxillae and mandibles (Fig. 2B).
Embryos carrying both the P0-Cre transgene and the caAcvr1 transgene showed Cre-dependent recombination evidenced by PCR amplification of the expected size of band (approx. 350-bp) for both line L35 and line A11 (Fig. 2C). Levels of BMP signaling activity was measured by quantification of phospho-Smad1/5/9 (P-Smad1/5/9) using protein lysate prepared from frontal heads of E10.5 embryos including the nasal process and branchial arches. Both line L35 and A11 samples showed significantly higher levels of BMP-Smad signaling compared with controls, but L35 showed more robust augmentation (Fig. 2D). These facts may explain, at least in part, more severe craniofacial phenotypes developed in L35 mut depicted in Fig. 2.
To gain insight how these structure abnormalities developed, we looked at several stages of embryos. Histologic observation revealed that in L35 mut at E13.5 the palatal shelves were formed but smaller than those in controls (Fig. 3 top). In contract, A11 mut developed comparable palatal shelves at E13.5 with these in controls (Fig. 3 top). Tongue and Meckel’s cartilage developed normally at this stage in A11 mut (Fig. 3 top). At E15.5, the palatal shelves in A11 mut were shorter and remained unfused (Fig. 3, middle). Similar palatal structural abnormalities were observed at E17.5 along with a deformed tongue in A11 mut (Fig. 3, bottom). Palatal shelves in L35 remained small at E15.5 and 17. 5 and did not fuse (Fig 3, middle and bottom).
FIGURE 3.
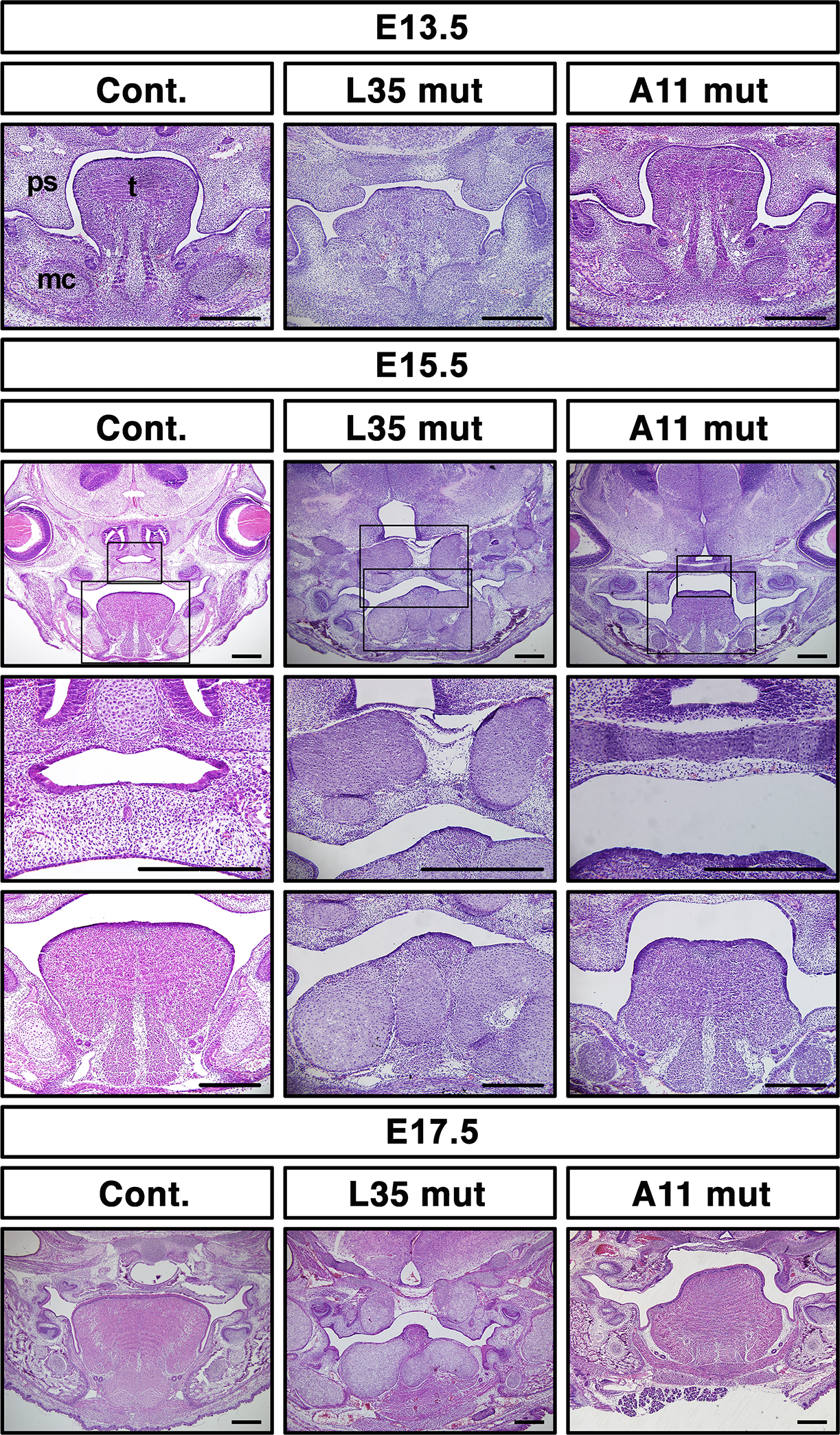
Abnormal palatogenesis in A11 mut embryos. Frontal sections at eye levels were made from control and two mutant lines of embryos at the indicated stages and stained with hematoxylin and eosin. For E15.5 samples, enlarged images for the palate and the tongue, respectively, are also shown. mc, Meckel’s cartilage, ps, palatal shelf, t, tongue. Bar = 200 μm.
Histologic observation of L35 mut revealed abnormal tissue condensations in the tongue and surrounding tissues (Fig. 3, middle column) indicating formation of ectopic cartilage as we recently reported (Yang et al. 2021). We stained the tissues with Safranin O with fast green to confirm formation of ectopic cartilage in L35 mut while the Meckel’s cartilage was hypomorphic (Fig. 4A, B, middle column). In contrast, no ectopic cartilage was identified in A11 mut embryos (Fig. 4A, B, right column). Whole mount cartilage staining at E17.5 confirms this notion, i.e. massive ectopic cartilage was developed in L35 mut embryos with undistinctive Meckel’s cartilage, while abnormal shaped nasal cartilage and shorter Meckel’s cartilage were identified in A11 mut embryos (Fig. 4C).
