Abstract
Vaccines against Japanese encephalitis (JE) have been available for decades. Currently, most JE-endemic countries have vaccination programs for their at-risk populations. Even so, JE remains the leading recognized cause of viral encephalitis in Asia. In 2018, the U.S. Centers for Disease Control and Prevention and PATH co-convened a group of independent experts to review JE prevention and control successes, identify remaining scientific and operational issues that need to be addressed, discuss opportunities to further strengthen JE vaccination programs, and identify strategies and solutions to ensure sustainability of JE control during the next decade. This paper summarizes the key discussion points and recommendations to sustain and expand JE control.
Subject terms: Infectious diseases, Public health
Introduction
Despite the availability of safe and effective vaccines against Japanese encephalitis (JE) for several decades (Table 1), JE virus (JEV) remains the leading recognized cause of viral encephalitis in Asia1. JEV is transmitted by mosquitoes and is sustained in an enzootic cycle with pigs and wading birds as amplifying hosts. Human cases typically occur in children and in rural areas where people live and work in proximity to elements in the transmission cycle, such as near rice fields or domestic pigs. There is a risk of JEV transmission in 24 countries in Asia and the Western Pacific, either nationally or in endemic areas2. While some regions have nearly eliminated JE through comprehensive immunization programs, the incidence in other areas remains high3.
Table 1.
Licensed Japanese encephalitis (JE) vaccines.
| Vaccine type | Substrate | Viral strains | WHO-prequalified manufacturers | WHO recommended vaccine schedule | Other manufacturers (domestic and traveler markets) |
|---|---|---|---|---|---|
| Inactivated | Mouse brain | Nakayama | n/a | n/a | Vietnam: Vabiotech |
| Vero | Beijing-1 | n/a | n/a | Japan: Biken (Research Foundation for Microbial Diseases of Osaka University), Kaketsuken (The Chemo-Sero-Therapeutic Research Institute, Korea: Boryung Pharmaceutical Co., Ltd (fill-finish of Kaketsuken vaccine) | |
| Vero | Beijing P-3 | n/a | n/a | China: Liaoning Chengda Biotechnology Co | |
| Vero | SA 14-14-2 | India: Biological E | Two doses 28 days apart, from 1 year of age | Austria: Valneva Austria GmbH | |
| Vero | Kolar strain (JEV 821564XY) | n/a | n/a | India: Bharat Biotech | |
| Live attenuated | Hamster kidney cells | SA 14-14-2 | China: Chengdu Institute of Biological Products | Single dose at ≥8 months of age | Wuhan Institute of Biological Products |
| Live chimeric | Vero | JE SA 14-14-2/yellow fever 17D | Thailand: Government Pharmaceutical Organization-Merieux Biological Products Co. | Single dose at ≥9 months of agea | n/a |
Adapted from Halstead et al.1.
aManufacturer recommends pediatric booster 1–2 years after the primary dose.
While most human JEV infections are asymptomatic, severe disease occurs in about 1 per 250 JEV infections1. JEV infection can rapidly progress to encephalitis, with an estimated case fatality rate of 20–30%4,5. Common symptoms include sudden onset of high fever, chills, headache, myalgias, mental confusion, and convulsions in pediatric patients6. Among survivors, about 30–50% have long-term neurologic sequelae with intellectual or physical disabilities7,8. Although there is no specific treatment for JE, supportive care improves outcome. JE burden estimates suffer from poor surveillance resulting from the difficulty of diagnosing JE without cerebrospinal fluid (CSF) samples and because cases generally occur in rural areas where surveillance and laboratory capacity might be limited.
The World Health Organization (WHO) has long recommended JE vaccination programs in areas where JE is a public health problem9. However, poor recognition of the burden of disease, prioritization of other vaccines and public health interventions over JE vaccination, and the high cost and multiple-dose regimen of the older, inactivated mouse brain-derived vaccine limited JE vaccine introduction. Since 2003, PATH and multiple stakeholder organizations have worked to expand the use of WHO-prequalified JE vaccines primarily through the support of Gavi, the Vaccine Alliance10. Substantial impact on JE-associated deaths and disability has been shown in countries administering JE vaccine in childhood vaccination schedules. For example, large vaccination campaigns targeting variable age ranges were implemented in 31 districts in Nepal from 2006 through 2011. JE incidence after the campaigns was 78% lower than the pre-campaign incidence, resulting in over 3000 JE cases estimated to have been prevented through 201411,12.
In 2018, the U.S. Centers for Disease Control and Prevention and PATH co-convened a group of independent experts to review JE prevention and control successes, identify remaining scientific and operational issues that need to be addressed, discuss opportunities to further strengthen JE vaccination programs, and identify strategies and solutions to ensure sustainability of JE control during the next decade. This paper summarizes the key discussion points and recommendations to sustain and expand JE control (Box 1).
Box 1. Key recommendations for optimal JE control beyond 2020.
Reliable JE Vaccine Supply
-
Optimize and ensure a reliable global supply of affordable JE vaccines
Global Coordination and Policy Optimization
Establish and support an international coalition of partners with responsibility for global coordination of JE control
Maintain dedicated opportunities for information exchange and discussion of emerging issues across country JE programs
-
Support advocacy efforts to ensure momentum for JE control is not lost
JE Surveillance, Vaccine Utilization, and Diagnostics
Provide technical support to countries for JE surveillance and vaccine monitoring (e.g., coverage surveys, immunization registries)
Support country efforts for surveillance and vaccine introduction, and quality assurance for laboratory testing
-
Monitor JEV geographic spread as land use and climate change might affect mosquito distribution
Other Country Programmatic Support
Troubleshoot vaccine supply issues
When possible, integrate surveillance and vaccination programs into other programs for efficiency and sustainability
Conduct community advocacy and communication for maximal vaccine acceptance
-
Provide guidance for the adverse event following immunization investigations of special interest for JE
Research Priorities
Monitor duration of protection following a single dose of live JE vaccines
Assess vaccine safety in pregnant and immunocompromised persons vaccinated with live JE vaccines
Standardize and validate plaque reduction neutralization test reagents and procedures
Ensure reliable supply of JE diagnostic kits
Facilitate the development of JE treatments
Estimate JE vaccine impact globally to highlight the success of JE vaccine programs and the need to sustain progress made
Study safety and immunogenicity of JE vaccine co-administration with any new vaccines that might be co-administered with JE vaccine
Achievements in JE vaccine development, prequalification, and policy advancement
The first inactivated mouse brain-derived vaccines were developed in the 1930s. Vaccine manufacturers in several Asian countries developed these vaccines primarily for domestic use. Studies of early JE vaccines have shown that human illnesses can be eliminated with high vaccination coverage even though JEV continues to circulate in the enzootic cycle. For example, in Japan, universal vaccination with inactivated mouse brain-derived vaccine virtually eliminated JE in the early 1990s12.