FIGURE 4.
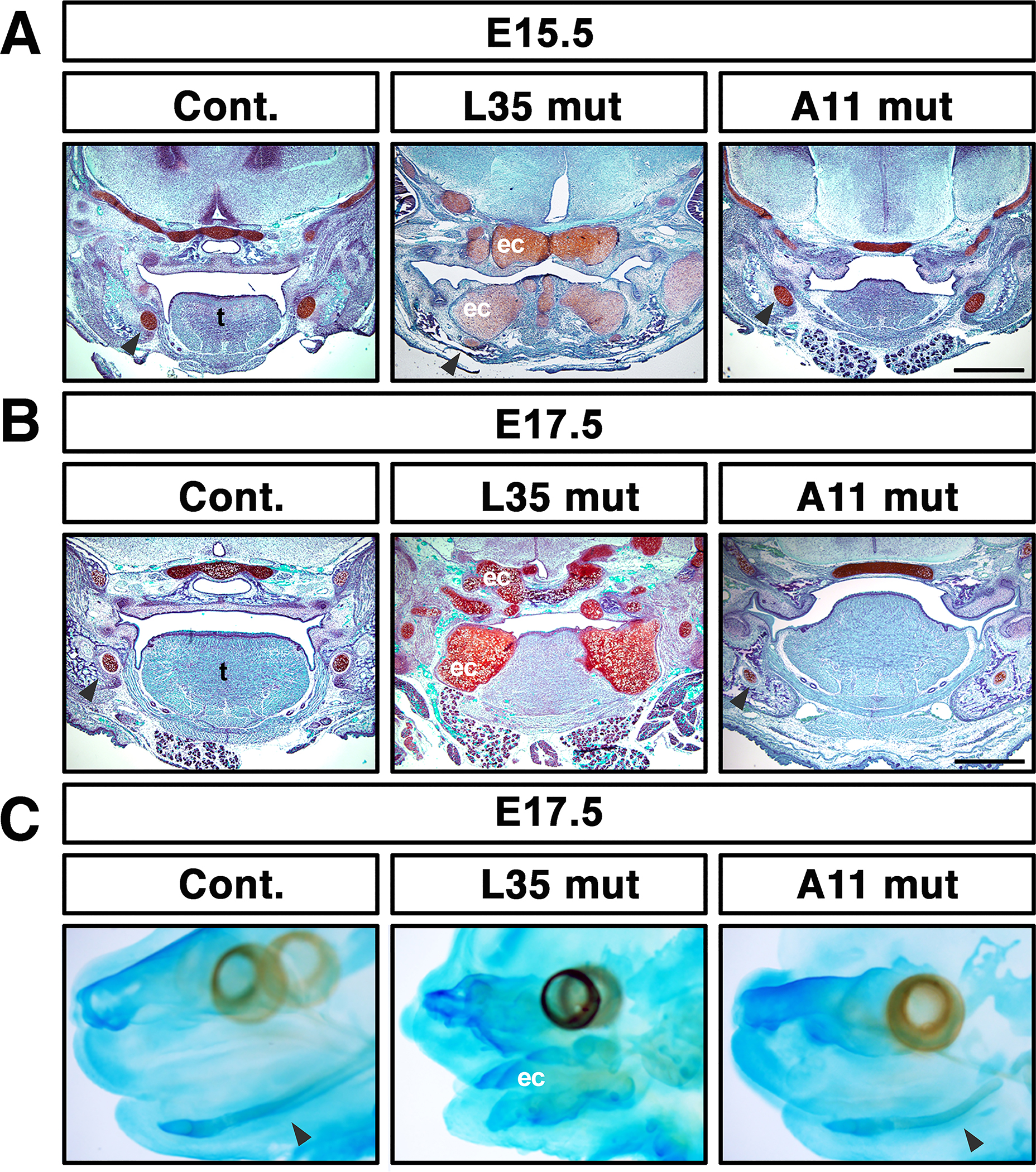
Ectopic cartilage formation in L35 mut embryos, but not in A11 mut embryos. A-B: Frontal sections at eye levels were made and stained with safranin O (red) and counterstained with Fast Green (blue) at E15.5 or E17.5. Formation of massive ectopic cartilage is visualized in L35 mut embryos. Bar = 500 μm. C: Whole mount alcian blue staining at E17.5. Arrowheads, Meckel’s cartilage, ec, ectopic cartilage, t, tongue.
Because BMP signaling play an important role in cardiac neural crest cells as we and others reported that conditional disruption of type 1 BMP receptors in neural crest cells results in defects in outflow tracts and volve function(Kaartinen et al. 2004; Stottmann et al. 2004; Morikawa et al. 2009; Nomura-Kitabayashi et al. 2009), we sought potential phenotypes in cardiac tissues in these 2 mutant lines. Histologic observation at E14.5 revealed that abnormally shaped superior atrioventricular cushions (SAC) in one of five A11 mut embryos (Fig. 5 O, P). L35 mut embryos showed comparable morphology with control embryos (Fig. 5 A–H, n=3 for each). SAC is known to be neural crest origin (Jiang et al. 2000), thus it is likely this abnormality is caused by increased BMP signaling in this tissue.
FIGURE 5.