In the 1980s, virologists in China developed SA 14-14-2 (CD-JEV), a live attenuated JE vaccine grown on primary hamster kidney cells. This vaccine was introduced province by province and markedly reduced JE incidence in China13. Recognizing the potential impact with greater use of CD-JEV, in 2003, PATH partnered with the Chengdu Institute of Biological Products (CDIBP) to export CD-JEV internationally. CDIBP and PATH developed a new manufacturing facility compliant with good manufacturing practices (GMP) and greater production capacity, which opened in 2012. As part of this partnership, CDIBP also made CD-JEV available to low- and lower middle-income countries at a low public sector price to facilitate countries’ abilities to fund JE vaccine introduction.
Defining an immune correlate of protection (i.e., neutralizing antibody titer ≥1:10) in 2005 was a critical step in JE vaccine development14. In a landmark vaccine efficacy study of mouse brain-derived JE vaccine conducted in Thailand, more than 65,000 children were vaccinated15. Due to the high cost of clinical trials, any subsequent efficacy trial would be expensive and likely infeasible due to the need to vaccinate and clinically follow so many children. However, defining an immune correlate of protection allowed manufacturers to develop new JE vaccines for commercial use without large and logistically challenging vaccine efficacy trials. Several Asian and European manufacturers developed inactivated Vero cell culture-based JE vaccines that had a superior safety profile and were easier to manufacture than mouse brain vaccine, but, like earlier inactivated vaccines, still required multiple doses and periodic boosting to maintain immunity. Another manufacturer developed a recombinant chimeric vaccine (JE-CV) by inserting the E and prM genes of SA 14-14-2 JEV into a yellow fever 17D vaccine backbone1. Thus, in a few decades, the JE vaccine portfolio expanded to multiple products using a variety of vaccine technologies.
WHO prequalification is a process to validate a product’s quality, safety, and efficacy, and to ensure a manufacturer is compliant with WHO and international quality standards16. Prequalification is needed for vaccines procured through UNICEF, procured using Gavi support, or intended for use in WHO and other United Nations programs. Biological E. Ltd’s JEEV, a Vero cell culture-based vaccine, was prequalified in 2013, but initially was indicated for adults only. Later in 2013, CD-JEV became the first JE vaccine prequalified for children and the first Chinese vaccine to be WHO prequalified, an accomplishment that may help additional Chinese-manufactured vaccines enter the global markets.
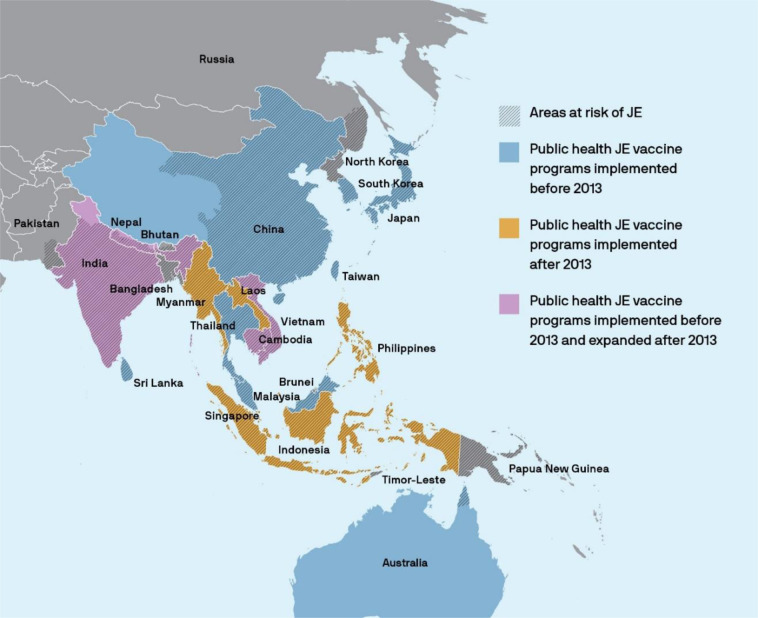
After WHO prequalification of these JE vaccines, Gavi committed to financing catch-up vaccination campaigns followed by routine vaccination within the childhood vaccination schedule, allowing Gavi-eligible countries to introduce JE vaccine. New vaccine introductions and expansions of JE vaccination programs were also enabled by the WHO recommendation that JE vaccine be introduced in all areas where JE is a public health priority, improved JE awareness at country and community level, government commitment, and financial and/or technical support by organizations such as WHO, PATH, Gavi, and the Bill and Melinda Gates Foundation3. Guidance and training materials were developed to support country decision-making and vaccine introduction as well as JE surveillance and measurement of vaccine impact17–19. With products available and funding mechanisms in place, accelerated JE control became one of eight immunization goals in the Regional Framework for Implementation of the Global Vaccine Action Plan in the Western Pacific, highlighting JE as a regional priority and defining operational targets for accelerated control of JE to be achieved by 203020. As of June 2020, 15 countries have national or subnational public health vaccination programs in JE-endemic areas. These include Australia (Outer Torres Strait Islands), Malaysia (Sarawak), Japan, Republic of Korea, Thailand, Cambodia, Lao PDR, Myanmar, Indonesia (Bali), Philippines (three high-incidence regions), China, India (about 40–50% of districts), Nepal, Sri Lanka, and Vietnam (Fig. 1).
Fig. 1. Areas with risk of Japanese encephalitis (JE) transmission and JE vaccine introduction relative to the WHO JE vaccine prequalification year (2013).
While some countries have implemented national public health JE vaccine programs, others may have only introduced in high risk areas.
Status of JE surveillance and control
JEV is a positive-stranded, enveloped RNA flavivirus. Because of low virus titers in infected humans and the neurotropism of JEV, the virus is rarely isolated from human cases. Therefore the gold standard for JE diagnosis is the presence of JE immunoglobulin M (IgM) antibody in the CSF1. However, as a disease that occurs predominantly in rural areas, the capacity for CSF collection, especially in children, is often limited and lumbar punctures are not always performed, especially in settings where treatment for encephalitis is limited or not available.
Access to diagnostics is important because JE cannot be clinically differentiated from other viral causes of acute central nervous system infection such as enterovirus 7121. Important progress in improving JE surveillance and defining disease burden came with the development of a range of JE diagnostic kits22–25. While three commercial JE IgM ELISA assays were previously available in JE-endemic countries, only the InBios JE DetectTM IgM antibody capture ELISA is still being manufactured. CSF can be tested with the InBios JE assay alone, but, because of cross reactivity, serum samples must also be tested with a dengue IgM assay and results interpreted according to a CDC-devised algorithm26.
Even when diagnostics are available, interpreting serum IgM results in areas with co-circulation of other flaviviruses can be complicated because antibodies against other flaviviruses often cross react with JEV. In addition, it is well-recognized that IgM following JEV infection can be detectable for months or years, so there is sometimes uncertainty when JE IgM is detected in serum whether it truly reflects JEV as the cause of a patient’s presentation or could be persisting IgM following a previous asymptomatic infection or mild febrile illness unrecognized as JE. A recent analysis of IgM following vaccination with CD-JEV in a clinical trial suggests serum IgM is common at 28 days following vaccination but rare at 6 months following vaccination (~40% vs. 3% of vaccinees with positive or equivocal test results by ELISA, respectively)27. While this finding suggests that serum IgM is unlikely to be related to vaccination administered at least six months prior, it does not negate the importance of CSF collection for proper JE diagnosis.