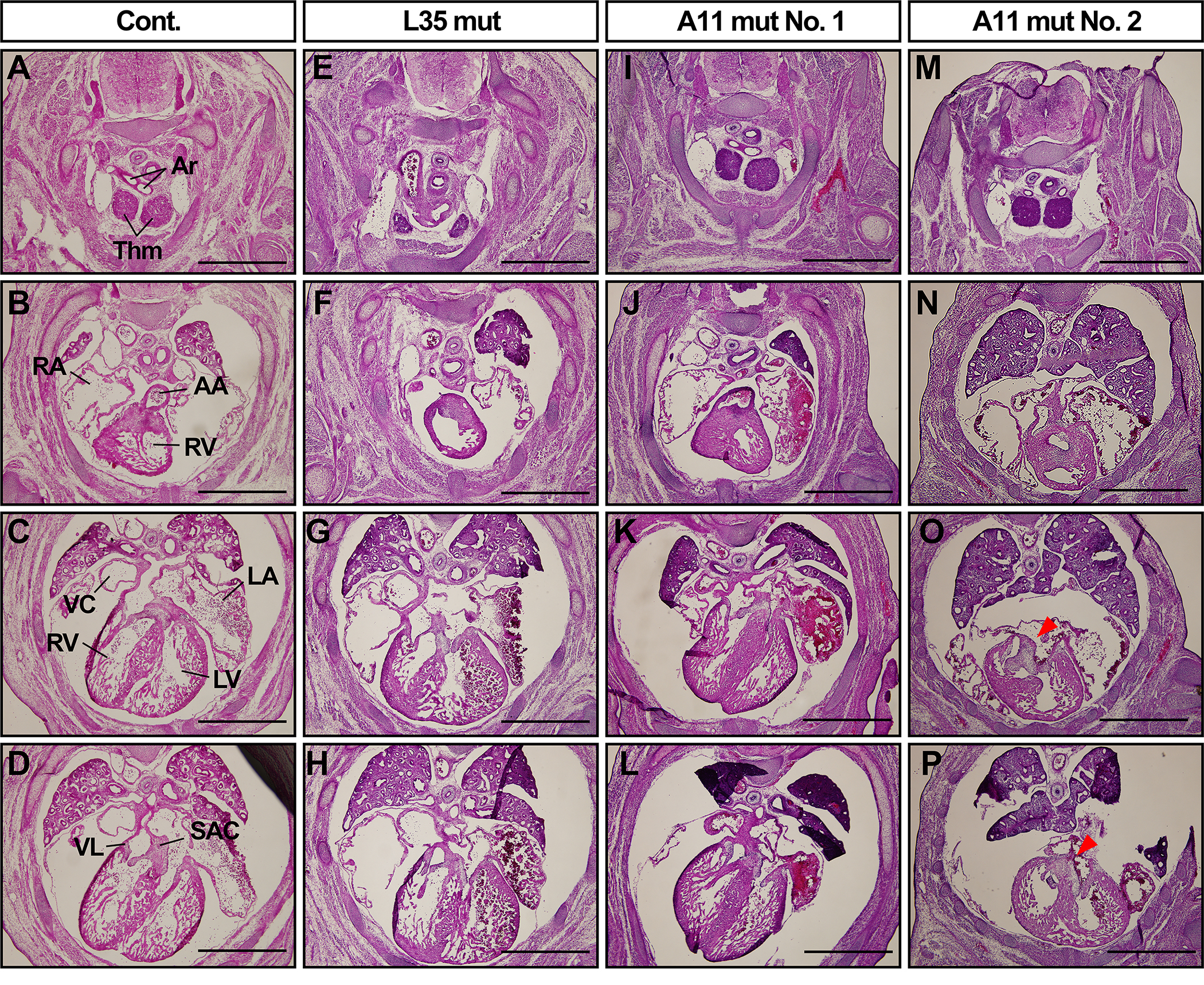
Abnormal cardiac development in A11 mut embryos but not in L35 mut embryos. Serial transverse sections for the cardiac part of control and two mutant lines of embryos at E14.5 were prepared and stained with hematoxylin and eosin. N=3 for controls and L35 mut, and n=5 for A11 mut. Two A11 mut embryos are shown. Arrowheads, an abnormal cushion tissue in A11 mut embryo. Ar, artery, Thm, thymus, AA, ascending aorta, RA, right atrium, RV, right ventricle, LA, left atrium, LV, left ventricle, VC, vena cava, VL, valve leaflet, SAC, superior atrioventricular cushion tissue. Bar = 1 mm.
Next, we bred both lines with Prrx1-Cre transgenic line (Logan et al. 2002) to evaluate a potential difference of the two lines in limb mesenchyme. At E10.5, formation of both forelimb buds and hindlimb buds in A11 mutant embryos were comparable with these in littermate controls (Fig. 6A, C), while nearly no signs of forelimb buds were identified in L35 mutant embryos (Fig. 6B). At E14.5, control embryos established distinctive limb structures (Fig. 6E1, autopod (a), zeugopod (b), stylopod (c) and scapula (d) for forelimbs, and autopod (e), zeugopod (f), and stylopod (g) for hindlimbs. In L35 mutant embryos, cartilage primordia were highly disorganized in both types of limbs with a greater degree in forelimb buds (Fig. 6F, G, G1). At E15.5, L35 mut embryos showed very little mineralization in the appendicular bones and became necrotic by E17.5 (Supplementary Fig. 1). In A11 mutant embryos, cartilage primordia were relatively normal at E14.5 (Fig. 6H, I, I1). A11 mutants were able to survive postnatally, however, skeletal components in arms and legs were significantly smaller than those in littermate controls at postnatal day 20 (PN20, Fig. 6J, K). Because body sizes of A11 mutant mice were smaller, we normalized the length of each segment of appendicular bones by the length of spine (from cervical 1 to sacral 4) and compared between genotypes. The zeugopods, stylopods and scapulae of A11 mutant mice were unproportionally shorter than those in controls while the autopods were less affected (Fig. 6 L, M).
FIGURE 6.
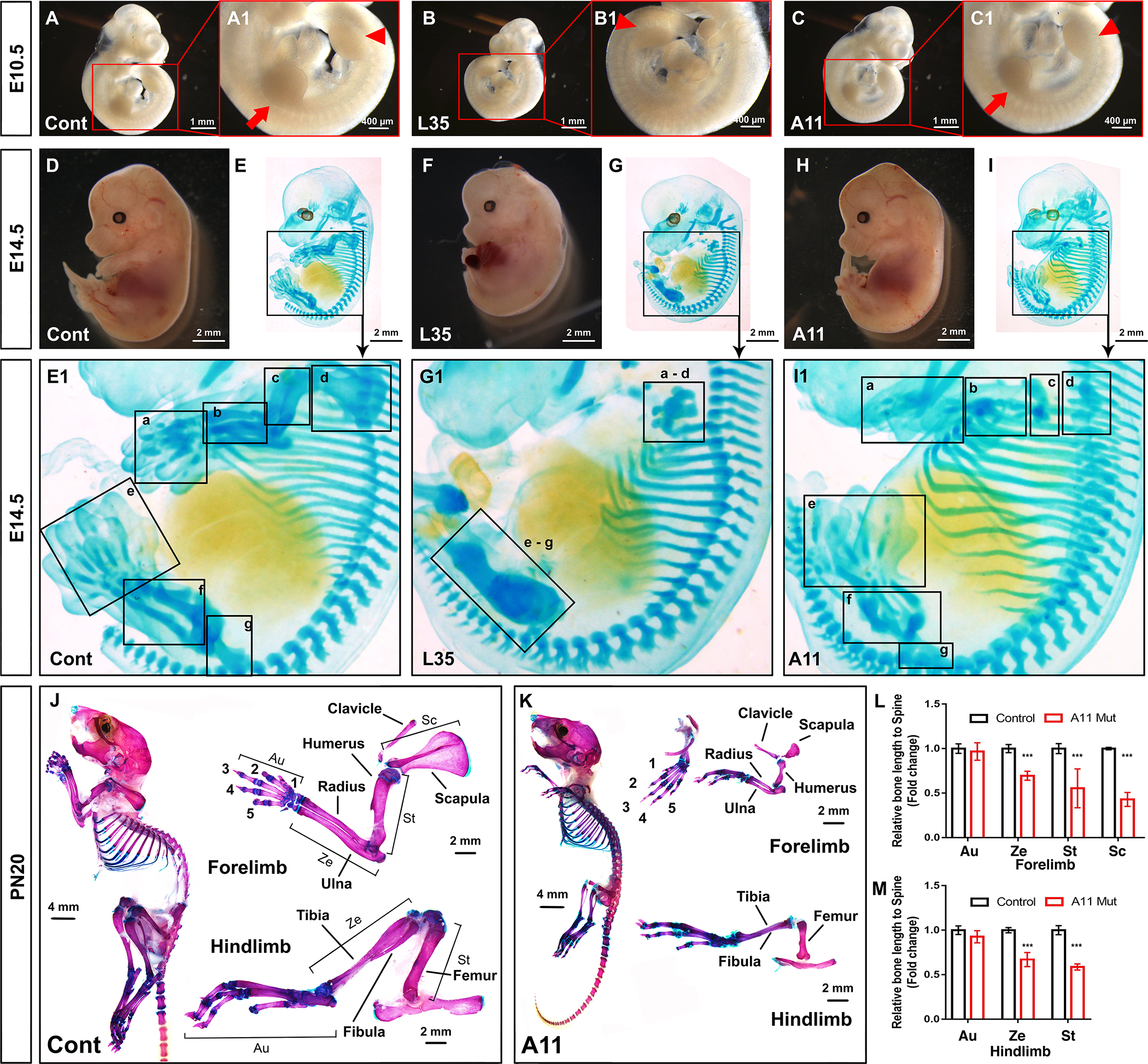
Different limb phenotypes in L35 and A11 mouse lines driven by Prrx1-Cre. Both line L35 and A11 were bred with the Prrx1-Cre mouse line and embryos were harvested at the stages indicated. Skeletal patterns in limbs were visualized either by whole mount cartilage staining (E14.5) or Alcian blue/alizarin red staining (PN20). Bar = 400 μm (A1, B1, C1), 1 mm (A, B, C), or 2 mm (D-I, J-K). For PN20, the length of each segment of limbs are normalized by length of the spine (from cervical 1 to sacral 4) and compared between two genotypes (6L, M). Au, autopod, Ze, zeugopod, St, stylopod, Sc, scapula. N=3. ***, p<0.001.
The line L35 caAcvr1 mice have been used as the first genetic mouse model for heterotopic ossification (HO) (Yu et al. 2008; Shimono et al. 2011; Agarwal et al. 2016; Wang et al. 2016; Valer et al. 2019). Standard protocol for HO formation is to locally inject recombinant adenovirus expressing Cre recombinase to increase BMP signaling along with cardiotoxin for local tissue injury (Pan et al. 2020), however, this protocol failed to induce HO in line A11 mice (data not shown). Recently, we reported that breeding with NFATc1-Cre mice develop HO without trauma or tissue injury (Agarwal et al. 2015). At PN10, L35 mice carrying NFATc1-Cre transgene developed HO around the ankle joint as expected (Fig. 7B, B1), while there is no sign of HO formation in A11 mice carrying NFATc1-Cre transgene at PN7 (data not shown) or PN10 (Fig. 7C, C1). We also injected cardiotoxin locally into A11 mice carrying NFATc1-Cre transgene at PN3, 6 and 9 to induce tissue injury expecting to prompt HO formation, however, there was no signs of HO at PN17 (Fig. 7D, E).
FIGURE 7.
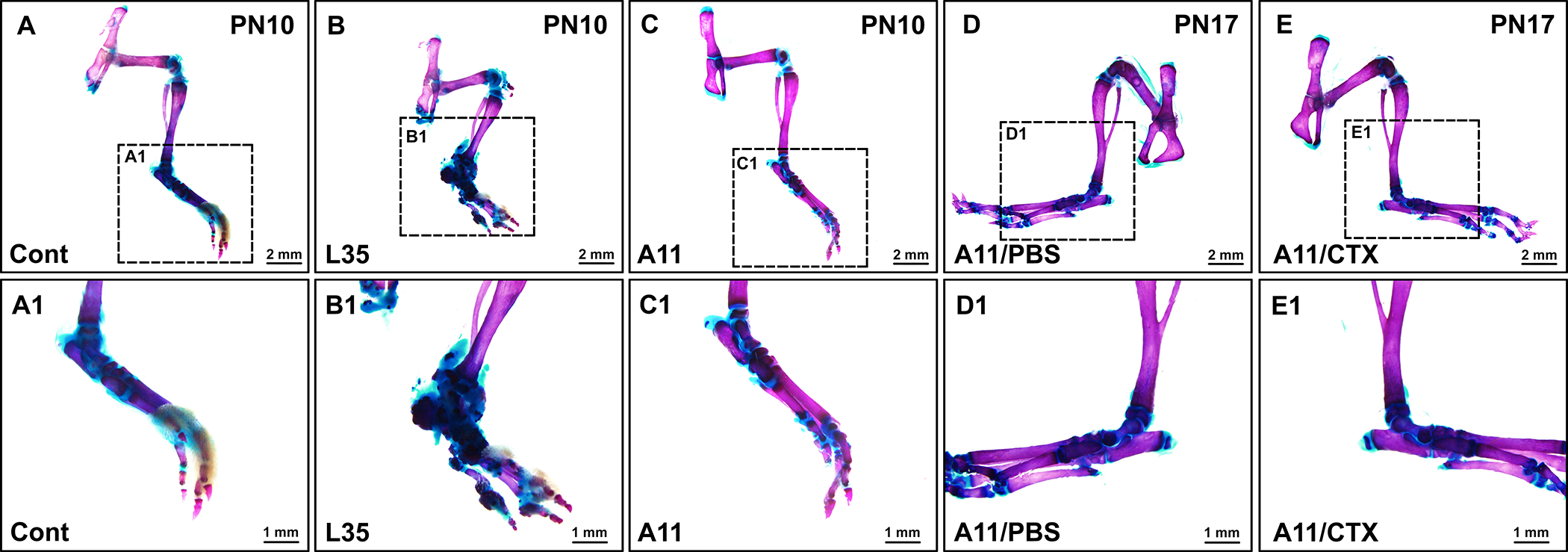
Line A11 does not generate heterotopic bones. Both line L35 and A11 were bred with the NFATc1-Cre mouse line and formation of heterotopic ossification was assessed at PN10. A11 mice also carrying the NFATc1-Cre transgene were treated with cardiotoxin (CTX) to induce tissue injury and formation of heterotopic ossification was assessed at PN17. Bar = 2 mm (A-E) or 1 mm (A1-E1).