To improve and standardize JE diagnosis in JE-endemic countries, PATH worked with partners to establish WHO JE Laboratory Networks in WHO’s South-East Asia and Western Pacific Regions during 2006–2008. These networks have substantially increased the frequency and quality of JE laboratory testing and continue to provide support to countries for confirmatory testing, quality assurance, and other JE testing issues28.
Despite progress with JE diagnostics and laboratory testing, because of the difficulty diagnosing JE, reported JE case counts are generally considered to be underestimates of true JE incidence. A 2011 estimate of global JE burden, in which high-quality surveillance data from limited areas in Asia were extrapolated to other endemic regions, calculated that 67,900 JE cases occurred annually, of which 75% occurred in children <15 years of age29. Based on the reported case fatality rate of 20–30% and long-term neurological sequelae among 30–50% of survivors, it was estimated that JE caused 13,600–20,400 fatalities each year and long-term neuropsychological sequelae in 14,300–27,200 people. In contrast to the estimate of case numbers, only 4402 JE cases were reported through the WHO-UNICEF Joint Reporting Form on Immunization in 2018, suggesting substantial under-reporting2. In a vaccine impact assessment conducted in Nepal using JE and acute encephalitis syndrome (AES) surveillance data, AES incidence was 59% lower than expected had no JE vaccination campaign occurred, with the absolute reduction in AES cases substantially greater than the reduction in confirmed JE cases, suggesting many AES cases were undiagnosed JE cases and again highlighting the unrecognized burden of JE11.
In addition to having better burden of disease estimates, JE surveillance is critical to inform vaccine introduction, monitor vaccination programs and identify alternative causes of encephalitis, evaluate vaccine effectiveness in different epidemiological settings, and monitor JEV expansion into new areas. WHO first published JE surveillance standards in 2008, and they were updated in 201830,31. Still, there continues to be substantial variability in approach, quality, and extent of surveillance in JE-endemic countries, based on local capacity and resources and the need for integration into pre-existing systems2.
JE has been considered a disease of children in JE-endemic countries; immunity to JE by natural infection is widespread by adulthood and generally believed to be lifelong, at least in the context of natural boosting in endemic areas32. While vaccination has contributed to a relative shift in cases to older age groups in some countries, other countries without vaccination programs have also shown a substantial proportion of cases in adults, raising the question of the need for adult vaccination32,33. JE vaccination programs in Nepal that targeted all age groups showed the rate of AES in individuals ≥15 years after vaccination was 77% lower than expected, suggesting an unrecognized burden of JE in this age group. While strong pediatric vaccination programs are the foundation of a JE vaccination program, some countries, based on the burden of disease and prioritization, may consider vaccinating adults as well.
Mosquito-borne transmission is of greatest concern, although transplacental transmission and transmission through blood transfusion have been documented34,35. As a mosquito-borne disease, JE incidence is highly variable based on temperature, elevation, and rainfall. JEV is transmitted primarily by the mosquito Culex tritaeniorhynchus. There have been limited reports of JEV outside of Asia. In Italy, one pool of Culex pipiens was found to contain JEV as determined by RT-PCR of a small JEV RNA fragment, although no virus isolation was performed. In 2016, a patient with confirmed yellow fever infection in Angola was found to be co-infected with a JEV genotype III virus based on deep sequencing technology36,37. Further data are needed to confirm JEV transmission outside of Asia; however, with the global presence of Culex vectors and amplifying hosts, it is possible JEV could emerge outside of the traditional geographic boundaries due to population movement into nonendemic areas that results in changes in local farming practices and animal husbandry, expanded rice irrigation to meet population demand, and possibly global climate change. Although less likely, the transport of infected mosquito vectors or their infected eggs, the importation of viremic vertebrate hosts, or changes in bird migration patterns due to global climate change has the potential to expand the range of JEV38,39.
Status of JE vaccines and vaccination
There have been ~15 JE vaccine products commercialized in Asia, including several manufactured for use only in the country of manufacture1,40. JE vaccines fall into four categories: (1) live attenuated vaccine (i.e., CD-JEV); (2) live recombinant (chimeric) vaccine (i.e., JE-CV); (3) inactivated Vero cell-derived vaccine; and (4) inactivated mouse brain-derived vaccine. WHO recommends both CD-JEV and JE-CV be administered on a single-dose schedule starting at age 8 months and 9 months, respectively, although the JE-CV vaccine package insert recommends a pediatric booster dose. WHO recommends administering Vero cell JE vaccines per the package insert, which is generally 2–3 doses with a booster dose at variable time intervals by product. CD-JEV holds the greatest global market share with more than 300 million doses to date administered outside of China. WHO recommends countries move away from mouse brain vaccines given the favorable profile of second-generation products, and no mouse brain vaccines are WHO prequalified3.
PATH chose CDIBP’s CD-JEV to target for global use because it could be administered as a single-dose vaccine and marketed at an affordable price in low-income countries in Asia. Data from China supported high vaccine effectiveness with a strong safety profile and PATH led additional clinical trials in other Asian countries in accordance with international standards, as did other manufacturers using CD-JEV as a comparison vaccine41–44. Studies using CD-JEV vaccine generally showed seroprotection (i.e., neutralizing antibody titer ≥1:10) in more than 90% of vaccinees 28 days after primary vaccination, although one study using WHO-prequalified CD-JEV found lower seroprotection rates43–48. Most case-control studies of CD-JEV vaccine demonstrated vaccine effectiveness at around 95% up to five years post-vaccination, although other studies have found lower point estimates49–56. Poor ascertainment of JE cases and accurate vaccination status is a challenge for these studies. A vaccine effectiveness study of the WHO-prequalified CD-JEV vaccine is currently underway. Safety of CD-JEV has been assessed both in clinical trials and through post-licensure passive reporting of adverse events and the vaccine has been found to have a robust safety profile57. Reports of encephalitis post-vaccination with CD-JEV are rare and a causal relationship with vaccination has not been established58,59. The WHO Global Advisory Committee on Vaccine Safety reviewed data on CD-JEV and determined it has a strong safety profile based on available data60.
Countries use different vaccines in country-supported JE immunization programs (implemented either nationally or regionally): 11 countries use CD-JEV (Cambodia, China, India, Indonesia (Bali), Laos, Myanmar, Nepal, Philippines, South Korea, Sri Lanka, Thailand), four use JE-CV vaccine (Australia, Malaysia, Thailand, and China’s Taiwan province), and three use Vero cell vaccines (Japan, South Korea, and China’s Taiwan province). Vietnam continues to use mouse brain vaccines. Brunei and North Korea have used JE-CV and CD-JEV, respectively, but do not have sustained programs, and Singapore has determined routine vaccination is not required based on surveillance data. Bangladesh, Bhutan, Pakistan, Papua New Guinea, Russia, and Timor Leste do not have JE immunization programs, but have recent or past evidence of JEV transmission61. Several countries co-administer CD-JEV vaccine with the measles-containing vaccines, which has been shown convincingly not to cause immune interference46,47,62.
Programmatic challenges and threats to JE vaccination
Despite great advances in JE vaccination and control over the last 15 years, there continue to be challenges that put these gains at risk and may impede further progress. These challenges fall into the categories of (1) maintaining high JE vaccine coverage, (2) maintaining a stable JE vaccine supply, and (3) maintaining surveillance and vaccination data.