In conclusion, the newly generated ca-Acvr1 line A11 model offers an additional model to understand context-dependent function of BMP-Smad signaling during embryogenesis and after birth. Conditional activation of BMP-Smad signaling can avoid early embryonic lethality caused by ubiquitously increased BMP signaling (Hu et al. 2004; Fukuda et al. 2006). By crossing with three different Cre lines, line A11 mutants show distinct phenotypes from line L35, which we previously reported (Fukuda et al. 2006). There would be several explanations for distinct phenotypes between the two lines generated using the same construct. One is the levels of BMP-Smad signaling. Despite higher copy number of the transgene in line A11, levels of Smad signaling after Cre recombination is lower than that of L35 as shown in Figure 2D. Another speculation is the difference in cell types that express the transgene. Despite our attempts to achieve ubiquitous and strong expression of the transgenes by employing the CAG promoter, expression levels of the transgene are low and restricted for L35 (Fukuda et al. 2006) or under detection for A11. We speculate that the transgene in line L35 is expressed in cells including progenitor and/or multi-potent population such as neural crest cells and fibro adipogenic progenitor cells, and thus ectopic cartilage and ectopic bones are developed after Cre recombination (Yu et al. 2008; Shimono et al. 2011; Agarwal et al. 2016; Wang et al. 2016; Valer et al. 2019; Yang et al. 2021). In contrast, the transgene in line A11 may be expressed in committed and or more differentiated cells.
For the potential molecular mechanisms of ectopic cartilage formation found in L35 mut embryos, we recently demonstrated that increased BMP signaling suppresses autophagic activity to lower degradation of β-catenin (Yang et al. 2021). Unlike progenitor cells in limb mesenchyme, reduction in Wnt canonical signaling prompt chondrogenic differentiation of cranial neural crest cells (Yang et al. 2021). Based on the timing of a requirement of Wnt signaling, we speculate that formation of ectopic cartilage is due to aberrant cell fate specification of multi-potent population rather than prompting chondrogenesis after lineage commitment (Yang et al. 2021). Taken together with the data that we did not see formation of HO in A11 mut but unproportionally shorter bones in limb, the newly developed line A11 would be suitable to investigate functions of BMP signaling in committed cells for their proliferation, differentiation, migration and/or cell death.
We previously generated conditional constitutively activated Bmpr1a mice, which generate BMP type 1A receptor with a Q233G mutation, using the same transgenic vector (Komatsu et al. 2013). When crossed with P0-Cre mice, ca-Bmpr1a mice develop skull deformity due to the premature fusion of cranial sutures while the mutant mice can survive over one year (Komatsu et al. 2013; Hayano et al. 2015; Pan et al. 2017). Another neural crest conditional ca-Bmpr1a mice developed by another group displays ectopic cartilage formation in craniofacial region along with perinatal lethality (Li et al. 2013). It would be an interesting future endeavor to examine the levels and patterns of phospho-Smad1/5/9 among those gain-of-function mutant mice for BMP-Smad signaling to understand highly context-dependent BMP functions.
Materials & Methods
Vector Construction
Construction of the transgenic basic vector, CAG-Z-EGFP, was described previously (Fukuda et al. 2006). In brief, a 0.9kb fragment of the neo cassette that contains a triple poly A signal and a 4.0-kb fragment of NTR-lacZ cassette were inserted into the EcoRV site of CAG-LITMUS28 (CAG-lacZ-LITMUS28) and a 2.5-kb fragment of IRES-EGFP cassette was further inserted to CAG-lacZ-LITMUS28. The cDNA fragment of a gene of interest can be inserted into PstI site of CAG-Z-EGFP (Fukuda et al. 2006). The plasmid that contains a constitutively active form of human ACVR1 (ACVR1-Q207D, ca-AVCR1) having an HAtag at the C-terminus was kindly obtained from Dr. Takashi Imamura (Cancer Institute of Japan, Tokyo). This protein tag will allow specific detection of the transgene product at the protein level (Noda et al. 2016). A 1.6-kb EcoRI-XbaI fragment of ca-ACVR1 was inserted into PstI site of CAG-Z-EGFP (CAG-Z-EGFP-ca-Acvr1) (Fig. 1A). All ligation reactions were carried out by blunt end ligation with modification after blunt-ended reactions with T4 DNA polymerase. A single nucleotide overhang was added with Taq DNA polymerase to increase the efficiency of ligation (Marchuk et al. 1991).
Transgenesis and Mouse Breeding
One μg of the transgenic vector was linearized with AflII and electroporated into 1.0 × 10^7 AB2.2 ES cells (Lexicon Genetics) along with linearized 0.2 μg of Pgk-neo-bpA (Fig. 1A, B). Ninety-Six G418 resistant colonies were picked up, two replica plates were made and one was stained with X-gal for beta-galactosidase activity (Fig. 1C). The other replica plates were treated with recombinant Adenovirus expressing Cre to confirm these cells were capable for Cre-dependent DNA recombination (data not shown). Two clones (A11 and D9) that showed strong beta-galactosidase activity were picked up, propagated and injected to blastocysts obtained from C57BL6/J. The resulting chimeras were bred to C57BL6/J females and F1 agouti off spring were genotyped by PCR as described below. Both clones underwent germline and we focused on founders generated from clone A11 (line A11) since this line developed craniofacial phenotypes as described in the result section.