Maintaining high JE vaccine coverage
Because of the enzootic cycle of JEV involving birds and mosquitoes, JEV elimination from the environment is impossible. Sustained high vaccination coverage must be maintained to prevent human cases. There is also evidence JEV is moving into new geographic areas, including evidence of occasional urban transmission; consequently target areas where vaccination is needed may shift and grow63–66. Careful diagnoses and surveillance are needed to monitor JE geographic spread as human migration, land use and climate change may affect mosquito distribution in the future. Furthermore, general vaccine hesitancy among parents and healthcare providers is on the rise, including in countries affected by JE67–69. In 2019, WHO named vaccine hesitancy as one of the top 10 threats to global health70. Confidence in vaccination programs is essential to maintain high JE vaccine coverage.
Maintaining a stable JE vaccine supply
CDIBP’s CD-JEV is the most widely used JE vaccine and several low- and middle-income countries rely on it. This reliance on a single product from one site operated by a lone manufacturer presents numerous threats to vaccine supply. Catastrophic events, such as infection among the pathogen-free colony of hamsters that are a source of cells for vaccine virus growth, natural disasters (e.g., earthquakes), or human disease outbreaks (e.g., pandemic influenza) could threaten the global vaccine supply. A vaccine supply continuity plan to help ensure CD-JEV global supply and availability is needed, using methods such as off-site bulk storage and identification of alternative sources of manufacturing materials.
There are issues of supply and demand due to the need for manufacturers to grow virus for either live or inactivated vaccines. Thus, countries must assess their need for vaccines many months in advance of usage and are unable to acquire WHO-prequalified JE vaccines in a rapid ad hoc fashion, which makes ordering vaccines as a response to a JE outbreak unfeasible. None of the three WHO-prequalified vaccine producers have stockpiles of finished products with which to respond to requests during outbreaks. For example, the time required to produce and release a batch of CD-JEV can be up to nine months; as a result, countries must order at least nine months in advance. Another challenge with CD-JEV is the short shelf life of 24 months (compared to 36 months for the other two WHO-prequalified products). This means CD-JEV must be used expeditiously once it arrives in countries.
WHO currently recommends that CD-JEV be given as a single-dose vaccine without need for a booster dose. India and China, large consumers of CD-JEV, administer the vaccine as a two-dose primary series for programmatic reasons, which has an impact on vaccine supply. Because CDIBP has established a public sector price for specific JE-endemic countries, CD-JEV is currently the most viable vaccine product for many low- and lower middle-income countries. The cost and/or multiple-dosing regimens of other JE vaccine products have made them less desirable in many settings. Nonetheless, diversity is needed in the global supply of JE vaccine.
Poor surveillance and vaccination data
Other important challenges exist with respect to disease surveillance and vaccine monitoring. Poor surveillance for JE disease has made the burden difficult to quantify in many countries, hindering decisions about vaccine introduction. When JE vaccination has been introduced, poor surveillance has limited availability of data to demonstrate impact and support advocacy efforts to maintain vaccine programs. Lack of immunization registries makes determination of vaccination status difficult over the longer term; monitoring vaccine failures and determining vaccination status in vaccine effectiveness studies are both critical for studying the duration of vaccine protection and the assessment of the eventual need of a booster dose. Challenges with the laboratory component of surveillance include suboptimal coordination between laboratory and epidemiology staff in some countries, and general challenges such as sample quality on arrival in laboratories, and difficulties with sample transport internationally for testing at reference laboratories. The risk of an insufficient supply of commercial JE IgM kits because of the limited market is a large threat to routine JE diagnostics. There remains no ready alternative if the only company currently producing a commercial IgM ELISA discontinues production. Critically, any reduction in support to countries from the regional JE laboratory networks could threaten the gains made in capacity for, and quality of, JE diagnosis.
Vaccine safety concerns, especially around severe adverse events such as encephalitis, can trigger vaccine hesitancy. This was seen in India when a cluster of suspected encephalitis cases were reported shortly after a JE vaccination campaign in 2014, though on review the cases were determined to be unrelated to vaccination71. It is important to have robust investigations of adverse events following immunization (AEFI) without creating unsupported concerns. Investigation of post-vaccination encephalitis has not been systematic and requires good epidemiologic and laboratory resources to investigate properly. Enhanced AEFI surveillance following the initiation of a JE vaccine campaign is important. Working with stakeholders to maximize communication efforts while at the same time minimizing misinformation in the community is critical.
Decisions about vaccine introduction in resource-limited settings frequently require prioritization of multiple public health interventions for diseases that affect populations. Without good JE burden data, it has been difficult to contextualize the importance of JE vaccination relative to other high-priority vaccines competing for government resources, such as rotavirus and human papillomavirus vaccines. Continued advocacy is necessary for keeping JE on the list of priority vaccines for countries to consider.
Research gaps
Vaccine immunogenicity and need for booster doses
Much of the earlier research on CD-JEV effectiveness and immunogenicity was done using vaccines produced in an older CDIBP facility that did not meet good manufacturing practice (GMP) standards. To license and prequalify CD-JEV made in the newer, GMP-compliant facility, a non-inferiority study compared three lots of “new facility” vaccine with one lot of vaccine from the older facility48. Seroprotection rates 28 days after vaccination for recipients of the new facility vaccine were 80.2–84.5% compared to 86.3% among recipients of the vaccine made in the older facility. Although this was not statistically different, seroprotection rates were noted to be higher (i.e., 90.5 to 92.1%) in earlier clinical studies of CD-JEV47,72. Because plaque reduction neutralization tests in these studies were performed at different times, comparing results is difficult. Still, while the findings of the lot-to-lot consistency trial supported the use of “newer facility” CD-JEV, continued monitoring is required on the vaccine’s duration of protection, which potentially could have implications for booster doses.
With reasonable immunogenicity of CD-JEV over a sustained period of time and an absence of reported vaccine failures with programmatic use, WHO does not recommend JE vaccine booster doses unless future evidence warrants it40. Data on vaccine effectiveness studies and vaccine failure monitoring are important to inform the need for booster doses for all WHO-prequalified JE vaccines. Studies are in progress (e.g., a case-control study in Bali, Indonesia) that will provide longer-term vaccine effectiveness data (e.g., up to 5 years) and inform the duration of protection of a single dose of CD-JEV. Studies of antibody persistence alone are insufficient as undetectable neutralizing antibody might not indicate the absence of protective immunity. For example, data from a trial of JE-CV indicated that at two years post-primary vaccination some children who did not seroconvert with a primary JE-CV dose exhibited an anamnestic response following a receipt of a booster dose40. Similar findings were seen in a study of the other live JE vaccine, CD-JEV. Children who did not seroconvert initially or no longer had seroprotective antibody four years after immunization were given a booster dose of CD-JEV that resulted in a strong anamnestic response73. Anamnestic responses following revaccination with live JE vaccines suggest that despite undetectable antibodies, vaccines might be protected from clinical disease. Should booster doses of CD-JEV ultimately be recommended, this would have additional implications for vaccine supply and programmatic implementation. The anamnestic response has not been studied for inactivated JE vaccines.
Furthermore, data on safety and immunogenicity following vaccination of special populations, such as pregnant women and immunocompromised persons, are missing for both live vaccines, CD-JEV and JE-CV. Research priorities across vaccine platforms are available in WHO’s position paper on JE vaccines3.
Emerging genotypes
All licensed vaccine viruses are genotype III JEV. While studies suggest these vaccines provide good protection against multiple genotypes, there are questions pertaining to future vaccine effectiveness if a new JEV genotype emerges74,75. Two genotype V JEV isolates have been identified, one from a human and one from a mosquito, and genotype V sequences from Culex mosquitoes also have been recently identified76. Additional evidence is required to determine if this genotype is emerging. If so, the question has arisen if currently available vaccines might be less effective, based on neutralizing antibody data and the 9% amino acid difference between genotype V and genotype III viruses. This situation should be monitored.
Disease sequelae and treatment options
Although the expert meeting focused on JE vaccination, there are other research gaps related to JE and treatment. While disability following encephalitis has long been recognized, the extent of sequelae following mild, non-neurologic JE illness is poorly understood. Such data could provide a better understanding of the full burden of JE morbidity and a more comprehensive understanding of the benefits of vaccination. Better information on the comparative severity of JE in adults and children would also be useful. Finally, as there is no specific treatment for JE currently available, investigation of possible JE treatments is another important avenue for further research. Limited clinical trials to date have not yielded any successful compounds, although promising targets exist77. There is a potential role of inflammation as a contributor to poor clinical outcomes from JEV infections, thus anti-inflammatory agents in combination with antiviral agents might be effective; this deserves further study77.
Recommendations to optimize JE control
The challenges defined above highlight that despite major achievements in JE control, efforts are required to continue and sustain progress. Experts at the meeting identified key recommendations in the following categories: (1) vaccine supply, (2) global coordination and policy optimization, (3) JE surveillance and diagnostics, (4) country programmatic support, and (5) research priorities.
Reliable JE vaccine supply
An adequate supply of JE vaccine is essential to JE control and is currently at risk as discussed above. A reliable JE vaccine supply requires vaccination schedules be optimized, countries improve lead-time for ordering JE vaccine, and vaccine manufacturers develop contingency plans for events such as natural disasters or other disruptions of vaccine supply. JE vaccine manufacturers with pre-qualified products currently in limited use globally are encouraged to make their products available and affordable to affected countries. At a minimum, country licensure of more than one product will allow product switching if the need arises.
Global coordination and policy optimization
A coalition of key partners should be established that is responsible for exchange of information, best practices, and coordinated actions among JE control programs. This coalition would serve as a technical resource for countries and should maintain a web-based library of materials to support vaccine introduction and monitoring. As national and international partners have highlighted the utility of biannual WHO JE biregional (i.e., South-East Asia and Western Pacific regions) meetings to share experiences and lessons learned, these meetings should continue under the coordination of the coalition. Biregional meetings are important for information exchange and discussion of new or emerging issues. Advocacy for JE vaccine introduction is still needed to ensure that the momentum achieved through the efforts of PATH, WHO and its regional offices, and other partners, WHO prequalification of JE vaccines, and Gavi support, is not lost. As additional data become available (see Research Priorities), re-evaluation of global JE vaccine policies (e.g., the need for booster doses) might be warranted.
JE surveillance, vaccine utilization, and diagnostics
The need for strong surveillance and diagnostics does not end when a vaccine is introduced in the country. Many JE-endemic countries will still need support in the areas of JE surveillance, diagnostics, and vaccination program monitoring after JE vaccine has been introduced. JE surveillance support will help decision-making for countries planning to introduce JE vaccine to understand disease burden and target vaccination geographically and by age. In countries with JE vaccination, JE surveillance and immunization registries are important to help identify program deficiencies, assess long-term vaccine protection, and monitor the geographic spread of JE. JE vaccination program monitoring should be improved through vaccine coverage surveys and better completion of the WHO/UNICEF Joint Reporting Form on Immunization.
Other country programmatic support
WHO Regional offices need to be supported as they directly assist countries with these issues—surveillance and vaccine introduction, quality assurance for laboratory testing, and troubleshooting vaccine supply issues. To maximize efficiencies across programs and ensure stable funding, efforts in surveillance, diagnostics, and vaccine coverage monitoring should be combined for JE and other vaccine-preventable diseases; this will also help integrate JE into routine immunization activities. Community advocacy and communication must be supported for maximal vaccine acceptance, especially during vaccination campaigns. To help address vaccine safety concerns and vaccine hesitancy, all reported serious AEFIs should be investigated promptly and properly, but with the care that the investigations do not create inappropriate concern that they are caused by vaccination if other etiologies are more likely. Readily available resources to guide AEFI investigations, particularly of encephalitis post-vaccination, would be helpful.
High-priority research needs
The potential need for a JE vaccine booster dose to achieve life-long protection against JE. Longer-term vaccine effectiveness studies and documentation of vaccine failures through careful JE surveillance will be the most convincing data to indicate if booster doses are required for CD-JEV or JE-CV and the frequency of boosting for inactivated JE vaccines.
Vaccine safety in special populations. To address research gaps on vaccine safety in immunocompromised persons and pregnant women who are knowingly or unknowingly vaccinated with CD-JEV and JE-CV, vaccine registries for these populations are needed.
Laboratory and diagnostic issues. Additional diagnostic tests are urgently needed. Market shaping for JE ELISA kits will likely be needed through Gavi and other agencies, as other manufacturers have not shown interest in the development of similar kits due to the limited market. The use of new technologies and platforms for diagnostics could hold promise in the future but would require investments. Standardized and validated plaque reduction neutralization test reagents and procedures may allow comparison of immunogenicity results across laboratories.
JE treatment. To help facilitate JE treatment research, expert consultation is needed with the aim of identifying the next drugs that should be tested in humans as well as to discuss the design of therapeutic trials, including types of studies, suitable locations, and outcomes to be measured.
JE vaccine impact. To document the importance of JE vaccination and the need for sustained efforts, it will be important to conduct a retrospective impact study of the number of JE cases, disabilities, and deaths averted and to estimate the total cost savings from JE vaccination. Mathematical modeling of expected JE cases in the absence of vaccination could be a potential approach to overcome the deficiencies in JE surveillance. Such studies could consolidate country support for existing JE immunization programs and place JE in proper context alongside other vaccine-preventable diseases to support decision-making for JE vaccine introduction.
Safety and immunogenicity of JE vaccine co-administration with new vaccines. Ongoing safety and immunogenicity studies of co-administration of JE vaccines with newly introduced vaccines (e.g., typhoid conjugate vaccine) should be conducted when there is a programmatic desire to co-administer vaccines.
Conclusions
JE control in Asia through immunization programs is a public health success. Together with affected country drive to develop new vaccines and control JE, international investments into an Asian domestic vaccine manufacturer enabled an affordable product and expanded access to this life-saving vaccine. However, more work is needed to secure the gains made and accelerate control of JE by expanding the use of JE vaccines in JE-endemic areas that do have immunization programs or do not have adequate programs.