Following transgenic mouse strains were bred with ca-Acvr1 line A11 mice to assess tissue-specific outcomes of augmented BMP signaling activity; P0-Cre (C57BL/6J-Tg(P0-Cre)94Imeg (ID 148)), Prrx1-Cre, (Tg(Prrx1-cre)1Cjt), and NFATc1-Cre (Ntatc1tm1.1(cre)Bz) (Yamauchi et al. 1999; Logan et al. 2002; Wu et al. 2012). For tissue injury, 0.9 μg of cardiotoxin (L8102, Latoxan) dissolved in 10 μL of phosphate buffered saline was injected per leg.
All mouse experiments were performed in accordance with institutional guidelines covering the humane care and use of animals in research. The animal protocols were approved by the Institutional Animal Care and Use Committees at the National Institute of Environmental Health Sciences (while TF, YK and YM were affiliated) and the University of Michigan. The authors are current exploring possibilities to deposit the newly developed mice to public repositories and are happy to share them upon request.
Genomic PCR and genomic Q-PCR
Genotypes were determined by genomic PCR. Sequences for each primer were shown in Supplementary Table 1. For the Cre transgene, we used Cre F and Cre R of which target size is 696-bp, and for the ca-Acvr1 transgene we used TF41 and TF61 of which target size is 580-bp (Fig. 1A). CJ-Green was used along with TF41 to detect Cre induced DNA recombination (the target size; approximately 350-bp). Primers for the endogenous genes were used as internal controls as follow; Alk2/Acvr1 locus for Cre: A2–5 and A2–3 (the target size is 330-bp), Evc2/Limbin locus for caAlk2: LbnFR1 and LbnRev3 (the target size; 334 bp), and Tak1 locus for Cre-recombined ca-Acvr1: TAK1 G1 and TAK1 G2 (the target size is 200-bp) (Zhang et al. 2015; Liu et al. 2018). Genomic real-time quantitative PCR was performed using TaqMan Gene Expression Assays to quantify copy numbers of the Egfp cassette with a custom designed TaqMan primer set (Supplemental Table 2). A TaqMan primer set for Gapdh (Mm99999915_g1, cat# 4351368) was used for an internal control. Homozygous mice for mT/mG Cre reporter mice (Gt(ROSA)26Sortm4(ACTB-tdTomato, -EGFP)Luo) were used as a reference (2 copies per animal) (Muzumdar et al. 2007).
Micro Computed Tomography (μCT) Evaluation
Whole heads from E18.5 embryos were dissected, fixed with 70 % ethanol, and then scanned using a μCT system (μCT100 Scanco Medical, Bassersdorf, Switzerland). Scan settings were voxel size 10 μm, medium resolution, energy 70 kV, intensity 114 μA, 0.5 mm AL filter, and integration time 500 μs. Analysis was performed using the manufacturer’s software. 3D models of embryos were reconstructed through MicroView (http://www.parallax-innovations.com/microview.html).
Histology, cartilage staining and skeletal staining
Embryos were fixed in 4% paraformaldehyde (PFA), embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E) or Safranin O & Fast Green according to a standard procedure. For cardiac observations, embryos at E14.5 were immersed in 30 % sucrose in phosphate buffered saline (PBS) after PFA fixation, then embedded in optimal cutting temperature (OCT) compound (Fisher Healthcare). Serial transverse sections were prepared with 10 μm thickness subjected for H&E staining. For whole cartilage staining, embryos were fixed with Bouin’s solution, stained with alcian blue and cleared with 1:1 mixture of benzyl benzoate and benzyl alcohol (Jegalian and De Robertis 1992). For skeletal staining, skinned pups were stained with alcian blue and alizarin red (Hayano et al. 2015).
Western blot analyses
For protein extraction, harvested embryonic tissues were washed with PBS containing a protease inhibitor cocktail (Roche) and homogenized in NP40 lysis buffer (20mM pH 8.0 Tris-HCl, 137mM NaCl, 1% NP40, 10% Glycerol, 1mM Na3VO4) using a Bio-Gen Pro200 homogenizer (Pro Scientific, Oxford, CT, USA). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot were carried out according to standard protocol. Antigen detection was performed using antibodies directed against total Samd1 (9743S, Cell signaling Technology), P-Smad1/5/9 (13820, Cell signaling Technology) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; 5174, Cell Signaling Technology). Bound primary antibodies were detected with horseradish peroxidase-conjugated species-specific secondary antibodies (Cell signaling Technology) and the immunoreactive bands were quantified using ImageJ. The mean ratios of the indicated protein from 3 independent experiments are shown on the right of the figures.
Statistical analyses
Morphometric measurements were made using ImageJ 1.50i (NIH) at defined anatomical landmarks. Statistical analyses were performed using Prism. All values were expressed as mean ± s.d. ANOVA were used to analyze the differences between or among groups. A P-value of less than 0.05 was considered statistically significant. All representative experiments shown were repeated 3 or more times. Experiments and analyses were performed in a blinded manner.
Supplementary Material
Supplementary Figure 1
Abnormal limb development in L35 mouse line bred with Prrx1-Cre line.
Acknowledgement
We thank Drs. Manas Ray and Greg Scott for initial stage of mouse breeding; Ms. Christine Joseph for excellent technical assistance; Drs. Kenichi Yamamura and Bin Zhao for mouse lines, and Drs. Vesa Kaartinen, Maiko Omi, and Kaitrin Kramar for critical reading and editing of this manuscript. This study is supported by the National Institutes of Health (R01DE020843 to YM, R01DE025897 to YK, R03DE027456 to HZ), International Fibrodysplasia Ossificans Progressiva Association (YM), and the grant-in-aid from the National Natural Science Foundation of China (31500788 to JY). The micro-CT core at the University of Michigan School of Dentistry is funded in part by NIH/ NCRR S10RR026475–01. The molecular biology core at the School of Dentistry is funded by NIH/P30AR069620. MT is funded by Fukuoka Dental College Foreign Exchange Program.
Footnotes
Data Availability
The data that supports the findings of this study are available in the supplementary material of this article.