Acknowledgements
This meeting was supported by Bill & Melinda Gates Foundation grants to US Centers for Disease Control and Prevention and PATH. In addition, the authors are grateful to the Bill and Melinda Gates Foundation for hosting the expert consultation. The findings and conclusions in this report are those of the authors and do not represent the official position of the Centers for Disease Control and Prevention or the World Health Organization.
Author contributions
All authors contributed to the analysis and recommendations outlined in the manuscript. K.V., S.H., and A.M. conceived the outline of the manuscript. K.V. wrote the first draft of the manuscript. All authors critically revised and approved the final version of the manuscript.
Data availability
No datasets were generated or analyzed during the current study.
Competing interests
No conflicts of interest: G.W.L., J.M.H., J.H., S.H., L.M.S., K.V., A.A.M. T.S. is supported by the National Institute for Health Research (NIHR) Health Protection Research Unit in Emerging and Zoonotic Infections (Grant No. IS-HPU-1112-10117), NIHR Global Health Research Group on Brain Infections (No. 17/63/110), and the European Union’s Horizon 2020 research and innovation program ZikaPLAN (Preparedness Latin America Network), grant agreement No. 734584. A.D.T.B. is Editor in Chief of npj Vaccines.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A list of authors and their affiliations appears at the end of the paper.
Contributor Information
Anthony A. Marfin, Email: aamarfin@path.org
the Japanese encephalitis vaccination experts panel:
Katie Anderson, Marc Fischer, Kim Fox, Julie Jacobson, Jayantha Liyanage, Florian Marks, Ike Ogbuanu, and Piyanit Tharmaphornpilas
References
- 1.Halstead, S. B., Hills, S. L. & Dubischar, K. in Plotkin’s Vaccines (Seventh Edition) (Eds. Plotkin, S. A. et al.) 511–548.e12 (Elsevier, 2018).
- 2.Heffelfinger JD, et al. Japanese encephalitis surveillance and immunization — Asia and Western Pacific Regions, 2016. Mmwr. Morbidity Mortal. Wkly. Rep. 2017;66:579–583. doi: 10.15585/mmwr.mm6622a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Weekly epidemiological record Relevé épidémiologique hebdomadaire. WHO Weekly epidemiological record90, 69–87 (2015).
- 4.Schneider RJ, Firestone MH, Edelman R, Chieowanich P, Pornpibul R. Clinical sequelae after japanese encephalitis: a one year follow-up study in Thailand. Southeast Asian J. Trop. Med Public Health. 1974;5:560–568. [PubMed] [Google Scholar]
- 5.Kamala CS, Rao MV, George S, Prasanna NY. Japanese encephalitis in children in Bellary Karnataka. Indian Pediatr. 1989;26:445–452. [PubMed] [Google Scholar]
- 6.Solomon T, Vaughn DW. Pathogenesis and clinical features of Japanese encephalitis and West Nile virus infections. Curr. Top. Microbiol. Immunol. 2002;267:171–194. doi: 10.1007/978-3-642-59403-8_9. [DOI] [PubMed] [Google Scholar]
- 7.Huy BV, Tu HC, Luan TV, Lindqvist R. Early mental and neurological sequelae after Japanese B encephalitis. Southeast Asian J. Tropical Med. Public Health. 1994;25:549–553. [PubMed] [Google Scholar]
- 8.Pieper SJL, Kurland LT. Sequelae of Japanese B and mumps encephalitis. Am. J. Tropical Med. Hyg. 1958;7:481–490. doi: 10.4269/ajtmh.1958.7.481. [DOI] [PubMed] [Google Scholar]
- 9.Japanese encephalitis vaccines: WHO Position Paper. WHO Wkly. Epidemiol. Rec. 325–40 (2006).
- 10.Solomon T. Control of Japanese encephalitis — Within Our Grasp? N. Engl. J. Med. 2006;355:869–871. doi: 10.1056/NEJMp058263. [DOI] [PubMed] [Google Scholar]
- 11.Upreti Shyam Raj, et al. Updated estimation of the impact of a Japanese encephalitis immunization program with live, attenuated SA 14-14-2 vaccine in Nepal. PLOS Neglected Tropical Dis. 2017;11:1–9. doi: 10.1371/journal.pntd.0005866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arai S, et al. Japanese encephalitis: surveillance and elimination effort in Japan from 1982 to 2004. Jpn. J. Infect. Dis. 2008;61:333–338. [PubMed] [Google Scholar]
- 13.Chen P, et al. Observation of epidemiological effect of Japanese encephalitis (JE) live vaccine strain SA 14-14-2. Chin. J. Biologicals. 1992;3:135–137. [Google Scholar]
- 14.Hombach J, Solomon T, Kurane I, Jacobson J, Wood D. Report on a WHO consultation on immunological endpoints for evaluation of new Japanese encephalitis vaccines, WHO, Geneva, 2–3 September, 2004. Vaccine. 2005;23:5205–5211. doi: 10.1016/j.vaccine.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Hoke CH, et al. Protection against Japanese encephalitis by inactivated vaccines. N. Engl. J. Med. 1988;319:608–614. doi: 10.1056/NEJM198809083191004. [DOI] [PubMed] [Google Scholar]
- 16.Prequalification of medicines by WHO. World Health Organizationhttps://www.who.int/news-room/fact-sheets/detail/prequalification-of-medicines-by-who (2013).
- 17.PATH. Navigating vaccine introduction: a guide for decision-makers—Japanese encephalitis. https://www.path.org/resources/navigating-vaccine-introduction-a-guide-for-decision-makersjapanese-encephalitis/ (2017).
- 18.World Health Organization & PATH. JE vaccines, health worker training materials. https://www.who.int/immunization/diseases/japanese_encephalitis/training-package/en/ (2016).
- 19.World Health Organization. Measuring effectiveness and impact of Japanese encephalitis vaccination. https://apps.who.int/iris/bitstream/handle/10665/255651/WHO-IVB-16.03-eng.pdf (2016).
- 20.Regional Framework for Implementation of the Global Vaccine Action Plan in the Western Pacific. https://iris.wpro.who.int/bitstream/handle/10665.1/10921/9789290617099_eng.pdf (2015).
- 21.Ooi MH, Wong SC, Lewthwaite P, Cardosa MJ, Solomon T. Clinical features, diagnosis, and management of enterovirus 71. Lancet Neurol. 2010;9:1097–105. doi: 10.1016/S1474-4422(10)70209-X. [DOI] [PubMed] [Google Scholar]
- 22.Solomon T, et al. Rapid diagnosis of Japanese encephalitis by using an immunoglobulin M dot enzyme immunoassay. J. Clin. Microbiol. 1998;36:2030. doi: 10.1128/JCM.36.7.2030-2034.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cuzzubbo AJ, et al. Comparison of PanBio dengue Duo Igm and IgG capture ELISA and venture technologies dengue IgM and IgG dot blot. J. Clin. Virol. 2000;16:135–144. doi: 10.1016/S1386-6532(99)00076-1. [DOI] [PubMed] [Google Scholar]
- 24.Lewthwaite P, et al. Evaluation of two commercially available ELISAs for the diagnosis of Japanese encephalitis applied to field samples. Tropical Med. Int. Health. 2010;15:811–818. doi: 10.1111/j.1365-3156.2010.02537.x. [DOI] [PubMed] [Google Scholar]
- 25.Khalakdina A, et al. Field evaluation of commercial Immunoglobulin M antibody capture ELISA diagnostic tests for the detection of Japanese encephalitis virus infection among encephalitis patients in Nepal. Int. J. Infect. Dis. 2010;14:e79–e84. doi: 10.1016/j.ijid.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 26.Johnson BW, Goodman CH, Jee Y, Featherstone DA. Differential diagnosis of japanese encephalitis virus infections with the inbios JE detect TM and DEN detect TM MAC-ELISA kits. Am. J. Tropical Med. Hyg. 2016;94:820–828. doi: 10.4269/ajtmh.15-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hills, S. et al. Persistence of IgM antibodies after vaccination with live attenuated Japanese encephalitis vaccine. Am. J. Tropical Med. Hygiene tpmd201132 10.4269/ajtmh.20-1132 (2020). [DOI] [PMC free article] [PubMed]
- 28.Mulders MN, et al. Expansion of surveillance for vaccine-preventable diseases: building on the global polio laboratory network and the global measles and rubella laboratory network platforms. J. Infect. Dis. 2017;216:S324–S330. doi: 10.1093/infdis/jix077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campbell G, et al. Estimated global incidence of Japanese encephalitis: a systematic review. Bull. World Health Organization. 2011;89:766–774. doi: 10.2471/BLT.10.085233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hills S, et al. Evidence and rationale for the World Health Organization recommended standards for Japanese encephalitis surveillance. BMC Infect. Dis. 2009;9:214. doi: 10.1186/1471-2334-9-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization. Surveillance Standards for Vaccine-Preventable Diseases, Second Edition. https://apps.who.int/iris/bitstream/handle/10665/275754/9789241513920-eng.pdf (2018).
- 32.Yoocharoan P, A-neugoolpipat A, Anantapreecha S, Tharmaphornpilas P. Seroprevalence of immunity against Japanese encephalitis in Thai population. Dis. Control J. 2009;35:276–284. [Google Scholar]
- 33.Montgomery S, et al. Hospital-based surveillance for Japanese encephalitis at four sites in Bangladesh, 2003–2005. Am. J. Tropical Med. Hyg. 2010;82:344–349. doi: 10.4269/ajtmh.2010.09-0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chaturvedi UC, et al. Transplacental infection with Japanese encephalitis virus. J. Infect. Dis. 1980;141:712–715. doi: 10.1093/infdis/141.6.712. [DOI] [PubMed] [Google Scholar]
- 35.Cheng VCC, et al. Japanese encephalitis virus transmitted via blood transfusion, Hong Kong, China. Emerg. Infect. Dis. J. 2018;24:49. doi: 10.3201/eid2401.171297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ravanini P, et al. Japanese encephalitis virus RNA detected in Culex pipiens mosquitoes in Italy. Eurosurveillance. 2012;17:1–4. doi: 10.2807/ese.17.28.20221-en. [DOI] [PubMed] [Google Scholar]
- 37.Simon-Loriere E, et al. Autochthonous Japanese encephalitis with yellow fever coinfection in Africa. N. Engl. J. Med. 2017;376:1483–1485. doi: 10.1056/NEJMc1701600. [DOI] [PubMed] [Google Scholar]
- 38.Oliveira ARS, et al. Perspectives regarding the risk of introduction of the Japanese encephalitis virus (JEV) in the United States. Front. Vet. Sci. 2020;7:1–8. doi: 10.3389/fvets.2020.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nett RJ, Campbell GL, Reisen WK. Potential for the emergence of Japanese encephalitis virus in California. Vector-Borne Zoonotic Dis. 2009;9:511–517. doi: 10.1089/vbz.2008.0052. [DOI] [PubMed] [Google Scholar]
- 40.SAGE Working Group on Japanese encephalitis vaccines. Background Paper on Japanese Encephalitis Vaccines. https://www.who.int/immunization/sage/meetings/2014/october/1_JE_Vaccine_Background_Paper.pdf (2014).
- 41.Zhou B, Jia L, Xu X. A large-scale study on the safety and epidemiological efficacy of Japanese encephalitis (JE) live vaccine (SA14-14-2) in the JE endemic areas. Chin Journal of Epidem. 1999;20:38–41. [PubMed] [Google Scholar]
- 42.Zhou B, Zhang M, Chen P, Jiang X, Zhang L. An 11 Year Follow-up of Epidemiological Effect of Japanese Encephalitis Vaccine, Live (SA 14-14-2) Chin. J. Biologicals. 2001;14:183–185. [Google Scholar]
- 43.Kim DS, et al. A randomized study of the immunogenicity and safety of Japanese encephalitis chimeric virus vaccine (JE-CV) in comparison with SA14-14-2 vaccine in children in the Republic of Korea. Hum. Vaccines Immunother. 2014;10:2656–2663. doi: 10.4161/hv.29743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feroldi E, et al. Primary immunization of infants and toddlers in Thailand with Japanese encephalitis chimeric virus vaccine in comparison with SA14-14-2: a randomized study of immunogenicity and safety. Pediatr. Infect. Dis. J. 2014;33:643–649. doi: 10.1097/INF.0000000000000276. [DOI] [PubMed] [Google Scholar]
- 45.Tsai TF, et al. Immunogenicity of live attenuated SA14-14-2 Japanese encephalitis vaccine—A comparison of 1- and 3-month immunization schedules. J. Infect. Dis. 1998;177:221–223. doi: 10.1086/517358. [DOI] [PubMed] [Google Scholar]
- 46.Victor JC, et al. Corrigendum to “Comparison of the immunogenicity and safety of measles vaccine administered alone or with live, attenuated Japanese encephalitis SA 14-14-2 vaccine in Philippine infants” [Vaccine 26 (2008) 2234–2241] Vaccine. 2014;32:306–308. doi: 10.1016/j.vaccine.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 47.Wijesinghe PR, et al. Safety and immunogenicity of live-attenuated Japanese encephalitis SA 14-14-2 vaccine co-administered with measles vaccine in 9-month-old infants in Sri Lanka. Vaccine. 2014;32:4751–4757. doi: 10.1016/j.vaccine.2014.06.036. [DOI] [PubMed] [Google Scholar]
- 48.Zaman K, et al. Lot-to-lot consistency of live attenuated SA 14-14-2 Japanese encephalitis vaccine manufactured in a good manufacturing practice facility and non-inferiority with respect to an earlier product. Vaccine. 2014;32:6061–6066. doi: 10.1016/j.vaccine.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 49.Bista MB, et al. Efficacy of single-dose SA 14-14-2 vaccine against Japanese encephalitis: a case control study. Lancet. 2001;358:791–5. doi: 10.1016/S0140-6736(01)05967-0. [DOI] [PubMed] [Google Scholar]