References
- Agarwal S, Loder S, Brownley C, Cholok D, Mangiavini L, Li J, Breuler C, Sung HH, Li S, Ranganathan K et al. 2016. Inhibition of Hif1alpha prevents both trauma-induced and genetic heterotopic ossification. Proc Natl Acad Sci U S A 113: E338–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal S, Loder SJ, Brownley C, Eboda O, Peterson JR, Hayano S, Wu B, Zhao B, Kaartinen V, Wong VC et al. 2015. BMP signaling mediated by constitutively active Activin type 1 receptor (ACVR1) results in ectopic bone formation localized to distal extremity joints. Dev Biol 400: 202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagarova J, Vonner AJ, Armstrong KA, Borgermann J, Lai CS, Deng DY, Beppu H, Alfano I, Filippakopoulos P, Morrell NW et al. 2013. Constitutively active ALK2 receptor mutants require type II receptor cooperation. Mol Cell Biol 33: 2413–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazil DP, Church RH, Surae S, Godson C, Martin F. 2015. BMP signalling: agony and antagony in the family. Trends Cell Biol 25: 249–264. [DOI] [PubMed] [Google Scholar]
- Chen G, Ishan M, Yang J, Kishigami S, Fukuda T, Scott G, Ray MK, Sun C, Chen SY, Komatsu Y et al. 2017. Specific and spatial labeling of P0-Cre versus Wnt1-Cre in cranial neural crest in early mouse embryos. Genesis 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva Madaleno C, Jatzlau J, Knaus P. 2020. BMP signalling in a mechanical context - Implications for bone biology. Bone 137: 115416. [DOI] [PubMed] [Google Scholar]
- Dudas M, Sridurongrit S, Nagy A, Okazaki K, Kaartinen V. 2004. Craniofacial defects in mice lacking BMP type I receptor Alk2 in neural crest cells. Mech Dev 121: 173–182. [DOI] [PubMed] [Google Scholar]
- Fukuda T, Mishina Y, Walker MP, DiAugustine RP. 2005. Conditional transgenic system for mouse aurora a kinase: degradation by the ubiquitin proteasome pathway controls the level of the transgenic protein. Mol Cell Biol 25: 5270–5281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda T, Scott G, Komatsu Y, Araya R, Kawano M, Ray MK, Yamada M, Mishina Y. 2006. Generation of a mouse with conditionally activated signaling through the BMP receptor, ALK2. Genesis 44: 159–167. [DOI] [PubMed] [Google Scholar]
- Grafe I, Alexander S, Peterson JR, Snider TN, Levi B, Lee B, Mishina Y. 2018. TGF-beta Family Signaling in Mesenchymal Differentiation. Cold Spring Harb Perspect Biol 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z, Reynolds EM, Song J, Lei H, Feijen A, Yu L, He W, MacLaughlin DT, van den Eijnden-van Raaij J, Donahoe PK et al. 1999. The type I serine/threonine kinase receptor ActRIA (ALK2) is required for gastrulation of the mouse embryo. Development 126: 2551–2561. [DOI] [PubMed] [Google Scholar]
- Hayano S, Komatsu Y, Pan H, Mishina Y. 2015. Augmented BMP signaling in the neural crest inhibits nasal cartilage morphogenesis by inducing p53-mediated apoptosis. Development 142: 1357–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q, Ueno N, Behringer RR. 2004. Restriction of BMP4 activity domains in the developing neural tube of the mouse embryo. EMBO Rep 5: 734–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jegalian BG, De Robertis EM. 1992. Homeotic transformations in the mouse induced by overexpression of a human Hox3.3 transgene. Cell 71: 901–910. [DOI] [PubMed] [Google Scholar]
- Jiang X, Rowitch DH, Soriano P, McMahon AP, Sucov HM. 2000. Fate of the mammalian cardiac neural crest. Development 127: 1607–1616. [DOI] [PubMed] [Google Scholar]
- Kaartinen V, Dudas M, Nagy A, Sridurongrit S, Lu MM, Epstein JA. 2004. Cardiac outflow tract defects in mice lacking ALK2 in neural crest cells. Development 131: 3481–3490. [DOI] [PubMed] [Google Scholar]
- Kamiya N, Kaartinen VM, Mishina Y. 2011. Loss-of-function of ACVR1 in osteoblasts increases bone mass and activates canonical Wnt signaling through suppression of Wnt inhibitors SOST and DKK1. Biochem Biophys Res Commun 414: 326–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishigami S, Yoshikawa S, Castranio T, Okazaki K, Furuta Y, Mishina Y. 2004. BMP signaling through ACVRI is required for left-right patterning in the early mouse embryo. Dev Biol 276: 185–193. [DOI] [PubMed] [Google Scholar]
- Klingensmith J, Matsui M, Yang YP, Anderson RM. 2010. Roles of bone morphogenetic protein signaling and its antagonism in holoprosencephaly. Am J Med Genet C Semin Med Genet 154C: 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu Y, Kaartinen V, Mishina Y. 2011. Cell cycle arrest in node cells governs ciliogenesis at the node to break left-right symmetry. Development 138: 3915–3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu Y, Yu PB, Kamiya N, Pan H, Fukuda T, Scott GJ, Ray MK, Yamamura K, Mishina Y. 2013. Augmentation of Smad-dependent BMP signaling in neural crest cells causes craniosynostosis in mice. J Bone Miner Res 28: 1422–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Wang Y, Lin M, Yuan G, Yang G, Zheng Y, Chen Y. 2013. Augmented BMPRIA-mediated BMP signaling in cranial neural crest lineage leads to cleft palate formation and delayed tooth differentiation. PLoS One 8: e66107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Hayano S, Pan H, Inagaki M, Ninomiya-Tsuji J, Sun H, Mishina Y. 2018. Compound mutations in Bmpr1a and Tak1 synergize facial deformities via increased cell death. Genesis 56: e23093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan M, Martin JF, Nagy A, Lobe C, Olson EN, Tabin CJ. 2002. Expression of Cre Recombinase in the developing mouse limb bud driven by a Prxl enhancer. Genesis 33: 77–80. [DOI] [PubMed] [Google Scholar]
- Marchuk D, Drumm M, Saulino A, Collins FS. 1991. Construction of T-vectors, a rapid and general system for direct cloning of unmodified PCR products. Nucleic Acids Res 19: 1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishina Y, Crombie R, Bradley A, Behringer RR. 1999. Multiple roles for activin-like kinase-2 signaling during mouse embryogenesis. Dev Biol 213: 314–326. [DOI] [PubMed] [Google Scholar]
- Morikawa Y, Zehir A, Maska E, Deng C, Schneider MD, Mishina Y, Cserjesi P. 2009. BMP signaling regulates sympathetic nervous system development through Smad4-dependent and -independent pathways. Development 136: 3575–3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. 2007. A global double-fluorescent Cre reporter mouse. Genesis 45: 593–605. [DOI] [PubMed] [Google Scholar]
- Noda K, Mishina Y, Komatsu Y. 2016. Constitutively active mutation of ACVR1 in oral epithelium causes submucous cleft palate in mice. Dev Biol 415: 306–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura-Kitabayashi A, Phoon CK, Kishigami S, Rosenthal J, Yamauchi Y, Abe K, Yamamura K, Samtani R, Lo CW, Mishina Y. 2009. Outflow tract cushions perform a critical valve-like function in the early embryonic heart requiring BMPRIA-mediated signaling in cardiac neural crest. Am J Physiol Heart Circ Physiol 297: H1617–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H, Fleming N, Hong CC, Mishina Y, Perrien DS. 2020. Methods for the reliable induction of heterotopic ossification in the conditional Alk2(Q207D) mouse. J Musculoskelet Neuronal Interact 20: 149–159. [PMC free article] [PubMed] [Google Scholar]
- Pan H, Zhang H, Abraham P, Komatsu Y, Lyons K, Kaartinen V, Mishina Y. 2017. BmpR1A is a major type 1 BMP receptor for BMP-Smad signaling during skull development. Dev Biol 429: 260–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath TK, Reddi AH. 2020. Discovery of bone morphogenetic proteins - A historical perspective. Bone 140: 115548. [DOI] [PubMed] [Google Scholar]
- Sanchez-Duffhues G, Williams E, Goumans MJ, Heldin CH, Ten Dijke P. 2020. Bone morphogenetic protein receptors: Structure, function and targeting by selective small molecule kinase inhibitors. Bone 138: 115472. [DOI] [PubMed] [Google Scholar]
- Shi C, Mandair GS, Zhang H, Vanrenterghem GG, Ridella R, Takahashi A, Zhang Y, Kohn DH, Morris MD, Mishina Y et al. 2018. Bone morphogenetic protein signaling through ACVR1 and BMPR1A negatively regulates bone mass along with alterations in bone composition. J Struct Biol 201: 237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimono K, Tung WE, Macolino C, Chi AH, Didizian JH, Mundy C, Chandraratna RA, Mishina Y, Enomoto-Iwamoto M, Pacifici M et al. 2011. Potent inhibition of heterotopic ossification by nuclear retinoic acid receptor-gamma agonists. Nat Med 17: 454–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore EM, Xu M, Feldman GJ, Fenstermacher DA, Cho TJ, Choi IH, Connor JM, Delai P, Glaser DL, LeMerrer M et al. 2006. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat Genet 38: 525–527. [DOI] [PubMed] [Google Scholar]
- Stottmann RW, Choi M, Mishina Y, Meyers EN, Klingensmith J. 2004. BMP receptor IA is required in mammalian neural crest cells for development of the cardiac outflow tract and ventricular myocardium. Development 131: 2205–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzawa T, Yoshida H, Takahashi M, Itose M, Takimoto R, Sasama Y, Tanaka M, Ikezaki K, Shirora T, Maki K et al. 2020. Prospects of neural crest-derived cells from oral and dentofacial tissues for application in regenerative medicine. Oral Science International 17: 115–124. [Google Scholar]
- Valer JA, Sanchez-de-Diego C, Gamez B, Mishina Y, Rosa JL, Ventura F. 2019. Inhibition of phosphatidylinositol 3-kinase alpha (PI3Kalpha) prevents heterotopic ossification. EMBO Mol Med 11: e10567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Lindborg C, Lounev V, Kim JH, McCarrick-Walmsley R, Xu M, Mangiavini L, Groppe JC, Shore EM, Schipani E et al. 2016. Cellular Hypoxia Promotes Heterotopic Ossification by Amplifying BMP Signaling. J Bone Miner Res 31: 1652–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrana JL, Attisano L, Wieser R, Ventura F, Massague J. 1994. Mechanism of activation of the TGF-beta receptor. Nature 370: 341–347. [DOI] [PubMed] [Google Scholar]
- Wu B, Zhang Z, Lui W, Chen X, Wang Y, Chamberlain AA, Moreno-Rodriguez RA, Markwald RR, O’Rourke BP, Sharp DJ et al. 2012. Endocardial cells form the coronary arteries by angiogenesis through myocardial-endocardial VEGF signaling. Cell 151: 1083–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi Y, Abe K, Mantani A, Hitoshi Y, Suzuki M, Osuzu F, Kuratani S, Yamamura K. 1999. A novel transgenic technique that allows specific marking of the neural crest cell lineage in mice. Dev Biol 212: 191–203. [DOI] [PubMed] [Google Scholar]
- Yang J, Kitami M, Pan H, Nakamura MT, Zhang H, Liu F, Zhu L, Komatsu Y, Mishina Y. 2021. Augmented BMP signaling commits cranial neural crest cells to a chondrogenic fate by suppressing autophagic beta-catenin degradation. Sci Signal 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Mishina Y. 2019. Generation and Identification of Genetically Modified Mice for BMP Receptors. Methods Mol Biol 1891: 165–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Ueharu H, Mishina Y. 2020. Energy metabolism: A newly emerging target of BMP signaling in bone homeostasis. Bone 138: 115467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu PB, Deng DY, Lai CS, Hong CC, Cuny GD, Bouxsein ML, Hong DW, McManus PM, Katagiri T, Sachidanandan C et al. 2008. BMP type I receptor inhibition reduces heterotopic ossification. Nat Med 14: 1363–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Takeda H, Tsuji T, Kamiya N, Rajderkar S, Louie K, Collier C, Scott G, Ray M, Mochida Y et al. 2015. Generation of Evc2/Limbin global and conditional KO mice and its roles during mineralized tissue formation. Genesis 53: 612–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao GQ. 2003. Consequences of knocking out BMP signaling in the mouse. Genesis 35: 43–56. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1
Abnormal limb development in L35 mouse line bred with Prrx1-Cre line.