- 50.Ohrr H, et al. Effect of single dose of SA 14-14-2 vaccine 1 year after immunisation in Nepalese children with Japanese encephalitis: a case-control study. Lancet. 2005;366:1375–8. doi: 10.1016/S0140-6736(05)67567-8. [DOI] [PubMed] [Google Scholar]
- 51.Tandan JB, et al. Single dose of SA 14-14-2 vaccine provides long-term protection against Japanese encephalitis: a case–control study in Nepalese children 5 years after immunization. Vaccine. 2007;25:5041–5045. doi: 10.1016/j.vaccine.2007.04.052. [DOI] [PubMed] [Google Scholar]
- 52.Kumar R, Tripathi P, Rizvi A. Effectiveness of one dose of SA 14-14-2 vaccine against Japanese encephalitis. N. Engl. J. Med. 2009;360:1465–1466. doi: 10.1056/NEJMc0808664. [DOI] [PubMed] [Google Scholar]
- 53.Hennessy S, et al. Effectiveness of live-attenuated Japanese encephalitis vaccine (SA14-14-2): a case-control study. Lancet. 1996;347:1583–1586. doi: 10.1016/S0140-6736(96)91075-2. [DOI] [PubMed] [Google Scholar]
- 54.Murhekar MV, et al. Low coverage and acceptable effectiveness of single dose of Japanese encephalitis vaccine, Gorakhpur division, Uttar Pradesh, India, 2013. J. Infect. 2014;69:517–20. doi: 10.1016/j.jinf.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 55.Gurav YK, et al. A large outbreak of Japanese encephalitis predominantly among adults in northern region of West Bengal, India. J. Med. Virol. 2016;88:2004–2011. doi: 10.1002/jmv.24556. [DOI] [PubMed] [Google Scholar]
- 56.Tandale BV, et al. Effectiveness of Japanese encephalitis SA 14-14-2 live attenuated vaccine among Indian children: retrospective 1:4 matched case-control study. J. Infect. Public Health. 2018;11:713–719. doi: 10.1016/j.jiph.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 57.Ginsburg AS, Meghani A, Halstead SB, Yaich M. Use of the live attenuated Japanese encephalitis vaccine SA 14–14–2 in children: a review of safety and tolerability studies. Hum. Vaccines Immunother. 2017;13:2222–2231. doi: 10.1080/21645515.2017.1356496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jia N, et al. Encephalitis temporally associated with live attenuated Japanese encephalitis vaccine: four case reports. BMC Infect. Dis. 2011;11:344. doi: 10.1186/1471-2334-11-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu Y, et al. Safety of Japanese encephalitis live attenuated vaccination in post-marketing surveillance in Guangdong, China, 2005–2012. Vaccine. 2014;32:1768–1773. doi: 10.1016/j.vaccine.2013.11.107. [DOI] [PubMed] [Google Scholar]
- 60.WHO | Global Advisory Committee on Vaccine Safety, report of meeting held on 11-12 December 2013. WHO Weekly Epidemiological Record on 14 February 2014 (2014).
- 61.World Health Organization, R. O. for S.-E. A. Prevention and Control of Japanese encephalitis (JE). (World Health Organization, 2015).
- 62.Li Y, et al. Immunogenicity and safety of measles-rubella vaccine co-administered with attenuated Japanese encephalitis SA 14-14-2 vaccine in infants aged 8 months in China: a non-inferiority randomised controlled trial. Lancet Infect. Dis. 2019;19:402–409. doi: 10.1016/S1473-3099(18)30650-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li Y-X, et al. Japanese encephalitis, Tibet, China. Emerg. Infect. Dis. J. 2011;17:934. doi: 10.3201/eid1705.101417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bhattachan A, Amatya S, Sedai TR, Upreti SR, Partridge J. Japanese encephalitis in Hill and Mountain Districts. Nepal. Emerg. Infect. Dis. J. 2009;15:1691. doi: 10.3201/eid1510.081641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Partridge J, Ghimire P, Sedai T, Bista MB, Banerjee M. Endemic Japanese encephalitis in the Kathmandu Valley, Nepal. Am. J. Trop. Med. Hyg. 2007;77:1146–1149. doi: 10.4269/ajtmh.2007.77.1146. [DOI] [PubMed] [Google Scholar]
- 66.Kumari R, et al. First indigenous transmission of Japanese encephalitis in urban areas of National Capital Territory of Delhi, India. Tropical Med. Int. Health. 2013;18:743–749. doi: 10.1111/tmi.12104. [DOI] [PubMed] [Google Scholar]
- 67.Larson HJ, Jarrett C, Eckersberger E, Smith DMD, Paterson P. Understanding vaccine hesitancy around vaccines and vaccination from a global perspective: A systematic review of published literature, 2007–2012. Vaccine. 2014;32:2150–2159. doi: 10.1016/j.vaccine.2014.01.081. [DOI] [PubMed] [Google Scholar]
- 68.Larson HJ, Hartigan-Go K, de Figueiredo A. Vaccine confidence plummets in the Philippines following dengue vaccine scare: why it matters to pandemic preparedness. Hum. Vaccines Immunother. 2019;15:625–627. doi: 10.1080/21645515.2018.1522468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Larson HJ, Wilson R, Hanley S, Parys A, Paterson P. Tracking the global spread of vaccine sentiments: The global response to Japan’s suspension of its HPV vaccine recommendation. Hum. Vaccines Immunother. 2014;10:2543–2550. doi: 10.4161/21645515.2014.969618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ten threats to global health in 2019. World Health Organizationhttps://www.who.int/news-room/feature-stories/ten-threats-to-global-health-in-2019.
- 71.Singh AK, Joshi J, Shewale A, Gupta N. Lesson learned from investigating cluster adverse event following immunization in mass campaign of Japanese encephalitis vaccine in India. Int. J. Infect. Dis. 2016;45:425. doi: 10.1016/j.ijid.2016.02.904. [DOI] [Google Scholar]
- 72.Gatchalian S, et al. Comparison of the immunogenicity and safety of measles vaccine administered alone or with live, attenuated Japanese encephalitis SA 14-14-2 vaccine in Philippine infants. Vaccine. 2008;26:2234–2241. doi: 10.1016/j.vaccine.2008.02.042. [DOI] [PubMed] [Google Scholar]
- 73.Marfin, A. A. Antibody Persistence and the Immunologic Response to Booster Vaccination with Live Attenuated SA 14-14-2 Japanese Encephalitis Vaccine. in 68th Annual Meeting, American Society of Tropical Medicine and Hygiene (2019).
- 74.Erra EO, et al. Cross-protective capacity of Japanese encephalitis (JE) vaccines against circulating heterologous JE virus genotypes. Clin. Infect. Dis. 2013;56:267–270. doi: 10.1093/cid/cis883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Trent DW, Minor P, Jivapaisarnpong T, Shin J. WHO working group on the quality, safety and efficacy of Japanese encephalitis vaccines (live attenuated) for human use, Bangkok, Thailand, 21–23 February 2012. Biologicals. 2013;41:450–457. doi: 10.1016/j.biologicals.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 76.Li M-H, et al. Genotype V Japanese encephalitis virus is emerging. PLoS Neglected Tropical Dis. 2011;5:e1231. doi: 10.1371/journal.pntd.0001231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Turtle L, Solomon T. Japanese encephalitis — the prospects for new treatments. Nat. Rev. Neurol. 2018;14:298–313. doi: 10.1038/nrneurol.2018.30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analyzed during the current study.